Abstract
Numerous strategies have been suggested to reduce dependence on synthetic products, such as physical, microbial, and natural methods. Among the natural remedies, plant extracts have emerged as a popular option owing to their eco-friendly character, ease of degradation, and harmless nature to humans. In our study, we used the acetone and hexane extracts of Rhaphiolepis indica fruit to combat two fungal pathogens that were isolated from infected bean plants and showed root rot symptoms. The two pathogens were confirmed to be pathogenic by pathogenicity assays conducted in vivo. The morphological and molecular identification by ITS-region sequencing revealed that the two isolates were Rhizoctonia solani and Fusarium solani, and they were assigned accession numbers OQ880457 and OQ820158, respectively. Our data showed that both hexane and acetone extracts caused a significant decrease in the linear growth of F. solani at all concentrations used (1%, 2%, and 3%), compared to the control. However, at a concentration of 3%, the hexane extract caused much greater inhibition than the acetone extract. For R. solani, the hexane extract, shows a significant inhibition percentage at all concentrations, which further increases to 85.24% at 3% concentration. The HPLC of both extracts indicated the presence and absence of phenolic and flavonoid compounds. The obtained results revealed that five acetonic phenolic extract compounds were ferulic, p-coumaric, gallic, p-OH benzoic, and cinnamic, with concentrations of 5.31, 10.36, 7.24, 6.08, and 0.89 mg/mL, respectively. On the other hand, the five hexanoic phenolic compounds were catechol, caffeic, chlorogenic, p-OH benzoic, and cinnamic acids, with concentrations of 3.66, 5.14, 0.69, 6.31, and 13.47 mg/mL, respectively. The identified acetonic flavonoid extract compounds, namely rutin, chrysin, quercetin, kaempferol, chrysoeriol, 7-OH flavone, and naringin, had respective concentrations of 5.36, 10.23, 4.32, 15.33, 1.06, 0.087, and 0.069 mg/mL, respectively. In contrast, it was observed that the seven hexanoic flavonoid extracts comprised of rutin, quercetin, kampferol, luteolin, chrysoeriol, 7-OH flavone, and catechin exhibited concentrations of 5.36, 7.15, 18.20, 6.04, 2.04, 10.24, and 13.43 mg/mL, respectively. The results of the study suggest that plant extracts may be a useful natural remedy for combating fungal pathogens and reducing dependence on synthetic products.
Keywords:
Rhaphiolepis; extract; bean; Fusarium solani; ITS; hexane; antifungal; HPLC; cinnamic; rutin 1. Introduction
Annually, plant diseases result in substantial economic losses in global crop production [1]. Fungal-induced diseases are prevalent among plant species and have posed a continuous challenge to the sustenance of food and feed security since the inception of agricultural plant domestication [2]. Plant pathogenic fungi can affect a wide range of crops both during growth in the field and after harvesting, resulting in reduced agricultural productivity and shorter shelf life for many agricultural products [3]. The main method to protect plants from fungal infections is to use synthetic fungicides. However, resistance development and the non-biodegradability of these chemicals are concerning. They can accumulate in soil, plants, and water, posing potential harm to human health via the food chain [3]. As a result, the risks to health and the environment associated with chemical fungicide use have increased the demand for safer, effective, and environmentally friendly alternatives, as noted by various sources [4,5].
Various approaches have been proposed to minimize the reliance on synthetic products, including physical, microbial, and natural treatments [6]. In terms of natural agents, plant extracts have received considerable attention due to their environmentally friendly nature, ease of decomposition, and non-toxicity to humans [7,8]. The antimicrobial and antifungal properties of numerous plant extracts have garnered significant attention and interest in the scientific community [9]. Natural antifungal compounds found in plant chemicals are deemed safer and more desirable than their synthetic counterparts [10]. Several plant extracts have been studied for their antimycotic properties [11,12,13]. As a result, utilizing plant extracts that have inhibitory effects on fungal plant pathogens could pave the way for the creation of eco-friendly fungicides that rely on naturally occurring substances. The Rhaphiolepis indica (Indian hawthorn) plant is a sizable, evergreen tree belonging to the Maloideae subfamily of the Rosaceae family [14]. Its origins can be traced back to China, where it has been cultivated for over two millennia. According to Rajalakshmi et al. [15], the cultivation of this plant has been introduced to over 30 countries worldwide, such as Japan, India, Mediterranean countries in Europe, Australia, Madagascar, New Zealand, Kenya, and South Africa. However, commercial cultivation of the plant is limited to only a select few countries. The medicinal and dietary benefits of these plants were ascertained through the identification of certain active chemical constituents present in their composition [16]. The botanical specimens possess significant medicinal and nutritional properties, rendering them highly advantageous in the domains of medicine and therapy.
Phaseolus vulgaris L., commonly known as the common bean, is a leguminous plant belonging to the Fabaceae family. It is noteworthy for being the most extensively distributed and consumed legume species [17]. Most of the legumes grown are beans, which make up 46% of the total. With a production share of 22%, which is almost half that of beans, chickpeas are the second most common grain legume. People all over the world know that the common bean is an important source of proteins and carbs. Also, it is good for human health, including being anti-diabetic, protecting the heart, and protecting against different types of cancer [18]. The protein level of common beans is almost twice as high as that of cereals, which is a good sign of their nutritional value [19]. They also have less fat than other beans and contain a lot of minerals [20]. Beans are vulnerable to several soil-borne fungi that attack them at different growth stages, causing root rot, collar rot, and wilt diseases. Among these pathogens, Fusarium solani and Rhizoctonia solani are the most significant, causing damping-off and root-rot diseases that decrease the quality and quantity of crop yield, thereby reducing productivity [21,22]. Thus, this research aimed to assess the antifungal properties of R. indica extract, which is derived from Rhaphiolepis fruits, against F. solani and R. solani under laboratory conditions. As well as the best solvent for separating the active compounds from the fruits of R. indica by comparing two solvents (acetone and hexane). Furthermore, HPLC analysis was utilized to determine the primary phytochemical constituents present in the acetone and hexane fruit extracts of R. indica.
2. Materials and Methods
2.1. Sample Collection, Isolation, and Purification of the Pathogen
The common bean plant specimens were collected from the nearby uncovered fields situated in Borg El Arab city, located in the Alexandria Governorate of Egypt. Bean plants exhibiting symptoms such as chlorosis, wilting, damping off, and root rot were carefully selected and subsequently transported to the laboratory in sterilized plastic bags. After that, the distressed plants were meticulously dissected into their subterranean and above-ground components using a sanitized surgical instrument. The samples were subjected to a mild washing procedure using tap water, followed by fragmentation into small segments measuring approximately 0.5–1.0 cm in length using scissors. The samples underwent a brief sterilization process lasting one minute, utilizing a 0.5% NaOCl (w/v) solution. Following this, they were rinsed multiple times with sterile double-distilled water and subsequently dried. Subsequently, the aseptic fragments were subjected to cultivation on potato dextrose agar (PDA) plates and maintained for 7 days at a temperature of 28 ± 2 °C. The hyphal tip technique was utilized to achieve the purification of the fungus isolate. Subsequently, each fungal colony was subjected to a 7-day incubation period at 28 ± 2 °C on PDA plates to verify its purity. The fungal specimen, which underwent decontamination, was subsequently transferred onto PDA slants and kept in storage for future investigations.
2.2. In Vivo Test of the Isolated Fungal Isolates
The pathogenicity of the obtained fungal isolates on bean plants in a greenhouse was determined by following these steps:
- a.
- The fungi were cultured on potato dextrose agar (PDA) plates at 28 °C for 7–10 days. Once the fungi had grown, five plates of each fungus were scraped from the surface of the agar with a sterile loop and suspended in 250 mL of sterile distilled water.
- b.
- Sterile soil of equal parts peat moss, sand, and clay was put in 1 L pots in a greenhouse. After that, each pot received 50 mL of the fungal inoculum and was watered with 250 mL of water. The pots were kept for a week before two bean seeds (cv. Nebraska) were sown in each pot. Control plants were watered with sterile distilled water only.
- c.
- The plants were monitored regularly for symptoms of the disease. Symptoms on bean plants included wilting, yellowing, damping-off of the lower stem, and root rot.
- d.
- Finally, Koch’s postulates were used to re-isolate and identify the fungi by placing the infected cut sections on an agar medium.
2.3. Morphological and Molecular Characterization of Fungal Isolates
The first step in identifying each isolate was to look at its physical and behavioral characteristics, as described previously [23,24]. A lactophenol-cotton blue solution stain was used to figure out what kind of fungus it was by looking at its shape. The slide was first stained, and then the mycelia growth was put on it. The spot and mycelia were then both covered with a slip. After that, the slide that had been made was looked at through a microscope [25]. The fungal DNA isolation was conducted as described before [1,26]. The genomic DNA of the fungus was analyzed using a 1% agarose gel made in tris-borate EDTA (0.5X TBE) as previously described [27]. The DNA was then seen on an ultraviolet transilluminator after being stained with ethidium bromide. Using a primer, the DNA-ITS region of the fungus isolate was found and made bigger. The PCR reaction mixture had a master mix of 10 µL, 1 µL of DNA (50 ng), universal primer ITS (forward TCCGTAGGTGAA CCTGCGG and reverse TCCTCCGCTTATTGATATG), 1 µL for each primer (10 pmol/µL), and sterile dH2O up to a final volume of 20 µL. The PCR technique included an initial denaturation step at 95 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 1 min, and extension at 72 °C for 1 min. The final part of stretching was done at a temperature of 72 °C for 5 min. The amplified PCR products were cleaned up with a PCR clean-up column kit (Koma Biotech, Seoul, Republic of Korea), following the directions from the manufacturer. After the sequencing process, the DNA sequence was deposited into the NCBI Gene Bank database, where it was compared to other strains of the same species available in the database, and subsequently, a phylogenetic tree was generated using MEGA 11 software [28].
2.4. Preparation of Rhaphiolepis indica Fruit Samples for Analysis
Rhaphiolepis indica (Family: Rosaceae) fruit samples were collected and identified by the Floriculture, Ornamental Horticulture and Garden Design Department, Faculty of Agriculture (El-Shatby), Alexandria University, Alexandria, Egypt. The collected samples were left to dry out at room temperature for two weeks. The dried fruits were then put through a grinder mill to make a fine powder. After that, 50 g of dried fruit powder were soaked in 500 mL of acetone. Also, 50 g of the same powder were mixed with 500 mL of hexane in an Erlenmeyer flask and shaken on a rotating shaker at 100 turns per minute for 48 h at room temperature. The mixtures were filtered with Whatman filter paper No. 1, and then the solvents acetone and hexane were taken out of the extract by using a rotating evaporator to concentrate it. The Rhaphiolepis fruit extract was kept at a temperature of 4 °C until it was needed for further testing.
2.5. In Vitro Evaluation of the Antifungal Effect of Rhaphiolepis indica Fruit Extracts
The linear growth of Rhizoctonia solani and Fusarium solani was measured in response to extracts of Rhaphiolepis fruits in 1%, 2%, and 3% acetone and hexane. The poisoned media method was employed for the current assay. Various concentrations of Rhaphiolepis fruit extract were formulated namely 1%, 2%, and 3%. PDA plates were utilized to generate varying concentrations of Rhaphiolepis fruit extract, which were subsequently contrasted with the negative control (PDA plates devoid of any additives). Discs of fungus measuring 5 mm in diameter were excised from a culture that was 6 days old. These discs were subsequently transferred to the centers of Petri dishes that had been poured with varying concentrations of the extract. The dishes were then incubated for one week at a temperature of 25 °C. Three replicates were utilized for each treatment. The study documented the effectiveness of Rhaphiolepis fruit extract in promoting the linear growth of the fungus. As per the report of Heflish et al. [29], the computation of growth inhibition was expressed as a percentage using the formula (%) = (A − B/A) × 100. Here, A denotes the growth length of the fungus on the control plate, while B represents the growth of the fungus on the treated plates.
2.6. HPLC Analysis of R. indica Fruit Extracts
The identification of the polyphenolic components present in the Rhaphiolepis fruits extract (acetone and hexane) was carried out using an HPLC analysis with an Agilent 1260 series. The extraction process was conducted as previously described [30] and Fiorito et al. [31]. The chromatographic separation was conducted utilizing an Eclipse C18 column with dimensions of 4.6 mm inner diameter, 250 mm length, and a particle size of 5 µm. The aqueous component (A) and trifluoroacetic acid in acetonitrile 0.05% (B) are present in the mobile phase, which is flowing at a rate of 0.9 mL/min. The mobile phase was subjected to a linear gradient and a multi-wavelength detector was used at a wavelength of 280 nm. Each sample was subjected to the utilization of 5 µL. The temperature of the column was maintained at a constant value of 40 °C. The polyphenolic components were identified through the utilization of a standardized group of compounds, which consisted of 7-OH flavone, caffeic, catechin, catechol, chlorogenic, chrysin, chrysoeriol, cinnamic, ferulic, gallic, kaempferol, luteolin, p-coumaric, p-OH benzoic, quercetin, and rutin.
2.7. Statistical Analysis
The experiments were conducted using a randomized design, and the data was analyzed through the application of analysis of variance with the assistance of “CoSTAT” software. The results are reported in terms of mean ± standard deviation and were deemed statistically significant if the p-value was less than or equal to 0.05.
3. Results
3.1. Isolation and Pathogenicity
Yellowing, damping-off, and root rot signs were seen in the bean plants and picked for testing. From infected tissues, we isolated the pathogens on PDA media (Figure 1).
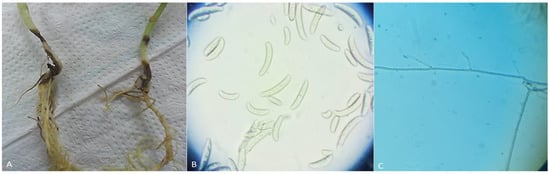
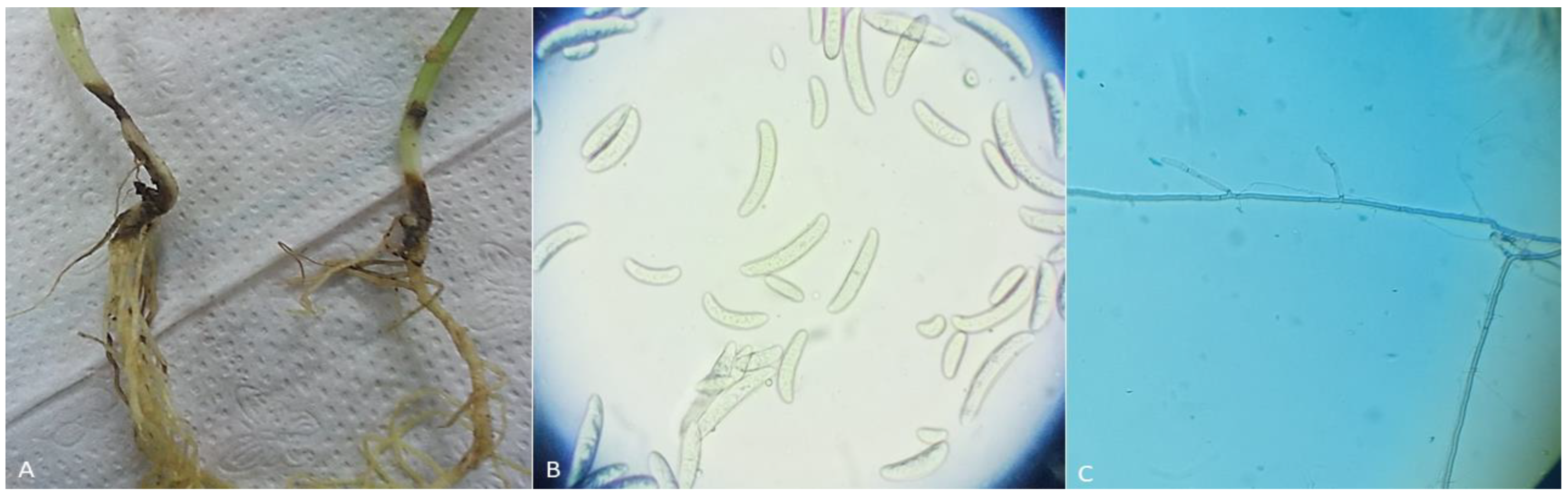
Figure 1.
(A) Infected stem and root rot symptoms in bean plants; (B) Fusarium solani macroconidia and microconidia spores, which are typically cylindrical or fusiform in shape; and (C) hyphae of Rhizoctonia solani, which are branched and irregular in shape.
3.2. Phenotypic Identification of the Fungal Isolates
Different structures of Rhizoctonia and Fusarium species were observed, allowing the genus to be determined (Figure 1). As our results initially characterize R. solani as a filamentous fungus with long, branching, and multinucleate hyphae.
- a.
- Mycelium: R. solani can grow as mycelium (fungal threads) that are initially white, but can turn brown or gray as they age. The mycelium can be either thick or thin and can form mats or strands, and it grows rapidly on nutrient-rich media.
- b.
- Sclerotia: The fungus produces dark, hard, irregularly shaped sclerotia. The size and shape of the sclerotia vary depending on the strain of R. solani.
- c.
- Hyphae: The hyphae of R. solani are septate, and they are often branched and irregular in shape.
- d.
- Spores: R. solani does not produce any distinctive conidial spores, and its mode of reproduction is primarily through vegetative means, such as mycelial growth and sclerotia formation.
While F. solani is a filamentous fungus that belongs to the Fusarium genus. Its morphology was identified based on several characteristics, including:
- a.
- Colony appearance: F. solani colonies typically appear cottony or woolly in texture and are initially white, becoming yellow or tan as they mature.
- b.
- Growth rate: F. solani grows rapidly on potato dextrose agar (PDA) and other media, forming dense mycelial mats.
- c.
- Hyphae: The hyphae of Fusarium solani are septate, meaning they are divided into distinct segments by cross-walls called septa.
- d.
- Sporulation: F. solani produces a variety of spores, including macroconidia and microconidia, which are typically cylindrical or fusiform in shape.
- e.
- Microscopic characteristics: When viewed under a microscope, F. solani hyphae appear smooth, and the conidia are generally single-celled and have a distinct shape.
As a result, the identification of R. solani and F. solani based on morphology requires careful observation and analysis of these various characteristics. However, we used the ITS molecular technique in the next section for accurate identification.
3.3. Identification of the Fungal Isolates by ITS-PCR
The ITS region of fungal DNA has become a popular molecular marker for use in fungal taxonomy, classification, and evolutionary studies. This is due to its abundance of reference sequences, its high degree of sequence diversity within and across fungal species, and the conservation of its surrounding regions. To this end, ITS has been called a “fungal barcode” and is widely employed in several fungal research applications. Using polymerase chain reaction (PCR) and the internal transcribed spacer (ITS) region primers, we amplified the genomic DNA of the fungal isolate; the resulting PCR product measured 550–600 base pairs (bp) in length.
The ITS DNA nucleotide sequence was received from the Seoul, Korea-based Macrogen Corporation. The BLAST program was then used to check the nucleotide sequences against the NCBI’s worldwide database. Rhizoctonia solani and Fusarium solani exhibited the highest degree of similarity among the fungal isolates. The nucleotide sequences of the fungal isolates were subsequently uploaded to the GenBank database submission site, where they were assigned the accession numbers OQ880457 and OQ820158, respectively.
3.4. Phylogenetic Tree Construction
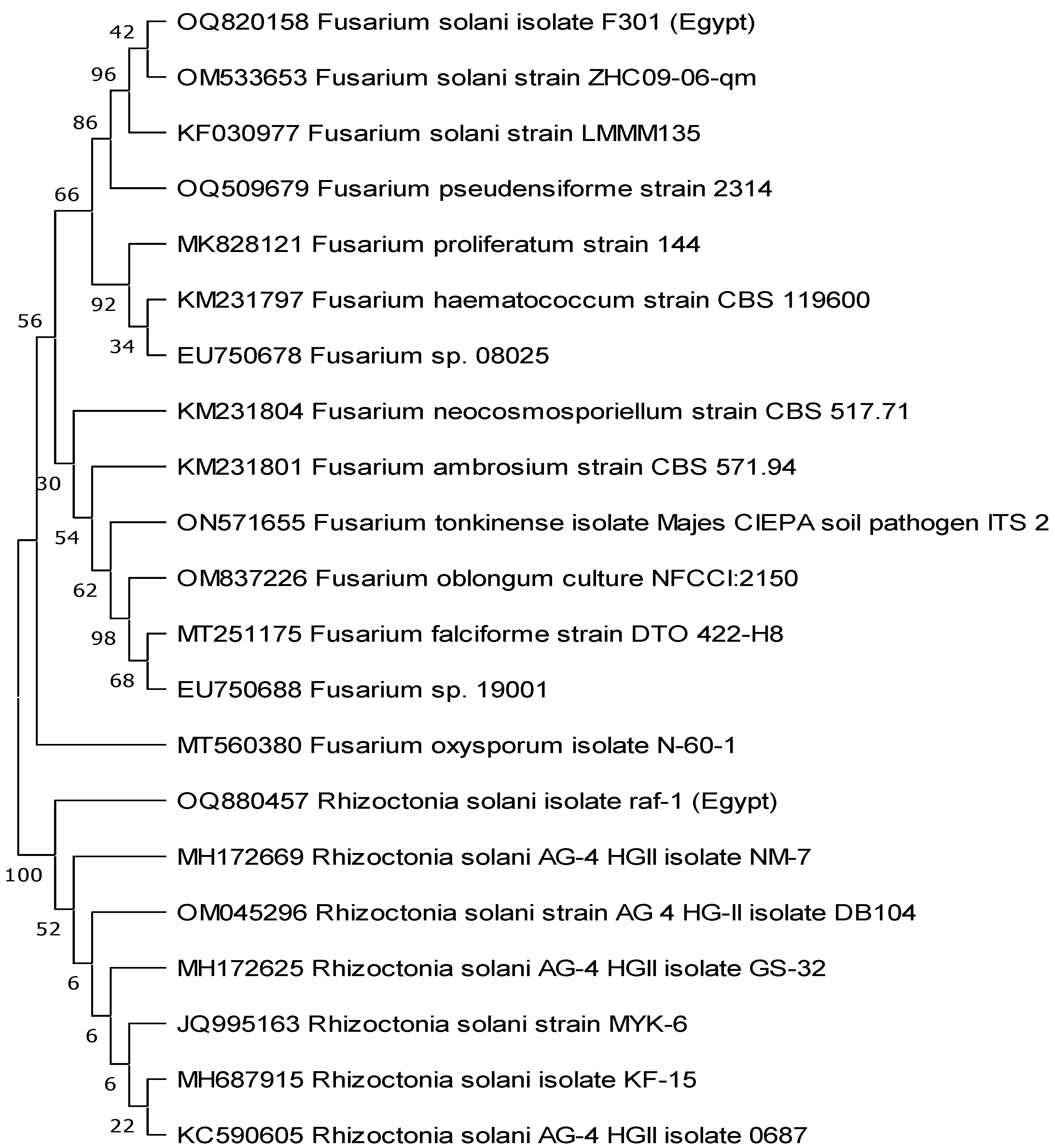
Using GenBank sequences of fungi that were most closely related to the individual fungal isolates, we created a phylogenetic tree of the fungi’s nucleotides (ITS). As can be seen in Figure 2, the created tree separated all of the species into two groups: those consisting of R. solani isolates and those consisting of F. solani isolates that were genetically identical to the studied isolates, albeit at varying percentages.
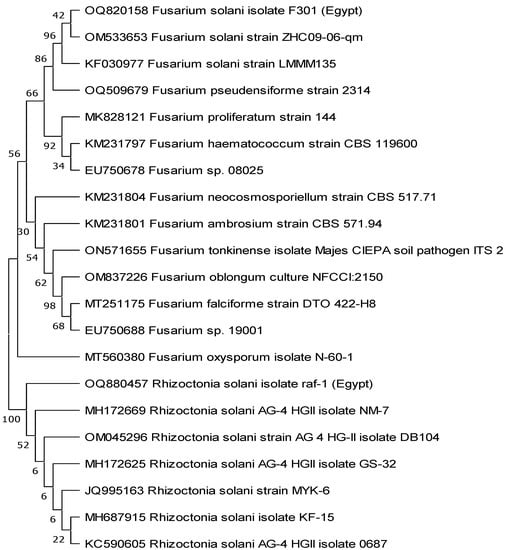
Figure 2.
The phylogentic tree was constructed using nucleotide sequences of Egyptian Rhizoctonia solani and Fusarium solani fungal pathogens represented by accession numbers OQ880457 and OQ820158, respectively. The tree was bootstrapped and inferred from 1000 replicates using the maximum likelihood method by MEGA 11 software.
3.5. Effect of Rhaphiolepis Fruit Extracts on the Growth of F. solani and R. solani In Vitro
The data in Table 1 present the effect of different concentrations of hexane and acetone extracts of Rhaphiolepis fruits on the linear growth of two fungal pathogens, F. solani and R. solani. The data showed that both hexane and acetone extracts have a significant effect on the growth of the two fungal pathogens in a concentration-dependent manner. For F. solani, both hexane and acetone extracts caused a significant decrease in linear growth at all concentrations used (1%, 2%, and 3%), compared to the control. However, at a concentration of 3%, the hexane extract caused a much greater inhibition percentage than the acetone extract. At 1% concentration, the hexane extract shows a 44.76% inhibition percentage, which increases to 57.62% at 2% concentration, and further increases to 85.24% at 3% concentration. The acetone extract also shows an increase in inhibition percentage with an increase in the concentration of the extract. At 1% concentration, the acetone extract shows a 46.19% inhibition percentage, which increases to 55.24% at 2% concentration and then increased slightly to 60.00% at 3% concentration. For R. solani, the hexane extract shows a significant inhibition percentage at all used concentrations, which further increases to 85.24% at 3% concentration. At 1% concentration, the hexane extract shows a lower inhibition percentage of 13.81%. The acetone extract at 2% shows a 70.95% inhibition percentage, which increases to 79.52% at 3% concentration, while the acetone extract shows no inhibitory effect on the growth of R. solani at 1% tested concentrations. The efficacy of the extracts varies with the fungal species, with F. solani being more susceptible to both extracts than R. solani.

Table 1.
Inhibition percentage (%) of Fusarium solani and Rhizoctonia solani fungal isolates in response to Rhaphiolepis indica fruit acetone and hexane extracts in vitro.
3.6. Polyphenolic Compounds in R. indica Fruit Extracts
3.6.1. Phenolic Compound Profiles
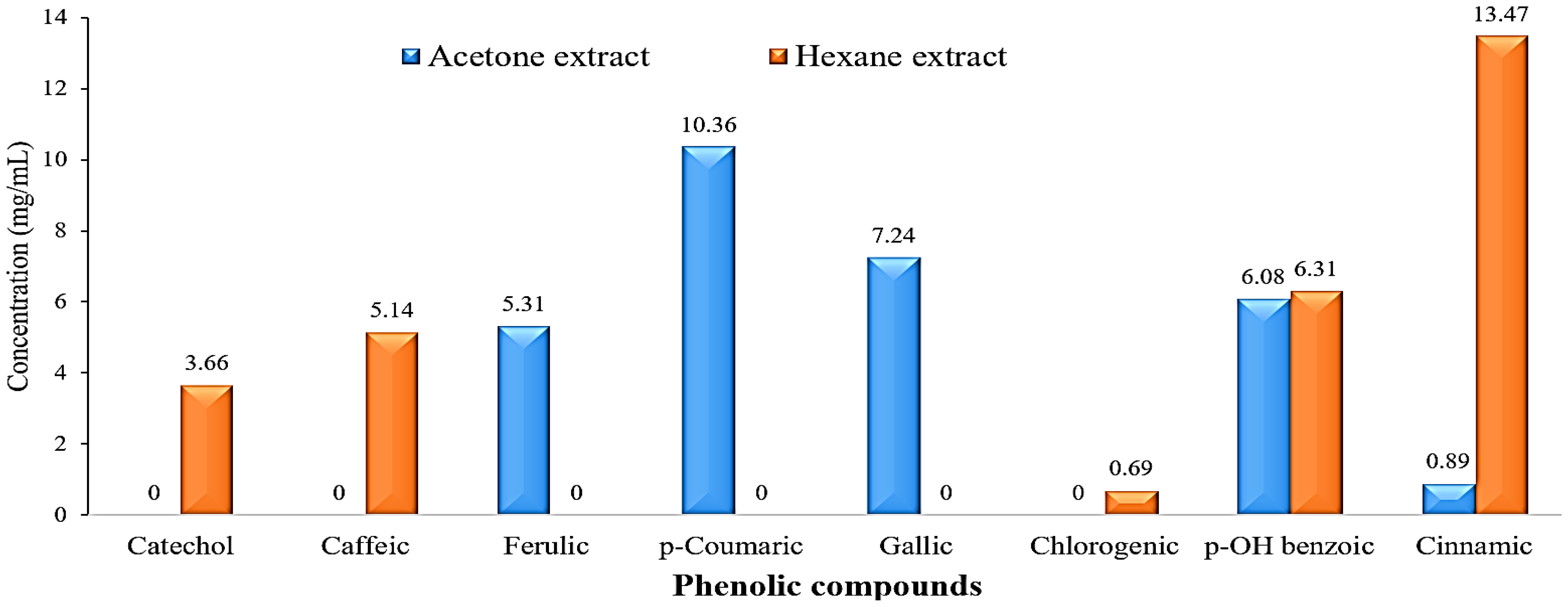
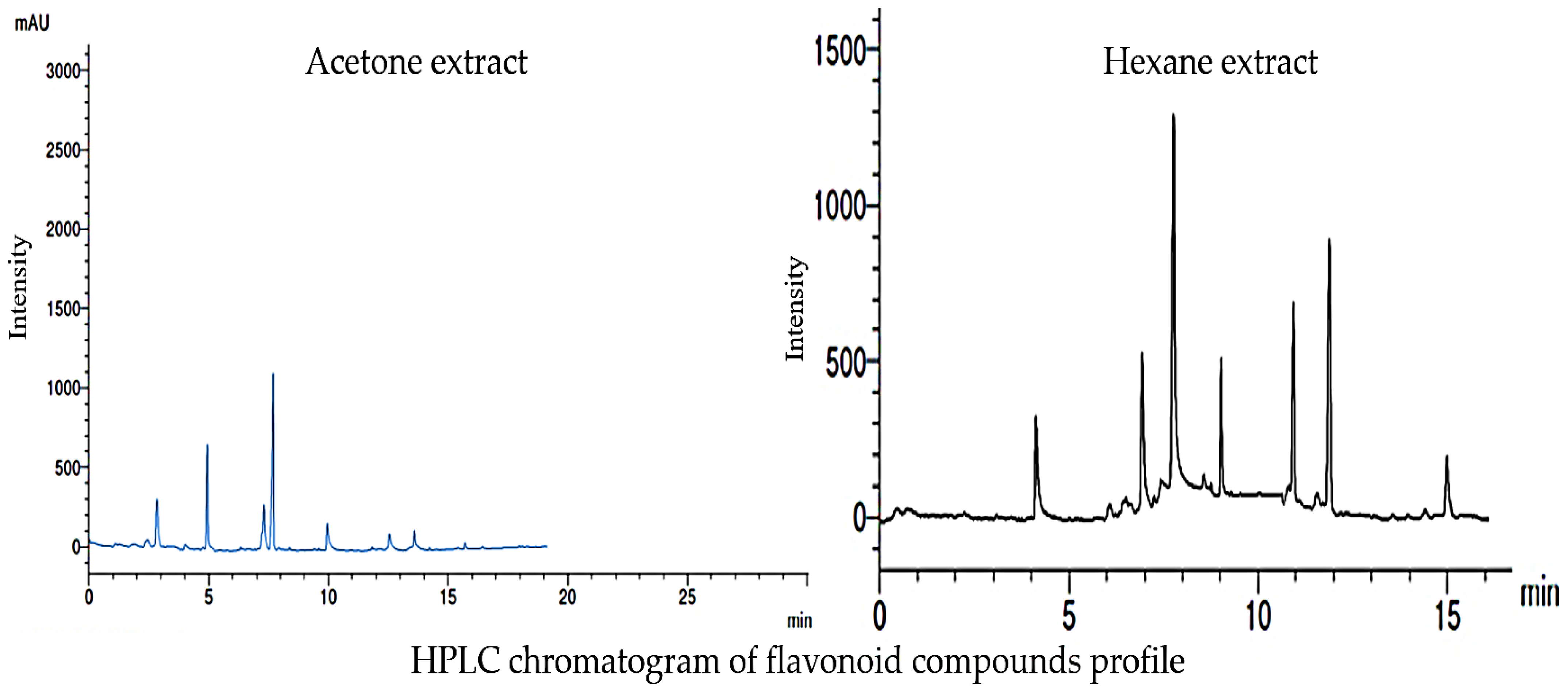
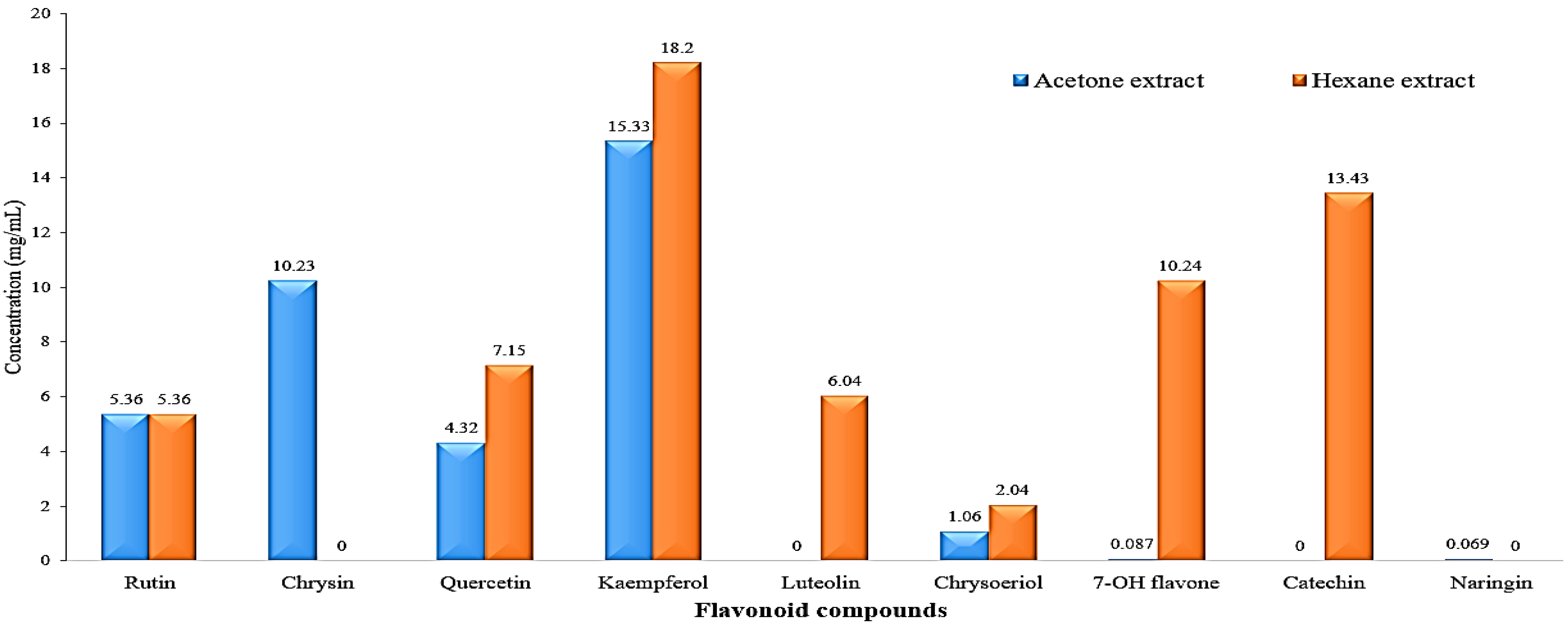
The HPLC chromatograms of the phenolic compound profile of acetone and hexane extracts are presented in Figure 3. The obtained results revealed that five phenolic compounds were detected in both extracts. The five acetonic extract compounds were ferulic, p-coumaric, gallic, p-OH benzoic, and cinnamic with concentrations of 5.31, 10.36, 7.24, 6.08, and 0.89 mg/mL, respectively (Figure 4). On the other hand, the five hexanoic compounds were catechol, caffeic, chlorogenic, p-OH benzoic, and cinnamic acids with oncentrations of 3.66, 5.14, 0.69, 6.31, and 13.47 mg/mL, respectively (Figure 4).
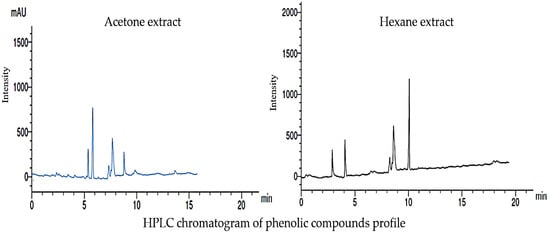
Figure 3.
Line graphs of the phenolic compounds present in the acetone and hexane extracts of Rhaphiolepis indica fruit using HPLC instrument.
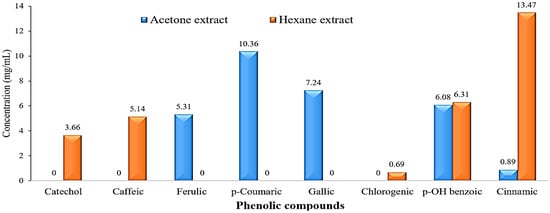
Figure 4.
Bar charts of phenolic compounds concentration (mg/mL) in the acetone and hexane extracts of Rhaphiolepis indica fruit.
3.6.2. Flavonoid Compound Profiles
Figure 5 displays the HPLC chromatogram of the flavonoid compound profile obtained from the acetone and hexane extracts. The results obtained from the analysis indicate that a total of seven distinct flavonoid compounds were detected in both of the extracts. The identified acetonic extract compounds, namely rutin, chrysin, quercetin, kaempferol, chrysoeriol, 7-OH flavone, and naringin, with respective concentrations of 5.36, 10.23, 4.32, 15.33, 1.06, 0.087, and 0.069 mg/mL, respectively, as depicted in Figure 6. In contrast, it was observed that the seven hexanoic extracts comprised of rutin, quercetin, kampferol, luteolin, chrysoeriol, 7-OH flavone, and catechin, exhibited concentrations of 5.36, 7.15, 18.20, 6.04, 2.04, 10.24, and 13.43 mg/mL, respectively, as depicted in Figure 6.
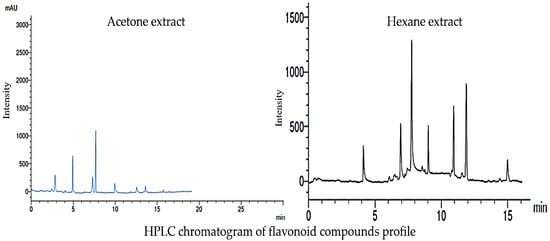
Figure 5.
Line graphs of the flavonoid compounds present in the acetone and hexane extracts of Rhaphiolepis indica fruit HPLC instrument.
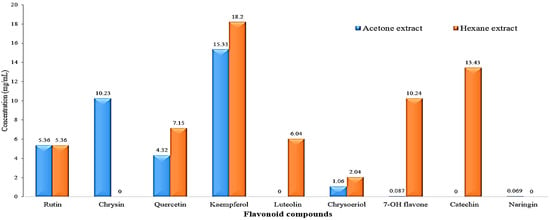
Figure 6.
Bar charts of flavonoid compounds concentration (mg/mL) present in the acetone and hexane extracts of Rhaphiolepis indica fruit.
4. Discussion
On a global scale, several plant crops are being adversely affected by a variety of infections that have a destructive effect on the productivity and total yield of plants [32]. Accordingly, one of the primary causative agents of these diseases is fungi [5]. Numerous strategies are utilized to regulate and administer the escalation of fungal diseases in plants on a global scale. Natural extracts have been proposed as a safer and more cost-effective alternative to synthetic chemical antifungal substances for achieving a chemically clean and uncontaminated environment. Plant extracts are known for their varied active compounds, minerals, secondary metabolites, and antioxidants, which contribute to their effectiveness [2,8]. Since screening and identifying new plant extracts with potent antifungal activities for agricultural applications is crucial, the current study aimed to assess the inhibitory activity of different R. indica fruit extracts on the growth of F. solani and R. solani in vitro. The investigation of the morphology of R. solani isolate derived from bean plant roots was consistent with the characteristics of the Rhizoctonia genus, given that it is a septate anamorphic mycelial fungus that did not generate asexual spores, as reported by Heflish et al. [29]. The NCBI-Blast and phylogenetic tree analysis confirmed the morphological identification, and subsequently, the ITS nucleotide sequence was deposited in GenBank as R. solani isolate raf-1. Also, F. solani isolate exhibited certain morphological characteristics, such as mycelium texture, colony color and pigmentation, long monophialides, cream sporodochia, and abundant chlamydospores, which were described previously [33]. In our study, the selection of the extraction solvents is a crucial factor to take into consideration. For the extraction of numerous secondary metabolites from plants, acetone, hexane, ethyl acetate, petroleum ether, chloroform, ethanol, methanol, and water are frequently utilized. When compared to water extract made from the same plant species, organic solvents like acetone, ethyl acetate, and hexane showed greater antifungal efficacy against various Fusarium infections [34]. This conclusion was consistent with the findings of other authors who claimed that, in comparison to non-polar extracts, aqueous extracts typically demonstrated little to no antimicrobial action [35,36,37]. Metabolites vary greatly, which affects both their solubility during extraction and the extracts’ antifungal effectiveness afterward [38]. The large differences in polarity between the constituent metabolites affect both how soluble they are during extraction and how effective the extracts are at inhibiting the growth of fungi.
The findings of our recent research were corroborated by this study, which demonstrated the antifungal properties of the R. indicia fruit extract against F. solani and R. solani in vitro. According to Ashmawy et al. [39], the growth of R. solani, Botrytis cinerea, and Fusarium culmorum was inhibited by 64.4%, 100%, and 38.5%, respectively, when treated with the ethanolic extract of Coccoloba uvifera. Furthermore, the findings align with those reported by various other researchers [40,41]. Our previous study indicated that the growth of R. solani was inhibited to a significant extent when exposed to Plantago lagopus extract at concentrations ranging from 2 to 10 µg/mL. This inhibition of growth was found to be comparable to the effect of fluconazole, which is a commonly used antifungal drug [8]. Herbal plants, such as thyme, oregano, garlic, and sage, are rich sources of biologically active compounds. These plants have remarkable biosynthetic capabilities that allow them to produce natural bioactive compounds [42], which can serve as alternatives to synthetic chemicals. These natural compounds, called natural fungicides, are broad-spectrum and have a comprehensive effect on pathogens. The efficacy of these natural fungicides, which are derived from plant extracts, depends mainly on the presence of phenols, terpenes, and alkaloids. Phenols possess antioxidant and anti-radical properties, while certain types of phenolic compounds, such as phenolic acids, flavonoids, and tannins, have direct anti-fungal effects [43,44]. In 2012, Surender [45] conducted an assessment of the antifungal properties of the aqueous extract obtained from 20 plants. The study was focused on the impact of the extracts on the mycelial growth inhibition of F. solani, which is responsible for potato dry rot. Results revealed that various plant extracts exhibited different levels of effectiveness against mycelial growth inhibition. Debjani et al. [46] conducted an experiment in which they examined the inhibitory effect of three plant extracts, namely ginger, polyalthi, and clerodendrum, on R. solani in vivo. The results demonstrated that the three plant extracts exhibited considerable inhibitory activity against R. solani. According to a study by Yavuz et al. [47], ethanol extracts from Pyrus serikensis’ leaves and fruit exhibit biofungicide efficacy against Monillinia fructigena, Sclerotinia sclerotiorum, R. solani, and F. oxysporum f. sp. cucumerinum. Also, in the same way Muscari aucheri (Boiss) Baker plant methanol extract (Flower + flower stalk) was examined against five different plant diseases, according to Onaran and Başaran [48]. It demonstrated antifungal activity against F. oxysporum f. sp. cucumerinum, Alternaria solani, Verticillium dahliae, R. solani, and Botrytis cinerea.
In our study, benzoic acid was identified in the acetone and hexane extracts of R. indica fruit. This compound has been found to provide a permeability barrier to the cell membrane, which is crucial for various cellular processes, such as maintaining energy levels, solute transport, metabolic regulation, and membrane-coupled energy transducing processes [49,50] Additionally, cinnamic acid and its hydroxylated derivatives have antifungal properties due to their ability to inhibit antityrosinase enzyme activity and fungal spore germination [51]. These compounds have been shown to interact with benzoate 4-hydroxylase enzyme, which is responsible for aromatic detoxification and can cause fungal growth inhibition [52]. El Sawi et al. [53] previously reported that the presence of flavonoids and kaempferol derivatives in plant extracts may be responsible for their antimicrobial activity. Other studies have also identified several phenolic compounds in medicinal plants, which are known to contribute to their antibacterial and antioxidant properties [13,54]. Overall, the results suggest that the hexane extract has a stronger inhibitory effect on both fungal pathogens than the acetone extract.
5. Conclusions
The Rhaphiolepis indica fruit extracts, particularly the hexane extract, showed significant inhibitory effects on the growth of Rhizoctonia solani and Fusarium solani in a concentration-dependent manner. The HPLC analysis of the extracts identified several phenolic and flavonoid compounds, which may be responsible for the observed antifungal properties. These findings suggest that natural plant extracts could serve as an eco-friendly and non-toxic alternative to synthetic fungicides for controlling fungal diseases in agriculture. In conclusion, this study highlights the potential of natural plant extracts as effective agents for controlling fungal pathogens that cause root-rot symptoms in bean plants.
Author Contributions
Conceptualization, methodology, software and investigation A.A.H., A.A., A.A.A.-A., Y.S., S.I.B. and M.K.G.; writing—original draft preparation, A.A.H., A.A. and S.I.B.; project administration, A.A. and A.A.A.-A.; funding acquisition, A.A.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Researchers Supporting Project number (RSP2023R505), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
Not applicable.
Acknowledgments
The authors express their sincere thanks to the City of Scientific Research and Technological Applications (SRTA-City) and the Faculty of Agriculture (Saba Basha), Alexandria University, Egypt, for providing the necessary research facilities. The authors would like to extend their appreciation to the Researchers Supporting Project number (RSP2023R505), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soliman, S.A.; Al-Askar, A.A.; Sobhy, S.; Samy, M.A.; Hamdy, E.; Sharaf, O.A.; Su, Y.; Behiry, S.I.; Abdelkhalek, A. Differences in Pathogenesis-Related Protein Expression and Polyphenolic Compound Accumulation Reveal Insights into Tomato—Pythium aphanidermatum Interaction. Sustainability 2023, 15, 6551. [Google Scholar]
- Sobhy, S.; Al-Askar, A.A.; Bakhiet, E.K.; Elsharkawy, M.M.; Arishi, A.A.; Behiry, S.I.; Abdelkhalek, A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations 2023, 10, 189. [Google Scholar]
- Mdee, L.K.; Masoko, P.; Eloff, J.N. The activity of extracts of seven common invasive plant species on fungal phytopathogens. S. Afr. J. Bot. 2009, 75, 375–379. [Google Scholar]
- Hostettmann, K.; Marston, A.; Ndjoko, K.; Wolfender, J.-L. The potential of African plants as a source of drugs. Curr. Org. Chem. 2000, 4, 973–1010. [Google Scholar]
- Behiry, S.; Soliman, S.A.; Massoud, M.A.; Abdelbary, M.; Kordy, A.M.; Abdelkhalek, A.; Heflish, A. Trichoderma pubescens Elicit Induced Systemic Resistance in Tomato Challenged by Rhizoctonia solani. J. Fungi 2023, 9, 167. [Google Scholar] [CrossRef]
- Koch, E.; Roberts, S.J. Non-chemical seed treatment in the control of seed-borne pathogens. In Global Perspectives on the Health of Seeds and Plant Propagation Material; Springer: Berlin/Heidelberg, Germany, 2014; pp. 105–123. [Google Scholar]
- Choudhury, D.; Dobhal, P.; Srivastava, S.; Saha, S.; Kundu, S. Role of botanical plant extracts to control plant pathogens-A review. Indian J. Agric. Res. 2018, 52, 341–346. [Google Scholar]
- Behiry, S.I.; Soliman, S.A.; Al-Askar, A.A.; Alotibi, F.O.; Basile, A.; Abdelkhalek, A.; Elsharkawy, M.M.; Salem, M.Z.M.; Hafez, E.E.; Heflish, A.A. Plantago lagopus extract as a green fungicide induces systemic resistance against Rhizoctonia root rot disease in tomato plants. Front. Plant Sci. 2022, 13, 2818. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Bhat, M.Y.; Malik, A.R.; Koka, J.A.; Ashraf, N. Antimycotic potential of some phytoextracts on some pathogenic fungi. J. Biopestic. 2017, 10, 60–65. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. De Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar]
- Behiry, S.I.; Philip, B.; Salem, M.Z.M.; Amer, M.A.; El-Samra, I.A.; Abdelkhalek, A.; Heflish, A. Urtica dioica and Dodonaea viscosa leaf extracts as eco-friendly bioagents against Alternaria alternata isolate TAA-05 from tomato plant. Sci. Rep. 2022, 12, 16468. [Google Scholar]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar]
- Youssef, N.H.; Qari, S.H.; Behiry, S.I.; Dessoky, E.S.; El-Hallous, E.I.; Elshaer, M.M.; Kordy, A.; Maresca, V.; Abdelkhalek, A.; Heflish, A.A. Antimycotoxigenic Activity of Beetroot Extracts against Altenaria alternata Mycotoxins on Potato Crop. Appl. Sci. 2021, 11, 4239. [Google Scholar] [CrossRef]
- Dong, Z.; Qu, S.; Landrein, S.; Yu, W.-B.; Xin, J.; Zhao, W.; Song, Y.; Tan, Y.; Xin, P. Increasing taxa sampling provides new insights on the phylogenetic relationship between Eriobotrya and Rhaphiolepis. Front. Genet. 2022, 13, 831206. [Google Scholar]
- Rajalakshmi, P.; Code, Q.R. Nutraceutical studies on Eriobotrya japonica (Thunb.) Lindl. (Fruits & Seeds). Int. J. Adv. Sci. Res. 2017, 3, 44–48. [Google Scholar]
- Ngugi, M.P.; Maingi, J. Phytochemical Screening of Aqueous Leaf, Stem Bark and Root Extracts of Rhaphiolepis bibas (Lour.). Int. J. Innov. Sci. Res. Technol. 2013, 8, 1140–1144. [Google Scholar]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Thompson, M.D.; Brick, M.A.; McGinley, J.N.; Thompson, H.J. Chemical composition and mammary cancer inhibitory activity of dry bean. Crop Sci. 2009, 49, 179–186. [Google Scholar]
- Siddiq, M.; Ravi, R.; Harte, J.B.; Dolan, K.D. Physical and functional characteristics of selected dry bean (Phaseolus vulgaris L.) flours. LWT-Food Sci. Technol. 2010, 43, 232–237. [Google Scholar]
- Kutoš, T.; Golob, T.; Kač, M.; Plestenjak, A. Dietary fibre content of dry and processed beans. Food Chem. 2003, 80, 231–235. [Google Scholar]
- Zitnick-Anderson, K.; Oladzadabbasabadi, A.; Jain, S.; Modderman, C.; Osorno, J.M.; McClean, P.E.; Pasche, J.S. Sources of resistance to Fusarium solani and associated genomic regions in common bean diversity panels. Front. Genet. 2020, 11, 475. [Google Scholar]
- Elewa, I.S.; Ahmed, M.A.; Moustafa, A.M. Grouping of Rhizoctonia solani Kuhn in Egypt and their pathogenic effect on different host plants [corn, broad bean, cabbage, squash, clover, cucumber]. Ann. Agric. Sci. 1994, 123–146. [Google Scholar]
- Sneh, B.; Burpee, L.; Qgoshi, A. Identification of Rhizoctonia Species; APS Press: St. Paul, MN, USA, 1991. [Google Scholar]
- Van der Plaats-Niterink, A.J. Monogrpah of the Genus Pythium. In Studies Mycology No. 21; Centra Albareau vor Schimmel Cultures: Baarh, The Netherlands, 1981. [Google Scholar]
- Leck, A. Lactophenol Cotton Blue Slide Mounts. Community Eye Health 1999, 12, 24. [Google Scholar] [PubMed]
- Tiwari, K.L.; Jadhav, S.K.; Gupta, S. Modified CTAB technique for isolation of DNA from some medicinal plants. Res. J. Med. Plant 2012, 6, 65–73. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; University of Texas South Western Medical Center: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar]
- El-Bilawy, E.H.; Al-Mansori, A.-N.A.; Soliman, S.A.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Sabry, A.E.-N.; Elsharkawy, M.M.; Heflish, A.A.; Behiry, S.I. Antifungal, Antiviral, and HPLC Analysis of Phenolic and Flavonoid Compounds of Amphiroa anceps Extract. Sustainability 2022, 14, 12253. [Google Scholar]
- Fiorito, S.; Ianni, F.; Preziuso, F.; Epifano, F.; Scotti, L.; Bucciarelli, T.; Genovese, S. UHPLC-UV/Vis quantitative analysis of hydroxylated and O-prenylated coumarins in pomegranate seed extracts. Molecules 2019, 24, 1963. [Google Scholar]
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 403–421. [Google Scholar]
- Gerlach, W.; Nirenberg, H. The Genus Fusarium: A Pictorial Atlas (Mitteilungen aus der Biologischen Bundesanstalt fur Land-und Forstwirtschaft Berlin-Dahlem); Kommissionsverlag P. Parey: Berlin, Germany, 1982. [Google Scholar]
- Seepe, H.A.; Amoo, S.O.; Nxumalo, W.; Adeleke, R.A. Antifungal activity of medicinal plant extracts for potential management of Fusarium pathogens. Res. Crops 2019, 20, 399–406. [Google Scholar]
- Parekh, J.; Chanda, S. In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 2007, 31, 53–58. [Google Scholar]
- Van Vuuren, S.F.; Naidoo, D. An antimicrobial investigation of plants used traditionally in southern Africa to treat sexually transmitted infections. J. Ethnopharmacol. 2010, 130, 552–558. [Google Scholar]
- Kitonde, C.K.; Fidahusein, D.S.; Lukhoba, C.W.; Jumba, M.M. Antimicrobial activity and phytochemical screening of Senna didymobotrya used to treat bacterial and fungal infections in Kenya. Int. J. Educ. Res. 2014, 2, 1–12. [Google Scholar]
- Bhattacharjee, I.; Chatterjee, S.K.; Ghosh, A.; Chandra, G. Antibacterial activities of some plant extracts used in Indian traditional folk medicine. Asian Pac. J. Trop. Biomed. 2011, 1, S165–S169. [Google Scholar]
- Ashmawy, N.A.; Salem, M.Z.M.; El Shanhorey, N.; Al-Huqail, A.; Ali, H.M.; Behiry, S.I. Eco-friendly wood-biofungicidal and antibacterial activities of various Coccoloba uvifera L. leaf extracts: HPLC analysis of phenolic and flavonoid compounds. BioResources 2020, 15, 4165–4187. [Google Scholar]
- Deena, M.J.; Thoppil, J.E. Antimicrobial activity of the essential oil of Lantana camara. Fitoterapia 2000, 71, 453–455. [Google Scholar]
- Valarini, P.J.; de Melo, I.S.; Morsoletto, R.V. Alternative control of root rot of common beans (Phaseolus vulgaris L.). Summa Phytopathol. 2003, 29, 334–339. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic plants as a source of bioactive compounds. Agriculture 2012, 2, 228–243. [Google Scholar]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Activity of phenolic compounds from plant origin against Candida species. Ind. Crops Prod. 2015, 74, 648–670. [Google Scholar]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M. Status and prospects of botanical biopesticides in Europe and Mediterranean countries. Biomolecules 2022, 12, 311. [Google Scholar]
- Bhardwaj, S.K. Evaluation of plant extracts as antifungal agents against Fusarium solani (Mart.) Sacc. World J. Agric. Sci. 2012, 8, 385–388. [Google Scholar]
- Choudhury, D.; Anand, Y.R.; Kundu, S.; Nath, R.; Kole, R.K.; Saha, J. Effect of plant extracts against sheath blight of rice caused by Rhizoctonia solani. J. Pharmacogn. Phytochem. 2017, 6, 399–404. [Google Scholar]
- Yavuz, A.; Onaran, A.; Bayar, Y. Endemik Serik Armudu (Pyrus serikensis)’nun Yaprak ve Meyve Ekstraktlarının Bazı Bitki Patojeni Funguslara Karşı Biyofungusidal Aktivitesi. Türk Tarım ve Doğa Bilimleri Dergisi 2022, 9, 256–262. [Google Scholar]
- Onaran, A.; Başaran, M. Determination of antifungal activity and phenolic compounds of endemic Muscari aucheri (Boiss.) baker extract. Gaziosmanpaşa Üniversitesi Ziraat Fakültesi Dergisi 2018, 35, 60–67. [Google Scholar]
- Sikkema, J.A.N.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [PubMed]
- Bajpai, V.K.; Sharma, A.; Baek, K.-H. Antibacterial mode of action of Ginkgo biloba leaf essential oil: Effect on morphology and membrane permeability. Bangladesh J. Pharmacol. 2015, 10, 337–350. [Google Scholar]
- Wu, H.-S.; Raza, W.; Fan, J.-Q.; Sun, Y.-G.; Bao, W.; Shen, Q.-R. Cinnamic acid inhibits growth but stimulates production of pathogenesis factors by in vitro cultures of Fusarium oxysporum f. sp. niveum. J. Agric. Food Chem. 2008, 56, 1316–1321. [Google Scholar]
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N. Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP 53). J. Appl. Microbiol. 2014, 116, 955–966. [Google Scholar]
- El Sawi, S.; Moawad, D.; El Alfy, S. Activity of Chorisia insignis HBK. against larynx carcinoma and chemical investigation of its polar extracts. J. Appl. Sci. Res. 2012, 8, 5564–5571. [Google Scholar]
- Mohamed, A.A.; Behiry, S.I.; Ali, H.M.; EL-Hefny, M.; Salem, M.Z.M.; Ashmawy, N.A. Phytochemical compounds of branches from P. halepensis oily liquid extract and S. terebinthifolius essential oil and their potential antifungal activity. Processes 2020, 8, 330. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).