Utilizing Subcritical Methanol Extraction for Catechin and Epicatechin Recovery from Peanut Skin as Agricultural Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Material
2.2. Chemicals
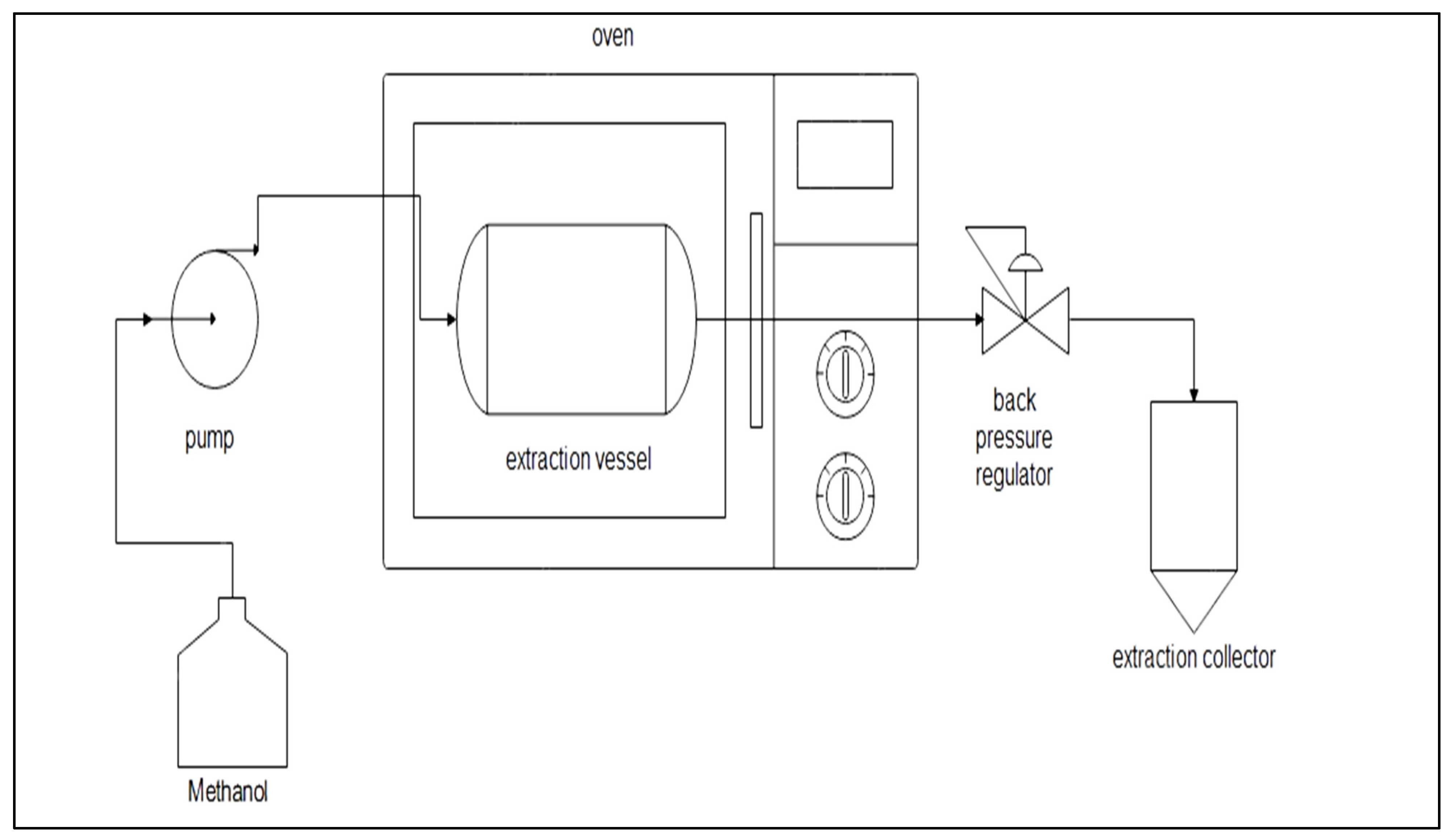
2.3. Subcritical Methanol Extraction (SME)
2.4. Catechin and Epicatechin Analysis
2.5. Experimental Design for Optimization
2.6. Semi-Emperical Modeling
2.6.1. Calculation of the Solubility of Catechin and Epicatechin
2.6.2. Chrastil Model
2.6.3. Del Valle Aguilera
2.6.4. Average Absolute Relative Deviation (AARD) and Coefficient of Determination (R2)
3. Results and Discussions
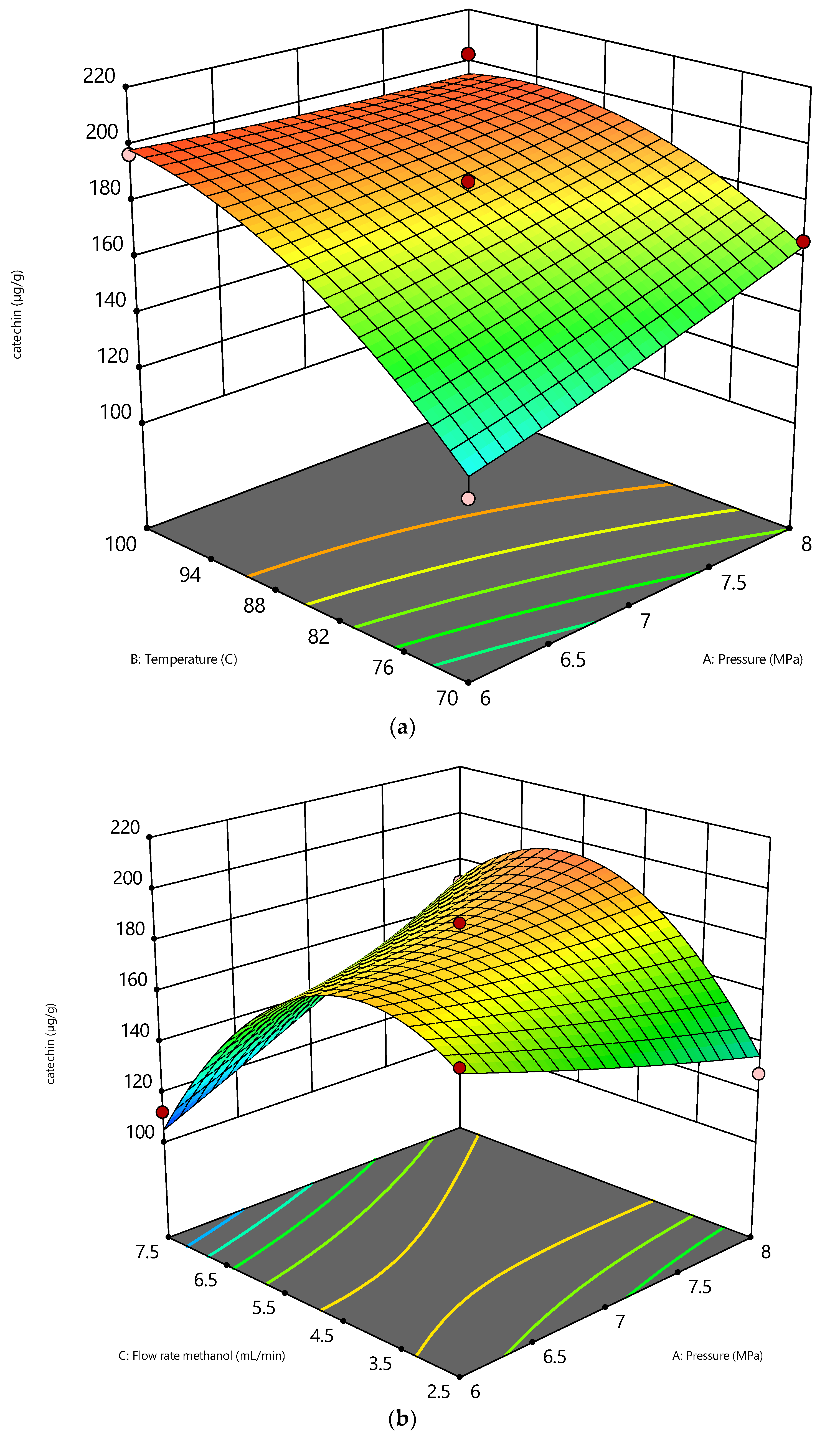
3.1. Process Effects and Statistical Data Regarding Catechin Recovery
3.2. Process Effects and Statistical Data on Epicatechin Recovery
3.3. Multiple Responses Optimization and Comparison with the Previous Study
3.4. Semi-Empirical Models for the Solubility of Catechin and Epicatechin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nepote, V.; Grosso, N.; Guzman, C.A. Extraction of antioxidant components from peanut skins. Grasas Aceites 2007, 53. [Google Scholar] [CrossRef]
- Putra, N.R.; Yunus, M.A.C.; Ruslan, M.S.H.; Idham, Z.; Idrus, F.N. Comparison extraction of peanut skin between CO2 supercritical fluid extraction and soxhlet extraction in term of oil yield and catechin. Pertanika J. Sci. Technol. 2018, 26, 799–810. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S.F. Optimizing the extraction of phenolic antioxidants from peanut skins using response surface methodology. J. Agric. Food Chem. 2009, 57, 3064–3072. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Rodriguez-Amado, I.; Agregán, R.; Munekata, P.E.; Vázquez, J.A.; Barba, F.J.; Lorenzo, J.M. Optimization of antioxidants extraction from peanut skin to prevent oxidative processes during soybean oil storage. LWT 2018, 88, 1–8. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Ruslan, M.S.H.; Mohd Azizi, C.; Idham, Z.; Morad, N.A.; Ali, A. Parametric evaluation for extraction of catechin from Areca catechu Linn seeds using supercritical CO2 extraction. J. Teknol. 2015, 74, 87–92. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2004, 86, 593–600. [Google Scholar] [CrossRef]
- Daud, N.M.; Putra, N.R.; Jamaludin, R.; Norodin, N.S.M.; Sarkawi, N.S.; Hamzah, M.H.S.; Nasir, H.M.; Zaidel, D.N.A.; Yunus, M.A.C.; Salleh, L.M. Valorisation of plant seed as natural bioactive compounds by various extraction methods: A review. Trends Food Sci. Technol. 2022, 119, 201–214. [Google Scholar] [CrossRef]
- Benito-Román, Ó.; Blanco, B.; Sanz, M.T.; Beltrán, S. Subcritical water extraction of phenolic compounds from onion skin wastes (Allium cepa cv. Horcal): Effect of temperature and solvent properties. Antioxidants 2020, 9, 1233. [Google Scholar] [CrossRef]
- Casazza, A.A.; Aliakbarian, B.; Mantegna, S.; Cravotto, G.; Perego, P. Extraction of phenolics from Vitis vinifera wastes using non-conventional techniques. J. Food Eng. 2010, 100, 50–55. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Faizal, A.N.M.; Che Yunus, M.A. Methods and Potential in Valorization of Banana Peels Waste by Various Extraction Processes: In Review. Sustainability 2022, 14, 10571. [Google Scholar] [CrossRef]
- Putra, N.R.; Aziz, A.H.A.; Yian, L.N.; Ramli, W.D.; Yunus, M.A.C. Optimization of supercritical carbon dioxide and co-solvent ethanol extraction of wasted peanut skin using response surface methodology. In Proceedings of the MATEC Web of Conferences, Semarang, Indonesia, 14 March 2018; p. 02005. [Google Scholar]
- Bai, L.-S.; Yang, Y.; Lv, D.-D. Microwave extraction of total flavonoids in peanut skins. Zhong Yao Cai Zhongyaocai J. Chin. Med. Mater. 2012, 35, 977–980. [Google Scholar]
- Ballard, T.S.; Mallikarjunan, P.; Zhou, K.; O’Keefe, S. Microwave-assisted extraction of phenolic antioxidant compounds from peanut skins. Food Chem. 2010, 120, 1185–1192. [Google Scholar] [CrossRef]
- Marcus, Y. Extraction by subcritical and supercritical water, methanol, ethanol and their mixtures. Separations 2018, 5, 4. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lim, S.-B. Kinetic study of subcritical water extraction of flavonoids from citrus unshiu peel. Sep. Purif. Technol. 2020, 250, 117259. [Google Scholar] [CrossRef]
- Wu, G.; Dong, H.; Li, J.; Guo, L.; Cheng, Y.; Geng, Y.; Wang, X. Extraction of parishin B and parishin C from Gastrodiae Rhizoma by subcritical water technology. J. Ind. Eng. Chem. 2022, 108, 280–287. [Google Scholar] [CrossRef]
- Kubátová, A.; Jansen, B.; Vaudoisot, J.-F.; Hawthorne, S.B. Thermodynamic and kinetic models for the extraction of essential oil from savory and polycyclic aromatic hydrocarbons from soil with hot (subcritical) water and supercritical CO2. J. Chromatogr. A 2002, 975, 175–188. [Google Scholar] [CrossRef]
- Putra, N.R.; Idham, Z.B.; Machmudah, S.; Ruslan, M.S.H.b.; Che Yunus, M.A. Extraction of peanut skin oil by modified supercritical carbon dioxide: Empirical modelling and optimization. Sep. Sci. Technol. 2018, 53, 2695–2703. [Google Scholar] [CrossRef]
- Esquıvel, M.; Bernardo-Gil, M.; King, M. Mathematical models for supercritical extraction of olive husk oil. J. Supercrit. Fluids 1999, 16, 43–58. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction: An Introduction to Fundamentals of Supercritical Fluids and the Application to Separation Processes; Springer Science & Business Media: Berlin, Germany, 2013; Volume 4. [Google Scholar]
- Chrastil, J. Solubility of solids and liquids in supercritical gases. J. Phys. Chem. 1982, 86, 3016–3021. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. An improved equation for predicting the solubility of vegetable oils in supercritical carbon dioxide. Ind. Eng. Chem. Res. 1988, 27, 1551–1553. [Google Scholar] [CrossRef]
- Poon, G. Analysis of catechins in tea extracts by liquid chromatography–electrospray ionization mass spectrometry. J. Chromatogr. A 1998, 794, 63–74. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Zaini, A.S.; Machmudah, S.; Yunus, M.A.C. Solubility of catechin and epicatechin from Arachis Hypogea skins wastes by using supercritical carbon dioxide-ethanol and its optimization. J. Food Meas. Charact. 2021, 15, 2031–2038. [Google Scholar] [CrossRef]
- Gbashi, S.; Adebo, O.A.; Piater, L.; Madala, N.E.; Njobeh, P.B. Subcritical water extraction of biological materials. Sep. Purif. Rev. 2017, 46, 21–34. [Google Scholar] [CrossRef]
- Abdul Aziz, A.H.; Putra, N.R.; Kong, H.; Che Yunus, M.A. Supercritical carbon dioxide extraction of sinensetin, isosinensetin, and rosmarinic acid from Orthosiphon stamineus leaves: Optimization and modeling. Arab. J. Sci. Eng. 2020, 45, 7467–7476. [Google Scholar] [CrossRef]
- Rasidek, N.A.M.; Nordin, M.F.M.; Tokuyama, H.; Nagatsu, Y.; Mili, N.; Zaini, A.S.; Idham, Z.; Yunus, M.A.C. Subcritical water-based pectin from banana peels (Musa Paradisiaca Cv. Tanduk) as a natural gelation agent. Mater. Today Proc. 2021, 47, 1329–1335. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Veza, I.; Jumakir, J.; Waluyo, W.; Suparwoto, S.; Qomariyah, L.; Yunus, M.A.C. Solubilization and Extraction of Valuable Compounds from Peanut skin in Subcritical Water. J. Food Process. Preserv. 2022, 46, e17005. [Google Scholar] [CrossRef]
- Zaini, A.S.; Putra, N.R.; Idham, Z.; Mohd Faizal, A.N.; Che Yunus, M.A.; Mamat, H.; Abdul Aziz, A.H. Comparison of alliin recovery from Allium sativum L. using Soxhlet extraction and subcritical water extraction. ChemEngineering 2022, 6, 73. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sa-Nogueira, I.; Simoes, P.; Paiva, A.; Barreiros, S. Valorization of white wine grape pomace through application of subcritical water: Analysis of extraction, hydrolysis, and biological activity of the extracts obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Zaini, A.; Putra, N.; Idham, Z.; Norodin, N.M.; Rasidek, N.M.; Yunus, M.C. Mini Review: Extraction of Allicin from Allium sativum using Subcritical Water Extraction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 932, 012023. [Google Scholar] [CrossRef]
- Ciftci, D.; Saldaña, M.D. Hydrolysis of sweet blue lupin hull using subcritical water technology. Bioresour. Technol. 2015, 194, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Martinez-Jimenez, F.; Rezende, C.; Tompsett, G.; Timko, M.; Forster-Carneiro, T. Subcritical water hydrolysis of sugarcane bagasse: An approach on solid residues characterization. J. Supercrit. Fluids 2016, 108, 69–78. [Google Scholar] [CrossRef]
- Ko, M.-J.; Cheigh, C.-I.; Chung, M.-S. Relationship analysis between flavonoids structure and subcritical water extraction (SWE). Food Chem. 2014, 143, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Griest, E.M.; Webb, W.; Schiessler, R.W. Effect of pressure on viscosity of higher hydrocarbons and their mixtures. J. Chem. Phys. 1958, 29, 711–720. [Google Scholar] [CrossRef]
- Pillot, M.; Lebeau, B.; Nouali, H.; Daou, T.J.; Patarin, J.; Ryzhikov, A. High pressure intrusion of water and LiCl aqueous solutions in hydrophobic KIT-6 mesoporous silica: Influence of the grafted group nature. Microporous Mesoporous Mater. 2019, 280, 248–255. [Google Scholar] [CrossRef]
- Plaza, M.; Marina, M.L. Pressurized hot water extraction of bioactives. TrAC Trends Anal. Chem. 2019, 116, 236–247. [Google Scholar] [CrossRef]
- Vatai, T.; Škerget, M.; Knez, Ž.; Kareth, S.; Wehowski, M.; Weidner, E. Extraction and formulation of anthocyanin-concentrates from grape residues. J. Supercrit. Fluids 2008, 45, 32–36. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Rambabu, K.; AlYammahi, J.; Thanigaivelan, A.; Bharath, G.; Sivarajasekar, N.; Velu, S.; Banat, F. Sub-critical water extraction of reducing sugars and phenolic compounds from date palm fruit. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Aziz, A.H.A.; Idham, Z.; Qomariyah, L.; Yunus, M.A.C. Extraction rate of Valuable Compounds from Peanut Skin Waste by Ethanol-Assisted Supercritical Carbon Dioxide: Modelling and Optimization. Malays. J. Fundam. Appl. Sci. 2022, 18, 157–170. [Google Scholar] [CrossRef]
- Bodoira, R.; Rossi, Y.; Montenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using water-ethanol at high pressure and temperature conditions. J. Supercrit. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Redzuan, S.; Ho, C.Y.; Idham, Z.; Yusuf, S.; Putra, N.R.; Yunus, M.A.C.; Mansor, S.; Ruslan, M.S.H. Optimization of Anthocyanins Extracts from Roselle (Hibiscus sabdarifa) Petals Using Ultrasonic-Assisted Extraction Method. In Proceedings of the 3rd International Conference on Separation Technology, Johor, Malaysia, 15–16 August 2020; Springer: Berlin/Heidelberg, Germany, 2021; pp. 295–309. [Google Scholar]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Idham, Z.; Zaini, M.A.A.; Yunus, M.A.C.; Aziz, A.H.A. Optimization and solubilization of interest compounds from roselle in subcritical ethanol extraction (SEE). Alex. Eng. J. 2022, 65, 59–74. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N. A review of subcritical water as a solvent and its utilisation for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Putra, N.R.; Rizkiyah, D.N.; Idham, Z.; Veza, I.; Qomariyah, L.; Yunus, M.A.C. Optimization and modelling in flavonoid and phenolic compounds recovery from peanut skin by subcritical water. Biomass Convers. Biorefinery 2022, 30, 1–11. [Google Scholar] [CrossRef]
- Mayanga-Torres, P.; Lachos-Perez, D.; Rezende, C.; Prado, J.; Ma, Z.; Tompsett, G.; Timko, M.; Forster-Carneiro, T. Valorization of coffee industry residues by subcritical water hydrolysis: Recovery of sugars and phenolic compounds. J. Supercrit. Fluids 2017, 120, 75–85. [Google Scholar] [CrossRef]
- Zaidul, I.S.M.; Nik Norulaini, N.A.; Mohd Omar, A.K.; Smith, R.L. Supercritical carbon dioxide (SC-CO2) extraction of palm kernel oil from palm kernel. J. Food Eng. 2007, 79, 1007–1014. [Google Scholar] [CrossRef]


| Run | A: Pressure | B: Temperature | C: Flow Rate | Catechin | Epicatechin |
|---|---|---|---|---|---|
| MPa | C | mL/min | µg/g | µg/g | |
| 1 | 7 | 70 | 7.5 | 102.52 | 159.11 |
| 2 | 6 | 85 | 7.5 | 112.22 | 106.2 |
| 3 | 8 | 85 | 7.5 | 170.85 | 91.62 |
| 4 | 7 | 100 | 2.5 | 166.26 | 49.4 |
| 5 | 8 | 70 | 5 | 166.19 | 353.83 |
| 6 | 7 | 85 | 5 | 183.26 | 208.51 |
| 7 | 6 | 100 | 5 | 196.71 | 35.32 |
| 8 | 6 | 70 | 5 | 122.19 | 66.06 |
| 9 | 6 | 85 | 2.5 | 172.77 | 12.06 |
| 10 | 7 | 70 | 2.5 | 115.94 | 353.26 |
| 11 | 7 | 100 | 7.5 | 140.57 | 248.9 |
| 12 | 8 | 100 | 5 | 202.72 | 189.84 |
| 13 | 8 | 85 | 2.5 | 127.77 | 417.91 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 15,163.30 | 9 | 1684.81 | 27.44 | 0.0010 |
| A—Pressure | 506.26 | 1 | 506.26 | 8.25 | 0.035 |
| B—Temperature | 4971.04 | 1 | 4971.04 | 80.97 | 0.0003 |
| C—Flow rate methanol | 400.16 | 1 | 400.16 | 6.52 | 0.05 |
| AB | 360.81 | 1 | 360.81 | 5.88 | 0.06 |
| AC | 2684.79 | 1 | 2684.79 | 43.73 | 0.0012 |
| BC | 37.64 | 1 | 37.64 | 0.61 | 0.46 |
| A2 | 6.09 | 1 | 6.09 | 0.09 | 0.76 |
| B2 | 652.68 | 1 | 652.68 | 10.63 | 0.02 |
| C2 | 5715.92 | 1 | 5715.92 | 93.11 | 0.0002 |
| Residual | 306.95 | 5 | 61.39 | ||
| Cor Total | 288.27 | 3 | 96.09 | 10.29 | 0.09 |
| Std. Dev. | 7.84 | R2 | 0.98 | ||
| Mean | 156.57 | Adjusted R2 | 0.94 | ||
| C.V. % | 5.00 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Coefficient |
|---|---|---|---|---|---|---|
| Model | 2.094 × 105 | 9 | 23,263.93 | 15.62 | 0.0037 | 207.39 (intercept) |
| A—Pressure | 86,852.78 | 1 | 86,852.78 | 58.31 | 0.0006 | 104.19 |
| B—Temperature | 20,889.68 | 1 | 20,889.68 | 14.02 | 0.013 | −51.10 |
| C—Flow rate methanol | 6429.78 | 1 | 6429.78 | 4.32 | 0.09 | −28.35 |
| AB | 4438.89 | 1 | 4438.89 | 2.98 | 0.14 | −33.31 |
| AC | 44,190.35 | 1 | 44,190.35 | 29.67 | 0.002 | −105.11 |
| BC | 38,740.08 | 1 | 38,740.08 | 26.01 | 0.003 | 98.41 |
| A2 | 7787.04 | 1 | 7787.04 | 5.23 | 0.07 | −45.92 |
| B2 | 0.1533 | 1 | 0.1533 | 0.0001 | 0.99 | −0.2 |
| C2 | 75.39 | 1 | 75.39 | 0.0506 | 0.83 | −4.52 |
| Residual | 7447.92 | 5 | 1489.58 | |||
| Cor Total | 7422.92 | 3 | 2474.31 | 197.93 | 0.005 | |
| Std. Dev. | 38.60 | R2 | 0.96 | |||
| Mean | 180.38 | Adjusted R2 | 0.90 | |||
| C.V. % | 21.40 |
| Parameters | Values | Responses | Predicted | Observed | Error (%) |
|---|---|---|---|---|---|
| Pressure, MPa | 8 | Catechin, µg/g | 178.62 | 194.32 | 8.07 |
| Temperature, °C | 79.6 | ||||
| Flow rate, mL/min | 4.39 | Epicatechin, µg/g | 336.41 | 372.64 | 9.73 |
| Run | Experimental Data | Chrastil Model | DVa Model | |||||
|---|---|---|---|---|---|---|---|---|
| Sexp Catechin × 10−3 (g/L) | Sexp Epicatechin × 10−3 (g/L) | Ln Sexp Catechin | Ln Sexp Epicatechin | Ln Smod Catechin | Ln Smod Epicatechin | Ln Smod Catechin | Ln Smod Epicatechin | |
| 1 | 4.10 | 6.36 | −5.50 | −5.06 | −5.91 | −4.89 | −5.81 | −5.81 |
| 2 | 2.24 | 2.12 | −6.10 | −6.15 | −5.91 | −5.12 | −5.81 | −5.81 |
| 3 | 2.28 | 1.22 | −6.08 | −6.71 | −5.91 | −5.12 | −5.81 | −5.81 |
| 4 | 6.65 | 1.98 | −5.01 | −6.23 | −5.94 | −5.96 | −5.67 | −6.22 |
| 5 | 6.65 | 14.2 | −5.01 | −4.26 | −5.89 | −4.27 | −5.97 | −5.35 |
| 6 | 2.44 | 2.78 | −6.01 | −5.89 | −5.94 | −5.75 | −5.67 | −6.22 |
| 7 | 3.93 | 0.706 | −5.54 | −7.26 | -5.94 | -5.96 | -5.67 | -6.22 |
| 8 | 2.44 | 1.32 | −6.01 | −6.63 | −5.89 | −4.27 | −5.97 | −5.35 |
| 9 | 3.46 | 2.41 | −5.67 | −8.33 | −5.94 | −5.75 | −5.67 | −6.22 |
| 10 | 1.55 | 4.71 | −6.47 | −5.36 | −5.89 | −4.27 | −5.97 | −5.35 |
| 11 | 5.62 | 9.96 | −5.18 | −4.61 | −5.91 | −5.33 | −5.81 | −5.81 |
| 12 | 2.70 | 2.53 | −5.91 | −5.98 | −5.91 | −5.33 | −5.81 | −5.81 |
| 13 | 2.56 | 8.36 | −5.97 | −4.78 | −5.89 | −4.51 | −5.97 | −5.35 |
| 14 | 4.10 | 6.36 | −5.50 | −5.06 | −5.91 | −4.89 | −5.81 | −5.81 |
| Bioactive Compounds | Model | k1 | a | b | c | AARD |
|---|---|---|---|---|---|---|
| (%) | ||||||
| Catechin | Chrastil | 0.48 | −4.34 | −9.31 | - | 6.86 |
| DVa | −0.38 | −1102 | −15.71 | −0.03 | 5.97 | |
| Epicatechin | Chrastil | 12.15 | 1996 | −95.71 | 14.44 | |
| DVa | −278 | 4916 | −15.69 | −0.02 | 12.69 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putra, N.R.; Rizkiyah, D.N.; Yunus, M.A.C.; Abdul Aziz, A.H.; Pamungkas, A. Utilizing Subcritical Methanol Extraction for Catechin and Epicatechin Recovery from Peanut Skin as Agricultural Waste. Separations 2023, 10, 82. https://doi.org/10.3390/separations10020082
Putra NR, Rizkiyah DN, Yunus MAC, Abdul Aziz AH, Pamungkas A. Utilizing Subcritical Methanol Extraction for Catechin and Epicatechin Recovery from Peanut Skin as Agricultural Waste. Separations. 2023; 10(2):82. https://doi.org/10.3390/separations10020082
Chicago/Turabian StylePutra, Nicky Rahmana, Dwila Nur Rizkiyah, Mohd Azizi Che Yunus, Ahmad Hazim Abdul Aziz, and Ade Pamungkas. 2023. "Utilizing Subcritical Methanol Extraction for Catechin and Epicatechin Recovery from Peanut Skin as Agricultural Waste" Separations 10, no. 2: 82. https://doi.org/10.3390/separations10020082
APA StylePutra, N. R., Rizkiyah, D. N., Yunus, M. A. C., Abdul Aziz, A. H., & Pamungkas, A. (2023). Utilizing Subcritical Methanol Extraction for Catechin and Epicatechin Recovery from Peanut Skin as Agricultural Waste. Separations, 10(2), 82. https://doi.org/10.3390/separations10020082









