Concurrent Optimization of Ultrasonic-Assisted Extraction of Total Phenolic Compounds and In Vitro Anticancer and Antioxidant Potential of Pulicaria schimperi (Aerial Parts) Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Apparatus and Reagents
2.3. Extraction Process
2.3.1. Ultrasound-Assisted Extraction of Aerial Parts of P. schimperi
2.3.2. Conventional Solvent Extraction (CSE)
2.4. Determination of Total Phenolic Content (TPC)
2.5. Antioxidant Activity
2.5.1. Scavenging Activity of DPPH Radical
2.5.2. ABTS Radical Scavenging Activity
2.6. Anticancer Activity
2.7. Box–Behnken Design (BBD) Experimental Design
2.7.1. Single Factor Experimental Design
2.7.2. Optimization of Extraction Variables Using the BBD Method and Method Validity Testing
2.8. Statistical Analysis
3. Results
3.1. Single Extraction Factor Effect on Total Phenol Content (TPC)
3.2. BBD Optimization of Extraction Conditions
3.2.1. Model Fitting
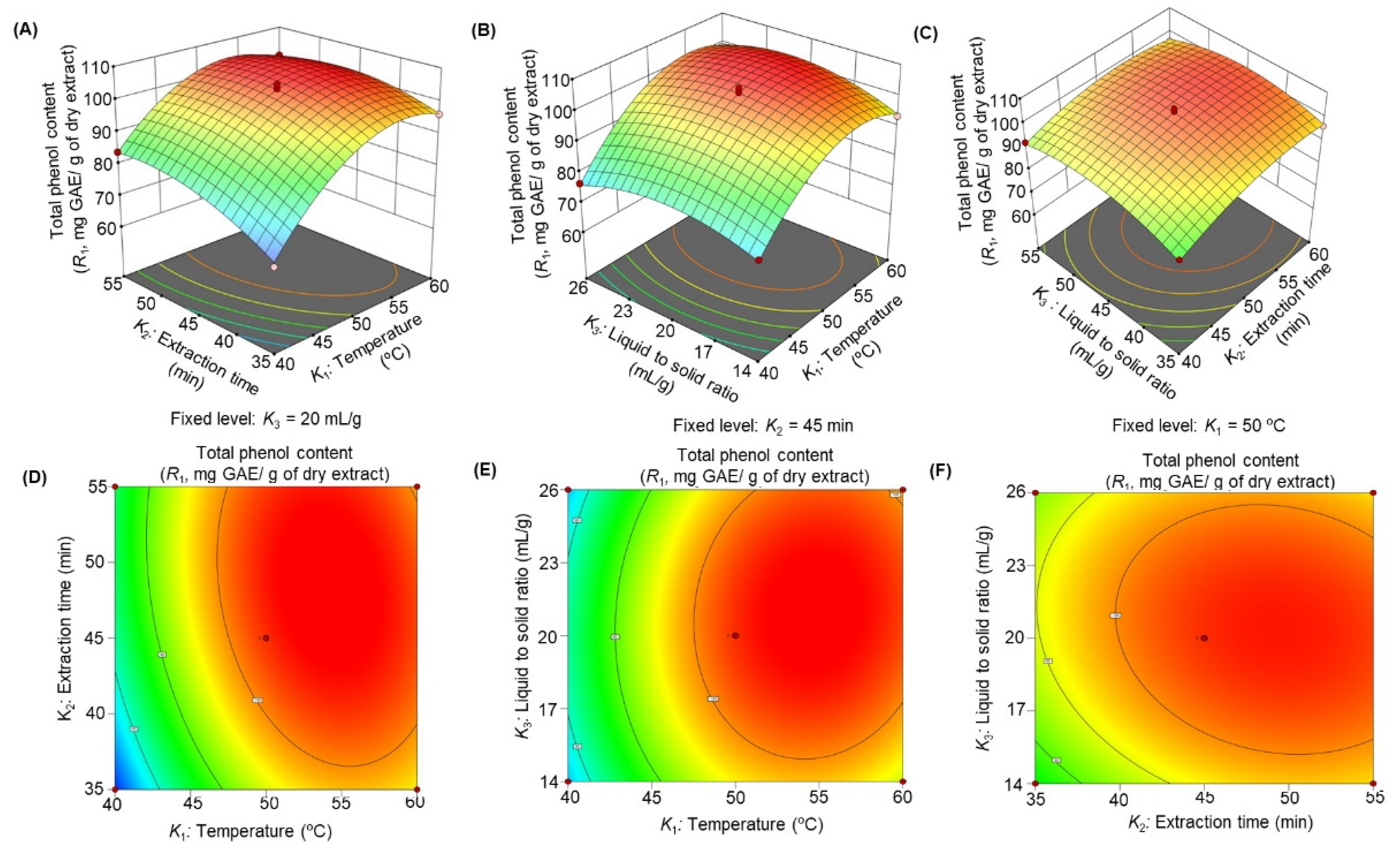
3.2.2. Influence of Extraction Parameters on Total Phenolic Content (TPC)
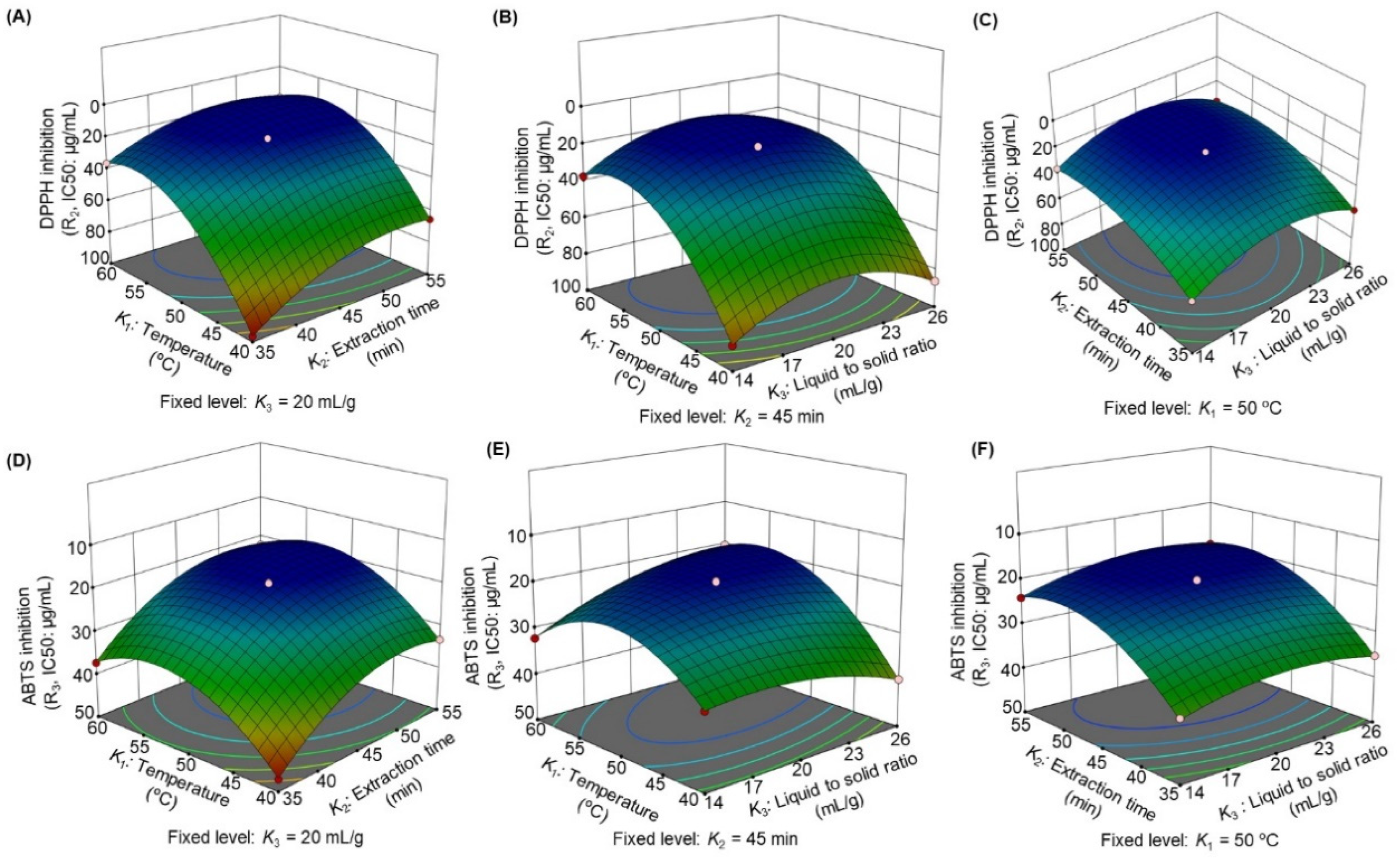
3.2.3. Influence of the Extraction Parameters on Antioxidant Activity
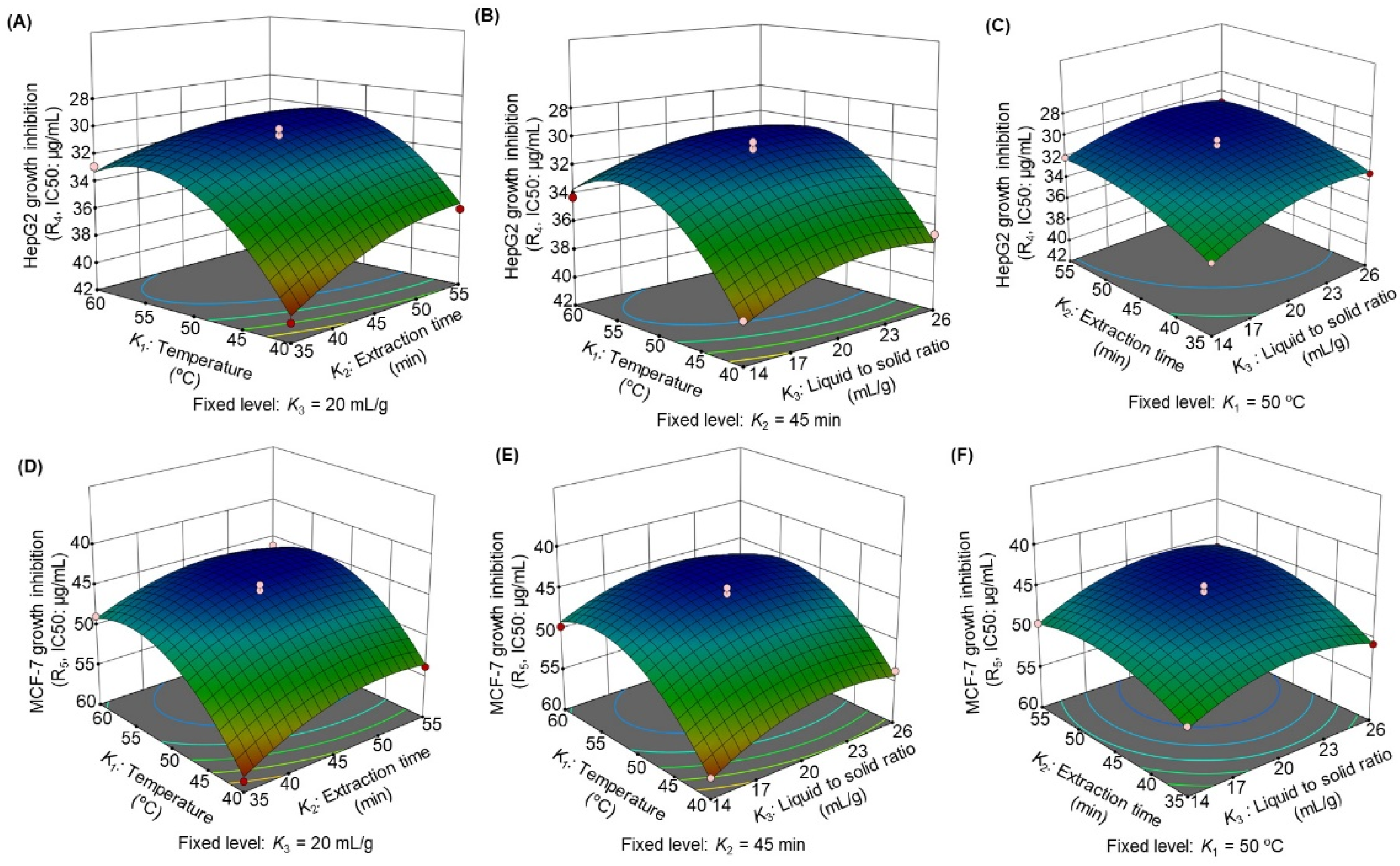
3.2.4. Influence of the Extraction Parameters on Anticancer Activity
3.2.5. Optimization of Extraction Conditions and Verification of Predictive Model
3.2.6. Comparison of UAE with CSE
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, H.Q.; Lu, G.; Liu, Y.; Bauer, R.; Tao, O.; Gong, J.T.; Zhao, L.Y.; Li, J.H.; Ren, Z.Y.; Yan, Y.H. Is it possible to rapidly and noninvasively identify different plants from Asteraceae using electronic nose with multiple mathematical algorithms? J. Food Drug Anal. 2015, 23, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Salleh, W.M.N.H.W.; Kassim, H.; Tawang, A. Volatile components and biological activities of Pulicaria essential oils. A review. Riv. Ital. Sostanze Grasse 2021, 98, 49–58. [Google Scholar]
- Wang, Y.-H.; Al-Rehaily, A.J.; Yousaf, M.; Ahmad, M.S.; Khan, I.A. Characterization and discrimination of different Pulicaria species using UHPLC-UV-MS QTOF (quadrupole time-of-flight mass spectrometer). J. Chem. Soc. Pak. 2015, 37, 967–973. [Google Scholar]
- AlQathama, A.; Bader, A.; Al-Rehaily, A.; Gibbons, S.; Prieto, J.M. In vitro cytotoxic activities of selected Saudi medicinal plants against human malignant melanoma cells (A375) and the isolation of their active principles. Eur. J. Integr. Med. 2022, 49, 102083. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef]
- Rodiah, M.H.; Nur Asma Fhadhila, Z.; Noor Asiah, H.; Aziah, M.Y.; Kawasaki, N. Ultrasound-assisted extraction of natural colourant from husk of Cocos nucifera: A comparison with agitated-bed extraction. Pertanika J. Sci. Technol. 2018, 26, 1039–1052. [Google Scholar]
- Yang, J.; Li, N.; Wang, C.; Chang, T.; Jiang, H. Ultrasound-homogenization-assisted extraction of polyphenols from coconut mesocarp: Optimization study. Ultrason. Sonochem. 2021, 78, 105739. [Google Scholar] [CrossRef]
- Alam, P.; Noman, O.M.; Herqash, R.N.; Almarfadi, O.M.; Akhtar, A.; Alqahtani, A.S. Efficient Extraction of an Anthraquinone Physcion Using Response Surface Methodology (RSM) Optimized Ultrasound-Assisted Extraction Method from Aerial Parts of Senna occidentalis and Analysis by HPLC-UV. Separations 2022, 9, 142. [Google Scholar] [CrossRef]
- Alam, P.; Noman, O.M.; Herqash, R.N.; Almarfadi, O.M.; Akhtar, A.; Alqahtani, A.S. Response Surface Methodology (RSM)-Based Optimization of Ultrasound-Assisted Extraction of Sennoside A, Sennoside B, Aloe-Emodin, Emodin, and Chrysophanol from Senna alexandrina (Aerial Parts): HPLC-UV and Antioxidant Analysis. Molecules 2022, 27, 298. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Alam, P.; Alrehaily, A.J.; Alqahtani, A.S.; Akhtar, A.; Alhowiriny, T.A.; Almarfadi, O.M.; Mothana, R.A. Optimization of ultrasound-assisted parthenolide extraction from Tarchonanthus camphoratus leaves using response surface methodology: HPTLC and cytotoxicity analysis. Arab. J. Chem. 2021, 14, 103194. [Google Scholar] [CrossRef]
- Alam, P.; Siddiqui, N.A.; Alqahtani, A.S.; Haque, A.; Basudan, O.A.; Alqasoumi, S.I.; AL-Mishari, A.A.; Khan, M.U. Response surface methodology-based optimization of ultrasound-assisted extraction of β-sitosterol and lupeol from Astragalus atropilosus (roots) and validation by HPTLC method. Asian Pac. J. Trop. Biomed. 2020, 10, 281–292. [Google Scholar] [CrossRef]
- Singleton, C.P.; Rossi, J.A. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Alqahtani, A.S.; Noman, O.M.; Rehman, M.T.; Siddiqui, N.A.; Alajmi, M.F.; Nasr, F.A.; Shahat, A.A.; Alam, P. The influence of variations of furanosesquiterpenoids content of commercial samples of myrrh on their biological properties. Saudi Pharm. J. 2019, 27, 981–989. [Google Scholar] [CrossRef]
- Almarfadi, O.M.; Siddiqui, N.A.; Shahat, A.A.; Alqahtani, A.S.; Alam, P.; Nasr, F.A.; Alshahrani, S.S.; Noman, O.M. Quantification of biomarkers and evaluation of antioxidant, anti-inflammatory, and cytotoxicity properties of Dodonaea viscosa grown in Saudi Arabia using HPTLC technique. Open Chem. 2022, 20, 559–569. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct. Food Health Dis. 2011, 1, 232–244. [Google Scholar] [CrossRef]
- Alam, P.; Al-Yousef, H.M.; Siddiqui, N.A.; Alhowiriny, T.A.; Alqasoumi, S.I.; Amina, M.; Hassan, W.H.B.; Abdelaziz, S.; Abdalla, R.H. Anticancer activity and concurrent analysis of ursolic acid, β-sitosterol and lupeol in three different Hibiscus species (aerial parts) by validated HPTLC method. Saudi Pharm. J. 2018, 26, 1060–1067. [Google Scholar] [CrossRef]
- Lee, M.H.; Lin, C.C. Comparison of technique for extraction of isoflavones from the root of Radix Puerariae: Ultrasonic and pressurised solvent extraction. Food Chem. 2007, 105, 223–228. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y.; Canada, A. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Garrido, T.; Gizdavic-Nikolaidis, M.; Leceta, I.; Urdanpilleta, M.; Guerrero, P.; de la Caba, K.; Kilmartin, P.A. Optimizing the extraction process of natural antioxidants from chardonnay grape marc using microwave-assisted extraction. Waste Manag. 2019, 88, 110–117. [Google Scholar] [CrossRef]
- Pandey, A.; Belwal, T.; Sekar, K.C.; Bhatt, I.D.; Rawal, R.S. Optimization of ultrasonic-assisted extraction (UAE) of phenolics and antioxidant compounds from rhizomes of Rheum moorcroftianum using response surface methodology (RSM). Ind. Crops Prod. 2018, 119, 218–225. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Roman, A.; Pereira, R.N.; Teixeira, J.A.; Domingues, L. Bioactive compounds recovery optimization from vine pruning residues using conventional heating and microwave-assisted extraction methods. Ind. Crops Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef]
- Hussein, K.; Ahmed, A.H.; Al-Maqtari, M.A. Composition and Radical Scavenging Activity of Edible Wild Pulicaria jaubertii (Asteraceae) volatile Oil. PSM Biol. Res. 2017, 2, 21–29. [Google Scholar]

| Independent Variable | Factor Level | Dependent Variables | Goal | ||||||
|---|---|---|---|---|---|---|---|---|---|
| −1 | 0 | +1 | Total phenolic content (mg GAE/g of dry extract) (R1) | DPPH inhibition (IC50: µg/mL) (R2) | ABTS inhibition (IC50: µg/mL) (R3) | HepG2 growth inhibition (IC50: µg/mL) (R4) | MCF-7 growth inhibition (IC50: µg/mL) (R5) | Maximized | |
| Extraction temperature (°C) (K1) | 40 | 50 | 60 | ||||||
| Extraction time (min) (K2) | 35 | 45 | 55 | ||||||
| Liquid-to-solid ratio (mL/g) (K3) | 14 | 20 | 26 | ||||||
| Run | Coded Variables | Actual Variables | Total Phenolic Content | Antioxidant Activity | Anticancer Activity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (K1) (°C) | (K2) (min) | (K3) (mL/g) | (K1) (°C) | (K2) (min) | (K3) (mL/g) | (mg AE/g of Dry Extract) (R1) | DPPH IC50 (µg/mL) (R2) | ABTS IC50 (µg/mL) (R3) | HepG2 IC50 (µg/mL) (R4) | MCF-7 IC50 (µg/mL) (R5) | |
| 1 | 0 | 0 | 0 | 50 | 45 | 20 | 103.53 ± 4.91 | 18.3 ± 0.79 | 19.4 ± 0.77 | 30.28 ± 1.17 | 44.27 ± 1.94 |
| 2 | −1 | −1 | 0 | 40 | 35 | 20 | 69.19 ± 2.85 | 96.8 ± 4.95 | 47.3 ± 1.74 | 40.66 ± 1.78 | 58.72 ± 2.51 |
| 3 | 1 | 1 | 0 | 60 | 55 | 20 | 100.64 ± 4.21 | 20.8 ± 0.96 | 22.1 ± 0.81 | 31.48 ± 1.15 | 46.14 ± 2.13 |
| 4 | 1 | −1 | 0 | 60 | 35 | 20 | 96.08 ± 3.78 | 36.1 ± 0.15 | 37.1 ± 1.51 | 32.89 ± 1.37 | 48.82 ± 1.71 |
| 5 | −1 | 1 | 0 | 40 | 55 | 20 | 83.85 ± 3.59 | 65.2 ± 2.54 | 33.2 ± 1.26 | 36.33 ± 1.56 | 53.66 ± 1.97 |
| 6 | 0 | 0 | 0 | 50 | 45 | 20 | 104.82 ± 4.49 | 18.1 ± 0.67 | 19.2 ± 0.58 | 30.76 ± 1.11 | 44.99 ± 2.03 |
| 7 | 1 | 0 | 1 | 60 | 45 | 26 | 98.57 ± 4.66 | 32.3 ± 1.35 | 20.3 ± 0.71 | 32.33 ± 1.32 | 47.44 ± 1.95 |
| 8 | 0 | 1 | −1 | 50 | 55 | 14 | 96.35 ± 4.51 | 35.9 ± 1.79 | 24.1 ± 0.77 | 32.1 ± 1.37 | 49.5 ± 2.12 |
| 9 | 1 | 0 | −1 | 60 | 45 | 14 | 93.47 ± 4.29 | 37.3 ± 1.61 | 32.1 ± 1.48 | 34.27 ± 1.61 | 49.63 ± 2.09 |
| 10 | −1 | 0 | 1 | 40 | 45 | 26 | 76.54 ± 3.72 | 86.2 ± 3.26 | 39.7 ± 1.65 | 36.63 ± 1.53 | 53.62 ± 2.49 |
| 11 | 0 | −1 | 1 | 50 | 35 | 26 | 92.11 ± 4.45 | 57.1 ± 2.33 | 35.1 ± 1.22 | 33.2 ± 1.18 | 50.61 ± 2.32 |
| 12 | −1 | 0 | −1 | 40 | 45 | 14 | 76.49 ± 2.88 | 87.3 ± 3.73 | 33.9 ± 1.11 | 39.1 ± 1.68 | 57.56 ± 2.52 |
| 13 | 0 | 0 | 0 | 50 | 45 | 20 | 102.11 ± 4.05 | 18.7 ± 0.77 | 19.8 ± 0.72 | 30.98 ± 1.37 | 45.13 ± 2.24 |
| 14 | 0 | 0 | 0 | 50 | 45 | 20 | 102.51 ± 3.88 | 18.5 ± 0.86 | 19.6 ± 0.71 | 30.77 ± 1.13 | 45.39 ± 2.08 |
| 15 | 0 | −1 | −1 | 50 | 35 | 14 | 87.19 ± 3.75 | 60.4 ± 2.79 | 36.5 ± 1.57 | 35.6 ± 1.66 | 52.04 ± 2.14 |
| 16 | 0 | 1 | 1 | 50 | 55 | 26 | 96.18 ± 3.52 | 35.1 ± 1.76 | 20.1 ± 0.87 | 31.1 ± 1.29 | 46.3 ± 1.68 |
| 17 | 0 | 0 | 0 | 50 | 45 | 20 | 103.18 ± 4.51 | 18.4 ± 0.59 | 19.5 ± 0.69 | 31.03 ± 1.35 | 45.36 ± 1.95 |
| Quercetin | 7.46 ± 0.26 | ||||||||||
| Ascorbic Acid | 7.74 ± 0.29 | ||||||||||
| Vinblastine | 2.3 ± 0.07 | 2.8 ± 0.05 | |||||||||
| Dependent Variables | Source | R2 | Adjusted R2 | Predicted R2 | SD | Sequential p-Value | Lack of Fit p-Value | |
|---|---|---|---|---|---|---|---|---|
| R1 | Linear | 0.5321 | 0.4242 | 0.2862 | 8.22 | 0.0168 | 0.0003 | |
| 2FI | 0.5526 | 0.2841 | −0.1948 | 9.16 | 0.9259 | 0.0002 | ||
| Quadratic | 0.9938 | 0.9858 | 0.9347 | 1.29 | <0.0001 | 0.2312 | Suggested | |
| Cubic | 0.9977 | 0.9906 | 1.05 | 0.2312 | ||||
| R2 | Linear | 0.5690 | 0.4695 | 0.3648 | 19.56 | 0.0101 | <0.0001 | |
| 2FI | 0.5752 | 0.3203 | −0.0724 | 22.14 | 0.9849 | <0.0001 | ||
| Quadratic | 0.9999 | 0.9998 | 0.9990 | 0.35 | <0.0001 | 0.0854 | Suggested | |
| Cubic | 1.0000 | 0.9999 | 0.22 | 0.0854 | ||||
| R3 | Linear | 0.4834 | 0.3642 | 0.1925 | 7.26 | 0.0308 | <0.0001 | |
| 2FI | 0.5432 | 0.2692 | −0.2084 | 7.78 | 0.7317 | <0.0001 | ||
| Quadratic | 0.9995 | 0.9990 | 0.9948 | 0.29 | <0.0001 | 0.1793 | Suggested | |
| Cubic | 0.9998 | 0.9994 | 0.22 | 0.1793 | ||||
| R4 | Linear | 0.5281 | 0.4192 | 0.2446 | 2.39 | 0.0177 | 0.0003 | |
| 2FI | 0.5453 | 0.2724 | −0.3702 | 2.67 | 0.9427 | 0.0001 | ||
| Quadratic | 0.9872 | 0.9708 | 0.8279 | 0.53 | <0.0001 | 0.0542 | Suggested | |
| Cubic | 0.9978 | 0.9910 | 0.29 | 0.0542 | ||||
| R5 | Linear | 0.5188 | 0.4078 | 0.2797 | 3.43 | 0.0200 | 0.0004 | |
| 2FI | 0.5281 | 0.2450 | −0.2317 | 3.88 | 0.9768 | 0.0002 | ||
| Quadratic | 0.9919 | 0.9815 | 0.9081 | 0.61 | <0.0001 | 0.1712 | Suggested | |
| Cubic | 0.9974 | 0.9896 | 0.45 | 0.1712 |
| Factor | Coefficient (β) | ||||
|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | |
| Intercept | 103.23 | 18.40 | 19.50 | 30.76 | 45.03 |
| Linear | |||||
| K1 | 10.34 | −26.12 | −5.31 | −2.72 | −3.94 |
| K2 | 4.06 | −11.68 | −7.06 | −1.42 | −1.82 |
| K3 | 1.24 | −1.27 | −1.42 | −0.98 | −1.35 |
| Interaction | |||||
| K1 K2 | −2.52 | 4.07 | −0.23 | 0.73 | 0.59 |
| K1 K3 | 1.26 | −0.97 | −4.40 | 0.13 | 0.44 |
| K2 K3 | −1.27 | 0.62 | −0.65 | 0.35 | −0.44 |
| Quadratic | |||||
| K12 | −11.24 | 24.99 | 8.99 | 3.58 | 4.63 |
| K22 | −4.55 | 11.34 | 6.44 | 0.99 | 2.18 |
| K32 | −5.72 | 17.39 | 3.01 | 1.24 | 2.41 |
| F-value (model) | 124.78 | 9968.65 | 1697.08 | 60.09 | 95.39 |
| p-value (model) | <0.0001 s | <0.0001 s | <0.0001 s | <0.0001 s | <0.0001 s |
| F-value (lack of fit) | 2.19 ns | 4.67 ns | 2.72 ns | 6.26 ns | 2.82 ns |
| CV(%) | 1.38 | 0.82 | 1.05 | 1.60 | 1.23 |
| Adeq precision | 33.09 | 284.35 | 122.05 | 23.1 | 28.29 |
| Residual | 11.62 | 0.91 | 0.6075 | 2.01 | 2.58 |
| Pure error | 4.39 | 0.21 | 0.20 | 0.35 | 0.8277 |
| Dependent Variables | Independent Variables | SS a | DF b | MS c | F-Value | p-Value d |
|---|---|---|---|---|---|---|
| R1 | Linear effects | |||||
| K1 | 854.70 | 1 | 854.70 | 514.68 | <0.0001 | |
| K2 | 131.63 | 1 | 131.63 | 79.26 | <0.0001 | |
| K3 | 12.25 | 1 | 12.25 | 7.38 | 0.0299 | |
| Quadratic effects | ||||||
| K1K2 | 25.50 | 1 | 25.50 | 15.36 | 0.0058 | |
| K1K3 | 6.38 | 1 | 6.38 | 3.84 | 0.0909 | |
| K2K3 | 6.48 | 1 | 6.48 | 3.90 | 0.0888 | |
| Interaction effects | ||||||
| K12 | 531.95 | 1 | 531.95 | 320.33 | <0.0001 | |
| K22 | 87.17 | 1 | 87.17 | 52.49 | 0.0002 | |
| K32 | 137.88 | 1 | 137.88 | 83.03 | <0.0001 | |
| R2 | Linear effects | |||||
| K1 | 5460.12 | 1 | 5460.12 | 42467.64 | <0.0001 | |
| K2 | 1090.44 | 1 | 1090.44 | 8481.24 | <0.0001 | |
| K3 | 13.01 | 1 | 13.01 | 101.15 | <0.0001 | |
| Quadratic effects | ||||||
| K1K2 | 66.42 | 1 | 66.42 | 516.62 | <0.0001 | |
| K1K3 | 3.80 | 1 | 3.80 | 29.57 | 0.0010 | |
| K2K3 | 1.56 | 1 | 1.56 | 12.15 | 0.0102 | |
| Interaction effects | ||||||
| K12 | 2628.95 | 1 | 2628.95 | 20447.37 | <0.0001 | |
| K22 | 541.22 | 1 | 541.22 | 4209.46 | <0.0001 | |
| K32 | 1272.95 | 1 | 1272.95 | 9900.71 | <0.0001 | |
| R3 | Linear effects | |||||
| K1 | 225.78 | 1 | 225.78 | 2601.59 | <0.0001 | |
| K2 | 399.03 | 1 | 399.03 | 4597.89 | <0.0001 | |
| K3 | 16.24 | 1 | 16.24 | 187.19 | <0.0001 | |
| Quadratic effects | ||||||
| K1K2 | 0.2025 | 1 | 0.2025 | 2.33 | 0.1705 | |
| K1K3 | 77.44 | 1 | 77.44 | 892.31 | <0.0001 | |
| K2K3 | 1.69 | 1 | 1.69 | 19.47 | 0.0031 | |
| Interaction effects | ||||||
| K12 | 340.11 | 1 | 340.11 | 3918.92 | <0.0001 | |
| K22 | 174.49 | 1 | 174.49 | 2010.59 | <0.0001 | |
| K32 | 38.21 | 1 | 38.21 | 440.29 | <0.0001 | |
| R4 | Linear effects | |||||
| K1 | 59.13 | 1 | 59.13 | 206.53 | <0.0001 | |
| K2 | 16.07 | 1 | 16.07 | 56.14 | 0.0001 | |
| K3 | 7.62 | 1 | 7.62 | 26.63 | 0.0013 | |
| Quadratic effects | ||||||
| K1K2 | 2.13 | 1 | 2.13 | 7.44 | 0.0294 | |
| K1K3 | 0.0702 | 1 | 0.0702 | 0.2453 | 0.6356 | |
| K2K3 | 0.4900 | 1 | 0.4900 | 1.71 | 0.2321 | |
| Interaction effects | ||||||
| K12 | 53.94 | 1 | 53.94 | 188.40 | <0.0001 | |
| K22 | 4.18 | 1 | 4.18 | 14.61 | 0.0065 | |
| K32 | 6.47 | 1 | 6.47 | 22.58 | 0.0021 | |
| R5 | Linear effects | |||||
| K1 | 124.27 | 1 | 124.27 | 337.49 | <0.0001 | |
| K2 | 26.61 | 1 | 26.61 | 72.26 | <0.0001 | |
| K3 | 14.47 | 1 | 14.47 | 39.30 | 0.0004 | |
| Quadratic effects | ||||||
| K1K2 | 1.42 | 1 | 1.42 | 3.85 | 0.0907 | |
| K1K3 | 0.7656 | 1 | 0.7656 | 2.08 | 0.1925 | |
| K2K3 | 0.7832 | 1 | 0.7832 | 2.13 | 0.1881 | |
| Interaction effects | ||||||
| K12 | 90.20 | 1 | 90.20 | 244.98 | <0.0001 | |
| K22 | 19.98 | 19.98 | 54.27 | 0.0002 | ||
| K32 | 24.37 | 1 | 24.37 | 66.20 | <0.0001 | |
| Response Variables | Optimum Extraction Condition | Maximum Value | |||
|---|---|---|---|---|---|
| K1 (°C) | K2 (min) | K3 (mL/g) | Experimental Value | Predicted Value | |
| TPC (mg GAE/g) (R1) | 54.4 | 48 | 20.72 | 107.93 ± 3.28 | 107.23 |
| DPPH [IC50 (µg/mL)] (R2) | 15.7 ± 0.51 | 15.1 | |||
| ABTS [IC50 (µg/mL)] (R3) | 17.1 ± 0.68 | 16.5 | |||
| HepG2 [IC50 (µg/mL)] (R4) | 25.67 ± 1.07 | 26.2 | |||
| MCF-7 [IC50 (µg/mL)] (R5) | 31.87 ± 1.33 | 31.3 | |||
| Extraction Method | Temp (°C) | Time (min) | Methanol (mL/g) | TPC (mg GAE/g) | DPPH IC50 (µg/mL) | ABTS IC50 (µg/mL) | HepG2 IC50 (µg/mL) | MCF-7 [IC50 (µg/mL)] |
|---|---|---|---|---|---|---|---|---|
| UAE | 54.4 | 48 | 20.72 | 107.16 ± 4.96 | 15.7 ± 0.51 | 17.1 ± 0.68 | 25.67 ± 1.07 | 31.87 ± 1.33 |
| CSE | 75 | 60 | 25 | 74.29 ± 3.21 | 47.3 ± 2.17 | 54.1 ± 2.52 | 57.11 ± 2.64 | 61.28 ± 2.77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, P.; Fantoukh, O.I.; Asaker, M.; Almarfadi, O.M.; Akhtar, A.; Al-Hamoud, G.A.; Hawwal, M.F.; Alqahtani, A.S. Concurrent Optimization of Ultrasonic-Assisted Extraction of Total Phenolic Compounds and In Vitro Anticancer and Antioxidant Potential of Pulicaria schimperi (Aerial Parts) Using Response Surface Methodology. Separations 2023, 10, 208. https://doi.org/10.3390/separations10030208
Alam P, Fantoukh OI, Asaker M, Almarfadi OM, Akhtar A, Al-Hamoud GA, Hawwal MF, Alqahtani AS. Concurrent Optimization of Ultrasonic-Assisted Extraction of Total Phenolic Compounds and In Vitro Anticancer and Antioxidant Potential of Pulicaria schimperi (Aerial Parts) Using Response Surface Methodology. Separations. 2023; 10(3):208. https://doi.org/10.3390/separations10030208
Chicago/Turabian StyleAlam, Perwez, Omer I. Fantoukh, Mohammed Asaker, Omer M. Almarfadi, Ali Akhtar, Gadah A. Al-Hamoud, Mohammed F. Hawwal, and Ali S. Alqahtani. 2023. "Concurrent Optimization of Ultrasonic-Assisted Extraction of Total Phenolic Compounds and In Vitro Anticancer and Antioxidant Potential of Pulicaria schimperi (Aerial Parts) Using Response Surface Methodology" Separations 10, no. 3: 208. https://doi.org/10.3390/separations10030208
APA StyleAlam, P., Fantoukh, O. I., Asaker, M., Almarfadi, O. M., Akhtar, A., Al-Hamoud, G. A., Hawwal, M. F., & Alqahtani, A. S. (2023). Concurrent Optimization of Ultrasonic-Assisted Extraction of Total Phenolic Compounds and In Vitro Anticancer and Antioxidant Potential of Pulicaria schimperi (Aerial Parts) Using Response Surface Methodology. Separations, 10(3), 208. https://doi.org/10.3390/separations10030208








