HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Collection
2.3. Instrumentation and Chromatographic Conditions
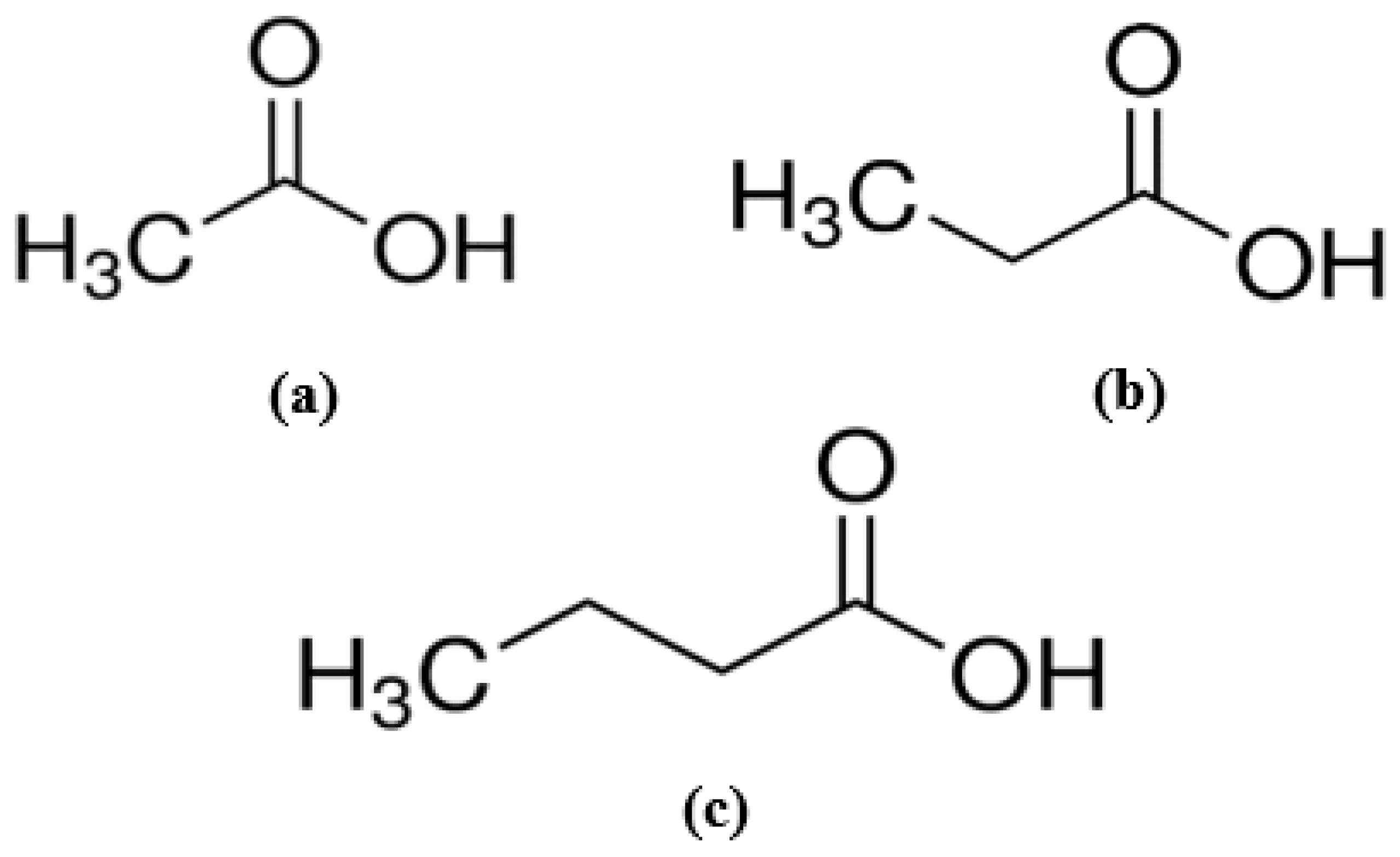
2.4. Preparation of Standard Stock Solution
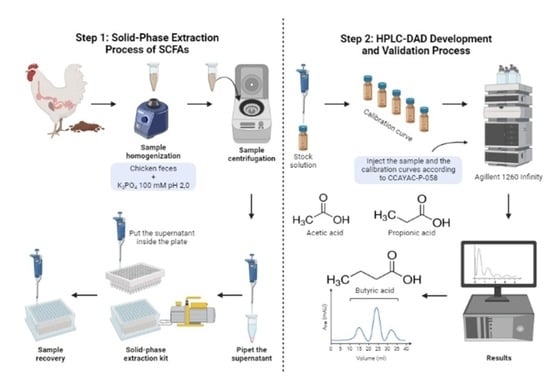
2.5. Solid-Phase Extraction (SPE)
2.6. Validation Method
2.6.1. Linearity and Range
2.6.2. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.6.3. Accuracy Study
2.6.4. Precision Study
Repeatability
Reproducibility
2.6.5. Robustness
2.6.6. Recovery
3. Results
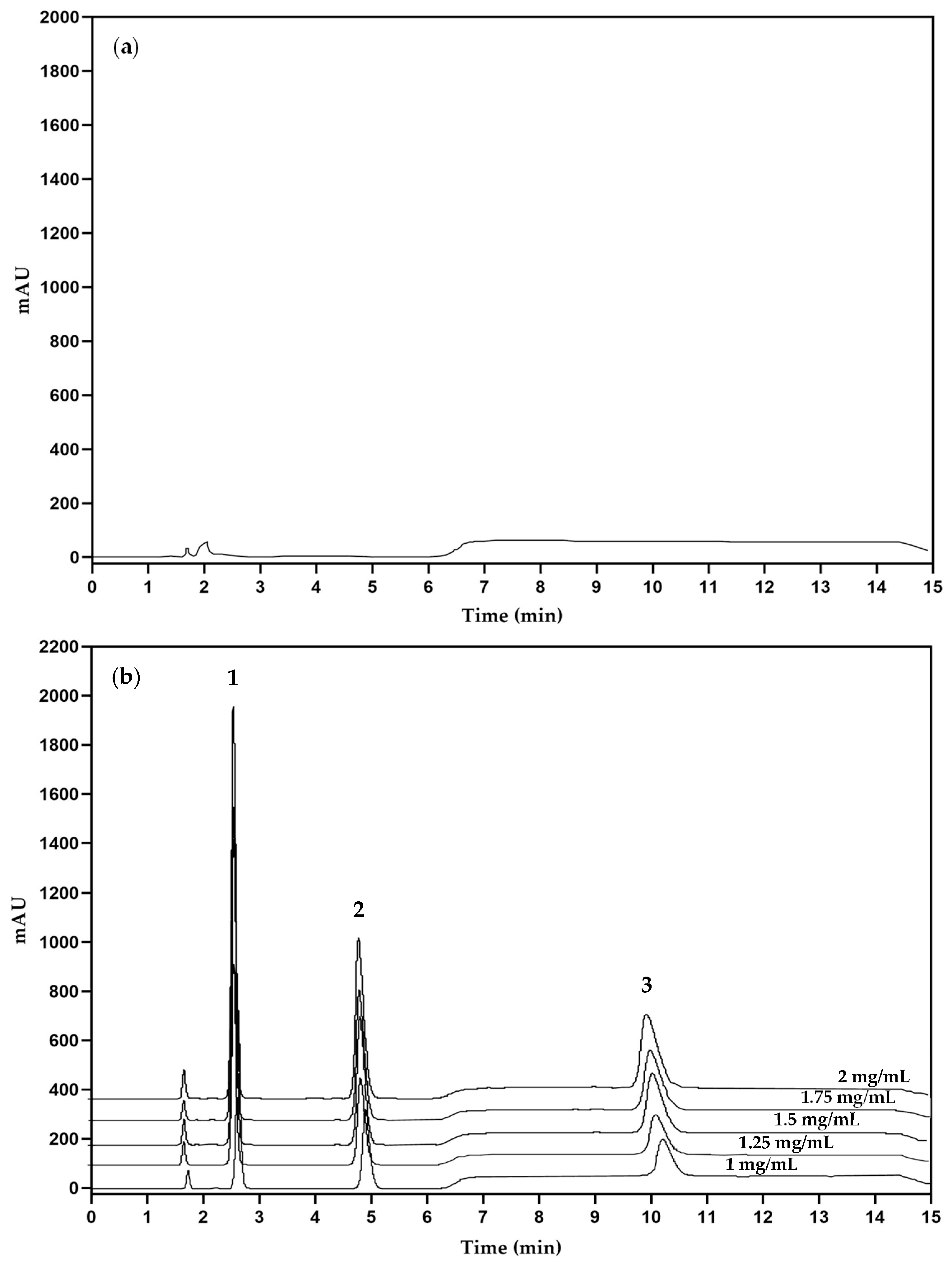
3.1. Solid-Phase Extraction Process
3.2. Validation Method
3.2.1. Linearity and Range
3.2.2. LOD and LOQ
3.2.3. Accuracy
3.2.4. Precision
3.2.5. Robustness
3.2.6. Recovery Analysis of Chicken Feces Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, J.; Lin, S.; Zheng, B.; Cheung, P.C.K. Short-chain fatty acids in control of energy metabolism. Crit. Rev. Food Sci. Nutr. 2018, 58, 1243–1249. [Google Scholar] [CrossRef]
- Ali, Q.; Ma, S.; La, S.; Guo, Z.; Liu, B.; Gao, Z.; Farooq, U.; Wang, Z.; Zhu, X.; Cui, Y.; et al. Microbial short-chain fatty acids: A bridge between dietary fibers and poultry gut health: A review. Anim. Biosci. 2022, 35, 1461–1478. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Primec, M.; Mičetić-Turk, D.; Langerholc, T. Analysis of short-chain fatty acids in human feces: A scoping review. Anal. Biochem. 2017, 526, 9–21. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Yang, Y.; Guo, A. Biological Function of Short-Chain Fatty Acids and Its Regulation on Intestinal Health of Poultry. Front. Vet. Sci. 2021, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Yu, Y.N.; Wang, J.L.; Lin, Y.W.; Kong, X.; Yang, C.Q.; Yang, L.; Liu, Z.J.; Yuan, Y.Z.; Liu, F.; et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am. J. Clin. Nutr. 2013, 97, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; González, S. Fiber from a regular diet is directly associated with fecal short-chain fatty acid concentrations in the elderly. Nutr. Rsch. 2013, 33, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Weir, T.L.; Manter, D.K.; Sheflin, A.M.; Barnett, B.A.; Heuberger, A.L.; Ryan, E.P. Stool Microbiome and Metabolome Differences between Colorectal Cancer Patients and Healthy Adults. PLoS ONE 2013, 8, e70803. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Critical analysis of methods for the measurement of volatile fatty acids. Crit. Rev. Environ. Sci. Technol. 2016, 46, 209–234. [Google Scholar] [CrossRef]
- Torii, T.; Kanemitsu, K.; Wada, T.; Itoh, S.; Kinugawa, K.; Hagiwara, A. Measurement of short-chain fatty acids in human faeces using high-performance liquid chromatography: Specimen stability. Ann. Clin. Biochem. Int. J. Lab. Med. 2010, 47, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Kotani, A.; Miyaguchi, Y.; Kohama, M.; Ohtsuka, T.; Shiratori, T.; Kusu, F. Determination of Short-chain Fatty Acids in Rat and Human Feces by High-Performance Liquid Chromatography with Electrochemical Detection. Anal. Sci. 2009, 25, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Salzman, N.H.; Bennett, S.H.; Barman, M.; Mills, D.; Marcobal, A.; Tancredi, D.J.; Bevins, C.L.; Sherman, M.P. A Randomized Placebo-controlled Comparison of 2 Prebiotic/Probiotic Combinations in Preterm Infants: Impact on Weight Gain, Intestinal Microbiota, and Fecal Short-chain Fatty Acids. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Taverniti, V.; Milani, C.; Fiore, W.; Laureati, M.; De Noni, I.; Stuknyte, M.; Chouaia, B.; Riso, P.; Guglielmetti, S. Modulation of Fecal Clostridiales Bacteria and Butyrate by Probiotic Intervention with Lactobacillus paracasei DG Varies among Healthy Adults. J. Nutr. 2014, 144, 1787–1796. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Chouinard, Y.P.; Jacques, H.; Venkatramanan, S.; Maf, A.A.; Defnoun, S.; Jones, P.J. The effect of drinking milk containing conjugated linoleic acid on fecal microbiological profile, enzymatic activity, and fecal characteristics in humans. Nutr. J. 2007, 6, 1787–1796. [Google Scholar] [CrossRef]
- Okazaki, M.; Matsukuma, S.; Suto, R.; Miyazaki, K.; Hidaka, M.; Matsuo, M.; Noshima, S.; Zempo, N.; Asahara, T.; Nomoto, K. Perioperative synbiotic therapy in elderly patients undergoing gastroenterological surgery: A prospective, randomized control trial. Nutrition 2013, 29, 1224–1230. [Google Scholar] [CrossRef]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Matsushima, A.; Hayakawa, K.; Ikegawa, H.; Tasaki, O.; Kuwagata, Y.; Shimazu, T. Gut microbiota and environment in patients with major burns—A preliminary report. Burns 2015, 41, e28–e33. [Google Scholar] [CrossRef]
- Wysocki, J.; Dong, M. Ultraviolet detectors: Perspectives, principles, and practices. LCGC N. Am. 2019, 37, 750–759. [Google Scholar]
- Zhao, G.; Nyman, M.; Åke-Jönsson, J. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef]
- Zheng, J.; Kuang, Y.; Zhou, S.; Gong, X.; Ouyang, G. Latest Improvements and Expanding Applications of Solid-Phase Microextraction. Anal. Chem. 2023, 95, 218–237. [Google Scholar] [CrossRef]
- Hu, T.; Chen, R.; Wang, Q.; He, C.; Liu, S. Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis. J. Sep. Sci. 2021, 44, 274–309. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Hua, Y.; Xin, W.; Wenrui, X.; Wentao, L.; Yingping, X.; Xiaoting, Z. Early Intervention with Cecal Fermentation Broth Regulates the Colonization and Development of Gut Microbiota in Broiler Chickens. Front. Microbiol. 2019, 10, 1422. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.; Hu, J.; Peng, H.; Li, B.; Xu, J.; Song, X.; Kong, F. Research Note: The gut microbiota varies with dietary fiber levels in broilers. Poult. Sci. 2022, 101, 101922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.; Gu, X.Y.; Xu, W.; Jiao, H.Y.; Zhao, H.C.; Lin, H. Different effects of probiotics and antibiotics on the composition of microbiota, SCFAs concentrations and FFAR2/3 mRNA expression in broiler chickens. J. Appl. Microbiol. 2021, 131, 913–924. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, K.; Mishra, R.; Jha, R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020, 99, 4776–4785. [Google Scholar] [CrossRef]
- Hati, S.; Patel, M.; Mishra, B.K.; Das, S. Short-chain fatty acid and vitamin production potentials of Lactobacillus isolated from fermented foods of Khasi Tribes, Meghalaya, India. Ann. Microbiol. 2019, 69, 1191–1199. [Google Scholar] [CrossRef]
- De Baere, S.; Eeckhaut, V.; Steppe, M.; De Maesschalck, C.; De Backer, P.; Van Immerseel, F.; Croubels, S. Development of a HPLC–UV method for the quantitative determination of four short-chain fatty acids and lactic acid produced by intestinal bacteria during in vitro fermentation. J. Pharm. Biomed. Anal. 2013, 80, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Idroes, R.; Idroes, G.M.; Suhendra, R. The effect of column and temperature variation on the determination of the dead time in gas chromatographic systems using indirect methods. Heliyon 2020, 6, e03302. [Google Scholar] [CrossRef]
- Matysik, S.; Le Roy, C.I.; Liebisch, G.; Claus, S.P. Metabolomics of fecal samples: A practical consideration. Trends Food Sci. Technol. 2016, 57, 244–255. [Google Scholar] [CrossRef]
- Hurst, N.R.; Kendig, D.M.; Murthy, K.S.; Grider, J.R. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol. Motil. 2014, 26, 1586–1596. [Google Scholar] [CrossRef]
- Zidwick, M.J.; Chen, J.S.; Rogers, P. The Prokaryotes: Organic Acid and Solvent Production: Propionic and Butyric Acids and Ethanol, 4th ed.; Springer Reference: New York, NY, USA, 2013; pp. 135–167. [Google Scholar]
- Wang, H.Y.; Wang, C.; Guo, L.X.; Zheng, Y.F.; Hu, W.H.; Dong, T.T.; Tsim, K.W. Simultaneous determination of short-chain fatty acids in human feces by HPLC with ultraviolet detection following chemical derivatization and solid-phase extraction segmental elution. J. Sep. Sci. 2019, 42, 2500–2509. [Google Scholar] [CrossRef]
- Zhang, L.; Tsui, T.H.; Loh, K.C.; Dai, Y.; Tong, Y.W. Acidogenic Fermentation of Organic Wastes for Production of Volatile Fatty Acids in Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–366. [Google Scholar] [CrossRef]
- Dobrowolska-Iwanek, J.; Lauterbach, R.; Huras, H.; Paśko, P.; Prochownik, E.; Woźniakiewicz, M.; Zagrodzki, P. HPLC-DAD method for the quantitative determination of short-chain fatty acids in meconium samples. Microchemical. J. 2020, 155, 104671. [Google Scholar] [CrossRef]


| Time (min) | Flow (mL/min) | Sulfuric Acid (5 mM) | Acetonitrile |
|---|---|---|---|
| 0.00 | 1.0 | 100 | 0 |
| 4.00 | 1.0 | 100 | 0 |
| 4.20 | 1.2 | 98 | 2 |
| 11.90 | 1.2 | 98 | 2 |
| 15.00 | 1.2 | 100 | 0 |
| Operational Parameter | Low | Optimal | High |
|---|---|---|---|
| Wavelength (nm) | 205 | 210 | 215 |
| Mobile phase H2SO4 concentration (mM) | 2 | 5 | 8 |
| Column temperature (°C) | 30 | 40 | 50 |
| H2SO4 % change time on gradient (min) | 3.7 | 4.2 | 4.7 |
| Parameters | Acetic Acid | Propionic Acid | Butyric Acid |
|---|---|---|---|
| Linearity (R2) | 0.9987 | 0.9985 | 0.9966 |
| Range (R, m) 1 | 0.9993, 0.9987 | 0.9992, 0.9985 | 0.9983, 0.9966 |
| LOD (mg/mL) | 0.14 | 0.14 | 0.14 |
| LOQ (mg/mL) | 0.44 | 0.45 | 0.43 |
| Accuracy (%) | 99.98 ± 1.58% | 99.98 ± 1.27% | 99.98 ± 1.40% |
| Acid | Acetic | Propionic | Butyricid | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/mL) | 1.0 | 1.5 | 2.0 | 1.0 | 1.5 | 2.0 | 1.0 | 1.5 | 2.0 |
| Intraday (n = 6) | |||||||||
| Recovery (%) | 99.42 | 100.0 | 99.67 | 99.46 | 99.95 | 99.71 | 99.49 | 99.93 | 99.72 |
| RSD 1 (%) | 1.11 | 1.05 | 0.69 | 1.44 | 1.45 | 0.95 | 1.62 | 1.30 | 1.16 |
| SD 2 | 1.10 | 1.05 | 0.69 | 1.43 | 1.45 | 0.95 | 1.61 | 1.30 | 1.15 |
| Interday (n = 9) | |||||||||
| Recovery (%) | 99.26 | 100.2 | 99.61 | 99.32 | 100.1 | 99.66 | 99.3 | 100.1 | 99.68 |
| RSD 1 (%) | 1.44 | 1.74 | 1.63 | 1.38 | 1.27 | 1.53 | 1.35 | 1.44 | 1.62 |
| SD 2 | 1.43 | 1.74 | 1.62 | 1.37 | 1.27 | 1.53 | 1.34 | 1.44 | 1.61 |
| Parameter | Modification | Retention Time (min) | Accuracy (%) | RSD (%) | DE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Optimal condition 1 | - | AA | PA | BA | AA | PA | BA | AA | PA | BA | AA | PA | BA |
| 2.62 | 4.88 | 9.90 | 97.19 | 98.04 | 97.83 | 0.00 | 0.03 | 0.16 | 0.00 | 0.03 | 0.15 | ||
| Wavelength (nm) | 205 | 2.62 | 4.88 | 9.89 | 105.9 | 102.3 | 98.60 | 0.08 | 0.12 | 0.47 | 0.09 | 0.13 | 0.46 |
| 215 | 2.62 | 4.88 | 9.87 | 78.49 | 82.35 | 84.32 | 0.02 | 0.00 | 0.06 | 0.02 | 0.00 | 0.05 | |
| Mobile phase H2SO4 concentration (mM) | 2 | 2.62 | 4.87 | 9.66 | 97.06 | 98.13 | 97.90 | 0.01 | 0.01 | 0.03 | 0.01 | 0.01 | 0.03 |
| 8 | 2.61 | 4.85 | 9.66 | 96.91 | 97.65 | 96.74 | 0.02 | 0.04 | 0.15 | 0.02 | 0.04 | 0.14 | |
| Column temperature (°C) | 30 | 2.73 | 5.23 | 10.6 | 96.39 | 97.23 | 96.26 | 0.02 | 0.01 | 0.06 | 0.02 | 0.01 | 0.06 |
| 50 | 2.53 | 4.55 | 9.06 | 97.14 | n.q. | n.q. | 0.01 | n.q. | n.q. | 0.01 | n.q. | n.q. | |
| H2SO4 % change time on gradient (min) | 1.7 | 2.62 | 4.77 | 9.57 | 96.94 | 97.88 | 97.01 | 0.02 | 0.00 | 0.10 | 0.02 | 0.00 | 0.09 |
| 4.7 | 2.61 | 4.93 | 10.0 | 96.88 | 98.03 | 97.17 | 0.00 | 0.01 | 0.10 | 0.00 | 0.01 | 0.10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Corona, L.R.; Parra-Saavedra, K.J.; Mora-Alonzo, R.S.; Macías-Rodríguez, M.E.; Martínez-Preciado, A.H.; Guevara-Martínez, S.J.; Zamudio-Ojeda, A.; Macias-Lamas, A.M. HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction. Separations 2023, 10, 308. https://doi.org/10.3390/separations10050308
Díaz-Corona LR, Parra-Saavedra KJ, Mora-Alonzo RS, Macías-Rodríguez ME, Martínez-Preciado AH, Guevara-Martínez SJ, Zamudio-Ojeda A, Macias-Lamas AM. HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction. Separations. 2023; 10(5):308. https://doi.org/10.3390/separations10050308
Chicago/Turabian StyleDíaz-Corona, Lenin Rodolfo, Karina Jeanette Parra-Saavedra, Renata Sofia Mora-Alonzo, María Esther Macías-Rodríguez, Alma H. Martínez-Preciado, Santiago José Guevara-Martínez, Adalberto Zamudio-Ojeda, and Adriana Macaria Macias-Lamas. 2023. "HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction" Separations 10, no. 5: 308. https://doi.org/10.3390/separations10050308
APA StyleDíaz-Corona, L. R., Parra-Saavedra, K. J., Mora-Alonzo, R. S., Macías-Rodríguez, M. E., Martínez-Preciado, A. H., Guevara-Martínez, S. J., Zamudio-Ojeda, A., & Macias-Lamas, A. M. (2023). HPLC-DAD Development and Validation Method for Short-Chain Fatty Acids Quantification from Chicken Feces by Solid-Phase Extraction. Separations, 10(5), 308. https://doi.org/10.3390/separations10050308