Abstract
A new multi-residue method using gas chromatography–mass spectrometry electron ionization selective ion monitoring mode (SIM) has been developed for the simultaneous determination of eight 1,3,5-triazine herbicides such as 1,3,5-triazine-2,4-diamine (atrazine), ametryn, prometryn, propazine, terbuthylazine, terbutryn, and simazine simetryn in water and soil samples. Quantification is done using lindane (gamma benzene hexachloride) as an internal standard. A specific Capillary DB-Wax column of 30 m length, 0.32 mm internal diameter, and 0.25 µm film thickness is used for the separation of eight 1,3,5-triazine-2,4-diamine. The method was applied for the determination of residues in groundwater and soil samples. The lowest detection limit by GC-MS-SIM (selective ion monitoring mode) is 0.1 pg/mL. Recovery in water samples is in the range of 93–103%, and in soil samples, 91–102% for different individual compounds. Forty-five groundwater and soil samples were collected in and around Kancheepuram district in Tamilnadu (India), and they were analyzed for the respective residues. A detailed discussion of the GC-MS analysis results has been presented.
1. Introduction
Herbicides are agro-chemical substances that inhibit or interrupt plant growth and cause huge losses for agricultural businesses. They are widely used in weed management. Herbicides are cost-effective in weed control with minimum labor [1]. However, improper herbicide use can always result in crop injury, environmental contamination, and pose a threat to the applicator. For many years, triazine herbicides have been used in the agriculture industry for corn and sugarcane fields and have shown that herbicides are more persistent in the water body. The environmental fate of residues was found in a number of authors as listed in water [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20], in soil [16,17,18,19,20,21,22,23,24], and in plants determined [21,22,23,24,25], blood/plasma [26,27,28,29,30,31], saliva/urine [9,10], milk [5], mode of action, metabolism, photostability [32], decontamination [33], bioaccumulation [34], and mammalian toxicity [15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] have been widely described by different researchers [35,36,37,38,39,40].
The majority of the reports say that the most heavily used triazine herbicide, atrazine, is relatively persistent in the environment and has been found in drinking water supplies. This is of significant concern because atrazine is a mammalian carcinogen [22] endocrine disrupter and suppresses immune function in males [38]. Numerous analytical methods are available for determining residues of different individual triazine herbicides in various crops, water, and soil. Nevertheless, reported work clearly shows that many of the monitoring studies are based on individual herbicides. In view of the greater demand for miniaturized techniques in monitoring human health and environment exposure [39,40,41,42,43,44,45,46] and in continuation of our investigations on occupational exposure of herbicides in soil and water bodies.
The residual nature of herbicide atrazine and its metabolites was determined simultaneously using the LCMS/MS method in soil and water [43] from agricultural production, leading to environmental contamination. The method was sensitive to detecting the sample and concluded that the soil contamination was higher than in water due to the strong adsorption of atrazine into the soil, compared to the leaching in water with various pH and climatic changes. The method recovery of LCMS analysis of soil, surface water, and groundwater was in the range of 75% to 94%, 78% to 90%, and 83% to 106% in three difference fortified levels of 0.1 µg/kg, 0.5 µg/kg, and 1.0 µg/kg, respectively.
The relevance of the abovementioned eight herbicides was present in the environment from the wastewater treatment and led to the impact on sensitive aquatic species in the food chain [44]. The determination of the presence of the triazine herbicide was assessed by using the GCMS method. The toxicity to algae and daphnia was evaluated for the 11 triazine herbicides, proving the treated wastewater still has the contamination of residual level to affect such aquatic species by GCMS. The solid phase extraction of the matrix (wastewater) was done for the GC-MS analysis.
The crop safety and soil degradation of triazine compared with chloro sulfuron and its derivatives were evaluated by the proton and carbon-13 NMR techniques [45]. The newly synthesized triazine herbicides in the 4,6 di-substitution accelerated the high degradation rate on acidic and alkaline soil. The alkaline soil resulted in a 22 times more degradation rate compared with the chloro sulfuron herbicide. Pyrimidine and triazine herbicides were also confirmed with HRMS for the purity of molecular mass and structural relationship for high degradation pattern compared with existing sulfonylurea herbicides. The efficacy of the herbicide and crop safety was maintained by the substitute triazine herbicide and the pyrimidine in soil [46].
We exhaustively reviewed the removal of atrazine and evaluated the sample by different analytical methods, including a review of different techniques to remove atrazine in contaminated soil and water samples [46]. Ozone oxidation and photocatalysis of the atrazine were also studied, and we quantified the amount of atrazine degradation. The TiO2 photocatalyst is employed as the proven catalyst in the removal of atrazine herbicide in the environmental soil and water. In the biological method, different biological strains were used to degrade the atrazine at 9 mg to 300 mg/L from 3 hr to 30 hrs. Compared to the phytoremediation method using effective biological removal was obtained in the material combined with the biological strains. There were several methods used to achieve the removal of atrazine from the environmental samples. Chemical and biological, including photochemical and photobiological methods, are reviewed for the removal of atrazine in the soil and water samples.
The cost-effective analytical method was developed based on bio-enzymatic sorption and detection. Furthermore, biosensors for the control of pesticide contamination in the environmental samples by amperometric detectors used were developed [47]—the photosystem II affective triazine herbicide with the LOD of 2 nM to 10 nM. Atrazine and simazine triazine herbicides are the major PSII system inhibited for synechococcous bi granualtus photosynthetic enzyme. By using the fluorescence detector, the whole cells of the chlamydomonas reinhardt enzyme inhibition were detected for atrazine and propazine at 0.5 nM. The method was validated with a 0.2 ppt–2 ppb linear range in the amperometric detection method.
Atrazine concentration and the microbial growth of the water circulation body and the stagnant water body were evaluated [48]. In both cases, the atrazine dissipation and microbial growth were very significant, as per the statistical analysis. The validated HPLC (high-pressure liquid chromatography) method was detected at LOD from 2.0 to 2.6 µM. The atrazine degradation was quantified in the sediment slurries as low as 0.8–1.9 mg/L. The liquid phase of atrazine concentration was 2.6 µg/L, and the sediment contained 8.6 µg/g. The degradation compounds of atrazine were not found in the sediments. The sensitivity of the method of detection was very low in comparison with the advanced LCMS method.
The paper by Barry J. Allred titled “Batch Test Screening of Industrial Product/Byproduct Filter Materials for Agricultural Drainage Water Treatment” investigates the effectiveness of various industrial filter materials in treating agricultural drainage water [49]. The study used a batch testing method to evaluate six different filter materials, including coal slag, crushed glass, sand, zeolite, clinoptilolite, and biochar. The objective was to identify filter materials that could remove nutrients and sediment from agricultural drainage water before being discharged into surface waters. The results showed that all the filter materials effectively removed nutrients and sediment from the water. However, some materials performed better than others. Zeolite, clinoptilolite, and biochar were the most effective filter materials for removing nutrients, while coal slag, crushed glass, and sand were more effective in removing sediment. The study also found that the performance of the filter materials was affected by factors such as pH, contact time, and flow rate. Overall, the study concludes that industrial filter materials can be an effective and affordable solution for treating agricultural drainage water. The findings suggest that a combination of filter materials may be necessary to achieve optimal results, and further research is needed to evaluate the long-term performance of these materials. The study provides important insights into sustainable water management practices for agricultural systems, essential for protecting the environment and human health.
The paper “Effect of the Presence of Nonionic Surfactant Brij35 on the Mobility of Metribuzin in Soil” investigates the impact of the nonionic surfactant Brij35 [50] on the mobility of the herbicide metribuzin in soil. The study aimed to understand the factors affecting the behavior and fate of metribuzin in the environment, as it is a commonly used herbicide in agricultural systems. The results of the study showed that the addition of Brij35 to soil significantly increased the mobility of metribuzin. This was attributed to the increased solubility of metribuzin in the presence of the surfactant, leading to increased leaching and potential groundwater contamination. The study also found that the mobility of metribuzin was influenced by soil properties such as organic matter content and pH. The study findings have important implications for pesticide management and environmental protection. The use of surfactants in pesticide formulations can significantly impact the behavior and fate of pesticides in the environment. The results suggest that adding surfactants to herbicide formulations should be carefully considered to minimize the potential for groundwater contamination. Overall, the study provides important insights into the factors affecting the mobility of metribuzin in soil and highlights the importance of sustainable pesticide management practices to protect the environment and human health.
The preparation and application of simetryn-imprinted nanoparticles (SINPs) for analyzing triazine herbicide residues were discussed in [51]. Synthesized SINPs used a precipitation polymerization method with simetryn as the template molecule, methacrylic acid as the functional monomer, and ethylene glycol dimethacrylate as the cross-linker. The synthesized SINPs were characterized using scanning electron microscopy, Fourier-transform infrared spectroscopy, and thermogravimetric analysis. The results showed that the SINPs had a uniform size distribution and high selectivity for simetryn. The synthesized SINPs were then applied to determine simetryn residues in soil and water samples. The samples were extracted using a Quenchers method, and the extracted simetryn was purified using the SINPs. The purified samples were then analyzed using high-performance liquid chromatography. The results showed that the SINPs had a high selectivity for simetryn and could effectively remove interference from other triazine herbicides. The method was found to have good linearity, sensitivity, and accuracy. In conclusion, the authors demonstrated that SINPs could be used as effective sorbents to analyze simetryn residues in soil and water samples. The method has the advantages of high selectivity, sensitivity, and accuracy and can be used for the rapid detection of simetryn residues in environmental samples.
The paper presents a streamlined method for the determination of triazine herbicides and their metabolites in multiple medicinal parts of traditional Chinese medicines using ultra-fast liquid chromatography-electrospray ionization tandem mass spectrometry (UFLC-ESI-MS/MS) [52]. The authors developed an extraction method that uses a mixture of acetonitrile and water for the pretreatment of samples. The extracted samples were then cleaned up using solid-phase extraction cartridges before being analyzed by UFLC-ESI-MS/MS. The developed method was validated by analyzing different medicinal parts of traditional Chinese medicines, including roots, stems, leaves, flowers, and fruits. The results showed that the developed method had good linearity, accuracy, precision, and sensitivity. The limits of detection and quantification for the analytes ranged from 0.01 to 0.05 ng/g and 0.03 to 0.17 ng/g, respectively. The recoveries of the analytes ranged from 76.6% to 104.9%, and the relative standard deviations were below 10%. The developed method was then applied to the analysis of real traditional Chinese medicine samples. The method has the advantages of high sensitivity, accuracy, and precision and can be used to analyze other complex samples.
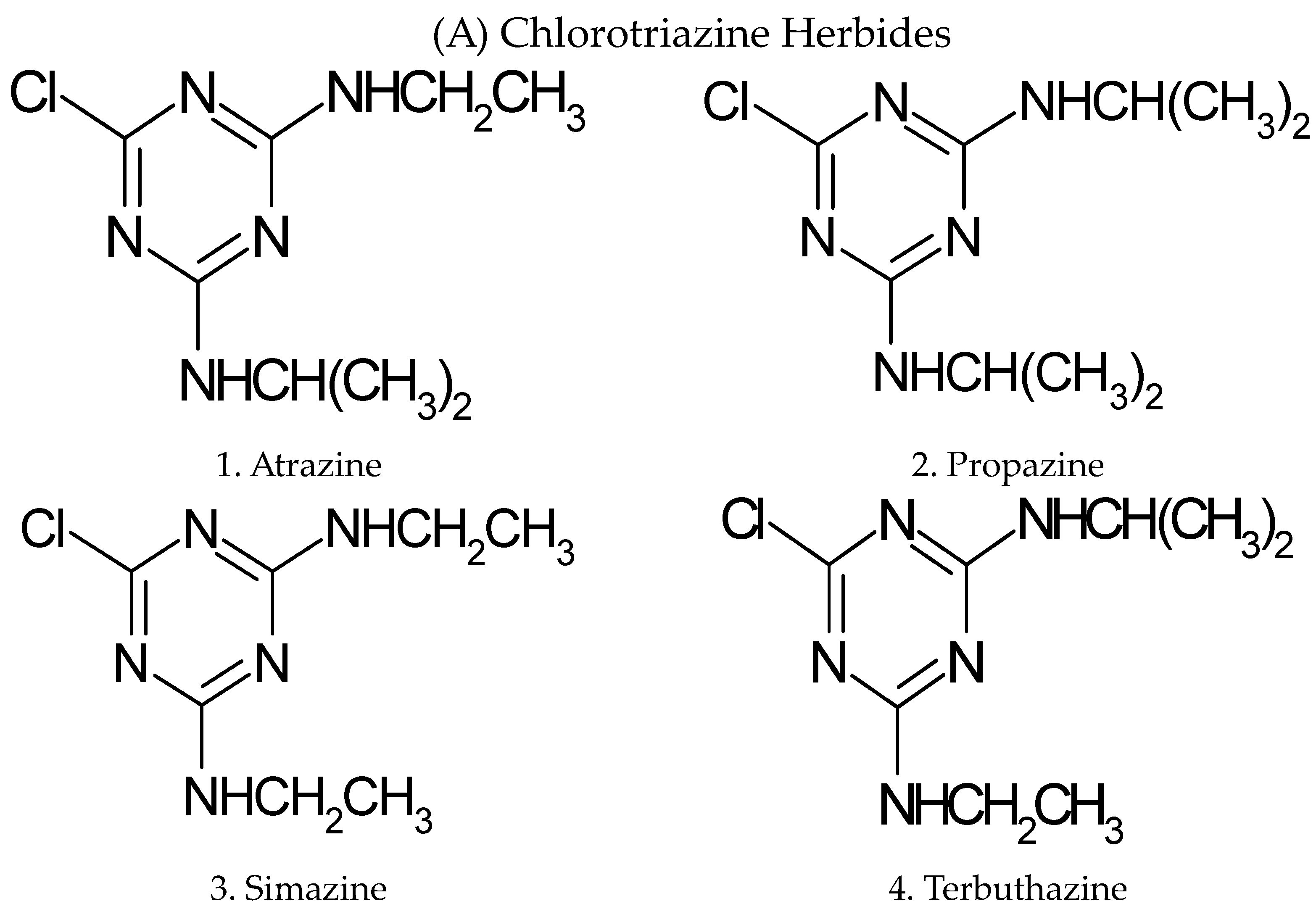
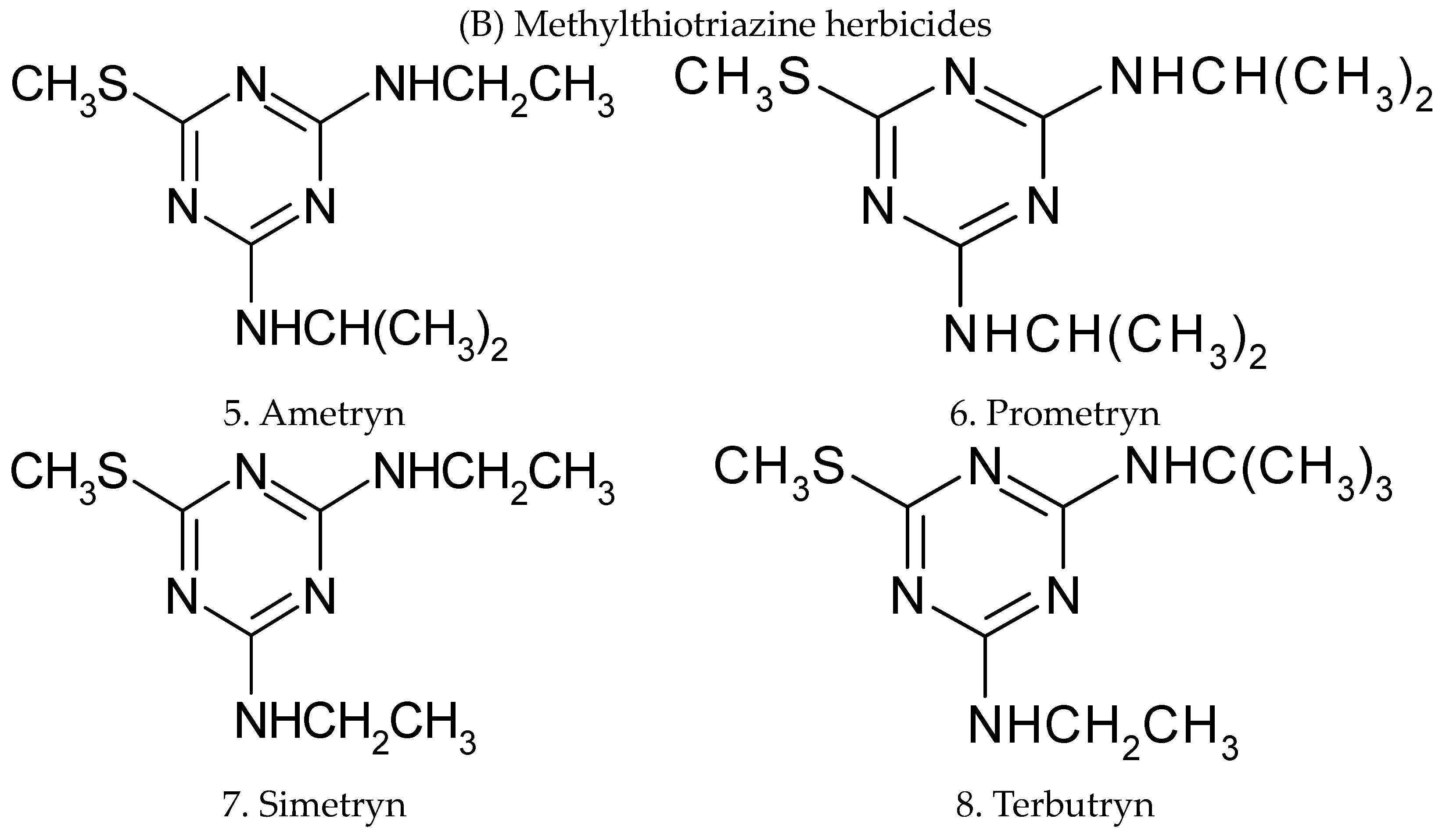
A new rapid and highly sensitive analytical technique has been developed for the sequential determination of residues of eight triazine herbicides in water and soil. The method has been successfully applied to evaluate residual contamination groundwater and soil in Kancheepuram district, Tamilnadu state, India. The selected 1,3,5-triazine 2,4 diamine herbicides are classified under the heading chlorotriazine herbicides (atrazine, propazine, simazine, and terbuthalazine) and methylthiotriazine herbicides (ametryn, prometryn, simetryn, and terbutryn), they are shown in Figure 1.


Figure 1.
Structural representation of 1,3,5-triazine-2,4-diamine herbicides.
2. Materials and Methods
2.1. Reagents and Chemicals
The chemicals used in the study were pure and organic trace analysis grade (E.merck, Darmstadt, Germany). The details of analytical reference standards of different triazine herbicides, atrazine (99.0%), propazine (99.0%), terbuthalazine (98.1%), terbutryn (98.2%), simazine (99.0%), prometryn (99.5%), and simetryn (98.0%) were obtained from Chem. service, (West Chester, PA, USA). Ametryn (98.2%) and lindane (IS) (99.9%) were obtained from PESTANAL, Riedel de-Haen., (Seelze, Germany).
2.2. Methods
Gas Chromatograph–Mass Spectrometry
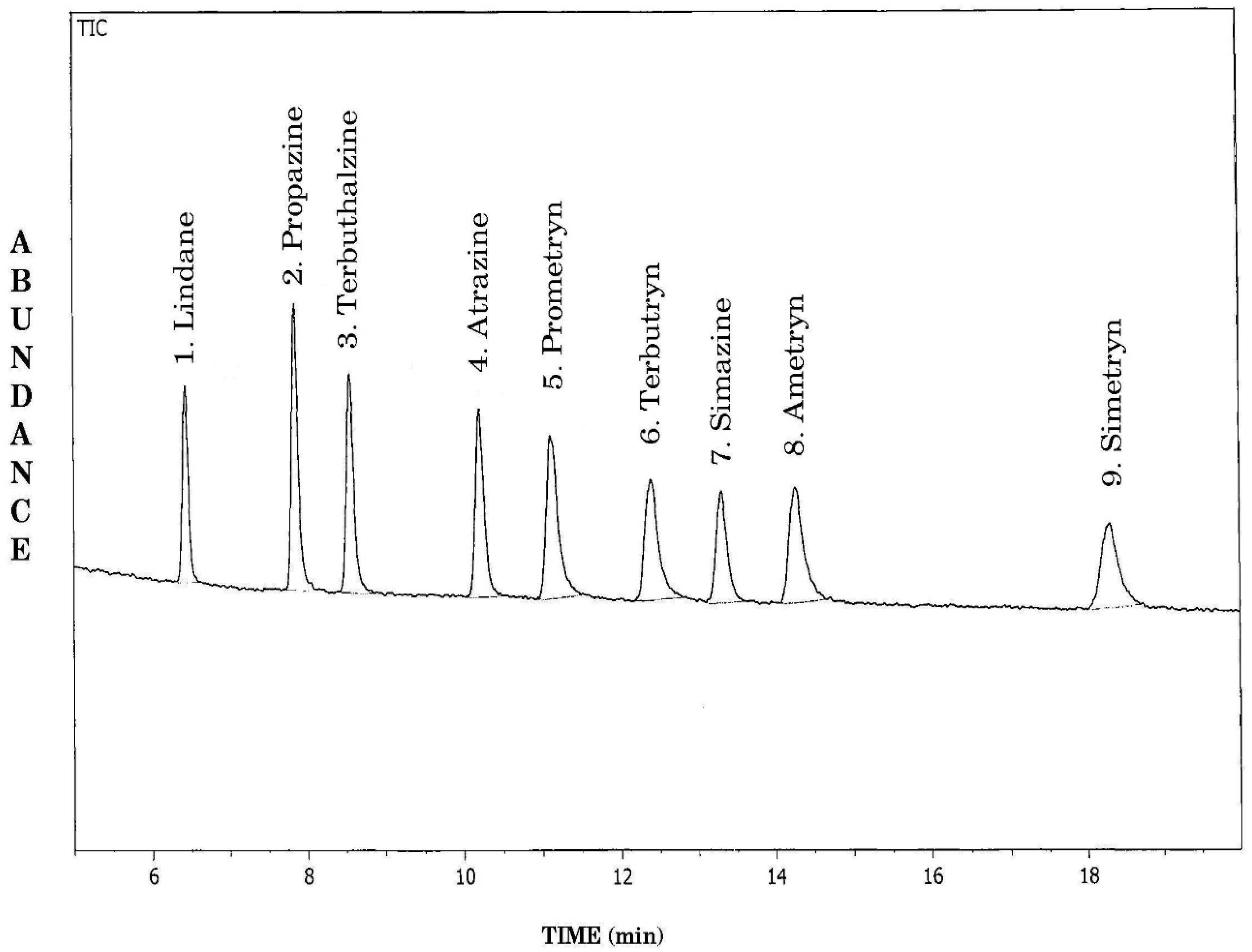
A gas chromatograph–mass spectrometer (GC-MS-EI) attached with an AOC 20 i auto-injector (Shimadzu Corporation, Tokyo, Japan), model GC-MS QP-5050 A interfaced to a computer for data acquisition supported by Class-5000 software was used. A Shimadzu GC-17 A gas chromatograph, a DB-Wax capillary column of length 30 m, 0.32 mm internal diameter, and 0.25 µm film thickness (J&W scientific, Folsom, CA, USA), was used for the quantification purpose. Helium was used as the carrier gas at a constant column flow rate of 1.0 mL min−1. The temperature conditions were: column oven—initial 210 °C for isothermal, for 20 min; injector and interface temperature were kept at 220 °C and 240 °C, respectively. Split ratio was 1:5. The total ion chromatogram (TIC) of GC-MS scan spectra (Figure 2) recorded in electron impact ionization mode shows the following retention times; atrazine (10.07 min), ametryn (14.07 min), prometryn (10.94 min), propazine (7.72 min), terbuthalazine (8.43 min), terbutryn (12.19 min), simazine (13.14 min), simetryn (18.03 min), and lindane (γ-BHC or gamma benzene hexachloride) (6.43 min) was used as the internal standard. The selective ion monitoring (SIM) method was adopted to determine triazine residues. Details of ions selected for quantification proposes are presented in Table 1.

Figure 2.
GCMS-EI total ion chromatogram of different 1,3,5-triazine herbicide standards in scan mode. 1-lindane (internal standard), 2-propazine, 3-terbuthalazine, 4-atrazine, 5-prometryn, 6-terbutryn, 7-simazine, 8-ametryn, 9-simetryn.

Table 1.
Ions used for detection and quantification of 1, 3, and 5-triazines.
2.3. Preparation of Reference Analytical Standard Stock Solutions
The 1000 mg L−1 stock solutions of all the individual triazine herbicides were prepared in separate volumetric flasks using trace analytical grade acetone. The different working standard solutions were prepared by suitable aliquots of the standard solutions and diluted with acetone. An Artic 380 deep freezer (Froilabo, Meyzieue, France), with an automatic temperature recorder and display facility, was used for storing the stock solutions (at −25 °C). The pretreatment procedure is to sample concentration with Buchi Rotavapor and resolve in acetonitrile and then filter through a 0.22-micron syringe filter before each analysis.
2.4. Collection of Water and Soil Samples
Groundwater and soil (0–15 cm depth) samples are collected in and around Kancheepuram, Tamilnadu, India.
2.5. Extraction of Residues from Water
A 50 mL water sample was taken in a 500 mL separatory flask, and dilute sodium hydroxide solution was added until the solution reached pH 8–9 (indicate paper) and was extracted twice with 100 mL of chloroform in a separating funnel. The chloroform extract was dried with anhydrous sodium sulfate and evaporated to dryness in a rotary vacuum evaporator.
2.6. Extraction of Residues from Soil
A total of 50 g of representative soil sample (≈20–30% moisture) was taken in a 500 mL extraction flask with added 100 mL methanol and shaken for 2 h using a mechanical shaker. The total extracts were transferred into a 500 mL separating funnel. We diluted it with the same amount of water and added 20 mL of aqueous sodium chloride solution, and shook it with a 100 mL portion of chloroform. The combined total extract was dried with anhydrous sodium sulfate and evaporated to dryness in a rotary vacuum evaporator.
2.7. Column Chromatography
A total of 10 g of alumina (grade V) was filled in a chromatographic column using n-hexane, and the sample was dissolved in 5 mL of benzene poured into the column. We washed the column with 40 mL of n-hexane. Finally, we eluted the residues with 150 mL of n-hexane and ethyl acetate (2:1 v/v). Then, we evaporated the solvent to dryness in a rotary vacuum evaporator and added 5 mL of n-hexane: ethanol (1:1 v/v) to the residue and added a known concentration (20 µL) of lindane as the internal standard and analyzed by GC-MS-EI in selective ion monitoring mode. The DB wax is highly stable at high temperatures and has a good resolution of the compound without affecting the target molecules.
2.8. Recovery Process and Methodology Study
A mixture of triazines atrazine, ametryn, prometryn, propazine, terbuthalazine, terbutryn, simazine, and simetryn were spiked with control samples from the stock solutions. All experiments were conducted at six concentration levels: 10, 20, 40, 60, 80, and 100 pg mL−1 of water and pg g−1 soil. Water and soil samples were collected and fortified with a known amount of 1,3,5-triazine 2,4 diamine standards with regular shakings. The samples were allowed to equilibrate and extracted by the above methods.
2.9. Method Validation and Demonstration
After the identification of the respective triazines in the EI scan mode, two characteristic fragments of individual analytes were monitored in selective ion monitoring mode (SIM).
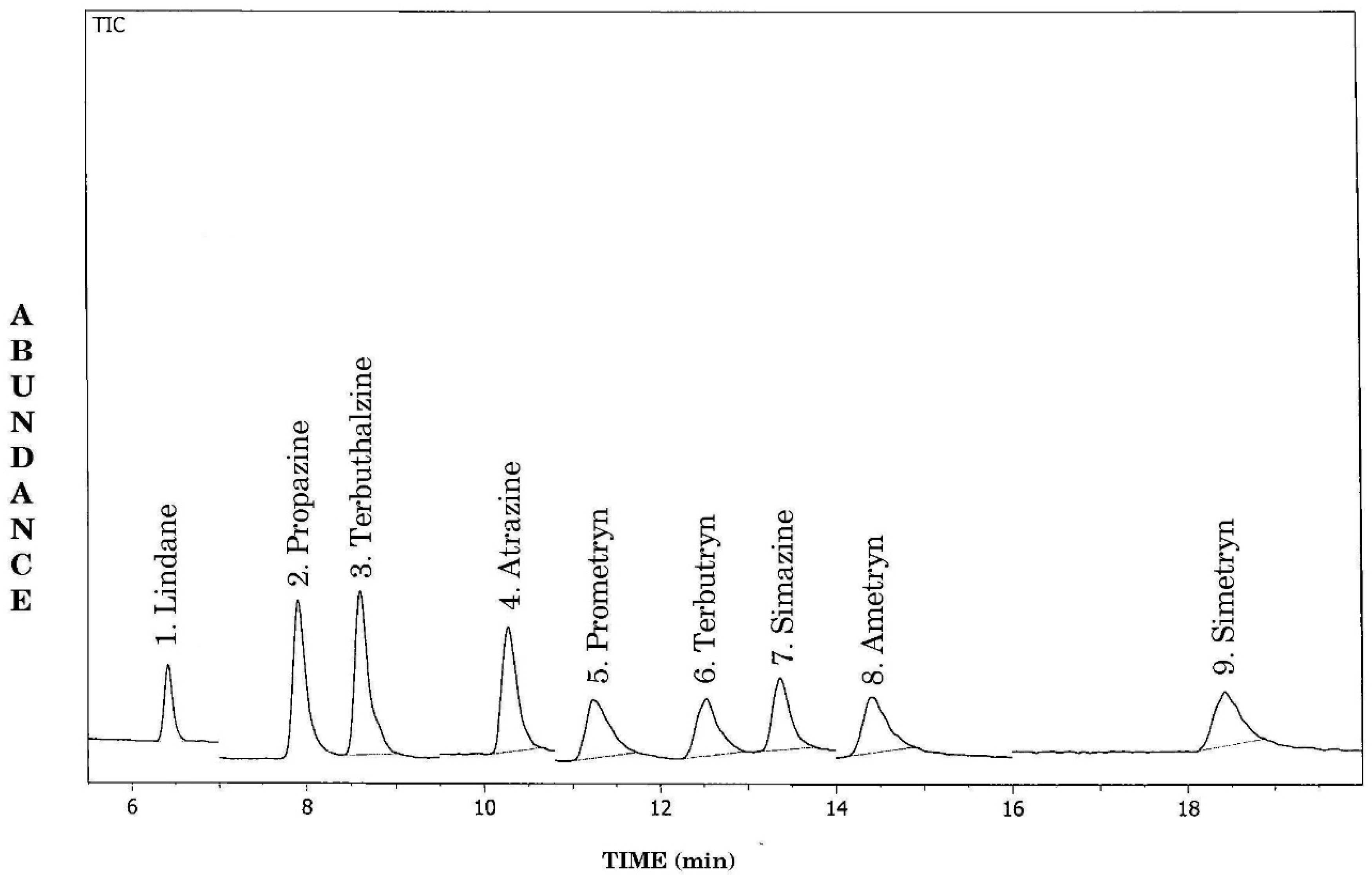
GCMS SIM mode is used at positive and negative electron ionization (GCMS-EI-SIM) for detecting the target molecules. Evolution was done by the peak area calculation of the active substance (Figure 3 and Figure 4). The linear regression analysis method is adopted for each analyte over the concentration range between 1 and 100 pg mL−1. The linearity of the method was investigated by calculation of the regression line using least squares and expressed as correlation coefficient R2. Once R2 had been achieved between the 0.99 and 1.00 range, linear regression equations were used to quantify the analytes in samples. The linearity of each compound was measured at six levels. The limit of detection (LOD) and limit of quantification (LOQ) parameters were used to study the sensitivity of the method. The lowest LOD was measured as the quantity of analyte needed to give a response of three times higher than the baseline noise at the expected retention time of the analyte in the chromatogram of a non-fortified water extract. The LOQ was measured by the lowest standard with a signal:noise ratio of at least five. Table 2 demonstrates the results of the method validation as a mean of six replicate analyses.

Figure 3.
GCMS-EI total ion chromatogram of different 1,3,5-triazine2,4 diamine herbicide standards in SIM mode 1—lindane (internal standard), 2—propazine, 3—terbuthalazine, 4—atrazine, 5—prometryn, 6—terbutryn, 7—simazine, 8—ametryn, 9—simetryn.

Figure 4.
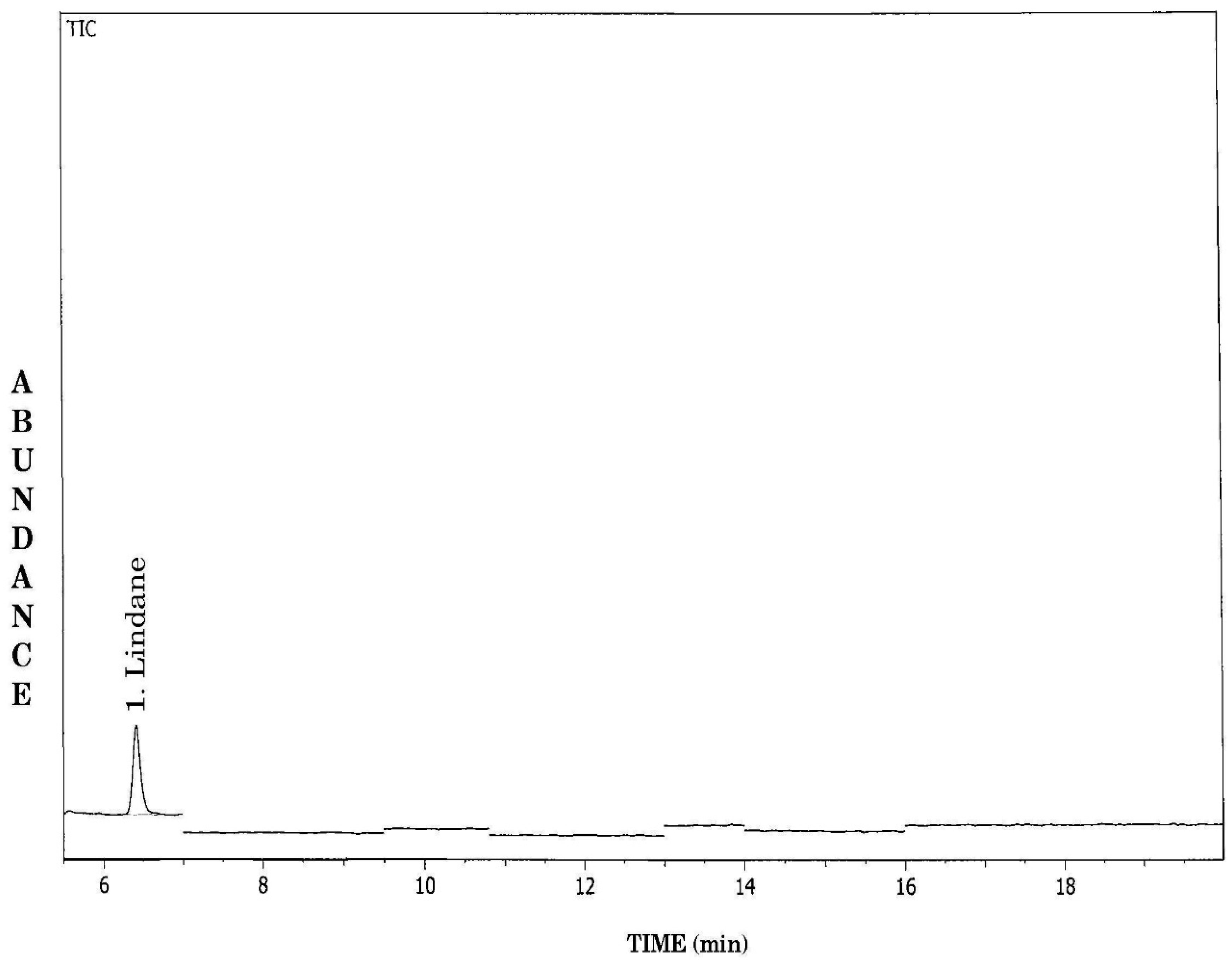
GCMS-EI total ion chromatogram of water sample in SIM mode, 1-lindane (internal standard).

Table 2.
LODs, LOQs, and calibration results.
3. Results and Discussion
Simultaneous and rapid determination of 1, 3, 5-triazine herbicides is more difficult when compared with other herbicides because the majority of the compounds structurally contain 1,3,5-triazine2,4 diamine content. Hence, this study focuses on the simultaneous determination of eight triazine herbicide residues that were quantified as a mixture. Additional chromatographic peaks do not appear in the sample analysis. The result clearly shows that the analysis of water samples showed a recovery of 93–103%, and soil samples showed a recovery of 91–102% (Table 3 and Table 4). The data clearly shows that the deviations in the measurements are within 4%. The study was tested by analyzing real samples using the optimized recommended procedure described earlier. Different water and soil samples were collected from vulnerable sites in and around Kancheepuram, Tamilnadu, India, where extensive agriculture is practiced; the results are tested for the residues. Samples were collected during mid-summer. The maximum temperature during the day is 41 °C. Further emphasis was given to collecting the water sample nearer to small ponds/lakes where acute water shortage is experienced. The results of the residue analysis are shown in Table 5.

Table 3.
Recovery percentage range of 1, 3, 5-triazine herbicides in water samples *.

Table 4.
Recovery percentage range of 1, 3, 5-triazine herbicides in soil samples *.

Table 5.
Analysis of groundwater samples collected in and around Kancheepuram *.
A modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) method for the determination of triazine herbicides in fish and seafood using ultra-high-performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). The authors developed a modified QuEChERS method [53] that uses ethyl acetate as the extraction solvent and magnesium sulfate, sodium chloride, and primary–secondary amine as the sorbents for the cleanup of the extracted samples. The developed method was validated using spiked fish and seafood samples, and the results showed that the method had good linearity, accuracy, precision, and sensitivity. The limits of detection and quantification for the analytes ranged from 0.004 to 0.008 mg/kg and 0.01 to 0.02 mg/kg, respectively. The recoveries of the analytes ranged from 76.8% to 110.2%, and the relative standard deviations were below 10%. The developed method was then applied to analyze real fish and seafood samples. The results showed that none of the samples were contaminated with triazine herbicides, indicating that the risk to human health from these contaminants in fish and seafood is low. In conclusion, the developed method provides a simple, fast, and reliable approach for determining triazine herbicides in fish and seafood using UHPLC-MS/MS. The method has the advantages of high sensitivity, accuracy, and precision and can be used to analyze other complex samples.
The paper reports on a study of the occurrence and health risks of triazine herbicides in drinking water in the Yangtze River Delta region of China [54]. The authors collected water samples from 20 drinking water treatment plants and analyzed them for the presence of 10 triazine herbicides using solid-phase extraction and liquid chromatography-tandem mass spectrometry. The results showed that six of the ten triazine herbicides were detected in the drinking water samples, with the most frequently detected herbicides being atrazine and simazine. The concentration of the detected herbicides ranged from below the limit of quantification to 7.92 ng/L, with atrazine and simazine being the most abundant. The authors also calculated the health risk associated with ingesting the detected herbicides and found that the risk was low for most drinking water samples but exceeded the acceptable level in one sample. The study highlights the potential risk to human health from the presence of triazine herbicides in drinking water in the Yangtze River Delta region. The authors suggest that further monitoring and management of these herbicides in the region’s water sources is necessary to ensure the safety of drinking water.
This paper investigates the occurrence, fate, seasonal variability, and risk assessment of twelve triazine herbicides and eight related derivatives in the water supply of Wuhan, Central China [55]. The study found that six of the twelve herbicides and three of the eight derivatives were detected in the water samples, with the most frequently detected herbicides being atrazine, simazine, and propazine. The concentrations of the detected compounds varied with the seasons, with higher concentrations observed in summer and autumn. The risk assessment of the detected compounds indicated low risk to human health, with hazard quotient values below 1.0. The study highlights the need for further monitoring and management of these compounds in the region’s water sources to ensure the safety of drinking water. The simultaneous determination of six triazine herbicides and their eight metabolites in shellfish using high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) combined with quadrupole/exactive-Orbitrap high-resolution mass spectrometry [56] (Q/E-Orbitrap HRMS). The authors extracted the target analytes from shellfish samples using a modified QuEChERS method and detected them using the developed HPLC-MS/MS method. The results showed that all six triazine herbicides and seven of the eight metabolites were detected in the shellfish samples. The most frequently detected herbicides were atrazine and simazine, while the most frequently detected metabolites were deethylatrazine and hydroxyatrazine. The concentrations of the detected compounds ranged from 0.011 to 10.16 µg/kg wet weight. The authors also evaluated the method’s accuracy, precision, and recovery rate, which were satisfactory for detecting the target analytes in shellfish samples. Overall, the developed method demonstrated high sensitivity and selectivity for determining triazine herbicides and their metabolites in shellfish. The study provides useful information on the occurrence and levels of triazine herbicides and their metabolites in shellfish and highlights the importance of monitoring these compounds in seafood to ensure food safety. The developed method has the potential for application in the routine monitoring of these compounds in shellfish and other seafood.
This paper aims to develop a simple, sensitive, and reliable method for determining herbicide residues in soil using a gas chromatography flame ionization detector (GC-FID). The authors used three commonly used herbicides, namely acetochlor, atrazine, and imidacloprid [57], as test analytes. The extraction and cleanup of soil samples were optimized using different solvent systems and solid-phase extraction cartridges, respectively. The GC-FID method was validated in terms of linearity, limit of detection, and accuracy and was found to be suitable for the analysis of herbicide residues in soil samples. The results showed that the proposed method could detect low levels of herbicide residues in soil, with recoveries ranging from 77.1% to 96.7%. The authors concluded that the GC-FID method is a reliable and effective technique for determining herbicide residues in soil, which could be useful in environmental monitoring, risk assessment, and regulatory compliance.
This study aimed to develop a sensitive and reliable method for determining herbicide residues in food using gas chromatography with flame ionization detection (GC-FID) [58]. The authors analyzed six commonly used herbicides, namely atrazine, simazine, metolachlor, alachlor, acetochlor, and propisochlor, in food samples. The extraction and cleanup of food samples were optimized using different solvent systems and solid-phase extraction cartridges. The GC-FID method was validated in terms of linearity, limit of detection, and accuracy and was found to be suitable for analyzing herbicide residues in food samples. The results showed that the proposed method could detect low levels of herbicide residues in food, with recoveries ranging from 70.6% to 103.5%. The authors concluded that the GC-FID method is a reliable and effective technique for determining herbicide residues in food, which could be useful in food safety monitoring, risk assessment, and regulatory compliance.
A sensitive and reliable method was developed for determining herbicide residues in environmental water samples using gas chromatography with flame ionization detection (GC-FID). The authors focused on analyzing five commonly used herbicides, namely acetochlor, atrazine, simazine, propazine, and alachlor, in water samples [59]. The extraction and cleanup of water samples were optimized using different solvent systems and solid-phase extraction cartridges. The GC-FID method was validated in terms of linearity, limit of detection, and accuracy and was found to be suitable for the analysis of herbicide residues in environmental water samples. The results showed that the proposed method could detect low levels of herbicide residues in water, with recoveries ranging from 77.6% to 103.5%. The authors concluded that the GC-FID method is a reliable and effective technique for determining herbicide residues in environmental water samples, which could be useful in environmental monitoring, risk assessment, and regulatory compliance.
A sensitive and reliable method for the determination of herbicides in water and soil using gas chromatography with flame ionization detection (GC-FID) and mass spectrometry (GC-MS) was developed [60] using six commonly used herbicides, namely acetochlor, alachlor, metolachlor, atrazine, simazine, and propazine, as test analytes. The extraction and cleanup of water and soil samples were optimized using different solvent systems and solid-phase extraction cartridges, respectively. The GC-FID and GC-MS methods were validated in terms of linearity, limit of detection, and accuracy and were found to be suitable for the analysis of herbicides in water and soil samples. The results showed that the proposed methods could detect low levels of herbicide residues in water and soil, with recoveries ranging from 70% to 120%. The authors concluded that the combined GC-FID and GC-MS methods are reliable and effective techniques for determining herbicides in water and soil, which could be useful in environmental monitoring, risk assessment, and regulatory compliance.
Quantitative analysis of herbicides in soil using gas chromatography with flame ionization detection and mass spectrometry by Thijs Koelmans et al. presents a study aimed at developing a sensitive and reliable method for the quantitative analysis of herbicides in soil using gas chromatography with flame ionization detection (GC-FID) and mass spectrometry (GC-MS) [61]. The authors focused on five commonly used herbicides, namely atrazine, simazine, diuron, terbuthylazine, and metolachlor, as test analytes. The extraction and cleanup of soil samples were optimized using a Quenchers (quick, easy, cheap, effective, rugged, and safe) approach. The GC-FID and GC-MS methods were validated in terms of linearity, limit of detection, and accuracy and were found to be suitable for the quantitative analysis of herbicides in soil samples. The results showed that the proposed methods were able to detect low levels of herbicide residues in soil, with recoveries ranging from 70.9% to 116.6%. The authors concluded that the combined GC-FID and GC-MS methods are reliable and effective techniques for the quantitative analysis of herbicides in soil, which could be useful in environmental monitoring, risk assessment, and regulatory compliance.
Synthesis and herbicidal activity of novel pyridine carboxylic acid derivatives containing 1,3,4-thiadiazole moiety by Chen et al., published in the Journal of Agricultural and Food Chemistry in 2019, reports on the design, synthesis, and herbicidal activity of a new series of pyridine carboxylic acid derivatives containing a 1,3,4-thiadiazole moiety [62]. The authors aimed to synthesize novel herbicides that could exhibit higher efficiency and lower toxicity than the currently used herbicides. The synthesized compounds were characterized by spectroscopic techniques, and their herbicidal activities were evaluated against several weeds. The results showed that some of the synthesized compounds exhibited excellent herbicidal activities, with EC50 values ranging from 1.32 to 14.26 μg/mL, which were better than the commercial herbicide bispyribac-sodium. The structure–activity relationship (SAR) analysis indicated that the substitution patterns of the pyridine ring and the thiadiazole moiety were crucial factors affecting the herbicidal activities of the synthesized compounds. In conclusion, the study demonstrates that the new series of pyridine carboxylic acid derivatives containing a 1,3,4-thiadiazole moiety have the potential to be developed as new herbicides with high efficiency and low toxicity. Further research could be conducted to optimize the structure and improve the herbicidal activity of these compounds.
The effect of soil organic matter on the adsorption and leaching of imazethapyr and pendimethalin herbicides in soils by Xu et al., published [63] in Environmental Science and Pollution Research in 2019, investigates the effect of soil organic matter (SOM) on the adsorption and leaching behavior of two commonly used herbicides, imazethapyr and pendimethalin, in soils. The study showed that SOM significantly affects the adsorption and leaching of these herbicides, with higher SOM content leading to higher adsorption and lower leaching of the herbicides. The findings suggest that SOM content should be considered when predicting the environmental fate and behavior of herbicides in soils.
They developed a SERS-based immunoassay using gold nanoparticles functionalized with specific antibodies to detect traces of herbicides [64,65,66,67,68], such as atrazine and simazine, in soil and water samples. The study demonstrated that the SERS-based immunoassay had a low detection limit, high specificity, and excellent reproducibility for detecting herbicides in environmental samples. The method could detect concentrations as low as 0.05 μg/L in water samples and 0.1 μg/kg in soil samples, lower than the maximum residue limits set by regulatory agencies. The authors concluded that the SERS-based immunoassay is a promising technique for the rapid and sensitive detection of herbicide residues in environmental samples. The method has the potential to be used as a reliable tool for monitoring herbicide contamination in the environment and ensuring food safety.
A recent paper investigates the adsorption and degradation behavior of commonly used herbicides, including atrazine, bentazon, and simazine, in the paddy soils of Thailand [65]. The authors conducted laboratory experiments to determine the adsorption capacity and degradation rates of these herbicides in soils with varying physical and chemical properties. The study found that the adsorption capacity of the herbicides varied significantly among different soil types, with clay soils exhibiting higher adsorption capacity than sandy soils. Additionally, the degradation rates of the herbicides were affected by soil pH and microbial activity. The authors also found that the herbicides were degraded more quickly in paddy soils compared to upland soils due to the presence of waterlogged conditions in paddy fields. The findings of this study suggest that the fate and behavior of herbicides in paddy soils are influenced by several factors, including soil properties, environmental conditions, and microbial activity. The results could be used to improve the management practices for herbicide use in paddy fields, minimize the risk of contamination, and protect the environment and public health.
To investigate the adsorption behavior of commonly used herbicides, including atrazine, simazine, and metolachlor, on various soil minerals [66], including kaolinite, goethite, and gibbsite, the authors conducted laboratory experiments to determine the adsorption capacity of the herbicides on these soil minerals and compared their adsorption behavior. The study found that the adsorption capacity of the herbicides varied significantly among different soil minerals, with clay minerals exhibiting higher adsorption capacity than iron oxide minerals. Additionally, the authors found that the adsorption behavior of the herbicides was influenced by several factors, including pH, ionic strength, and the presence of organic matter. The findings of this study suggest that the fate and behavior of herbicides in soils are influenced by the mineral composition of the soil and other physicochemical factors. The results could be used to improve the understanding of the adsorption behavior of herbicides in soils and their potential impact on the environment and public health.
A novel analytical method for detecting herbicides in soil and water samples analysis of glyphosate, glufosinate, and aminomethylphosphonic acid in soil and water samples [67] using high-performance liquid chromatography with post-column derivatization was developed, using high-performance liquid chromatography (HPLC) with post-column derivatization to analyze glyphosate, glufosinate, and aminomethyl phosphonic acid (AMPA) in soil and water samples. The method involves the addition of a derivatization reagent to the eluent, which converts the analytes into fluorescent derivatives, allowing for their detection and quantification. The study found that the developed method was highly sensitive, with detection limits in the range of 0.2–0.4 μg/L for the three herbicides. The method also showed good reproducibility and accuracy, with recovery rates ranging from 79.3% to 98.3%. The findings of this study suggest that the developed method can be used for the reliable detection and quantification of glyphosate, glufosinate, and AMPA in soil and water samples. The method could be useful in monitoring the levels of these herbicides in the environment and assessing their potential impact on public health and ecosystems.
In our present work, almost all samples showed atrazine residues over the range of 15–32 pg mL−1 in water and 3–7 pg g−1 in soil samples, simazine showed 6–14 pg mL−1 of residues in water and 9 pg gL−1 in soil, while other triazine herbicides (prometryn, propazine, terbuthalazine, tertbutyl, simazine, and simetryn) established less than the lowest detection limits. The results are confirmed by suitably spiking a few exposed samples and subsequently by their positive detection. Thus, the method is found suitable for the analysis of residues in water and soil samples and is also applicable to the plant and blood samples.
From the above discussion of the reported studies, it can be concluded that the present method, which involves the gas chromatographic–mass-spectrometric electron impact ionization mode, simultaneous and rapid determination, has many advantages over the previously reported individual methods [8,9,10,11]. Data presented in Table 6 show the method to be a simultaneous determination of eight 1,3,5-triazine2,4 diamine herbicides and more sensitive than other techniques as reported in the literature [68,69,70,71,72,73,74,75,76,77,78,79]. In the present study, a high recovery rate and very low detection limits fill the gap towards an analytical method development for the simultaneous determination of residues of different structurally similar 1, 3, and 5-triazine compounds present in the water bodies.

Table 6.
Comparison of methods related to 3,5-triazine 2,4 diamine herbicides detection in water and soil.
4. Conclusions
In environmental samples in the study, mass spectrometry is the pre-eminent technique for characterizing small quantities of pesticides. This paper describes an isolation procedure and capillary column GC-MS-EI detection of structurally similar compounds with high selectivity and accuracy. The method is rapid and economical and provides a high degree of cleanup and determination for GCMS-EI selective ion monitoring. The developed method in the present study clearly achieved the aim and showed the potential and simultaneous determination of multiple 3,5-triazine 2,4 diamine herbicides. This simple method could be adopted without any matrix interferences up to 0.1 pg mL−1.
Author Contributions
Conceptualization, J.R.R.; methodology, P.E.T.; software, S.J.; validation, P.E.R. and P.E.T.; formal analysis, S.J., data curation, M.K.; writing—original draft preparation, P.E.T. and J.R.R.; writing—review and editing, H.A.-L.; funding acquisition, H.A.-L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the financial support through the Researchers Supporting Project number (RSP2023R54), King Saud University, Riyadh 11451, Saudi Arabia.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgments
Authors are thankful to the management of IIBAT, India, for their immense support in conducting this study. The author (Hamad Al-Lohedan) acknowledge the financial support through the Researchers Supporting Project number (RSP2023R54), King Saud University, Riyadh 11451, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bridges, D.C. A Simulation Analysis of the Use and Benefits of Triazine Herbicides. ACS Symposium SERIES 683: Triazine Herbicides: Risk Assessment; Ballantine, L.G., McFarland, J.E., Hackett, D., Eds.; American Chemical Society: Washington, DC, USA, 1998; p. 24. [Google Scholar]
- Bardalaye, P.C.; Wheeler, W.B. Gas chromatographic determination of ametryn and its metabolites in tropical root crops. J. Assoc. Off. Anal. Chem. 1984, 67, 280. [Google Scholar] [CrossRef] [PubMed]
- Battista, M.; Di Corcia, A.; Marchetti, M. Extraction and isolation of triazine herbicides from water and vegetables by a double trap tandem system. Anal. Chem. 1989, 61, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Brzezicki, J.M.; Andersen, M.E.; Cranmer, B.K.; Tessari, J.D. Quantitative identification of atrazine and its chlorinated metabolites in plasma. J. Anal. Toxicol. 2003, 27, 569. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, B.A.; Fultz, E.; Haack, K.W.; Vogel, J.S.; Gilman, S.D.; Gee, S.J.; Hammock, B.D.; Hui, X.; Wester, R.C.; Maibach, H.I. HPLC-accelerator MS measurement of atrazine metabolites in human urine after dermal exposure. Anal. Chem. 1999, 71, 3519–3525. [Google Scholar] [CrossRef]
- Balduini, L.; Matoga, M.; Cavalli, E.; Seilles, E.; Riethmuller, D.; Thomassin, M.; Guillaume, Y.C. Triazine herbicide determination by gas chromatography-mass spectrometry in breast milk. J. Chromatogr. B 2003, 794, 389–395. [Google Scholar] [CrossRef]
- Bouaid, A.; Martin-Esteban, A.; Fernandez, P.; Camara, F.C. Microwave-assisted extraction method for the determination of atrazine and four organophosphorus pesticides in oranges by gas chromatography (GC). J. Anal. Chem. 2000, 367, 291–394. [Google Scholar] [CrossRef]
- Bailey, R.; LeBel, G.; Lawrence, J.F. Gas-liquid chromatography of triazine herbicides as heptafluorobutyryl derivatives and some applications to analysis in foods. J. Chromatogr. A 1978, 161, 251–257. [Google Scholar] [CrossRef]
- Bardalaye, P.C.; Wheeler, W.B.; Meister, C.W.; Templeton, J.L. Capillary gas chromatographic determination of terbutryn and its degradation products in sorghum grain and confirmation of the compounds by mass spectrometry. Food Addit. Contam. 1985, 2, 283–294. [Google Scholar] [CrossRef]
- Bardalaye, P.C.; Wheeler, W.B. Capillary gas chromatographic determination of prometryn and its degradation products in parsley. J. Assoc. Off. Anal. Chem. 1985, 68, 750–753. [Google Scholar] [CrossRef]
- Bardalaye, P.C.; Wheeler, W.B.; Templeton, J.L. Gas chromatographic and mass spectrometric determination of ametryn and its N-dealkylated products. J. Assoc. Off. Anal. Chem. 1984, 67, 904–909. [Google Scholar] [CrossRef]
- Calderon, M.J.; Ortega, M.; Hermosin, M.C.; Garcia-Baudin, J.; Cornejo, J. Hexazinone and simazine dissipation in forestry field nurseries. Chemosphere 2004, 54, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dopico, M.S.; Gonzalez, M.V.; Castro, J.M.; Gonzalez, E.; Perez, J.; Rodríguez, M.; Calleja, A. Determination of triazines in water samples by high-performance liquidchromatography with diode-array detection. J. Chromatogr. Sci. 2002, 40, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Jesudoss, S.K.; Vijaya, J.J.; Kennedy, L.J.; Rajan, P.L.; Al-Lohedan, H.A.; Ramalingam, R.J.; Kaviyarasu, K.; Bououdina, M. Studies on the efficient dual performance of Mn1–xNixFe2O4 spinel nanoparticles in photodegradation and antibacterial activity. J. Photochem. Photobiol. B Biol. 2016, 165, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Dich, J.; Zahm, S.H.; Hanberg, A.; Adami, H.O. Pesticides and cancer. Cancer Causes Control. Cancer Causes Control. 1997, 8, 420–423. [Google Scholar] [CrossRef]
- Denovan, L.A.; Lu, C.; Hines, C.J.; Fenske, R.A. Saliva biomonitoring of atrazine exposure among herbicide applicators. Int. Arch. Occup. Environ. Health 2000, 73, 457–462. [Google Scholar] [CrossRef]
- Erickson, M.D.; Frank, C.W.; Morgan, D.P. Determination of s-triazine herbicide residues in urine: Analytical method development. J. Agric. Food Chem. 1979, 27, 740–743. [Google Scholar] [CrossRef]
- Ferrari, R.; Nilsson, T.; Arena, R.; Arlati, P.; Bartolucci, G.; Basla, R.; Cioni, F.; DelCarlo, G.; Dellavedova, P.; Fattore, E.; et al. Inter-laboratory validation of solid-phase microextraction for the determination of triazine herbicides and their degradation products at ng/l level in water samples. J. Chromatogr. A 1998, 795, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Funari, E.; Brambilla, A.L.; Camoni, I.; Canuti, A.; Cavallaro, A.; Chierici, S.; Cialella, G.; Donati, G.; Jaforte, A.; Prandi, L. Extensive atrazine pollution of drinking water in the Lombardia region and related public health aspects. Biomed. Environ. Sci. 1998, 1, 350–355. [Google Scholar]
- Ferris, I.G.; Haigh, B.M. A rapid and sensitive HPLC procedure for the determination of atrazine residues in soil-water extracts. J. Chromatogr. Sci. 1987, 25, 170–173. [Google Scholar] [CrossRef]
- Hequet, V.; Gonzalez, C.; Le Cloirec, P. Photochemical processes for atrazine degradation: Methodological approach. Water Res. 2001, 35, 4253–4260. [Google Scholar] [CrossRef]
- Friedmann, A.S. Atrazine inhibition of testosterone production in rat males following peripubertal exposure. Reprod. Toxicol. 2002, 16, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.D.; Gee, S.J.; Hammock, B.D.; Vogel, J.S.; Haack, K.; Buchholz, B.A.; Freeman, S.P.; Wester, R.C.; Hui, X.; Maibach, H.I. Analytical performance of accelerator mass spectrometry and liquid scintillation counting for detection of 14C-labeled atrazine metabolites in human urine. Anal. Chem. 1998, 70, 3463–3469. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Wintersteiger, R. Rapid screening of triazines and quantitative determination in drinking water. J. Biochem. Biophys. Methods 2002, 53, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Q. Simultaneous determination of alachlor, metolachlor, atrazine, and simazine in water and soil by isotope dilution gas chromatography/mass spectrometry. J. Assoc. Off. Anal. Chem. 1989, 72, 349–354. [Google Scholar] [CrossRef]
- Hernandez, F.; Hidalgo, C.; Sancho, J.V.; Lopez, F. Coupled-column liquid chromatography applied to the trace-level determination of triazine herbicides and some of their metabolites in water samples. J. Anal. Chem. 1998, 70, 3322–3328. [Google Scholar] [CrossRef]
- Harman, W.L.; Wang, E.; Williams, J.R. Reducing atrazine losses: Water quality implications of alternative runoff control practices. J. Environ. Qual. 2004, 33, 7. [Google Scholar] [CrossRef]
- Holland, D.C.; Munns, R.K.; Roybal, J.E.; Hurlbut, J.A.; Long, A.R. Liquid chromatographic determination of simazine, atrazine, and propazine residues in catfish. J. AOAC Int. 1995, 78, 1067–1071. [Google Scholar] [CrossRef]
- Hayes, T.B.; Collins, A.; Lee, M.; Mendoza, M.; Noriega, N.; Stuart, A.A.; Vonk, A. Hermaphroditic demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc. Natl. Acad. Sci. USA 2002, 99, 5476–5480. [Google Scholar] [CrossRef]
- Hernandez, F.; Beltran, J.; Lopez, F.J.; Gaspar, J.V. Use of solid-phase microextraction for the quantitative determination of herbicides in soil and water samples. Anal. Chem. 2000, 72, 2313–2322. [Google Scholar] [CrossRef]
- Hormann, W.D.; Formica, G.; Ramsteiner, K.; Eberle, D.O. Pesticide residues. Automated method for extraction; cleanup; and gas chromatographic determination of triazine herbicides in soil. J. Assoc. Off. Anal. Chem. 1972, 55, 1031–1038. [Google Scholar]
- Ismaili, L.; Andre, C.; Nicod, L.; Truong, T.T.; Mollet, J.; Thomassin, M.; Cavalli, E.; Chaumont, J.P.; Xicluna, A.; Guillaume, Y.C. Triazine-human serum albumin association: Thermodynamic approach and sodium effect. J. Chromatogr. B. Analyt Technol. Biomed. Life Sci. 2002, 780, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, M.; Sancho, J.V.; Pozo, O.J.; Hernandez, F. Use of quadrupole time-of-flight mass spectrometry in environmental analysis: Elucidation of transformation products of triazine herbicides in water after UV exposure. Anal. Chem. 2004, 76, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Jandera, P.; Spacek, M. Method for characterization of selectivity in reversed-phase liquid chromatography. III. Retention behavior in gradient-elution chromatography: Application to the chromatography of pesticide compounds. J. Chromatogr. 1986, 366, 107–126. [Google Scholar] [CrossRef]
- Kettles, M.K.; Browning, S.R.; Prince, T.S.; Horstman, S.W. Triazine herbicide exposure and breast cancer incidence: An ecologic study of Kentucky counties. Environ. Health Perspect. 1997, 105, 1222–1227. [Google Scholar] [CrossRef]
- Kim, K.R.; Son, E.W.; Hee-Um, S.; Kim, B.O.; Rhee, D.K.; Pyo, S. Immune alterations in mice exposed to the herbicide simazine. J. Toxicol. Environ. Health A 2003, 66, 1159–1173. [Google Scholar] [CrossRef]
- Kumazawa, T.; Sato, K.; Seno, H.; Suzuki, O. Rapid isolation with Sep-Pak C18 cartridges and capillary gas chromatography of triazine herbicides in human body fluids. Forensic Sci. Int. 1992, 54, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Anderson, L.C.; Fenske, R.A. Determination of atrazine levels in whole saliva and plasma in rats: Potential of salivary monitoring for occupational exposure. J. Toxicol. Environ. Health 1997, 50, 101–111. [Google Scholar] [PubMed]
- Loos, R.; Niessner, R. Analysis of atrazine terbutylazine and their N-dealkylated chloro and hydroxyl metabolites by solid-phase extraction and gas chromatography-mass spectrometry and capillary electrophoresis-ultraviolet detection. J. Chromatogr. A 1999, 835, 217–229. [Google Scholar] [CrossRef]
- Muir, D.C. Determination of terbutryn and its degradation products in water; sediments; aquatic plants; and fish. J. Agric. Food Chem. 1980, 28, 714–719. [Google Scholar] [CrossRef]
- Ma, W.T.; Fu, K.K.; Cai, Z.; Jiang, G.B. Gas chromatography/mass spectrometry applied for the analysis of triazine herbicides in environmental waters. Chemosphere 2003, 52, 1627–1632. [Google Scholar] [CrossRef]
- Mazanti, L.; Rice, C.; Bialek, K.; Sparling, D.; Stevenson, C.; Johnson, W.E.; Kangas, P.; Rheinstein. Aqueous-phase disappearance of atrazine; metolachlor and chlorpyrifos in laboratory aquaria and outdoor macrocosms. J. Arch. Environ. Contam. Toxicol. 2003, 44, 67280–67284. [Google Scholar] [CrossRef] [PubMed]
- Mendas, G.; Drevenkar, V.; Zupancic-Kralj, L. Solid-phase extraction with styrene-divinylbenzene sorbent for high-performance liquid or gas chromatographic determination of urinary chloro- and methylthiotriazines. J. Chromatogr. A 2001, 918, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Large solar plant photocatalytic water decontamination: Degradation of atrazine. Solar Energy 1996, 56, 411–419. [Google Scholar] [CrossRef]
- Moretti, M.; Marcarelli, M.; Villarini, M.; Fatigon, C.; Scassellati-Sforzolin, G.; Pasquini, R. In vitro testing for genotoxicity of the herbicide terbutryn: Cytogenetic and primary DNA damage. Toxicology In Vitro 2002, 16, 81–88. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, P.A.; Delzell, E.; Sathiakumar, N.; Myers, S.L. Mortality among triazine herbicide manufacturing workers. J. Toxicol. Environ. Health A 2003, 66, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, M.L.; Speth, T.F.; Kelty, C.A. Determination of interfering triazine degradation products by gas chromatography-ion traps mass spectrometry. J. Chromatogr. A 2000, 868, 115–119. [Google Scholar] [CrossRef]
- Mueller, T.C.; Senseman, S.A.; Carson, K.H.; Sciumbato, A.S. Stability and recovery of triazine and chloroacetamide herbicides from pH adjusted water samples by using empore solid-phase extraction disks and gas chromatography with ion trap mass spectrometry. J. AOAC Int. 2001, 84, 1070–1073. [Google Scholar] [CrossRef]
- Navarro, S.; Vela, N.; Garcia, C.; Navarro, G. Persistence of simazine and terbuthylazine in a semiarid soil after organic amendment with urban sewage sludge. J. Agric. Food Chem. 2003, 51, 7359–7365. [Google Scholar] [CrossRef]
- Navarro, S.; Oliva, J.; Barba, A.; Garcia, C. Determination of simazine; terbuthylazine; and their dealkylated chlorotriazine metabolites in soil using sonication micro extraction and gas chromatography. J. AOAC Int. 2000, 83, 1239–1243. [Google Scholar]
- O’Connor, J.C.; Plowchalk, D.R.; Van Pelt, C.S.; Davis, L.G.; Cook, J.C. Role of prolactin in chloro-S-triazine rat mammary tumorigenesis. Drug. Chem. Toxicol. 2000, 23, 575–601. [Google Scholar] [CrossRef]
- Okihashi, M.; Akutsu, K.; Obana, H.; Hori, S. Determination of triazine herbicides in foods with liquid chromatography mass spectrometry. Analyst 2000, 125, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Purcell, M.; Neault, J.F.; Malonga, H.; Arakawa, H.; Carpentier, R.; Tajmir-Riahi, H.A. Interactions of atrazine and 2,4-D with human serum albumin studied by gel and capillary electrophoresis, and FTIR spectroscopy. Biochim. Biophys. Acta 2001, 1548, 129. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, J.W.; Fiddler, W.; Donoghue, D.J. Supercritical fluid extraction of atrazine and other triazine herbicides from fortified and incurred eggs. J. Agric. Food Chem. 2000, 48, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, R.H.; Lanchote, V.L.; Bonato, P.S.; Tozato, E.; de Carvalho, D.; Gomes, M.A.; Cerdeira, A.L. Determination of ametryn herbicide by bioassay and gas chromatography-mass spectrometry in analysis of residues in drinking water. Boll. Chim. Farm. 1999, 138, 249–253. [Google Scholar] [PubMed]
- Ritter, W.F. Pesticide contamination of ground water in the United States--a review. J. Environ. Sci. Health B 1990, 25, 1–29. [Google Scholar] [CrossRef]
- Ramsteiner, K.; Hormann, W.D.; Eberle, D.O. Multiresidue method for the determination of triazine herbicides in field-grown agricultural crops; water; and soils. J. Assoc. Off. Anal. Chem. 1974, 57, 192–201. [Google Scholar] [CrossRef]
- Rivas, J.; Beltran, F.J.; Garcia-Araya, J.F.; Navarret, V. Simazine removal from water in a continuous bubble column by O3 and O3/H2O2. J. Environ. Sci. Health B. 2001, 36, 809–819. [Google Scholar] [CrossRef]
- Rooney, A.A.; Matulk, R.A.; Luebke, R.W. Developmental atrazine exposure suppresses immune function in male, but not female Sprague-Dawley rats. Toxicol. Sci. 2003, 76, 366–375. [Google Scholar] [CrossRef]
- Rayner, J.L.; Wood, C.; Fenton, S.E. Exposure parameters necessary for delayed puberty and mammary gland developmentin Long-Evans rats exposed in utero to atrazine. Toxicol. Appl. Pharmacol. 2004, 195, 23–34. [Google Scholar] [CrossRef]
- Ramesh, A.; Ravi, P.E. Application of solid-phase microextraction (SPME) in the determination of residues of certain herbicides at trace levels in environmental samples. J. Environ. Monit. 2001, 3, 505. [Google Scholar] [CrossRef]
- Ramesh, A.; Ravi, P.E. Electron ionization gas chromatography-mass spectrometric determination of residues of thirteen pyrethroid insecticides in whole blood. J. Chromatogr. B 2004, 802, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Ravi, P.E. Negative Ion Chemical Ionization Gas Chromatographic-Mass Spectrometric Determination of Residues of Different Pyrethroid Insecticides in Whole Blood and serum. J. Anal. Toxicol. 2004, 28, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.; Knopp, D.; Niessner, R. Sol-gel glass immunosorbent-based determination of s-triazines in water and soil samples using gas chromatography with a nitrogen phosphorus detection system. Environ. Sci. Technol. 2002, 36, 3372–3377. [Google Scholar] [CrossRef] [PubMed]
- Sabik, H.; Jeannot, R.; Rondeau, B. Multiresidue methods using solid-phase extraction techniques for monitoring priority pesticides; including triazines and degradation products; in ground and surface waters. J. Chromatogr. A 2000, 885, 217–236. [Google Scholar] [CrossRef]
- Silva, E.; Fialho, A.M.; Sa-Correi, I.; Burns, R.G.; Shaw, L.J. Combined bioaugmentation and biostimulation to cleanup soil contaminated with high concentrations of atrazine. Environ. Sci. Technol. 2004, 38, 632–637. [Google Scholar] [CrossRef]
- Stevens, J.T.; Breckenridge, C.B.; Wetzel, L. A risk characterization for atrazine: Oncogenicity profile. J. Toxicol. Environ. Health A 1999, 56, 69–109. [Google Scholar] [CrossRef]
- Shen, G.; Lee, H.K. Determination of triazines in soil by microwave-assisted extraction followed by solid-phase microextraction and gas chromatography-mass spectrometry. J. Chromatogr. A 2003, 985, 167–174. [Google Scholar] [CrossRef]
- Tanabe, A.; Kawata, K. Determination of triazine pesticides and related compounds in environmental water by liquid chromatography-mass spectrometry. Anal. Sci. 2004, 20, 227–230. [Google Scholar] [CrossRef]
- Tucker, S.P.; Reynolds, J.M.; Wickman, D.C.; Hines, C.J.; Perkins, J.B. Development of sampling and analytical methods for concerted determination of commonly used chloroacetanilide, chlorotriazine, and 2; 4-D herbicides in hand-wash; dermal-patch; and air samples. Appl. Occup. Environ. Hyg. 2001, 16, 698–707. [Google Scholar] [CrossRef]
- Tavera-Mendoza, L.; Ruby, S.; Brousseau, P.; Fournier, M.; Cyr, D.; Marcogliese, D. Response of the amphibian tadpole xenopus laevis to atrazine during sexual di.erentiation of the ovary. Environ. Toxicol. Chem. 2002, 21, 1264–1267. [Google Scholar] [CrossRef]
- Tuzimski, T.; Soczewinski, E. Correlation of retention parameters of pesticides in normal- and reversed-phase systems and their utilization for the separation of a mixture of 14 triazines and urea herbicides by means of two-dimensional thin-layer chromatography. J. Chromatogr. A 2002, 961, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, B.A.; Ilgner, R.H. Determination of atrazine and four organophosphorus pesticides in ground water using solid phase microextraction (SPME) followed by gas chromatography with selected-ion monitoring. J. Chromatogr. A 2002, 972, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Vryzas, Z.; Papadopoulou-Mourkidou, E. Determination of triazine and chloroacetanilide herbicides in soils by microwave-assisted extraction (MAE) coupled to gas chromatographic analysis with either GC-NPD or GC-MS. J. Agric. Food Chem. 2002, 50, 5026–5033. [Google Scholar] [CrossRef] [PubMed]
- Viden, I.; Rathouska, Z.; Davidek, J.; Hajslova, J. Use of gas liquid chromatography/mass spectrometry for triazine herbicide residues analysis in forage and milk. Z. Lebensm. Unters. Forsch. 1987, 185, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, K.C. Herbicide resistance work in the United States Department of Agriculture-Agricultural Research Service. Pest. Manag. Sci. 2003, 59, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Duh, J.R.; Liang, Y.F.; Chen, Y.L. Dissipation of three s-triazine herbicides, atrazine, simazine, and ametryn, in subtropical soils. Bull. Environ. Contam. Toxicol. 1995, 55, 351–358. [Google Scholar] [CrossRef]
- Winklmair, M.; Weller, M.G.; Mangler, J.; Schlosshauer, B.; Niessner, R. Monitoring carbamazepine in surface and wastewaters by an immunoassay based on a monoclonal antibody. J. Anal. Chem. 1997, 358, 614. [Google Scholar]
- Yokley, R.A.; Cheung, M.W. Analytical method for the determination of atrazine and its dealkylated chlorotriazine metabolites in water using gas chromatography/mass selective detection. J. Agric. Food Chem. 2000, 48, 4500–4507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).