Study on Homogeneous Reduction Technology in Gas Samples for Oil and Gas Loss
Abstract
1. Introduction
2. Experimental Scheme and Design
2.1. Laboratory Equipment
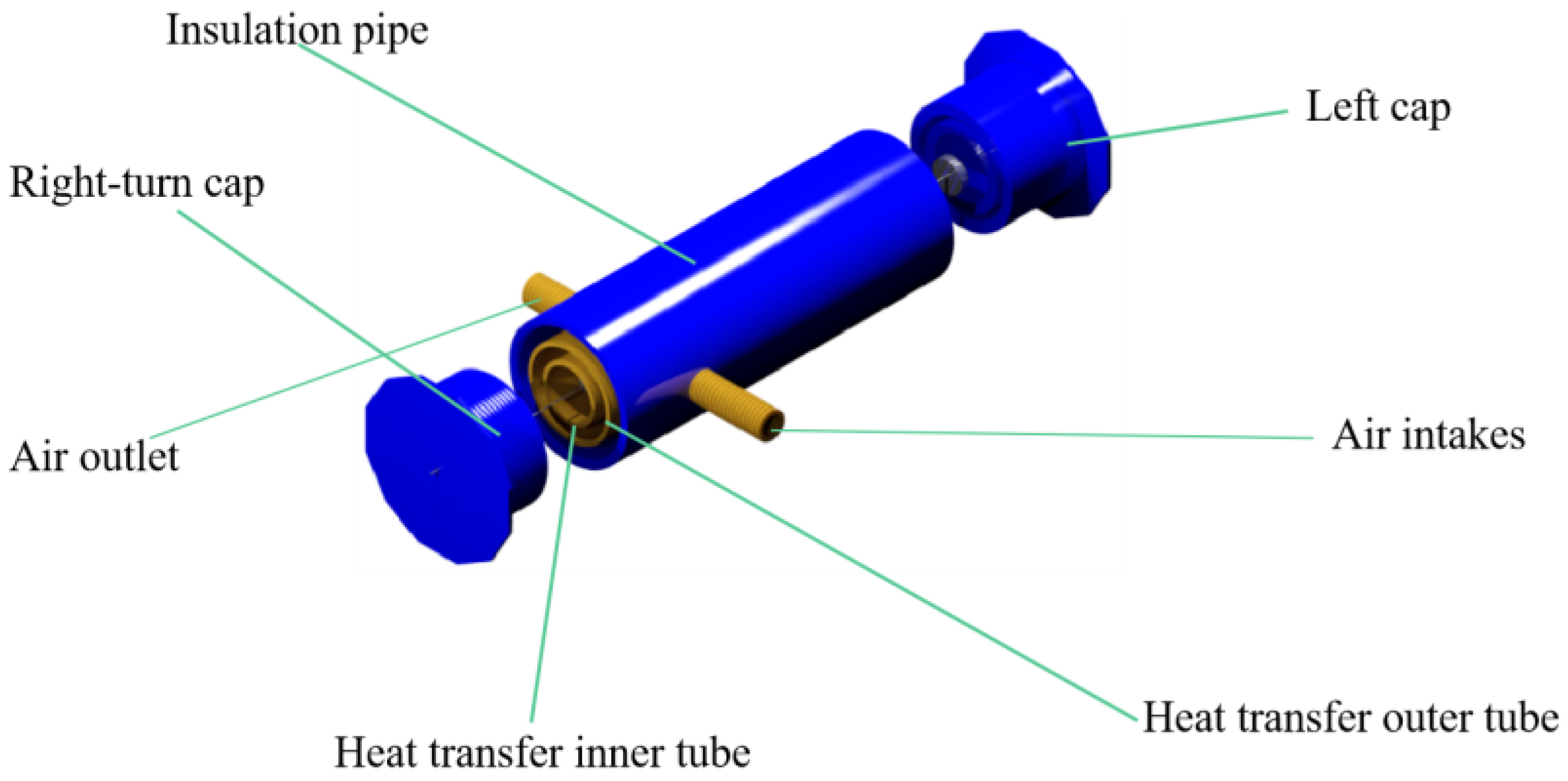
2.2. Schematic Structure Design of Heating Box
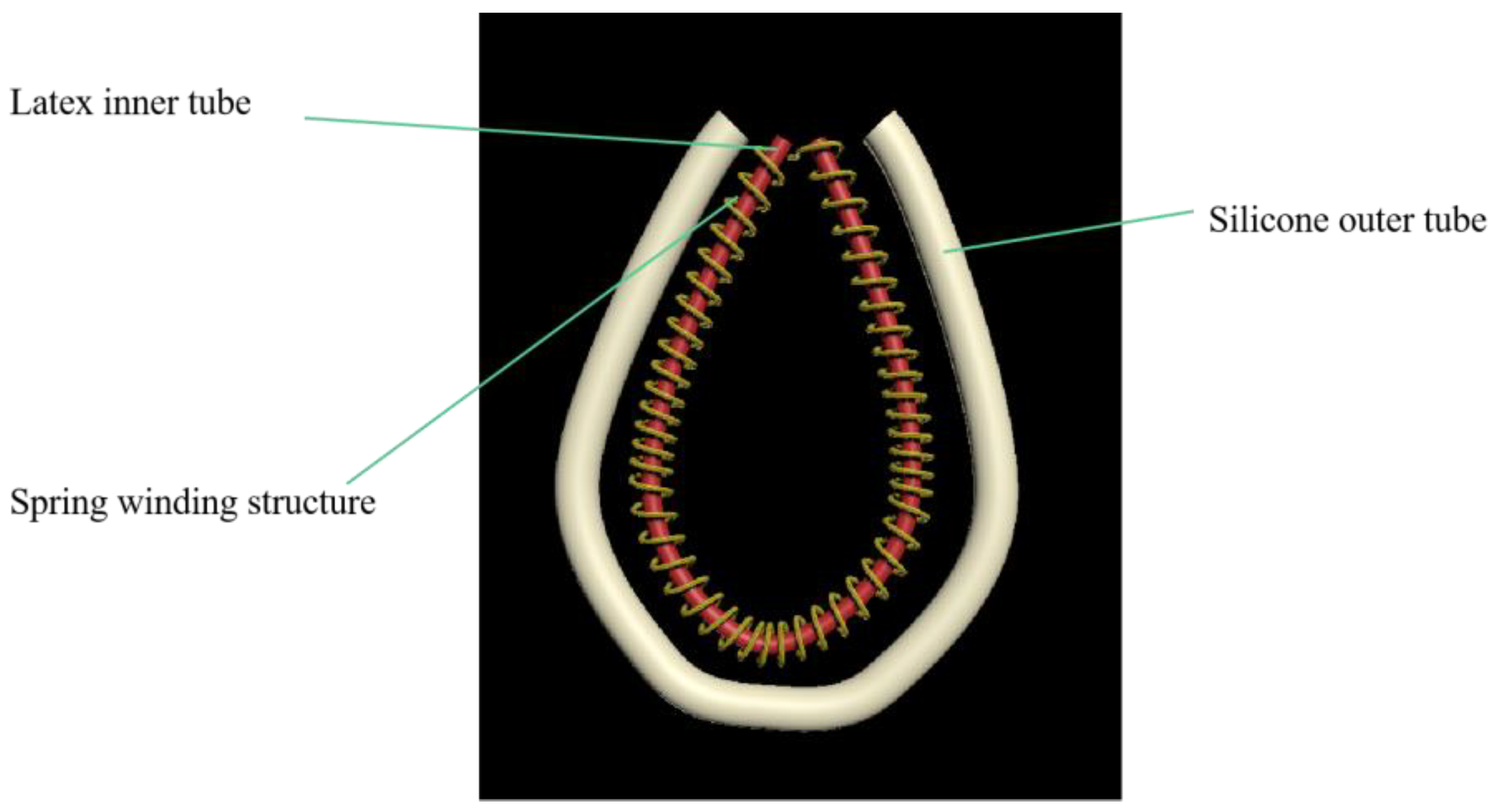
2.3. Transfer Device Design
2.4. Constant-Temperature Gas Transmission Pipeline Design
3. Experimental Parameter Design
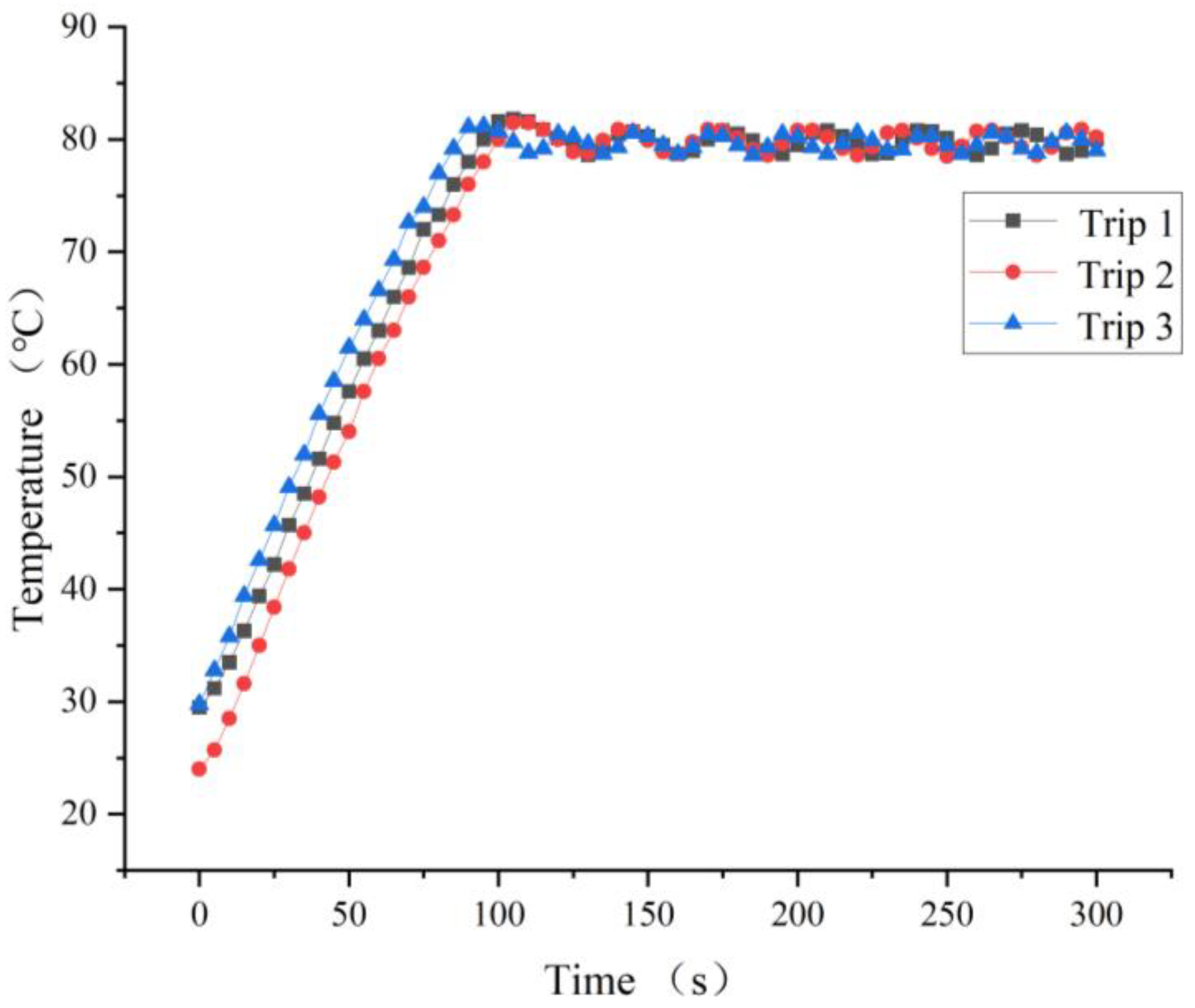
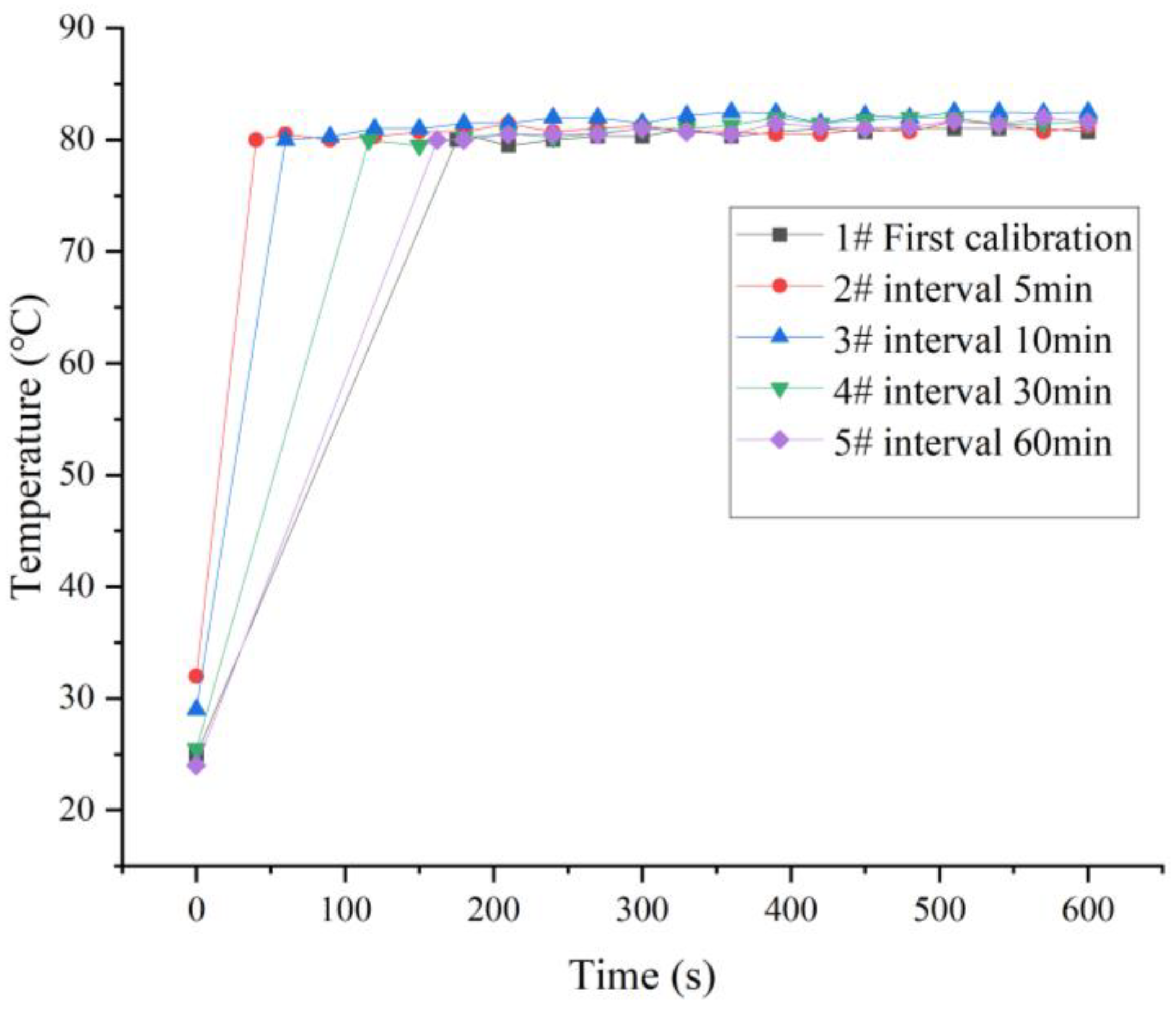
3.1. Heating Temperature of the Transmitter
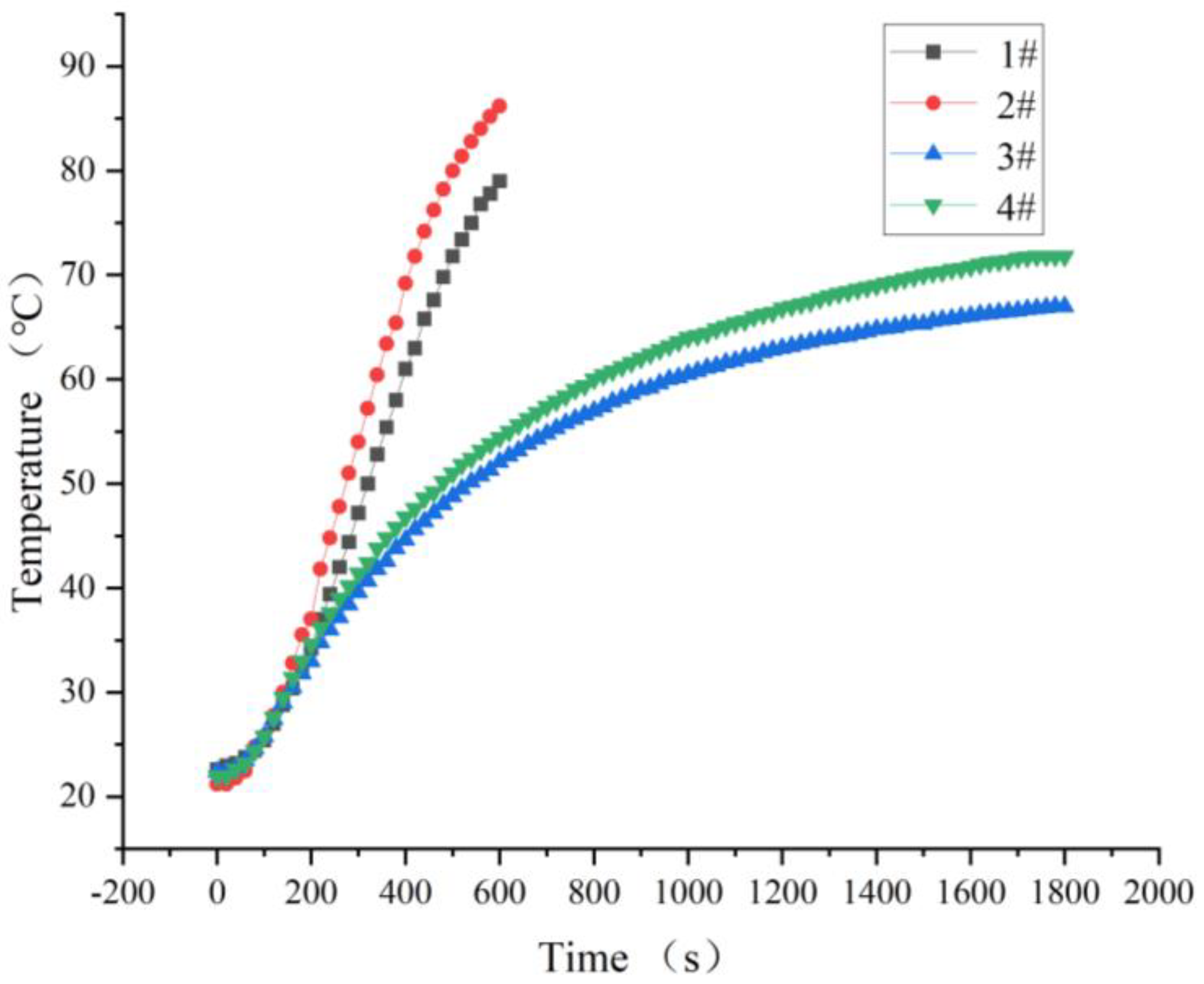
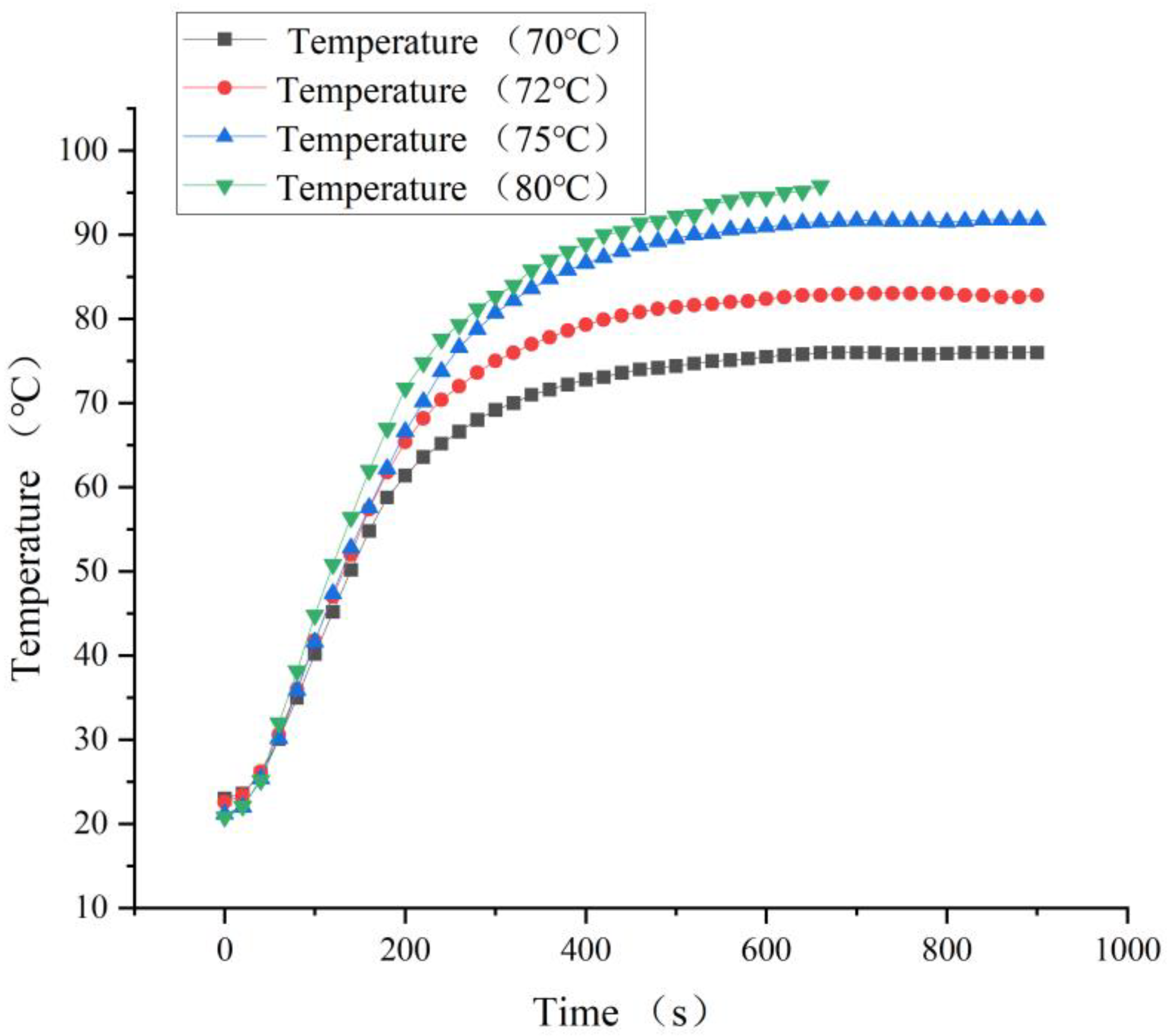
3.2. Hot Air System Temperature
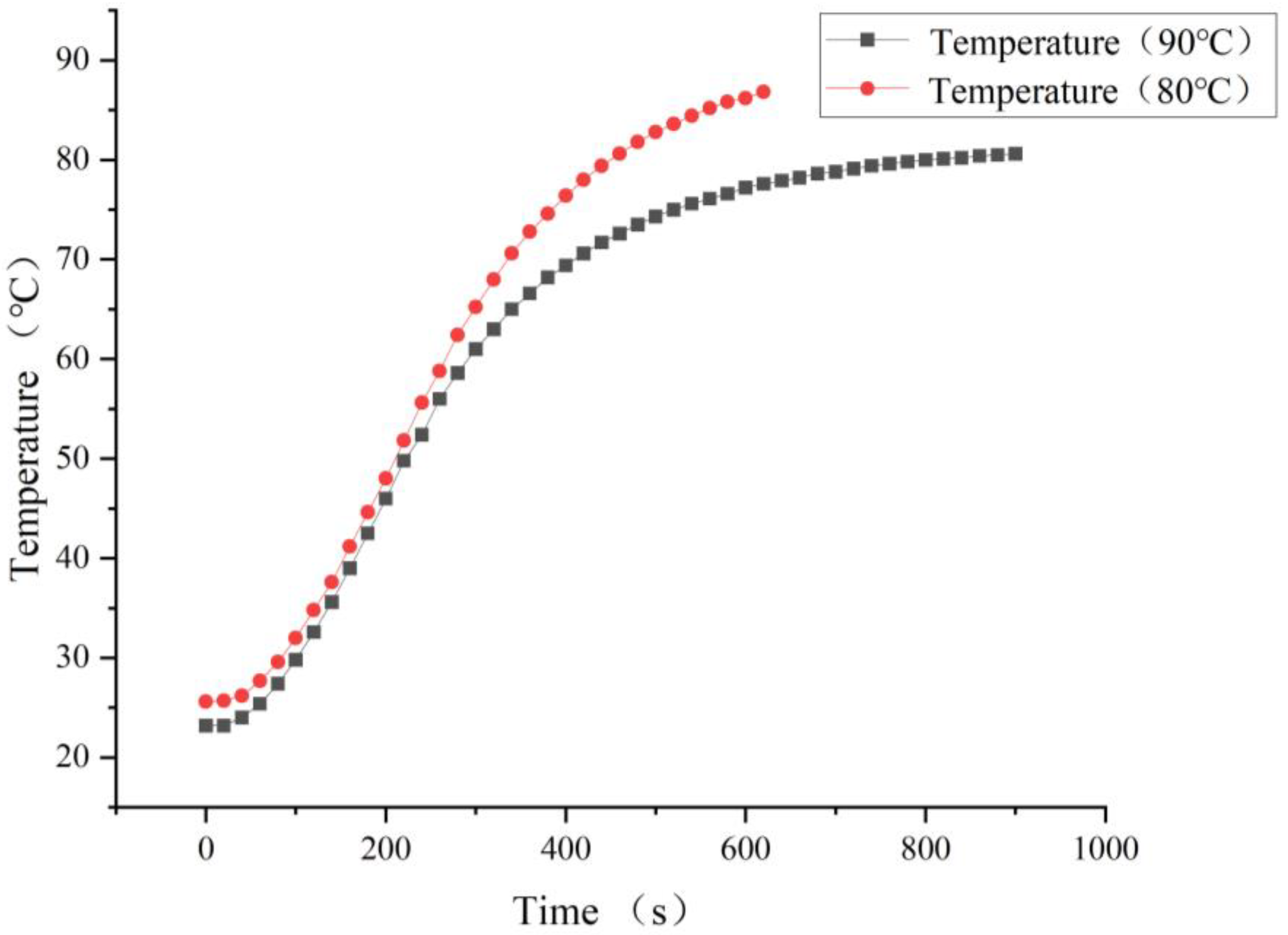
3.3. Heating Temperature of the Constant-Temperature Conveying Gas Pipe
3.4. The Experimental Scheme
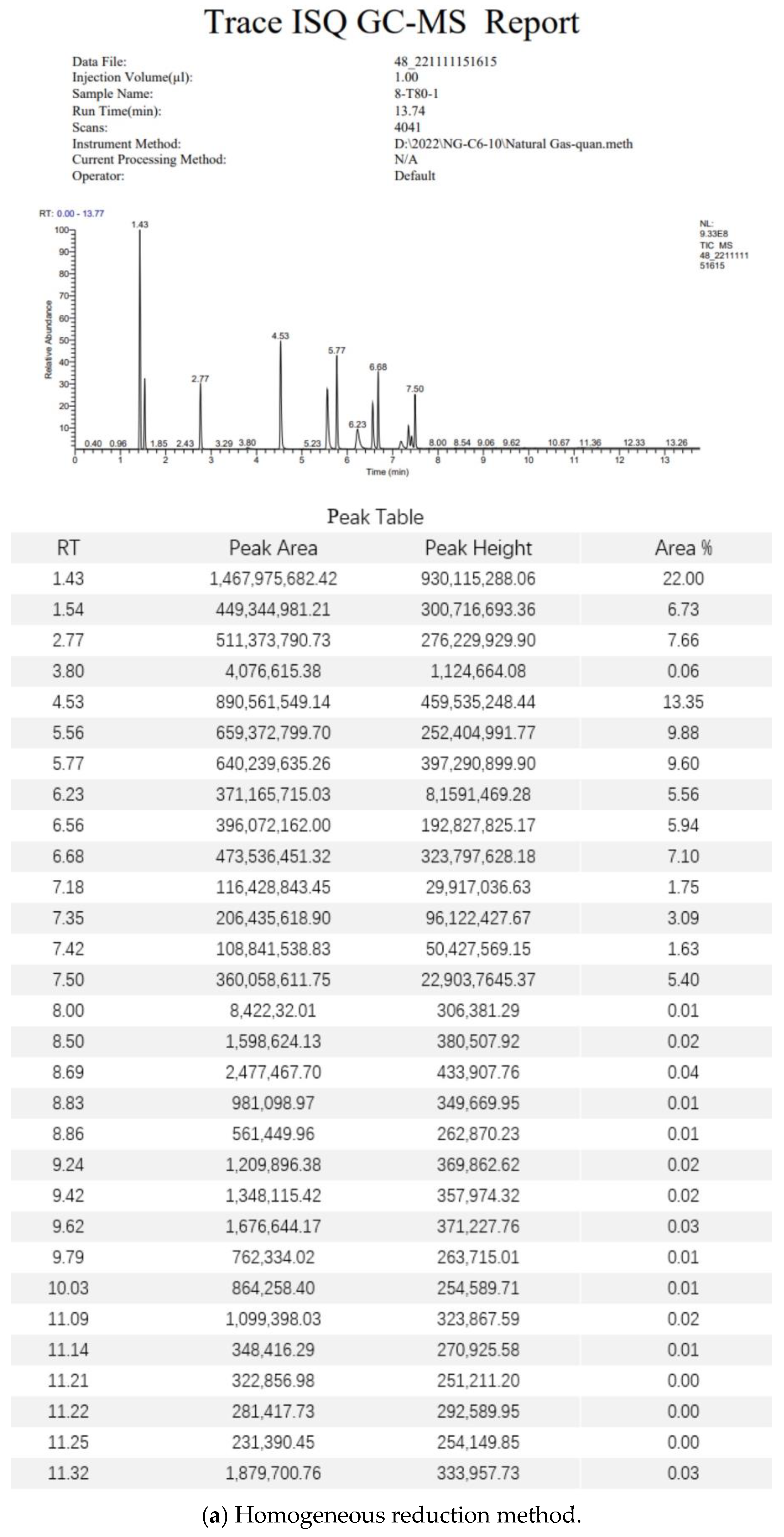
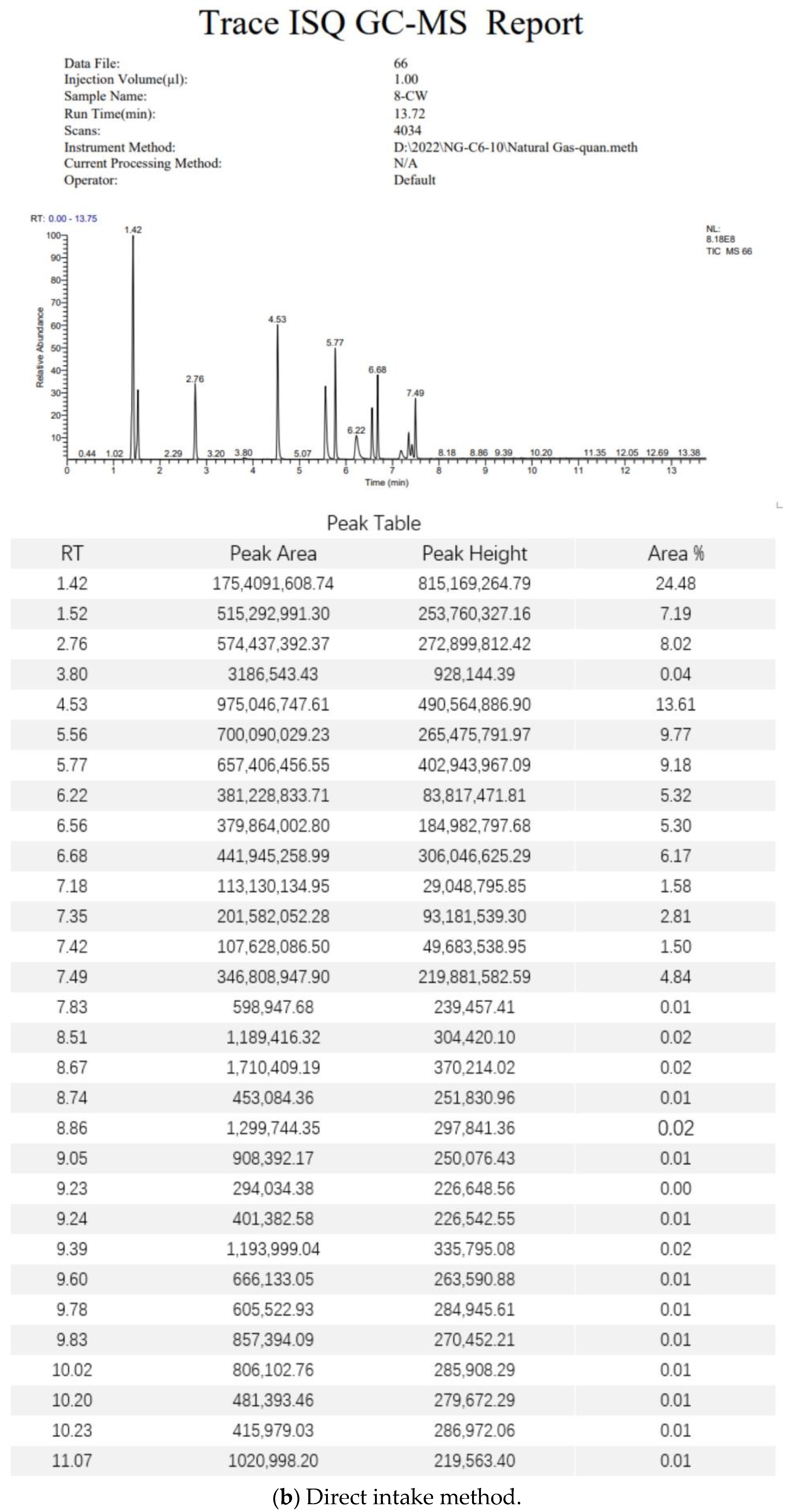
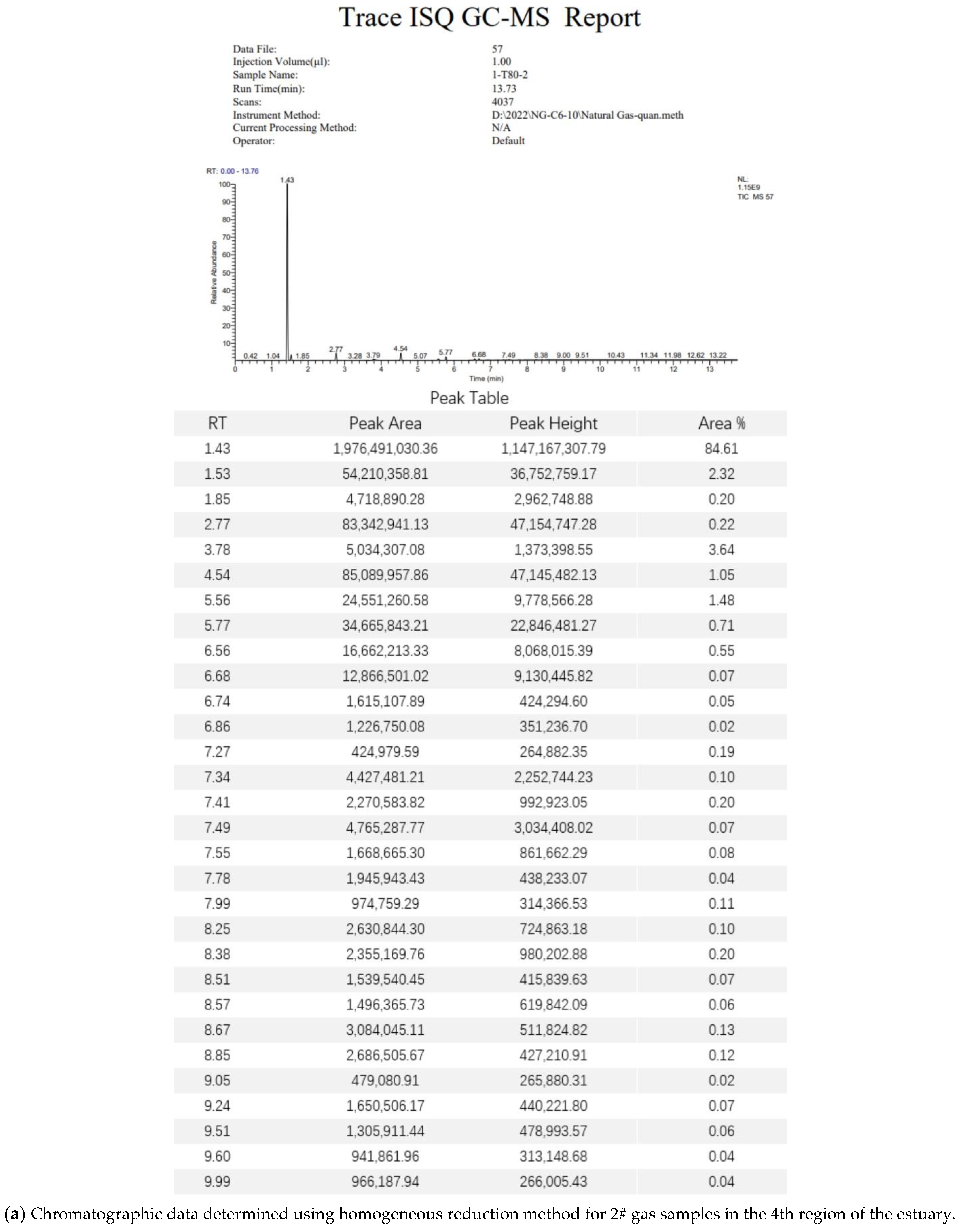
3.5. Gas Chromatography–Mass Spectrometry Analysis Methods
3.6. Standard Gas Test Data Analysis
3.7. Field Gas Loss Measurement Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Tissot, B.P.; Welte, D.H. Composition of Crude Oils. In Petroleum Formation and Occurrence: A New Approach to Oil and Gas Exploration; Springer: Berlin/Heidelberg, Germany, 1978; pp. 333–368. [Google Scholar]
- Yang, S. Chemical Composition and Properties of Reservoir Fluids. In Fundamentals of Petrophysics; Springer: Berlin/Heidelberg, Germany, 2017; pp. 3–26. [Google Scholar]
- Faramawy, S.; Zaki, T.; Sakr, A.A.E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Barker, C. Origin, composition and properties of petroleum. In Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 1985; Volume 17, pp. 11–45. [Google Scholar]
- Jurušs, M.; Seile, E. Application of Loss Rates for Petroleum Products Due to Natural Wastage in Customs Procedures. Procedia Eng. 2017, 178, 377–383. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, X.; Li, G.; Ma, X.; Zhang, K.; Gu, J.; Yao, J.; Wang, J.; Sun, H. An automatic history matching method based on ensemble and neural architecture search. J. China Univ. Pet. (Nat. Sci. Ed.) 2022, 46, 127–136. [Google Scholar]
- Zhang, K.; Zhao, X.; Zhang, L.; Zhang, H.; Wang, H.; Chen, G.; Zhao, M.; Jiang, Y.; Yao, J. Current status and prospect for the research and application of big data and intelligent optimization methods in oilfield development. J. China Univ. Pet. (Nat. Sci. Ed.) 2020, 44, 28–38. [Google Scholar]
- Zhang, K.; Zhang, H.; Zhang, L.; Yao, J. Construction and optimization method of adaptive well pattern based on reservoir uncertainty. Chin. Sci. Pap. 2017, 12, 2438–2444. [Google Scholar]
- Zhang, K.; Chen, G.; Xue, X.; Zhang, L.; Sun, H.; Yao, C. A reservoir production optimization method based on principal component analysis and surrogate model. J. China Univ. Pet. (Nat. Sci. Ed.) 2020, 44, 90–97. [Google Scholar]
- Wang, S.; Zhang, L.; Wang, J.; Wu, Y.; Zhang, K. Fault-Block reservoir production optimization based on Multi-Objective algorithm. Spec. Oil Gas Reserv. 2019, 26, 124–129. [Google Scholar]
- Yin, F.; Xue, X.; Zhang, C.; Zhang, K.; Han, J.; Liu, B.; Wang, J.; Yao, J. Multifidelity genetic transfer: An efficient framework for production optimization. SPE J. 2021, 26, 1614–1635. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, J.; Ma, X.; Yao, C.; Zhang, L.; Yang, Y.; Wang, J.; Yao, J.; Zhao, H. History matching of naturally fractured reservoirs using a deep sparse autoencoder. SPE J. 2021, 26, 1700–1721. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Z.; Chen, G.; Zhang, L.; Yang, Y.; Yao, C.; Wang, J.; Yao, J. Training effective deep reinforcement learning agents for real-time life-cycle production optimization. J. Pet. Sci. Eng. 2022, 208, 109766. [Google Scholar] [CrossRef]
- Sharma, Y.K.; Majhi, A.; Kukreti, V.S.; Garg, M.O. Stock loss studies on breathing loss of gasoline. Fuel 2010, 89, 1695–1699. [Google Scholar] [CrossRef]
- Jian, S.; Han, K.; Xu, X. Risk-based inspection for large-scale crude oil tanks. J. Loss Prev. Process Ind. 2012, 25, 166–175. [Google Scholar]
- Howari, F.M. Evaporation losses and dispersion of volatile organic compounds from tank farms. Environ. Monit. Assess. 2015, 187, 273. [Google Scholar] [CrossRef]
- Adekojo Waheed, M.; Henschke, M.; Pfennig, A. Mass transfer by free and forced convection from single spherical liquid drops. Int. J. Heat Mass Transf. 2002, 45, 4507–4514. [Google Scholar] [CrossRef]
- Haelssig, J.B.; Tremblay, A.Y.; Thibault, J.; Etemad, S.G. Direct numerical simulation of interphase heat and mass transfer in multicomponent vapour–liquid flows. Int. J. Heat Mass Transf. 2010, 53, 3947–3960. [Google Scholar] [CrossRef]
- Boychenko, S.; Vovk, O.; Chernyak, L.; Akinina, K. Quality and ecological safety of motor fuels. Chem. Chem. Technol. 2007, 1. [Google Scholar] [CrossRef]
- Busahmin, B.; Maini, B.B. Measurements of Surface Tension for Mineral and Crude Oil Systems; Defect and Diffusion Forum; Trans Tech Publ: Stafa-Zurich, Switzerland, 2019; pp. 106–113. [Google Scholar]
- Hassanvand, A.; Hashemabadi, S.H.; Bayat, M. Evaluation of gasoline evaporation during the tank splash loading by CFD techniques. Int. Commun. Heat Mass Transf. 2010, 37, 907–913. [Google Scholar] [CrossRef]
- Farzaneh-Gord, M.; Nabati, A.; Rasekh, A.; Saadat-Targhi, M. The Effect of Crude Oil Type on Evaporation Loss from Khark Island Storage Tanks. Pet. Sci. Technol. 2013, 31, 866–879. [Google Scholar] [CrossRef]
- Liang, Y. A Study on Regula Pattern of Evaporation Loss in the Fixed-roof tank. Master’s Thesis, Xi’an Petroleum University, Xi’an, China, 2013. [Google Scholar]
- Gao, J. Measures for reduce the evaporation loss in ground vertical oil tank. Petrochem. Saf. Environ. Technol. 2007, 5, 31–34+68. [Google Scholar]
- Bariha, N.; Mishra, I.M.; Srivastava, V.C. Hazard analysis of failure of natural gas and petroleum gas pipelines. J. Loss Prev. Process Ind. 2016, 40, 217–226. [Google Scholar] [CrossRef]
- Zuo, M.; Huan, H.; Ma, Y.; Rao, D.; Liu, S. The Comparison and Application of Fixed-Roof Oil Tank’s Small Breathing Losses Calculation Formula. Guangdong Chem. Ind. 2014, 41, 171–172. [Google Scholar]
- Huang, W.; Liu, H.; Zhong, J.; Tao, M.; Wang, J. Study of Evaporation Loss in Loading Gasoline into Tank. J. Jiangsu Polytech. Univ. 2016, 67, 4994–5005. [Google Scholar]
- Huang, W.; Wang, Z.; Ji, H.; Zhao, L.; Li, A.; Xu, X.; Wang, Y. Experimental determination and numerical simulation of vapor diffusion and emission in loading gasoline into tank. CIESC J. 2016, 67, 4994–5005. [Google Scholar]
- Huang, W.; Gao, X.; Liu, X.; Wang, Y. Experimental Determination on Gasoline Evaporation loss from a Laboratory-Sized Cone-Roof Metal Tank. J. Jiangsu Inst. Petrochem. Technol. 1997, 3, 1–6. [Google Scholar]
- Zhu, L.; Chen, J.; Liu, Y.; Geng, R.; Yu, J. Experimental analysis of the evaporation process for gasoline. J. Loss Prev. Process Ind. 2012, 25, 916–922. [Google Scholar] [CrossRef]
- Okamoto, K.; Watanabe, N.; Hagimoto, Y.; Miwa, K.; Ohtani, H. Changes in evaporation rate and vapor pressure of gasoline with progress of evaporation. Fire Saf. J. 2009, 44, 756–763. [Google Scholar] [CrossRef]
- Abdelmajeed, M.A.; Onsa, M.H.; Rabah, A.A. Management of evaporation losses of gasoline’s storage tanks. Sudan. Eng. Soc. J. 2009, 55, 39–45. [Google Scholar]
- Gargano, M.; Cavaliere, F.; Viganò, D.; Galli, A.; Ludwig, N. A new spherical scanning system for infrared reflectography of paintings. Infrared Phys. Technology. 2017, 81, 128–136. [Google Scholar] [CrossRef]
- Gao, C.; Shi, J.; Zhao, F. Successful polymer flooding and surfactant-polymer flooding projects at Shengli Oilfield from 1992 to 2012. J. Pet. Explor. Prod. Technol. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Bai, Y.; Lv, L.; Wang, T. The application of the semi-quantitative risk assessment method to urban natural gas pipelines. J. Eng. Sci. Technol. Rev. 2013, 6, 74–77. [Google Scholar] [CrossRef]
- Blomberg, J.; Schoenmakers, P.; Brinkman, U. Gas chromatographic methods for oil analysis. J. Chromatogr. A 2002, 972, 137–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Acree, T.; Butts, R. Determination of methyl alcohol in wine by gas chromatography. Anal. Chem. 1975, 47, 747–748. [Google Scholar] [CrossRef]
- Yung, K.W. A practical study of the application of gas chromatography in the analysis of oil products. China Pet. Chem. Ind. Stand. Qual. 2019, 39, 154–155. [Google Scholar]
- Saeid, N.H.; Busahmin, B.S.; Khalid, A.A. Mixed convection jet impingement cooling of a moving plate. J. Mech. Eng. Sci. 2019, 13, 5528–5541. [Google Scholar] [CrossRef]
- Zimmerle, D.; Vaughn, T.; Bell, C.; Bennett, K.; Denshmukh, P.; Thoma, E. Detection limits of optical gas imaging for natural gas leak detection in realistic controlled conditions. Environ. Sci. Technol. 2020, 54, 11506–11514. [Google Scholar] [CrossRef]

| Serial Number | Component | Homogeneous Reduction Method | Direct Intake Method | ||
|---|---|---|---|---|---|
| Test results | Deviation | Test results | Deviation | ||
| 1 | N2 | 0.57258 | 6.95% | 0.59347 | 10.86% |
| 2 | CH4 | 0.19924 | 0.63% | 0.19819 | 0.10% |
| 3 | CH3CH3 | 0.05238 | −11.53% | 0.05103 | −13.79% |
| 4 | CH3CH2CH3 | 0.05265 | −11.06% | 0.05000 | −15.53% |
| 5 | CH3CHCH3 | 0.02742 | −7.35% | 0.02526 | −14.67% |
| 6 | CH3CH2CH2CH3 | 0.02943 | −0.92% | 0.02621 | −11.75% |
| 7 | CH3(CH2)2CH3 | 0.01420 | −6.55% | 0.01266 | −16.74% |
| 8 | CH3CH(CH3)CH2CH3 | 0.01516 | 3.11% | 0.01261 | −14.22% |
| 9 | CH3CH2CH2CH2CH3 | 0.01700 | −13.69% | 0.01377 | −30.13% |
| 10 | CH3C(CH3)2CH2CH3 | 0.00293 | −40.42% | 0.00247 | −49.78% |
| 11 | CH3CHCHCH3 | 0.00520 | 5.86% | 0.00440 | −10.33% |
| 12 | CH3CH2CH2CH(CH3)2 | - | - | - | - |
| 13 | CH3CH2CH(CH3)CH2CH3 | 0.00274 | −44.19% | 0.00235 | −52.12% |
| 14 | CH3CH2CH2CH2CH2CH3 | 0.00907 | −53.98% | 0.00757 | −61.55% |
| Total | - | 1.0000 | 1.0000 | ||
| Mean | - | −3.45% | −11.62% | ||
| Serial Number | Component | Estuary District 4–2# | Yongyi Lian Primary Settling Tank | Yongyi Lian Secondary Settlement Tank | Yongyi Lian Export Tank | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Homogeneous Reduction Method | Direct Intake Method | Boost Performance | Homogeneous Reduction Method | Direct Intake Method | Boost Performance | Homogeneous Reduction Method | Direct Intake Method | Boost Performance | Homogeneous Reduction Method | Direct Intake Method | Boost Performance | ||
| 1 | N2 | 0.9473 | 0.9448 | 0.26% | 0.5487 | 0.7704 | −40.40% | 0.8449 | 0.9426 | −11.56% | 0.7836 | 0.9762 | −24.58% |
| 2 | CH4 | 0.0295 | 0.0317 | −7.46% | 0.2816 | 0.1669 | 40.73% | 0.0677 | 0.0140 | 79.32% | 0.1279 | 0.0015 | 98.83% |
| 3 | CO2 | 0.0009 | 0.0010 | −11.11% | 0.0027 | 0.0023 | 14.81% | 0.0016 | 0.0008 | 50.00% | 0.0021 | 0.0009 | 57.14% |
| 4 | CH3CH3 | 0.0105 | 0.0112 | −6.67% | 0.0181 | 0.0094 | 48.07% | 0.0070 | 0.0015 | 78.57% | 0.0084 | 0.0005 | 94.05% |
| 5 | CH3CH2CH3 | 0.0062 | 0.0065 | −4.84% | 0.0455 | 0.0177 | 61.10% | 0.0220 | 0.0069 | 68.64% | 0.0258 | 0.0025 | 90.31% |
| 6 | C(CH3)4 | 0.0013 | 0.0013 | 0.00% | 0.0137 | 0.0051 | 62.77% | 0.0070 | 0.0031 | 55.71% | 0.0083 | 0.0015 | 81.93% |
| 7 | CH3CH2CH2CH3 | 0.0020 | 0.0019 | 5.00% | 0.0363 | 0.0125 | 65.56% | 0.0195 | 0.0098 | 49.74% | 0.0196 | 0.0049 | 75.00% |
| 8 | CH3CH(CH3)CH2CH3 | 0.0008 | 0.0007 | 12.50% | 0.0195 | 0.0057 | 70.77% | 0.0106 | 0.0068 | 35.85% | 0.0101 | 0.0037 | 63.37% |
| 9 | CH3CH2CH2CH2CH3 | 0.0006 | 0.0005 | 16.67% | 0.0150 | 0.0044 | 70.67% | 0.0084 | 0.0057 | 32.14% | 0.0067 | 0.0031 | 53.73% |
| 10 | C5H10 | 0.0000 | 0.0000 | 0.0010 | 0.0003 | 70.00% | 0.0005 | 0.0004 | 20.00% | 0.0004 | 0.0002 | 50.00% | |
| 11 | C10H10 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 100.00% | 0.0000 | 0.0000 | - | 0.0000 | 0.0000 | - | |
| 12 | CH3CH(CH3)CH2CH3 | 0.0001 | 0.0001 | 0.00% | 0.0035 | 0.0009 | 74.29% | 0.0019 | 0.0016 | 15.79% | 0.0015 | 0.0008 | 46.67% |
| 13 | CH3CH2CH(CH3)CH3 | 0.0001 | 0.0000 | 100.00% | 0.0019 | 0.0005 | 73.68% | 0.0011 | 0.0009 | 18.18% | 0.0008 | 0.0005 | 37.50% |
| 14 | CH3CH2CH2CH2CH2CH3 | 0.0001 | 0.0001 | 0.00% | 0.0039 | 0.0012 | 69.23% | 0.0022 | 0.0020 | 9.09% | 0.0015 | 0.0010 | 33.33% |
| 15 | C5H9CH3 | 0.0001 | 0.0000 | 100.00% | 0.0020 | 0.0006 | 70.00% | 0.0011 | 0.0010 | 9.09% | 0.0007 | 0.0006 | 14.29% |
| 16 | C6H6 | 0.0000 | 0.0000 | - | 0.0002 | 0.0001 | 50.00% | 0.0001 | 0.0001 | 0.00% | 0.0001 | 0.0000 | 100.00% |
| 17 | C6H12 | 0.0000 | 0.0000 | - | 0.0010 | 0.0003 | 70.00% | 0.0006 | 0.0006 | 0.00% | 0.0004 | 0.0003 | 25.00% |
| 18 | CH3CH2CH2CH(CH3)CH2CH3 | 0.0001 | 0.0000 | 100.00% | 0.0023 | 0.0006 | 73.91% | 0.0015 | 0.0011 | 26.67% | 0.0009 | 0.0007 | 22.22% |
| 19 | CH3CH2CH2CH2CH2CH2CH3 | 0.0001 | 0.0000 | 100.00% | 0.0011 | 0.0003 | 72.73% | 0.0007 | 0.0004 | 42.86% | 0.0004 | 0.0003 | 25.00% |
| 20 | C6H11CH3 | 0.0000 | 0.0000 | - | 0.0010 | 0.0003 | 70.00% | 0.0007 | 0.0004 | 42.86% | 0.0004 | 0.0003 | 25.00% |
| 21 | C7H8 | 0.0000 | 0.0000 | - | 0.0003 | 0.0001 | 66.67% | 0.0002 | 0.0001 | 50.00% | 0.0001 | 0.0000 | 100.00% |
| 22 | CH3CH(CH3)CH2CH2CH2CH3 | 0.0000 | 0.0000 | - | 0.0004 | 0.0001 | 75.00% | 0.0003 | 0.0001 | 66.67% | 0.0002 | 0.0001 | 50.00% |
| 23 | CH3CH2CH2CH2CH2CH2CH2CH3 | 0.0000 | 0.0000 | - | 0.0002 | 0.0000 | 100.00% | 0.0002 | 0.0000 | 100.00% | 0.0001 | 0.0000 | 100.00% |
| Total | - | 0.9997 | 0.9998 | 620.08% | 1.0000 | 1.0000 | 1229% | 1.0000 | 1.0000 | 876.3% | 1.0000 | 1.0000 | 1274.2% |
| Mean | - | - | 0.0435 | 26.96% | 0.0435 | 0.0435 | 53.46% | 0.0435 | 0.0435 | 38.16% | 0.0435 | 0.0435 | 55.40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Yue, Y.; Song, H.; Zhang, X.; Hu, X.; Dai, Y. Study on Homogeneous Reduction Technology in Gas Samples for Oil and Gas Loss. Separations 2023, 10, 294. https://doi.org/10.3390/separations10050294
Fan L, Yue Y, Song H, Zhang X, Hu X, Dai Y. Study on Homogeneous Reduction Technology in Gas Samples for Oil and Gas Loss. Separations. 2023; 10(5):294. https://doi.org/10.3390/separations10050294
Chicago/Turabian StyleFan, Lu, Yu Yue, Honglin Song, Xiaohan Zhang, Xinyun Hu, and Yongshou Dai. 2023. "Study on Homogeneous Reduction Technology in Gas Samples for Oil and Gas Loss" Separations 10, no. 5: 294. https://doi.org/10.3390/separations10050294
APA StyleFan, L., Yue, Y., Song, H., Zhang, X., Hu, X., & Dai, Y. (2023). Study on Homogeneous Reduction Technology in Gas Samples for Oil and Gas Loss. Separations, 10(5), 294. https://doi.org/10.3390/separations10050294





