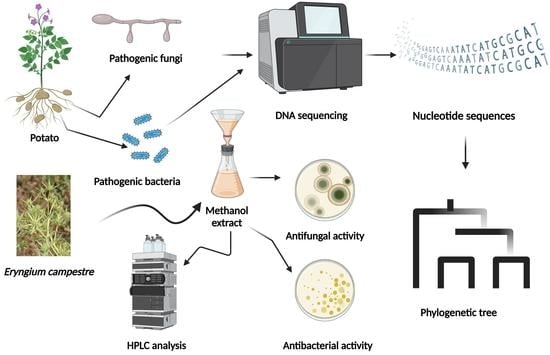
Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium campestre
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals
2.3. Plant Material and Extractions Setting Up
2.4. Phytopathogenic Fungi
2.4.1. Isolation Trails
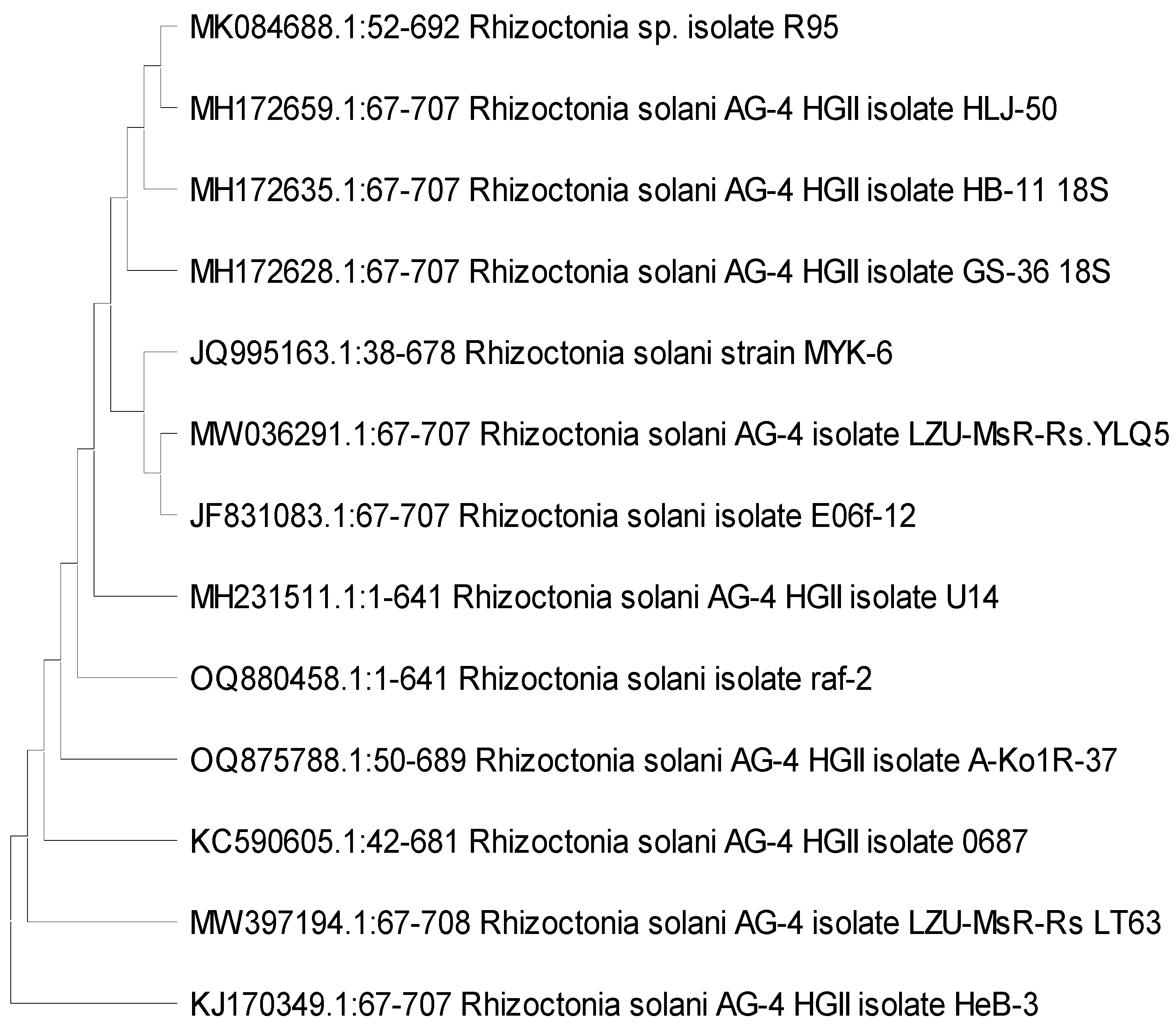
2.4.2. DNA Isolation, PCR Amplification, Sequence Analysis, and Phylogenetic Tree
2.4.3. Antifungal Activity of the Extract
2.5. Phytopathogenic Bacteria
2.5.1. Isolation Methods
2.5.2. DNA Extraction, Performed PCR, Sequence Analysis, and Phylogenetic Tree
2.5.3. Antibacterial Activity of the Extract
2.6. HPLC Conditions
- HPLC column: C18 column (150 mm × 4.6 mm, 5 μm particle size)
- Mobile phase composition:
- Solvent A: Water with 0.1% formic acid
- Solvent B: Acetonitrile
- Gradient elution:
- Initial conditions: 95% A/5% B
- Time (min)/% A/% B:
- 0/95/5
- 10/85/15
- 15/75/25
- 20/50/50
- 25/40/60
- 30/20/80
- 35/5/95
- 40/5/95 (hold for 5 min)
- 45/95/5 (re-equilibration, hold for 5 min)
- Flow rate: 1.0 mL/min
- Detection wavelength: UV multi-wave detector
- Injection volume: 10 μL
- Column temperature: 25 °C
- Retention time calibration: Analyze a standard mixture of phenolic compounds including (pyrogallol, quinol, gallic acid, catechol, p-hydroxy benzoic acid, catechin, chlorogenic acid, vanillic acid, caffeic acid, syringic acid, p-coumaric acid, benzoic acid, ferulic acid, rutin, ellagic acid, o-coumaric acid, resveratrol, cinnamic acid, quercetin, rosmarinic acid, naringenin, and myricetin) to determine the retention times of the target compounds.
2.7. Statistical Analysis
3. Results
3.1. Identification of the Fungal Isolates
3.2. Antifungal Properties
3.3. Bacterial Strain Identification
3.4. Antibacterial Properties
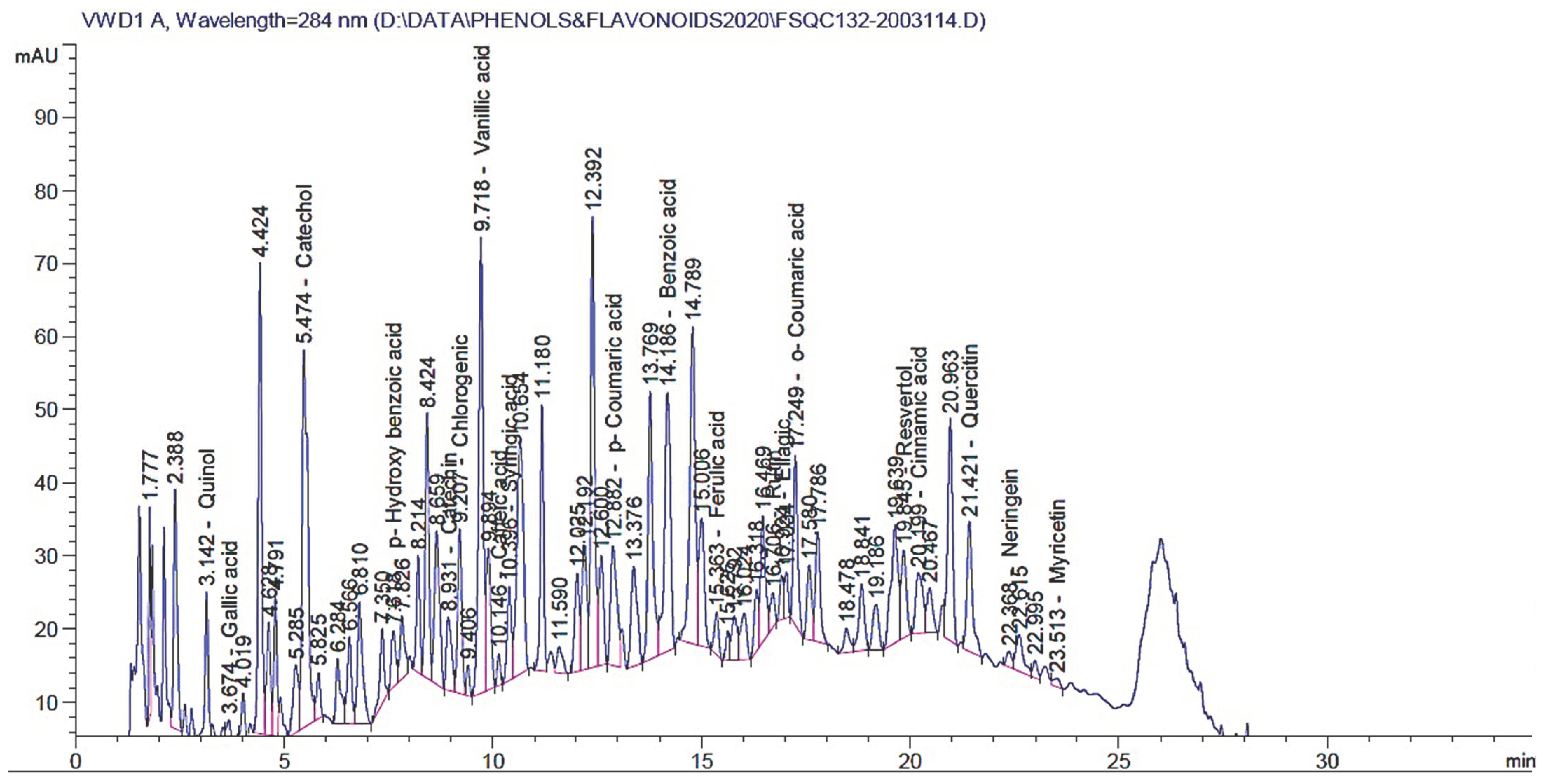
3.5. HPLC of the Yielded Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stokstad, E. The new potato. Science 2019, 363, 574–577. [Google Scholar] [CrossRef]
- Rabia, A.H.; Mohamed, A.; Abdelaty, E.F.; Shahin, S.F.; Yacout, D.M.M. Investigating Adaptation Strategies Developed by Potato Farmers to Cope with Climate Change Impacts in Egypt. Alex. Sci. Exch. J. 2021, 42, 871–881. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Muturi, P.; Yu, J.; Maina, A.N.; Kariuki, S.; Mwaura, F.B.; Wei, H. Bacteriophages isolated in China for the control of Pectobacterium carotovorum causing potato soft rot in Kenya. Virol. Sin. 2019, 34, 287–294. [Google Scholar] [CrossRef]
- Behiry, S.I.; Ashmawy, N.A.; Abdelkhalek, A.A.; Younes, H.A.; Khaled, A.E.; Hafez, E.E. Compatible- and incompatible-type interactions related to defense genes in potato elucidation by Pectobacterium carotovorum. J. Plant Dis. Prot. 2018, 125, 197–204. [Google Scholar] [CrossRef]
- Curland, R.D.; Mainello, A.; Perry, K.L.; Hao, J.; Charkowski, A.O.; Bull, C.T.; McNally, R.R.; Johnson, S.B.; Rosenzweig, N.; Secor, G.A. Species of Dickeya and Pectobacterium isolated during an outbreak of blackleg and soft rot of potato in northeastern and north Central United States. Microorganisms 2021, 9, 1733. [Google Scholar] [CrossRef]
- Trabelsi, B.M.; Abdallah, R.A.B.; Ammar, N.; Kthiri, Z.; Hamada, W. Bio-suppression of Fusarium wilt disease in potato using nonpathogenic potatoassociated fungi. J. Plant Pathol. Microbiol. 2016, 7, 2. [Google Scholar]
- Tapwal, A.; Garg, S.; Gautam, N.; Kumar, R. In vitro antifungal potency of plant extracts against five phytopathogens. Braz. Arch. Biol. Technol. 2011, 54, 1093–1098. [Google Scholar] [CrossRef]
- Pinhero, R.G.; Coffin, R.; Yada, R.Y. Post-harvest storage of potatoes. In Advances in Potato Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 339–370. [Google Scholar]
- Jiménez-Reyes, M.F.; Carrasco, H.; Olea, A.F.; Silva-Moreno, E. Natural compounds: A sustainable alternative to the phytopathogens control. J. Chil. Chem. Soc. 2019, 64, 4459–4465. [Google Scholar] [CrossRef]
- Šernaitė, L. Plant extracts: Antimicrobial and antifungal activity and appliance in plant protection. Sodinink. Daržinink 2017, 36, 58–68. [Google Scholar]
- Nebija, F.; Stefkov, G.; Karapandzova, M.; Stafilov, T.; Panovska, T.K.; Kulevanova, S. Chemical characterization and antioxidant activity of Eryngium campestre L., Apiaceae from Kosovo. Maced. Pharm. Bull. 2009, 55, 22–32. [Google Scholar] [CrossRef]
- Küpeli, E.; Kartal, M.; Aslan, S.; Yesilada, E. Comparative evaluation of the anti-inflammatory and antinociceptive activity of Turkish Eryngium species. J. Ethnopharmacol. 2006, 107, 32–37. [Google Scholar] [CrossRef]
- Gugliucci, A.; Bastos, D.H.M. Chlorogenic acid protects paraoxonase 1 activity in high density lipoprotein from inactivation caused by physiological concentrations of hypochlorite. Fitoterapia 2009, 80, 138–142. [Google Scholar] [CrossRef]
- Behiry, S.I.; Soliman, S.A.; Al-Askar, A.A.; Alotibi, F.O.; Basile, A.; Abdelkhalek, A.; Elsharkawy, M.M.; Salem, M.Z.M.; Hafez, E.E.; Heflish, A.A. Plantago lagopus extract as a green fungicide induces systemic resistance against Rhizoctonia root rot disease in tomato plants. Front. Plant Sci. 2022, 13, 966929. [Google Scholar] [CrossRef]
- Behiry, S.I.; Philip, B.; Salem, M.Z.M.; Amer, M.A.; El-Samra, I.A.; Abdelkhalek, A.; Heflish, A. Urtica dioica and Dodonaea viscosa leaf extracts as eco-friendly bioagents against Alternaria alternata isolate TAA-05 from tomato plant. Sci. Rep. 2022, 12, 16468. [Google Scholar] [CrossRef]
- Elbanoby, N.E.; El-Settawy, A.A.A.; Mohamed, A.A.; Salem, M.Z.M. Phytochemicals derived from Leucaena leucocephala (Lam.) de Wit (Fabaceae) biomass and their antimicrobial and antioxidant activities: HPLC analysis of extracts. Biomass Convers. Biorefinery 2022, 1–17. [Google Scholar] [CrossRef]
- Wang, P.; Su, Z.; Yuan, W.; Deng, G.; Li, S. Phytochemical constituents and pharmacological activities of Eryngium L.(Apiaceae). Pharm. Crops 2012, 3, 99–120. [Google Scholar] [CrossRef]
- Le Claire, E.; Schwaiger, S.; Banaigs, B.; Stuppner, H.; Gafner, F. Distribution of a new rosmarinic acid derivative in Eryngium alpinum L. and other Apiaceae. J. Agric. Food Chem. 2005, 53, 4367–4372. [Google Scholar] [CrossRef]
- Hohmann, J.; Pall, Z.; Günther, G.; Mathe, I. Flavonolacyl glycosides of the aerial parts of Eryngium campestre. Planta Med. 1997, 63, 96. [Google Scholar] [CrossRef]
- Kholkhal, W.; Ilias, F.; Bekhechi, C.; Bekkara, F.A. Eryngium maritimum: A rich medicinal plant of polyphenols and flavonoids compounds with antioxidant, antibacterial and antifungal activities. Curr. Res. J. Biol. Sci. 2012, 4, 437–443. [Google Scholar]
- Zhang, Z.; Li, S.; Ownby, S.; Wang, P.; Yuan, W.; Zhang, W.; Beasley, R.S. Phenolic compounds and rare polyhydroxylated triterpenoid saponins from Eryngium yuccifolium. Phytochemistry 2008, 69, 2070–2080. [Google Scholar] [CrossRef]
- Erdelmeier, C.A.J.; Sticher, O. Coumarin Derivatives from Eryngium campestre1. Planta Med. 1985, 51, 407–409. [Google Scholar] [CrossRef]
- Ingram, M. Species Account: Eryngium Campestre; Bot. Soc. Br. Isles: Durham, UK, 2006. [Google Scholar]
- Baytop, T. Türkiye’de Bitkilerle Tedavi–Geçmişten Bugüne, 2nd ed.; Nobel Tıp Basımevi: İstanbul, Türkiye, 1999; pp. 348–349. [Google Scholar]
- Soumia, B. Eryngium campestre L.: Polyphenolic and flavonoid compounds. applications to health and disease; In Polyphenols: Mechanisms of Action in Human Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 69–79. [Google Scholar]
- Güneş, M.G.; İşgör, B.S.; İşgör, Y.G.; Moghaddam, N.S.; Geven, F.; Yildirim, Ö. The effects of Eryngium campestre extracts on glutathione-s-transferase, glutathione peroxidase and catalase enzyme activities. Turk. J. Pharm. Sci. 2014, 11, 339–346. [Google Scholar]
- Bouzidi, S.; Benkiki, N.; Hachemi, M.; Haba, H. Investigation of In Vitro Antioxidant Activity and In Vivo Antipyretic and Anti-Inflammatory Activities of Algerian Eryngium campestre L. Curr. Bioact. Compd. 2017, 13, 340–346. [Google Scholar] [CrossRef]
- Kartnig, T. Flavonoids from the aerial parts of Eryngium campestre. Planta Med. 1993, 59, 285. [Google Scholar] [CrossRef]
- Thiem, B.; Goslinska, O.; Kikowska, M.; Budzianowski, J. Antimicrobial activity of three Eryngium L. species (Apiaceae). Herba Pol. 2010, 56, 52–58. [Google Scholar]
- Hawas, U.W.; El-Kassem, L.A.T.; Awad, H.M.; Taie, H.A.A. Anti-Alzheimer, antioxidant activities and flavonol glycosides of Eryngium campestre L. Curr. Chem. Biol. 2013, 7, 188–195. [Google Scholar] [CrossRef]
- Abd-Elmonem, A.R.; Shehab, N.G. Study of the volatile oil of Eryngium campestre L. growing in Egypt. Bull. Fac. Pharm. Cairo. Univ. 2008, 44, 3379–3388. [Google Scholar]
- Medbouhi, A.; Benbelaïd, F.; Djabou, N.; Beaufay, C.; Bendahou, M.; Quetin-Leclercq, J.; Tintaru, A.; Costa, J.; Muselli, A. Essential oil of Algerian Eryngium campestre: Chemical variability and evaluation of biological activities. Molecules 2019, 24, 2575. [Google Scholar] [CrossRef]
- Azizkhani, M.; Tooryan, F. Antioxidant and antimicrobial activities of rosemary extract, mint extract and a mixture of tocopherols in beef sausage during storage at 4C. J. Food Saf. 2015, 35, 128–136. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Afroz, T.; Lee, Y.-G.; Kim, B.-S. Morphological and molecular characterization of Fusarium tricinctum causing postharvest fruit rot of pumpkin in Korea. J. Gen. Plant Pathol. 2018, 84, 407–413. [Google Scholar] [CrossRef]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115. [Google Scholar] [CrossRef]
- Soliman, S.A.; Al-Askar, A.A.; Sobhy, S.; Samy, M.A.; Hamdy, E.; Sharaf, O.A.; Su, Y.; Behiry, S.I.; Abdelkhalek, A. Differences in Pathogenesis-Related Protein Expression and Polyphenolic Compound Accumulation Reveal Insights into Tomato–Pythium aphanidermatum Interaction. Sustainability 2023, 15, 6551. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Thomas, P.; Upreti, R. Significant effects due to peptone in Kelman medium on colony characteristics and virulence of Ralstonia solanacearum in tomato. Open Microbiol. J. 2014, 8, 95. [Google Scholar] [CrossRef]
- El-Gendi, H.; Al-Askar, A.A.; Király, L.; Samy, M.A.; Moawad, H.; Abdelkhalek, A. Foliar Applications of Bacillus subtilis HA1 Culture Filtrate Enhance Tomato Growth and Induce Systemic Resistance against Tobacco mosaic virus Infection. Horticulturae 2022, 8, 301. [Google Scholar] [CrossRef]
- Moni, Z.R.; Ali, M.A.; Alam, M.S.; Rahman, M.A.; Bhuiyan, M.R.; Mian, M.S.; Iftekharuddaula, K.M.; Latif, M.A.; Khan, M.A.I. Morphological and genetical variability among Rhizoctonia solani isolates causing sheath blight disease of rice. Rice Sci. 2016, 23, 42–50. [Google Scholar] [CrossRef]
- Desvani, S.D.; Lestari, I.B.; Wibowo, H.R.; Supyani; Poromarto, S.H. Hadiwiyono Morphological characteristics and virulence of Rhizoctonia solani isolates collected from some rice production areas in some districts of Central Java. In Proceedings of the International Conference on Science and Applied Science (ICSAS) 2018, Surakarta, Indonesia, 12 May 2018; Volume 2014, p. 020068. [Google Scholar]
- Ke, X.; Lu, M.; Wang, J. Identification of Fusarium solani species complex from infected zebrafish (Danio rerio). J. Vet. Diagn. Investig. 2016, 28, 688–692. [Google Scholar] [CrossRef]
- Al-Abedy, A.N.; Al-Fadhal, F.A.; Karem, M.H.; Al–Masoudi, Z.; Al-Mamoori, S.A. Genetic variability of different isolates of Rhizoctonia solani Kühn isolated from Iranian imported potato tubers (Solanum tuberosum L.). Int. J. Agric. Statatistic Sci. 2018, 14, 587–598. [Google Scholar]
- Khan, M.; Wang, R.; Li, B.; Liu, P.; Weng, Q.; Chen, Q. Comparative evaluation of the LAMP assay and PCR-based assays for the rapid detection of Alternaria solani. Front. Microbiol. 2018, 9, 2089. [Google Scholar] [CrossRef]
- Schocha, C.L.; Seifertb, K.A.; Huhndorfc, S.; Robertd, V.; Spougea, J.L.; Levesqueb, C.A.; Chenb, W.; Consortiuma, F.B. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Dickie, I.A. Does host plant richness explain diversity of ectomycorrhizal fungi? Re-evaluation of Gao et al. (2013) data sets reveals sampling effects. Mol. Ecol. 2014, 23, 992–995. [Google Scholar] [CrossRef]
- Kikowska, M.; Chanaj-Kaczmarek, J.; Derda, M.; Budzianowska, A.; Thiem, B.; Ekiert, H.; Szopa, A. The evaluation of phenolic acids and flavonoids content and antiprotozoal activity of Eryngium species biomass produced by biotechnological methods. Molecules 2022, 27, 363. [Google Scholar] [CrossRef]
- Azizkhani, M.; Sodanlo, A. Antioxidant activity of Eryngium campestre L., Froriepia subpinnata, and Mentha spicata L. polyphenolic extracts nanocapsulated in chitosan and maltodextrin. J. Food Process. Preserv. 2021, 45, e15120. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Savin, S.; Lucian Radu, G. Chemical and bioactivity evaluation of Eryngium planum and Cnicus benedictus polyphenolic-rich extracts. Biomed Res. Int. 2019, 2019, 3692605. [Google Scholar] [CrossRef]
- Kikowska, M.; Budzianowski, J.; Krawczyk, A.; Thiem, B. Accumulation of rosmarinic, chlorogenic and caffeic acids in in vitro cultures of Eryngium planum L. Acta Physiol. Plant. 2012, 34, 2425–2433. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Krawczyk, A.; Więckowska, B.; Sliwinska, E. Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tissue Organ Cult. 2013, 114, 197–206. [Google Scholar] [CrossRef]

| Concentrations (µg/mL) | Growth Inhibition % | Group | ||
|---|---|---|---|---|
| Rhizoctonia solani | Fusarium oxysporum | F. solani | ||
| 100 | 78.52 | 64.81 | 61.85 | C |
| 200 | 87.04 | 72.96 | 65.56 | B |
| 300 | 88.89 | 77.04 | 68.52 | A |
| NC (negative control) | 0.00 | 0.00 | 0.00 | D |
| p ≤ 0.05 | 0.0000 *** | 0.0000 *** | 0.0000 *** | Significant |
| Eryngium campestre Extract Concentrations (µg/mL) | Inhibition Zone Diameter (mm) | ||
|---|---|---|---|
| Ralstonia solanacerum | Dickeya solani | Pectobacterium carotovorum | |
| 100 | 7.33 d | 8.67 b | 8.67 bc |
| 200 | 7.67 cd | 8.67 b | 8.67 bc |
| 300 | 7.67 cd | 9.33 ab | 9.33 b |
| 400 | 8.33 c | 8.67 b | 9.00 bc |
| 600 | 9.67 b | 9.33 ab | 9.00 bc |
| 800 | 9.67 b | 9.67 ab | 8.67 bc |
| 1000 | 9.33 b | 9.67 ab | 8.33 c |
| 2000 | 9.33 b | 10.00 a | 9.00 bc |
| 3000 | 9.33 b | 9.67 ab | 9.00 bc |
| Positive control (Augmentin 25 µg/disc) | 11.67 a | 10.00 a | 10.67 a |
| Negative control | 0.00 e | 0.00 c | 0.00 d |
| Retention Time (min) | Amount (mg/kg) | Compounds |
|---|---|---|
| 2.900 | * ND | Pyrogallol |
| 3.142 | 128.77 | Quinol |
| 3.674 | 9.180 | Gallic acid |
| 5.474 | 626.728 | Catechol |
| 7.618 | 66.487 | p-Hydroxy benzoic acid |
| 8.931 | 16.205 | Catechin |
| 9.207 | 65.205 | Chlorogenic acid |
| 9.718 | 356.489 | Vanillic acid |
| 10.146 | 5.494 | Caffeic acid |
| 10.396 | 30.84 | Syringic acid |
| 12.882 | 36.65 | p-Coumaric acid |
| 14.186 | 2135.53 | Benzoic acid |
| 15.363 | 12.846 | Ferulic acid |
| 16.706 | 49.17 | Rutin |
| 16.924 | 16.38 | Ellagic acid |
| 17.249 | 30.24 | o-Coumaric acid |
| 19.845 | 323.41 | Resveratrol |
| 20.199 | 16.55 | Cinnamic acid |
| 21.421 | 579.048 | Quercetin |
| 22.000 | ND | Rosmarinic acid |
| 22.368 | 153.038 | Naringenin |
| 23.513 | 73.35 | Myricetin |
| 24.447 | ND | Kaempferol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Askar, A.A.; Bashir, S.; Mohamed, A.E.; Sharaf, O.A.; Nabil, R.; Su, Y.; Abdelkhalek, A.; Behiry, S.I. Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium campestre. Separations 2023, 10, 362. https://doi.org/10.3390/separations10060362
Al-Askar AA, Bashir S, Mohamed AE, Sharaf OA, Nabil R, Su Y, Abdelkhalek A, Behiry SI. Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium campestre. Separations. 2023; 10(6):362. https://doi.org/10.3390/separations10060362
Chicago/Turabian StyleAl-Askar, Abdulaziz A., Shimaa Bashir, Abdallah E. Mohamed, Omaima A. Sharaf, Rokaia Nabil, Yiming Su, Ahmed Abdelkhalek, and Said I. Behiry. 2023. "Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium campestre" Separations 10, no. 6: 362. https://doi.org/10.3390/separations10060362
APA StyleAl-Askar, A. A., Bashir, S., Mohamed, A. E., Sharaf, O. A., Nabil, R., Su, Y., Abdelkhalek, A., & Behiry, S. I. (2023). Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium campestre. Separations, 10(6), 362. https://doi.org/10.3390/separations10060362