Box–Behnken Design Based Development of UV-Reversed Phase High Performance Liquid Chromatographic Method for Determination of Ascorbic Acid in Tablet Formulations
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Standards
2.2.1. Preparation of Buffer Solutions for HPLC Method Development
2.2.2. Preparation of Mobile Phases for HPLC Method Development
2.2.3. Preparation of Standard Solutions of Ascorbic Acid
2.3. Procedure to Determine λmax of Ascorbic Acid by UV Specrophotometry
2.4. Optimization of Variables for HPLC Method Development
2.5. Procedure for the Determination of Ascorbic Acid by Proposed Method
2.6. Procedure for the Determination of Ascorbic Acid by Reference Method [61]
2.7. Procedure for the Assay of Ascorbic Acid in Tablets
3. Results and Discussion
3.1. BBD Design for HPLC Optimization
3.2. Response Surface Plots
3.3. Method Validation
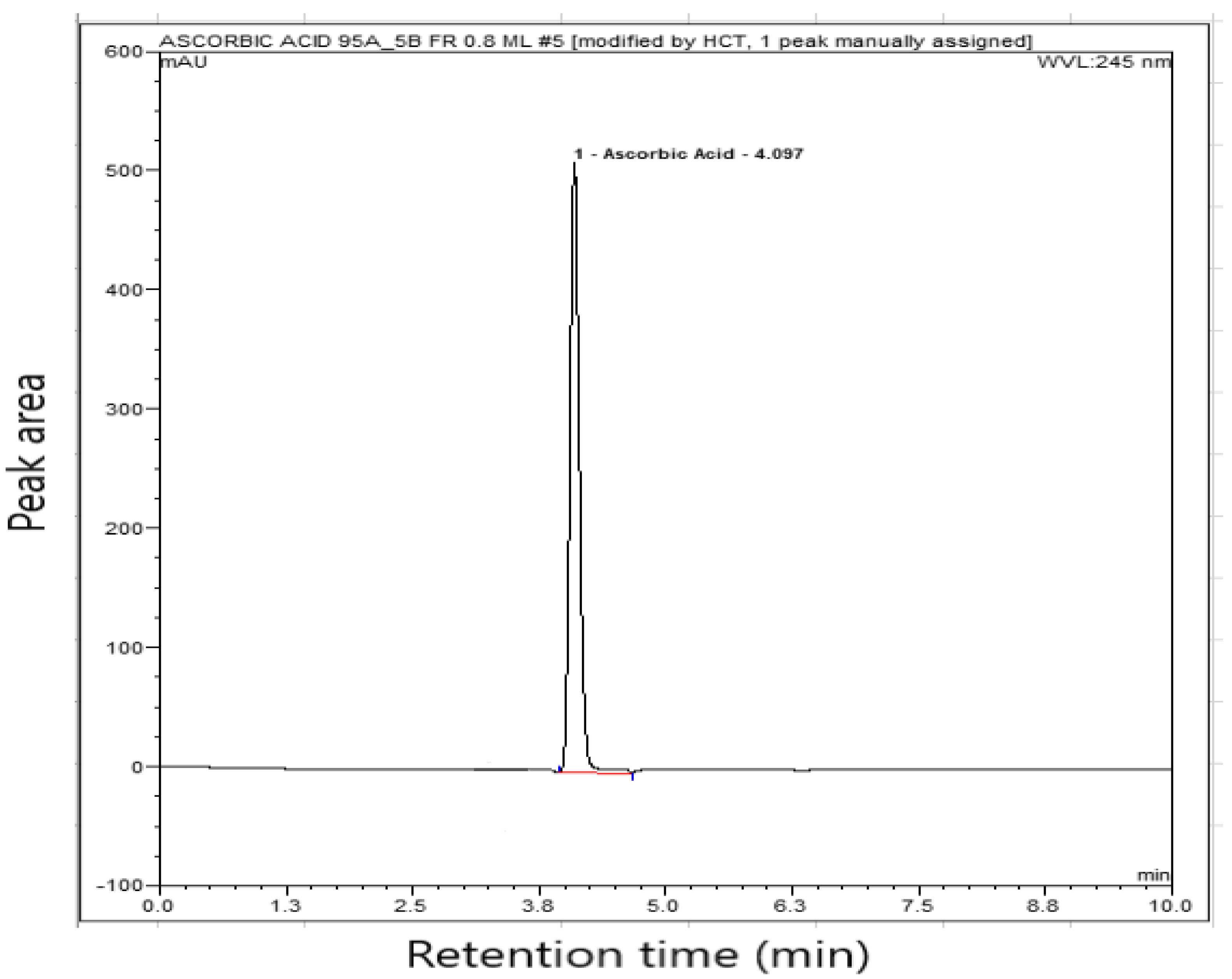
3.3.1. System Suitability
3.3.2. Robustness
3.3.3. Linearity and Range
3.3.4. Limits of Detection and Quantitation
3.3.5. Precision
3.3.6. Specificity
3.3.7. Accuracy
3.4. Comparison of the Proposed Method with Other Published HPLC Methods
3.5. Greenness Profile of the Proposed Method
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valente, A.; Albuquerque, T.G.; Sanches-Silva, A.; Costa, H.S. Ascorbic acid content in exotic fruits: A contribution to produce quality data for food composition databases. Food Res. Int. 2011, 44, 2237–2242. [Google Scholar] [CrossRef]
- British Pharmacopoeia Commission. British Pharmacopoeia, Vol. I; Her Majesty Stationary Office: London, UK, 2018; pp. 203–204. [Google Scholar]
- USP 42. In United States Pharmacopoeia; The United States Pharmacopeial Convention: Rockville, MD, USA, 2018; pp. 377–378.
- European Pharmacopoeia. European Pharmacopoeia, 9th ed.; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2017; pp. 1760–1761. [Google Scholar]
- NHS. British National Formulary 81, Chapter 9: Blood and Nutrition; Royal Pharmaceutical Society: London, UK, 2021; p. 1132. [Google Scholar]
- Baker, E.M.; Hodges, R.E.; Hood, J.; Sauberlich, H.E.; March, S.C. Metabolism of ascorbic-1–14C acid in experimental human scurvy. Am. J. Clin. Nutr. 1969, 22, 549–558. [Google Scholar] [CrossRef]
- Krebs, N.A.; Peters, R.A.; Coward, K.H. Vitamin C requirement of human adults: Experimental study of vitamin C deprivation in man. Lancet 1948, 254, 853–858. [Google Scholar]
- World Health Organization and Food and Agriculture Organization of the United Nations. Vitamin C. In Vitamin and Mineral Requirements in Human Nutrition, 2nd ed.; World Health Organization and Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2004; p. 132. [Google Scholar]
- Rossetti, C.A.; Real, J.P.; Palma, S.D. High dose of ascorbic acid used in Sars COVID-19 treatment: Scientific and clinical support for is therapeutic implementation. Ars Pharm. 2020, 61, 145–148. [Google Scholar]
- Hiedra, R.; Lo, K.B.; Elbashabsheh, M.; Gul, F.; Wright, R.M.; Albano, J.; Azmaiparashvili, Z.; Patarroyo Aponte, G. The use of IV vitamin C for patients with COVID-19: A case series. Expert Rev. Anti Ther. 2020, 18, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Klein, K. Chapter 15—Antioxidant Vitamins and Minerals, in Antioxidants. In Food, Vitamins and Supplements; Elsevier: San Diego, CA, USA, 2014; pp. 277–294. [Google Scholar]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1209. [Google Scholar]
- Azmi, S.N.H.; Iqbal, B.; Al Humaimi, N.S.H.; Al Salmani, I.R.S.; Al Ghafri, N.A.S.; Rahman, N. Quantitative analysis of cefixime via complexation with palladium (II) in pharmaceutical formulations by spectrophotometry. J. Pharm. Anal. 2013, 3, 248–256. [Google Scholar] [CrossRef]
- Husain, A.; Iram, F.; Siddiqui, A.A.; Almutairi, S.M.; Mohammed, O.B.; Khan, S.A.; Azmi, S.N.H.; Rahman, N. Identification of metabolic pathways involved in the biotransformation of eslicarbazepine acetate using UPLC-MS/MS, human microsomal enzymes and in silico studies. J. King Saud Univ. Sci. 2021, 33, 101281. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Al-Othman, Z.A.; Rahman, N. Analytical techniques in pharmaceutical analysis: A review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Al Othman, Z.A.; Rahman, N.; Siddiqui, M.R. Review on pharmaceutical impurities, stability studies and degradation products. Rev. Adv. Sci. Eng. 2013, 2, 155–166. [Google Scholar] [CrossRef]
- Rahman, N.; Azmi, S.N.H.; Wu, H.F. The importance of impurity analysis in pharmaceutical products: An integrated approach. Accredit. Qual. Assur. 2006, 11, 69–74. [Google Scholar] [CrossRef]
- Qureshi, S.Z.; Saeed, A.; Haque, S.; Rahman, N. Spectrophotometric method for the estimation of Vitamin C in drug formulations. Anal. Lett. 1990, 23, 995–1003. [Google Scholar] [CrossRef]
- Salkić, M.; Selimović, A. Spectrophotometric determination of L-Ascorbic Acid in pharmaceuticals based on its oxidation by potassium peroxymonosulfate and hydrogen peroxide. Croat. Chem. Acta 2015, 88, 73–79. [Google Scholar] [CrossRef]
- Shishehbore, M.R.; Aghamiri, Z. A highly sensitive kinetic spectrophotometric method for the determination of ascorbic acid in pharmaceutical samples. Iran. J. Pharm. Res. 2014, 13, 373–382. [Google Scholar]
- Hussein, A.Z.M. Spectrophotometric determination of ascorbic acid in aqueous solutions and in pharmaceuticals formulations. J. Al-Nahrain Univ. 2013, 16, 65–71. [Google Scholar] [CrossRef]
- Mowry, S.; Ogren, P.J. Kinetics of methylene blue reduction by ascorbic acid. J. Chem. Educ. 1999, 76, 970–973. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Sugawara, T. Thermodynamic parameters of complexation of lanthanoid (III) with ascorbic acid. Inorg. Chim. Acta 1988, 143, 277–280. [Google Scholar] [CrossRef]
- Pyka-Pajak, A.; Dołowy, M.; Parys, W.; Bober, K.; Janikowska, G. A simple and cost-effective TLC-densitometric method for the quantitative determination of acetylsalicylic acid and ascorbic acid in combined effervescent tablets. Molecules 2018, 23, 3115. [Google Scholar] [CrossRef]
- Akasaka, K. Simple determination of L-ascorbic acid on TLC by visual detection using autocatalytic reaction. Anal. Sci. 2013, 29, 505–509. [Google Scholar] [CrossRef][Green Version]
- Polak, B.; Pajurek, E. Separation of some vitamins in reversed-phase thin-layer chromatography and pressurized planar electrochromatography with eluent containing surfactant. Sci. Rep. 2021, 11, 21851. [Google Scholar] [CrossRef]
- Alam, P.; Kamal, Y.T.; Alqasoumi, S.I.; Foudah, A.I.; Alqarni, M.H.; Yusufoglu, H.S. HPTLC method for simultaneous determination of ascorbic acid and gallic acid biomarker from freeze dry pomegranate juice and herbal formulation. Saudi Pharm. J. 2019, 27, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.L.; Kaligirwa, A.; Lukwago, T. Stability indicating high-performance thin-layer chromatography method for estimation of ascorbic acid in Hibiscus sabdariffa L. aqueous extract. J. Complement. Med. Res. 2019, 10, 50–57. [Google Scholar] [CrossRef]
- Koh, E.V.; Bissell, M.G.; Ito, R.K. Measurement of vitamin C by capillary. electrophoresis in biological fluids and fruit beverages using a stereoisomer as an internal standard. J. Chromatogr. 1993, 633, 245–250. [Google Scholar] [CrossRef]
- Amayreh, M.; Hourani, W.; Hourani, M.K. Voltammetric determination of ascorbic acid in pharmaceutical formulations using modified iodine-coated platinum electrode. J. Vitae 2021, 28, 346228. [Google Scholar] [CrossRef]
- Nojavan, S.; Khalilian, F.; Kiaie, F.M.; Rahimi, A.; Arabanian, A.; Chalavi, S. Extraction and quantitative determination of ascorbic acid during different maturity stages of Rosa canina L. fruit. J. Food Compos. Anal. 2008, 21, 300–305. [Google Scholar] [CrossRef]
- Russel, L.F. Food Analysis by HPLC. In Quantitative Determination of Water-Soluble Vitamins, 2nd ed.; Nollet, L.M.L., Ed.; Marcel Dekker: New York, NY, USA, 2000; pp. 403–476. [Google Scholar]
- Ibrahim, F.; El-Enany, N.; El-Shaheny, R.N.; Mikhail, I.E. Development and validation of a new HPLC method for the simultaneous determination of paracetamol, ascorbic acid, and pseudoephedrine HCl in their co-formulated tablets. Application to in vitro dissolution testing. Anal. Sci. 2015, 31, 943–947. [Google Scholar] [CrossRef] [PubMed]
- El-Din, M.S.; Eid, M.; Zeid, A.M. Development and validation of RP-HPLC method for simultaneous determination of ascorbic acid and salicylamide in their binary mixtures: Application to combined tablets. J. Chromatogr. Sep. Tech. 2012, 3, 1000137. [Google Scholar] [CrossRef]
- Tsvetkova, B.; Pencheva, I.; Zlatkov, A.; Peikov, P. Simultaneous high-performance liquid chromatography determination of paracetamol and ascorbic acid in tablet dosage forms. Afr. J. Pharm. Pharmacol. 2012, 6, 1332–1336. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.G. HPLC-UV method for the simultaneous determinations of ascorbic acid and dehydroascorbic acid in human plasma. Transl. Clin. Pharmacol. 2016, 24, 37–42. [Google Scholar] [CrossRef]
- Hu, L.; Li, L.; Luo, Z.; Yang, J.; Liu, W. Determination of trace vitamin C by ion-pair HPLC with UV detection in calcium gluconate and vitamin C compound oral solution. J. Chromatogr. Sci. 2012, 50, 102–107. [Google Scholar] [CrossRef]
- Mahmood, H.S.; Ahmad, R.R. High performance liquid chromatographic determination of ascorbic acid in pharmaceutical preparations, fruit juices and human Serum. Rafidain J. Sci. 2018, 27, 79–92. [Google Scholar]
- Stana, M.; Sorana, M.L.; Marutoiub, C. Extraction and HPLC Determination of the ascorbic acid content of three indigenous spice plants. J. Anal. Chem. 2014, 69, 998–1002. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Jayaprakasha, G.K.; Yoo, K.S.; Jifon, J.L.; Patil, B.S. An improved sample preparation method for quantification of ascorbic acid and dehydroascorbic acid by HPLC. Food Sci. Technol. 2012, 47, 443–449. [Google Scholar] [CrossRef]
- Gennaro, M.C.; Bertolo, P.L. L-Ascorbic acid determination in fruits and medical formulations by ion interaction reagent reverse phase HPLC technique. J. Liq. Chromatogr. 1990, 13, 1419–1434. [Google Scholar] [CrossRef]
- Lloyd, L.L.; Warner, F.P.; White, C.A.; Kennedy, J.F. Quantitative reversed phase HPLC analysis of L-ascorbic acid (vitamin C) and identification of its degradation products. Chromatographia 1987, 24, 371–376. [Google Scholar] [CrossRef]
- Garnero, C.; Longhi, M. Development of HPLC and UV spectrophotometric methods for the determination of ascorbic acid using hydroxypropyl-β-cyclodextrin and triethanolamine as photostabilizing agents. Anal. Chim. Acta 2010, 659, 159–166. [Google Scholar] [CrossRef]
- Maia, A.M.; Baby, A.R.; Yasaka, W.J.; Suenaga, E.; Kaneko, T.M.; Velasco, M.V.R. Validation of HPLC stability-indicating method for Vitamin C in semisolid pharmaceutical/cosmetic preparations with glutathione and sodium metabisulfite, as antioxidants. Talanta 2007, 71, 639–643. [Google Scholar] [CrossRef]
- Novakova, L.; Solichova, D.; Pavlovicova, S.; Solich, P. Hydrophilic interaction liquid chromatography method for the determination of ascorbic acid. J. Sep. Sci. 2008, 31, 1634–1644. [Google Scholar] [CrossRef]
- Kand’ar, R.; Zakova, P. Determination of ascorbic acid in human plasma with a view to stability using HPLC with UV detection. J. Sep. Sci. 2008, 31, 3503–3508. [Google Scholar] [CrossRef]
- Ahmida, M.H.S. Determination of ascorbic acid in vitamin C (tablets) by high-performance liquid chromatography. Asian J. Chem. 2009, 21, 6463–6467. [Google Scholar]
- Mitić, S.S.; Kostić, D.A.; Nasković-Dokić, D.C.; Mitic, M.N. Rapid and reliable HPLC method for the determination of vitamin C in pharmaceutical samples. Trop. J. Pharm. Res. 2011, 10, 105–111. [Google Scholar] [CrossRef]
- Abe-Matsumoto, L.T.; Sampaio, G.R.; Bastos, D.H.M. Is titration as accurate as HPLC for determination of vitamin C in supplements? Am. J. Anal. Chem. 2020, 11, 269–279. [Google Scholar] [CrossRef]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.D. Improved extraction methods for avoiding the interference of copper in the LC determination of ascorbic acid in multivitamin-mineral tablets. J. Pharm. Biomed. Anal. 2001, 25, 985–994. [Google Scholar] [CrossRef]
- Koblová, P.; Sklenářová, H.; Brabcová, I.; Solich, P. Development and validation of a rapid HPLC method for the determination of ascorbic acid, phenylephrine, paracetamol and caffeine using a monolithic column. Anal. Methods 2012, 4, 1588–1591. [Google Scholar] [CrossRef]
- Rahman, N.; Sameen, S.; Kashif, M. Application of Box–Behnken design and desirability function in the optimization of spectrophotometric method for the quantification of WADA banned drug: Acetazolamide. J. Mol. Liq. 2019, 274, 270–277. [Google Scholar] [CrossRef]
- Rahman, N.; Khan, S. Experimental design approach in the optimization of potentiometric method for lansoprazole determination using lansoprazole-tungstate based ion-selective electrode. Ind. Eng. Chem. Res. 2018, 57, 9351–9361. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al-Jassasi, B.M.H.; Al-Sawafi, H.M.S.; Al-Shukaili, S.H.G.; Rahman, N.; Nasir, M. Optimization for synthesis of silver nanoparticles through response surface methodology using leaf extract of Boswellia sacra and its application in antimicrobial activity. Environ. Monit. Assess. 2021, 193, 497. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al-Masrouri, Z.N.; Al-Lamki, I.R.; Al-Jabri, A.K.; Rahman, N.; Nasir, M.; Abdelrahman, K.; Fnais, M.S.; Alam, M. Development and validation of spectrophotometric method for determination of imipramine hydrochloride in tablets (solid materials). J. King Saud Univ.-Sci. 2022, 34, 101823. [Google Scholar] [CrossRef]
- Afzal, M.; Muddassir, M.; Alarifi, A.; Ansari, M.T. Box-Behnken assisted validation and optimization of an RP-HPLC method for simultaneous determination of domperidone and lansoprazole. Separations 2021, 8, 8010005. [Google Scholar] [CrossRef]
- International Council for Harmonization. ICH Q14, Analytical Procedure Development and Revision of Q2(R1) Analytical Validation (Concept Paper); ICH: Geneva, Switzerland, 2018. [Google Scholar]
- Demirel, C.; Kabutey, A.; Herák, D.; Sedlacek, A.; Mizera, C.; Dajbych, O. Using Box–Behnken design coupled with response surface methodology for optimizing rapeseed oil expression parameters under heating and freezing conditions. Processes 2022, 10, 490. [Google Scholar] [CrossRef]
- Azmi, S.N.H.; Al Lawati, W.M.; Al Hoqani, U.H.A.; Al Aufi, E.; Al Hatmi, K.; Al Zadjali, J.S.; Rahman, N.; Nasir, M.; Rahman, H.; Khan, S.A. Development of a Citric-Acid-Modified Cellulose Adsorbent Derived from Moringa peregrina Leaf for Adsorptive Removal of Citalopram HBr in Aqueous Solutions. Pharmaceuticals 2022, 15, 760. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.M.P.; Vi, N.T.T.; Van, N.T.N. Determination of vitamin C in multivitamin effervescent tablets by high-performance liquid chromatography. Can Tho J. Med. Pharm. 2019, 5, 108–117. [Google Scholar]
- Rahman, N.; Ahmad, Y.; Azmi, S.N.H. Kinetic spectrophotometric method for the determination of norfloxacin in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2004, 57, 359–367. [Google Scholar] [CrossRef]
- Miller, J.C.; Miller, J.N. Errors in instrumental analysis; regression and correlation. In Statistics for Analytical Chemistry, 3rd ed.; Ellis Horwood: Chichester, UK; Prentice Hall: Hoboken, NJ, USA, 1993; p. 119. [Google Scholar]
- Canada Health Protection Branch. Drugs Directorate guidelines, Acceptable Methods; Draft; Ministry of National Health and Welfare: Ottawa, ON, Canada, 1994. [Google Scholar]
- Rahman, N.; Haque, S.M.; Azmi, S.N.H.; Rahman, H. Optimized and validated spectrophotometric methods for the determination of amiodrone hydrochloride in commercial dosage forms using N-bromosuccinimide and bromothymol blue. J. Saudi Chem. Soc. 2017, 21, 25–34. [Google Scholar] [CrossRef]
- Rahman, N.; Sameen, S.; Kashif, M. Spectroscopic study on the interaction of haloperidol and 2,4-dinitrophenylhydrazine and its application for the quantitation in drug formulations. Anal. Chem. Lett. 2016, 6, 874–885. [Google Scholar] [CrossRef]
- Hartmann, C.; Smeyers-Verbeke, J.; Pinninckx, W.; Heyden, Y.V.; Vankeerberghen, P.; Massart, D.L. Reappraisal of hypothesis testing for method validation: Detection of systematic error by comparing the means of two methods or of two laboratories. Anal. Chem. 1995, 67, 4491–4499. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta 2018, 181, 204–209. [Google Scholar] [CrossRef]





| S.No. | Solvent/Mobile Phase | Peak (nm) | Absorbance |
|---|---|---|---|
| 1 | Distilled water (pH 7) | 260 | 1.106 |
| 2 | 0.4% NaH2PO4 (pH 3 maintained with H3PO4)-Acetonitrile (90:10 v/v) | 245 | 1.226 |
| 3 | 0.4% NaH2PO4 (pH 3.5 maintained with H3PO4)-Acetonitrile (90:10 v/v) | 247 | 1.277 |
| 4 | 0.4% NaH2PO4 (pH 4.0 maintained with H3PO4)-Acetonitrile (90:10 v/v) | 254 | 1.230 |
| 5 | 0.4% NaH2PO4 (pH 4.65)-Acetonitrile (90:10 v/v) | 261 | 1.742 |
| Factor A | Factor B | Factor C | Experimental | Predicted | ||
|---|---|---|---|---|---|---|
| Std | Run | Acetonitrile Fraction | Flow Rate | Column Temp. | Peak Area | Peak Area |
| % | mL min−1 | °C | mAU | mAU | ||
| 3 | 1 | 2.5 | 0.9 | 25 | 35.50 | 35.40 |
| 14 | 2 | 5.0 | 0.8 | 25 | 53.42 | 53.42 |
| 12 | 3 | 5.0 | 0.9 | 30 | 52.09 | 52.07 |
| 10 | 4 | 5.0 | 0.9 | 20 | 52.96 | 53.04 |
| 7 | 5 | 2.5 | 0.8 | 30 | 35.10 | 35.19 |
| 4 | 6 | 7.5 | 0.9 | 25 | 36.74 | 36.75 |
| 2 | 7 | 7.5 | 0.7 | 25 | 35.34 | 35.41 |
| 9 | 8 | 5.0 | 0.7 | 20 | 50.10 | 50.12 |
| 8 | 9 | 7.5 | 0.8 | 30 | 35.34 | 35.41 |
| 1 | 10 | 2.5 | 0.7 | 25 | 33.90 | 33.89 |
| 15 | 11 | 5.0 | 0.8 | 25 | 53.42 | 53.42 |
| 13 | 12 | 5.0 | 0.8 | 25 | 53.42 | 53.42 |
| 5 | 13 | 2.5 | 0.8 | 20 | 33.50 | 33.49 |
| 11 | 14 | 5.0 | 0.7 | 30 | 52.27 | 52.20 |
| 16 | 15 | 5.0 | 0.8 | 25 | 53.42 | 53.42 |
| 17 | 16 | 5.0 | 0.8 | 25 | 53.42 | 53.42 |
| 6 | 17 | 7.5 | 0.8 | 20 | 36.20 | 36.11 |
| S. No. | Source | Std. Dev. | R2 | Adjusted R2 | Predicted R2 | PRESS | |
|---|---|---|---|---|---|---|---|
| 1 | Linear | 1.00 × 101 | 0.0067 | −0.2225 | −0.7869 | 2.35 × 103 | |
| 2 | 2FI | 1.14 × 103 | 0.0095 | −0.5849 | −2.8738 | 5.10 × 103 | |
| 3 | Quadratic | 7.46 × 10−2 | 1.0000 | 0.9999 | 0.9995 | 6.22 × 10−1 | Suggested |
| S. No. | Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|---|
| 1 | Model | 1.32 × 103 | 9 | 1.46 × 102 | 2.64×104 | <0.0001 | Significant |
| 2 | A-Acetonitrile fraction | 4.32 | 1 | 4.32 | 7.78×102 | <0.0001 | Significant |
| 3 | B-Flow rate | 3.89 | 1 | 3.89 | 7.01×102 | <0.0001 | Significant |
| 4 | C-Column Temp. | 0.605 | 1 | 0.605 | 1.09×102 | <0.0001 | Significant |
| 5 | AB | 2.6 × 10−3 | 1 | 2.6 × 10−3 | 0.4592 | 0.5198 | Insignificant |
| 6 | AC | 1.32 | 1 | 1.32 | 2.38×102 | <0.0001 | Significant |
| 7 | BC | 2.31 | 1 | 2.31 | 4.16×102 | <0.0001 | Significant |
| 8 | A2 | 1.28 × 103 | 1 | 1.28 × 103 | 2.30×105 | <0.0001 | Significant |
| 9 | B2 | 1.76 | 1 | 1.76 | 3.18×102 | <0.0001 | Significant |
| 10 | C2 | 3.54 | 1 | 3.54 | 6.37×102 | <0.0001 | Significant |
| Residual | 3.89 × 10−2 | 7 | 5.6 × 10−3 | ||||
| Lack of Fit | 3.89 × 10−2 | 3 | 1.3 × 10−2 | ||||
| Pure Error | 0 | 4 | 0 | ||||
| Cor Total | 1.32 × 103 | 16 |
| Parameters | Unit | Range & Level | ||
|---|---|---|---|---|
| Low | Medium | High | ||
| Variables | ||||
| Acetonitrile: buffer | % | 2.5:97.5 (v/v) | 5.0:95.0 (v/v) | 7.5:92.5 (v/v) |
| Flow rate | mL min−1 | 0.8 | 0.8 | 0.8 |
| Column temperature | °C | 25 | 25 | 25 |
| Ascorbic acid | μg mL−1 | 50 | 50 | 50 |
| Responses | ||||
| Peak area | mAU | 34.51 | 53.42 | 37.10 |
| Retention time | min | 4.5 | 4.1 | 2.43 |
| Theoretical plate | 7000 | 13,785 | 9000 | |
| Asymmetry | 1.15 | 1.15 | 1.15 | |
| Parameters | HPLC Method | |
|---|---|---|
| Proposed | Reference | |
| UV detection wavelength (nm) | 245 | 248 |
| Retention time (min) | 4.1 | 4.1 |
| No. of theoretical plates | 13,785 | 13,781 |
| Asymmetry factor | 1.15 | 0.96 |
| Beer’s law range (µg mL−1) | 10–180 | 15–120 |
| Linear regression equation | PA = 2.57 × 10−3 + 1.069 C | PA = 0.1046 + 1.064 C |
| Standard deviation of intercept, Sa | 0.124 | 8.23 × 10−2 |
| Confidence limit of the intercept, ±tSa | 0.319 | 0.212 |
| Standard deviation of the slope, Sb | 1.09 × 10−3 | 1.13 × 10−3 |
| Confidence limit of the slope, ±tSb | 2.80 × 10−3 | 2.91 × 10−3 |
| Standard deviation of the calibration line (So) | 0.143 | 9.71 × 10−2 |
| Variance (So2) | 2.05 × 10−2 | 9.43 × 10−3 |
| Correlation coefficient ® | 0.999 | 0.999 |
| Limit of detection, LOD (µg mL−1) | 0.44 | 0.30 |
| Limit of quantification, LOQ (µg mL−1) | 1.34 | 0.91 |
| Number of concentration levels (n) | 7 | 7 |
| Initial Concentration of Imipramine HCl (µg mL−1) | Intra-day Assay and Inter-day Precisions | |||||
|---|---|---|---|---|---|---|
| Measured Concentration ± SD (µg mL−1) | RSD a (%) | Recovery a (%) | ||||
| Intra-day | Inter-day | Intra-day | Inter-day | Intra-day | Inter-day | |
| 40.0 | 39.97 ± 0.122 | 40.04 ± 0.131 | 0.305 | 0.326 | 99.92 | 100.11 |
| 120.0 | 119.96 ± 0.059 | 120.16 ± 0.074 | 0.050 | 0.62 | 99.97 | 100.11 |
| 160.0 | 160.13 ± 0.095 | 159.91 ± 0.162 | 0.059 | 0.102 | 100.08 | 99.94 |
| Tablet Formulations | Concentration Found ± SD (µg mL−1) a | |||||
|---|---|---|---|---|---|---|
| Method | t-Value b | F-Value b | θL c | θU c | ||
| Proposed | Reference | |||||
| Redoxon 1000 mg (Bayer, Switzwerland) | 99.98 ± 0.11 | 100.04 ± 0.07 | 1.04 | 2.10 | 0.999 | 1.002 |
| C-Tamin 500 mg (Vital Health, USA) | 99.98 ± 0.11 | 100.04 ± 0.07 | 1.12 | 2.28 | 0.999 | 1.002 |
| S. No. | Mobile Phase | Column | Temperature (°C) and UV Detection Wavelength (nm) | Linear Range (μg mL−1) & Retention Time (min) | Flow Rate (mL min−1) | EcoScale Greenness Point | References |
|---|---|---|---|---|---|---|---|
| 1 | Methanol−0.05 M sodium dihydrogen phosphate (35:65 v/v), pH 2.5 adjusted with orthophosphoric acid. | Promosil C18, LC column, 4.6 (id) × 250 mm, 5 μm particle size (Agela Technologies Inc., Torrance, CA, USA) | 37 & 220 | 3.0–60 & 2.4 | 1.0 | 61 | [33] |
| 2 | Methanol−0.03 M sodium dihydrogen phosphate (55:45 v/v), pH 4 adjusted with orthophosphoric acid. | CLC Shim-pack C8 column, 4.6 (id) × 250 mm, 5 µm particle size (Shimadzu Corporation, Kyoto, Japan) | 25 & 255 | 0.5–10 & 3.1 | 1.0 | 71 | [34] |
| 3 | 1 mM sodium pentane sulphonate in (0.4 mL of formic acid + 25 mL of methanol + 75 mL distilled water). | LiChrosorb C18, 4.6 (id) × 250 mm, 5 μm particle size, (Merck, Darmstadt, Germany) | 25 & 254 | 5–40 & 3.53 | 1.0 | 61 | [35] |
| 4 | 5 mM cetyl trimethyl ammonium bromide + 50 mM KH2PO4 | Symmetry C18 column, 4.6 (id) × 280 mm, particle size 5 μm (Waters, Milford, MA, USA). | 25 & 254 | 1–100 & 8.1 | 1.2 | 56 | [36] |
| 5 | 5 mM Tetrabutyl ammonium hydroxide (pH 6, adjusted with H3PO4)-methanol (80:20 v/v) | Venusil XBP C18 column, 4.6 (id) × 250 mm, 5 μm particle size (Agela Technologies Inc., USA) | 25 & 245 | 10–100 & 3.1 | 0.9 | 71 | [37] |
| 6 | Acetonitrile-dichloromethane- 0.25% KH2PO4 (5:5:95 v/v/v) | Cosmosil 5C18-MS-II column, 4.6 (id) ×250 mm, 5 µm particle size (Nacalai Tesque Inc., Kyoto, Japan) | 25 & 246 | 0.05–30 & 2.54 | 1.0 | 66 | [38] |
| 7 | A: Phosphate buffer (pH 2.7, adjusted with H3PO4)-B: Methanol (A 90–80% for 5 min: B 10–20% for 5 min v/v) (gradient mode) | Alltima C18 column, 3 (id) × 100 mm, 3 µm particle size (Grace company, Salt Lake City, UT, USA) | 30 & 243 | 0.3–1.0 & 1.85 | 0.4 | 62 | [39] |
| 8 | 0.01 M dihydrogen ammonium phosphate (pH 2.6, adjusted with H3PO4) | Spherisorb C18 column, 4.6 (id) × 150 mm, 3 µm particle size, (Waters, USA) | 25 & 254 | 1.9–125 & 2.81 | 1.0 | 51 | [40] |
| 9 | Octylamine + salicylic acid (0.005 M) | LiChrospher RP-18 HPLC column, 4(id) × 250 mm, 5 µm particle size, (Merck, Germany) | 25 & 254 | 0.2–10.0 & 5.97 | 1.0 | 51 | [41] |
| 10 | 0.2 M NaH2PO4 (pH 2.14 adjusted with 0.5 MHCl | 4.6 (id) × 250 mm, 5 µm particle size (Agilent, Santa Clara, CA, USA) | 40 & 220 | 0.02–10.0 & 7.1 | 0.5 | 61 | [42] |
| 11 | Methanol-phosphate buffer (0.01 M KH2PO4, pH 2.0) 35:65 (v/v) | Gemini C18 LC column, 4.6 (id) × 250 mm, 5 µm particle size (Phenomenex, USA) | 25 & 245 | 12.2–21.4 & 2.5 | 1.5 | 61 | [43] |
| 12 | Metaphosphoric acid (0.2%)-methanol-acetonitrile (90:8:2, v/v/v) | LiChrospher® 100–RP18, 4.6 (id) × 250 mm, 5 µm particle size (Phenomenex, USA) | 254 & 24.0 ± 2.0 | 1.0–12.0 & 3.4 | 1.0 | 71 | [44] |
| 13 | Acetonitrile and 50 mM ammnonium acetate buffer pH 6.8 (78:22 v/v) | SeQuant ZIC HILIC, 2.1 (id) × 150 mm, 3.5 µm particle size (Merck, Germany) | 268 & 23 | 0.1–100 & 4.66 | 0.3 | 61 | [45] |
| 14 | 5% methanol in 25 mmolL−1 sodium dihydrogen phosphate pH 4.8 | Discovery C18, 4 (id) × 250 mm, 5 µm particle size (Merck, Germany) | 265 & 25 | 0.35–44.03 & 4.2 | 0.5 | 61 | [46] |
| 15 | Water is brought to pH 2.2 with sulphuric acid:methanol (80:20) | Haisil C18 100 Å column (25 cm × 4.6 mm (Higgins Analytical, Mountain View, CA, USA) | 243 & 25 | 1.0–80.0 & 3.18 | 1.0 | 61 | [47] |
| 16 | 1.5 g of 1-hexanesulfonic acid sodium salt dissolved in 500 mL acetic acid (pH 2.6) | Superspher C18 column, 4 (id) ×125 mm, 4µm particle size (Merck, Germany) | 20 & 280 | 150–250 & 3.5 | 0.7 | 56 | [48] |
| 17 | Methanol−0.05 M sodium dihydrogen phosphate buffer (gradient mode) | Shim-pack C18 VP-ODS-2 RP column, 4.6 (id) × 150 mm, 5 µm particle size (Shimadzu, Japan) | 35 & 254 | 29.8–476.9 & 3.0 | 0.8 | 61 | [49] |
| 18 | Methanol—KH2PO4 (5 mmolL−1), pH 2.65 (gradient mode) | LiChrospher® 100–RP18, 4.6 (id) × 250 mm, 5 µm particle size (Phenomenex, USA) | 25 & 245 | 0–300 & 4.01 | 0.8 | 61 | [50] |
| 19 | Ion-pair solution-acetonitrile (98:2 v/v) | Hypersil C18 BDS column. 4 (id) × 250 mm, 5 µm particle size (Thermo Fisher Scientific, USA) | 25 & 275 | 0–200 & 2.2 | 1.0 | 76 | [51] |
| 20 | Acetonitrile + phosphate buffer (pH 6.5) (10:90 v/v) | Onyx Monolithic C18, LC column, 4.6 (id) × 100 mm, (Phenomenex, USA) | 25 & 235 | 150–450 & 1.6 | 1.0 | 60 | [52] |
| 21 | Methanol + 0.4% KH2PO4 (pH 3.0); (5.0:95 v/v) | Lunar C18 column, 4.6 (id) × 250 mm, 5µm particle size (Phenomenex, USA) | 25 & 248 | 15–120 & 4.05 | 1.0 | 61 | Reference method [61] |
| 22 | Acetonitrile + 0.4% NaH2PO4 (pH 3.0); (5.0:95 v/v) | AcclaimTM 120 C18 column, 4.6 (id) × 250 mm, 5µm particle size (Dionex, USA). | 25 & 245 | 10–180 & 4.1 | 0.8 | 66 | Proposed work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azmi, S.N.H.; Al Hoqani, U.; Al Mamari, J.O.S.; Al Mamari, B.M.S.; Al Jassasi, B.S.A.R.; Al Rubaiai, A.S.S.; Rahman, N.; Nasir, M.; Haque, S.M.; Khan, S.A.; et al. Box–Behnken Design Based Development of UV-Reversed Phase High Performance Liquid Chromatographic Method for Determination of Ascorbic Acid in Tablet Formulations. Separations 2022, 9, 361. https://doi.org/10.3390/separations9110361
Azmi SNH, Al Hoqani U, Al Mamari JOS, Al Mamari BMS, Al Jassasi BSAR, Al Rubaiai ASS, Rahman N, Nasir M, Haque SM, Khan SA, et al. Box–Behnken Design Based Development of UV-Reversed Phase High Performance Liquid Chromatographic Method for Determination of Ascorbic Acid in Tablet Formulations. Separations. 2022; 9(11):361. https://doi.org/10.3390/separations9110361
Chicago/Turabian StyleAzmi, Syed Najmul Hejaz, Umaima Al Hoqani, Juhaina Obaid Said Al Mamari, Buthaina Mohamed Salim Al Mamari, Balaqis Sultan Ali Rashid Al Jassasi, Aziza Saleh Saif Al Rubaiai, Nafisur Rahman, Mohd Nasir, Sk Manirul Haque, Shah Alam Khan, and et al. 2022. "Box–Behnken Design Based Development of UV-Reversed Phase High Performance Liquid Chromatographic Method for Determination of Ascorbic Acid in Tablet Formulations" Separations 9, no. 11: 361. https://doi.org/10.3390/separations9110361
APA StyleAzmi, S. N. H., Al Hoqani, U., Al Mamari, J. O. S., Al Mamari, B. M. S., Al Jassasi, B. S. A. R., Al Rubaiai, A. S. S., Rahman, N., Nasir, M., Haque, S. M., Khan, S. A., Ahmed, Q. U., & Zakaria, Z. A. (2022). Box–Behnken Design Based Development of UV-Reversed Phase High Performance Liquid Chromatographic Method for Determination of Ascorbic Acid in Tablet Formulations. Separations, 9(11), 361. https://doi.org/10.3390/separations9110361









