Abstract
Effective risk management and control methods for potentially genotoxic impurities (PGIs), including alkyl halides, are of significant importance in the medicinal (pharmaceutical) sector. The three alkyl halides in posaconazole are PGIs. The detection and assessment of genotoxic substances is a top priority for all regulatory organizations. Quantifying PGIs at trace levels using standard analytical techniques, such as gas chromatography (GC) and high-performance liquid chromatography (HPLC), is challenging for the pharmaceutical manufacturing industry. Thus, the detection of trace quantities of PGIs in posaconazole is essential for developing sensitive analytical methodologies. The objective of this study was to establish an analytical technique for quantifying the three PGIs (alkyl halides) in posaconazole and its intermediate. These alkyl halides are 1-(2,4-difluorophenyl) ethan-1-one (PGI-1), (Z)-1-(1-bromoprop-1-en-2-yl)- 2,4-difluorobenzene (PGI-2), and 1-bromo-2-(2,4-difluorophenyl) propan-2-ol (PGI-3). To identify trace quantities (parts per million (ppm)) of these impurities, we employed a gas chromatography (GC-MS/MS) equipped with a triple quadrupole mass spectrometry detector. The GC column was a USP phase G43, which is a mid-polar 6% cyanopropyl; 94% polydimethylsiloxane, with a 60 m length, 0.32 mm inner diameter, and 1.8 μm film thickness. Helium (He) was used as the carrier gas, with a flow rate of 1.5 mL/min. A thermal gradient elution program was used for this procedure. The method was calibrated for the three PGIs with limits of detection (LOD) and quantification (LOQ) of 0.01 and 0.025 ppm, respectively. The linear range of concentrations (25–150%) was maintained with respect to the specification level. This method was validated according to the ICH regulations and was shown to be specific, rugged, robust, precise, sensitive, accurate, linear, and stable. Therefore, in this newly developed method, the combination of suitable analytical techniques, such as GC-MS/MS and proper chromatographic conditions and column selection with the lowest LOD and LOQ, have allowed the induction of excellent ionization. These conditions have successfully facilitated the identification of PGI-1, PGI-2, and PGI-3 in posaconazole and its intermediate during routine analysis.
1. Introduction
Alkanes and halogens react to form alkyl halides. Owing to their high reactivity, simplicity of use, low cost, and wide commercial availability, these compounds are mostly utilized in alkylation processes via nucleophilic substitution in the synthesis of active pharmaceutical ingredients (APIs). Alkyl halides are potential genotoxic impurities (PGIs) owing to their ability to alkylate DNA bases (on N-7 of guanine and N-3 of adenine) [1,2,3,4,5].
Production safety is a top priority for scientists, chemists, engineers, and formulators when manufacturing pharmaceutical products for use in industry or clinical trials. APIs [6,7,8] perform a prominent role in the safety, purity, and quality of raw materials. Various low-level impurities are frequently present in pharmacological substances and must be investigated and controlled at permissible levels (parts per million (ppm)). When compared with the expected health benefits, it is possible to accept a certain amount of patient risk, even if it is doubtful that the pharmaceutical material itself is completely safe.
Pharmaceutical companies and regulatory agencies must carefully assess this risk-to-reward trade-off. However, impurities are considered to only be harmful and to have no beneficial effects. To eliminate genotoxic impurities, manufacturers must create and implement their own analytical strategies and limits [9,10,11,12,13]. Human cancer due to genetic mutations, chromosomal breakages, or chromosomal rearrangements [14,15,16] was observed as a result of pharmaceutical PGIs. Serious toxicological consequences occur owing to exposure to trace amounts of PGIs present in the final drug products. Therefore, chemical scientists should consider methods to reduce the production and use of genotoxic compounds [11,17,18,19]. It may not always be possible to stop using these drugs entirely or to stop producing pollutants with DNA reactivity. Although present in small concentrations, PGIs are essential for drug evolution [20] and, if appropriately addressed, could lead to a delay in clearance by regulatory authorities [21].
Analytical scientists must develop the required techniques to precisely analyze and regulate the amounts of PGIs in drugs [22,23,24,25,26]. Appropriate analytical methods are necessary to develop reliable manufacturing processes and ensure patient safety. In addition to the contamination of drugs during processing, PGIs can be produced by them during formulation or storage. Genotoxic substances, including hydrolytic substances, pose numerous obstacles to the development of new drugs [27,28,29,30,31]. Genotoxicity is defined as an adverse destructive effect on the DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) of a cell, jeopardizing the health of the cell. Genotoxic chemicals are also known as genotoxins. Teratogens, mutagens, and carcinogens are substances that can cause birth deformities, create mutations, and cause cancer, respectively [32,33,34,35].
The EMEA, ICH Q3A/B, and USFDA guidelines are followed to restrict the genotoxic impurities in pharmaceutical substances. Four types of impurities are listed by the FDA, ICH, and USP [36,37,38], respectively. Owing to certain reactions, including the removal of carbon dioxide, dehydration, and oxidation, the first category of impurities associated with APIs is further divided into two groups. Due to the relationship between their structure and activity, impurities associated with APIs may be carcinogenic, mutagenic, or genotoxic [39,40].
Genotoxic impurities may originate from different sources; however, they are often introduced by the starting materials used to synthesize pharmaceuticals and genotoxic impurities, by-products, or intermediates. Additionally, because solvents, catalysts, and reagents are used in pharmaceutical synthesis, genotoxic impurities are present in pharmacological compounds. Drug impurities accumulate owing to the degradation of drugs during storage, air oxidation, hydrolysis, and exposure to light. During the manufacturing of stereoselective pharmaceuticals, chiral impurities are produced in pharmacological compounds [41,42,43].
Genotoxicity statistics are helpful for evaluating the risks associated with pharmaceutical substances, food, consumer goods, and industrial products. Information on genotoxicity is essential for determining the risks posed by naturally occurring environmental toxins. Genetic alterations have severe adverse consequences for health, even at modest levels of vulnerability. Roto-oncogenes, tumor suppressor genes, and DNA damage response genes can be mutated by a variety of carriers, including both chemical and physical agents. Somatic cells with damaged DNA also contribute to degenerative conditions, such as accelerated aging, lowered immunity, and cardiovascular and neurological problems. The assessment of mutagenic potential is an essential component of chemical risk evaluation to prevent the negative effects of genetic alteration on human health [44,45,46].
Regulatory bodies worldwide require managerial data on the genotoxic potential of pharmaceutical products to evaluate the products and procedures for safety. Therefore, pre-symptomatic investigations are often conducted to assess basic toxicological data of new chemical entities (NCE). Additionally, such data helps identify genotoxicity risks that can cause DNA damage and fixation [47,48,49].
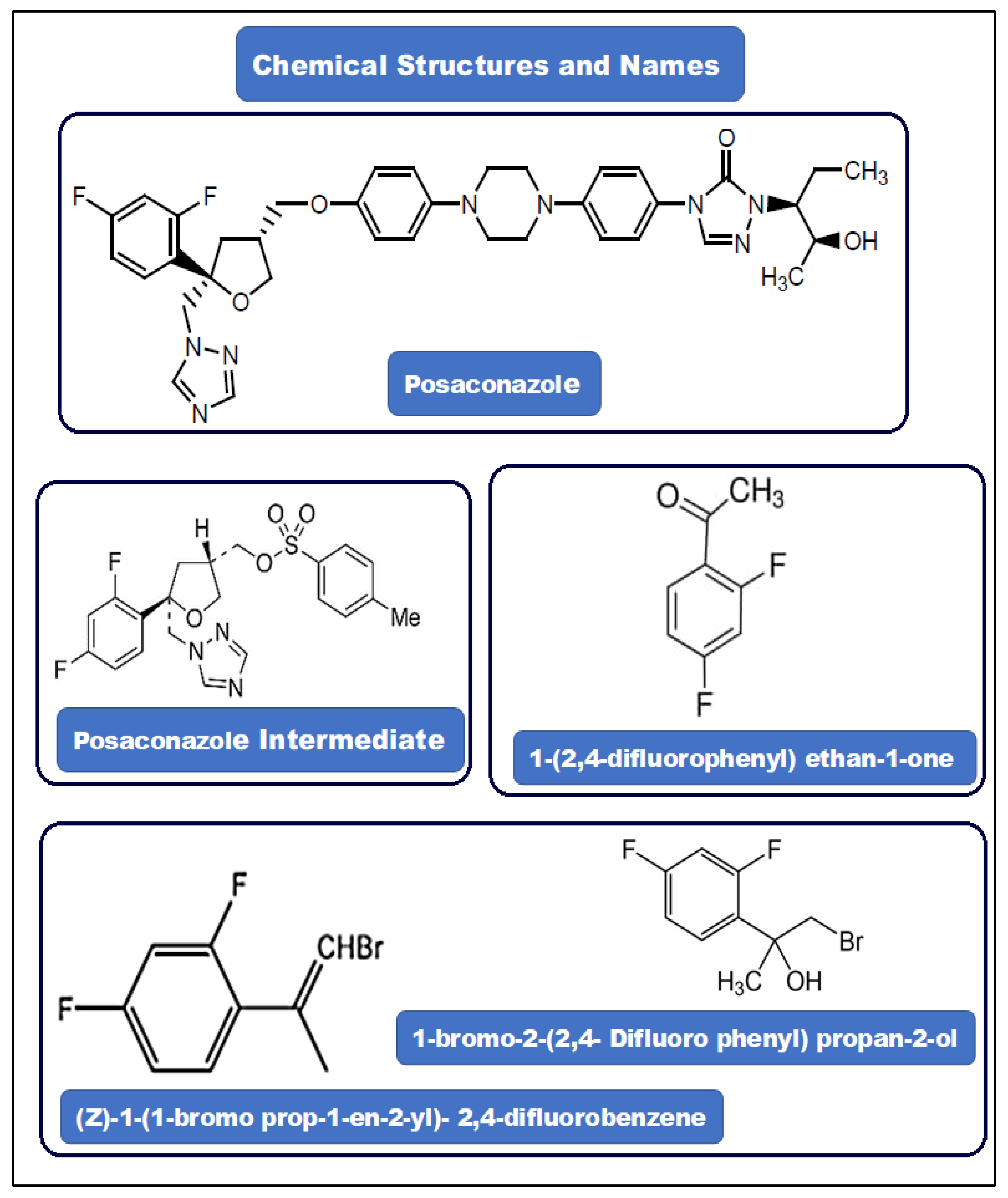
The posaconazole intermediate, (5R-cis)-toluene-4-sulfonic acid 5-(2,4 -difluorophenyl)-5-(1H-1,2,4-triazol-1-yl) methyl tetra hydrofuran-3-ylmethyl ester, is a key compound in its synthesis (Figure 1). Posaconazole is sold under the trade name, Noxafil® and is an azole antifungal agent (Figure 1). It has been approved for the treatment and prophylaxis of invasive Aspergillus and Candida infections in patients in the United States [50,51]. In high-risk immunity individuals, posaconazole is often used to prevent invasive yeast and mold infections, such as invasive aspergillosis.

Figure 1.
Structures of posaconazole, posaconazole intermediate, PGI-1, PGI-2, and PGI-3.
Impurities must be assessed to establish upper limits for those that are considered PGIs. According to the ICH Q3A guidelines, the ideal limits for PGIs must be well below those for common impurities, and it is necessary to create improved analytical methods that can detect and assess PGIs at the ppm level. Class-1–5 impurities are distinguished based on their ability to cause cancer and mutations. According to the toxicological concern-based (TTC-based) threshold, the preferred daily intake of PGIs is 1.5 μg per person per day; this value can be used, along with the length of pharmacological treatment, to establish an acceptable limit for impurities in pharmaceutical products according to ICH M7.
The impurities PGI-1 (C8H6F2O), PGI-2 (C9H7BrF2), and PGI-3 (C9H9BrF2O) (Figure 1) in antifungal agents are toxic. These PGIs are Class-3 (ICH M7) impurities and are alkyl halides [2]. Any PGIs should be measured in accordance with the recommendations of regulatory bodies; otherwise, they would become hazardous over time. To detect and measure these contaminants, a sensitive and reliable analytical technique is needed. Although there are numerous methods for posaconazole analysis in the prior literature, including HPLC [52], HPLC/UV and bioassay [53], HPLC-DAD [54], and LC-MS/MS [55,56], these methods described the content of posaconazole in other substances and have not described for trace-level analysis and cannot be used to analyze the low content of PGIs in posaconazole [57]. The established LOD and LOQ are higher. Additionally, in a previously published study, Chen et al., have applied HR/MS/MS and online H/D exchange LC/MS methods to study the degradation product of posaconazole. In this study, the accurate mass value has significantly improved the possibility of the identification of unknown structures formed due to the degradation of posaconazole, whereas the online H/D exchange LC-HR/MS experiments have facilitated the structural identifications of four degradants during the degradation process [58]. Similarly, in a recently published study, Li et al. have successfully identified the degradation products of lurasidone using LC-PDA/UV-MS technique, and they have suggested that this technique can also be readily applied to rationalize the formation of posaconazole degradant [59].
Neither the detection of PGIs nor their quantification in posaconazole and its intermediate has been disclosed by prior methods. In contrast, a more precise method is more suitable for the detection of PGIs in trace levels. Additionally, the proposed method uses less solvent and has a shorter overall quantification time. This approach was evaluated in accordance with the ICH guidelines, and the analysis method was straightforward, sensitive, and repeatable.
No new approach was disclosed for the quantification of the three PGIs in posaconazole after reviewing the reported methodologies. To determine these three PGIs, a specific and sensitive approach using GC-MS/MS was evaluated and validated according to the ICH Q2 (R1) guidelines [60]. The current GC-MS/MS method for the identification and quantification of the three PGIs is novel, advanced, and industrially feasible (Scheme 1). This method is highly sensitive with the lowest LOD and LOQ detection.
Scheme 1.
Graphical representation of the GC-MS/MS method for the identification and quantification of PGIs.
2. Results
2.1. Optimization of Mass Spectrometric Parameters
The Q1 and Q3 values were determined for PGI-1, PGI-2, and PGI-3 through mass tuning. The solubility of each analyte was evaluated to identify the impurities present in the posaconazole and its intermediate. Posaconazole and its impurities and intermediates are soluble in alcohol.
The mass parameters were obtained by tuning the mass spectrometry with diluted solutions of each PGI. The EI acts as an ion source to establish mass detection and ascertain both the Q1 and Q3 values. MRM-1 (m/z) and MRM-2 (m/z) were established for each impurity.
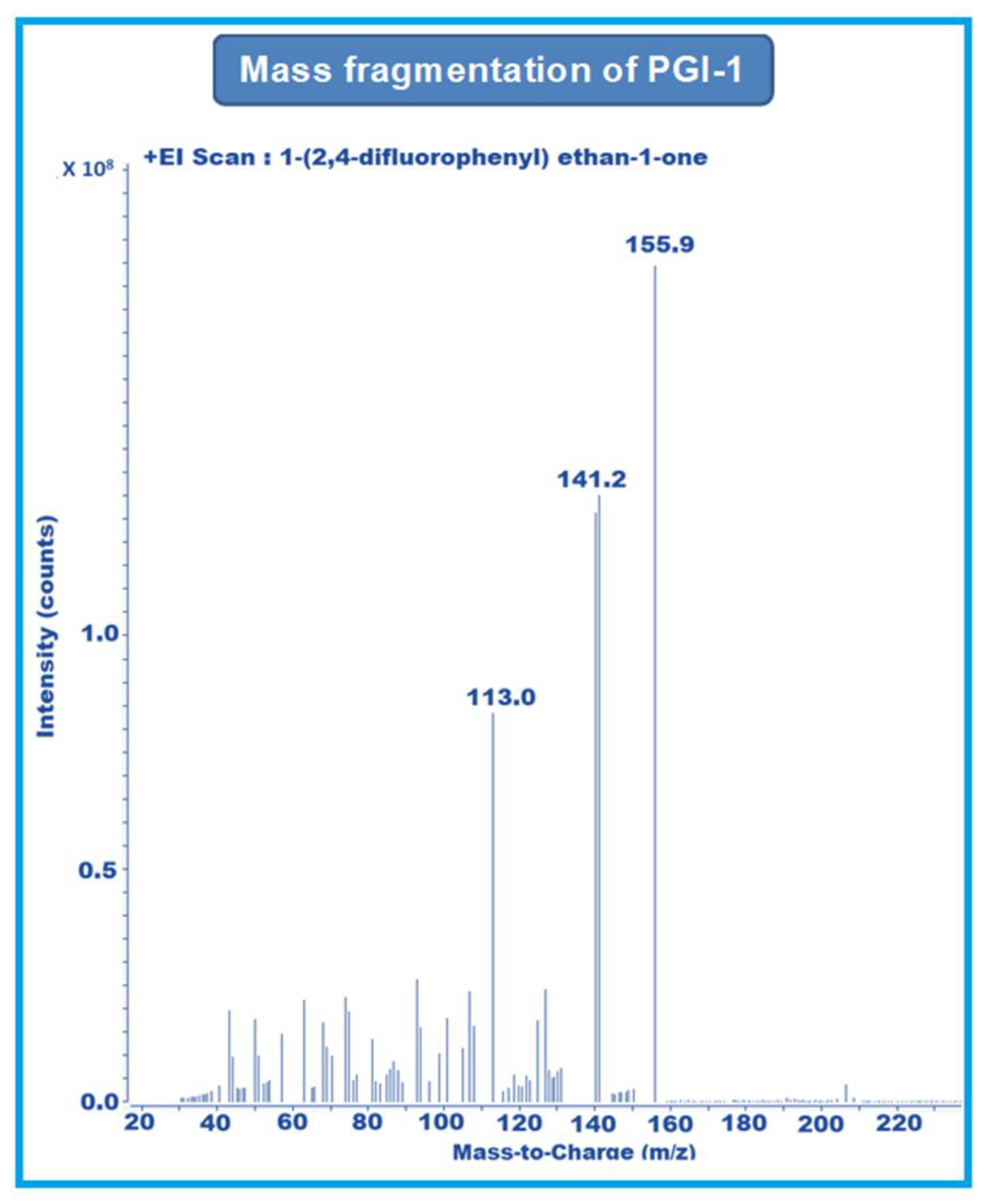
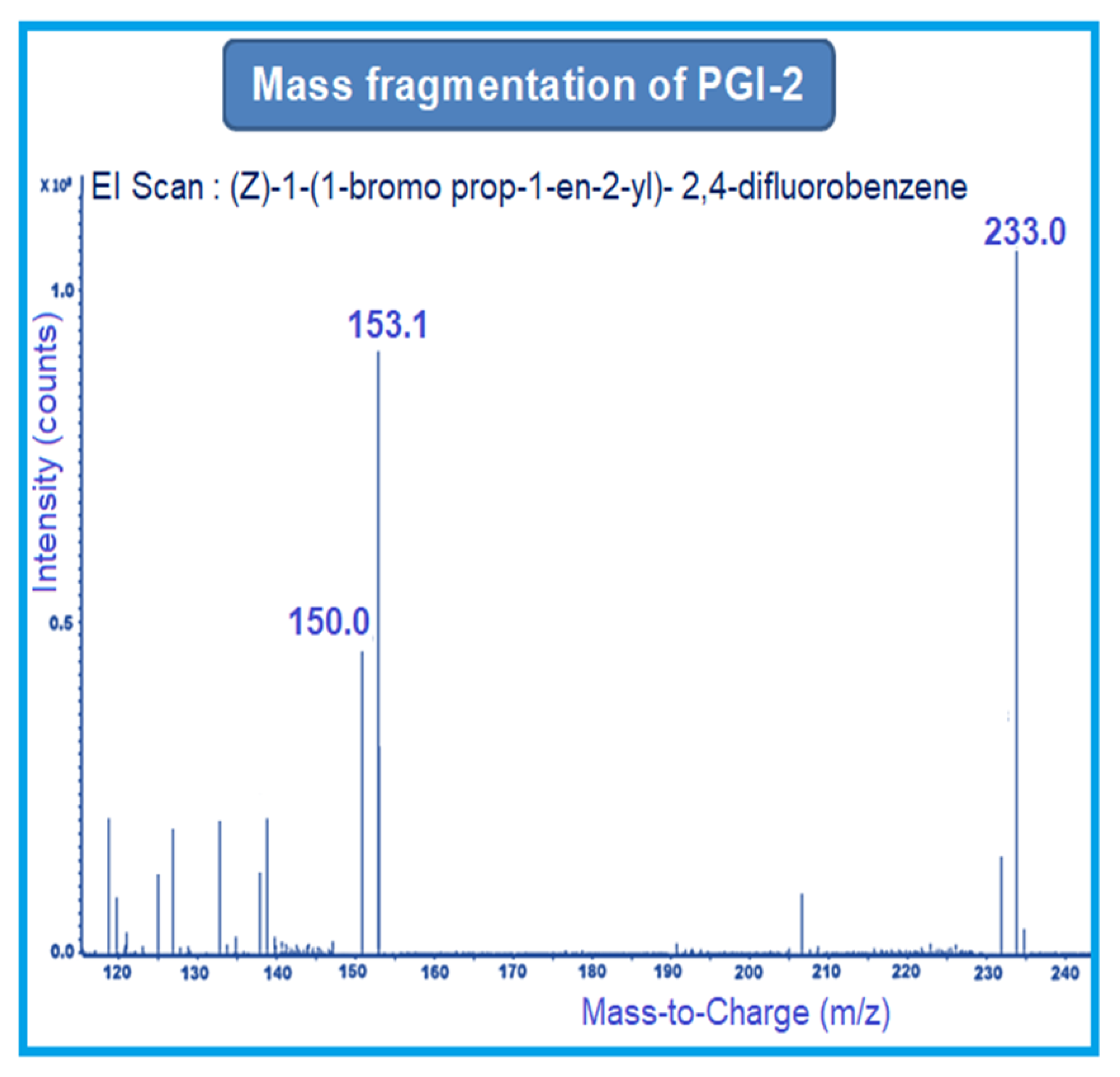
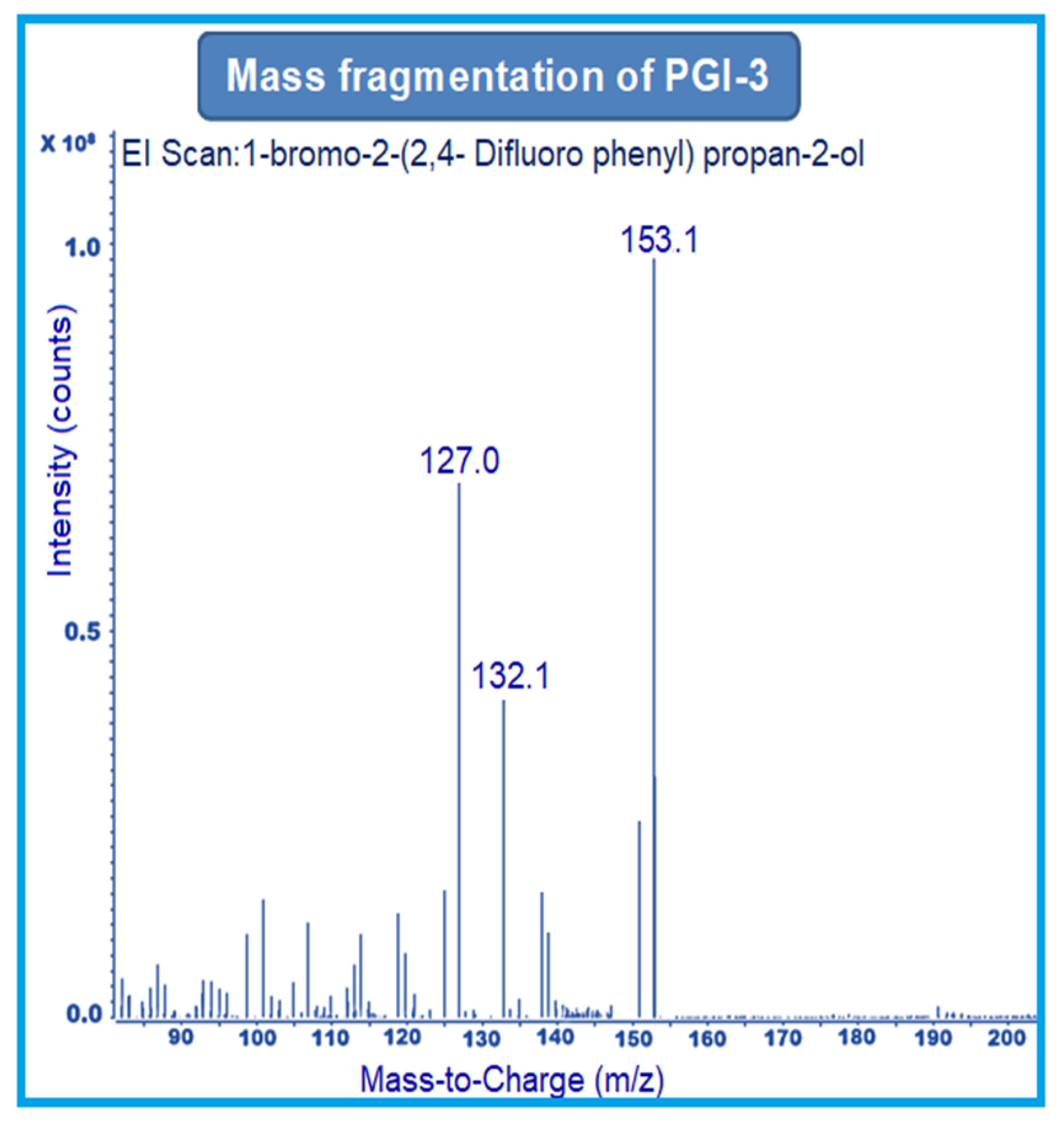
MRM-1 used 156–141 amu, 233–153 amu, and 152–127 amu for the quantification of PGI-1, PGI-2, and PGI-3, respectively. MRM-2 used 156–113 amu, 233–150 amu, and 152–132 amu for the qualification of PGI-1, PGI-2, and PGI-3, respectively (Figure 2, Figure 3 and Figure 4).
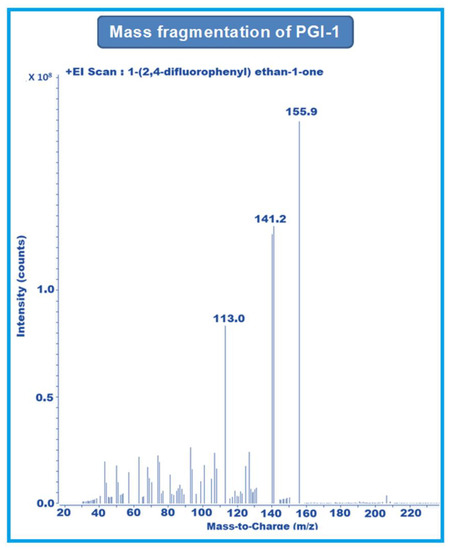
Figure 2.
Mass fragmentation of PGI-1.

Figure 3.
Mass fragmentation of PGI-2.
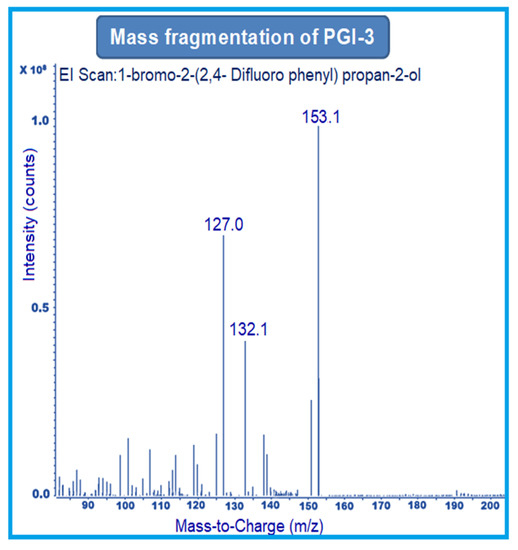
Figure 4.
Mass fragmentation of PGI-3.
2.2. Optimization of Chromatographic Conditions
Different solvents were used as diluents for the preparation of the standards and samples. To conduct the diluent compatibility study, polar and nonpolar solvents and low and high boiler solvents were considered, including dimethyl sulfoxide, dimethylformamide, N-methyl-2-pyrrolidone, ethanol, dichloromethane, acetonitrile, methanol, and hexane.
We observed solvent interference, split peaks, broad peak shapes, and poor responsiveness of PGI impurities to various diluents during development. Methanol was the most suitable diluent. In methanol, no interference was observed, and each PGI responded very well at ppm concentrations.
Choosing the right column was crucial for developing this method. Different column chemistries, such as DB-wax, DB-5, DB-624, and DB-1, were employed for optimization during development. We observed that DB-624 was most suitable because each PGI peak was very sharp and well ionized, with good resolution.
The final method was improved by using helium as the carrier gas. The Detector off (MS-off) program was used before and after PGI peak elution. The retention times for PGI-1, PGI-2, and PGI-3 were approximately 13, 20, and 21 min, respectively.
2.3. Method Validation Study
To demonstrate that the established analytical method was suitable for its intended purpose, validation was conducted in compliance with the ICH Q2 (R1) requirements. The method was validated in terms of system suitability, specificity, the limit of detection (LOD), limit of quantification (LOQ), LOQ precision, linearity/range, method precision, intermediate precision, accuracy/recovery, robustness, and solution stability to ascertain the presence of PGIs in posaconazole and its intermediate.
2.4. System Specificity and Suitability
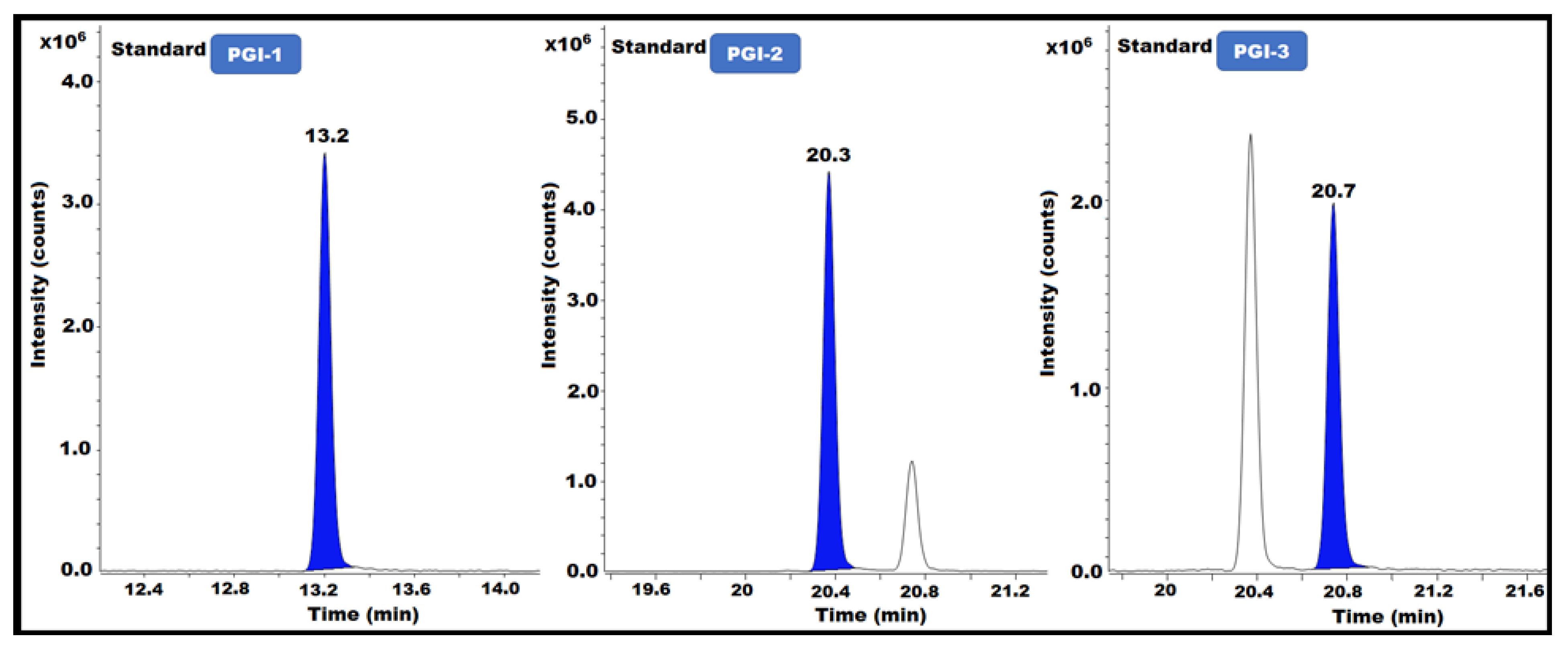
The capacity of an analytical method to evaluate a target constituent precisely and selectively within a mixture of contaminants is known as specificity. The resolution between neighboring peaks in the reference solution must be measured. Specificity is a key component of this strategy because multiple PGIs must be studied concurrently. In this method, specificity refers to the ability to quantify the analyte response in the presence of impurities (PGI-1, PGI-2, and PGI-3) in the posaconazole and its intermediate. To assess the specificity, all impurity solutions (PGI-1, PGI-2, and PGI-3) were independently produced and injected into the GC-MS instrument to determine the retention time. Additionally, according to the methodology, blank, sample, and spiked sample solutions were created, then injected into the GC-MS/MS (Table 1) (Figure 5).

Table 1.
Detailed results and their validation.
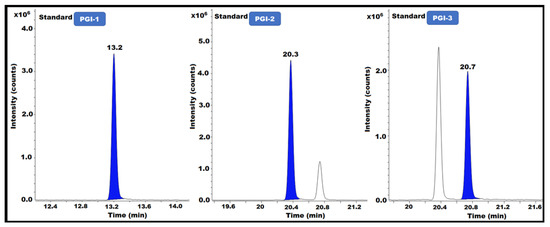
Figure 5.
PGI-1, PGI-2, and PGI-3 standard GC-MS/MS chromatograms.
2.5. LOQ, LOD, and Precision at LOQ
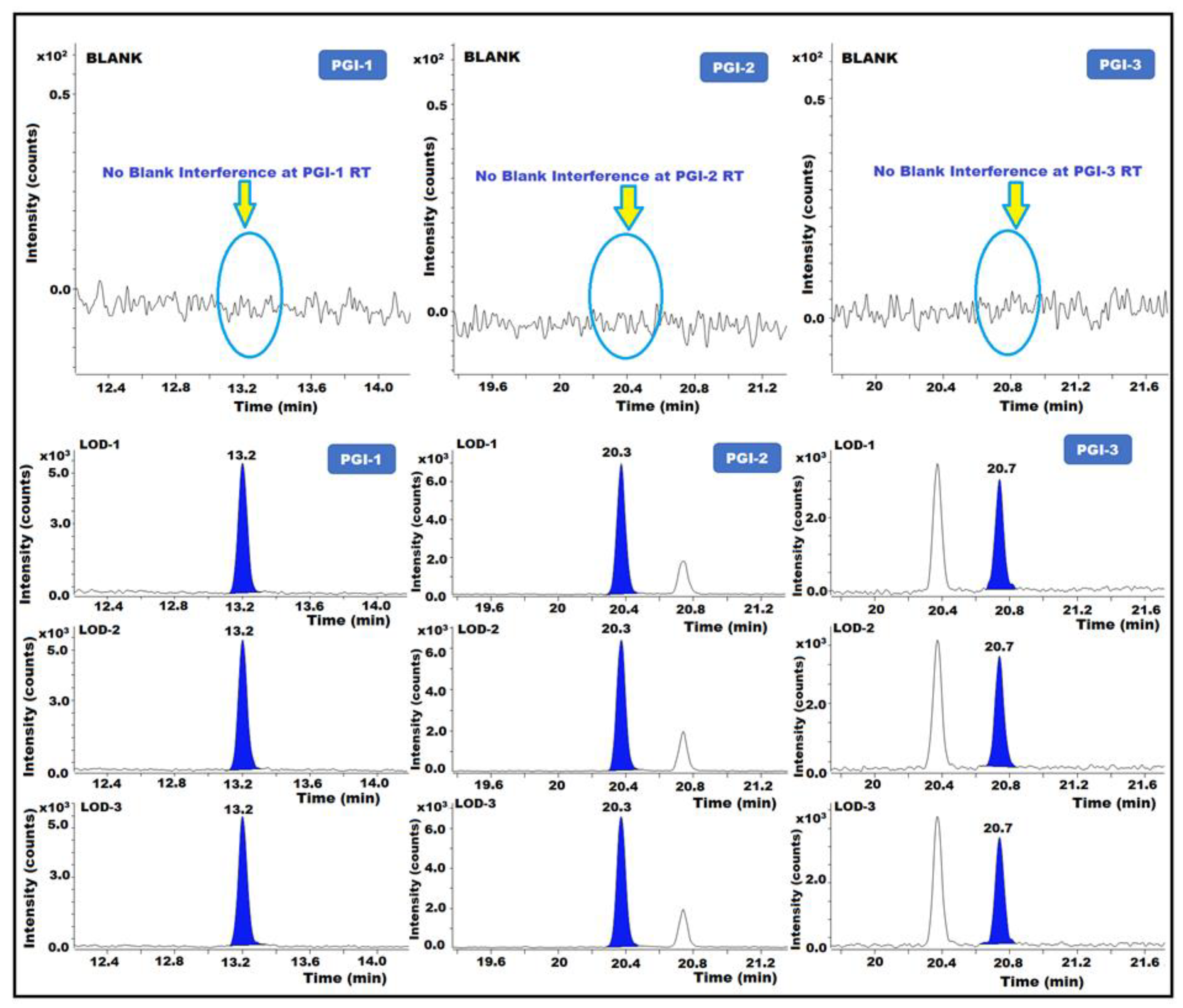
The minimum quantity of analyte required for validation was used to calculate the LOQ for each sample. The LOD is the lowest measurable concentration of any analyte in the samples. The signal-to-noise (s/n) ratios of the test were approximately 3 and 10, which allowed the separation of the LOD and LOQ. The LOD and LOQ were determined by injecting diluted PGI solutions in triplicates and measuring the known levels of each impurity. To determine LOQ precision, replicate injections of the LOQ solution (n = 6) were employed. The results showed that for the LOD and LOQ solutions, the s/n ratios were greater than 3 and 10, respectively. For each PGI, the % RSD of duplicate injections with LOQ precision was <15.0%. The technique accurately determined the amounts of PGI-1, PGI-2, and PGI-3 in posaconazole and its intermediate at the LOQ level (Table 2) (Figure 6).

Table 2.
Final instrument conditions for the GC-MS/MS technique.
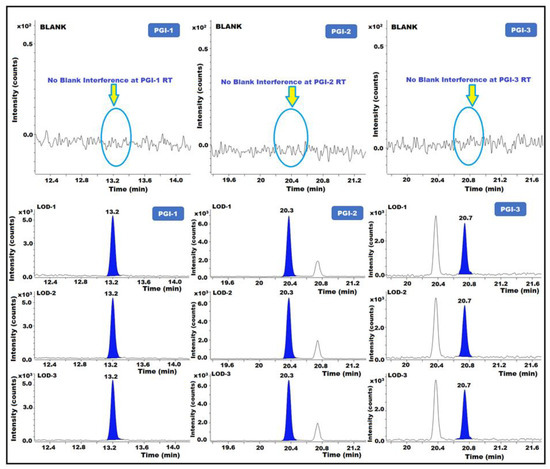
Figure 6.
GC-MS/MS chromatograms of PGI-1, PGI-2, and PGI-3 show the limit of detection (LOD).
2.6. Linearity
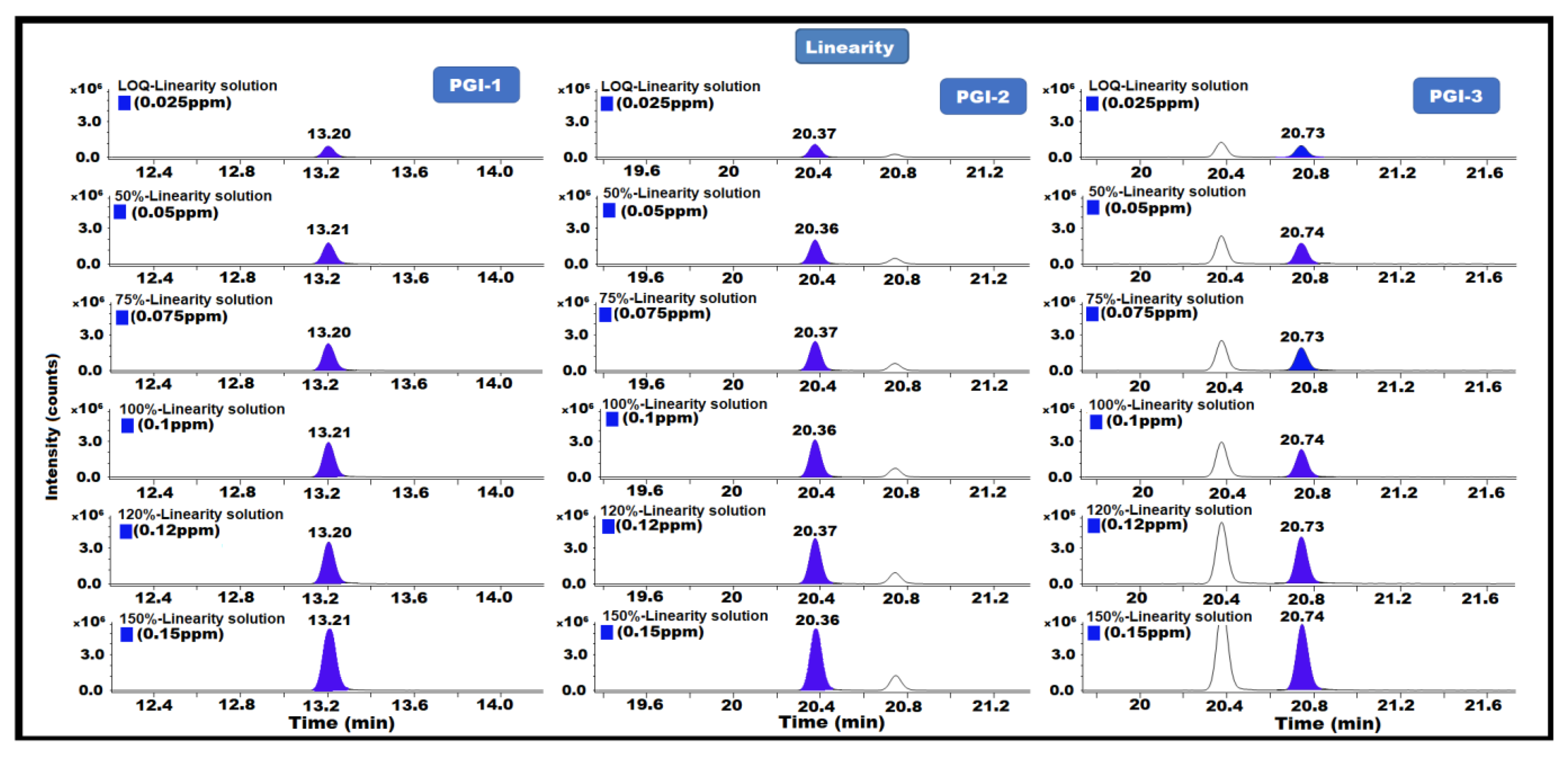
The linearity of a test method is defined as its ability to obtain a linear measurement value within a specified range in relation to the amount (or concentration) of each analyte. Linearity was performed from the LOQ level to 0.15 ppm. With respect to the sample concentrations, the linearity solution concentrations were LOQ (0.025 ppm), 50% (0.05 ppm), 75% (0.075 ppm), 100% (0.1 ppm), 120% (0.12 ppm), and 150% (0.15 ppm). Each solution was injected in duplicate into the GC-MS/MS system. We also established the range and correlation coefficient by plotting the peak area responses versus the concentration (R). The analytical method was demonstrated to be linear for each PGI, with all findings being no less than 0.99 for the correlation coefficient (R) for each PGI, indicating that the method is linear for the determination of PGI-1, PGI-2, and PGI-3 content in posaconazole and its intermediate (Table 1) (Figure 7).
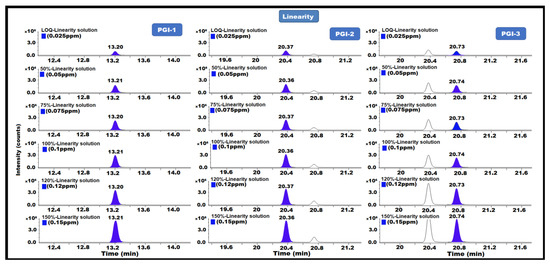
Figure 7.
GC-MS/MS chromatograms of PGI-1, PGI-2, and PGI-3 show linearity.
2.7. Repeatability (Method Precision) (MP)
The repeatability of the approach was investigated in terms of method precision. The effectiveness of the procedure was assessed through repeated injections of the standard, sample, and spiked sample solutions. Six analyses of the standard solution were performed to evaluate the performance of the GC-MS/MS system on the day of the test, using the parameters of the test method (system precision). For each PGI, the system precision experimental results for the relative standard deviation were provided in the system suitability parameters. Six sample solutions were prepared using a single batch of Posaconazole, and each PGI was added at a specified level for the MP experiment. These solutions were injected into the GC-MS/MS apparatus. Each prepared solution was administered only once. In the sample solution, the impurity content and % RSD were calculated; the findings showed that % RSD ≤ 20.0% (Table 1).
2.8. Intermediate Precision
The term “intermediate precision” (IP) refers to the consistency of results from variances within the laboratory caused by unpredictable occurrences, such as different days, analysts, or equipment, that may occur during the procedure. Different analysts, days, and columns were used to establish an IP in line with the MP. The PGI content and % RSD of the sample and spiked solutions were calculated. The % RSD for the spiked sample solution was <15.0% (n = 6). The % RSD for the MP- and IP-spiked sample solutions at the prescribed level was <20.0% (n = 12). The findings show that this technique is reliable for determining the amounts of PGI-1, PGI-2, and PGI-3 in posaconazole and its intermediate (Table 1).
2.9. Accuracy
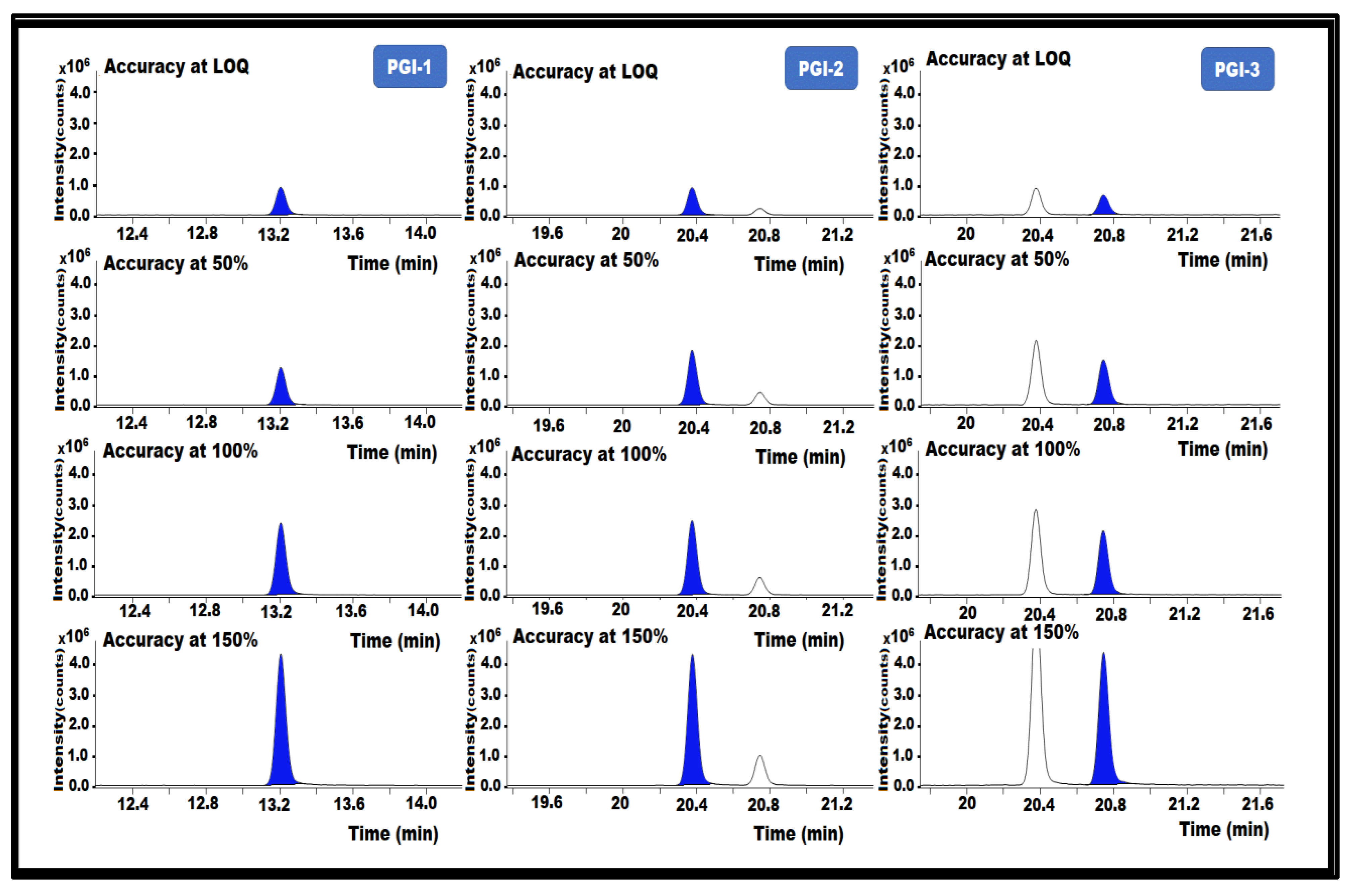
Accuracy is the degree to which a measured value is within a certain range of true or standard values. Recovery analysis of a matrix API that had been spiked with a PGI standard was used to assess the accuracy. Therefore, PGI-1, PGI-2, and PGI-3 were added to the posaconazole at the following concentrations: LOQ (0.025 ppm), 50% (0.05 ppm), 100% (0.1 ppm), and 150% (0.15 ppm). Posaconazole samples without impurities were prepared in triplicate for the accuracy experiment, then injected into the GC-MS/MS.
The % recoveries were calculated after analyzing the control and spiked samples. The proposed analytical method was used to determine the analyte content in the spiked sample solutions, and the recovery was estimated for each solution. All obtained results passed the acceptance requirements and were within 80–120% recovery for all PGIs. The results indicate that the method is accurate for the determination of PGI-1, PGI-2, and PGI-3 content in the posaconazole and in its intermediate (Table 1) (Figure 8).
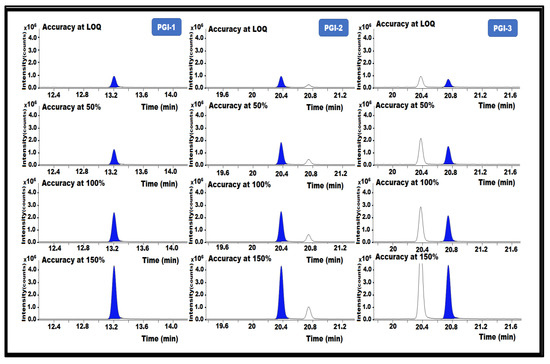
Figure 8.
GC-MS/MS chromatograms of PGI-1, PGI-2, and PGI-3 show the accuracy of the method used in this study.
2.10. Robustness
The robustness of a method is its capacity to survive small, deliberate modifications to its input parameters. Together with the original column oven temperatures of +122 and −118 °C, the actual column flow rate was modified to plus (+) (1.65 mL/min) and minus (−) (1.35 mL/min) flows. The results for the spike, standard, and MP data were compared with the solutions for concentration and retention time (RT). The RT of each impurity showed no irregularities, and there was a discrepancy of <10% in the impurity content between the MP and the robustness study results. The findings show that this technique is reliable for determining the amounts of PGI-1, PGI-2, and PGI-3 in Posaconazole and in its intermediate (Table 1).
2.11. Solution Stability
Solution stability studies were performed for up to 24 h at ambient laboratory temperature (25 ± 5 °C) and under refrigeration (8 ± 2 °C) using secondary intermediate stock solutions of PGI-1, PGI-2, and PGI-3 impurities and spiked samples with impurities at 100% concentration levels (0.10 ppm). Stability studies were performed using the percent recovery of freshly prepared primary standard solutions of the impurities and spiked samples. The findings demonstrate that these solutions are reliable for determining the amounts of PGI-1, PGI-2, and PGI-3 in the posaconazole intermediate and posaconazole (Table 1). Additional method validation chromatograms and FT-IR and NMR data are provided in the Supplementary Materials (Figures S1–S25).
3. Discussion
Gas chromatography with electron ionization mass spectrometry is a powerful analytical technique in the pharmaceutical industry for the highly specific and quantitative measurement of trace levels of analytes and impurities. Among various commonly encountered genotoxic impurities, alkylating agents, such as alkyl halides, alkyl sulfonates, and other related structures, poses significant challenge to analytical scientists for the development of suitable analytical methodologies for their accurate measurement at trace level [61]. These impurities, particularly alkyl halides, are typically generated during the chemical synthesis and processing of APIs, including Posaconazole, which often result in cytotoxicity. For instance, Posaconazole is a member of triazole derivatives, which potentially involve the utilization of alkyl halide during their synthesis process [62]. An optimized GC-MS/MS method was developed to determine the PGI-1, PGI-2, and PGI-3 content in posaconazole and in its intermediate. Due to the molecular mass and fragmentation are specific to each compound and impurity, there was no interference with the impurity retention time because of the sample and blank solutions. This method has the advantage of detecting impurities at the trace and ppm levels, whereas previous methods [54,55,56,57,58], such as HPLC, HPLC/UV and bioassay, HPLC-DAD, and LC-MS/MS, are not focused on the content and determination of impurities. These methods are complex and describe the content of posaconazole in other substances.
In view of the prior arts and analytes polarity, there was a probability of developing an analytical method by using different analytical techniques, including HPLC-PDA and LC-MS/MS. We conducted a few experiments by changing different diluents and chromatographic conditions in LC-MS/MS. Different ion sources, including ESI positive and negative and APCI positive and negative, were used for mass tuning. All ionization conditions were found to produce extremely poor fragmentation and response. The development trials were conducted by using C8 and C18 columns with various lengths, diameters, and particle sizes and different pH buffers (acidic, basic, and neutral) to measure these PGIs; however, poor peak response was noticed. We concluded from the findings that the LC-MS/MS approach would not be suitable for quantifying these three PGIs. In HPLC experiments, the PGI-1, PGI-2, and PGI-2 peaks detected more than 100 ppm concentration standard solution; hence, we concluded from the findings that the HPLC approach would not be suitable for quantifying these three PGIs in trace levels.
To measure these three PGIs, a specific and sensitive approach using GC-MS/MS with a triple quadrupole mass spectrometry detector was evaluated. Different column chemistries, such as DB-wax, DB-5, DB-624, and DB-1, were employed for optimization during development. We observed that DB-624 (USP phase G43) was suited to one since each PGI peak was very sharp and well ionized with good resolution. Furthermore, the use of helium as the carrier gas improved the method. To enhance the response rate of these PGIs, we conducted the diluent study with different solvents, such as dimethyl sulfoxide, dimethylformamide, N-methyl-2-pyrrolidone, ethanol, dichloromethane, acetonitrile, methanol, and hexane. However, we observed that other than methanol, the response rate of the PGI impurities was poor and found split peaks and broad peak shapes.
The developed method has the following advantages over the other methods mentioned. Detection via GC-MS-MS would be more accurate and reliable. The sensitivity was assessed using the LOQ. For each PGI, the LOQ was determined to be 0.025 ppm. This method is as good as or better than the methods described in the other published articles.
The developed method was used for the study of validation to sleuth its performance characteristics.
4. Experimental
4.1. Materials and Reagents
PGI-1, PGI-2, and PGI-3 were purchased from HTS Biopharma Pvt. Ltd. (ALEAPIndustrial Estate, near Pragathi Nagar, Hyderabad, India). Methanol of GC grade was obtained from Merck (India). Posaconazole and its intermediate were received as gifts from Jisai Pharma Pvt. Ltd. (Phase-4, Plot No. 12, IDA, Cherlapally, Hyderabad, India).
4.2. Equipment
Using an Agilent 7890 B GC system (Agilent, Santa Clara, CA, USA) coupled to an Agilent 7010 B GC/TQ triple quadrupole outfitted with electron impact ionization (EI) as the MSD ion source and multiple reaction monitoring (MRM) modes, data were gathered using Mass Hunter software. Method development and validation were performed using this GC-MS/MS device. The standards and samples were weighed using an analytical balance (Mettler Toledo ME204E, Zürich, Switzerland). Samples and standards were mixed using a vortex mixer (Remi, India). A Thermo Scientific variable micropipette, Finn pipette F2, was used for dilution (Thermo Scientific, Vantaa, Finland).
4.3. Chromatographic Conditions
The GC-MS/MS system was optimized using USP phase G43, a mid-polar 6% cyanopropyl, 94% polydimethylsiloxane with a length of 60 m, inner diameter of 0.32 mm, and film thickness of 1.8 μm. The temperature in the column oven was initially set to 120 °C and held for 5 min. The temperature was gradually increased to 250 °C at a rate of 5 °C/min and was maintained for 6 min. As the carrier gas, 1.5 mL/min of helium was selected. The injector heater was kept at a temperature of 200 °C, and a split ratio of 1:1 was used for the injection volume of 2 µL.
4.4. Mass Spectrometer Conditions
The multiple reaction monitoring (MRM) mode of the GC-MS/MS system was used while considering each PGI precursor ion (Q1) and production (Q3). MRM-1 (m/z) and MRM-2 (m/z) were established for each PGI based on its fragmentation pattern and response, and MRM-1 was used to quantify each PGI. MRM-1 (m/z) values were 156–141 amu for PGI-1, 233–153 amu for PGI-2, and 152–127 amu for PGI-3. The mass source and quad temperatures were 240 and 150 °C, respectively (Table 2).
4.5. Impurity Standard and Test Sample Solution Preparation
Each PGI standard (0.1 ppm with respect to sample concentration) was prepared in methanol (diluent). A posaconazole or posaconazole intermediate test sample (200 mg/mL) was prepared and diluted. The solutions were thoroughly incorporated after 5 min in a vortex. The specified concentration for each PGI was 0.1 ppm/(µg/mL) with respect to sample concentration is 100% of the specification limit. The sample concentration was optimized based on the accuracy findings obtained during technique development. Recovery was attained when the impurity was spiked at different sample concentrations, using a sample concentration of 200 mg/mL.
5. Conclusions
A sensitive and simultaneous GC-MS analytical method for three alkyl halides as PGIs in posaconazole and posaconazole intermediate was successfully developed. This study emphasizes the utility and efficiency of implementing an advanced analytical approach for the development of an analytical method using GC-MS/MS to quantify PGIs with low detection limits. This work presents a sensitive, effective, and reproducible GC-MS/MS method that is useful for determining and quantifying traces of PGI-1, PGI-2, and PGI-3 in posaconazole intermediate and posaconazole. The proposed method was validated according to the ICH guidelines; it met the criteria of acceptance for analytical parameters, such as specificity and system suitability, LOD, LOQ, LOQ precision, linearity and range, method precision, accuracy, ruggedness, robustness, and solution stability. This method can detect 0.01 ppm and quantify each PGI at 0.025 ppm and, thus, is useful for determining these PGIs in the routine analysis of posaconazole and its intermediate.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10050295/s1, Figure S1: 1-(2,4-difluorophenyl) ethan-1-one (PGI-1) standard certificate of analysis; Figure S2: PGI-1 Purity by HPLC Chromatogram; Figure S3: PGI-1 standard 1H-NMR spectrum data; Figure S4: PGI-1 standard 13C-NMR spectrum data; Figure S5: PGI-1 standard FT-IR spectrum; Figure S6: PGI-1 standard TGA graph; Figure S7: (Z)-1-(1-bromo prop-1-en-2-yl)-2,4-difluorobenzene (PGI-2) standard certificate of analysis; Figure S8: PGI-2 standard purity by HPLC chromatogram; Figure S9: PGI-2 standard 1H-NMR spectrum graph; Figure S10: PGI-2 standard 13C-NMR spectrum graph; Figure S11: PGI-2 standard FT-IR spectrum; Figure S12: PGI-2 standard TGA graph; Figure S13: 1-bromo-2-(2,4-Difluoro phenyl) propan-2-ol (PGI-3) standard certificate of analysis; Figure S14: PGI-3 standard purity by HPLC chromatogram; Figure S15: PGI-3 standard 1H-NMR spectrum; Figure S16: PGI-3 standard 13C-NMR spectrum; Figure S17: PGI-3 standard FT-IR spectrum; Figure S18: PGI-3 standard TGA graph; Figure S19: Blank solution chromatogram; Figure S20: Standard solution chromatogram; Figure S21: LOQ precision chromatogram; Figure S22: Method precision chromatogram; Figure S23: Sample solution chromatogram; Figure S24: Intermediate precision chromatogram; Figure S25: Linearity chromatogram; Figure S26: Method development experiments result; Figure S27: Plausible Fragmentation pattern of PGI-1, PGI-2, and PGI-3 impurities.
Author Contributions
Conceptualization, H.N.P.R.C. and J.V.S.K.; methodology, H.N.P.R.C. and J.V.S.K.; formal analysis, M.R.S., T.H.O., M.K. (Mujeeb Khan), M.K. (Merajuddin Khan) and B.S.; investigation, H.N.P.R.C., J.V.S.K. and M.R.S.; resources, H.N.P.R.C. and J.V.S.K.; data curation, H.N.P.R.C., J.V.S.K. and M.R.S.; validation, A.B. and M.K. (Mujeeb Khan); writing—original draft preparation, H.N.P.R.C., J.V.S.K. and A.B.; writing—review and editing, H.N.P.R.C. and M.R.S.; supervision, J.V.S.K.; project administration, J.V.S.K.; funding acquisition, M.R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project number (RSPD2023R665), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data contained within the article and supplementary file.
Acknowledgments
The authors acknowledge funding from the Researchers Supporting Project number (RSPD2023R665), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eder, E.; Henschler, D.; Neudecker, T. Mutagenic properties of allylic and α, β-unsaturated compounds: Consideration of alkylating mechanisms. Xenobiotica 1982, 12, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Sobol, Z.; Engel, M.; Rubitski, E.; Ku, W.; Aubrecht, J.; Schiestl, R. Genotoxicity profiles of common alkyl halides and esters with alkylating activity. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2007, 633, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Szekely, G.; Amores de Sousa, M.C.; Gil, M.; Castelo Ferreira, F.; Heggie, W. Genotoxic impurities in pharmaceutical manufacturing: Sources, regulations, and mitigation. Chem. Rev. 2015, 115, 8182–8229. [Google Scholar] [CrossRef]
- Bolt, H.M.; Gansewendt, B. Mechanisms of carcinogenicity of methyl halides. Crit. Rev. Toxicol. 1993, 23, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Yoo, W.; Jeong, J.H. Analytical Method Development for 19 Alkyl Halides as Potential Genotoxic Impurities by Analytical Quality by Design. Molecules 2022, 27, 4437. [Google Scholar] [CrossRef]
- Stauffer, F.; Vanhoorne, V.; Pilcer, G.; Chavez, P.; Rome, S.; Schubert, M.; Aerts, L.; De Beer, T. Raw material variability of an active pharmaceutical ingredient and its relevance for processability in secondary continuous pharmaceutical manufacturing. Eur. J. Pharm. Biopharm. 2018, 127, 92–103. [Google Scholar] [CrossRef]
- Burcham, C.L.; Florence, A.J.; Johnson, M.D. Continuous manufacturing in pharmaceutical process development and manufacturing. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 253–281. [Google Scholar] [CrossRef]
- Ozawa, S.; Chen, H.-H.; Lee, Y.-F.A.; Higgins, C.R.; Yemeke, T.T. Characterizing medicine quality by active pharmaceutical ingredient levels: A systematic review and meta-analysis across low-and middle-income countries. Am. J. Trop. Med. Hyg. 2022, 106, 1778. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Deshpande, M.; Zaheer, Z.; Shinde, D.B.; Arote, R. Quality by design approach: Regulatory need. Arab. J. Chem. 2017, 10, S3412–S3425. [Google Scholar] [CrossRef]
- Müller, L.; Mauthe, R.J.; Riley, C.M.; Andino, M.M.; De Antonis, D.; Beels, C.; DeGeorge, J.; De Knaep, A.G.; Ellison, D.; Fagerland, J.A. A rationale for determining, testing, and controlling specific impurities in pharmaceuticals that possess potential for genotoxicity. Regul. Toxicol. Pharm. 2006, 44, 198–211. [Google Scholar] [CrossRef]
- Bercu, J.P.; Dobo, K.L.; Gocke, E.; McGovern, T.J. Overview of genotoxic impurities in pharmaceutical development. Int. J. Toxicol. 2009, 28, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.; Prasad, A.; Reddy, K.R. Strategies for the identification, control and determination of genotoxic impurities in drug substances: A pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 2011, 55, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Looker, A.R.; Ryan, M.P.; Neubert-Langille, B.J.; Naji, R. Risk assessment of potentially genotoxic impurities within the framework of quality by design. Org. Process Res. Dev. 2010, 14, 1032–1036. [Google Scholar] [CrossRef]
- Bolt, H.M.; Foth, H.; Hengstler, J.G.; Degen, G.H. Carcinogenicity categorization of chemicals—New aspects to be considered in a European perspective. Toxicol. Lett. 2004, 151, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA damage as a source of genomic instability in cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef] [PubMed]
- International Council for Harmonisation Guideline M7 (R1) on Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk Step 4 Version. 2017. Available online: https://database.ich.org/sites/default/files/M7_R1_Guideline.pdf (accessed on 3 May 2022).
- Kondo, K.; Watanabe, A.; Iwanaga, Y.; Abe, I.; Tanaka, H.; Nagaoka, M.H.; Akiyama, H.; Maitani, T. Determination of genotoxic phenylhydrazine agaritine in mushrooms using liquid chromatography–electrospray ionization tandem mass spectrometry. Food Addit. Contam. 2006, 23, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Humfrey, C.D. Recent developments in the risk assessment of potentially genotoxic impurities in pharmaceutical drug substances. Toxicol. Sci. 2007, 100, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Giordani, A.; Kobel, W.; Gally, H.U. Overall impact of the regulatory requirements for genotoxic impurities on the drug development process. Eur. J. Pharm. Sci. 2011, 43, 1–15. [Google Scholar] [CrossRef]
- Dow, L.K.; Hansen, M.M.; Pack, B.W.; Page, T.J.; Baertschi, S.W. The assessment of impurities for genotoxic potential and subsequent control in drug substance and drug product. J. Pharm. Sci. 2013, 102, 1404–1418. [Google Scholar] [CrossRef]
- Food and Drug Administration; HHS. International Conference on Harmonisation; revised guidance on Q3B (R) Impurities in New Drug Products; Availability. Notice. Fed. Regist. 2003, 68, 64628–64629. [Google Scholar]
- Ramachandra, B. Development of impurity profiling methods using modern analytical techniques. Crit. Rev. Anal. Chem. 2017, 47, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Basniwal, P.K. Forced degradation and impurity profiling: Recent trends in analytical perspectives. J. Pharm. Biomed. Anal. 2013, 86, 11–35. [Google Scholar] [CrossRef] [PubMed]
- Chittireddy, H.N.P.R.; Kumar, J.S.; Bhimireddy, A.; Shaik, M.R.; Shaik, A.H.; Alwarthan, A.; Shaik, B. Development and Validation for Quantification of Cephapirin and Ceftiofur by Ultraperformance Liquid Chromatography with Triple Quadrupole Mass Spectrometry. Molecules 2022, 27, 7920. [Google Scholar] [CrossRef] [PubMed]
- Chittireddy, H.N.P.R.; Kumar, J.S.; Bhimireddy, A.; Shaik, M.R.; Khan, M.; Adil, S.F.; Khan, M.; Aldhuwayhi, F.N. Development and Validation for Quantification of 7-Nitroso Impurity in Sitagliptin by Ultraperformance Liquid Chromatography with Triple Quadrupole Mass Spectrometry. Molecules 2022, 27, 8581. [Google Scholar] [CrossRef] [PubMed]
- Chittireddy, H.N.P.R.; Kumar, J.S.; Bhimireddy, A.; Shaik, M.R.; Hatshan, M.R.; Khan, M.; Alwarthan, A.; Shaik, B. Development and Validation for Quantitative Determination of Genotoxic Impurity in Gemfibrozil by Gas Chromatography with Mass Spectrometry. Separations 2023, 10, 145. [Google Scholar] [CrossRef]
- Nanda, K.K.; Mozziconacci, O.; Small, J.; Allain, L.R.; Helmy, R.; Wuelfing, W.P. Enrichment of relevant oxidative degradation products in pharmaceuticals with targeted chemoselective oxidation. J. Pharm. Sci. 2019, 108, 1466–1475. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, G.G.; George, K.L.S.; Zhou, D. A novel accelerated oxidative stability screening method for pharmaceutical solids. J. Pharm. Sci. 2011, 100, 3529–3538. [Google Scholar] [CrossRef]
- Roberto de Alvarenga Junior, B.; Lajarim Carneiro, R. Chemometrics approaches in forced degradation studies of pharmaceutical drugs. Molecules 2019, 24, 3804. [Google Scholar] [CrossRef]
- Gomes, A.R.; Varela, C.L.; Tavares-da-Silva, E.J.; Roleira, F.M. Epoxide containing molecules: A good or a bad drug design approach. Eur. J. Med. Chem. 2020, 201, 112327. [Google Scholar] [CrossRef]
- Ensign, S.A.; Allen, J.R. Aliphatic epoxide carboxylation. Annu. Rev. Biochem 2003, 72, 55–76. [Google Scholar] [CrossRef]
- Wilson, S.C.; Howard, P.W.; Forrow, S.M.; Hartley, J.A.; Adams, L.J.; Jenkins, T.C.; Kelland, L.R.; Thurston, D.E. Design, synthesis, and evaluation of a novel sequence-selective epoxide-containing DNA cross-linking agent based on the pyrrolo [2, 1-c][1, 4] benzodiazepine system. J. Med. Chem. 1999, 42, 4028–4041. [Google Scholar] [CrossRef]
- Schuch, A.P.; Menck, C.F.M. The genotoxic effects of DNA lesions induced by artificial UV-radiation and sunlight. J. Photochem. Photobiol. B 2010, 99, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Douki, T.; Reynaud-Angelin, A.; Cadet, J.; Sage, E. Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochemistry 2003, 42, 9221–9226. [Google Scholar] [CrossRef] [PubMed]
- Savale, S.K. Genotoxicity of drugs: Introduction, prediction and evaluation. Asian J. Biomater. Res. 2018, 4, 1–29. [Google Scholar]
- Guideline, E. Guideline on the Limits of Genotoxic Impurities. CPMP/SWP/5199/02. 2006. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-limits-genotoxic-impurities_en.pdf (accessed on 1 January 2023).
- Guidance, F. Genotoxic and Carcinogenic Impurities in Drug Substances and Products: Recommended Approaches; US Food and Drug Administration, Department of Health and Human Services: Washington, DC, USA, 2008.
- Guideline, I.H.T. Impurities in New Drug Substances Q3A (R2). In Proceedings of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland, 25 October 2006. [Google Scholar]
- Liu, K.-T.; Chen, C.-H. Determination of impurities in pharmaceuticals: Why and how? In Quality Management and Quality Control-New Trends and Developments; IntechOpen: London, UK, 2019; pp. 1–17. [Google Scholar]
- Gerding, J.; Anhäuser, L.; Eickmann, U.; Nienhaus, A. A simple approach to assess the cancer risk of occupational exposure to genotoxic drugs in healthcare settings. J. Occup. Med. Toxicol. 2022, 17, 1–9. [Google Scholar] [CrossRef]
- Gaunt, M.J.; Johansson, C.C.; McNally, A.; Vo, N.T. Enantioselective organocatalysis. Drug Discov. Today 2007, 12, 8–27. [Google Scholar] [CrossRef]
- Casado, N.; Valimaña-Traverso, J.; García, M.Á.; Marina, M.L. Enantiomeric determination of drugs in pharmaceutical formulations and biological samples by electrokinetic chromatography. Crit. Rev. Anal. Chem. 2020, 50, 554–584. [Google Scholar] [CrossRef]
- Mwamwitwa, K.W.; Kaibere, R.M.; Fimbo, A.M.; Sabitii, W.; Ntinginya, N.E.; Mmbaga, B.T.; Shewiyo, D.H.; Shearer, M.C.; Smith, A.D.; Kaale, E.A. A retrospective cross-sectional study to determine chirality status of registered medicines in Tanzania. Sci. Rep. 2020, 10, 17834. [Google Scholar] [CrossRef]
- Phillips, D.H.; Arlt, V.M. Genotoxicity: Damage to DNA and its consequences. In Molecular, Clinical and Environmental Toxicology: Volume 1: Molecular Toxicology; Springer: Basel, Switzerland, 2009; pp. 87–110. [Google Scholar]
- Vogel, E.; Natarajan, A. DNA damage and repair in somatic and germ cells in vivo. Mutat. Res./Fundam. Mol. Mech. Mutagen. 1995, 330, 183–208. [Google Scholar] [CrossRef]
- Rotter, S.; Beronius, A.; Boobis, A.; Hanberg, A.; Van Klaveren, J.; Luijten, M.; Machera, K.; Nikolopoulou, D.; Van Der Voet, H.; Zilliacus, J. Overview on legislation and scientific approaches for risk assessment of combined exposure to multiple chemicals: The potential EuroMix contribution. Crit. Rev. Toxicol. 2018, 48, 796–814. [Google Scholar] [CrossRef] [PubMed]
- Committee, E.S. Scientific Opinion on genotoxicity testing strategies applicable to food and feed safety assessment. EFSA J. 2011, 9, 2379. [Google Scholar]
- Charoo, N.A.; Ali, A.A. Quality risk management in pharmaceutical development. Drug Dev. Ind. Pharm. 2013, 39, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Suprin, M.; Chow, A.; Pillwein, M.; Rowe, J.; Ryan, M.; Rygiel-Zbikowska, B.; Wilson, K.J.; Tomlin, I. Quality risk management framework: Guidance for successful implementation of risk management in clinical development. Ther. Innov. Regul. Sci. 2019, 53, 36–44. [Google Scholar] [CrossRef]
- Noxafil® Approval Label by United States Food and Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022003s018s020,0205053s002s004,0205596s001s003lbl.pdf (accessed on 1 January 2023).
- Chen, L.; Krekels, E.H.; Verweij, P.E.; Buil, J.B.; Knibbe, C.A.; Brüggemann, R.J. Pharmacokinetics and pharmacodynamics of posaconazole. Drugs 2020, 80, 671–695. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.V.; Costa, G.R.; Mendez, A.S. Stability-indicating HPLC method for posaconazole bulk assay. Sci. Pharm. 2012, 80, 317–328. [Google Scholar] [CrossRef]
- Cendejas-Bueno, E.; Forastiero, A.; Rodriguez-Tudela, J.; Cuenca-Estrella, M.; Gomez-Lopez, A. HPLC/UV or bioassay: Two valid methods for posaconazole quantification in human serum samples. Clin. Microbiol. Infect. 2012, 18, 1229–1235. [Google Scholar] [CrossRef]
- Santana, A.C.S.G.V.; Danda, L.J.d.A.; Nunes, L.C.C.; Soares Sobrinho, J.L. Simultaneous Quantification of Benznidazole and Posaconazole by HPLC-DAD Using QbD Approach. J. Chromatogr. Sci. 2019, 57, 156–162. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, Y.-K.; Pattengale, P.; O’Gorman, M.R.; Fu, X. A rapid high-performance LC-MS/MS method for therapeutic drug monitoring of voriconazole, posaconazole, fluconazole, and itraconazole in human serum. J. Appl. Lab. Med. 2017, 1, 626–636. [Google Scholar] [CrossRef]
- Cunliffe, J.M.; Noren, C.F.; Hayes, R.N.; Clement, R.P.; Shen, J.X. A high-throughput LC–MS/MS method for the quantitation of posaconazole in human plasma: Implementing fused core silica liquid chromatography. J. Pharm. Biomed. Anal. 2009, 50, 46–52. [Google Scholar] [CrossRef]
- Milind, G.; Vivek, K.; Srinivas Reddy, S.; Ganesh, C.; Jitendra, V.; Mubeen Ahmed, K. Process for preparation of posaconazole and crystalline polymorphic form v of posaconazole, WO2011158248A2 World Intellectual Property Organization (Patent Corperation Treaty). 2011. Available online: https://patents.google.com/patent/WO2011158248A3/da (accessed on 12 March 2023).
- Bethanne, W.; Angela, G.; Guodong, C. Analysis of Impurities and Degradants in Pharmaceuticals by High Resolution Tandem Mass Spectrometry and On-line H/D Exchange LC/MS. Am. Pharm. Rev. 2010, 13, 20–27. [Google Scholar]
- Wang, K.C.; Guo, Q.; Kuang, Z.; Jin, J.; Li, D.; Chen, W.; Zhu, W.; Li, M. Structural elucidation of two novel degradants of lurasidone and their formation mechanisms under free radical-mediated oxidative and photolytic conditions via liquid chromatography-photodiode array/ultraviolet-tandem mass spectrometry and one-dimensional/two-dimensional nuclear magnetic resonance spectroscopy. J. Mass Spectrom. 2022, 57, e4871. [Google Scholar] [PubMed]
- Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. [Google Scholar] [CrossRef]
- Reddy, A.V.B.; Jaafar, J.; Umar, K.; Majid, Z.A.; Aris, A.B.; Talib, J.; Madhavi, G. Identification, control strategies, and analytical approaches for the determination of potential genotoxic impurities in pharmaceuticals: A comprehensive review. J. Sep. Sci. 2015, 38, 764–779. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ni, T.; Chai, X.; Wang, T.; Wang, H.; Chen, J.; Jin, Y.; Zhang, D.; Yu, S.; Jiang, Y. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives. Eur. J. Med. Chem. 2018, 143, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).