Galactolipids from Launaea capitata (Spreng.) Dandy with In Vitro Anti-Inflammatory and Neuroprotective Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Instruments
2.3. Extraction and Purification
2.4. Enzyme Inhibition Assays
2.4.1. BchE Inhibition Assay
2.4.2. COX-2 Inhibition Assay
2.4.3. 5-LOX Inhibition Assay
2.5. Statistical Analysis
2.6. Docking Study
3. Results and Discussion
3.1. Identification of the Isolated Compounds
3.1.1. Identification of Compound 1
3.1.2. Identification of Compound 2
3.2. Enzymes Inhibitory Activities
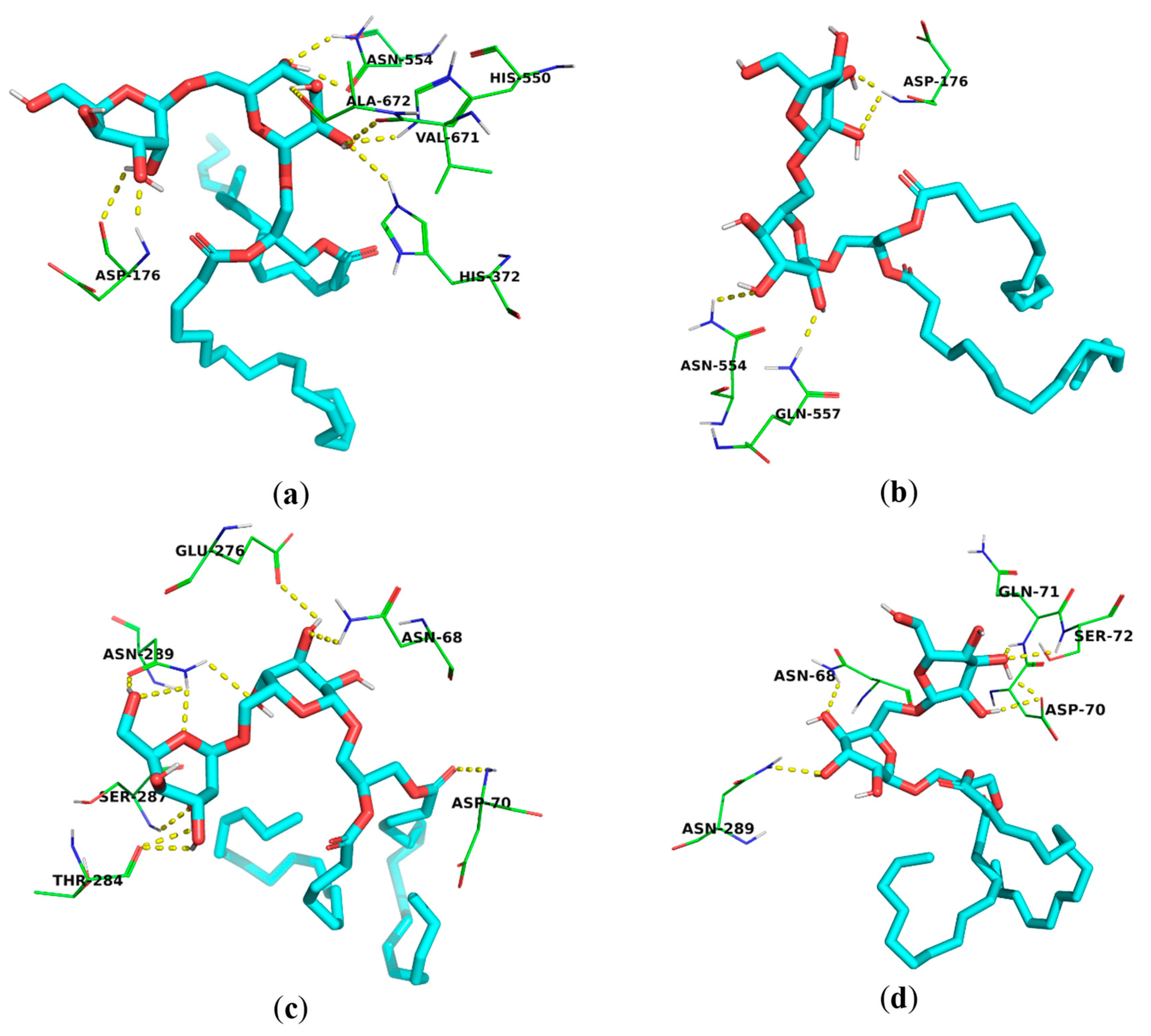
3.3. Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the war against inflammation with natural products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef]
- Gorlenko, C.L.; Kiselev, H.Y.; Budanova, E.V.; Zamyatnin, A.A., Jr.; Ikryannikova, L.N. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiotics 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Kilian, N. Revision of Launaea Cass. (Compositae, Lactuceae, Sonchinae). Englera 1997, 17, 1–478. [Google Scholar] [CrossRef]
- Daur, I. Plant flora in the rangeland of western Saudi Arabia. Pak. J. Bot. 2012, 44, 23–26. [Google Scholar]
- Elsharkawy, E.R. Isolation of phytoconstituents and evaluation of anticancer and Antioxidant potential of Launaea mucronata (Forssk.) Muschl. subsp. Pak. J. Pharm. Sci. 2017, 30, 399–405. [Google Scholar]
- Al-Fatimi, M. Wild edible plants traditionally collected and used in southern Yemen. J. Ethnobiol. Ethnomed. 2021, 17, 49. [Google Scholar] [CrossRef]
- Khalil, H.E.; Aldakheel, T.S.; AlAhmed, A.; Emeka, P.M.; Kandeel, M. Anti-proliferative activity of leaves of Launaea capitata Asteraceae: Phytochemical, cytotoxicity and in silico studies. Trop. J. Pharm. Res. 2020, 19, 2129–2136. [Google Scholar] [CrossRef]
- Mansour, R.M.A.; Ahmed, A.A.; Saleh, N.A.M. Flavone glycosides of some Launaea species. Phytochemistry 1983, 22, 2630–2631. [Google Scholar] [CrossRef]
- Emad, F.; Khalafalah, A.K.; El Sayed, M.A.; Mohamed, A.H.; Stadler, M.; Helaly, S.E. Three new polyacetylene glycosides (PAGs) from the aerial part of Launaea capitata (Asteraceae) with anti-biofilm activity against Staphylococcus aureus. Fitoterapia 2020, 143, 104548. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Parveen, S.; Riaz, N.; Tahir, M.N.; Ashraf, M.; Afzal, I.; Ali, M.S.; Malik, A.; Jabbar, A. New bioactive natural products from Launaea nudicaulis. Phytochem. Lett. 2012, 5, 793–799. [Google Scholar] [CrossRef]
- Cheriti, A.; Belboukhari, M.; Belboukhari, N.N.B.; Djeradi, H.H.D. Phytochemical and biological studies on Launaea Cass. Genus ( Asteracea) from Algerian sahara. Curr. Top. Phytochem. 2012, 11, 67–80. [Google Scholar]
- Hossain, J.S.; El-Sayed, M.; Aoshima, H. Antioxidative and anti-α-amylase activities of four wild plants consumed by pastoral nomads in Egypt. Orient. Pharm. Exp. Med. 2009, 9, 217–224. [Google Scholar] [CrossRef]
- Tariq, M.; Mossa, J.S.; Al-yahya, M.A.; Al-meshal, I.A.; Al-badr, A.A. Phytochemical and Biological Screening of Saudi Medicinal Plants Part-10* A Study on Saudi Plants of Family Compositae. Int. J. Crude Drug Res. 1987, 25, 17–25. [Google Scholar] [CrossRef]
- Nguyen, T.Q.C.; Binh, T.D.; Kusunoki, R.; Pham, T.L.A.; Nguyen, Y.D.H.; Nguyen, T.T.; Kanaori, K.; Kamei, K. Effects of Launaea sarmentosa Extract on Lipopolysaccharide-Induced Inflammation via Suppression of NF-κB/MAPK Signaling and Nrf2 Activation. Nutrients 2020, 12, 2586. [Google Scholar] [CrossRef]
- Asif, M.; Mahrukh; Saadullah, M.; Yaseen, H.S.; Saleem, M.; Yousaf, H.M.; Khan, I.U.; Yaseen, M.; Shams, M.U. Evaluation of in vivo anti-inflammatory and anti-angiogenic attributes of methanolic extract of Launaea spinosa. Inflammopharmacology 2020, 28, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Akimat, E.K.; Omwenga, G.I.; Moriasi, G.A.; Ngugi, M.P. Antioxidant, Anti-Inflammatory, Acute Oral Toxicity, and Qualitative Phytochemistry of The Aqueous Root Extract of Launaea cornuta (Hochst. Ex Oliv. & Hiern.). J. Evid. Based Complement. Altern. Med. 2021, 26, 2515690X211064585. [Google Scholar] [CrossRef]
- Lamia, S.; Belboukhari, N.; Aminata, K.; Sulaiman, M.; Yakoubi, M.; Sekkoum, K.; Abdelkrim, C. Investigation of The Analgesic and Anti-Inflammatory Activities of Launaea Nudicaulis From Southwest of Algeria. Biomed. J. Sci. Technol. Res. 2021, 23, 17173–17178. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Proschak, E.; Steinhilber, D.; Rovati, G.E. Two-pronged approach to anti-inflammatory therapy through the modulation of the arachidonic acid cascade. Biochem. Pharmacol. 2018, 158, 161–173. [Google Scholar] [CrossRef]
- Sharma, J.N.; Jawad, N.M. Adverse effects of COX-2 inhibitors. Sci. World J. 2005, 5, 629–645. [Google Scholar] [CrossRef]
- Rainsford, K. Anti-inflammatory drugs in the 21st century. In Inflammation in The Pathogenesis of Chronic Diseases; Springer: Berlin/Heidelberg, Germany, 2007; pp. 3–27. [Google Scholar]
- El-Malah, A.A.; Gineinah, M.M.; Deb, P.K.; Khayyat, A.N.; Bansal, M.; Venugopala, K.N.; Aljahdali, A.S. Selective COX-2 inhibitors: Road from success to controversy and the quest for repurposing. Pharmaceuticals 2022, 15, 827. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Hu, C.; Ma, S. Recent development of lipoxygenase inhibitors as anti-inflammatory agents. Medchemcomm 2018, 9, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Manju, S.; Ethiraj, K.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Meshram, M.A.; Bhise, U.O.; Makhal, P.N.; Kaki, V.R. Synthetically-tailored and nature-derived dual COX-2/5-LOX inhibitors: Structural aspects and SAR. Eur. J. Med. Chem. 2021, 225, 113804. [Google Scholar] [CrossRef]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets (Formerly Curr. Drug Targets CNS Neurol. Disord.) 2014, 13, 1432–1439. [Google Scholar]
- Ramachandra, G.; Lakshmi, G. Influence of butyrylcholinesterase on the course of COVID-19. Biomed. Rev. 2021, 32, 37–46. [Google Scholar]
- Dighe, S.N.; Deora, G.S.; De la Mora, E.; Nachon, F.; Chan, S.; Parat, M.-O.; Brazzolotto, X.; Ross, B.P. Discovery and structure–activity relationships of a highly selective butyrylcholinesterase inhibitor by structure-based virtual screening. J. Med. Chem. 2016, 59, 7683–7689. [Google Scholar] [CrossRef] [PubMed]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; Kiewert, C.; Duysen, E.G.; Lockridge, O.; Greig, N.H.; Klein, J. Excessive hippocampal acetylcholine levels in acetylcholinesterase-deficient mice are moderated by butyrylcholinesterase activity. J. Neurochem. 2007, 100, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Abdel Bar, F.M.; Sameti, M.; Foudah, A.I.; Haque, A.; Elsbaey, M. In vitro and in silico inhibition of COX-2 and 5-LOX by beta-carboline alkaloids from the seeds of Peganum harmala L. S. Afr. J. Bot. 2022, 147, 926–936. [Google Scholar] [CrossRef]
- Soliman, A.F.; Abdel Bar, F.M.; Sallam, A.; Galala, A.A. New neuroprotective sesquiterpene lactate esters from carotol biotransformation. S. Afr. J. Bot. 2023, 153, 163–171. [Google Scholar] [CrossRef]
- Obregon, A.D.; Schetinger, M.R.; Correa, M.M.; Morsch, V.M.; da Silva, J.E.; Martins, M.A.; Bonacorso, H.G.; Zanatta, N. Effects per se of organic solvents in the cerebral acetylcholinesterase of rats. Neurochem. Res. 2005, 30, 379–384. [Google Scholar] [CrossRef]
- Atatreh, N.; Al Rawashdah, S.; Al Neyadi, S.S.; Abuhamdah, S.M.; Ghattas, M.A. Discovery of new butyrylcholinesterase inhibitors via structure-based virtual screening. J. Enzym. Inhib. Med. Chem. 2019, 34, 1373–1379. [Google Scholar] [CrossRef]
- Lee, C.; Liao, J.; Chen, S.; Yen, C.; Lee, Y.; Huang, S.; Huang, S.; Lin, C.; Chang, V.H. Fluorine-Modified Rutaecarpine Exerts Cyclooxygenase-2 Inhibition and Anti-inflammatory Effects in Lungs. Front. Pharmacol. 2019, 10, 91. [Google Scholar] [CrossRef]
- Yoon, S.H.; Cho, D.Y.; Choi, S.R.; Lee, J.Y.; Choi, D.K.; Kim, E.; Park, J.Y. Synthesis and biological evaluation of salicylic acid analogues of celecoxib as a new class of selective cyclooxygenase-1 inhibitor. Biol. Pharm. Bull. 2021, 44, 1230–1238. [Google Scholar] [CrossRef]
- Yarla, N.S.; Pathuri, G.; Gali, H.; Terzyan, S.; Panneerselvam, J.; Chandrakesan, P.; Scotti, M.T.; Houchen, C.; Madka, V.; Rao, C.V. Discovery and Development of a Novel mPGES-1/5-LOX Dual Inhibitor LFA-9 for Prevention and Treatment of Chronic Inflammatory Diseases. J. Inflamm. Res. 2020, 13, 1261–1278. [Google Scholar] [CrossRef]
- Shaaban, M.; Kamal, A.; Faggal, S.; Farag, N.; Aborehab, N.; El-Sahar, A.; Mohamed, K. Design, synthesis, and biological evaluation of new pyrazoloquinazoline derivatives as dual COX-2/5-LOX inhibitors. Archiv. Pharm. 2020, 353, 2000027. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Malkowski, M.G. Substrate-selective Inhibition of Cyclooxygeanse-2 by Fenamic Acid Derivatives Is Dependent on Peroxide Tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Rui, Z.; Neau, D.B.; Waight, M.T.; Bartlett, S.G.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. Conversion of human 5-lipoxygenase to a 15-lipoxygenase by a point mutation to mimic phosphorylation at Serine-663. FASEB J. 2012, 26, 3222–3229. [Google Scholar] [CrossRef] [PubMed]
- Rosenberry, T.L.; Brazzolotto, X.; Macdonald, I.R.; Wandhammer, M.; Trovaslet-Leroy, M.; Darvesh, S.; Nachon, F. Comparison of the Binding of Reversible Inhibitors to Human Butyrylcholinesterase and Acetylcholinesterase: A Crystallographic, Kinetic and Calorimetric Study. Molecules 2017, 22, 2098. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Guella, G.; Frassanito, R.; Mancini, I. A new solution for an old problem: The regiochemical distribution of the acyl chains in galactolipids can be established by electrospray ionization tandem mass spectrometry. Rapid. Commun. Mass Spectrom. 2003, 17, 1982–1994. [Google Scholar] [CrossRef]
- Bartels, D.; Dörmann, P. Plant Lipids: Methods and Protocols. In Methods in Molecular Biology; Walker, J.M., Ed.; Springer Nature Humana Press: Bonn, Germany, 2021; Volume 2295. [Google Scholar]
- Dörmann, P. Galactolipids in Plant Membranes; eLS, Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2013; pp. 1–7. [Google Scholar]
- Douce, R.; Joyard, J. Plant galactolipids. In Lipids: Structure and Function; Elsevier: Amsterdam, The Netherlands, 1980; pp. 321–362. [Google Scholar]
- Cateni, F.; Falsone, G.; Zilic, J.; Bonivento, P.; Zacchigna, M.; Žigon, D.; Sosa, S.; Altinier, G. Glyceroglycolipids from Euphorbia nicaeensis All. with antiinflamatory activity. ARKIVOC 2004, 2004, 54–65. [Google Scholar] [CrossRef]
- Wu, J.; Long, L.; Song, Y.; Zhang, S.; Li, Q.; Huang, J.; Xiao, Z. A new unsaturated glycoglycerolipid from a cultured marine dinoflagellate Amphidinium carterae. Chem. Pharm. Bull. 2005, 53, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Seki, K.; Ohnishi, M.; Ito, S.; Fujino, Y. Structure of novel glyceroglycolipids in Adzuki bean (Vigna angularis) seeds. Biochem. Cell Biol. 1990, 68, 59–64. [Google Scholar] [PubMed]
- Tanaka, R.; Sakano, Y.; Nagatsu, A.; Shibuya, M.; Ebizuka, Y.; Goda, Y. Synthesis of digalactosyl diacylglycerols and their structure-inhibitory activity on human lanosterol synthase. Bioorg. Med. Chem. Lett. 2005, 15, 159–162. [Google Scholar] [CrossRef]
- Christensen, L.P. Galactolipids as potential health promoting compounds in vegetable foods. Recent Pat. Food Nutr. Agric. 2009, 1, 50–58. [Google Scholar] [CrossRef]
- Watanabe, D.; Kerakawati, R.; Morita, T.; Nakamura, T.; Ueno, K.; Kumamoto, T.; Nakanishi, W.; Ishikawa, T.; Uzawa, J.; Seki, H.; et al. Isolation of β-Sitosterol and Digalactopyranosyl-diacylglyceride from Citrus hystrix, a Thai Traditional Herb, as Pancreatic Lipase Inhibitors. Heterocycles 2009, 78, 1497–1505. [Google Scholar] [CrossRef]
- Fan, G.-j.; Kim, S.; Han, B.H.; Han, Y.N. Glyceroglycolipids, a novel class of platelet-activating factor antagonists from Kalimeris indica. Phytochem. Lett. 2008, 1, 207–210. [Google Scholar] [CrossRef]
- Seo, E.J.; Wu, C.F.; Ali, Z.; Wang, Y.H.; Khan, S.I.; Walker, L.A.; Khan, I.A.; Efferth, T. Both Phenolic and Non-phenolic Green Tea Fractions Inhibit Migration of Cancer Cells. Front. Pharmacol. 2016, 7, 398. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-m.; Yu, K.; Xia, Y.; Shine, M.B.; Wang, C.; Navarre, D.; Kachroo, A.; Kachroo, P. Mono- and Digalactosyldiacylglycerol Lipids Function Nonredundantly to Regulate Systemic Acquired Resistance in Plants. Cell Rep. 2014, 9, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Singh, S.P.; Misra, S.; Gupta, J.; Misra-Bhattacharya, S. Galactolipids from Bauhinia racemosa as a new class of antifilarial agents against human lymphatic filarial parasite, Brugia malayi. Eur. J. Med. Chem. 2012, 50, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Marcus, J.; Popko, B. Galactolipids are molecular determinants of myelin development and axo–glial organization. Biochim. Biophys. Acta Gen. Subj. 2002, 1573, 406–413. [Google Scholar] [CrossRef]
- Chew, H.; Solomon, V.A.; Fonteh, A.N. Involvement of lipids in Alzheimer’s disease pathology and potential therapies. Front. Physiol. 2020, 11, 598. [Google Scholar] [CrossRef] [PubMed]
- Dupree, J.L.; Suzuki, K.; Popko, B. Galactolipids in the formation and function of the myelin sheath. Microsc. Res. Tech. 1998, 41, 431–440. [Google Scholar] [CrossRef]
- Barricklow, J.; Blatnik, M. 2-Arachidonoylglycerol is a substrate for butyrylcholinesterase: A potential mechanism for extracellular endocannabinoid regulation. Arch. Biochem. Biophys. 2013, 536, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Saura, P.; Maréchal, J.D.; Masgrau, L.; Lluch, J.M.; González-Lafont, À. Computational insight into the catalytic implication of head/tail-first orientation of arachidonic acid in human 5-lipoxygenase: Consequences for the positional specificity of oxygenation. Phys. Chem. Chem. Phys. 2016, 18, 23017–23035. [Google Scholar] [CrossRef]
- Sobeh, M.; Mamadalieva, N.Z.; Mohamed, T.; Krstin, S.; Youssef, F.S.; Ashour, M.L.; Azimova, S.S.; Wink, M. Chemical profiling of Phlomis thapsoides (Lamiaceae) and in vitro testing of its biological activities. Med. Chem. Res. 2016, 25, 2304–2315. [Google Scholar] [CrossRef]
- Uzairu, S.M.; Tijani, Y.; Gadaka, M.A.; Modu, B.; Watafua, M.; Ahmad, H.A.; Zakariya, U.A.; Ibrahim, A.; Daja, A.; Zanna, H.; et al. Kinetics and computational study of butyrylcholinesterase inhibition by methylrosmarinate: Relevance to Alzheimer’s disease treatment. Heliyon 2022, 8, e10613. [Google Scholar] [CrossRef]

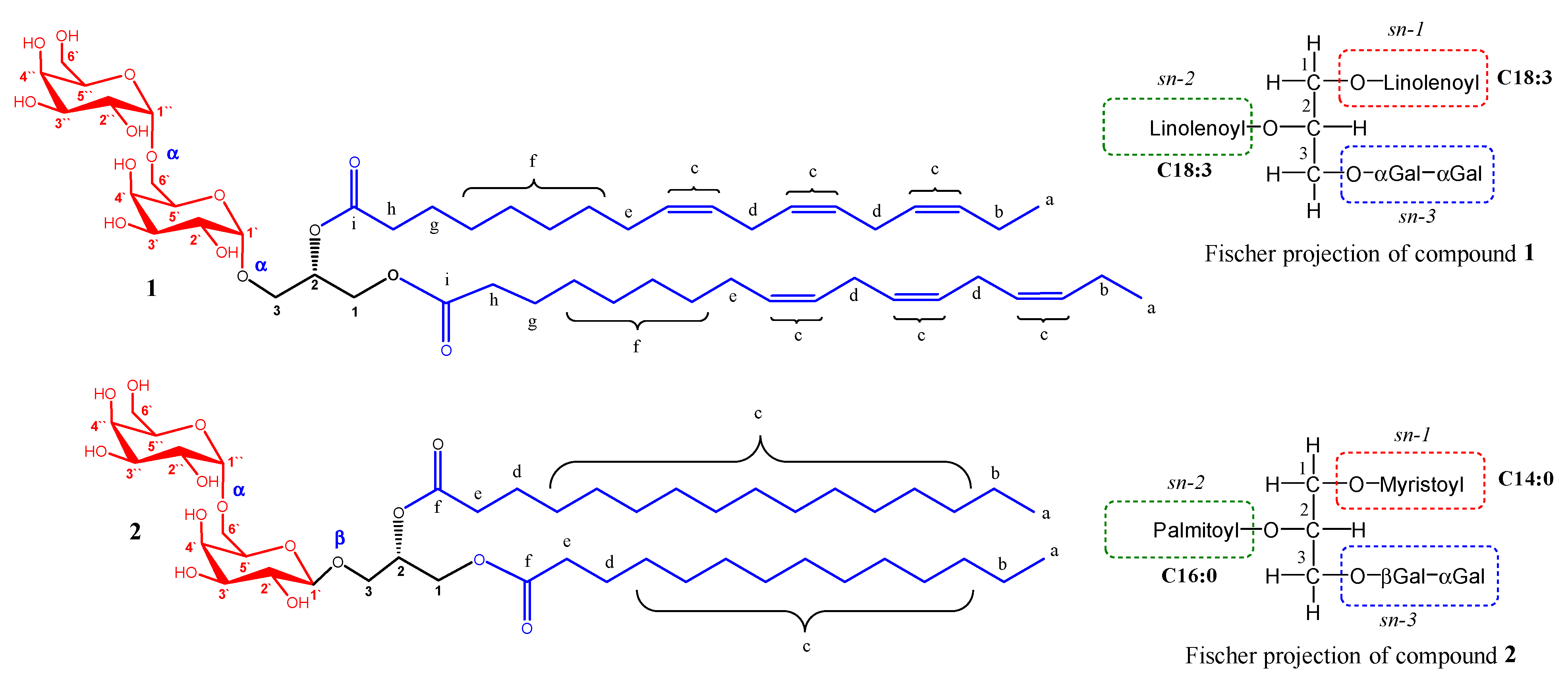
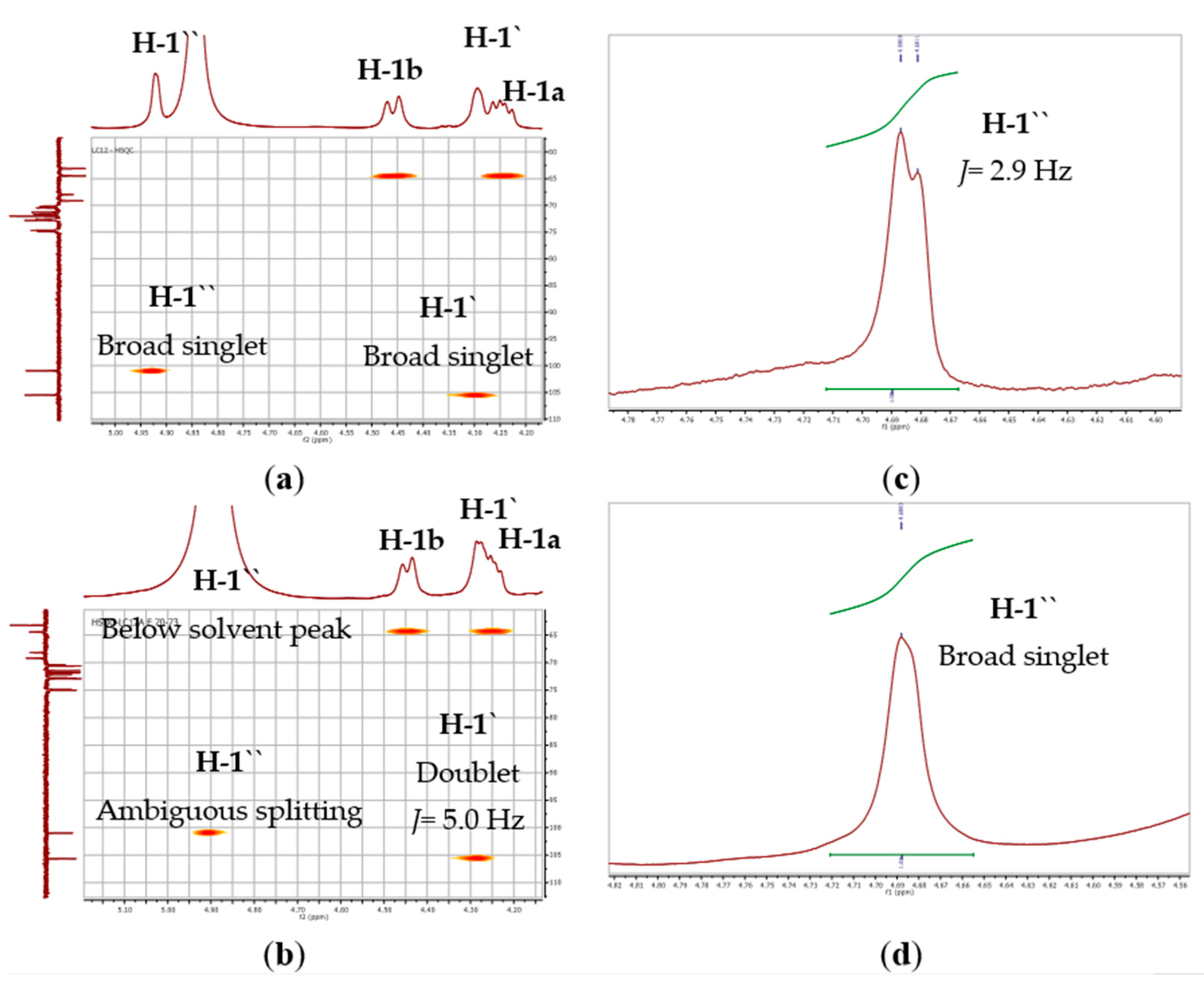
| C/H No. | C Type | 13C (δ, ppm) | 1H (δ, ppm) |
|---|---|---|---|
| Glycerol moiety | |||
| 1 | CH2 | 63.9 | Ha: 4.24, dd (11.6, 6.9) Hb: 4.45, d (11.7) |
| 2 | CH | 71.5 | 5.28, m |
| 3 | CH2 | 68.6 | Ha: 3.97, m Hb: 3.76, m |
| Galactose-1 | |||
| 1′ | CH | 105.0 | 4.29, brs |
| 2′ | CH | 72.1 | 3.55, m |
| 3′ | CH | 74.3 | 3.78, m |
| 4′ | CH | 70.7 | 3.95, m |
| 5′ | CH | 74.1 | 3.55, m |
| 6′ | CH2 | 67.4 | Ha: 3.90, m Hb: 3.72, m |
| Galactose-2 | |||
| 1′′ | CH | 100.4 | 4.92, brs |
| 2′′ | CH | 71.2 | 3.79, m |
| 3′′ | CH | 69.6 | 3.96, m |
| 4′′ | CH | 69.9 | 3.82, m |
| 5′′ | CH | 72.3 | 3.87, m |
| 6′′ | CH2 | 62.5 | 3.75, m |
| Diacyl (Linolenic acid moieties) | |||
| a | CH3 | 14.8 (2C) | 0.97, t (7.6) |
| b | CH2 | 21.4 (2C) | 2.06, m, overlapping |
| c | CH | 128.1 (2C), 128.7 (2C), 129.0 (2C), 129.1 (2C), 130.9 (2C), 132.6 (2C) | 5.31-5.41, m |
| d | CH2 | 26.4, 26.5 | 2.81, m |
| e | CH2 | 28.1 (2C) | 2.06, m, overlapping |
| f | CH2 | 30.1, 30.1, 30.2, 30.2, 30.3 (2C), 30.6 (2C) | 1.34-1.38, brs |
| g | CH2 | 25.9, 25.0 | 1.61, m |
| h | CH2 | 34.8, 35.0 | 2.33, m |
| i | C=O | 174.3, 174.6 | --- |
| C/H No. | C Type | 13C (δ, ppm) | 1H (δ, ppm) |
|---|---|---|---|
| Glycerol moiety | |||
| 1 | CH2 | 64.4 | Ha: 4.23, dd (11.5, 6.6) Hb: 4.44, brd (10.9) |
| 2 | CH | 72.1 | 5.27, brs |
| 3 | CH2 | 69.2 | Ha: 3.96, m Hb: 3.76, m |
| Galactose-1 | |||
| 1′ | CH | 105.6 | 4.28, d (5.0) |
| 2′ | CH | 72.8 | 3.53, m |
| 3′ | CH | 75.0 | 3.53, m |
| 4′ | CH | 71.4 | 3.92, m |
| 5′ | CH | 74.9 | 3.77, m |
| 6′ | CH2 | 68.1 | Ha: 3.91, m Hb: 3.69, m |
| Galactose-2 | |||
| 1′′ | CH | 101.0 | 4.90, brs* |
| 2′′ | CH | 70.6 | 3.79, m |
| 3′′ | CH | 70.4 | 3.90, m |
| 4′′ | CH | 71.8 | 3.74, m |
| 5′′ | CH | 72.9 | 3.86, m |
| 6′′ | CH2 | 63.2 | 3.74, m |
| Diacyl (Myristic and palmitic acids) | |||
| a | CH3 | 15.0 (2C) | 0.90, t (6.3) |
| b | CH2 | 24.2 (2C) | 1.31, brs, overlapping |
| c | CH2 | 30.5-33.5 (20C) | 1.31-1.36, brs |
| d | CH2 | 26.3, 26.4 | 1.62, brs |
| e | CH2 | 35.4, 35.5 | 2.34, q (6.7) |
| f | C=O | 175.1, 175.5 | --- |
| Compound | COX-2 a | 5-LOX a | BchE a |
|---|---|---|---|
| 1 | 110.44 ± 3.75 | 59.01 ± 3.04 | 13.37 ± 0.98 |
| 2 | 179.63 ± 8.14 | 21.67 ± 1.75 | 24.32 ± 1.29 |
| NDGA b | 1.42 ± 0.23 | 1.71 ± 0.27 | --- |
| Rivastigmine | --- | --- | 3.52 ± 0.37 |
| Donepezil | --- | --- | 2.19 ± 0.23 |
| Compound | Binding Energy (kcal/mol) | ||
|---|---|---|---|
| COX-2 | 5-LOX | BchE | |
| 1 | −7.360 | −8.124 | −8.313 |
| 2 | −5.723 | −8.634 | −7.502 |
| Co-crystallized ligand * | −8.659 | −5.830 | −8.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Bar, F.M.; Sherif, A.E.; ElNaggar, M.H. Galactolipids from Launaea capitata (Spreng.) Dandy with In Vitro Anti-Inflammatory and Neuroprotective Activities. Separations 2023, 10, 83. https://doi.org/10.3390/separations10020083
Abdel Bar FM, Sherif AE, ElNaggar MH. Galactolipids from Launaea capitata (Spreng.) Dandy with In Vitro Anti-Inflammatory and Neuroprotective Activities. Separations. 2023; 10(2):83. https://doi.org/10.3390/separations10020083
Chicago/Turabian StyleAbdel Bar, Fatma M., Asmaa E. Sherif, and Mai H. ElNaggar. 2023. "Galactolipids from Launaea capitata (Spreng.) Dandy with In Vitro Anti-Inflammatory and Neuroprotective Activities" Separations 10, no. 2: 83. https://doi.org/10.3390/separations10020083
APA StyleAbdel Bar, F. M., Sherif, A. E., & ElNaggar, M. H. (2023). Galactolipids from Launaea capitata (Spreng.) Dandy with In Vitro Anti-Inflammatory and Neuroprotective Activities. Separations, 10(2), 83. https://doi.org/10.3390/separations10020083








