Abstract
In recent years, the scientific community has turned its attention to the further study and application of green chemistry as well as to sustainable development in reducing the consumption of raw materials, solvents, and energy. The application of green chemistry aims to ensure the protection of the environment and to also, consequently, improve the quality of human life. It offers several benefits, both socially and economically. In the last few decades, new alternative non-conventional green extraction methodologies have been developed for the purposes of the extraction of active ingredient compounds from various raw products. The main objective of this literature review is to present the current knowledge and future perspectives regarding the green extraction of tea species in respect of the isolation of safe active biomolecules, which can be used as commercially available products—both as dietary supplements and pharmaceutical formulations. More specifically, in this literature review, the intention is to investigate several different extraction techniques, such as ultrasonic-assisted extraction, ultrasonic-assisted extraction with DESs, the microwave assisted-extraction method, and the reflux method. These are presented in respect of their role in the isolation of bioactive molecules regarding different tea species. Furthermore, following the literature review conducted in this study, the commonly used green extraction methods were found to be the ultrasound-assisted method and the microwave-assisted method. In addition to these, the use of a green solvent, in regard to its role in the maximum extraction yield of active ingredients in various species of tea, was emphasized. Catechins, alkaloids (such as caffeine), gallic acid, and flavonoids were the main extracted bioactive molecules that were isolated from the several tea species. From this literature review, it can be demonstrated that green tea has been widely studied at a rate of 52% in respect of the included research studies, followed by black tea at 26%, as well as white tea and oolong tea at 11% each. Regarding the determination of the bioactive molecules, the most utilized analytical method was found in the combination of high-performance liquid chromatography (HPLC) with a photodiode array detector (PDA) and mass spectrophotometry (MS) at a usage rate of about 80%. This method was followed by the utilization of UPLC and GC at 12% and 8%, respectively. In the future, it will be necessary to study the combination of green extraction techniques with other industry strategies, such as an encapsulation at the micro and nano scale, for the purposes of preparing stable final products with antioxidant properties where, finally, they can be safely consumed by humans.
1. Introduction
The term “Green Chemistry” appeared in the early 1990s. In addition, in the last two decades, it has subsequently been adopted worldwide. The goal of green chemistry is a reduction in environmental pollution, and support for the principles of sustainable development, in order to protect the health of workers. Furthermore, the expected results of the implementation of green chemistry include: reduction in environmental pollution; reduction in the use of toxic and hazardous chemicals; the use of renewable energy sources; energy-saving techniques; cost reductions; an increase in security; the improvement of human health, and a better quality of human life.
According to Anastas and Warner, the most important aspect of green chemistry is the design of novel technological methods and synthetic pathways. In this context, the scientists focus on the prevention of pollution by relying on scientific and technological achievements instead of waste disposal [1].
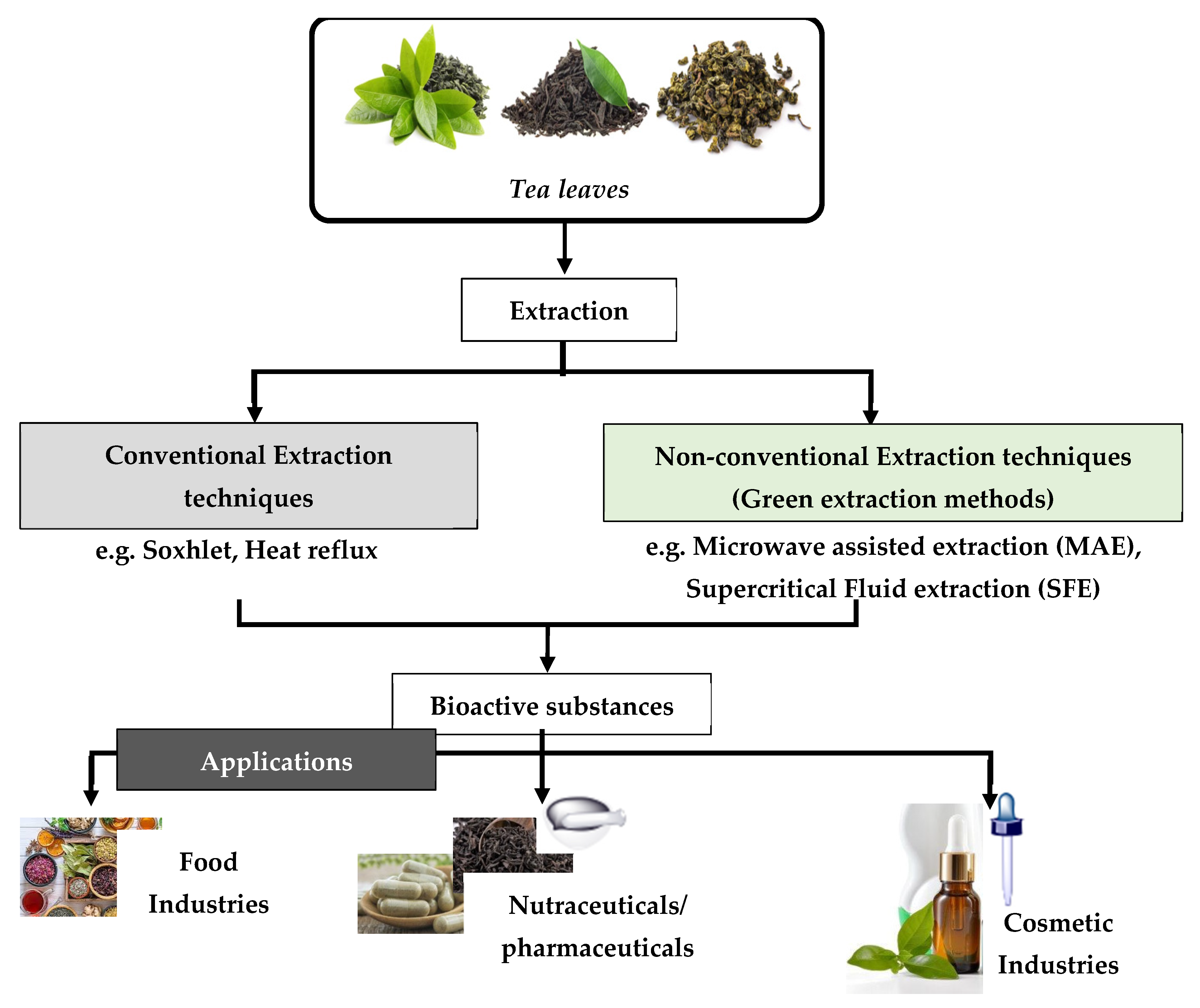
It must be mentioned that the isolation of natural products by the chemical industries has entailed negative effects on the environment. For example, such a process has required the consumption of 50% of the total energy of the ongoing industrial process. For this reason, the relevant industries turned their attention to a greener approach in order to produce natural products. This was achieved by developing new and pioneering technologies in order to save energy, as well as to decrease the usage of solvents, or to instead promote the usage of alternative solvents [2]. The desire to use alternative solvents arose following the widespread application of organic petrochemical solvents. This is due to the fact that such solvents caused negative effects not only to the environment, human health, and safety, but also to the global economy [3]. For this reason, the so-called “Green Extraction”—which is based on the design of different extraction processes for the reduction in energy consumption, as well as the usage of alternative solvents and renewable natural materials—was developed [3]. Figure 1 illustrates a flow diagram of the extraction methods of bioactive substances, as well as their application in the food, pharmaceutical, and cosmetic industries [4].
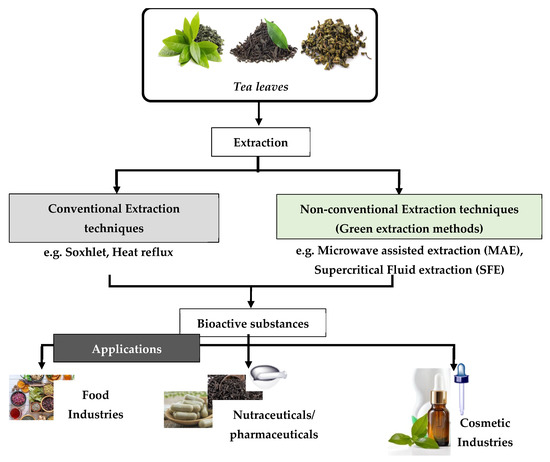
Figure 1.
Schematic diagram regarding the extraction of bioactive compounds from tea leaves and their applications.
Polyphenols, polysaccharides, amino acids, alkaloids, organic acids, proteins, and volatile components are the main bioactive compounds that have been determined in various tea species [4]. In recent years—in respect of the elimination of the disadvantages of conventional extraction methods—novel non-conventional extraction methods have been developed, such as: ultrasound-assisted; microwave-assisted; high-pressure; pressurized hot water; supercritical fluid; and deep eutectic extraction. All of this information, regarding bioactive compounds and the extraction techniques used, is discussed below in greater detail.
The main objective of this literature review is to present the current knowledge regarding the green extraction of tea species, as well as the future perspectives in respect of the extraction of safe active biomolecules from natural products. In the current literature review, the active ingredients contained in tea, the green extraction techniques, the selection criteria of green solvents, and the analytical techniques regarding the separation of the active ingredients of tea (wherein the last fifteen years of literature, although not exclusively, are focused upon) are summarized.
2. Active Ingredients Contained in Tea
Tea is the most popular daily beverage throughout the world, with an estimated daily consumption of more than 3 billion cups [5]. Its health benefits have also been confirmed by preclinical and epidemiological studies.
Tea is one of the main sources of caffeine intake through an average diet. Several species of tea, such as black, green, and white tea are prepared from the leaves of the Camellia sinensis plant, which is grown in at least 30 countries around the world [6,7]. Indeed, it is well known that the birthplace of tea is China [8]. Regarding the total amount of produced tea: 78% is black tea, which is consumed mainly in Western countries and certain Asian countries; 20% is green tea, which is consumed in Asia, North America, and Middle Eastern countries; and 2% is oolong tea, which is mainly produced in South China [9]. The Portuguese, on behalf of the Dutch, brought the tea to Europe for the first time in 1610. Rooibos tea, also known as red tea, is another tea species that is grown in South Africa. This type of tea contains aspalithin, which is a unique substance in contrast with the other types of tea [10]. This popular decoction is the subject of study for several scientists, due to its multiple uses and its benefits in respect of human health. Numerous research studies have proved that these extracts demonstrate antifungal, antiviral, antioxidant, antimutagenic, and anti-inflammatory properties [11]. These benefits derive from certain various contained compounds, such as polyphenols, alkaloids, vitamins, tannins, and flavonoids [12].
The type of tea is determined from the treatment of the Camelia sinensis leaves, which are collected within the first three years of the plant’s life. In order to produce black tea, the leaves are oxidized from two to three days, which is unlike green tea where the leaves are not oxidized. For this reason, green tea includes a high content of polyphenols [13]. In the initial stages of black tea production, the leaves contain both catechins and oxidation enzymes, which are found in different places on the leaf. The characteristic flavor and color of black tea is based on the production of theaflavins and thearubgins [9]. The production of white tea differs from green tea, due to the different time at which the leaves of Camellia sinensis are harvested. Regarding white tea, the collection of the leaves is carried out at the early stage of the plant’s development. This is in contrast to green tea, which involves the collection of leaves to be carried out in a later stage of the plant’s development [14]. Concerning oolong tea, the leaves are oxidized but not to the same degree of oxidation that occurs in relation to black tea leaves (which are semi-fermented) [15].
The main groups of biomolecules that are contained in a tea are: catechins, phenolic acids, and alkaloids. All these components show different bioactivity, while most of the time a mixture of them presents synergistic activity. It is worth noting that catechins and phenolic acids are responsible for the pharmacological action of tea. In detail, tea biomolecules mainly consist of non-protein amino acids, such as: theanine; free sugars; methylxanthine or purine alkaloids (such as caffeine); theobromine; theophylline and theacrine; as well as phenolic acids, such as gallic acid. This is in addition to eight other catechins. Moreover, the following catechins are also present: (+)-catechin (C); (−)-epicatechin (EC); (−)-gallocatechin (GC); (−)-epigallocatechin (EGC); (−)-catechin gallate (CG); (−)-gallocatechin gallate (GCG); (−)-epicatechin gallate (ECG); (−)-epigallocatechin gallate (EGCG); (−)-epicatechin (EC); (−)-epigallocatechin (EGC); (−)-epicatechin-3-gallate (ECG); and (−)-epigallocatechin-3-gallate (EGCG) [16,17].
The main groups of bioactive molecules contained in the various tea species are reported below.
2.1. Alkaloids
Methylxanthines
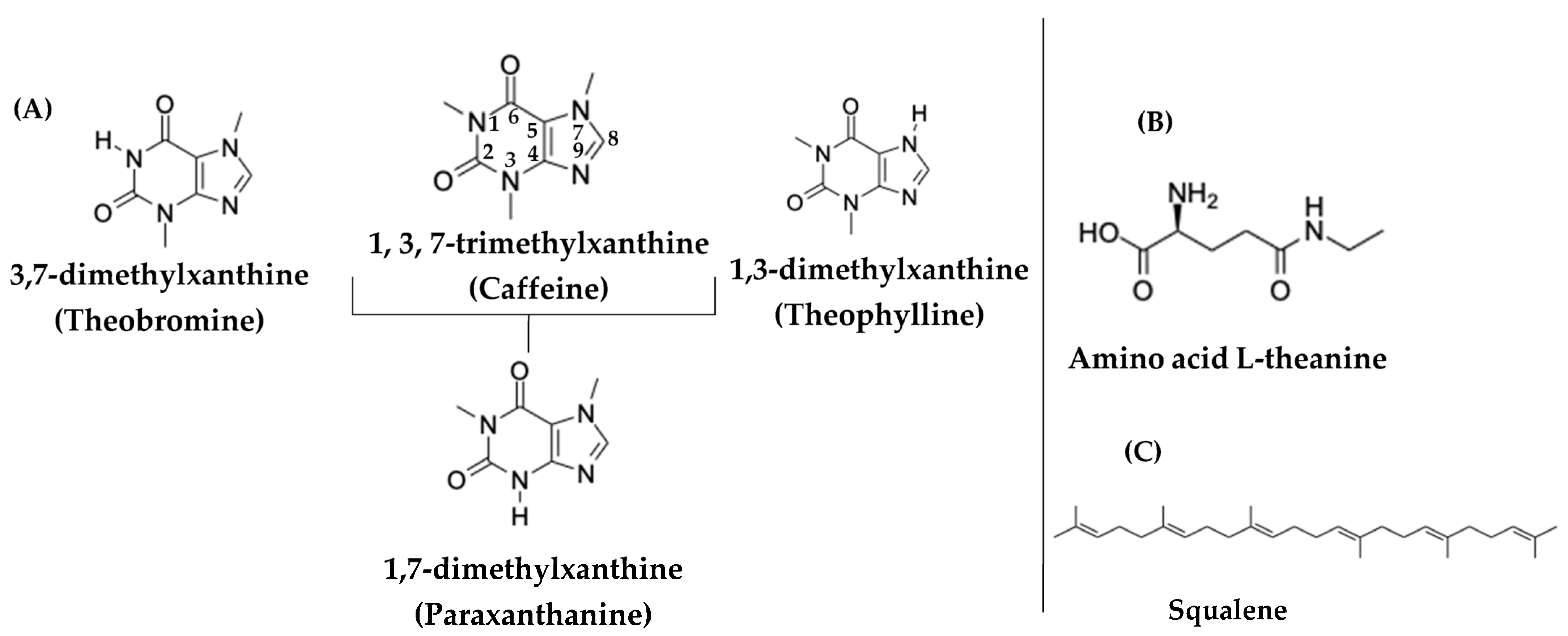
The category of methylxanthines—which are heterocyclic organic compounds and are methylated derivatives of xanthine—includes: theobromine, theophylline, and caffeine, which are all synthesized by plants through biosynthetic pathways [18]. In Figure 2, the three most basic methylxanthines are detailed, namely, caffeine, theobromine, theophylline, together with the major dimethylated metabolite of caffeine, i.e., paraxanthine [18].
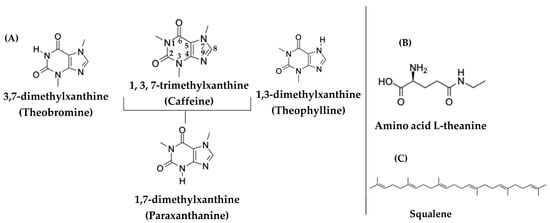
Figure 2.
(A) Chemical structures of the three main methylxanthines (caffeine, theophylline, and theobromine), as well as the caffeine metabolite, paraxanthine. (B) Chemical structure of the amino acid L-theanine in tea. (C) Chemical structure of squalene.
Methylxanthines cause various effects on the body’s physiology. They stimulate the respiratory, gastrointestinal, cardiovascular, and central nervous systems. They have been used not only for the treat of asthma and migraines, but also as antitussives and diuretics [19]. In addition, Aqel et al. reported that methylxanthines can reduce fatigue and improve learning ability. In contrast, when large doses are applied, they can also cause depression, hyperactivity, insomnia, headaches, nausea, dizziness, and anxiety [19].
Caffeine (1,3,7-trimethylxanthine) is a psychostimulant purine alkaloid that belongs to the class of methylxanthines (Figure 2A). The properties of caffeine arise through the three mechanisms of action [20]: (a) a mechanism in respect of the antagonism of adenosine receptors (mainly A1 and A2A receptors) in the central nervous system, thereby increasing the release of dopamine, noradrenaline, and glutamic acid; (b) a mechanism in respect of the induction of the release of intracellular calcium, which leads to the activation of NO synthase and NO synthesis; and finally (c) a mechanism in respect of the inhibition of phosphodiesterases and an increase in the cyclic adenosine monophosphate (cAMP) levels, which itself participates in many signaling pathways, including the adrenaline pathway. It is noted that the last two mechanisms are activated only at very high doses of caffeine, which a person cannot obtain solely through their diet [20].
2.2. Polyphenols
Catechins
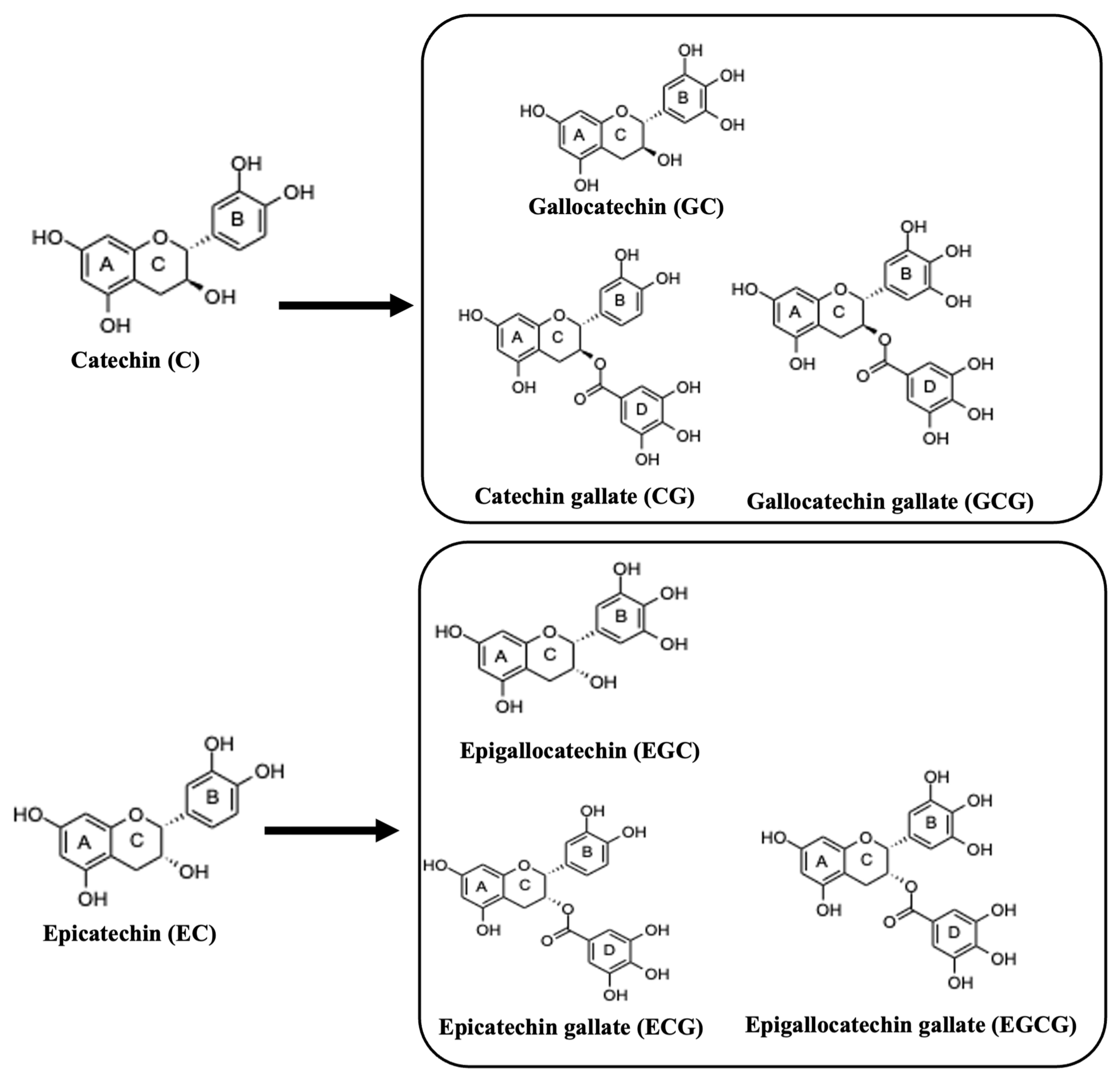
In addition to methylxanthines, tea contains a variety of other important bioactive molecules, such as catechins. Catechins are natural polyphenolic compounds (flavan-3-ols or flavanols), which belong to the flavonoid family. Catechins consist of two benzene rings, A and B, and a pyranic heterocyclic ring, C, with a hydroxyl group at carbon 3 (Figure 3). Figure 3 depicts the catechins and related conjugates of flavanols, which are created through esterification with the gallic acid groups, i.e., EG, EGC, and EGCG.
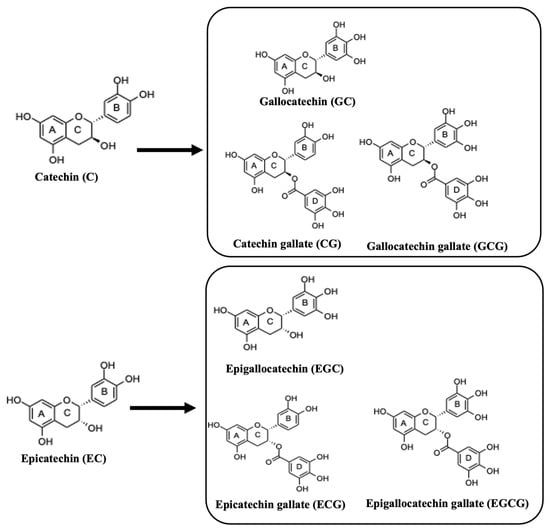
Figure 3.
The structures of catechins and the major derivatives of catechin in tea.
Figure 3 also shows EC, which is a stereoisomer of catechin. Catechins are found in high concentrations in fresh tea leaves. It has been reported that green tea shows the highest content of catechins when compared to other tea species, due to the different treatment techniques of tea leaves after harvesting [21]. According to Bernatoniene and Kopustinskiene, EGCG was found to be in large quantities in green tea [9,21,22]. The concentration of polyphenols in green tea (in one bag of green tea) was determined between 80 and 100 mg, while EGCG accounting for about 25 to 30 milligrams [9]. Moreover, catechins are mainly known for their antioxidant properties, while in recent years they have also shown their preventive abilities against cancer [9].
2.3. Flavonols
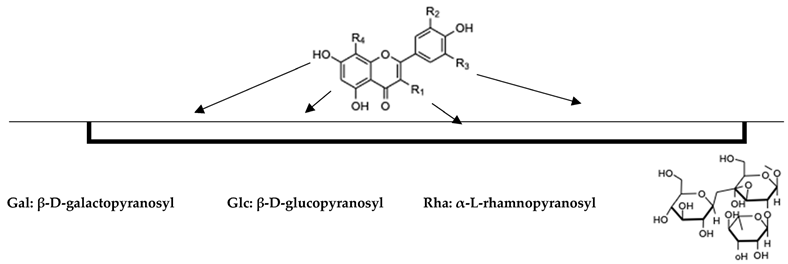
As is widely known, flavonols are also an important group of bioactive components in tea that are responsible for their antioxidant properties. Flavonols are mainly present in their glycosylated forms, as well as, to a lesser extent, in their non-glycosylated forms. Quercetin, kaempferol, and myricetin are the non-glycosylated moieties of the major flavonols that are found in tea leaves [23]. According to Chen et al., flavonols (including their glycosides) constitute 2–3% of the weight of dried tea leaves [23]. The most important flavonols in the Camellia sinensis plants were determined and are shown in Table 1.

Table 1.
The chemical structures of flavonols in the leaves and flowers of Camellia Sinensis.
2.4. Polysaccharides
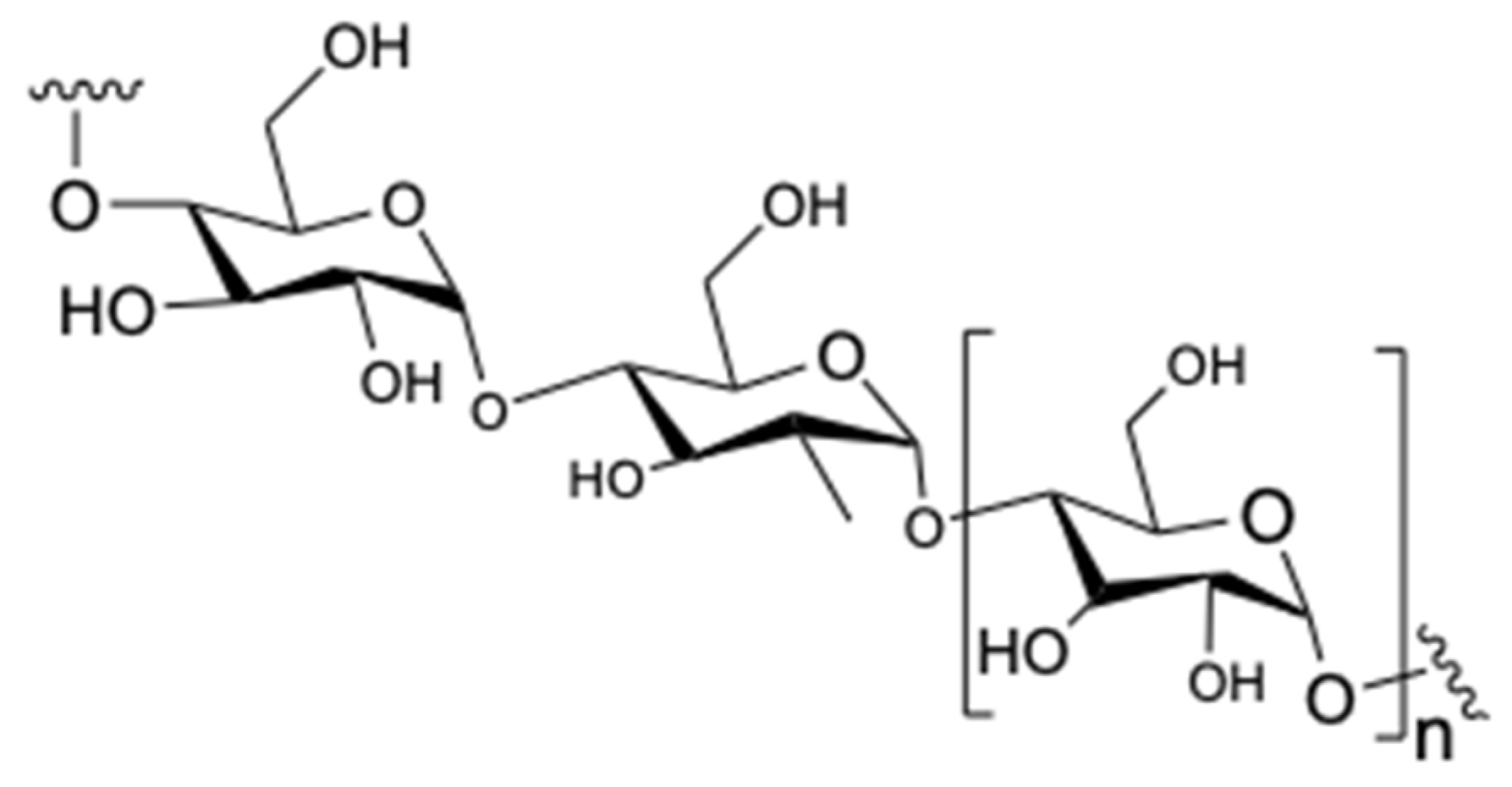
Polysaccharides are another group of compounds that are contained in tea species (Figure 4). In recent years, the scientific community has turned its attention to polysaccharides due to their excellent activity in these type of plants [6]. According to Nie and Xie, the tea leaves contain around 1.5–13% of polysaccharides [24,25]. Furthermore, polysaccharides contain various monosaccharides, such as glucose, rhamnose, arabinose, mannose, ribose, xylose, galactose, fucose, galacturonic acid, and glucuronic acid which are joined with different linkages [25].
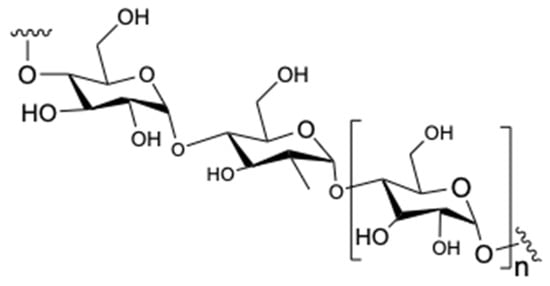
Figure 4.
Chemical structure of polysaccharides.
As is widely known, polysaccharides consist of 44.2% neutral sugar, 43.1% glyoxylate, and 3.5% protein [25]. Research shows that polysaccharides possess various biological activities, such as anti-tumor, anti-diabetic, antioxidant activities. [25,26,27].
Various tea plants contain different polysaccharide contents. Furthermore, it must be mentioned that different polysaccharides were determined by using different extraction methods in respect of the different parts of tea plants. The levels of tea-based polysaccharides that were found in tea leaves, tea flowers, and tea fruit peel were 3.64%, 5.24%, and 4.98%, respectively. Tea fruit peel contains 68.96% uronic acid, 14.25% protein, and seven monosaccharides, namely, mannose, glucose, galactose, arabinose, xylose, rhamnose, and fucose [25]. As Yao et al. reported, the tea leaves contain five monosaccharides: arabinose, xylose, fucose, glucose, and galactose; while tea seeds contain rhamnose, xylose, arabinose, glucose, galactose, galacturonic acid, and glucuronide [25]. Moreover, as Lv et al. reported, the polysaccharides of green tea consist of mannose, ribose, rhamnose, glucuronic acid, galacturonic acid, glucose, xylose, galactose, and arabinose [28].
The composition of polysaccharides is complex, which affects their biological activities. For this reason, sensitive and selective extraction methods of polysaccharides in respect of the different tea species must be developed.
2.5. Other Bioactive Molecules
Recent studies have shown that tea plants present protective properties against diseases, such as cardiovascular diseases, Alzheimer’s disease, and cancer [29,30]. According to Williams et al., the consumption of tea has been associated with improved cognitive, gastrointestinal, and cardiovascular function, as well as also improved metabolic health [30].
However, the beneficial properties of tea are not only due to the presence of flavonoids or catechins, but also to other bioactive molecules that are contained in tea. One of the bioactive molecules that appears to have a positive effect on health, is the amino acid L-theanine (γ-glutamylethylamide), which is abundant in the leaves of Camellia sinensis. The amino acid L-theanine has been associated with improved cognitive function, the protection of neurons from neurotoxic agents, the delay of neuronal death after transient ischemia, the compensation of the increase in pressure under stressful conditions, the enhancement of the effectiveness of anticancer drugs (such as idarubicin and doxorubicin) and the reduction in their toxicity, as well as the enhancement of function in respect of the immune system [30]. Figure 2B presents the chemical structure of the amino acid L-theanine (γ-glutamylethylamide).
According to Sheng et al., squalene is another bioactive compound that is contained in tea. However, the variation of squalene in various tea cultivars is unknown as of yet [31]. As shown in Figure 2C, squalene is a terpenoid (C30H50) and it is a precursor for the biosynthesis of sterols, such as cholesterol and steroid hormones in the human organism [31,32]. In recent years, several researchers have turned their attention to this bioactive compound due to its antioxidant, anticancer, antibacterial, and anti-inflammatory activities. This diverse range of biological activity in respect of squalene has led to its various applications in pharmaceutical and food industries [33,34]. As Wambulwa et al. reported, the concentration of squalene in tea leaves varied according to the development of tea shoots. In addition to this, the chemical composition of tea leaves depended on the different cultivars of tea [35].
3. Extraction of Natural Products
The most important stage of the process, in respect of the conventional extraction of natural products, is found in the usage of an appropriate solvent where the plant material will be in direct contact [36]. Often, the plant material is pulverized or crushed in order to increase the surface area that will be in contact with the solvent under stirring. The duration of the contact with the raw material in conjunction with the solvents is an important factor for the enhancement of extraction efficiency. Furthermore, it must be mentioned that the processes of most conventional extraction methods, such as maceration, are performed under stirring for the facilitating of mass transfer, which is achieved as a result of the enhancement of the extraction efficiency of bioactive substances.
Regarding extraction, both dried biomass or plant material, as well as fresh biomass (wet biomass) can be used. In the latter case, the sample preparation takes longer. However, minimal alteration of the isolated bioactive compounds is observed, via using fresh biomass. In the other hand, the fresh plant material contains a huge proportion of water, more than 70%. This often causes a problem during handling of the material due to the dilution of the extraction solvent. In contrast, the dried plant material is pulverized more easily to the appropriate size before extraction, which also helps to control the solvent/plant material ratio. This offers a greater reproducibility during experiments than the use of fresh plant material [36]. Extraction efficiency can be improved through the optimization of the time regarding: the contact with the plant material; the extraction solvent; the type of the plant; the fresh or dried material; the process of crushing or pulverization.
It is known that the traditional extraction methods encompass the solid–liquid extraction (SLE) techniques via the use of solvent and leaching [37]. The conventional extraction methods are found in: Soxhlet extraction, hydro-distillation, heat-reflux method, and maceration [38]. However, in recent years, the conventional techniques are not used very often due to certain disadvantages, such as the use of toxic solvents, as well as a huge time and energy consumption [38,39,40]. During the last few decades, due the disadvantages of the conventional methods, the scientific community has turned its attention to the development of more cost-effective and greener extraction methods of bioactive compounds from various natural products, such as tea plants [6,37].
Table 2 presents a brief description of the conventional, non-conventional, or green extraction methods of bioactive compounds from natural products. As shown in Table 2, the major experimental parameters for the efficient extraction of natural products are found in the type of solvent used and the temperature of extraction.

Table 2.
Conventional and non-conventional extraction methods of natural products.
Regarding the selection of the extraction solvents, various parameters—such as solubility of the active ingredients in them, their cost, and their security—should be considered. As is widely known, the polar bioactive compounds, such as flavonols, dissolve in polar solvents, while the non-polar ones, such as squalene, dissolve in non-polar solvents [39].
3.1. Conventional Extraction Methods
3.1.1. Conventional Maceration Extraction Using Water as Solvent
For the extraction of bioactive compounds using conventional maceration extraction, both hot and cold water have been used. The experimental factors that affect the concentration of the extracted compounds are found in the temperature of the extraction and the time of processing. A high temperature of extraction appears to increase the content of the bioactive compounds due to the probable promotion in the wetting of the tea samples and the increased of the solubility of the bioactive compounds. It is worthwhile to mention that the higher yield of bioactive compounds is associated with a longer extraction time. However, a longer extraction time in hot water causes thermal degradation of these biomolecules [41].
Although the extraction using cold water appears to present a higher yield for the extraction of bioactive molecules than hot water extraction, the process time when using cold water was much longer than the extraction time that is incurred when using hot water. Consequently, the longer extraction time that occurs when using cold water increases the risk of microbial contamination. This phenomenon may obstruct the utilization of these extracts within the food and the pharmaceutical industries [41].
As already mentioned above, the size of the tea particles is another important experimental factor that affects the extraction yield [39]. According to Bindes et al., a comparison of unground tea leaves and the ground tea leaves shows that the ground leaves increase the yields of bioactive compounds, especially polyphenols, by 14% [42]. Furthermore, the small particle diameter results the increase in the contact of tea samples with solvents. The decreased particle diameter is associated with the increased specific area, thus contributing to the increase in the contact area between tea samples and solvents [41]. It is noteworthy to mention that the particle size must be optimized. This is due to the fact that the tea leaves with particle sizes lower than 0.15 mm showed a decrease in the content of polyphenols, which was due to the formation of agglomerates. However, the optimization of the particle sizes of tea leaves should be studied in the future [42].
Conclusively, the usage of water as a solvent for the extraction of bioactive molecules is primarily determined by the issue of safety. However, as mentioned above, conducting extraction via using both hot and cold water shows several drawbacks. An extraction through using hot water causes a decomposition of the bioactive molecules; while, at the same time, the extraction time when using cold water is much longer than for hot-water-based extraction. Regarding the minimization of the drawbacks of the extraction method when using a water as a solvent, various organic solvents have been used.
3.1.2. Conventional Maceration Extraction Using Organic Solvents
The type of the used organic solvent for the purposes of extraction is dependent on the solubility of the bioactive compounds. For example, the comparison of chloroform, ethyl acetate, water, and methanol showed that methanol was the most appropriate solvent for the extraction of the polyphenols. It must be mentioned that the efficiency of the extraction in respect of the bioactive compounds increased up to an 8 h extraction time. In respect of a further extraction time, it was found that this slightly increased the efficiency, without substantial changes in the process. However, as is widely known, the longtime process of extraction should be prevented, in the interest of a lower cost and energy. Although extraction via using organic solvents has presented a lower cost and ease in respect of operations, there are, regardless, various disadvantages. These disadvantages may include: the existence of impurities in the extracts; the difficulty found in purifying the impurities; and the toxicity of the appropriate organic solvents. Therefore, one of the major drawbacks of using organic compounds is found in environmental pollution. For this reason, the development of greener extraction methods is a pressing requirement in order to address this issue [41].
In a general sense, maceration is characterized by the simplicity of the operation. However, the major disadvantages of such an approach are found in the low extraction yield and longer time that is required in completing the process [37].
3.1.3. Decoction
Decoction is considered another conventional method. This is where the extracts contain only substances that are soluble in water, due to the fact of water being used as a solvent. Although decoction presents a higher extraction efficiency for water-soluble substances, it cannot be used for the extraction of thermolabile substances [37]. As Zhang et al. reported, the high temperature of the extraction may enhance the dissolution of the water-soluble bioactive substances in comparison with approaches that utilize room temperature during the maceration process [37]. However, one of the major drawbacks of this method is that the high temperature used in the extraction can cause a transformation of certain bioactive compounds.
3.1.4. Hydro Distillation
The hydro distillation method is the oldest method that is still used for the extraction of essential oils and phenolics. Hydrolysis, hydro diffusion, and decomposition via heating are the main processes that are performed during the hydro distillation method. The disadvantages of such a method are found in the: long extraction time, high extraction temperature (which is, as a result, unsuitable for heat-sensitive compounds), and high energy consumption.
3.1.5. Reflux Extraction
The process of reflux extraction is conducted at a constant temperature, while the solvent is evaporated and condensed, repeatedly. The selection of the extraction temperature is dependent on the solvent that is used [39]. Indeed, when compared with percolation and maceration, the reflux extraction method demands a lesser volume of solvent and a smaller extraction time; however, the extraction efficiency is increased as a result [37].
3.1.6. Soxhlet Extraction
It is known that the Soxhlet extraction method is an automatic, continuous extraction method, which is based on the operating principle of the reflux method and siphoning. This conventional method incorporates the advantages of reflux extraction and percolation [37]. The selection of the extraction temperature depends on the used solvent, while process time can range from a few hours to up to 48 h. The main disadvantages of the Soxhlet method are found in low efficiency, the large volumes of the solvents that are required, the long extraction time, and the high extraction temperature. Due to the high extraction temperature, the Soxhlet method is not only unsuitable for thermolabile ingredients, but also increases the possibility of a thermal transformation of the bioactive compounds [39].
According to Zhang et al., the temperature of the extraction is one of the most important factors for the purposes of efficient extraction. The solubility and the diffusion of the active ingredients of the plants are enhanced via using a high temperature during the extraction. However, the high temperature may trigger a loss of the solvent, a creation of impurities in the extracts, and a possible decay of thermosensitive substances [37]. One of the drawbacks of the conventional extraction method is in the longer period of time that is required to conduct the extraction process, which thus results in a large energy cost. Based on all of the above, the development of greener extraction methods is thus encouraged.
3.2. Non-Conventional or Green Extraction Methods
During the few last decades, in order to overcome many of the drawbacks of the conventional extraction methods, green extraction techniques have been developed. Green extraction techniques present various advantages over the conventional approaches, such as a reduction in the consumption of the extraction solvent, the usage of non-hazardous substances, a reduction in the extraction time, and the consumption of less energy [37,39,40]. In recent years, the use of green extraction methods has become the new trend for the isolation of active ingredients from natural products, especially in respect of tea plants due to the fact that they promote a greater environmental sustainability [38]. The developed green extraction methods are: pressurized liquid extraction (PLE); microwave-assisted extraction (MAE); supercritical fluid extraction (SFE); ultrasound-assisted extraction (UAE); enzyme-assisted extraction (EAE); as well as the usage of eutectic mixtures and supercritical fluids that have been applied alone, or in a combination with the above mentioned techniques [7,37,39,40]. A brief description of the various green extraction methods used for natural products and, especially, for tea is provided in Table 2 and Table 3. Today, many scientific references highlight the advantages of the green extraction techniques over the conventional methods in terms of the extraction of bioactive compounds [7,37,38,39,40,41,42,43,44,45,46,47]. Table 3 details the advantages and the disadvantages of these advanced extraction techniques. In addition to their advantages, these methods also present their own disadvantages, such as: the non-uniformity in the distribution of ultrasound energy in respect of the UAE method; the lack of homogeneous results in respect of heating for the MAE method; the possible presence of inhibitors that effect the enzyme activity for the EAE method [40,43]. Moreover, the application of green extraction methods on an industrial scale is not easily achieved due to their disadvantages as reported in Table 3. Therefore, the challenge for the scientific community is to reduce their disadvantages and to implement all these green techniques on an industrial scale.

Table 3.
The main operative conditions, advantages, and disadvantages of the main green techniques for the purposes of extracting bioactive compounds from natural products.
3.3. Green Solvents
According to Choi and Verpoorte, an extraction in conjunction with a solvent is a crucial step in the sample preparation of natural products in the industrial production of antioxidants, aromas, antimicrobial compounds, etc. [48,49]. Although alcohols such as methanol and ethanol are the most common solvents used for the extraction of bioactive compounds from natural products, several other solvents are also used, such as chloroform, carbon tetrachloride, acetone, acetonitrile [4,39]. It is known that the extraction solvents that are produced from non-renewable resources are unsafe to the environment and thus are harmful to human health. In order to designate a solvent as a green solvent, there must be an account for not only the assessment of the environment, health, and safety, but also the energy demand. The main desired properties regarding the alternative solvents for green extraction are those of low toxicity, high solvency, and biodegradability. Moreover, the solvents used should originate from renewable resources and be recyclable—which, as a result, entails smaller environmental impacts [49]. The discovery of a suitable solvent is a challenge due to difficulty of appropriate selection. The factors to consider are dependent on the species of the plant, the target biomolecules, and the extraction method to be used.
Criteria of Green Solvent Selection
Some of the solvents used in the conventional methods are toxic, and as a result they pose a risk to both to the environment and to human health. For this reason, during the last few decades, more environmentally friendly solvents have been discovered and applied. According to Luczynska et al., the major principles of green chemistry are in the design of safer chemicals and more easily degradable products, as well as the use of renewable raw materials. The selection of extraction solvents must consider: low toxicity, the ability of quick mass transfer; and a low boiling point [2]. As Jessop reported, the main objective of the development of green solvents is in the reduction of toxic substances and the use of safer products [50]. In respect of this, certain green solvents are presented in Table 4.

Table 4.
Solvents for green extraction.
To date, ethanol, acetone, and chloroform are the most used solvents in the extraction of natural products, especially in respect of tea leaves [4,51]. As was mentioned above, various organic solvents may be toxic, necessitating the development of new “green” media with environmentally friendly requirements for the purposes of a sustainable environment.
The selection of the solvent is a crucial step for green extraction. It is worthwhile to mention that the good practices guidelines must be accounted for. At first, the evaluation of the possibility regarding the use of a “solvent-free” technique must be performed. Further, a 100% natural, agro-solvent must be used, especially when knowing the potentially related risks. The solvents that may be toxic and affect human health must be avoided. In addition, solvents with low volatile organic compounds and high-rate recyclability must be used. Moreover, solvents that reduce energy consumption and the cost of process must be used. One of the major issues is found in the suitability of the solvent within the respective industry that the extraction is being conducted for, as well as in respect of the maximal recovery of the solvent at the end of the procedure [49].
Water is considered a green solvent due to the fact that it is non-toxic, non-flammable, low cost, and environmentally friendly. As it is known as a polar solvent, it can extract polar compounds. However, it is not a good solvent for non-polar and semi-polar compounds [49,52]. An alternative solvent is subcritical water, which is water under different conditions of temperature, i.e., between the boiling point and critical point of water (which is found at 100 °C at 1 bar and 374 °C at 221 bar), as well in higher pressures where it is stable in a liquid state. The method that utilizes this solvent is the subcritical water extraction (SWE) technique. Indeed, subcritical water demonstrates a lower polarity, as a result of the hydrophobic organic compounds that are soluble in it [53]. Moreover, subcritical water can be used not only for the extraction of bioactive compounds, but also for the chromatographic analysis of the target analytes. Currently, the method of subcritical water extraction is popular due to the usage of such an excellent alternative solvent. Indeed, subcritical water—when compared to the conventional organic solvents—allows, through its application, for the extraction of various bioactive compounds, such as phenolic compounds and fatty acids [49,54].
Certain researchers have turned their attention to developing new solvents, such as DESs, which are considered to be promising substitutes of the more conventional solvents [4]. The usage of eutectic mixtures and supercritical fluids is considered to be a green solution and could eliminate the problem of using toxic solvents. Moreover, DESs are used for the extraction of bioactive compounds from tea species due their advantages over conventional solvents. For example, their low toxicity, thermal stability, low vapor pressure, biodegradability, and its low cost [4,49,55,56].
DESs are obtained from the combination of hydrogen bond donors (HBDs) and hydrogen bond acceptors (HBAs). The mixture of these components leads to the preparation of DESs with a lower melting point due to the hydrogen bonds and Van der Waals interactions that are formed [43,57]. In addition, DESs are mainly a mixture of quaternary ammonium salt (HBA) with metal salts (HBD). The mixing of specific HBA and HBD results in two types of DESs, i.e., hydrophilic and hydrophobic DESs. Hydrophilic DESs are created by the combination of quaternary salt with metal chloride or with a hydrated metal chloride. Hydrophobic DESs are a mixture of quaternary salt or metal chloride in conjunction with a hydrogen bond donor [43,56]. The most used DESs are a combination of choline chloride (ChCl) (as the HBA), with acetic acid and oxalic acid, combined with glycerol, xylitol, urea (as the HBD).
The implementation of non-conventional extraction methods—such as the ultrasound-assisted technique when using DESs as a solvent—have shown a higher extraction efficiency of bioactive compounds from natural products when compared to the UAE method when used with traditional solvents (e.g., ethanol). The advantages of a DES-based ultrasound-assisted extraction method are found in a decrease in the volume of solvents, a decrease in the consumption of energy, and an enhancement in the extraction yield of bioactive substances [43,58]. Moreover, according to Luo et al., the implementation of UAE-DES to the extraction of green tea has shown that the total phenolic content, the antioxidant activity, and the concentration of major catechins were enhanced when compared with those values achieved by more conventional methods [4].
In recent years, there has been an attempt to replace synthetic DESs with the corresponding natural sources of DESs, which are called natural deep eutectic solvents (NADESs). The formation of NADESs are a result of a combination of two or more natural substances, such as organic acids (e.g., lactic and citric acids), amino acids, sugars (e.g., sucrose and glucose), choline chloride. [59]. Similar to DESs, NADESs present a lower melting point than the traditional solvents. However, NADESs are also non-toxic, biodegradable, and sustainable when compared with synthetic DESs. Due to their advantages, the extracted bioactive compounds that are obtained as a result of using NADESs are considered safe to be used in the cosmetic and pharmaceutical industries [60,61,62].
In the effort of minimizing the use of solvents, solvent-free techniques have been developed. Indeed, solvent-free extraction techniques are popular as they can be utilized in the co-extraction of both lipophilic and hydrophilic substances, in addition to lipids, volatile and non-volatile substances. The advantages of solvent-free techniques are the decrease in risk associated with the organic solvents, the low cost, reduction in the risk of overpressure, and the ability to be scaled up [49,63]. Currently, various modern techniques based on solvent-free extraction, such as a new microwave solvent-free extraction technique called microwave hydro-diffusion and gravity (MHG), and pulsed electric fields (PEF) have been developed. These techniques improve the efficiency of the extraction as they reduce the extraction time and energy required, as well as eliminate the process step of treating wastewater. Moreover, according to Chemat et al., microwave hydro-diffusion and gravity (MHG) was developed for the extraction of various bioactive compounds, such as antioxidants and essential oils. In respect of this, the plant material is placed inside in a microwave reactor without any solvent. The microwaves increase the internal heating of the water of the plant material, thereby leading to the rupture of receptacles and glands due to the expansion of the cells [3].
4. Analytical Techniques for the Separation of Active Ingredients of Tea
Table 5 presents examples of the analytical methodologies that apply for the isolation of the bioactive molecules from the various species of tea. The specific study showed that catechins, alkaloids (such as caffeine), gallic acid, and flavonoids were the major extracted bioactive molecules that were isolated from several tea species. It is worth noting that the main isolated compound of Rooibos tea, which thrives in South Africa, was di-hydrochalcone aspalathin. In contrast, caffeine was not found in this tea [10]. Moreover, as has been demonstrated in this literature review, green tea has been widely studied at a rate of 52% of the available research studies, followed by black tea at 26%, and then white tea and oolong tea at 11% each. This, therefore, shows the importance of green tea due to certain valuable properties, such as its antiviral, antioxidant, antimutagenic, and anti-inflammatory traits.

Table 5.
Extraction and analysis methods for the determination of the bioactive molecules in various types of tea.
As can be seen from Table 5, several different extraction techniques—such as ultrasonic-assisted extraction, ultrasonic-assisted extraction with DESs, the microwave-assisted-extraction method, and the reflux method—have been used for the isolation of bioactive molecules on different tea species. Following the literature review in this study, the commonly used extraction methods were found to be the ultrasound-assisted and microwave-assisted methods. As mentioned above, a great effort has been made by scientists to develop green techniques for the extraction of bioactive molecules from several natural products. For this reason, in the last few decades, the application of green extraction methods, such as UAE-DESs, MAE, and SFE have been reported [4,8,43].
Table 5 also presents the analytical methods for the determination of the bioactive ingredients in the various types of tea. As is widely known, the substances in the extracts after using the different extraction methods are complex due to the fact that they contain a variety of bioactive molecules that require separation, purification, and identification [37]. From the literature review, it was clearly observed that chromatography and, more specifically, column chromatography are the most used analytical methods to identify the active ingredients of the tea. More specifically, regarding the determination of bioactive molecules, the most used analytical method is high-performance liquid chromatography (HPLC) combined with a photodiode array detector (PDA) and mass spectrophotometry (MS), at a usage rate of around 80%. This is followed by the UPLC and GC methods at 12% and 8%, respectively. Based on this, the selective chromatographic techniques and, mainly, liquid chromatography are widespread. Further, they are used for the determination of a cocktail of active ingredients that are extracted.
Sereshti et al. reported a simple ultrasound-assisted micro-solid phase extraction method based on graphene oxide (GO) nanoabsorbents (UAD-m-SPE); further, this was applied for the extraction of bioactive compounds, such as theophylline, theobromine, and caffeine from black, green, and white tea. The determination of these active ingredients was performed using HPLC-UV. Moreover, based on the specific research work, the following concentrations of active compounds were observed: For green tea: 30,0031.1 ng/mL caffeine, 462.4 ng/mL theophylline, and 1378.0 ng/mL theobromine; for black tea: 48,214.2 ng/mL caffeine, 471.4 ng/mL theophylline, and 6378.9 ng/mL theobromine; while for white tea: 18,161.5 ng/mL caffeine, 555.2 ng/mL theophylline, and 1641.5 ng/mL theobromine. These results clearly show the importance of the determination of the bioactive compounds in different tea species, as the type of bioactive compounds, their concentration, and thus their bioactivity are dependent on the tea species [12].
Novak et al. isolated, from green and black tea, the active compounds of gallic acid, EGC, C, EC, EGCG, and ECG while using the UAE method with water as the solvent. Japanese green tea showed: 1.435 mg/g gallic acid, 18.23 mg/g EGC, 0.785 mg/g C, 1.778 mg/g EC, 11.43 mg/g EGCG, and 3.058 mg/g ECG. Chinese green tea showed 0.711 mg/g gallic acid, 31.62 mg/g EGC, 5.764 mg/g C, 21.06 mg/g EC, 61.36 mg/g EGCG, and 33, 20 mg/g ECG [72]. In this research study, it was clearly seen that the concentration of the active ingredients depended on both the type of tea and the place of origin, which is also related to the climatic conditions.
Luo et al. reported the development of a green method, UAE-DES, which was used to extract the antioxidant polyphenols from green tea. Regarding this, DES ChCl-glycerol was selected as the green solvent. The application of the UAE-DES method demonstrated higher antioxidant activity and a concentration of catechins ((−)-EGC, (−)-EC, (−)-EGCG, and (−)-ECG)) when compared to the other extraction methods—i.e., UAE with ethanol, ethanol extraction, and hot water extraction. The results of this research support the use of this green technique for the extraction of antioxidant polyphenols from green tea, thereby showing its potential application in the food and pharmaceutical industries [4]. Moreover, according to Zhang et al., the use of DESs demonstrate a high extraction yield, i.e., 97% for epigallocatechin gallate and up to 82.7% for catechin [91].
According to Ghasemzadeh-Mohammadi et al., a comparison between the UAE and MAE methods in respect of a study of polyphenols, as well as of antioxidant activity, was performed. The utilization of the MAE method presented a higher efficiency than the UAE technique. Furthermore, the utilization of MAE showed a 125 ± 5 mg gallic acid/g dry weight and a 56 mg/g of phenol, while the UAE method showed a 96 ± 6 mg gallic acid/g dry weight of total polyphenols and 66 mg/g of phenol [94].
Thus, by taking the above into account, as well as the results of different research studies, the combination of MAE/UAE was shown to possess a higher extraction efficiency together instead of when each extraction technique was conducted alone. Moreover, for the combination of the MAE/ UAE techniques with a green solvent, the chitosan/ascorbic acid solvents appeared to be the best approach for the extraction of catechins from green tea [8].
5. Conclusions
Conventional methods—due to their drawbacks, such as high process time, as well as the high consumption of energy and organic solvents—have been proven to be damaging to the environment in comparison to alternative modern extraction methods. Consequently, researchers have turned their attention to the development of eco-friendly and sustainable green extraction methods for the efficient recovery of bioactive molecules from natural plants, especially in respect of various tea species.
In the last few decades, natural products, due to their antioxidant and antimicrobial activities, have been applied to the development of drugs. Nonetheless, the disadvantage regarding the time-consuming nature of the extraction of bioactive compounds prevents the widespread use of such natural products in the context of drug production.
In addition, following the literature review, several alternative green extraction methods were found to have been developed not only to improve the extraction efficiency of various bioactive substances and their bioactivity, but also in being friendly to the environment. The implementation of the non-conventional extraction methods has shown better extraction yields of non-structural modified bioactive molecules and lower extraction times [95,96]. In the long term, these methods are considered to be both ecological and cost-effective techniques. In addition to the former, these alternative green techniques present a decrease in the emissions of greenhouse gas, which, as a result, show a smaller environmental footprint [43].
Several different extraction techniques—such as ultrasonic-assisted extraction, ultrasonic-assisted extraction with DESs, the microwave assisted-extraction method, and the reflux method—were used for the isolation of bioactive molecules on different tea species. Following the literature review, it was found that the commonly used extraction methods were the ultrasound-assisted method and the microwave-assisted method. In the last few decades, the application of green extraction methods, such as UAE-DESs, MAE, and SFE, were reported.
Catechins, alkaloids (such as caffeine), gallic acid, and flavonoids were the major ex-traction bioactive molecules that were isolated from several tea species. In contrast with the other species of tea, the main isolated compound of Rooibos tea was dihydro-chalcone aspalathin, while caffeine was not found. It must be highlighted that the type of extracted bioactive molecules, the efficacy of the extraction, and the extraction kinetic properties depend on the nature of the tea species, the type of green extraction methods, and the extraction solvent.
Through this literature review, it was clearly shown that the scientific community continues in their research efforts to develop more sophisticated extraction methods due to the economic importance of commercial products that are rich with specific bioactive compounds, especially in respect of the pharmaceutical industry.
6. Future Perspectives
Further investigations are required for the optimization of the operation parameters of green extraction techniques. These future studies should investigate parameters such as the time and temperature of extraction; the pH; and the characteristics of the raw material, such as moisture, the particle size. All these parameters affect the mechanism of the interaction regarding the solute-solvent. In conclusion, the process regarding the green extraction of natural products is a novel concept that was developed not only for the protection of the environment and human health, but also for the enhancement of industries to be more ecological and economic.
As Calderón-Oliver and Ponce-Alquicira reported, the efficiency of the extraction must be validated for each material and extraction method separately [43]. However, the application of these processes in an industrial scale is not easily achieved. It is a challenge for the scientific community to implement all of these green techniques on an industrial scale. Consequently, researchers must investigate modern extraction methods at the pilot scale as well. Additionally, further study of the various mechanisms involved in green techniques is crucial to enhance their use in order to obtain extracts that are rich in bioactive compounds.
In the future, it will be necessary to study the combination of green extraction techniques with other industry strategies, such as the encapsulation at micro and nano scales. This is important for the purposes of creating stable final products with antioxidant properties, whereby they can be safely consumed by humans.
Author Contributions
E.H.: supervision, writing—original review and editing; I.M.K.: investigation, writing—review; Y.S.: investigation, editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to the University of Nicosia and especially to the Department of Health Sciences for their support to the implementation of this specific literature review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Luczynska, G.; Pena-Pereira, F.; Tobiszewski, M.; Namieśnik, J. Expectation-maximization model for substitution of missing values characterizing greenness of organic solvents. Molecules 2018, 28, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, J.R.; Li, H.B.; Wu, D.T.; Geng, F.; Corke, H.; Wei, X.L.; Ren-You Gan, R.Y. Green Extraction of Antioxidant Polyphenols from Green Tea (Camellia sinensis). Antioxidants 2020, 9, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, Z.X. Variations of main quality components of tea genetic resources (Camellia sinensis (L.) O. Kuntze) preserved in the China national germplasm tea repository. Plant Food Hum. Nutr. 2005, 60, 31–35. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.L.; Liang, L.; Huang, L.L.; Yu, G.Y.; Li, Q.H. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef]
- Banerjee, S.; Chatterjee, J. Efficient extraction strategies of tea (Camellia sinensis) biomolecules. J. Food Sci. Technol. 2015, 52, 3158–3168. [Google Scholar] [CrossRef]
- Fujioka, K.; Salaheldin, T.A.; Godugu, K.; Meyers, H.V.; Mousa, S.A. Edible Green Solvent for Optimized Catechins Extraction from Green Tea Leaves: Anti-Hypercholesterolemia. J Pharm. Pharmacol. Res. 2022, 6, 80–92. [Google Scholar] [CrossRef]
- Kanwar, J.; Taskeen, M.; Mohammad, I.; Huo, C.; Chan, T.H.; Dou, Q.P. Recent advances on tea polyphenols. Front Biosci. 2012, 4, 111–131. [Google Scholar] [CrossRef]
- van Heerden, F.R.; van Wyk, B.E.; Viljoen, A.M.; Steenkamp, P.A. Phenolic variation in wild populations of Aspalathus linearis (rooibos tea). Biochem. Syst. Ecol. 2003, 31, 885–895. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, B.; Row, K. Extraction of Catechin Compounds from Green Tea with a New Green Solvent. Chem. Res. Chin. Univ. 2014, 30, 37–41. [Google Scholar] [CrossRef]
- Sereshti, H.; Khosraviani, M.; Samadi, S.; Amini-Fazl, M.S. Simultaneous determination of theophylline, theobromine and caffeine in different tea beverages by graphene-oxide based ultrasonic-assisted dispersive micro solid-phase extraction combined with HPLC-UV. RSC Adv. 2014, 4, 47114–47120. [Google Scholar] [CrossRef]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural Properties of Green Tea Catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef]
- Chin, J.M.; Merves, M.L.; Goldberger, B.A.; Sampson-Cone, A.; Cone, E.J. Caffeine content of brewed teas. J. Anal. Toxicol. 2008, 32, 702–704. [Google Scholar] [CrossRef]
- Ng, K.W.; Cao, Z.J.; Chen, H.B.; Zhao, Z.Z.; Zhu, L.; Yi, T. Oolong tea: A critical review of processing methods, chemical composition, health effects, and risk. Crit. Rev. Food Sci. Nutr. 2017, 58, 2957–2980. [Google Scholar] [CrossRef]
- Unachukwu, U.J.; Ahmed, S.; Kavalier, A.; Lyles, J.T.; Kennelly, E.J. White and green teas (Camellia sinensis var. sinensis): Variation in phenolic, methylxanthine, and antioxidant profiles. J. Food Sci. 2010, 75, 541–548. [Google Scholar]
- Peng, L.; Song, X.; Shi, X.; Li, J.; Ye, C. An improved HPLC method for simultaneous determination of Phenolic compounds purine alkaloids and theanine in Camellia species. J. Food Compos. Anal. 2008, 21, 559–563. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Dias, T.R.; Alves, M.G.; Oliveira, P.F.; Monteiro, M.P.; Silva, B.M. Anti-obesity potential of natural methylxanthines. J. Funct. Foods 2018, 43, 84–94. [Google Scholar] [CrossRef]
- Aqel, A.; Almulla, A.; Al-Rifai, A.; Wabaidur, S.M.; ALOthman, Z.A.; Badjah-Hadj-Ahmed, A.-Y. Rapid and Sensitive Determination of Methylxanthines in Commercial Brands of Tea Using Ultra-High-Performance Liquid Chromatography-Mass Spectrometry. Int. J. Anal. Chem. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, P.; Dias, V.T.; Benvegnú, D.M.; Boufleur, N.; Barcelos, R.C.S.; Segat, H.J.; Pase, C.S.; dos Santos, C.M.M.; Flores, É.M.M.; Bürger, M.E. Antioxidant protection of gallic acid against toxicity induced by Pb in blood, liver and kidney of rats. Toxicol. Rep. 2016, 3, 351–356. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Zeng, L.; Dong, F.; Tu, Y.; Yang, Z. Occurrence of Functional Molecules in the Flowers of Tea (Camellia sinensis) Plants: Evidence for a Second Resource. Molecules 2018, 23, 790–806. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.P.; Xie, M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Yao, J.; Liu, H.; Ma, C.; Pu, L.; Yang, W.; Lei, Z. A Review on the Extraction, Bioactivity, and Application of Tea Polysaccharides. Molecules 2022, 27, 4679–4691. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Z.Y.; Li, T.Q.; Song, W.; Xiao, W.; Zheng, J.; Chen, H.; Chen, G.H.; Zou, H.Y. Anti-tumor activity and the mechanism of a green tea (Camellia sinensis) polysaccharide on prostate cancer. Int. J. Biol. Macromol. 2019, 122, 95–103. [Google Scholar] [CrossRef]
- Wang, H.S.; Chen, J.R.; Ren, P.F.; Zhang, Y.W.; Onayango, S.O. Ultrasound irradiation alters the spatial structure and improves the antioxidant activity of the yellow tea polysaccharide. Ultrason. Sonochem. 2021, 70, 105355. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, X.B.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z.Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A. Caffeine in tea Camellia sinensis-Content, absorption, benefits and risks of consumption. J. Nutr. Health Aging 2013, 18, 143–149. [Google Scholar] [CrossRef]
- Williams, J.; Sergi, D.; McKune, A.J.; Georgousopoulou, E.N.; Mellor, D.D.; Naumovski, N. The beneficial health effects of green tea amino acid l-theanine in animal models: Promises and prospects for human trials. Phytother. Res. 2019, 33, 571–583. [Google Scholar] [CrossRef]
- Sheng, Y.Y.; Xiang, J.; Wang, K.R.; Li, Z.Y.; Li, K.; Lu, J.L.; Ye, J.H.; Liang, Y.R.; Zheng, X.Q. Extraction of Squalene from Tea Leaves (Camellia sinensis) and Its Variations with Leaf Maturity and Tea Cultivar. Front Nutr. 2022, 9, 755514. [Google Scholar] [CrossRef]
- Kim, S.K.; Karadeniz, F. Biological importance and applications of squalene and squalane. Adv. Food Nutr. Res. 2012, 65, 223–233. [Google Scholar]
- Kotelevets, L.; Chastre, E.; Caron, J.; Mougin, J.; Bastian, G.; Pineau, A. A squalene-based nanomedicine for oral treatment of colon cancer. Cancer Res. 2017, 77, 2964–2975. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Martinez-Beamonte, R.; Sanclemente, T.; Surra, J.C.; Herrera-Marcos, L.V.; Sanchez-Marco, J. Current insights into the biological action of squalene. Mole Nutr. Food Res. 2018, 62, 1800136–1800152. [Google Scholar] [CrossRef]
- Wambulwa, M.C.; Meegahakumbura, M.K.; Kamunya, S.; Wachira, F.N. From the wild to the cup: Tracking footprints of the tea species in time and space. Front Nutr. 2021, 8, 706770–706778. [Google Scholar] [CrossRef]
- Mason, T.J.; Chemat, F.; Vinatoru, M. The Extraction of Natural Products using Ultrasound or Microwaves. Curr. Org. Chem 2011, 15, 237–247. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20–46. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- García, S.L.R.; Raghavan, V. Green extraction techniques from fruit and vegetable waste to obtain bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 6446–6466. [Google Scholar] [CrossRef]
- Mena- García, A.; Ruiz-Matute, A.I.; Soria, A.C.; Sanz, M.L. Green techniques for extraction of bioactive carbohydrates. TrAC Trend Anal. Chem. 2019, 119, 115612–115622. [Google Scholar]
- Li, H.; Guo, H.; Luo, Q.; Wu, D.T.; Zou, L.; Liu, Y.; Li, H.B.; Gan, R.Y. Current extraction, purification, and identification techniques of tea polyphenols: An updated review. Crit. Rev. Food Sci. Nutr. 2021, 1–19. [Google Scholar] [CrossRef]
- Bindes, M.M.M.; Cardoso, V.L.; Reis, M.H.M.; Boffito, D.C. Maximisation of the polyphenols extraction yield from green tea leaves and sequential clarification. J. Food Eng. 2019, 241, 97–104. [Google Scholar] [CrossRef]
- Calderón-Oliver, M.; Ponce-Alquicira, E. Environmentally Friendly Techniques and Their Comparison in the Extraction of Natural Antioxidants from Green Tea, Rosemary, Clove, and Oregano. Molecules 2021, 26, 1869–1889. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. Curr. Opin. Food Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Vinatoru, M.; Mason, T.J.; Calinescu, I. Ultrasonically assisted extraction (UAE) and microwave assisted extraction (MAE) of functional compounds from plant materials. Trend Anal. Chem. 2017, 97, 159–178. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trend Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Both, S.; Chemat, F.; Strube, J. Extraction of polyphenols from black tea—Conventional and ultrasound assisted extraction. Ultrason. Sonochem. 2014, 21, 1030–1034. [Google Scholar] [CrossRef]
- Choi, Y.H.; Verpoorte, R. Metabolomics: What you see is what you extract. Phytochem. Anal. 2014, 25, 289–290. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Tixier, A.S.F. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007–3034. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for green solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014–117025. [Google Scholar] [CrossRef]
- Mihaylova, D.; Lante, A. Water an Eco-Friendly Crossroad in Green Extraction: An Overview. Open Biotechnol. J. 2019, 13, 155–162. [Google Scholar] [CrossRef]
- Carr, A.G.; Mammucari, R.; Foster, N.R. A review of subcritical water as a solvent and its utilization for the processing of hydrophobic organic compounds. Chem. Eng. J. 2011, 172, 1–17. [Google Scholar] [CrossRef]
- Zakaria, S.M.; Kamal, S.M.M.; Harun, M.R.; Omar, R.; Siajam, S.I. Subcritical water technology for extraction of phenolic compounds from Chlorella sp. microalgae and assessment on its antioxidant activity. Molecules 2017, 22, 1105–1119. [Google Scholar] [CrossRef]
- Li, J.; Han, Z.; Zou, Y.; Yu, B. Efficient extraction of major catechins in Camellia sinensis leaves using green choline chloride-based deep eutectic solvents. RSC Adv. 2015, 5, 93937–93944. [Google Scholar] [CrossRef]
- Cai, C.; Li, F.; Liu, L.; Tan, Z. Deep eutectic solvents used as the green media for the efficient extraction of caffeine from Chinese dark tea. Sep. Purif. Technol. 2019, 227, 115723–115731. [Google Scholar] [CrossRef]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, M. Optimization of deep eutectic solvent-based ultrasound-assisted extraction of polysaccharides from Dioscorea opposita Thunb. Int. J. Biol. Macromol. 2017, 95, 675–681. [Google Scholar] [CrossRef]
- Espino, M.; Fernández, M.A.; Gomez, F.J.V.; Silva, M.F. Natural designer solvents for greening analytical chemistry. TRaC Trend Anal. Chem. 2016, 76, 126–136. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Neil Winterton, N. The green solvent: A critical perspective. Clean Technol. Environ. Policy 2021, 23, 2499–2522. [Google Scholar] [CrossRef]
- Lavaud, A.; Laguerre, M.; Bitric, S.; Fabiano Tixier, A.-S.; Roller, M.; Chemat, F. International. Patent WO 2016/162703 Al, 10 April 2015. Available online: https://worldwide.espacenet.com/publicationDetails/biblio?DB=EPODOC&II=4&ND=3&adjacent=true&locale=fr_EP&FT=D&date=20180406&CC=CN&NR=107889468A&KC=A# (accessed on 17 August 2019).
- Kerton, F.M.; Mariotte, R. Alternative Solvents for Green Chemistry, 2nd ed.; Royal Society of Chemistry: Croydon, UK, 2013; pp. 1–325. [Google Scholar]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catechins, caffeine and gallic acids in green, oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Komes, D.; Horžić, D.; Belščak, A.; Kovačević Ganič, K.; Baljak, A. Determination of caffeine content in tea and maté tea by using different methods. Czech J. Food Sci. 2009, 27, S213–S216. [Google Scholar] [CrossRef]
- Sereshti, H.; Samadi, S.; Jalali-Heravi, M. Determination of volatile components of green, black, oolong and white tea by optimized ultrasound-assisted extraction-dispersive liquid–liquid microextraction coupled with gas chromatography. J. Chromatogr. A 2013, 1280, 1–7. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Manchón, N.; D’Arrigo, M.; Guillamón, E.; Villares, A.; García-Lafuente, A.; Ramos, A.; Martínez, J.A. Fast and simultaneous determination of phenolic compounds and caffeine in teas, mate, instant coffee, soft drink and energetic drink by high-performance liquid chromatography using a fused-core column. Anal. Chim. Acta 2011, 685, 204–211. [Google Scholar] [CrossRef]
- Wei, K.; Wang, L.Y.; Zhou, J.; He, W.; Zeng, J.M.; Jiang, Y.W.; Cheng, H. Comparison of catechins and purine alkaloids in albino and normal green tea cultivars (Camellia sinensis L.) by HPLC. Food Chem. 2012, 130, 720–724. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, S.H. Extraction behavior of caffeine and EGCG from green and black tea. Biotechn. Bioprocess. Eng. 2008, 13, 646–649. [Google Scholar] [CrossRef]
- Bae, I.K.; Ham, H.M.; Jeong, M.H.; Kim, D.H.; Kim, H.J. Simultaneous determination of 15 phenolic compounds and caffeine in teas and mate using RP-HPLC/UV detection: Method development and optimization of extraction process. Food Chem. 2015, 172, 469–475. [Google Scholar] [CrossRef]
- Rahim, A.A.; Nofrizal, S.; Saad, B. Rapid tea catechins and caffeine determination by HPLC using microwave-assisted extraction and silica monolithic column. Food Chem. 2014, 147, 262–268. [Google Scholar] [CrossRef]
- Novak, I.; Šeruga, M.; Komorsky-Lovrić, Š. Characterisation of catechins in green and black teas using square-wave voltammetry and RP-HPLC-ECD. Food Chem. 2010, 122, 1283–1289. [Google Scholar] [CrossRef]
- Scoparo, C.T.; de Souza, L.M.; Dartora, N.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Analysis of Camellia sinensis green and black teas via ultra high performance liquid chromatography assisted by liquid–liquid partition and two-dimensional liquid chromatography (size exclusion × reversed phase). J. Chromatogr., A 2012, 1222, 29–37. [Google Scholar] [CrossRef]
- Yuda, N.; Tanaka, M.; Suzuki, M.; Asano, Y.; Ochi, H.; Iwatsuki, K. Polyphenols Extracted from Black Tea (Camellia sinensis) Residue by Hot-Compressed Water and Their Inhibitory Effect on Pancreatic Lipasein vitro. J. Food Sci. 2012, 77, H254–H261. [Google Scholar] [CrossRef]
- Jiang, H.; Engelhardt, U.H.; Thräne, C.; Maiwald, B.; Stark, J. Determination of flavonol glycosides in green tea, oolong tea and black tea by UHPLC compared to HPLC. Food Chem. 2015, 183, 30–35. [Google Scholar] [CrossRef]
- Fernando, C.D.; Soysa, P. Extraction Kinetics of phytochemicals and antioxidant activity during black tea (Camellia sinensis L.) brewing. Nutr. J. 2015, 31, 14–74. [Google Scholar] [CrossRef]
- Xu, L.L.; Chen, Y.; Chen, Z.Q.; Gao, X.D.; Wang, C.L.; Panichayupakaranant, P.; Chen, H.X. Ultrafiltration isolation, physicochemical characterization, and antidiabetic activities analysis of polysaccharides from green tea, oolong tea, and black tea. J. Food Sci. 2020, 85, 4025–4032. [Google Scholar] [CrossRef]
- Wang, H.; Helliwell, K.; You, X. Isocratic elution system for the determination of catechins, caffeine and gallic acid in green tea using HPLC. Food Chem. 2000, 68, 115–121. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Tan, S.P.; Stathopoulos, C.E.; Roach, P.D. Improved extraction of green tea components from teabags using the microwave oven. J. Food Compos. Anal. 2012, 27, 95–101. [Google Scholar] [CrossRef]
- He, X.; Li, J.; Zhao, W.; Liu, R.; Zhang, L.; Kong, X. Chemical fingerprint analysis for quality control and identification of Ziyang green tea by HPLC. Food Chem. 2015, 171, 405–411. [Google Scholar] [CrossRef]
- Demir, E.; Serdar, G.; Sökmen, M. Comparison of Some Extraction Methods for Isolation of Catechins and Caffeine from Turkish Green Tea. Int. J. Second. Metab. 2015, 2, 16–25. [Google Scholar] [CrossRef]
- Hwang, H.J.; Kim, Y.G.; Chung, M.S. Improving the Extraction of Catechins of Green Tea (Camellia sinensis) by Subcritical Water Extraction (SWE) Combined with Pulsed Electric Field (PEF) or Intense Pulsed Light (IPL) Pretreatment. Foods 2021, 10, 3092–3105. [Google Scholar] [CrossRef]
- Perva-Uzunalic, A.; Skerget, M.; Knez, Z.; Weinreich, B.; Otto, F.; Gruner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Selvi, I.K.; Nagarajan, S. Separation of catechins from green tea (Camellia sinensis L.) by microwave assisted acetylation, evaluation of antioxidant potential of individual components and spectroscopic analysis. Food Sci. Technol. 2018, 91, 391–397. [Google Scholar]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Ayyildiz, S.S.; Karadeniz, B.; Sagcan, N.; Bahar, B.; Abdullah, A.; Alasalvar, C. Optimizing the extraction parameters of epigallocatechin gallate using conventional hot water and ultrasound assisted methods from green tea. Food Bioprod. Process. 2018, 111, 37–44. [Google Scholar] [CrossRef]
- Jun, X.; Deji, S.; Shou, Z.; Bingbing, L.; Ye, L.; Rui, Z. Characterization of polyphenols from green tea leaves using a high hydrostatic pressure extraction. Int. J. Pharm. 2009, 382, 139–143. [Google Scholar]
- Das, P.R.; Eun, J.B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29. [Google Scholar] [CrossRef]
- Xiang, B.; Zhou, X.; Qin, D.; Xi, J. Vesicle-enhanced liquid-phase pulsed discharge extraction of polyphenols from green tea leaves. Innov. Food Sci. Emerg. 2021, 74, 102839. [Google Scholar] [CrossRef]
- Sökmen, M.; Demir, E.; Alomar, S.Y. Optimization of sequential supercritical fluid extraction (SFE) of caffeine and catechins from green tea. J. Supercrit. Fluids 2018, 133, 171–176. [Google Scholar] [CrossRef]
- Bermejo, D.V.; Ibánez, E.; Reglero, G.; Fornari, T. Effect of cosolvents (ethyl lactate, ethyl acetate and ethanol) on the supercritical CO2 extraction of caffeine from green tea. J. Supercrit. Fluids 2016, 107, 507–512. [Google Scholar] [CrossRef]
- Edwards, Q.A.; Hinkson, S.A.S.; Garner-O’Neale, L.D.; Kulikov, S.M. Quantification of caffeine in selected beverages via gas chromatography-mass spectroscopy. Int. J. Chem. Sci. 2015, 13, 133–142. [Google Scholar]
- Sharif, R.; Ahmad, S.W.; Anjum, H.; Ramzan, N.; Malik, S.R. Effect of infusion time and temperature on decaffeination of tea using liquid-liquid extraction technique. J. Food Process. Eng. 2014, 37, 46–52. [Google Scholar] [CrossRef]
- Ghasemzadeh-Mohammadi, V.; Zamani, B.; Afsharpour, M.; Mohammadi, A. Extraction of caffeine and catechins using microwave-assisted and ultrasonic extraction from green tea leaves: An optimization study by the IV-optimal design. Food Sci. Biotechnol. 2017, 26, 1281–1290. [Google Scholar] [CrossRef]
- Silva, A.S.; Reboredo-Rodríguez, P.; Sanchez-Machado, D.I.; López-Cervantes, J.; Barreca, D.; Pittala, V.; Samec, D.; Orhan, I.E.; Gulcan, H.O.; Forbes-Hernandez, T.Y.; et al. Evaluation of the status quo of polyphenols analysis: Part II—Analysis methods and food processing effects. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3219–3240. [Google Scholar] [CrossRef]
- Molina, G.A.; González-Fuentes, F.; Loske, A.M.; Fernández, F.; Estevez, M. Shock wave-assisted extraction of phenolic acids and flavonoids from Eysenhardtia polystachya heartwood: A novel method and its comparison with conventional methodologies. Ultrason. Sonochem. 2020, 61, 104809. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).