Abstract
Weeds are considered the main reason for crop yield loss in the world. Weed control and management include various treatments such as cultural, physical, chemical, and biological methods. Chemical control of weeds is the most common method; however, the application of commercial synthetic herbicides caused several dangerous hazards in the environment including the appearance of resistant weed biotypes. Prosopis farcta (Banks & Sol.) J.F.Macbr. (Family: Fabaceae), is a common weed plant in the Middle East, where it is hard to eliminate due to its deep and overlapped roots. On the other side, it has many traditional uses around the world. Herein, the essential oil (EO) of P. farcta above-ground parts was extracted via hydrodistillation techniques and then analyzed using gas chromatography-mass spectroscopy (GC-MS). From the GC-MS analysis, 47 compounds were identified with a relative concentration of 98.02%, including terpenes as the main components (95.08%). From overall identified compounds, cubenol (19.07%), trans-chrysanthenyl acetate (17.69%), torreyol (8.28%), davana ether (3.50%), camphor (3.35%), and farnesyl acetone (3.13%) represented the abundant constituents. Furthermore, the phytotoxic activity of the P. farcta EO was assessed against the weed Dactyloctenium aegyptium (L.) Willd. The EO of P. farcta, at a concentration of 100 µL L−1, significantly inhibited the germination, seedling shoot growth, and seedling root growth by 64.1, 64.0, and 73.4%, respectively. The results exhibited that the seedling root growth is the most affected followed by the seed germination and seedling shoot growth with respective IC50 at 64.5, 80.5, and 92.9 µL L−1. It can be concluded that weeds are not absolutely harmful, but they may have beneficial uses, such as, for example as a source of phytochemicals with application in weed control practices (bioherbicides). It is advised to conduct additional research to characterize the allelopathic action of the major chemicals in their pure form, either alone or in combination, against a variety of weeds.
1. Introduction
The uses of drugs from nature and medicinal plants have been the main targets for the treatment of diseases since the beginning of humanity. Around the world, essential oils (EOs) derived from different plant organs via various extraction processes are used in the perfume and food industries [1]. These phytochemicals are mixtures of chemical compounds, including mainly terpenes, along with other compounds like phenylpropanoid and hydrocarbons [2]. The EOs have been reported to exhibit many potent biological activities such as antiviral [3], antimicrobial [4], antiulcer [5], antipyretic, anti-inflammatory [6,7], antioxidant [8], and hepatoprotective [9], as well as allelopathic effects [10,11].
Prosopis L. genus includes around 45 plant species, and it is one of the genera of Fabaceae (Leguminosae) that is widely distributed in dry regions such as those in Asia, Africa, the Americas, and Australia [12,13]. Prosopis farcta (Banks & Sol.) J.F.Macbr. (Syrian mesquite) is a native plant to Asia and Northern Africa, while it is widespread as a weed in the Middle East [14]. It is the only shrub species of the genus Prosopis, less than 100 cm in height. This genus was documented to have a strong capability for many diverse series of complicated compounds. Many categories of chemical components were characterized by different Prosopis plants including flavonoids, phenolic acids, and alkaloids [15,16,17]. Due to the significant chemical composition of the plant belonging to this genus, many bioactivities were reported for the different extracts and isolated metabolites, such as antiinflammation, antimicrobial, antioxidant, and others [18,19,20]. Tryptamine, tyramine, β-phenethylamine, and piperidines were the most common components that were isolated and identified from a variety of Prosopis species [18].
Several traditional uses were documented for the different organs of P. farcta, such as the treatment of diarrhea, skin diseases, colds, diabetes, wound healing, measles, inflammation, cardiac pains, and prostate disorders [21]. In addition, the different extracts of this plant were documented to exhibit various biological activities like antimicrobial, antioxidant [19], neuroprotective [22], antidiabetic [23], and proliferative and angiogenic properties [20]. Several phenolic acids and flavonoid metabolites were characterized from the different extracts of this plant in addition to EOs, fatty acids, and proteins, while the EOs of P. farcta is poorly studied [24,25]. Several flavonoids were characterized from the ethyl acetate extract of P. farcta growing in Egypt including apigenin, kaempferol, and quercetin along with their glycosides. The analysis of the EO from the Tunisian ecospecies of P. farcta identified only 59.0% of the total mass of its EO [24], and only 42.6% of the EO extracted from the Egyptian ecospecies was assessed [25].
Weeds represent the most deleterious pests in crops causing around 30–50% loss of crop yields [26]. Weed removal and biomass reduction of growing areas cause the plant to develop vegetatively [27]. In addition to decreasing crop productivity, weeds can also pose a threat to livestock health and obstruct agricultural procedures. Herbicides are the most effective weed control method, but they cause serious environmental damage. Herbicide resistance reported in many species is an added concern as 166 conventional herbicides have been shown to be ineffective against nearly 500 species of weeds [28]. However, increasing crop production while reducing herbicide use is the biggest challenge. From the first year of production of commercial herbicide in 1940, a million tons of commercial synthetic herbicide were applied in agricultural practices that caused many tremendous and dangerous hazards for the environment [29] and created more resistant weed biotypes [30]. Therefore, many efforts from scientists and researchers were performed to find alternatives to manual control and weed biotypes resistant to traditional synthetic herbicides [31]. This strategy will reduce weed resistance to chemical herbicides, lower health, and environmental risks, and boost the country’s economy.
Natural products offer an eco-friendly and safe alternative for weed control, making them suitable for both organic and conventional farming methods [32]. The chemical composition of essential oils comprises a broad range of functionalized chemical classes, such as monoterpenoids, sesquiterpenoids, and phenylpropanoids, among others [2]. Plant essential oils are produced by specialized secretory structures, including glands, secretory hairs, resiniferous ducts, or secretory cavities, and are abundant in medicinal plants [33]. EOs derived from different plants showed potential phytotoxic activity against weeds which attract the attention of scientists and several scientific works around the world to be applied as eco-friendly bioherbicides [2,34]. In phytotoxicity, one species inhibits the development of another by producing natural chemical compounds that inhibit seed germination or growth. Among the worst weeds is Dactyloctenium aegyptium (L.) Willd.), which is an African and Asian house plant that requires little upkeep. It is a perennial weed that grows well in a variety of soils, especially moist soils, in moderate temperate and tropical regions [35]. It is a harmful weed that causes several problems during its growth and reproduction since it reduces crop vigor and leads to loss of yield [35].
As the EO of P. farcta is poorly studied, and up to our knowledge, neither study characterize the EO of the Saudi ecospecies nor their phytotoxicity, the current work’s goals were: (i) determine the chemical profiling of the EO of the Saudi ecospecies of P. farcta via gas chromatography-mass spectroscopy (GC-MS); and (ii) estimate the phytotoxic potentiality of P. farcta EO against the germination, seedling shoot growth, and seedling root growth of the weed D. aegyptium.
2. Materials and Methods
2.1. Collection, Authentication, and Preparation of Plant Materials
The above-ground parts of P. farcta were collected from three populations grown naturally in Rawadat Khuraim, 100 km north of Riyadh, Saudi Arabia (25°25′38.1′′ N, 47°14′12.0′′ E). The shrubs of P. farcta were found in high density at the periphery of the Sidr tree canopy (Ziziphus nummularia (Burm.f.) Wight & Arn. Numer) (Figure S1). The plant specimen was identified and authenticated according to the flora books of Saudi Arabia [36,37]. A voucher specimen of P. farcta was prepared, authenticated, and deposited in the National Herbarium and Genebank, Riyadh, Saudi Arabia with ID: RIY-236779. All the collected plant materials were carefully cleaned from the dust and then dried in the shade at room temperature at 25 ± 3 °C for a week, then grounded into powder by a grinder (IKA® MF 10 Basic Microfine Grinder Drive, Breisgau, Germany). The ground plant sample was packed in plastic bags and stored in the refrigerator at 4 °C until further analysis.
2.2. Essential Oil Extraction
About 200 g of the air-dried powder of P. farcta above-ground parts were subjected to hydro-distillation using the Clevenger apparatus including a glass round flask (5 L) for three hours. The extracted EO layer was then separated using 3–5 drops of n-hexane and immediately dried via 0.5 g of anhydrous Na2SO4. These procedures were performed for the three collected samples of P. farcta. The EO samples were kept in dark-brown glass vials and stored in a refrigerator adjusted at 4 °C till further GC-MS analysis and biological evaluations.
2.3. Gas Chromatography-Mass Spectroscopy (GC-MS) Analysis
Each EO sample was analyzed by GC-MS, and the chemical components were identified in accordance with the same methodology and under identical conditions described before by Abdelhameed, et al. [6]. Briefly, the Thermo Scientific ISQTM EC single quadrupole mass spectrometer and TRACE Ultra-Gas Chromatography (THERMO Scientific™ Corporate, Waltham, MA, USA) were both employed in the GC-MS analysis. A TR-5 MS column with an internal diameter of 30 m and a film with a thickness of 0.25 mm was installed in the GC-MS system. The carrier gas, Helium (He), was employed with a flow rate of (1.0 mL min−1) and a split ratio of (1:10). The temperature program was set for one minute at 60 °C, and then raised to 240 °C at one minute at a rate of 4.0 °C min−1. Each EO sample was injected into the injector and detector at 210 °C in a tiny amount (1 µL in hexane) at a concentration of 1:10 (v/v). Using a spectrum that ranges between m/z 40 and 450, the mass spectral data were obtained at 70 eV using electron ionization (EI). In addition to access to the Wiley Spectral Library collection and the NIST Library database (Gaithersburg, MD, USA; Wiley, Hoboken, NJ, USA), which were used for the determination of the retention indices relative to n-alkanes (C8–C22), or evaluation to the mass spectral data of authentic components, the chemical composition was identified using the AMDIS (automated mass spectral deconvolution and identification) software.
2.4. Phytotoxicity of P. farcta EO against D. aegyptium
The allelopathic potential of P. farcta’s EO against the weed D. aegyptium was examined. The weed D. aegyptium’s mature seeds were gathered from contaminated agricultural fields. Until further inspection, the seeds of equal size were chosen and stored in paper bags at room temperature (25 ± 3 °C). The seeds were surface sterilized for three minutes using 0.3% sodium hypochlorite (NaClO) prior to the setup of the experiment, three rounds of distilled water rinsing, and sterilized drying conditions [10,38]. The concentrations of 0, 25, 50, 75, and 100 µL L−1 were created by diluting the EO with the surfactant Tween 80® (Sigma-Aldrich, Darmstadt, Germany) in order to investigate their allelopathic activity. Twenty sterile weed seeds were placed uniformly over the Whatman Grade 1 filter paper that had been lined within the Petri plate (90 mm) and wetted with either 4 mL of each concentration or Tween (as control) [39]. From each concentration, three plates were prepared. To prevent EO leakage from plates, the three plates were sealed with Parafilm® (Sigma, St. Louis, MO, USA). The experiment was performed three times with the biological replicas using the three samples of P. farcta, i.e., the three populations. All the prepared Petri plates were arranged and incubated within a growth chamber adjusted at 27 °C and a 12 h light/12 h dark cycle. Daily counts of the 2 mm long radicle-germinated seeds were conducted, and on the tenth day of incubation, the lengths of all seedling roots and shoots were measured. The following equation was used to compute the germination and seedling growth inhibitions:
The IC50 values—the concentration of the EO needed for 50% inhibition—were computed based on the data of germination and seedling growth inhibition.
2.5. Statistical Analysis
Using the CoStat software, version 6.311 (CoHort Software, Monterey, CA, USA), the results of allelopathic bioassay tests with three replicas were subjected to one-way ANOVA at p < 0.05. In order to assess the significance of differences between pairs of group means, Tukey’s honestly significant difference (HSD) test was utilized as a post hoc analysis. Additionally, MS-EXCEL was used to compute exponentially the concentration of EO necessary for 50% inhibition of D. aegyptium.
3. Results and Discussion
3.1. P. farcta Essential Oil Chemical Characterization
The hydrodistillation of the Saudi P. farcta above-ground parts yielded 0.092% (v/w) of a pale yellow EO with a pungent smell. The yield of the oil in the present study was higher than the documented yields of the oils derived from the different organs of the same plant collected from Tunisia [24]. This EO was analyzed by GC-MS and the total ion chromatogram was presented in Figure S2.
The chemical components of the EO were authenticated and identified depending on the GC-MS analysis. The recognized chemical compounds are listed in Table 1, along with their relative concentrations, retention times, and Kovats indexes (both calculated and experimental). Forty-seven components were assigned representing 98.02% of overall oil mass. Five classes of compounds were categorized, including four oxygenated forms of compounds, mono- (23.70%), sesqui- (58.31%), di-terpenes (1.03%), and other oxygenated hydrocarbons (2.94%), in addition to only one non-oxygenated form of compounds, sesquiterpene hydrocarbons (12.04%) (Figure S3). These results revealed that this EO is very rich in oxygenated compounds (85.98%), while only 12.04% of non-oxygenated constituents were assessed. Previous data on Tunisian P. farcta EO showed that it is also rich in volatile oxygenated compounds [24].

Table 1.
The chemical profile of the essential oil extracted from the above-ground parts of Prosopis farcta collected from Saudi Arabia.
The sesquiterpenes represented the main constituents of EO of P. farcta with a relative concentration of 70.35% including the oxygenated compounds as majors with a considerable concentration of hydrocarbons. Twenty-three oxygenated sesquiterpenes were identified, comprising cubenol (19.07%), torreyol (8.28%), davana ether (3.50%), farnesyl acetone (3.13%), caryophyllene oxide (2.94%), and hexahydrofarnesyl acetone (2.87%) as main components (Figure S4); however, diepicedrene-1-oxide (0.18%) is the minor one, while δ-cadinene (2.05), α-amorphene (1.60%), α-calacorene (1.55%), and α-muurolene (1.37%) were characterized as the major identified sesquiterpene hydrocarbons that represented twelve compounds from all EO constituents. The abundance of the terpenes, and especially sesquiterpenes described in the current study, was not in harmony with the results of volatiles of the different organs of the Tunisian [24] and Egyptian P. farcta [25].
The monoterpenes were characterized with considerable relative concentration and represented as only oxygenated compounds with a complete absence of hydrocarbons. Eight oxygenated monoterpenes were identified in which trans-chrysanthenyl acetate (17.69%) and camphor (3.35%) are the leader compounds (Figure S4), while 1,8-cineole (0.14%) is the minor one. Additionally, the presence of the monoterpenes in the EO of P. farcta was not in agreement with the previous studies of the volatiles of the different organs of the Tunisian [24] and Egyptian ecospecies [25].
According to the published data, the existence of the diterpenes is very rare in the EOs derived from the different organs of plants, with some exceptions of plants, such as Araucaria heterophylla (Salisb.) Franco [40], Lactuca serriola L. [41], and Euphorbia mauritanica L. [42]. The current findings showed the rarity of diterpenes with only one common diterpene compound, phytol (1.03%). Phytol was also identified in the volatile oils derived from different organs of P. farcta collected from Tunisia [24] and from above-ground parts of Egyptian ecospecies [25]. In contrast to the published results of Harzallah-Skhiri et al. [24] and Saad et al. [25], the present EO analysis revealed the presence of the other hydrocarbons as traces. Only two hydrocarbons were assigned including one aromatic, α-hexyl-cinnamaldehyde (0.46%), and one fatty acid methyl ester (linolenic acid, methyl ester, 2.48%).
The clear variation between the current results and the previously published data of the same plant [24,25], could be attributed to the variations in the extraction techniques [43], genetic factors [44], as well as the environmental and climatic conditions [2,10,45]. The current findings showed that P. farcta is capable of biosynthesizing a number of oxygenated mono- and sesquiterpenes based on the chemical composition of its EO. Due to the value of these compounds, the plant’s capacity for the biosynthesis of these kinds of compounds supported the plant’s importance. Cubenol and its isomers were basically biosynthesized via the enzymatic reaction and rearrangement of germacrenyl cation inside the plant organs [46], while geranyl diphosphate, a pyrophosphate ester, was the starting and/or intermediate for the biosynthesis of many isoprenoids, including trans-chrysanthenyl acetate [47]. On the other side, farnesyl diphosphate was described as the main intermediate for the biosynthesis of terpenoids, including torreyol and sterols, in addition to carotenoid-derived compounds [48]. The other compounds were biosynthesized in the plant organs via different enzymatic reactions that are very difficult to chemically synthesize. Thus, this plant represented an important resource for these compounds, especially cubenol, trans-chrysanthenyl acetate, and torreyol
3.2. Phytotoxic Activity of P. farcta EO against D. aegyptium
The phytotoxic potential of the extracted EO from P. farcta above-ground parts was tested against the weed D. aegyptium. At the lowest concentration of the EO (25 µL L−1), the seed germination was reduced by 28.2%, while the seedling shoot and root growth were reduced by 16.6% and 31.0%, respectively. On the other side, the treatment of D. aegyptium with 100 µL L−1 retarded the germination, seedling shoot growth, and seedling root growth by 64.1, 64.0, and 73.4%, respectively (Figure 1A).
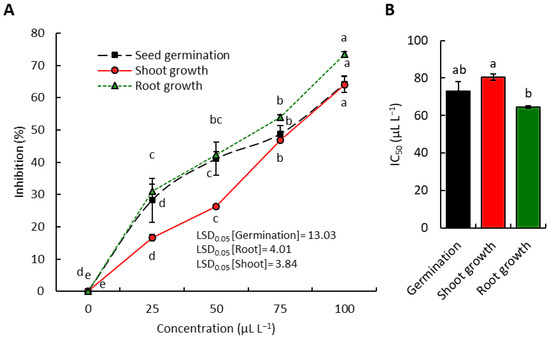
Figure 1.
Phytotoxic activity of Prosopis farcta EO against seed germination and seedling growth of the weed D. aegyptium. (A) Concentration-dependent inhibitory activity, and (B) the inhibitory concentration of 50% (IC50). Different letters within each line in figure A or among the columns in figure B shows significant variation at p < 0.05.
Based on the calculations of the IC50 values, the seedling root growth was the most affected by the EO application, where it showed an IC50 value of 64.5 µL L−1, while the seedling shoot growth and seed germination showed IC50 values of 92.9 µL L−1 and 80.5 µL L−1, respectively (Figure 1b). The current findings showed that D. aegyptium roots were more reduced by the P. farcta EO compared to the shoots. This observation was described in several documented works [4,10,49]. The permeability of the cell membrane and the roots’ direct contact with the phytotoxic EO chemicals in the medium resulted in more root inhibition [50,51].
The significant allelopathic potentiality of the P. farcta EO against the weed D. aegyptium might be directly attributed to the EO chemical components, especially the compounds with high relative consecration such as cubenol, trans-chrysanthenyl acetate, torreyol, davana ether, camphor, and farnesyl acetone. The rich EOs with terpenes, particularly the oxygenated terpenes, have been documented to possess strong toxic effects against weeds, including D. aegyptium [4,11]. The previous works proved that there is a strong and direct relationship between the phytotoxicity of the EOs derived from the plants and their content of oxygenated compounds [2,41,52]. Thus, the increase in the oxygenated constituents in EOs afforded strong inhibition of weed growth [52]. In the present study, the major compound, cubenol, was reported as allelochemical within the EO of Sinapis arvensis L. [53]. Additionally, the EO of Peucedanum ostruthium W.D.J. Koch, which is rich in cubenol (8.7%), reduced the seedling growth of Lolium multiflorum Lam. and Sinapis alba L. by 90.7% and 76.6%, respectively [54]. The rich EO with trans-chrysanthenyl acetate of Calotropis procera (Aiton) W.T. Aiton has been reported to exert strong phytotoxic activity against the weeds Bidens pilosa L. and D. aegyptium [4]. Moreover, the Argemone ochroleuca Sweet EO was reported with an abundance of major compound trans-chrysanthenyl acetate and it showed significant phytotoxic activity against the noxious weed Peganum harmala L. [55]. In this context, plants with EOs rich in camphor such as Salvia officinalis L. [56], Euphorbia heterophylla L. [41], and Tanacetum chiliophyllum Nábělek [57] showed considerable phytotoxicity against other plants. Summing up, these major identified compounds in the present study of P. farcta EO could act as allelochemicals either singularly or in combination. However, further study is recommended for confirmation of this observation and testing their mode(s) of action.
The modes of action of allelochemical include disruption of the cell membrane, photosynthetic pigments, enzymes, mitochondria, RNA, DNA, and respiration [50,58]. The oxidative activity of the EO is one of the actions that play a role in retardation of the plant growth due to their reactivity and production of the reactive oxygen species (ROS) which start with membrane dysfunction and loss of control of permeability [59,60].
Accordingly, the present chemical profiling of the P. farcta EO showed that the oxygenated and terpenoid components are the main constituents with the respective relative concentration of 85.98% and 95.08%. Therefore, these compounds in EOs could cause structural fractures and degradation in roots as well as hinder cell proliferation [49,60,61].
Due to the lack of thorough and systematic examinations into the functional mechanism underlying the phytotoxicity of P. farcta EO against weeds, further study is needed to evaluate the physiological and biochemical modes of action of P. farcta EO on a wide range of weeds. This can provide considerable information about using the EO of P. farcta EO, which could be used as a bioherbicide against weeds. Undoubtedly, this claim has to be supported by extensive field research as well as other phytotoxic tests involving weeds and other agricultural species.
4. Conclusions
The EO chemical composition and the phytotoxicity of the above-ground parts of P. farcta collected from Saudi Arabia were assessed for the first time. Forty-seven chemical compounds were determined in the EO P. farcta, mainly oxygenated form (85.98%). Cubenol, trans-chrysanthenyl acetate, torreyol, davana ether, camphor, and farnesyl acetone were determined as major compounds which represent 55% of the total EO mass. Additionally, the P. farcta EO exhibited significant phytotoxic effects on the germination and seedling growth of the weed D. aegyptium. This phytotoxicity could be attributed to the high concentration of terpenes and oxygenated constituents, particularly the major compounds that could act as allelochemicals either singularly or in combination. The present investigation showed that weeds are not an absolute nuisance, but they can be considered as a source of bioactive compounds with economic applications such as bioherbicides. Further study is recommended for assessment of the mode(s) of action of authentic pure form of the identified major compounds against a wide range of weeds as well as the accompanied crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10060361/s1, Figure S1: Prosopis farcta (Banks & Sol.) J.F.Macbr; Figure S2: GC-MS ion chromatogram of the essential oil of Prosopis farcta above-ground parts; Figure S3: chemical compounds grouping; Figure S4: structures of the major components (>3%) of the essential oil of Prosopis farcta.
Author Contributions
Conceptualization, A.M.A.-E., A.M.A. and A.I.E.; formal analysis, A.M.A.-E., B.A.D. and A.I.E.; investigation, A.M.A.-E., A.M.A., A.E.-N.E.G., L.P., B.A.D. and A.I.E.; writing—original draft preparation, A.M.A.-E. and A.I.E.; writing—review and editing, A.M.A.-E., A.M.A., A.E.-N.E.G., L.P., B.A.D. and A.I.E.; visualization, A.M.A.-E. and A.I.E. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia, research project no. IFKSUOR3-577-1.
Data Availability Statement
Not Applicable.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research and Innovation, “Ministry of Education” in Saudi Arabia for funding this research (IFKSUOR3-577-1).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Werrie, P.-Y.; Durenne, B.; Delaplace, P.; Fauconnier, M.-L. Phytotoxicity of essential oils: Opportunities and constraints for the development of biopesticides. A review. Foods 2020, 9, 1291. [Google Scholar] [CrossRef] [PubMed]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; Elshamy, A.I. Phytotoxic effects of plant essential oils: A systematic review and structure-activity relationship based on chemometric analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- El Gendy, A.E.-N.G.; Essa, A.F.; El-Rashedy, A.A.; Elgamal, A.M.; Khalaf, D.D.; Hassan, E.M.; Abd-ElGawad, A.M.; Elgorban, A.M.; Zaghloul, N.S.; Alamery, S.F. Antiviral potentialities of chemical characterized essential oils of Acacia nilotica bark and fruits against hepatitis A and herpes simplex viruses: In vitro, in silico, and molecular dynamics studies. Plants 2022, 11, 2889. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Amier, Y.A.; El Gendy, A.E.-N.G.; Al-Barati, S.A.; Dar, B.A.; Al-Rowaily, S.L.; Assaeed, A.M. Chemical composition variations, allelopathic, and antioxidant activities of Symphyotrichum squamatum (Spreng.) Nesom essential oils growing in heterogeneous habitats. Arab. J. Chem. 2020, 13, 4237–4245. [Google Scholar] [CrossRef]
- Ammar, N.M.; Hassan, H.A.; Ahmed, R.F.; El-Gendy, A.E.-N.G.; Abd-ElGawad, A.M.; Farrag, A.R.H.; Farag, M.A.; Elshamy, A.I.; Afifi, S.M. Gastro-protective effect of Artemisia sieberi essential oil against ethanol-induced ulcer in rats as revealed via biochemical, histopathological and metabolomics analysis. Biomarkers 2022, 27, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, M.F.; Asaad, G.F.; Ragab, T.I.; Ahmed, R.F.; El Gendy, A.E.-N.G.; El-Rahman, A.; Sahar, S.; Elgamal, A.M.; Elshamy, A.I. Oral and topical anti-inflammatory and antipyretic potentialities of Araucaria bidiwillii shoot essential oil and its nanoemulsion in relation to chemical composition. Molecules 2021, 26, 5833. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; Al-Rowaily, S.L.; Ragab, T.I.; El Gendy, A.E.-N.G.; Abd-ElGawad, A.M. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities. Ind. Crops Prod. 2020, 148, 112272. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Zaghloul, N.S.; Abd-ElGawad, A.M. Bioactive chemical constituents of Matthiola longipetala extract showed antioxidant, antibacterial, and cytotoxic potency. Separations 2023, 10, 53. [Google Scholar] [CrossRef]
- Damtie, D.; Braunberger, C.; Conrad, J.; Mekonnen, Y.; Beifuss, U. Composition and hepatoprotective activity of essential oils from Ethiopian thyme species (Thymus serrulatus and Thymus schimperi). J. Essent. Oil Res. 2019, 31, 120–128. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Assaeed, A.M.; Al-Rowaily, S.L.; Alshahri, M.S.; Bonanomi, G.; Elshamy, A.I. Influence of season and habitat on the essential oils composition, allelopathy, and antioxidant activities of Artemisia monosperma Delile. Separations 2023, 10, 263. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Assaeed, A.M.; El Gendy, A.E.-N.G.; Dar, B.A.; Elshamy, A.I. Volatile oils discrepancy between male and female ochradenus arabicus and their allelopathic activity on Dactyloctenium aegyptium. Plants 2023, 12, 110. [Google Scholar] [CrossRef]
- Burkart, A. A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). J. Arnold Arbor. 1976, 57, 450–525. [Google Scholar] [CrossRef]
- Quattrocchi, U. CRC World Dictionary of Plant Nmaes: Common Names, Scientific Names, Eponyms, Synonyms, and Etymology; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Pasiecznik, N.; Harris, P.J.; Smith, S.J. Identifying Tropical Prosopis Species: A Field Guide; Hdra: Coventry, UK, 2004. [Google Scholar]
- SivaKumar, T.; Srinivasan, K.; Rajavel, R.; Vasudevan, M.; Ganesh, M.; Kamalakannan, K.; Mallika, P. Isolation of chemical constituents from Prosopis juliflora bark and anti-inflammatory activity of its methanolic extracts. J. Pharm. Res. 2009, 2, 551–556. [Google Scholar]
- Ahmed, E.F.; Sleem, A.A.; Abbas, F.A.; El-Shafae, A.M.; El-Domiaty, M.M. Phytochemical constituents, HPLC-PDA-ESI-MS/MS profile and bioactivities of roots and rhizomes of Prosopis farcta (Banks & Sol.) JF Macbr. Nat. Prod. J. 2020, 10, 411–428. [Google Scholar]
- Sharifi-Rad, J.; Zhong, J.; Ayatollahi, S.A.; Kobarfard, F.; Faizi, M.; Khosravi-Dehaghi, N.; Suleria, H.A. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from Prosopis farcta (Banks & Sol.) JF Macbr. and their potential antioxidant activities. Cell. Mol. Biol. 2021, 67, 189–200. [Google Scholar] [PubMed]
- Schmeda-Hirschmann, G.; Theoduloz, C.; Jiménez-Aspee, F.; Echeverría, J. Bioactive constituents from south American Prosopis and their use and toxicity. Curr. Pharm. Des. 2020, 26, 542–555. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.A.F.; Etemadfard, H.; Zebarjad, Z. Antimicrobial and antioxidant characteristics of volatile components and ethanolic fruit extract of Prosopis farcta (Bank & Soland.). Trends Pharm. Sci. 2018, 4, 177–186. [Google Scholar]
- Noroozi, R.; Sadeghi, E.; Yousefi, H.; Taheri, M.; Sarabi, P.; Dowati, A.; Ayatallahi, S.A.; Noroozi, R.; Ghafouri-Fard, S. Wound healing features of Prosopis farcta: In vitro evaluation of antibacterial, antioxidant, proliferative and angiogenic properties. Gene Rep. 2019, 17, 100482. [Google Scholar] [CrossRef]
- Omidi, A.; Ansari nik, H.; Ghazaghi, M. Prosopis farcta beans increase HDL cholesterol and decrease LDL cholesterol in ostriches (Struthio camelus). Trop. Anim. Health Prod. 2013, 45, 431–434. [Google Scholar] [CrossRef]
- Mollashahi, M.; Tehranipour, M.; Khayyatzade, J.; Moosavi, B.Z. The neuroprotective effects of Prosopis farcta pod aqueous and ethanol extracts on spinal cord α-motoneurons neuronal density after sciatic nerve injury in rats. Life Sci. J. 2013, 10, 293–297. [Google Scholar]
- Agirman, E.; Celik, I.; Dogan, A. Consumption of the Syrian mesquite plant (Prosopis farcta) fruit and seed lyophilized extracts may have both protective and toxic effects in STZ-induced diabetic rats. Arch. Physiol. Biochem. 2022, 128, 887–896. [Google Scholar] [CrossRef]
- Harzallah-Skhiri, F.; Jannet, H.B.; Hammami, S.; Mighri, Z. Variation of volatile compounds in two Prosopis farcta (Banks et Sol.) Eig.(Fabales, Fabaceae = Leguminosae) populations. Flavour Fragr. J. 2006, 21, 484–487. [Google Scholar] [CrossRef]
- Saad, A.M.; Ghareeb, M.A.; Abdel-Aziz, M.S.; Madkour, H.M.F.; Khalaf, O.M.; El-Ziaty, A.K.; Abdel-Mogib, M. Chemical constituents and biological activities of different solvent extracts of Prosopis farcta growing in Egypt. J. Pharmacogn. Phytother. 2017, 9, 67–76. [Google Scholar]
- Soltani, N.; Dille, J.A.; Gulden, R.H.; Sprague, C.L.; Zollinger, R.K.; Morishita, D.W.; Lawrence, N.C.; Sbatella, G.M.; Kniss, A.R.; Jha, P. Potential yield loss in dry bean crops due to weeds in the United States and Canada. Weed Technol. 2018, 32, 342–346. [Google Scholar] [CrossRef]
- Seyyedi, S.M.; Moghaddam, P.R.; Mahallati, M.N. Weed competition periods affect grain yield and nutrient uptake of black seed (Nigella Sativa L.). Hortic. Plant J. 2016, 2, 172–180. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 1 April 2023).
- Wilson, C.; Tisdell, C. Why farmers continue to use pesticides despite environmental, health and sustainability costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef]
- Owen, M.D.; Zelaya, I.A. Herbicide-resistant crops and weed resistance to herbicides. Pest Manag. Sci. 2005, 61, 301–311. [Google Scholar] [CrossRef]
- Arafat, Y.; Shahida, K.; Lin, W.; Fang, C.; Sadia, S.; Ali, N.; Azeem, S. Allelopathic evaluation of selected plants extract against broad and narrow leaves weeds and their associated crops. Acad. J. Agric. Res. 2015, 3, 226–234. [Google Scholar]
- De Mastro, G.; El Mahdi, J.; Ruta, C. Bioherbicidal potential of the essential oils from Mediterranean Lamiaceae for weed control in organic farming. Plants 2021, 10, 818. [Google Scholar] [CrossRef]
- Nikolova, M.T.; Berkov, S.H. Use of essential oils as natural herbicides. Ecol. Balk. 2018, 10, 259–265. [Google Scholar]
- Assaeed, A.; Elshamy, A.; El Gendy, A.E.-N.; Dar, B.; Al-Rowaily, S.; Abd-ElGawad, A. Sesquiterpenes-rich essential oil from above ground parts of Pulicaria somalensis exhibited antioxidant activity and allelopathic effect on weeds. Agronomy 2020, 10, 399. [Google Scholar] [CrossRef]
- Riaz, S.; Basharat, S.; Ahmad, F.; Hameed, M.; Fatima, S.; Ahmad, M.S.A.; Shah, S.M.R.; Asghar, A.; El-Sheikh, M.A.; Kaushik, P. Dactyloctenium aegyptium (L.) Willd. (Poaceae) differentially responds to pre-and post-emergence herbicides through micro-structural alterations. Agriculture 2022, 12, 1831. [Google Scholar] [CrossRef]
- Chaudhary, S.A. Flora of the Kingdom of Saudi Arabia; Ministry of Agriculture and Water: Riyadh, Saudi Arabia, 1999; Volume 1. [Google Scholar]
- Collenette, S. Wildflowers of Saudi Arabia; National Commission for Wildlife Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999. [Google Scholar]
- Abd-ElGawad, A.M.; El-Amier, Y.A.; Bonanomi, G.; Gendy, A.E.-N.G.E.; Elgorban, A.M.; Alamery, S.F.; Elshamy, A.I. Chemical composition of Kickxia aegyptiaca essential oil and its potential antioxidant and antimicrobial activities. Plants 2022, 11, 594. [Google Scholar] [CrossRef]
- Macías, F.A.; Castellano, D.; Molinillo, J.M. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2000, 48, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.S.; Padalia, R.C.; Goswami, P.; Verma, S.K.; Chauhan, A.; Darokar, M.P. Chemical composition and antibacterial activity of foliage and resin essential oils of Araucaria cunninghamii Aiton ex D. Don and Araucaria heterophylla (Salisb.) Franco from India. Ind. Crops Prod. 2014, 61, 410–416. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; El-Nasser El Gendy, A.; Al-Rowaily, S.L.; Assaeed, A.M. Preponderance of oxygenated sesquiterpenes and diterpenes in the volatile oil constituents of Lactuca serriola L. revealed antioxidant and allelopathic activity. Chem. Biodivers. 2019, 16, e1900278. [Google Scholar] [CrossRef] [PubMed]
- Essa, A.F.; El-Hawary, S.S.; Abd-El Gawad, A.M.; Kubacy, T.M.; AM El-Khrisy, E.E.D.; Elshamy, A.I.; Younis, I.Y. Prevalence of diterpenes in essential oil of Euphorbia mauritanica L.: Detailed chemical profile, antioxidant, cytotoxic and phytotoxic activities. Chem. Biodivers. 2021, 18, e2100238. [Google Scholar] [CrossRef]
- Đurović, S.; Micić, D.; Pezo, L.; Radić, D.; Bazarnova, J.G.; Smyatskaya, Y.A.; Blagojević, S. The effect of various extraction techniques on the quality of sage (Salvia officinalis L.) essential oil, expressed by chemical composition, thermal properties and biological activity. Food Chem. X 2022, 13, 100213. [Google Scholar] [CrossRef]
- Ložienė, K.; Venskutonis, P. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Biochem. Syst. Ecol. 2005, 33, 517–525. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Abd-ElGawad, A.M.; El-Amier, Y.A.; El Gendy, A.E.N.G.; Al-Rowaily, S.L. Interspecific variation, antioxidant and allelopathic activity of the essential oil from three Launaea species growing naturally in heterogeneous habitats in Egypt. Flavour Fragr. J. 2019, 34, 316–328. [Google Scholar] [CrossRef]
- Bhaskar, P.; Sareen, D. Bioinformatics approach to understand nature’s unified mechanism of stereo-divergent synthesis of isoprenoid skeletons. World J. Microbiol. Biotechnol. 2020, 36, 142. [Google Scholar] [CrossRef] [PubMed]
- Houti, H.; Ghanmi, M.; Satrani, B.; Mansouri, F.E.; Cacciola, F.; Sadiki, M.; Boukir, A. Moroccan endemic Artemisia herba-alba essential oil: GC-MS analysis and antibacterial and antifungal investigation. Separations 2023, 10, 59. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.-L.; Zeng, J.; Zhang, L.; Ding, Z.-H.; Zeng, Y. Identification and characterization of a δ-cadinol synthase potentially involved in the formation of boreovibrins in Boreostereum vibrans of Basidiomycota. Nat. Prod. Bioprospecting 2016, 6, 167–171. [Google Scholar] [CrossRef] [PubMed]
- de Melo, S.C.; de Sa, L.E.C.; de Oliveira, H.L.M.; Trettel, J.R.; da Silva, P.S.; Gonçalves, J.E.; Gazim, Z.C.; Magalhães, H.M. Chemical constitution and allelopathic effects of Curcuma zedoaria essential oil on lettuce achenes and tomato seeds. Aust. J. Crop Sci. 2017, 11, 906–916. [Google Scholar] [CrossRef]
- El-Shora, H.M.; Abd El-Gawad, A.M. Response of Cicer arietinum to allelopathic effect of Portulaca oleracea root extract. Phyton-Ann. Rei Bot. 2015, 55, 215–232. [Google Scholar]
- M’barek, K.; Zribi, I.; Ullah, M.J.; Haouala, R. The mode of action of allelochemicals aqueous leaf extracts of some Cupressaceae species on lettuce. Sci. Hortic. 2019, 252, 29–37. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Miri, A.; Sharifi-Rad, M.; Sharifi-Rad, R.; Sharifi-Rad, M. Allelopathic effects of essential oils from Sinapis arvensis L. aerial part on germination and seedling growth of medicinal plants and weeds. Int. J. Biosci. 2014, 5, 135–140. [Google Scholar]
- Stefania, G.; Iriti, M.; Vitalini, S. Chemical composition, antiradical and phytotoxic activity of the essential oil from Peucedanum ostruthium WDJ Koch leaves. J. Phytomolecules Pharmacol. 2022, 1, 88–95. [Google Scholar]
- Abd-ElGawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Omer, E.A.; Dar, B.A.; Al-Taisan, W.a.A.; Elshamy, A.I. Essential oil enriched with oxygenated constituents from invasive plant Argemone ochroleuca exhibited potent phytotoxic effects. Plants 2020, 9, 998. [Google Scholar] [CrossRef]
- de Almeida, L.F.R.; Frei, F.; Mancini, E.; De Martino, L.; De Feo, V. Phytotoxic activities of Mediterranean essential oils. Molecules 2010, 15, 4309–4323. [Google Scholar] [CrossRef]
- Salamci, E.; Kordali, S.; Kotan, R.; Cakir, A.; Kaya, Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 2007, 35, 569–581. [Google Scholar] [CrossRef]
- El-Shora, H.M.; Abd El-Gawad, A.M. Physiological and biochemical responses of Cucurbita pepo L. mediated by Portulaca oleracea L. allelopathy. Fresenius Environ. Bull. 2015, 24, 386–393. [Google Scholar]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Arora, K.; Kohli, R.K. α-Pinene inhibits growth and induces oxidative stress in roots. Ann. Bot. 2006, 98, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Staszek, P.; Krasuska, U.; Ciacka, K.; Gniazdowska, A. ROS metabolism perturbation as an element of mode of action of allelochemicals. Antioxidants 2021, 10, 1648. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Ortega, R.; Lara-Núñez, A.; Anaya, A.L. Allelochemical stress can trigger oxidative damage in receptor plants: Mode of action of phytotoxicity. Plant Signal. Behav. 2007, 2, 269–270. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).