Abstract
Bearing in mind the centuries-old traditional use of chamomile, but also the increasing demand for its products in modern industry, oriented toward sustainable development, there are increasing efforts for the efficient extraction of high-value compounds of this plant, as well as obtaining its products with added value. With that goal, conventional and contemporary separation techniques were applied in this work. Both hydrodistillation processes (HD), conducted in a traditional manner and coupled with microwave irradiation (MWHD), were used for essential oil isolation. In parallel with those procedures, chamomile lipophilic extracts were obtained by Soxhlet extraction applying organic solvents and using supercritical fluid extraction as a greener approach. The obtained extracts and essential oils were characterized in terms of chemical composition (GC analysis, contents of total phenolics and flavonoids) and biological potential. GC analysis revealed that oxygenated sesquiterpenes and non-terpene compounds were the dominant compounds. α-Bisabolol oxide A (29.71–34.41%) and α-bisabolol oxide B (21.06–25.83%) were the most abundant individual components in samples obtained by distillation while in supercritical and Soxhlet extracts, major compounds were α-bisabolol oxide A and pentacosane. The biological potential of essential oils and extracts was tested by applying a set of analyzes to estimate the inhibition of biologically important enzymes (amylase, glucosidase, acetylcholinesterase, butyrylcholinesterase, tyrosinase) and antioxidant capacity (DPPH, ABTS, CUPRAC, FRAP, chelating and total antioxidant capacity). The results suggested essential oils as better antioxidants, while the extracts were proven to be better inhibitors of the tested enzymes. Principal Component Analysis was conducted using the experimental results of the composition of extracts and EOs of chamomile obtained by different separation techniques, showing clear discrimination between methods applied in correlation with the chemical profile. Molecular docking was applied for the identification of the main active principles present in the essential oil, among which α-bisabolol-oxide B (cp3) showed a higher affinity for tyrosinase.
1. Introduction
Chamomile essential oil is an aromatic liquid with intensive blue color that comes from azulenes (chamazulene). It is obtained by hydrodistillation from flower heads of Matricaria chamomila L. (Asteraceae). Due to its beneficial effects, it is widely used in liquid or semi-solid formulations or as a bath additive for skin inflammation, in products for oral hygiene, and make-up or shampoos [1]. Due to the pronounced biological activity and specific chemical composition of essential oils, there is a constant increase in their usage in modern pharmacy and medicine. Essential oils are plant products, mixtures of aromatic and volatile compounds of low molecular weight (usually below 500 Da) readily soluble in organic solvents and lipids. Their lipophilic nature, as well as theirlow molecular weight, enable them to facilitate passage through the cell membrane. After their entrance into cells, where may exhibit different biological effects, such as antimicrobial activity through the inhibition of cell membrane synthesis and disruption of the structure of certain enzyme systems or simply by reducing the concentration of enzyme systems (HMG-Co Reductase) [2]. In addition to antimicrobial activity, essential oils have been used in the treatment and control of various diseases such as cardiovascular disease, diabetes, Alzheimer’s disease, or cancer [3]. A pronounced anti-inflammatory activity in some chamomile essential oils is also reported [4].
The wide range of activities is a consequence of the significant content of various bioactive compounds in essential oils, with α-bisabolol and its oxides of α-bisabolol A and B, as well as chamazulene and farnesene, as main constituents [5]. On the other side, the concentration levels of these compounds depend on various factors. In order to obtain essential oils with a richer composition, the process of their isolation is extremely important. The basic method of obtaining essential oil involves steam distillation or hydrodistillation as the standard technique. In order to speed up the process, reduce energy consumption, preserve the thermolabile compounds at the same time, and enrich the composition of the oil, greener extraction techniques have been developed. This includes combining hydrodistillation with ultrasound or microwaves, but also the use of alternative solvents such as supercritical carbon dioxide or Natural Deep Eutectic Solvents (NADES solvents) [6]. A combination of hydrodistillation and microwave irradiation (MWHD) significantly saves time and has been successfully used to obtain essential oils from various plant matrices [7,8]. Lucchesi et al. [9] have performed a comparative study of solvent-free microwave extraction of essential oil from aromatic herbs with conventional hydrodistillation. The obtained results showed a higher yield of essential oil with higher amounts of more valuable oxygenated compounds, saving costs, energy, and plant material at the same time. In the study conducted by Pavlić et al. [10], peppermint was extracted by both conventional HD and MWHD techniques. The latter proved an excellent alternative to the traditional process, especially in terms of the content of terpenoids and bioactivity.
Besides pure essential oils, plant extracts that contain essential oils also attract a lot of attention. Such extracts represent lipophilic fractions and often express more diverse biological activity compared to isolated essential oils [10]. However, the use of volatile organic solvents is a drawback because of their negative effects on the environment and human health. Supercritical carbon dioxide is a “green” and safe solvent of the new generation that enables obtaining lipophilic fractions rich in essential oils. The obtained extracts are ready-to-use, without traces of solvents. The synergistic action of the components of essential oils and other bioactive compounds of a lipophilic nature is expected.
Inhibition of biologically important enzymes by natural products of chamomile, and medicinal herbs in general, is a challenging topic. By regulating the excessive activity of certain enzymes (such as amylase, glucosidase, tyrosinase, and cholinesterase), it is possible to influence the development of diseases that occur as a result of their excessive activity (diabetes, skin hyperpigmentation, Alzheimer’s disease, respectively) [10]. However, the development of medicinal products from essential oils and extracts is not an easy task. It needs a detailed analysis of the composition, and potential carrier of the given activity is necessary, as well as the determination of the most favorable orientation of the ligand (new chemical entity or drugs) that binds to a particular receptor of interest. This can be predicted by using computer-based models named Molecular Docking [11].
One of the objectives of this work was to examine the chemical composition and biological activity of essential oils and chamomile extracts obtained by conventional and greener extraction techniques. Furthermore, the advantages and disadvantages of traditional and contemporary approaches for obtaining chamomile essential oil and extracts were discussed. In order to highlight hidden relationships between chemical composition and biological activity chemometric approach was applied [12]. By molecular docking, an additional attempt to identify components that are responsible for the observed activities was made, as well.
2. Materials and Methods
2.1. Plant Material
Dried chamomile, i.e., dried flower heads of Matricaria chamomilla L. (Asteraceae), were donated by a local tea factory, Fructus doo (Bačka Palanka, Serbia). Plant material was harvested during flowering in the spring of 2018 on the territory of southern Bačka (Bačka Palanka, Serbia). Harvested inflorescences (mean diameter of 2.5 cm) were dried at the temperature of 40 °C in the solar dryer. The layer thickness of the plant material was 5 cm. The process of drying was completed after the moisture of the plant material was approximately 12%. Dried plant material was stored in paper bags and was used to obtain essential oils.
The essential oil was isolated from the plant material by traditional (hydrodistillation) and non-conventional (microwave-hydrodistillation) processes, while the extracts were obtained by Soxhlet extraction and by applying supercritical carbon dioxide extraction.
2.2. Distillation of Essential Oil
2.2.1. Conventional Hydrodistillation (HD)
The conventional process of hydrodistillation was performed in Clevenger-type apparatus according to the procedure described in pharmacopeia [13]. In a nutshell, 20 g of dried chamomile was placed into a round flask and topped with 400 mL of distilled water. The flask was placed in a heating mantle and connected to the Clevenger system. The power of heating was controlled and kept at 205 (HD-1) and 410 W (HD-2). The process duration, from the moment of boiling, is 2 h. The oils were collected in petroleum ether. Drying with anhydrous sodium sulfate was applied for the removal of moisture in a mixture of petroleum ether and essential oil, after which the mixture was filtered. The petroleum ether was removed by evaporation, and the isolated oils were kept in glass vials at 4 °C.
2.2.2. Microwave-Assisted Hydrodistillation (MWHD)
The non-conventional microwave-hydrodistillation process was performed using a modified microwave oven (Panasonic) with Unger glass apparatus. As in the case of hydrodistillation, 20 g of dried chamomile was placed into a round flask and filled up to 400 mL with distilled water. The flask was placed in a microwave oven and connected to the Unger system. The process was carried out at atmospheric pressure while the power of heating was controlled and kept at 200 W (MWHD-1) and 410 W (MWHD-2). Duration of each distillation, from the moment of boiling, was 2 h. The oils were collected in petroleum ether. Drying with anhydrous sodium sulfate was applied for the removal of moisture in a mixture of petroleum ether and essential oil, after which the mixture was filtered. The petroleum ether was removed by evaporation, and the isolated oils were kept in glass vials at 4 °C.
2.3. Soxhlet Extraction (Sox)
Chamomile (10.0 g) was separately extracted by methylene chloride and n-hexane (120 mL each) using the Soxhlet apparatus. Extraction was performed with fifteen exchanges of extract for approximately 6 h. The solvent was evaporated under a vacuum, and the extract was further dried at 40 °C for 24 h. Obtained extracts (Sox-Hex and Sox-MeCl) were put in glass vials, sealed, and stored at 4 °C prior to analysis.
2.4. Supercritical Fluid Extraction (SFE) by CO2
SFE of chamomile was performed on a laboratory-scale high-pressure extraction plant (HPEP, NOVA-Swiss, Effretikon, Switzerland). The main properties of the SFE plant were described by Pekić et al. (1995). Briefly, 70.0 ± 0.01 g of sample was measured and placed in an extractor. Extractions were performed at a fixed set temperature (40 °C), solvent flow rate (0.3 kg/h), and extraction time (3 h), while pressure was varied on 100, 200, 300, and 400 bar (samples SFE-1–SFE-4). The separator conditions were set at 15 bar and 25 °C. Extracts were collected in glass vials, sealed, and kept at −4 °C prior to analysis.
2.5. Chemical Composition of Essential Oils and Extracts
2.5.1. Analysis by GC-MS
GC-FID/MS analyses were carried out on an Agilent Gas Chromatograph model 6890N with flame ionization detector (FID) and split-splitless injector and coupled fluid to an Agilent 5975C mass (MS) detector. The sample solutions (2% in hexane) were chromatographed over HP-5MS capillary column (30 m × 0.25 mm, stationary phase thickness 0.25 μm). The injected volume was 1 μL, in split mode 10:1. The oven temperature was linearly programmed at the rate of 3 °C/min, starting from 60 °C up to 280 °C, and then held isothermal for 5 min. The carrier gas was He at the velocity of 1 mL/min. Injector and FID temperatures were 200 °C and 300 °C, respectively. The transfer line, ion source, and quadrupole temperatures were 250 °C, 230 °C, and 150 °C, respectively. The ionization was performed by electron impact (EI, 70 eV). The MS spectra were acquired at the mass range of 35–550 m/z.
The constituents were identified by comparing their mass spectra and linear retention indices (RI exp) to those found in the NIST/NBS and Wiley databases and the literature [14,15,16,17]. RI exp values were calculated in relation to a homologous series of n-alkanes (C8–C40) analyzed under the same operating conditions [18]. The relative percentages of the compounds were calculated from the FID area percent data using the normalization procedure.
2.5.2. The Total Phenolics and Flavonoids Contents
The total phenolics content was determined by applying reported but slightly modified methods [19]. The sample solution (0.25 mL) was mixed with diluted Folin-Ciocalteu reagent (1 mL, 1:9, v/v) and shaken vigorously. After 3 min, Na2CO3 solution (0.75 mL, 1%) was added, and the sample absorbance was read at 760 nm after a 2 h incubation at room temperature. The total phenolic content was expressed as milligrams of gallic acid equivalents (mg GAE/g extract).
The total flavonoid content was determined using the AlCl3 method. Briefly, the sample solution (1 mL) was mixed with the same volume of aluminum trichloride (2%) in methanol. Similarly, a blank was prepared by adding sample solution (1 mL) to methanol (1 mL) without AlCl3. The absorbance of samples and blank was read at 415 nm after 10 min incubation at room temperature. The absorbance of the blank was subtracted from that of the sample. The total flavonoid content was expressed in milligrams of rutin equivalents (mg RE/g extract) as a reference standard [20].
2.6. Biological Activity of Extracts and Essential Oils
2.6.1. Assays for Antioxidant Activity
For testing scavenging ability, 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radicals were used, and spectrophotometric analysis was conducted according to the procedures described by Grochowski et al. [21]. The antioxidant capacity of the essential oils and extracts was measured by applying different in vitro tests: FRAP, CUPRAC, and Total antioxidant capacity-TAC. The results obtained for TAC were expressed as mmol TE/g. The results of DPPH, ABTS, FRAP, and CUPRAC assays were expressed as trolox equivalents (mg TE/g), while for metal chelating assay, EDTA was used as a reference standard and results were expressed as EDTA equivalents (mg EDTAE/g) [22,23].
2.6.2. Neuroprotective Effects
Neuroprotective effects of chamomile extracts and essential oils were tested by their ability to inhibit Cholinesterase (acetylcholine esterase and butyrylcholine esterase) using Ellman’s method, described by Aktumsek et al. [24]. As a positive control, galantamine was used, and obtained results were expressed as galantamine equivalent per g of essential oil or extract (mg GALAE/g).
2.6.3. Skin-Whitening Ability
Skin-whitening ability of the chamomile essential oils and extracts was examined by their ability to inhibit tyrosinase. The activity was measured by the dopachrome method [21]. As a substrate, 10 mM L-DOPA (3,4-Dihydroxy-l-phenylalanine) was used, while kojic acid was used as the standard compound. The results are expressed as kojic acid equivalent per g of oil or extract (mg KAE/g).
2.6.4. Antidiabetic Activity
The antidiabetic activity of the extracts and essential oils was estimated according to their ability to inhibit α-glucosidase and α-amylase. The Caraway-Somogyi iodine/potassium iodide (IKI) method was used for measuring the ability of the oils to inhibit amylase, while in case of glucosidase method described by Zengin et al. [25] was used. In the case of both enzymes, the activity was expressed as acarbose equivalents per g of oil or extract (mmol ACAE/g).
2.7. Principal Component Analysis (PCA)
Principal component analysis (PCA), as an unsupervised pattern recognition technique, was used to reduce the dataset to a small number of independent components for analyzing relationships among the observed variables [12]. PCA was performed using Statistica 13.5.0. (StatSoft, Palo Alto, CA, USA).
2.8. Molecular Modelling
2.8.1. Receptors Preparation
The essential oil of chamomile was subjected to in silico evaluation studies so as to elucidate the binding mode to the enzymatic pocket of tyrosinase. The crystal structures of tyrosinase (pdb id: 2Y9X) in complex with tropolone have been downloaded from the PDB database [26]. The enzymes have been prepared for docking by the PrepWizard tool embedded in Maestro 2015 [27]. This software was utilized to neutralize the macromolecules at pH 7.4 by PROPKa to convert the seleno-cysteines and seleno-methionines, if present, to cysteines and methionines and to fix other errors present in the raw structure of the enzyme [28]. All the missing fragments and other errors present in the crystal structures were automatically and manually solved, and the hydrogens were added and minimized following the general method previously stated by our groups [29,30,31].
2.8.2. Preparation of Ligands
In the analyzed essential oils, the most abundant compounds were identified: α-bisabolol oxide B, α-bisabolol oxide A, α-bisabolone oxide A, (Z)-spiroether, chamazulene (CP1-5). These compounds, reported in Figure 1, were prepared by the LigPrep tool [32]. LigPrep returns a low-energy, single, 3D structure with right chirality; also, the ligands are neutralized at pH 7.4 by Epik [33] and minimized by the OPLS-3 force field [34].
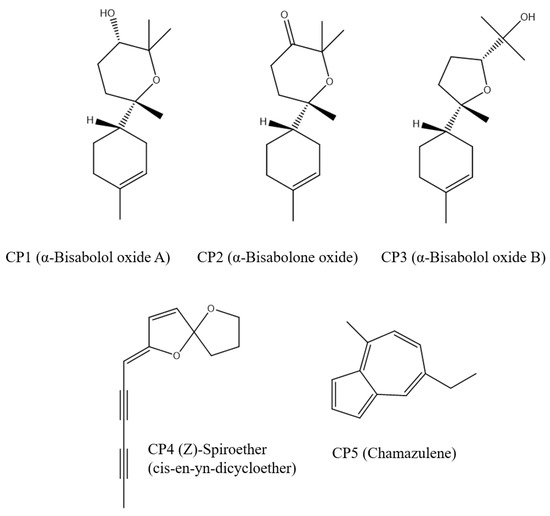
Figure 1.
Chemical structures of the most abundant components of the analyzed essential oils.
2.8.3. Molecular Docking
The docking experiments have been carried out on the selected compounds on tyrosinase by the software GOLD 5.5 [35] by performing a ChemScore scoring function (Development and Validation of a Genetic Algorithm for Flexible Docking) [36], which was validated for docking on this enzyme previously by our research group [37]. GOLD 5.5 provides the complete range of accuracy vs. speed choice. The grid for docking was determined automatically by centering the box on the crystallographic inhibitor, extended in a radius of 12 Angstroms around the ligand center. The best-ranked poses for each substance complexed with the selected protein are depicted in Figure 2 and Figure 3. The docking fitness values obtained for the essential oil components and tyrosinase are reported in Section 3.5.
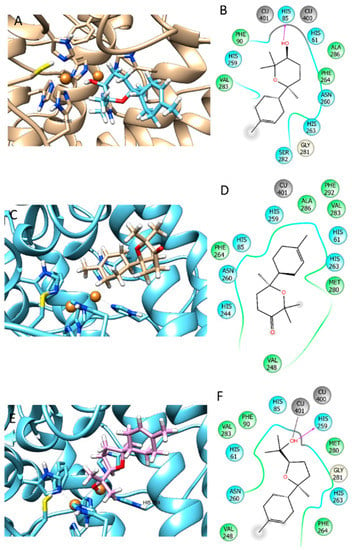
Figure 2.
Best docking pose of CP1 (A,B), CP2 (C,D) and CP3 (E,F) on tyrosinase.
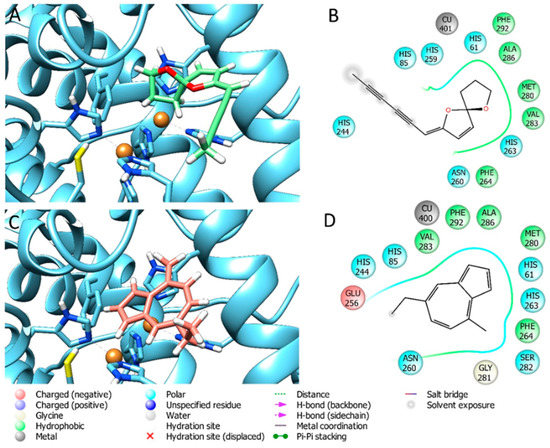
Figure 3.
Best docking pose of CP4 (A,B) and CP5 (C,D) on tyrosinase.
3. Results and Discussion
3.1. Chemical Composition of Extracts and Essential Oils
Considering the chemistry behind techniques, three techniques and four solvents were applied to isolate bioactive compounds from chamomile. Namely, the process of hydrodistillation performed in conventional (HD) and contemporary (MWHD) way were used to obtain the volatile fraction, i.e., essential oil, while Soxhlet extraction with two different solvents (methylene chloride (Sox-MeCl) and hexane (Sox-Hex)) together with supercritical fluid extraction (SFE) was applied to isolate lipophilic chamomile compounds. Qualitative analysis of the obtained samples was done using GC-FID/MS analysis and enabled the identification of 68 constituents in total, which constituted 89.3–96.6% of the investigated chamomile isolates (Table S1). The main class of constituents, as well as the main compounds in analyzed samples, are presented in Figure 4.
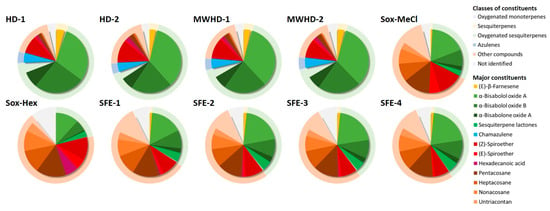
Figure 4.
The main class of constituents and the main compounds determined by GC analysis.
Oxygenated sesquiterpenes and non-terpene compounds dominated in all samples. On the other side, there were two different profiles obtained by (a) hydrodistillation and microwave-assisted hydrodistillation (samples HD-1, HD-2, MWHD-1and MWHD-2) that were all blue, and (b) Soxhlet extraction with organic solvents and subcritical fluid extraction with CO2 (samples Sox-MeCl, Sox-Hex, SFE-1, SFE-2, SFE-3, SFE-4). It is known that the blue color of chamomile essential oil comes from azulenes, which are formed by the breakdown of sesquiterpene lactones, i.e., proazulenes, during distillation. E.g., lactone matricin, under an acidic environment and elevated temperature during distillation, transforms into chamazulene through the formation of chamazulene carboxylic acid and its subsequent decarboxylation [38]. The yellow color of the SFE extracts is proof that the use of this type of extraction does not cause the thermal degradation of proazulene compounds.
In HD and MWHD samples, oxygenated sesquiterpenes (65.7–68.9%) and non-terpene compounds (17.1–22.3%) were the most abundant classes of constituents, while total sesquiterpene hydrocarbons and oxygenated monoterpenes were present in minor amounts. Within the oxygenated sesquiterpenes, 55.2–55.5% was the sum of bisabolol oxides A and B, while spiroethers (10.2–12.9%) comprised more than half of the number of compounds of non-terpene character. These samples also contained 4.6–5.2% of chamazulene, a blue hydrocarbon formed during a distillation process from sesquiterpene lactones as described above.
In the Sox and SFE samples, non-terpene compounds prevailed (52.1–68.1%), followed by oxygenated sesquiterpenes (21.2–39.0%) and minor amounts of sesquiterpene hydrocarbons and oxygenated monoterpenes. Among non-terpene compounds, hydrocarbons constituted 40.5–52.0% of the extracts, while the concentrations of spiroethers were similar as in distilled samples (9.2–11.8%), or somewhat higher (18.1% in Sox-MeCl extract). The sums of bisabolol oxides A and B (i.e., oxygenated sesquiterpenes) were lower than in HD/MWHD samples, but still, these samples could be regarded as rich in bisabolol oxides (17.4–30.8%). It is noteworthy that these extracts contained sesquiterpene lactones (leucodin, achilin, matricin, matricarin, acetoxyachillin) (2.8–5.3%), which were not detected in HD and MWHD samples due to their instability and the above-mentioned degradation and formation of azulenes.
Regarding individual compounds, all samples obtained by distillation (HD and MWHD) contained α-bisabolol oxide A and α-bisabolol oxide B as the most abundant constituents. α-Bisabolol oxide A was the major compound in all SFE samples and Sox-MeCl, followed by pentacosane, whereas pentacosane predominated over it in Sox-Hex extract (although the difference in percentages was only slight).
Apart from the fact that all samples analyzed in this study had a high content of bisabolol oxides, several more remarks regarding the composition could be made. The main difference between distilled and other samples is that the former contained a significant amount of chamazulene, whereas the latter contained a comparable amount of sesquiterpene lactones. The amount of (Z)-spiroether was significant in all samples (especially Sox-MeCl), while Sox extracts contained relatively high amounts of its E isomer. All these major compounds are significant because they have specific biological effects. For example, the study conducted by Taghizadeh et al. [39] confirmed the pronounced cytotoxic activity of these compounds, while Yoshinari et al. [40] showed that spiroethers of chamomile might inhibit the production of aflatoxin G by A. parasiticus and 3-acetyldeoxynivalenol (3-ADON) production by Fusarium graminearum.
Samples from this study correspond to the variety of chamomile and chamomile oil characterized by high contents of matricin/chamazulene and bisabolol oxides [41]. Bisabolol oxides-rich essential oil has been recognized for its medicinal purposes [42] and found its place in European Pharmacopoeia, in addition to the type rich in chamazulene and α-bisabolol. All HD and MWHD samples analyzed in this study fulfill the requirements for the Matricaria oil rich in bisabolol oxides [43], i.e., they contained 29–81% of the sum of bisabolol oxides A and B and ≥1% of chamazulene. On the other hand, among Sox and SFE extracts, only samples SFE-2, SFE-3, and SFE-4 contained the required amount of bisabolol oxides. It could be noted that the content of α-bisabolol oxides in SFE extracts was influenced by pressure, i.e., the increase in pressure led to the increase in their content. Previously, Kotnik et al. [44] noted this causality for α-bisabolol oxide A.
The presence of sesquiterpene lactones matricin and matricarin in chamomile was first confirmed as early as around the middle of the XX century [45,46]. In this study, matricin was identified in the Sox, and SFE extracts by mass spectral data library search only. On the other hand, we confirmed the presence of other sesquiterpene lactones, leucodin, achillin, matricarin, and acetoxyachillin based on the mass spectral and retention index data, having in mind that these compounds were previously found in the chamomile infusion [17]. Sesquiterpene lactone matricin was present in significant amounts in previously analyzed SFE extracts of chamomile as well, but its instability had a strong influence on the final content of this compound in the extracts [47]. A pressure of 250 bar was selected as the most optimal for efficient supercritical CO2 extraction of matricin, which is in accordance with literature data [44].
Polyphenol compounds are not included in the composition of essential oils, but they can be constituents of extracts obtained with non-polar or semi-polar solvents because of variations in their structure (polarity) [10]. Due to this, polyphenols and flavonoids were present in the extracts obtained with hexane and methylene chloride, as well as in supercritical extracts (Table 1). Regarding extraction with organic solvents, hexane, with its low dielectric constant (about 2), gave extracts with a lower content of phenols and flavonoids compared to methylene chloride (whose dielectric constant is four times higher, 8.93). The content of total phenols in SFE extracts was in the range of 15.56–32.49 mg GAE/g, while the content of flavonoids varied from 3.97 to 29.43 mg RE/g. The obtained results are in accordance with the literature and show a higher selectivity of hexane towards these compounds compared to methylene chloride and CO2.

Table 1.
Polyphenols content and antioxidant activity of chamomile essential oils, Soxhlet, and supercritical CO2 extracts.
3.2. Antioxidant Activity
On the basis of numerous beneficial effects of natural products, such as anti-inflammatory, anticarcinogenic, cardio- or neuroprotective activities, etc., is their antioxidant activity. In addition, this property is the reason for their increasingly frequent use not only in the pharmaceutical industry but also in the food industry, where they are successfully used to protect food from oxidation and premature spoilage. Components that contribute to the antioxidant power of natural products are mostly polyphenols, but also components contained in essential oils as well as other lipophilic compounds. All these components have a different mechanism of action as well as a different capacity, so the overall antioxidant activity of the product will be affected by different factors, among others, the technique of their extraction from the plant matrix. In order to determine the real antioxidant potential of natural products, it is necessary to apply different assays that rely on different mechanisms. In the frame of this paper, the antioxidant activity of essential oils and lipophilic extracts was determined by six tests whose parallel application provides a comprehensive insight into the power of chamomile. The obtained results are presented in Table 1.
With the exception of metal chelating ability, samples obtained by distillation processes provide a stronger antioxidant and antiradical ability than extracts. The difference between essential oil and extracts was notable, while on the other hand, essential oils did not differ significantly from each other. This means that microwave radiation does not have a strong influence on the activity of essential oil. Literature data have shown that polar chamomile extracts obtained by microwave extraction can be significantly different from extracts obtained by the traditional method [48]. Nevertheless, based on the obtained results, it can be concluded that in the case of essential oils and the hydrodistillation process, the use of microwave irradiation is not justified. The superiority of essential oils over extracts can be explained by low selectivity and co-extraction of interfering components (waxes and resins) whose presence is not desirable from the aspect of antioxidant activity. In the case of antiradical capacity, the data from Table 1 show that all analyzed samples had more capacity to neutralize ABTS (5.10–184.18 mg TE/g) than DPPH (1.49–32.44 mg TE/g) free radicals which were in accordance with literature data [10]. In the process of formation of reactive oxygen species, an important role is played by metal ions that have the ability to catalyze the oxidation process and promote the formation of radicals. For this reason, CUPRAC and FRAP tests were used, and the reducing potential of essential oils and extracts was defined. The obtained results showed an extremely high activity of essential oils both in the case of copper (409.05–485.84 mg TE/g) and ferric ions reduction (520.31–711.03 mg TE/g). A high reducing potential was shown by the extracts as well, where it was observed that in the case of the CUPRAC test, the extracts obtained with supercritical carbon dioxide (70.95–106.64 mg TE/g) showed higher activity than the extracts obtained with non-polar solvents (60.17–63.07 mg TE/g). It is noteworthy that in the case of supercritical extracts, a pressure of 350 bar leads to obtaining an extract with significant antioxidant potential, which was confirmed by all six applied antioxidant tests. In the case of metal ions chelation, a higher activity of extracts (28.80–63.98 mg EDTA/g) compared to the essential oils (16.86–41.29 mg EDTA/g) was observed, the most probably due to co-extraction of polyphenolic compounds.
3.3. Enzyme-Inhibitory Activity
Due to the importance of enzymes in the development and progression of various diseases, there is a growing trend of finding effective ways to regulate their activity. Namely, the progression of many diseases is associated with excessive activity of the responsible enzymes, so it has been observed that the progression of diabetes, which is becoming an increasingly frequent problem due to the lifestyle and diet that dominates in the modern world, can be controlled by regulating the activity of α-amylase and glucosidase [49]. Moreover, the occurrence of melanoma is closely related to tyrosinase activity. Regulation of these enzymes is possible by using well-known synthetic inhibitors; however, recently, there has been a growing awareness of the side effects of these inhibitors, especially if they are used for a long period. Due to this, an increasing number of studies are investigating natural inhibitors of biologically important enzymes. In this study, the possibility of using chamomile EOs and their extracts to inhibit cholinesterase, glycosidase, amylase, and tyrosinase was investigated.
3.3.1. Neuroprotective Activity
Alzheimer’s disease (AD) is a neurodegenerative disease and the most common cause of non-vascular dementia that affects brain regions involved in the control of thinking, memory, speech, reasoning, and other cognitive functions. Due to the importance of prevention and treatment of this disease with rapidly increasing prevalence, there is a growing interest in finding new approaches to treatment and controlling its progress. One of the possibilities for the control of Alzheimer’s disease is the inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE), key enzymes in the hydrolysis of the neurotransmitter acetylcholine. The property of chamomile essential oils and extracts to inhibit the activity of these enzymes is presented in Figure 5.
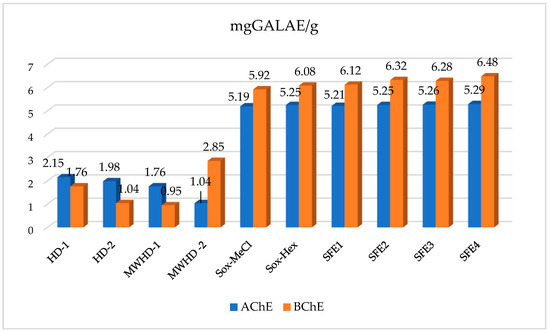
Figure 5.
Inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) by chamomile extracts obtained by various extraction and distillation techniques.
As seen in Figure 5, the extracts were more active than essential oils toward inhibition of both AChE and BChE. With the exception of MWHD-2, the higher activity for essential oils was recorded towards AChE, while the extracts expressed higher activity towards BChE. Although all SFE extracts showed an extremely high level of activity, differences were observed among them, which were influenced by the pressure that prevailed during extraction. Namely, with pressure increases, components that affect the inhibitory power of the extracts were extracted more. On the other hand, with essential oil, the use of higher heating is not justified, and the essential oil obtained at a lower heating power were more active. To provide a connection between chemical composition and cholinesterase abilities, we performed Pearson’s correlation analysis. From Table S1, several compounds, including achillin, tricosane, tetracosane, pentacosane, and hexacosane, correlated strongly with the cholinesterase inhibitory effects (R > 0.9). This indicates that hydrocarbons were the main contributors to the observed cholinesterase inhibitory effects, and SFE extracts contained more hydrocarbons when compared with other extracts. In this sense, the employment of SFE could be a useful approach to designing some effective anti-Alzheimer agents by using chamomile.
3.3.2. Anti-Diabetic Activity
Inhibitors of α-amylase and glucosidase are recognized as useful agents for the reduction of the increase in blood glucose levels [50]. The conventional approach involves the use of inhibitors such as acarbose [51], the effectiveness of which is only partial, while the side effects might be quite unpleasant (flatulence, diarrhea) or even serious (hepatitis) [52,53]. As alternatives, natural plant constituents with the ability to inhibit these enzymes can be successfully applied, whereby side effects could be avoided [54]. The ability of chamomile essential oils, as well as lipophilic extracts, to inhibit α-amylase and glucosidase was investigated, and obtained results are presented in Figure 6. Based on our results, HD and MWHD samples were active on amylase, but no activity on glucosidase was recorded. Soxhlet and SFE extracts were more active on glucosidase than on amylase. In the correlation analysis, some volatile compounds, including (E)-β-farnesene, dehydro-sesquicinole, β-selinene, and nerolidol oxide correlated strongly with anti-amylase inhibitory effects (R > 0.95). The compounds were not detected in SFE extracts and are likely to be more sensitive at high pressure. Consistent with our results, some authors reported that nerolidol, a compound structurally similar to nerolidol-oxide, was an effective antidiabetic agent [55,56]. In contrast to amylase inhibitory, SFE extracts exhibited stronger glucosidase inhibitory effects than those of other extracts. In the correlation assay, unlike amylase inhibition, different compounds had high correlation values. The compounds were achillin, tetracosane, pentacosane, and heptacosane. However, the complex nature of phytochemicals and their synergistic or antagonistic effects between them should not be forgotten. Therefore, further studies are needed to understand which compounds contribute to chamomile’s antidiabetic capabilities.
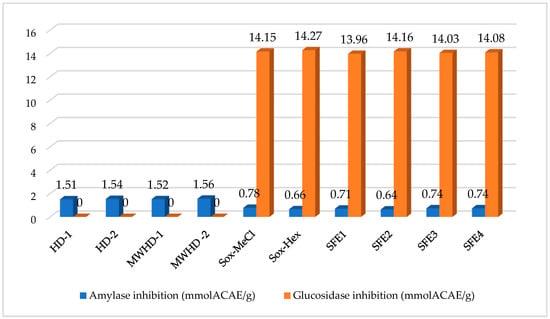
Figure 6.
Amylase and glucosidase inhibition activity of chamomile extracts and essential oils obtained by various extraction and distillation techniques.
3.3.3. Skin-Whitening Ability
Tyrosinase is one key enzyme in the synthesis of melanin, and the enzyme can modulate the level of melanin. At this point, the enzyme is considered an important checkpoint for hyperpigmentation disorders. To this end, several enzyme inhibitors have been chemically produced, but most of them have undesirable side effects. For example, kojic acid is one of the most common tyrosinase inhibitors, but it could lead to skin hypersensitivity in long-term use. For this reason, we investigated the anti-tyrosinase inhibitory effects of chamomile essential oils and lipophilic extracts (Table 2). Based on the results, SFE and Soxhlet extracts exhibited greater inhibitory effects when compared to other extracts. The weakest ability was found in MWHD-2 with 62.40 mg KAE/g. From Table S2, like AChE and BChE inhibitory effects, hydrocarbons showed a good correlation with the observed tyrosinase inhibitory effects. Taken together, SFE, which is a green extraction, could be useful for the preparation of cosmeceutical formulations using chamomile. Previously, several authors noted that chamomile had great potential as a cosmeceutical ingredient, and our results go in line with this [48,57,58].

Table 2.
Inhibition of tyrosinase by chamomile essential oils and extracts obtained by various distillation and extraction techniques.
3.4. Results of Principal Component Analysis (PCA)
PCA is used to reduce the dataset to a small number of independent components for analyzing relationships among the observed variables in a way to keep the highest percentage of explanatory variance. PCA was based on experimental results of the composition of extracts and EOs of chamomile obtained by different separation techniques. More precisely, experimental data on the content of target compounds (terpenoids and polyphenols) and bioactivity (antioxidant and enzyme-inhibitory activity) were subjected to chemometric evaluation. When PCA was applied to the data matrix, with Eigen analysis as an initial, two principal components (PCs) were extracted according to the Kaiser criterion, which explains up to 80.38% of the total variance (69.21 and 11.17%, respectively). Thus, the true dimensionality of the descriptor space is two. As a result, we obtained a space that can be described with two factors. Features with high positive or negative loadings essentially determine the factor.
Factor scores are derived from the PCs and are specific to individual objects, i.e., variables/compounds (Figure 7a) and EOs and extracts (Figure 7b).
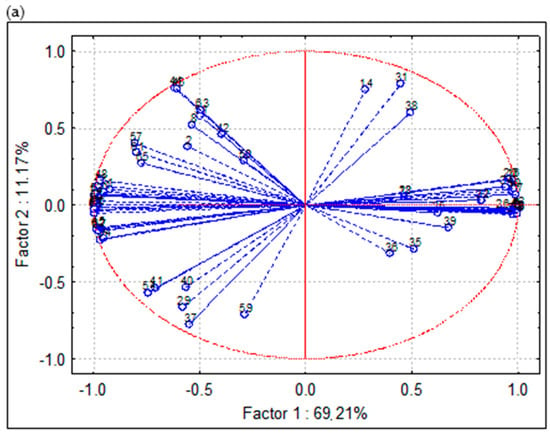
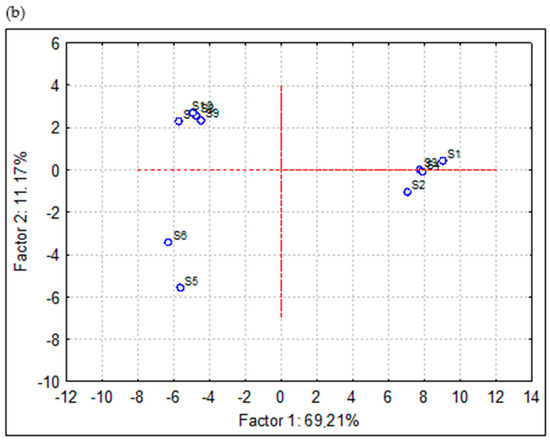
Figure 7.
Bi-plot distribution of F1 and F2 by PCA (a) variables/scores and (b) scores of chamomile extracts and essential oils.
PCA confirmed clear differentiation of F1 from F2. From Figure 7a, it can be observed the following: 1–2 were polyphenols content determined by spectrophotometric assays (TP, TF), 3–8 were in vitro antioxidant activity parameters (DPPH, ABTS, CUPRAC, FRAP, TAC, and MC), 9–13 were enzyme-inhibition activity (AChE, BChE, Tyr, Amyl, Gluc) and rest of the variables were individual compounds determined by GC-FID/MS. Another visualization presented in Figure 7b shows the following order of samples: HD-1 (S1), HD-2 (S2), MWHD-1 (S3), MWHD-2 (S4), Sox-MeCl (S5), Sox-Hex (S6), SFE-1 (S7), SFE-2 (S8), SFE-3 (S9) and SFE-4 (S10).
Total phenolics and flavonoid content are expressed by the moderate negative loadings in PC-1 and moderate positive in PC-2. All antioxidant activity attributes except metal chelating are expressed by strongly positive loadings in PC-1 (Figure 7a). Similar phenomena could be observed for all enzyme-inhibition activity parameters except FRAP, which was positively correlated in PC-1.
The grouping of the samples was strongly associated with the applied separation technique, which can be observed in Figure 7b. EOs obtained by HD and MWHD were affected by the strong positive loadings in PC-1 and very weak (positive and negative) in PC-2, which was similarly observed for the majority of terpenoids. This could be explained by the particularly high content of target terpenoids observed in the EOs. Chamomile extracts obtained by solvent extraction were evidently separated into two subgroups according to to applied solvent. Extracts obtained by Soxhlet extraction were correlated with the strong negative loadings in PC-1 and PC-2, while samples obtained with methylene chloride were distinguished from all other samples (Figure 7b). On the other hand, samples obtained by SFE were expressed by negative loadings in PC-1 and positive in PC-2. These samples were characterized by particularly high content of total phenols and flavonoids, as well as potent antioxidant activity.
3.5. Docking Results
Bearing in mind the more complex composition of extracts compared to essential oils, the docking experiment was performed in order to better indagate the biological effects of chamomile essential oils. The target enzyme was tyrosinase since essential oils exhibited the highest degree of inhibition against this enzyme. Docking experiments of the most abundant compounds (CP1–CP5) found in the essential oils have been carried out by Gold 5.5 by using the ChemScore scoring function, being able to reproduce the docking pose of the crystallographic ligand Tropolone present in the 2Y9X enzyme crystal structure with high precision. The docking fitness values found are reported in Table 3. It is possible to observe that the best values returned from the best docking pose were found for CP1 and CP3. Indeed, CP3 was able to establish one hydrogen bond with HIS85 and one coordinative bond to Copper401 (Figure 2E,F), and CP1 was able to form a hydrogen bond with HIS85 (Figure 2A,B). The other compounds, being very lipophilic, were only able to penetrate the tyrosinase binding pocket, but they do not seem to be able to form any hydrophilic interactions with the enzyme cavity (Figure 2C,D and Figure 3A,F). The results obtained are in agreement with the paper published by da Silva et al. (2017), in which it has been demonstrated that essential oil components are able to penetrate the enzymatic pocket of tyrosinase, and some of them may interact with the copper metals present in the deep of the cavity. Analogous to these results, we have found that CP3, and to a lesser extent CP1, are able to interact with the amino acidic environment of the tyrosinase enzymatic cavity, thus inhibiting the enzymatic activity of the enzyme by competing with the natural substrates. Even though MWHD-3 demonstrated to have a stronger inhibitory activity toward tyrosinase than the other three distilled samples, the relative presence of the tested compounds (CP1–CP5) is not significantly different among them; thus, the inhibitory activity recorded must be ascribed more to the plethora of volatile components than to the one specific compound.

Table 3.
Docking score of the components of the essential oil of chamomile expressed as ChemScore values.
4. Conclusions
Over the past decade, the development of extraction technology has gained interest in increasing yield and reducing the consumption of toxic solvents. With this in mind, we designed the present study to select how the different extraction methods affect the chemical composition and biological capabilities of Matricaria chamomila. Another goal was to employ greener and more sustainable approaches in line with the principles of green chemistry, especially those complemental to green analytical chemistry. Our results indicated that the extraction techniques affected the chemical profiles and biological capabilities of the samples tested. In all essential oils, the oxygenated sesquiterpenes were the major group, and bisabolol oxides A and B were reported as the main constituents. In the antioxidant assays, in general, the samples obtained by distillation methods provided a stronger antioxidant and anti-radical ability than extracts. Regarding enzyme inhibition assays, SFE samples showed a greater effect compared to other samples. In summary, our findings demonstrated a successful scientific story with a multivariate approach, starting from natural sources to functional applications, with M. chamomila. However, risk assessment on one side and testing the bioavailability in the gastrointestinal system on another could open a new road for other specialists in future studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10010018/s1, Table S1: Composition of chamomile essential oils and extracts determined by GC-FID/MS analysis; Table S2: Pearson’s correlation values between compounds and tested enzymes (red color indicates high correlation).
Author Contributions
A.C.K. and B.P. for conceptualization; A.C.K., J.A. and S.R. for methodology; G.Z., A.M., J.A., B.P., K.I.S. and A.C.K. for formal analysis; G.Z., A.M., J.A., K.I.S., L.Y. and A.C.K. for investigation; J.A., Z.Z., L.Y. and A.C.K. for resources; A.C.K. and S.R. for supervision; J.A. and S.R. for validation; A.C.K. and B.P. for writing the original draft; J.A. and S.R. for writing review & editing; A.C.K. and S.R. for project administration; Z.Z., L.Y., A.C.K. and S.R. for funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Projects No. 451-03-68/2022-14/200134, University of Novi Sad, Faculty of Technology and 451-03-68/2020-14/200161, University of Belgrade, Faculty of Pharmacy) as well as by Leadership Development Center Filip Moris within the project “Run for the Science”. The authors are also grateful to the Fundamental Research Funds for the Central Non-profit Research Institution of CAF for supporting investigation through the project “green extraction technology for functional, active ingredients isolation from agricultural and forestry wastes” (Project No. CAFYBB2018GB001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hänsel, R.; Sticher, O. Pharmakognosie-Phytopharmazie; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- De Laurentis, N.; Rosato, A.; Gallo, L.; Leone, L.; Milillo, M. Chemical composition and antimicrobial activity of Myrtus communis. Riv. Ital. EPPOS 2005, 39, 3–8. [Google Scholar]
- Li, Z.; Liu, A.; Du, Q.; Zhu, W.; Liu, H.; Naeem, A.; Guan, Y.; Chen, L.; Ming, L. Bioactive substances and therapeutic potential of camellia oil: An overview. Food Biosci. 2022, 49, 101855. [Google Scholar] [CrossRef]
- Chandrakanthan, M.; Handunnetti, S.M.; Premakumara, G.S.A.; Kathirgamanathar, S. Topical anti-Inflammatory activity of essential oils of Alpinia calcarata Rosc., its main constituents, and possible mechanism of action. Evid. Based Complement. Altern. Med. 2020, 2020, 2035671. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, S.; Maraschin, M.; Coimbra, M.A.; Rocha, S.M. In vitro and in vivo studies of natural products: A challenge for their valuation. The case study of chamomile (Matricaria recutita L.). Ind. Crops Prod. 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Analysis of volatile secondary metabolites from Colombian Xylopia aromatica (Lamarck) by different extraction and headspace methods and gas chromatography. J. Chromatogr. A 2004, 1025, 105–113. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) NE Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 2004, 1025, 93–103. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, R.; Kumar, A. Docking techniques in pharmacology: How much promising? Comput. Biol. Chem. 2018, 76, 210–217. [Google Scholar] [CrossRef]
- Ražić, S. Chemometrics in the Analysis of real Samples-From Theory to Application; Faculty of Pharmacy-University of Belgrade: Beograd, Serbia, 2011. [Google Scholar]
- Ph. Jug. IV. Pharmacopoea Jugoslavica Edito Qarta; Federal Office of Public Health: Belgrade, Serbia, 1984. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured publishing corporation Carol Stream: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Avonto, C.; Wang, M.; Chittiboyina, A.G.; Avula, B.; Zhao, J.; Khan, I.A. Hydroxylated bisabolol oxides: Evidence for secondary oxidative metabolism in Matricaria chamomilla. J. Nat. Prod. 2013, 76, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, I.; Schmolz, E.; Ruther, J. Cuticular lipids as trail pheromone in a social wasp. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tschiggerl, C.; Bucar, F. Guaianolides and volatile compounds in chamomile tea. Plant Foods Hum. Nutr. 2012, 67, 129–135. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Governatori, L.; Carlucci, G.; Genovese, S.; Mollica, A.; Epifano, F. Recent application of analytical methods to phase I and phase II drugs development: A review. Biomed. Chromatogr. 2012, 26, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Bizzarri, B.; Chimenti, P.; De Monte, C.; Mollica, A.; Rivanera, D.; Zicari, A.; Mari, E.; Zengin, G.; et al. Novel 1,3-thiazolidin-4-one derivatives as promising anti-Candida agents endowed with anti-oxidant and chelating properties. Eur. J. Med. Chem. 2016, 117, 144–156. [Google Scholar] [CrossRef]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Maestro; Version 12.2; Schrodinger LLC: New York, NY, USA, 2015.
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK a prediction and protonation state generation for drug-like molecules. J. Comput.-Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (-)-epicatechin source for food and medicinal applications. Phytomedicine 2016, 23, 979–988. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Aktumsek, A.; Mocan, A.; Mollica, A.; Locatelli, M.; Custodio, L.; Neng, N.R.; Nogueira, J.M.; Aumeeruddy-Elalfi, Z. Euphorbia denticulata Lam.: A promising source of phyto-pharmaceuticals for the development of novel functional formulations. Biomed. Pharmacother. 2017, 87, 27–36. [Google Scholar] [CrossRef]
- Ligprep; VERSION 2.3; Schrödinger, LLC: New York, NY, USA, 2009; pp. 1–116.
- Epik. Schrödinger, LLC: New York, NY, USA. Available online: https://www.schrodinger.com/epik (accessed on 21 March 2018).
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein— ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In vitro and in silico perspectives on biological and phytochemical profile of three halophyte species—A source of innovative phytopharmaceuticals from nature. Phytomedicine 2018, 38, 35–44. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Taghizadeh, S.F.; Azizi, M.; Rezaee, R.; Madarshahi, F.S.; Mehmandoust, M.; Karimi, G.; Asili, J. Cytotoxic activity of cis-(E)-and trans-(Z)-spiroethers isolated from various Arnebia species. S. Afr. J. Bot. 2021, 142, 114–123. [Google Scholar] [CrossRef]
- Yoshinari, T.; Yaguchi, A.; Takahashi-Ando, N.; Kimura, M.; Takahashi, H.; Nakajima, T.; Sugita-Konishi, Y.; Nagasawa, H.; Sakuda, S. Spiroethers of German chamomile inhibit production of aflatoxin G1 and trichothecene mycotoxin by inhibiting cytochrome P450 monooxygenases involved in their biosynthesis. FEMS Microbiol. Lett. 2008, 284, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.; Schilcher, H. Chamomile: Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Tomić, M.; Popović, V.; Petrović, S.; Stepanović-Petrović, R.; Micov, A.; Pavlović-Drobac, M.; Couladis, M. Antihyperalgesic and antiedematous activities of bisabolol-oxides-rich matricaria oil in a rat model of inflammation. Phytother. Res. 2014, 28, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2019; version 10.0.1836 (01/2008). [Google Scholar]
- Kotnik, P.; Škerget, M.; Knez, Ž. Supercritical fluid extraction of chamomile flower heads: Comparison with conventional extraction, kinetics and scale-up. J. Supercrit. Fluids 2007, 43, 192–198. [Google Scholar] [CrossRef]
- Čekan, Z.; Herout, V.; Šorm, F. Über terpene LXXX. Die struktur von matricin, ein guajanolid aus der kamille (Matricaria chamomilla L.). Collect. Czechoslov. Chem. Commun. 1957, 22, 1921–1929. [Google Scholar] [CrossRef]
- Čekan, Z.; Procházka, V.; Herout, V.; Šorm, F. On terpenes. CI. Isolation and constitution of matricarin, another guaianolide from camomile (Matricaria chamomilla L.). Collect. Czechoslov. Chem. Commun. 1959, 24, 1554–1557. [Google Scholar] [CrossRef]
- Kaiser, C.; Römpp, H.; Schmidt, P. Supercritical carbon dioxide extraction of chamomile flowers: Extraction efficiency, stability, and in-line inclusion of chamomile-carbon dioxide extract in β-cyclodextrin. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 249–256. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crops Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C. Antidiabetic drugs. Br. J. Cardiol. 2003, 10, 128–136. [Google Scholar]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Cagliero, C.; Marengo, A.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Bio-guided fractionation driven by in vitro α-amylase inhibition assays of essential oils bearing specialized metabolites with potential hypoglycemic activity. Plants 2020, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Comparative Radical Scavenging and Antidiabetic Activities of Methanolic Extract and Fractions from Achillea ligustica ALL. Biol. Pharm. Bull. 2005, 28, 1791–1794. [Google Scholar] [CrossRef]
- Sadiq, A.; Rashid, U.; Ahmad, S.; Zahoor, M.; AlAjmi, M.F.; Ullah, R.; Noman, O.M.; Ullah, F.; Ayaz, M.; Khan, I. Treating hyperglycemia from Eryngium caeruleum M. Bieb: In-vitro α-glucosidase, antioxidant, in-vivo antidiabetic and molecular docking-based approaches. Front. Chem. 2020, 8, 558641. [Google Scholar] [CrossRef]
- Fraihat, A.; Alatrash, L.; Abbasi, R.; Abu-Irmaileh, B.; Hamed, S.; Mohammad, M.; Abu-Rish, E.; Bustanji, Y. Inhibitory effects of methanol extracts of selected plants on the proliferation of two human melanoma cell lines. Trop. J. Pharm. Res. 2018, 17, 1081–1086. [Google Scholar] [CrossRef]
- Salem, M.A.; Radwan, R.A.; Mostafa, E.S.; Alseekh, S.; Fernie, A.R.; Ezzat, S.M. Using an UPLC/MS-based untargeted metabolomics approach for assessing the antioxidant capacity and anti-aging potential of selected herbs. RSC Adv. 2020, 10, 31511–31524. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).