The Role of Selective Flavonoids on Triple-Negative Breast Cancer: An Update
Abstract
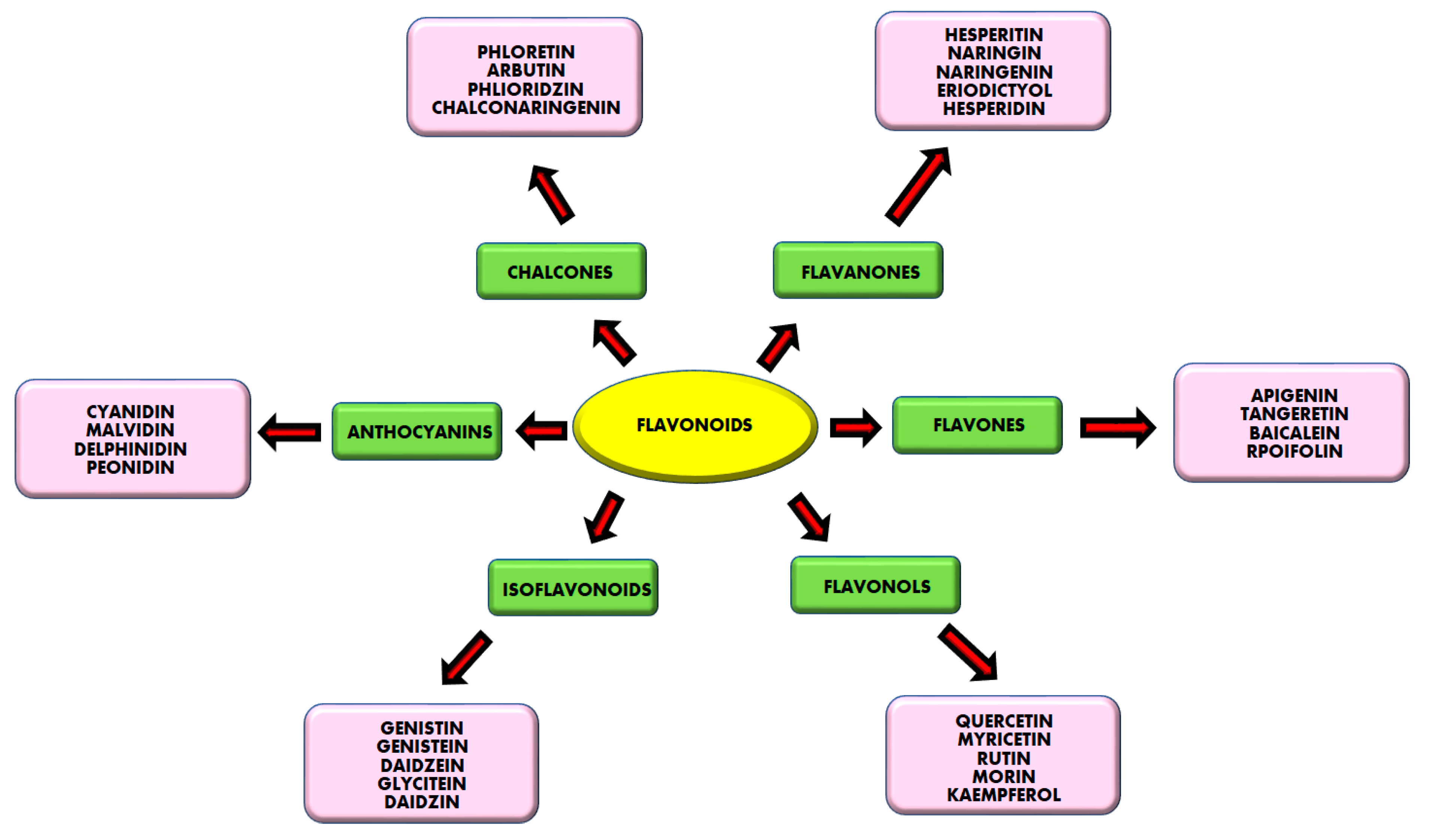
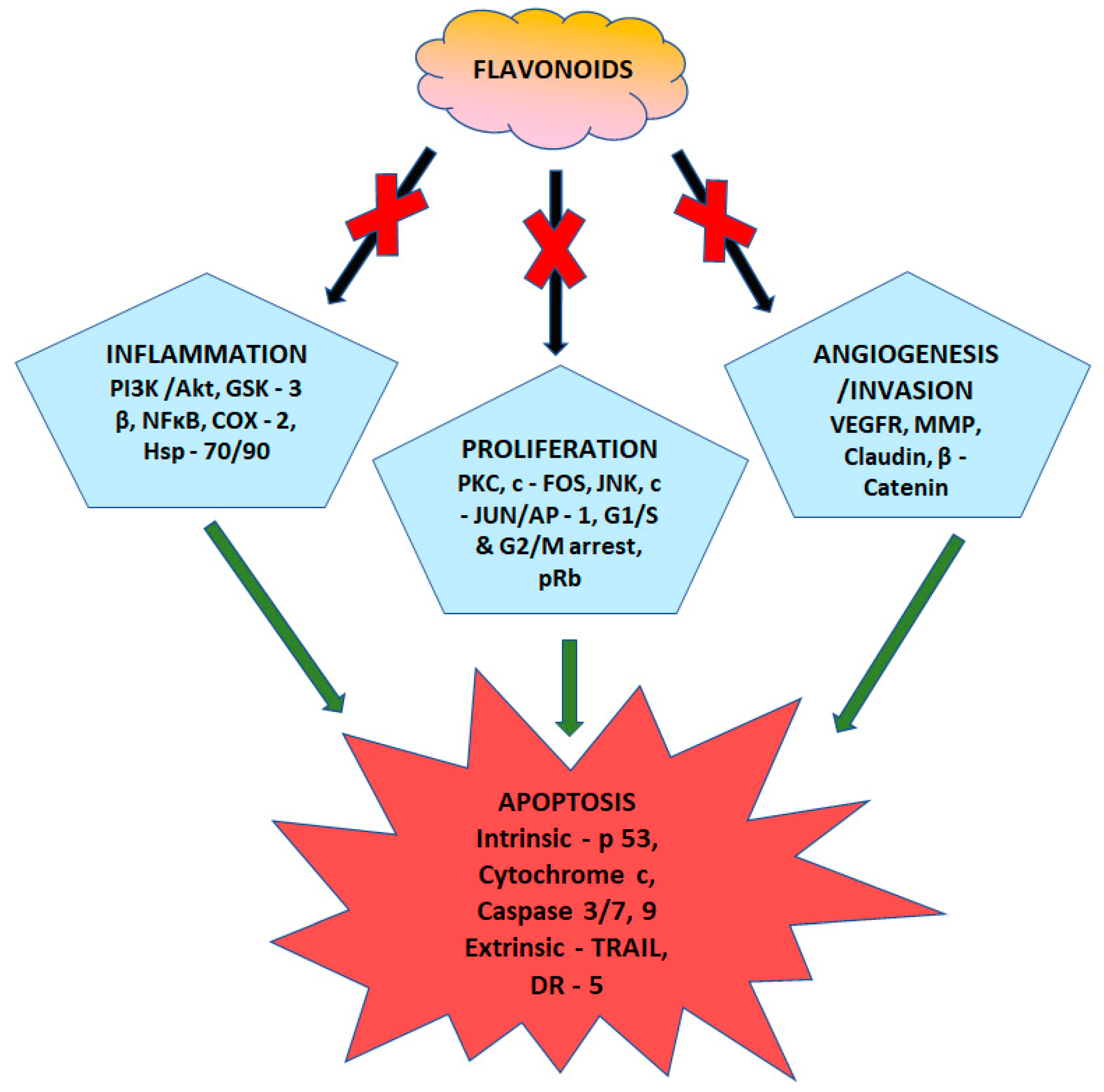
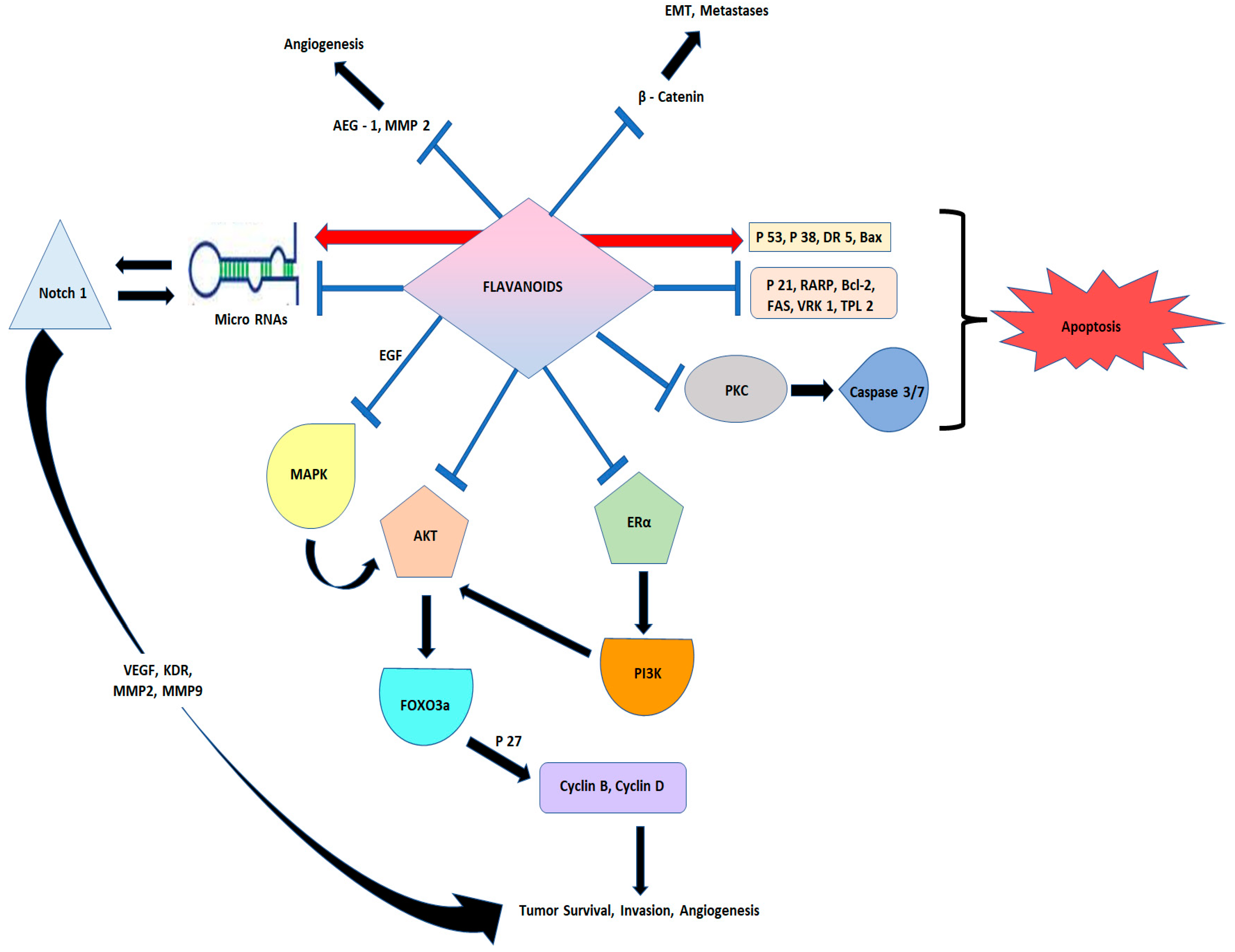
1. Introduction
2. Natural Products
2.1. Quercetin
2.2. Combined Effects of Quercetin and Curcumin
2.3. Epigallocatechin-3-Gallate (EGCG)
2.4. Fisetin
2.5. Myricetin
2.6. Silibinin
2.7. Luteolin
2.8. Genistein
2.9. Apigenin
2.10. Cyanidin-3-Glucoside
2.11. Norwogonin
2.12. Tangeretin
2.13. Sophoraflavanone G
2.14. Isorhamnetin
2.15. Genkwanin
2.16. Kaempferol
2.17. Icariin
2.18. General Extraction, Separation, and Purification Procedures for Flavonoids
2.19. Limitations
2.20. Future Perspectives of Flavonoid Research
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kroemer, G.; Pouyssegur, J. Tumor cell metabolism: Cancer’s Achilles’ heel. Cancer Cell 2008, 3, 472–482. [Google Scholar] [CrossRef]
- Graham, T.A.; Sottoriva, A. Measuring cancer evolution from the genome. J. Pathol. 2016, 241, 183–191. [Google Scholar] [CrossRef]
- Ferguson, J.L.; Turner, S.P. Bone Cancer: Diagnosis and Treatment Principles. Am. Fam. Physician 2018, 98, 205–213. [Google Scholar] [PubMed]
- Pasechnikov, V.; Chukov, S.; Fedorov, E.; Kikuste, I.; Leja, M. Gastric cancer: Prevention, screening and early diagnosis. World J. Gastroenterol. WJG 2014, 20, 13842. [Google Scholar] [CrossRef] [PubMed]
- Felder, M.; Kapur, A.; Gonzalez-Bosquet, J.; Horibata, S.; Heintz, J.; Albrecht, R.; Fass, L.; Kaur, J.; Hu, K.; Shojaei, H.; et al. MUC16 (CA125): Tumor biomarker to cancer therapy, a work in progress. Mol. Cancer 2014, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Kjällquist, U.; Acs, B.; Margolin, S.; Karlsson, E.; Kessler, L.E.; Garcia Hernandez, S.; Ekholm, M.; Lundgren, C.; Olsson, E.; Lindman, H.; et al. Real World Evaluation of the Prosigna/PAM50 Test in a Node-Negative Postmenopausal Swedish Population: A Multicenter Study. Cancers 2022, 14, 2615. [Google Scholar] [CrossRef]
- Prat Aparicio, A.; Schettini, F.; Chic, N.; Brasó Maristany, F.; Paré, L.; Pascual, T.; Conte, B.; Martínez Sáez, O.; Adamo, B.; Prat, A. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer 2021, 7, 1. [Google Scholar]
- Kao, T.-J.; Wu, C.C.; Phan, N.N.; Liu, Y.H.; Ta, H.D.; Anuraga, G.; Wu, Y.F.; Lee, K.H.; Chuang, J.Y.; Wang, C.Y. Prognoses and genomic analyses of proteasome 26S subunit, ATPase (PSMC) family genes in clinical breast cancer. Aging 2021, 13, 17970. [Google Scholar] [CrossRef]
- Park, J.H.; Ahn, J.-H.; Kim, S.-B. How shall we treat early triple-negative breast cancer (TNBC): From the current standard to upcoming immuno-molecular strategies. ESMO Open 2018, 3, e000357. [Google Scholar]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef]
- Kwa, M.J.; Adams, S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Lei, K.-F.; Han, F. Tumor microenvironment: Recent advances in various cancer treatments. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3855–3864. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.O. Cancer chemotherapy. Breastfeed. Med. 2016, 11, 164. [Google Scholar] [CrossRef]
- Liskova, A.; Koklesova, L.; Samec, M.; Smejkal, K.; Samuel, S.M.; Varghese, E.; Abotaleb, M.; Biringer, K.; Kudela, E.; Danko, J.; et al. Flavonoids in Cancer Metastasis. Cancers 2020, 12, 1498. [Google Scholar] [CrossRef]
- Hsu, Y.; Wu, L.Y.; Hou, M.F.; Tsai, E.M.; Lee, J.N.; Liang, H.L.; Jong, Y.J.; Hung, C.H.; Kuo, P.L. Glabridin, an isoflavan from licorice root, inhibits migration, invasion and angiogenesis of MDA-MB-231 human breast adenocarcinoma cells by inhibiting focal adhesion kinase/Rho signaling pathway. Mol. Nutr. Food Res. 2011, 55, 318–327. [Google Scholar] [CrossRef]
- Ci, Y.; Zhang, Y.; Liu, Y.; Lu, S.; Cao, J.; Li, H.; Zhang, J.; Huang, Z.; Zhu, X.; Gao, J.; et al. Myricetin suppresses breast cancer metastasis through down-regulating the activity of matrix metalloproteinase (MMP)-2/9. Phyther. Res. 2018, 32, 1373–1381. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, Q.; Yang, C.; Yu, P.; et al. Anti-Cancer Activities of Tea Epigallocatechin-3-Gallate in Breast Cancer Patients under Radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef]
- Noolu, B.; Gogulothu, R.; Bhat, M.; SYH Qadri, S.; Sudhakar Reddy, V.; Bhanuprakash Reddy, G.; Ismail, A. In Vivo Inhibition of Proteasome Activity and Tumour Growth by Murraya koenigii Leaf Extract in Breast Cancer Xenografts and by Its Active Flavonoids in Breast Cancer Cells. Anti-Cancer Agents Med. Chem. 2016, 16, 1605–1614. [Google Scholar] [CrossRef]
- ALaerjani, W.M.A.; Khan, K.A.; Al-Shehri, B.M.; Ghramh, H.A.; Hussain, A.; Mohammed, M.E.; Imran, M.; Ahmad, I.; Ahmad, S.; Al-Awadi, A.S. Chemical Profiling, Antioxidant, and Antimicrobial Activity of Saudi Propolis Collected by Arabian Honey Bee (Apis mellifera jemenitica) Colonies. Antioxidants 2022, 11, 1413. [Google Scholar] [CrossRef]
- Arzi, L.; Mollaei, H.; Hoshyar, R. Countering Triple Negative Breast Cancer via Impeding Wnt/β-Catenin Signaling, a Phytotherapeutic Approach. Plants 2022, 11, 2191. [Google Scholar] [CrossRef]
- Adinew, G.M.; Messeha, S.; Taka, E.; Soliman, K.F.A. The Prognostic and Therapeutic Implications of the Chemoresistance Gene BIRC5 in Triple-Negative Breast Cancer. Cancers 2022, 14, 5180. [Google Scholar] [CrossRef]
- Anuraga, G.; Wang, W.J.; Phan, N.N.; An Ton, N.T.; Ta, H.D.; Berenice Prayugo, F.; Minh Xuan, D.T.; Ku, S.C.; Wu, Y.F.; Andriani, V.; et al. Potential prognostic biomarkers of NIMA (Never in Mitosis, Gene A)-Related Kinase (NEK) family members in breast cancer. J. Pers. Med. 2021, 11, 1089. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Zakaryan, H.; Arabyan, E.; Oo, A.; Zandi, K. Flavonoids: Promising natural compounds against viral infections. Arch. Virol. 2017, 162, 2539–2551. [Google Scholar] [CrossRef]
- Pandey, M.K.; Gupta, S.C.; Karelia, D.; Gilhooley, P.J.; Shakibaei, M.; Aggarwal, B.B. Dietary nutraceuticals as backbone for bone health. Biotechnol. Adv. 2018, 36, 1633–1648. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, S. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Thangavel, C.; Liu, Y.; Shoyele, S.; Den, R.B.; Selvakumar, P.; Lakshmikuttyamma, A. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol. Carcinog. 2015, 55, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Lee, Y.H.; Sharma, A.R.; Park, J.B.; Jagga, S.; Sharma, G.; Lee, S.S.; Nam, J.S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef]
- Chen, W.-J.; Tsai, J.-H.; Hsu, L.-S.; Lin, C.-L.; Hong, H.-M.; Pan, M.-H. Quercetin blocks the aggressive phenotype of triple-negative breast cancer by inhibiting IGF1/IGF1R-mediated EMT program. J. Food Drug Anal. 2021, 29, 98. [Google Scholar] [CrossRef] [PubMed]
- Kundur, S.; Prayag, A.; Selvakumar, P.; Nguyen, H.; McKee, L.; Cruz, C.; Srinivasan, A.; Shoyele, S.; Lakshmikuttyamma, A. Synergistic anticancer action of quercetin and curcumin against triple-negative breast cancer cell lines. J. Cell Physiol. 2019, 234, 11103–11118. [Google Scholar] [CrossRef]
- Wang, H.; Lou, C.; Ma, N. Forskolin exerts anticancer roles in non-Hodgkin’s lymphomas via regulating Axin/β-catenin signaling pathway. Cancer Manag. Res. 2019, 11, 1685–1696. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2015, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, Q.; Liu, L.; Hu, Y.-Y.; Feng, Q. Potential Biological Effects of (−)-Epigallocatechin-3-gallate on the Treatment of Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2018, 62, 1–11. [Google Scholar] [CrossRef]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin—Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Cascella, M.; Barbieri, A.; Arra, C.; Cuomo, A. Current shreds of evidence on the anticancer role of EGCG in triple negative breast cancer: An update of the current state of knowledge. Infect. Agents Cancer 2020, 15, 1–6. [Google Scholar] [CrossRef]
- Grynkiewicz, G.; Demchuk, O.M. New Perspectives for Fisetin. Front. Chem. 2019, 7, 697. [Google Scholar] [CrossRef]
- Dharambir, K.; Vivek, K.G.; Hardeep, S.T.; Yerer, M.B.; Sak, K.; Anil, K.S.; Manoj, K.; Vaishali, A.; Sardul, S.S. Fisetin and Quercetin: Promising Flavonoids with Chemopreventive Potential. Biomolecules 2019, 9, 174. [Google Scholar]
- Jia, S.; Xu, X.; Zhou, S.; Chen, Y.; Ding, G.; Cao, L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress-and mitochondrial stress-dependent pathways. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Liou, C.-J.; Wei, C.-H.; Chen, Y.-L.; Cheng, C.-Y.; Wang, C.-L.; Huang, W.-C. Fisetin protects against hepatic steatosis through regulation of the Sirt1/AMPK and fatty acid β-oxidation signaling pathway in high-fat diet-induced obese mice. Cell Physiol. Biochem. 2018, 49, 1870–1884. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhu, Y.I.; McGowan, S.J.; Angelini, L.; Fuhrmann-Stroissnigg, H.; Xu, M.; Ling, Y.Y.; Melos, K.I.; Pirtskhalava, T.; Inman, C.L.; et al. Fisetin is a senotherapeutic that extends health and lifespan. Ebiomedicine 2018, 36, 18–28. [Google Scholar] [CrossRef]
- Li, J.; Gong, X.; Jiang, R.; Lin, D.; Zhou, T.; Zhang, A.; Li, H.; Zhang, X.; Wan, J.; Kuang, G.; et al. Fisetin Inhibited Growth and Metastasis of Triple-Negative Breast Cancer by Reversing Epithelial-to-Mesenchymal Transition via PTEN/Akt/GSK3β Signal Pathway. Front. Pharmacol. 2018, 9, 772. [Google Scholar] [CrossRef]
- Knickle, A.; Fernando, W.; Greenshields, A.L.; Rupasinghe, H.V.; Hoskin, D.W. Myricetin-induced apoptosis of triple-negative breast cancer cells is mediated by the iron-dependent generation of reactive oxygen species from hydrogen peroxide. Food Chem. Toxicol. 2018, 118, 154–167. [Google Scholar] [CrossRef]
- Wang, L.X.; Shi, Y.L.; Zhang, L.J.; Wang, K.R.; Xiang, L.P.; Cai, Z.Y.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Inhibitory Effects of (−)-Epigallocatechin-3-gallate on Esophageal Cancer. Molecules 2019, 24, 954. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, M.; Wang, L.; Yu, S. Anti-tumor effects and associated molecular mechanisms of myricetin. Biomed. Pharmacother. 2019, 120, 109506. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Luo, L. Effect of myricetin on primary open-angle glaucoma. Transl. Neurosci. 2018, 9, 132–141. [Google Scholar] [CrossRef]
- Sharma, P.; Khan, M.A.; Najmi, A.K.; Chaturvedi, S.; Akhtar, M. Myricetin-induced apoptosis in triple-negative breast cancer cells through inhibition of the PI3K/Akt/mTOR pathway. Med. Oncol. 2022, 39, 248. [Google Scholar] [CrossRef]
- Lingling, S.; Jianing, F.; Liu, W.; Toshihiko, H.; Yuheng, N.; Kazunori, M.; Shunji, H.; Hitomi, F.; Satoshi, O.; Takashi, I. Silibinin inhibits migration and invasion of breast cancer MDA-MB-231 cells through induction of mitochondrial fusion. Mol. Cell Biochem. 2019, 463, 189–201. [Google Scholar] [CrossRef]
- Zou, H.; Zhu, X.-X.; Zhang, G.-B.; Ma, Y.; Wu, Y.; Huang, D.-S. Silibinin: An old drug for hematological disorders. Oncotarget 2017, 8, 89307–89314. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Zhai, T.; You, J.; Chen, Y. Silibinin ameliorates hepatic lipid accumulation and oxidative stress in mice with non-alcoholic steatohepatitis by regulating CFLAR-JNK pathway. Acta Pharm. Sin. B 2019, 9, 745–757. [Google Scholar] [CrossRef]
- Prasad, R.R.; Paudel, S.; Raina, K.; Agarwal, R. Silibinin and non-melanoma skin cancers. J. Tradit. Complement. Med. 2020, 10, 236–244. [Google Scholar] [CrossRef]
- Molavi, O.; Narimani, F.; Asiaee, F.; Sharifi, S.; Tarhriz, V.; Shayanfar, A.; Hejazi, M.; Lai, R. Silibinin sensitizes chemo-resistant breast cancer cells to chemotherapy. Pharm. Biol. 2017, 55, 729–739. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.; Jeon, M.; You, D.; Lee, J.; Kim, H.J.; Bae, S.; Nam, S.J.; Lee, J.E. Silibinin inhibits triple negative breast cancer cell motility by suppressing TGF-β2 expression. Tumor Biol. 2016, 37, 11397–11407. [Google Scholar] [CrossRef]
- Ashokkumar, P.; Sudhandiran, G. Luteolin inhibits cell proliferation during Azoxymethane-induced experimental colon carcinogenesis via Wnt/ β-catenin pathway. Investig. New Drugs 2011, 29, 273–284. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Esa, N.M. Luteolin, a Bioflavonoid Inhibits Colorectal Cancer through Modulation of Multiple Signaling Pathways: A Review. Asian Pac. J. Cancer Prev. 2014, 15, 5501–5508. [Google Scholar] [CrossRef]
- Pandurangan, A.K.; Ganapasam, S. Cytotoxic effect of luteolin on human colorectal cancer cell line (HCT-15): Crucial involvement of reactive oxygen species. Middle East J. Cancer 2013, 4, 175–180. [Google Scholar]
- Pandurangan, A.K.; Sadagopan, S.K.A.; Dharmalingam, P.; Ganapasam, S. Luteolin, a Bioflavonoid, Attenuates Azoxymethane-Induced Effects on Mitochondrial Enzymes in Balb/c Mice. Asian Pac. J. Cancer Prev. 2013, 14, 6669–6672. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Zhang, B.-C.; Li, Z.; Xu, W.; Xiang, C.-H.; Ma, Y.-F. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264. 7 cells. Am. J. Transl. Res. 2018, 10, 265. [Google Scholar]
- Cook, M.T. Mechanism of metastasis suppression by luteolin in breast cancer. Breast Cancer: Targets Ther. 2018, 10, 89–100. [Google Scholar] [CrossRef]
- Kim, T.-H.; Custodio, R.J.; Cheong, J.H.; Kim, H.J.; Jung, Y.-S. Sleep Promoting Effect of Luteolin in Mice via Adenosine A1 and A2A Receptors. Biomol. Ther. 2019, 27, 584–590. [Google Scholar] [CrossRef]
- You, W.; Wu, Z.; Ye, F.; Wu, X. Cardamonin protects against adverse cardiac remodeling through mTORC1 inhibition in mice with myocardial infarction. Die Pharm. Int. J. Pharm. Sci. 2018, 73, 508–512. [Google Scholar]
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902. [Google Scholar] [CrossRef]
- Cao, D.; Zhu, G.Y.; Lu, Y.; Yang, A.; Chen, D.; Huang, H.J.; Peng, S.X.; Chen, L.W.; Li, Y.W. Luteolin suppresses epithelial-mesenchymal transition and migration of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Biomed. Pharmacother. 2020, 129, 110462. [Google Scholar] [CrossRef]
- Thangavel, P.; Puga-Olguín, A.; Rodríguez-Landa, J.F.; Zepeda, R.C. Genistein as Potential Therapeutic Candidate for Menopausal Symptoms and Other Related Diseases. Molecules 2019, 24, 3892. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R.; et al. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Xin, X.; Chen, C.; Hu, Y.-Y.; Feng, Q. Protective effect of genistein on nonalcoholic fatty liver disease (NAFLD). Biomed. Pharmacother. 2019, 117, 109047. [Google Scholar] [CrossRef]
- Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. Epigenetic Activation of BRCA1 by Genistein In Vivo and Triple Negative Breast Cancer Cells Linked to Antagonism toward Aryl Hydrocarbon Receptor. Nutrients 2019, 11, 2559. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, W.; He, W.; Liu, X.; Ding, Q.; Ling, L.; Zha, X.; Wang, S. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int. J. Mol. Med. 2012, 30, 337–343. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin: A Promising Molecule for Cancer Prevention. Pharm. Res. 2010, 27, 962–978. [Google Scholar] [CrossRef]
- Mafuvadze, B.; Benakanakere, I.; López Pérez, F.R.; Besch-Williford, C.; Ellersieck, M.R.; Hyder, S.M. Apigenin prevents development of medroxyprogesterone acetate-accelerated 7, 12-dimethylbenz (a) anthracene-induced mammary tumors in Sprague–Dawley rats. Cancer Prev. Res. 2011, 4, 1316–1324. [Google Scholar] [CrossRef]
- Way, T.-D.; Kao, M.-C.; Lin, J.-K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 2004, 279, 4479–4489. [Google Scholar] [CrossRef]
- Seo, H.-S.; Choi, H.S.; Kim, S.R.; Choi, Y.K.; Woo, S.M.; Shin, I.; Woo, J.K.; Park, S.Y.; Shin, Y.C.; Ko, S.K. Apigenin induces apoptosis via extrinsic pathway, inducing p53 and inhibiting STAT3 and NFκB signaling in HER2-overexpressing breast cancer cells. Mol. Cell. Biochem. 2012, 366, 319–334. [Google Scholar] [CrossRef]
- Chen, D.; Landis-Piwowar, K.R.; Chen, M.S.; Dou, Q.P. Inhibition of proteasome activity by the dietary flavonoid apigenin is associated with growth inhibition in cultured breast cancer cells and xenografts. Breast Cancer Res. 2007, 9, 1–8. [Google Scholar] [CrossRef]
- Chen, T.; Li, L.-P.; Lu, X.-Y.; Jiang, H.-D.; Zeng, S. Absorption and excretion of luteolin and apigenin in rats after oral administration of Chrysanthemum morifolium extract. J. Agric. Food Chem. 2007, 55, 273–277. [Google Scholar] [CrossRef]
- Gupta, S.; Afaq, F.; Mukhtar, H. Selective Growth-Inhibitory, Cell-Cycle Deregulatory and Apoptotic Response of Apigenin in Normal versus Human Prostate Carcinoma Cells. Biochem. Biophys. Res. Commun. 2001, 287, 914–920. [Google Scholar] [CrossRef]
- Kikuchi, H.; Yuan, B.; Hu, X.; Okazaki, M. Chemopreventive and anticancer activity of flavonoids and its possibility for clinical use by combining with conventional chemotherapeutic agents. Am. J. Cancer Res. 2019, 9, 1517–1535. [Google Scholar]
- Tan, J.; Li, Y.; Hou, D.-X.; Wu, S. The Effects and Mechanisms of Cyanidin-3-Glucoside and Its Phenolic Metabolites in Maintaining Intestinal Integrity. Antioxidants 2019, 8, 479. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, H.J.; Sparrow, J.R. Quercetin and cyanidin-3-glucoside protect against photooxidation and photodegradation of A2E in retinal pigment epithelial cells. Exp. Eye Res. 2017, 160, 45–55. [Google Scholar] [CrossRef]
- Liu, M.; Du, Y.; Li, H.; Wang, L.; Ponikwicka-Tyszko, D.; Lebiedzinska, W.; Pilaszewicz-Puza, A.; Liu, H.; Zhou, L.; Fan, H.; et al. Cyanidin-3-o-glucoside pharmacologically inhibits tumorigenesis via estrogen receptor β in melanoma mice. Front. Oncol. 2019, 9, 1–18. [Google Scholar] [CrossRef]
- Liang, L.; Liu, X.; He, J.; Shao, Y.; Liu, J.; Wang, Z.; Xia, L.; Han, T.; Wu, P. Cyanidin-3-glucoside induces mesenchymal to epithelial transition via activating Sirt1 expression in triple negative breast cancer cells. Biochimie 2019, 162, 107–115. [Google Scholar] [CrossRef]
- El-Hafeez, A.A.A.; Khalifa, H.O.; Mahdy, E.A.; Sharma, V.; Hosoi, T.; Ghosh, P.; Ozawa, K.; Montano, M.M.; Fujimura, T.; Ibrahim, A.R.; et al. Anticancer effect of nor-wogonin (5, 7, 8-trihydroxyflavone) on human triple-negative breast cancer cells via downregulation of TAK1, NF-κB, and STAT3. Pharmacol. Rep. 2019, 71, 289–298. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Zhou, L.; Liu, G.; Wu, Q.; Chen, C. Norwogonin flavone suppresses the growth of human colon cancer cells via mitochondrial mediated apoptosis, autophagy induction and triggering G2/M phase cell cycle arrest. J. Buon 2020, 25, 1449–1454. [Google Scholar]
- Choi, H.J.; Song, H.-H.; Lee, J.-S.; Ko, H.-J.; Song, J.-H. Inhibitory Effects of Norwogonin, Oroxylin A, and Mosloflavone on Enterovirus 71. Biomol. Ther. 2016, 24, 552–558. [Google Scholar] [CrossRef]
- Cheng, Y.-P.; Li, S.; Chuang, W.L.; Li, C.H.; Chen, G.J.; Chang, C.C.; Or, C.H.; Lin, P.Y.; Chang, C.C. Blockade of STAT3 signaling contributes to anticancer effect of 5-acetyloxy-6, 7, 8, 4′-tetra-methoxyflavone, a tangeretin derivative, on human glioblastoma multiforme cells. Int. J. Mol. Sci. 2019, 20, 3366. [Google Scholar] [CrossRef]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef]
- Surichan, S.; Arroo, R.R.; Tsatsakis, A.M.; Androutsopoulos, V.P. Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1–mediated metabolism to the product 4′ hydroxy tangeretin. Toxicol. Vitr. 2018, 50, 274–284. [Google Scholar] [CrossRef]
- Arivazhagan, L.; Pillai, S.S. Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7, 12-dimethylbenz (α) anthracene-induced rat mammary carcinoma. J. Nutr. Biochem. 2014, 25, 1140–1153. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, L.; Sun, Y.; Wang, T.; Wang, B. Tangeretin enhances radiosensitivity and inhibits the radiation-induced epithelial-mesenchymal transition of gastric cancer cells. Oncol. Rep. 2015, 34, 302–310. [Google Scholar] [CrossRef]
- Martínez Conesa, C.; Vicente Ortega, V.; Yáñez Gascón, M.J.; Alcaraz Baños, M.; Canteras Jordana, M.; Benavente-García, O.; Castillo, J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H.; Hanif, N.; Ikawati, M. Integrative bioinformatics study of tangeretin potential targets for preventing metastatic breast cancer. Evid.-Based Complement. Altern. Med. 2021, 2021, 2234554. [Google Scholar] [CrossRef]
- Huang, W.-C.; Gu, P.-Y.; Fang, L.-W.; Huang, Y.-L.; Lin, C.-F.; Liou, C.-J. Sophoraflavanone G from Sophora flavescens induces apoptosis in triple-negative breast cancer cells. Phytomedicine 2019, 61, 152852. [Google Scholar] [CrossRef]
- Guo, C.; Yang, L.; Wan, C.X.; Xia, Y.Z.; Zhang, C.; Chen, M.H.; Wang, Z.D.; Li, Z.R.; Li, X.M.; Geng, Y.D.; et al. Anti-neuroinflammatory effect of Sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways. Phytomedicine 2016, 23, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-L.; Sun, S.-C.; He, J.-M.; Lan, J.-E.; Gibbons, S.; Mu, Q. Synergism of sophoraflavanone G with norfloxacin against effluxing antibiotic-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents 2020, 56, 106098. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, D.; Guo, M.; Li, H.; Wang, Q. Sophoraflavanone G suppresses the progression of triple-negative breast cancer via the inactivation of EGFR–PI3K–AKT signaling. Drug Dev. Res. 2022, 83, 1138–1151. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Lin, Y.-C.; Lin, Y.-L.; Li, C.-C.; Chuang, C.-H. Comparing the metabolism of quercetin in rats, mice and gerbils. Eur. J. Nutr. 2016, 55, 413–422. [Google Scholar] [CrossRef]
- Zhang, H.-W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Jiang, X.; Zhang, H.; Gao, Z.; Li, Y.; Fu, R.; Li, L.; Li, J.; Cui, H.; et al. ROS-mediated activation and mitochondrial translocation of CaMKII contributes to Drp1-dependent mitochondrial fission and apoptosis in triple-negative breast cancer cells by isorhamnetin and chloroquine. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef]
- Luczak, E.D.; Anderson, M.E. CaMKII oxidative activation and the pathogenesis of cardiac disease. J. Mol. Cell. Cardiol. 2014, 73, 112–116. [Google Scholar] [CrossRef]
- Zhu, L.J.; Klutho, P.J.; Scott, J.A.; Xie, L.; Luczak, E.D.; Dibbern, M.E.; Prasad, A.M.; Jaffer, O.A.; Venema, A.N.; Nguyen, E.K.; et al. Oxidative activation of the Ca2+/calmodulin-dependent protein kinase II (CaMKII) regulates vascular smooth muscle migration and apoptosis. Vascul. Pharmacol. 2014, 60, 75–83. [Google Scholar] [CrossRef]
- Dilshara, M.G.; Bandara, M.M.; Jayawardana, B.C.; Vidanarachchi, J.K.; Jayasooriya, R.G.; Kim, G.Y. Silibinin sensitizes TRAIL-mediated apoptosis by upregulating DR5 through ROS-induced endoplasmic reticulum stress-Ca2+-CaMKII-Sp1 pathway. Oncotarget 2018, 9, 10324. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Liu, W.; Ji, Y.; Sun, Y.; Si, L.; Fu, J.; Hayashi, T.; Onodera, S.; Ikejima, T. Estrogen receptors participate in silibinin-caused nuclear translocation of apoptosis-inducing factor in human breast cancer MCF-7 cells. Arch. Biochem. Biophys. 2020, 689, 108458. [Google Scholar] [CrossRef]
- Vachetta, V.S.; Marder, M.; Troncoso, M.F.; Elola, M.T. Opportunities, obstacles and current challenges of flavonoids for luminal and triple-negative breast cancer therapy. Eur. J. Med. Chem. Rep. 2022, 6, 100077. [Google Scholar] [CrossRef]
- Zhou, R.; Chen, H.; Chen, J.; Chen, X.; Wen, Y.; Xu, L. Extract from Astragalus membranaceus inhibit breast cancer cells proliferation via PI3K/AKT/mTOR signaling pathway. BMC Complement. Altern. Med. 2018, 18, 83. [Google Scholar] [CrossRef]
- Rivera Rivera, A.; Castillo-Pichardo, L.; Gerena, Y.; Dharmawardhane, S. Anti-Breast Cancer Potential of Quercetin via the Akt/AMPK/Mammalian Target of Rapamycin (mTOR) Signaling Cascade. PLoS ONE 2016, 11, e0157251. [Google Scholar] [CrossRef]
- Won, Y.-S.; Seo, K.-I. Lupiwighteone induces caspase-dependent and -independent apoptosis on human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. Food Chem. Toxicol. 2020, 135, 110863. [Google Scholar] [CrossRef]
- Wang, X.; Song, Z.J.; He, X.; Zhang, R.Q.; Zhang, C.F.; Li, F.; Wang, C.Z.; Yuan, C.S. Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APCMin/+ mice. Int. Immunopharmacol. 2015, 29, 701–707. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alam, W.; Aschner, M.; Abdullah, U.; Alsharif, K.F.; Saso, L. Flavonoids Targeting the mTOR Signaling Cascades in Cancer: A Potential Crosstalk in Anti-Breast Cancer Therapy. Oxidative Med. Cell. Longev. 2022, 2022, 4831833. [Google Scholar] [CrossRef]
- Li, H.; Ji, H.S.; Kang, J.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Lee, C.H.; Lee, I.K.; Yun, B.S.; Jeong, T.S. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic β-cell function and suppressing hepatic lipid accumulation in db/db mice. J. Agric. Food Chem. 2015, 63, 7198–7210. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.V.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer Agents: From Plant Extracts to Phytochemicals in Healing Promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Pei, J.; Chen, A.; Zhao, L.; Cao, F.; Ding, G.; Xiao, W. One-Pot Synthesis of Hyperoside by a Three-Enzyme Cascade Using a UDP-Galactose Regeneration System. J. Agric. Food Chem. 2017, 65, 6042–6048. [Google Scholar] [CrossRef]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimaraes, J.T.; Keating, E.; Martel, F. The chemopreventive effect of the dietary compound kaempferol on the MCF-7 human breast cancer cell line is dependent on inhibition of glucose cellular uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef]
- Zhu, L.; Xue, L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 629–634. [Google Scholar] [CrossRef]
- Li, S.; Yan, T.; Deng, R.; Jiang, X.; Xiong, H.; Wang, Y.; Yu, Q.; Wang, X.; Chen, C.; Zhu, Y. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. Onco. Targets. Ther. 2017, 10, 4809. [Google Scholar] [CrossRef]
- Lee, S.-B.; Shin, J.-S.; Han, H.-S.; Lee, H.-H.; Park, C.; Lee, K.-T. Kaempferol 7-O-β-D-glucoside isolated from the leaves of Cudrania tricuspidata inhibits LPS-induced expression of pro-inflammatory mediators through inactivation of NF-κB, AP-1, and JAK-STAT in RAW 264.7 macrophages. Chem. Biol. Interact. 2018, 284, 101–111. [Google Scholar] [CrossRef]
- Lee, G.-A.; Choi, K.-C.; Hwang, K.-A. Treatment with phytoestrogens reversed triclosan and bisphenol A-induced anti-apoptosis in breast cancer cells. Biomol. Ther. 2018, 26, 503. [Google Scholar] [CrossRef]
- Yi, X.; Zuo, J.; Tan, C.; Xian, S.; Luo, C.; Chen, S.; Yu, L.; Luo, Y. Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in mcf-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 210–215. [Google Scholar] [CrossRef]
- Lee, G.-A.; Choi, K.-C.; Hwang, K.-A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef]
- Zheng, L.; Zhu, L.; Zhao, M.; Shi, J.; Li, Y.; Yu, J.; Jiang, H.; Wu, J.; Tong, Y.; Liu, Y.; et al. In Vivo Exposure of Kaempferol Is Driven by Phase II Metabolic Enzymes and Efflux Transporters. AAPS J. 2016, 18, 1289–1299. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Y.; Yang, D.; Yu, Y.; Guo, H.; Zhao, Z.; Zhang, B.; Yin, X. Inhibitory effects of kaempferol on the invasion of human breast carcinoma cells by downregulating the expression and activity of matrix metalloproteinase-9. Biochem. Cell Biol. 2015, 93, 16–27. [Google Scholar] [CrossRef]
- Sharma, A.; Sinha, S.; Keswani, H.; Shrivastava, N. Kaempferol and Apigenin suppresses the stemness properties of TNBC cells by modulating Sirtuins. Mol. Divers. 2022, 26, 3225–3240. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020, 111, 4242–4256. [Google Scholar] [CrossRef]
- Su, B.; Ye, H.; You, X.; Ni, H.; Chen, X.; Li, L. Icariin alleviates murine lupus nephritis via inhibiting NF-κB activation pathway and NLRP3 inflammasome. Life Sci. 2018, 208, 26–32. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Cole, A.; McLaughlin, S.; Du, W. Icariin improves Fanconi anemia hematopoietic stem cell function through SIRT6-mediated NF-kappa B inhibition. Cell Cycle 2018, 17, 367–376. [Google Scholar] [CrossRef]
- Tran, L.; Theodorescu, D. Determinants of resistance to checkpoint inhibitors. Int. J. Mol. Sci. 2020, 21, 1594. [Google Scholar] [CrossRef]
- Bi, Z.; Zhang, W.; Yan, X. Anti-inflammatory and immunoregulatory effects of icariin and icaritin. Biomed. Pharmacother. 2022, 151, 113180. [Google Scholar] [CrossRef]
- Köstlin, N.; Hofstädter, K.; Ostermeir, A.L.; Spring, B.; Leiber, A.; Haen, S.; Abele, H.; Bauer, P.; Pollheimer, J.; Hartl, D.; et al. Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype. J. Immunol. 2016, 196, 1132–1145. [Google Scholar] [CrossRef]
- Sadik, S.B.S.; Sivaprakasam, P.; Ramasami, N.; Pandurangan, A.K. The Molecular Mechanisms Involved in Suppressing Triple Negative Breast Cancer Using Natural Agents. In Handbook of Research on Natural Products and Their Bioactive Compounds as Cancer Therapeutics; IGI Global: Hershey, PA, USA, 2022; pp. 45–71. [Google Scholar] [CrossRef]
- Lopez, M.; Martınez, F.; Del Valle, C.; Orte, C.; Miro, M. Analysis of phenolic constituents of biological interest in red wines by high-performance liquid chromatography. J. Chromatogr. A 2001, 922, 359–363. [Google Scholar] [CrossRef]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Stevens, J.F.; Wollenweber, E.; Ivancic, M.; Hsu, V.L.; Sundberg, S.; Deinzer, M.L. Leaf surface flavonoids of Chrysothamnus. Phytochemistry 1999, 51, 771–780. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Nanos, C.G.; Vervoort, J.; Stalikas, C.D. Analytical procedure for the in-vial derivatization? Extraction of phenolic acids and flavonoids in methanolic and aqueous plant extracts followed by gas chromatography with mass-selective detection. J. Chromatogr. A 2004, 1041, 11–18. [Google Scholar] [CrossRef]
- Kempkes, M.; Golka, K.; Reich, S.; Reckwitz, T.; Bolt, H.M. Glutathione S-transferase GSTM1 and GSTT1 null genotypes as potential risk factors for urothelial cancer of the bladder. Arch. Toxicol. 1996, 71, 123–126. [Google Scholar] [CrossRef]
- Lahtinen, M.; Lempa, K.; Salminen, J.-P.; Pihlaja, K. HPLC analysis of leaf surface flavonoids for the preliminary classification of birch species. Phytochem. Anal. 2006, 17, 197–203. [Google Scholar] [CrossRef]
- Lin, M.-C.; Tsai, M.-J.; Wen, K.-C. Supercritical fluid extraction of flavonoids from Scutellariae Radix. J. Chromatogr. A 1999, 830, 387–395. [Google Scholar] [CrossRef]
- Scalia, S.; Giuffreda, L.; Pallado, P. Analytical and preparative supercritical fluid extraction of Chamomile flowers and its comparison with conventional methods. J. Pharm. Biomed. Anal. 1999, 21, 549–558. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Palma, M.; Barroso, C.G. Determination of catechins by means of extraction with pressurized liquids. J. Chromatogr. A 2004, 1026, 19–23. [Google Scholar] [CrossRef]
- Song, J.-Z.; Mo, S.-F.; Yip, Y.-K.; Qiao, C.-F.; Han, Q.-B.; Xu, H.-X. Development of microwave assisted extraction for the simultaneous determination of isoflavonoids and saponins in radix astragali by high performance liquid chromatography. J. Sep. Sci. 2007, 30, 819–824. [Google Scholar] [CrossRef]
- Gao, M.; Song, B.-Z.; Liu, C.-Z. Dynamic microwave-assisted extraction of flavonoids from Saussurea medusa Maxim cultured cells. Biochem. Eng. J. 2006, 32, 79–83. [Google Scholar] [CrossRef]
- Montedoro, G.; Servili, M.; Baldioli, M.; Miniati, E. Simple and hydrolyzable phenolic compounds in virgin olive oil. 1. Their extraction, separation, and quantitative and semiquantitative evaluation by HPLC. J. Agric. Food Chem. 1992, 40, 1571–1576. [Google Scholar] [CrossRef]
- Guillén, D.; Barroso, C.; Pérez-Bustamante, J. Automation of sample preparation as a preliminary stage in the high-performance liquid chromatographic determination of polyphenolic compounds in sherry wines. J. Chromatogr. A 1996, 730, 39–46. [Google Scholar] [CrossRef]
- Ragazzi, E.; Veronese, G. Quantitative analysis of phenolic compounds after thin-layer chromatographic separation. J. Chromatogr. A 1973, 77, 369–375. [Google Scholar] [CrossRef]
- Schulz, J.M.; Herrmann, K. Analysis of hydroxybenzoic and hydroxycinnamic acids in plant material: I. Sample preparation and thin-layer chromatography. J. Chromatogr. A 1980, 195, 85–94. [Google Scholar] [CrossRef]
- Watson, D.G.; Pitt, A.R. Analysis of flavonoids in tablets and urine by gas chromatography/mass spectrometry and liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 1998, 12, 153–156. [Google Scholar] [CrossRef]
- Queiroz, E.F.; Ioset, J.; Ndjoko, K.; Guntern, A.; Foggin, C.M.; Hostettmann, K. On-line identification of the bioactive compounds from Blumea gariepina by HPLC-UV-MS and HPLC-UV-NMR, combined with HPLC-micro-fractionation. Phytochem. Anal. An Int. J. Plant Chem. Biochem. Tech. 2005, 16, 166–174. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, L.; Luo, H.; Zhou, L.; Li, C.; Xu, X. Direct extraction of specific pharmacophoric flavonoids from gingko leaves using a molecularly imprinted polymer for quercetin. J. Chromatogr. A 2001, 934, 1–11. [Google Scholar] [CrossRef]
- Xiao, H.; Krucker, M.; Albert, K.; Liang, X. Determination and identification of isoflavonoids in Radix astragali by matrix solid-phase dispersion extraction and high-performance liquid chromatography with photodiode array and mass spectrometric detection. J. Chromatogr. A 2004, 1032, 117–124. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, U.; Zhang, T.; Yang, F.; Ito, Y. Separation of flavonoids from the seeds of Vernonia anthelmintica Willd by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1049, 219–222. [Google Scholar] [CrossRef]
- Soczewiński, E.; Wojciak-Kosior, M.; Matysik, G. Analysis of glycosides and aglycones of flavonoid compounds by double-development thin-layer chromatography. JPC–J. Planar Chromatogr. TLC 2004, 17, 261–263. [Google Scholar] [CrossRef]
- Jamshidi, A.; Adjvadi, M.; Husain, S.W. Determination of kaempferol and quercetin in an extract of Ginkgo biloba leaves by high-performance thin-layer chromatography (HPTLC). J. Planar Chromatogr. TLC 2000, 13, 57–59. [Google Scholar]
- Bagul, M.; Srinivasa, H.; Padh, H.; Rajani, M. A rapid densitometric method for simultaneous quantification of gallic acid and ellagic acid in herbal raw materials using HPTLC. J. Sep. Sci. 2005, 28, 581–584. [Google Scholar] [CrossRef]
- Głowniak, K.; Zgórka, G.; Kozyra, M. Solid-phase extraction and reversed-phase high-performance liquid chromatography of free phenolic acids in some Echinacea species. J. Chromatogr. A 1996, 730, 25–29. [Google Scholar] [CrossRef]
- Herrero, M.; Ibáñez, E.; Cifuentes, A. Analysis of natural antioxidants by capillary electromigration methods. J. Sep. Sci. 2005, 28, 883–897. [Google Scholar] [CrossRef]
- Lee, B.-L.; Ong, C.N. Comparative analysis of tea catechins and theaflavins by high-performance liquid chromatography and capillary electrophoresis. J. Chromatogr. A 2000, 881, 439–447. [Google Scholar] [CrossRef]
- Owades, J.L.; Rubin, G.; Brenner, M.W. Food tannins measurement, determination of food tannins by ultraviolet spectrophotometry. J. Agric. Food Chem. 1958, 6, 44–46. [Google Scholar] [CrossRef]
- Mabry, T.J.; Markham, R.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1970. [Google Scholar]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- OFolin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brune, M.; Hallberg, L.; Skånberg, A.-B. Determination of Iron-Binding Phenolic Groups in Foods. J. Food Sci. 1991, 56, 128–131. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Swain, T. Changes in tannins in ripening fruits. Phytochemistry 1963, 2, 371–383. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Carnart, A. Teneurs en principaux falvonoïdes des fleurs et des feuilles de Crataegus monogyna Jacq. et de Crataegus laevigata (Poiret) DC. en fonction de la période de vegetation. Plantes Médicinales Phytothérapie 1991, 25, 12–16. [Google Scholar]
- Zaporozhets, O.A.; Krushynska, O.A.; Lipkovska, N.A.; Barvinchenko, V.N. A New Test Method for the Evaluation of Total Antioxidant Activity of Herbal Products. J. Agric. Food Chem. 2004, 52, 21–25. [Google Scholar] [CrossRef]
- Amawi, H.; Ashby, C.R., Jr.; Tiwari, A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer 2017, 36, 50. [Google Scholar] [CrossRef]
- Zhou, J.; Du, G.; Chen, J. Novel fermentation processes for manufacturing plant natural products. Curr. Opin. Biotechnol. 2014, 25, 17–23. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. DIETARY FLAVONOIDS: Bioavailability, Metabolic Effects, and Safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Zhan, Y.; Liu, L.; Xu, Y.; Xu, T.; Liu, T. Preparative Isolation and Purification of Five Flavonoid Glycosides and One Benzophenone Galloyl Glycoside from Psidium guajava by High-Speed Counter-Current Chromatography (HSCCC). Molecules 2013, 18, 15648–15661. [Google Scholar] [CrossRef]
- Markham, K. Isolation techniques for flavonoids. In The Flavonoids; Springer: Berlin/Heidelberg, Germany, 1975; pp. 1–44. [Google Scholar]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Quattrocchio, F.; Baudry, A.; Lepiniec, L.; Grotewold, E. The regulation of flavonoid biosynthesis. In The Science of Flavonoids; Springer: Berlin/Heidelberg, Germany, 2006; pp. 97–122. [Google Scholar]
- Yu, C.-P.; Shia, C.-S.; Tsai, S.-Y.; Hou, Y.-C. Pharmacokinetics and Relative Bioavailability of Flavonoids between Two Dosage Forms of Gegen-Qinlian-Tang in Rats. Evid.-Based Complement. Altern. Med. 2012, 2012, 308018. [Google Scholar] [CrossRef]
- Cai, X.; Fang, Z.; Dou, J.; Yu, A.; Zhai, G. Bioavailability of Quercetin: Problems and Promises. Curr. Med. Chem. 2013, 20, 2572–2582. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F.; Tomás-Barberán, F.A. In Vitro Availability of Flavonoids and Other Phenolics in Orange Juice. J. Agric. Food Chem. 2001, 49, 1035–1041. [Google Scholar] [CrossRef]
- Mandalari, G.; Tomaino, A.; Rich, G.T.; Curto, R.L.; Arcoraci, T.; Martorana, M.; Bisignano, C.; Saija, A.; Parker, M.L.; Waldron, K.W.; et al. Polyphenol and nutrient release from skin of almonds during simulated human digestion. Food Chem. 2010, 122, 1083–1088. [Google Scholar] [CrossRef]
- King, R.A.; Bursill, D.B. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am. J. Clin. Nutr. 1998, 67, 867–872. [Google Scholar] [CrossRef]
- Gee, J.M.; DuPont, M.S.; Day, A.J.; Plumb, G.W.; Williamson, G.; Johnson, I.T. Intestinal Transport of Quercetin Glycosides in Rats Involves Both Deglycosylation and Interaction with the Hexose Transport Pathway. J. Nutr. 2000, 130, 2765–2771. [Google Scholar] [CrossRef]
- Aura, A.-M.; Martin-Lopez, P.; O’Leary, K.A.; Williamson, G.; Oksman-Caldentey, K.M.; Poutanen, K.; Santos-Buelga, C. In vitro metabolism of anthocyanins by human gut microflora. Eur. J. Nutr. 2005, 44, 133–142. [Google Scholar] [CrossRef]
- Gonthier, M.-P.; Verny, M.-A.; Besson, C.; Rémésy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jung, E.-A.; Sohng, I.-S.; Han, J.-A.; Kim, T.-H.; Han, M.J. Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch. Pharmacal Res. 1998, 21, 17–23. [Google Scholar] [CrossRef]
- Ueno, I.; Nakano, N.; Hirono, I. Metabolic fate of [14C] quercetin in the ACI rat. Jpn. J. Exp. Med. 1983, 53, 41–50. [Google Scholar]
- Boulton, D.W.; Walle, U.K.; Walle, T. Fate of the flavonoid quercetin in human cell lines: Chemical instability and metabolism. J. Pharm. Pharmacol. 1999, 51, 353–359. [Google Scholar] [CrossRef]
- Zhu, H.-J.; Brinda, B.J.; Chavin, K.D.; Bernstein, H.J.; Patrick, K.S.; Markowitz, J.S. An Assessment of Pharmacokinetics and Antioxidant Activity of Free Silymarin Flavonolignans in Healthy Volunteers: A Dose Escalation Study. Drug Metab. Dispos. 2013, 41, 1679–1685. [Google Scholar] [CrossRef]
- Dixit, N.; Baboota, S.; Kohli, K.; Ahmad, S.; Ali, J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J. Pharmacol. 2007, 39, 172. [Google Scholar] [CrossRef]
- Gawande, S.; Kale, A.; Kotwal, S. Effect of nutrient mixture and black grapes on the pharmacokinetics of orally administered (−) epigallocatechin-3-gallate from green tea extract: A human study. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 802–808. [Google Scholar] [CrossRef]
- Nunes, T.; Almeida, L.; Rocha, J.F.; Falcão, A.; Fernandes-Lopes, C.; Loureiro, A.I.; Wright, L.; Vaz-da-Silva, M.; Soares-da-Silva, P. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J. Clin. Pharmacol. 2009, 49, 1477–1482. [Google Scholar] [CrossRef]
- Decker, A. Phenolics: Prooxidants or Antioxidants? Nutr. Rev. 1997, 55, 396–398. [Google Scholar] [CrossRef]
- Fresco, P.; Borges, F.; Diniz, C.; Marques, M. New insights on the anticancer properties of dietary polyphenols. Med. Res. Rev. 2006, 26, 747–766. [Google Scholar] [CrossRef]
- Křížková, J.; Burdová, K.; Stiborová, M.; Křen, V.; Hodek, P. The effects of selected flavonoids on cytochromes P450 in rat liver and small intestine. Interdiscip. Toxicol. 2009, 2, 201–204. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, M. Mutual interactions between flavonoids and enzymatic and transporter elements responsible for flavonoid disposition via phase II metabolic pathways. RSC Adv. 2012, 2, 7948–7963. [Google Scholar] [CrossRef]
- Xiao, J.; Ni, X.; Kai, G.; Chen, X. A review on structure–activity relationship of dietary polyphenols inhibiting α-amylase. Crit. Rev. Food Sci. Nutr. 2013, 53, 497–506. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in dietary polyphenols as α-glucosidases inhibitors: A review on structure-activity relationship aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef]
- Xiao, J.B.; Huo, J.L.; Yang, F.; Chen, X.Q. Noncovalent Interaction of Dietary Polyphenols with Bovine Hemoglobin in Vitro: Molecular Structure/Property–Affinity Relationship Aspects. J. Agric. Food Chem. 2011, 59, 8484–8490. [Google Scholar] [CrossRef]
- Morris, M.E.; Zhang, S. Flavonoid–drug interactions: Effects of flavonoids on ABC transporters. Life Sci. 2006, 78, 2116–2130. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G. A review of dietary polyphenol-plasma protein interactions: Characterization, influence on the bioactivity, and structure-affinity relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef]
- Xiao, J.; Cao, H.; Wang, Y.; Zhao, J.; Wei, X. Glycosylation of Dietary Flavonoids Decreases the Affinities for Plasma Protein. J. Agric. Food Chem. 2009, 57, 6642–6648. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chinnikrishnan, P.; Aziz Ibrahim, I.A.; Alzahrani, A.R.; Shahzad, N.; Sivaprakasam, P.; Pandurangan, A.K. The Role of Selective Flavonoids on Triple-Negative Breast Cancer: An Update. Separations 2023, 10, 207. https://doi.org/10.3390/separations10030207
Chinnikrishnan P, Aziz Ibrahim IA, Alzahrani AR, Shahzad N, Sivaprakasam P, Pandurangan AK. The Role of Selective Flavonoids on Triple-Negative Breast Cancer: An Update. Separations. 2023; 10(3):207. https://doi.org/10.3390/separations10030207
Chicago/Turabian StyleChinnikrishnan, Pooja, Ibrahim Abdel Aziz Ibrahim, Abdullah R. Alzahrani, Naiyer Shahzad, Prathibha Sivaprakasam, and Ashok Kumar Pandurangan. 2023. "The Role of Selective Flavonoids on Triple-Negative Breast Cancer: An Update" Separations 10, no. 3: 207. https://doi.org/10.3390/separations10030207
APA StyleChinnikrishnan, P., Aziz Ibrahim, I. A., Alzahrani, A. R., Shahzad, N., Sivaprakasam, P., & Pandurangan, A. K. (2023). The Role of Selective Flavonoids on Triple-Negative Breast Cancer: An Update. Separations, 10(3), 207. https://doi.org/10.3390/separations10030207








