A Comparative Study of Chamomile Essential Oils and Lipophilic Extracts Obtained by Conventional and Greener Extraction Techniques: Chemometric Approach to Chemical Composition and Biological Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Distillation of Essential Oil
2.2.1. Conventional Hydrodistillation (HD)
2.2.2. Microwave-Assisted Hydrodistillation (MWHD)
2.3. Soxhlet Extraction (Sox)
2.4. Supercritical Fluid Extraction (SFE) by CO2
2.5. Chemical Composition of Essential Oils and Extracts
2.5.1. Analysis by GC-MS
2.5.2. The Total Phenolics and Flavonoids Contents
2.6. Biological Activity of Extracts and Essential Oils
2.6.1. Assays for Antioxidant Activity
2.6.2. Neuroprotective Effects
2.6.3. Skin-Whitening Ability
2.6.4. Antidiabetic Activity
2.7. Principal Component Analysis (PCA)
2.8. Molecular Modelling
2.8.1. Receptors Preparation
2.8.2. Preparation of Ligands
2.8.3. Molecular Docking
3. Results and Discussion
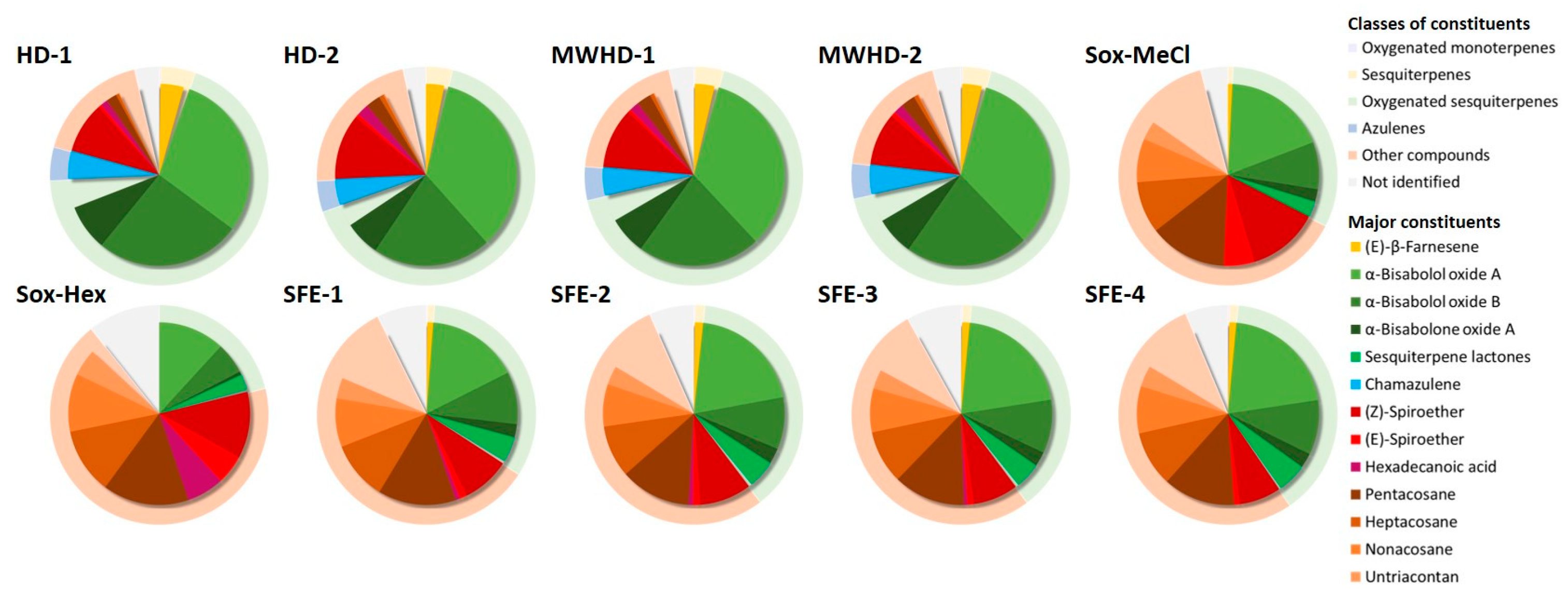
3.1. Chemical Composition of Extracts and Essential Oils
3.2. Antioxidant Activity
3.3. Enzyme-Inhibitory Activity
3.3.1. Neuroprotective Activity
3.3.2. Anti-Diabetic Activity
3.3.3. Skin-Whitening Ability
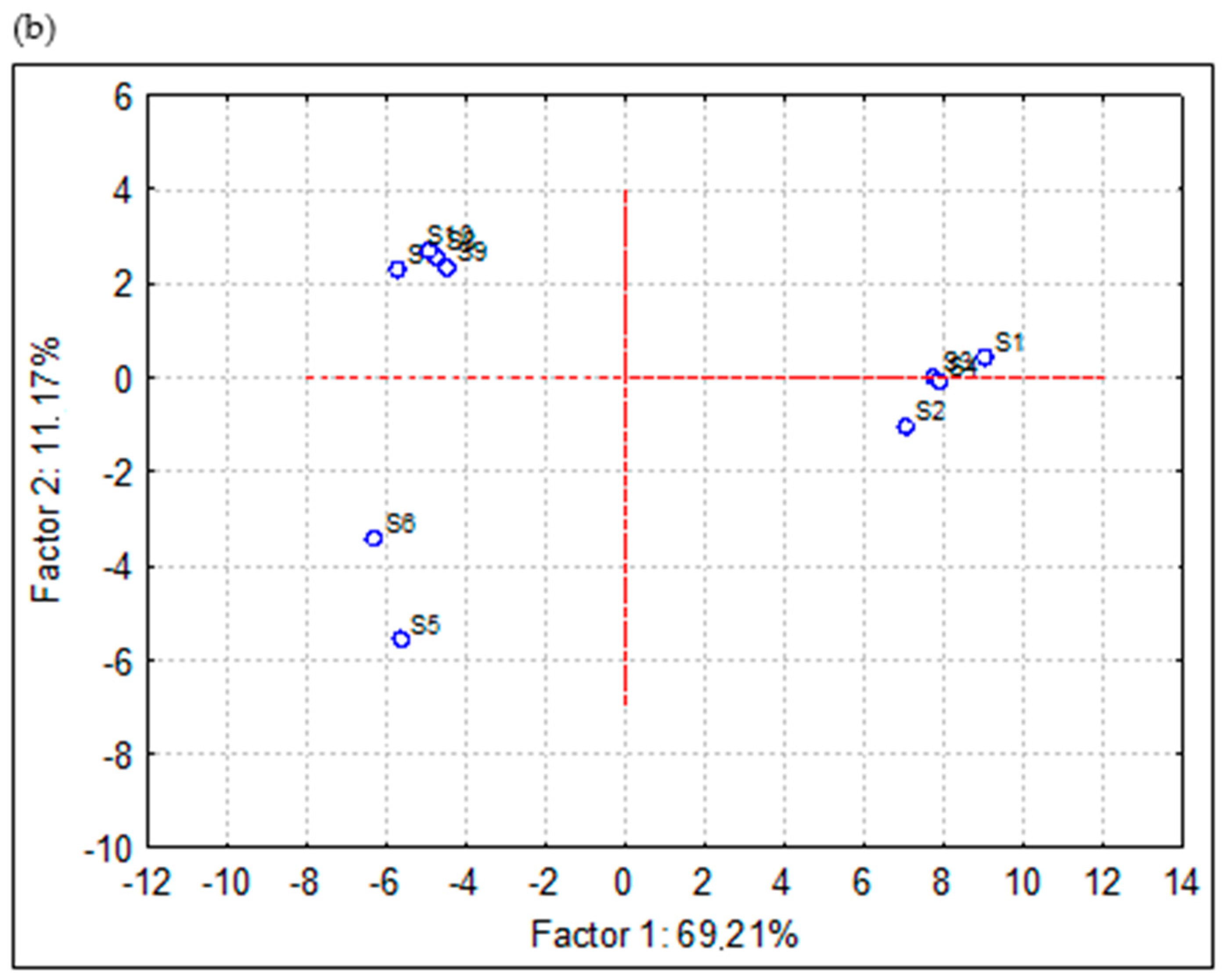
3.4. Results of Principal Component Analysis (PCA)
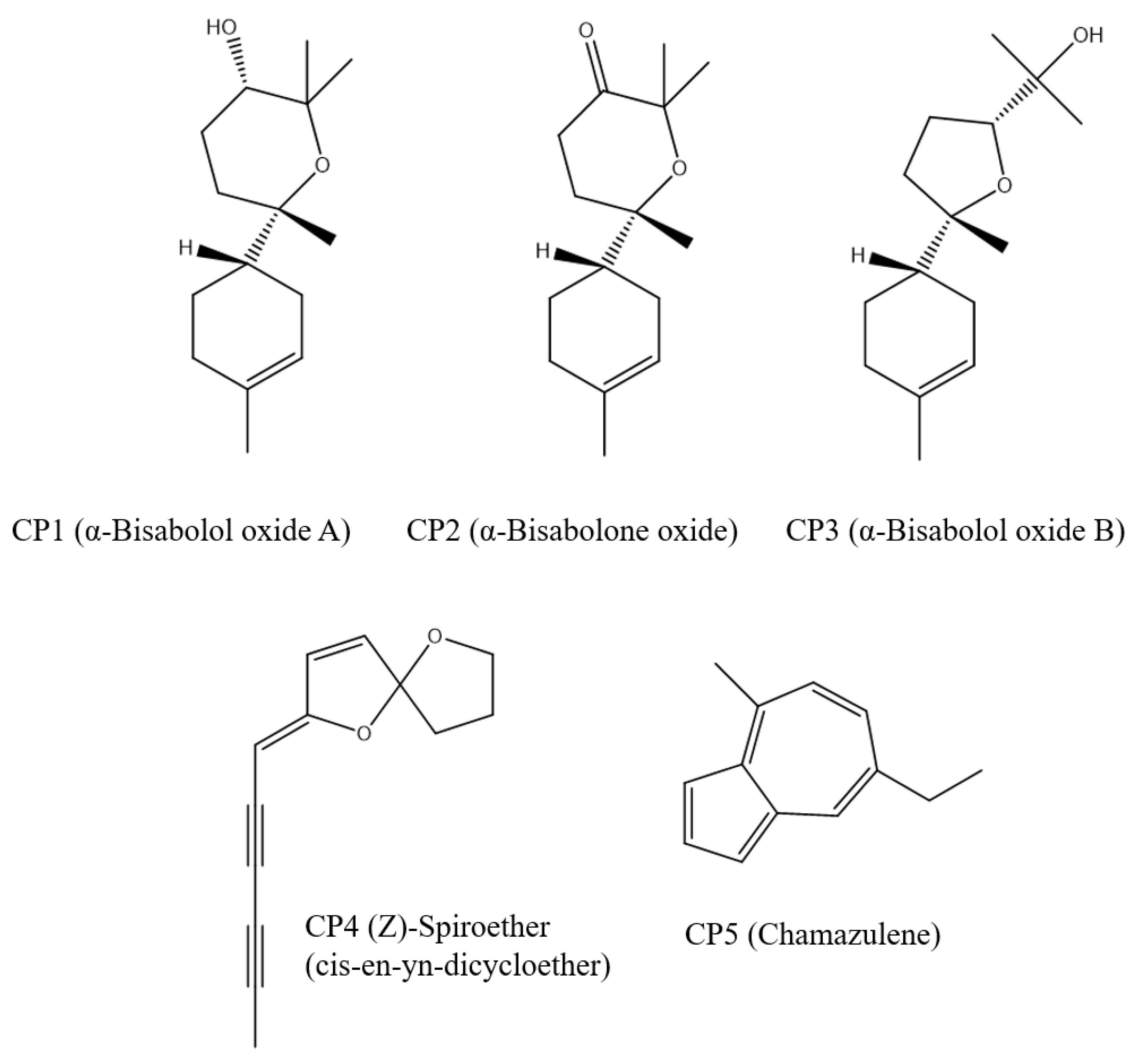
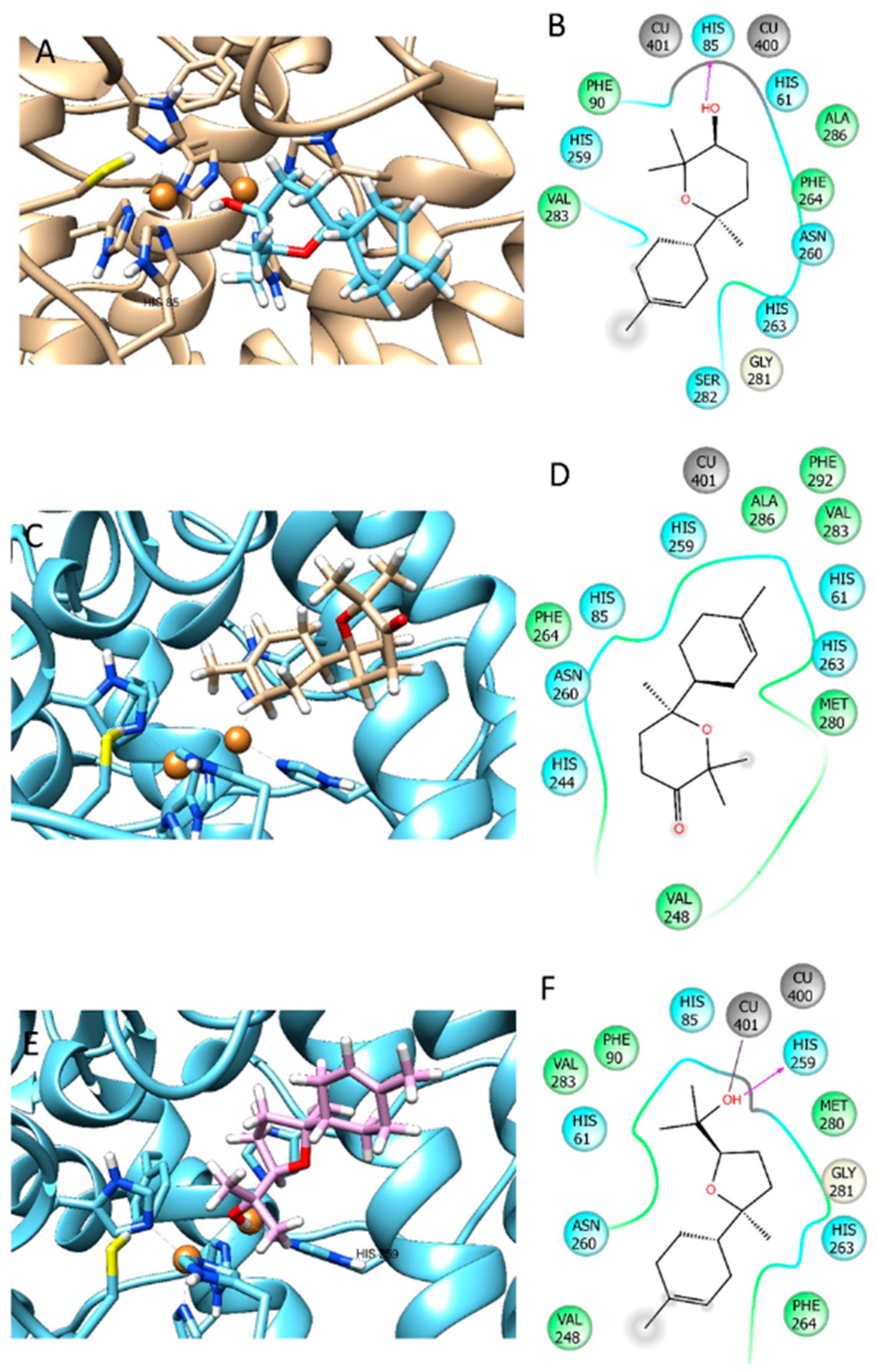
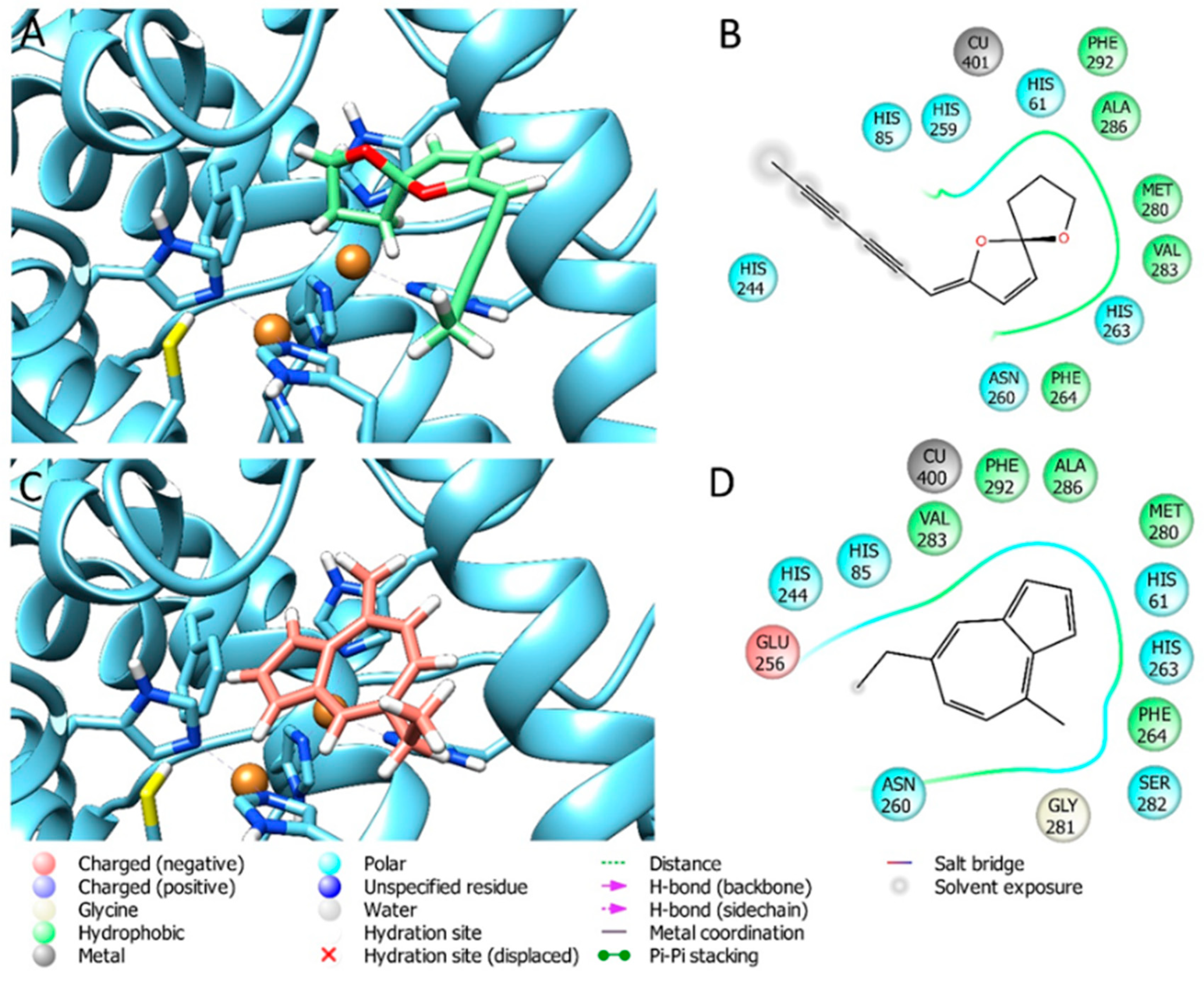
3.5. Docking Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hänsel, R.; Sticher, O. Pharmakognosie-Phytopharmazie; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- De Laurentis, N.; Rosato, A.; Gallo, L.; Leone, L.; Milillo, M. Chemical composition and antimicrobial activity of Myrtus communis. Riv. Ital. EPPOS 2005, 39, 3–8. [Google Scholar]
- Li, Z.; Liu, A.; Du, Q.; Zhu, W.; Liu, H.; Naeem, A.; Guan, Y.; Chen, L.; Ming, L. Bioactive substances and therapeutic potential of camellia oil: An overview. Food Biosci. 2022, 49, 101855. [Google Scholar] [CrossRef]
- Chandrakanthan, M.; Handunnetti, S.M.; Premakumara, G.S.A.; Kathirgamanathar, S. Topical anti-Inflammatory activity of essential oils of Alpinia calcarata Rosc., its main constituents, and possible mechanism of action. Evid. Based Complement. Altern. Med. 2020, 2020, 2035671. [Google Scholar] [CrossRef] [PubMed]
- Petronilho, S.; Maraschin, M.; Coimbra, M.A.; Rocha, S.M. In vitro and in vivo studies of natural products: A challenge for their valuation. The case study of chamomile (Matricaria recutita L.). Ind. Crops Prod. 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Stupar, A.; Šeregelj, V.; Ribeiro, B.D.; Pezo, L.; Cvetanović, A.; Mišan, A.; Marrucho, I. Recovery of β-carotene from pumpkin using switchable natural deep eutectic solvents. Ultrason. Sonochem. 2021, 76, 105638. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Analysis of volatile secondary metabolites from Colombian Xylopia aromatica (Lamarck) by different extraction and headspace methods and gas chromatography. J. Chromatogr. A 2004, 1025, 105–113. [Google Scholar] [CrossRef]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) NE Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 2004, 1025, 93–103. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: Comparison with conventional hydro-distillation. J. Chromatogr. A 2004, 1043, 323–327. [Google Scholar] [CrossRef]
- Pavlić, B.; Teslić, N.; Zengin, G.; Đurović, S.; Rakić, D.; Cvetanović, A.; Gunes, A.; Zeković, Z. Antioxidant and enzyme-inhibitory activity of peppermint extracts and essential oils obtained by conventional and emerging extraction techniques. Food Chem. 2021, 338, 127724. [Google Scholar] [CrossRef]
- Gupta, M.; Sharma, R.; Kumar, A. Docking techniques in pharmacology: How much promising? Comput. Biol. Chem. 2018, 76, 210–217. [Google Scholar] [CrossRef]
- Ražić, S. Chemometrics in the Analysis of real Samples-From Theory to Application; Faculty of Pharmacy-University of Belgrade: Beograd, Serbia, 2011. [Google Scholar]
- Ph. Jug. IV. Pharmacopoea Jugoslavica Edito Qarta; Federal Office of Public Health: Belgrade, Serbia, 1984. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured publishing corporation Carol Stream: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Avonto, C.; Wang, M.; Chittiboyina, A.G.; Avula, B.; Zhao, J.; Khan, I.A. Hydroxylated bisabolol oxides: Evidence for secondary oxidative metabolism in Matricaria chamomilla. J. Nat. Prod. 2013, 76, 1848–1853. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, I.; Schmolz, E.; Ruther, J. Cuticular lipids as trail pheromone in a social wasp. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tschiggerl, C.; Bucar, F. Guaianolides and volatile compounds in chamomile tea. Plant Foods Hum. Nutr. 2012, 67, 129–135. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Governatori, L.; Carlucci, G.; Genovese, S.; Mollica, A.; Epifano, F. Recent application of analytical methods to phase I and phase II drugs development: A review. Biomed. Chromatogr. 2012, 26, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Secci, D.; Carradori, S.; Bizzarri, B.; Chimenti, P.; De Monte, C.; Mollica, A.; Rivanera, D.; Zicari, A.; Mari, E.; Zengin, G.; et al. Novel 1,3-thiazolidin-4-one derivatives as promising anti-Candida agents endowed with anti-oxidant and chelating properties. Eur. J. Med. Chem. 2016, 117, 144–156. [Google Scholar] [CrossRef]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Aktumsek, A.; Zengin, G.; Guler, G.O.; Cakmak, Y.S.; Duran, A. Antioxidant potentials and anticholinesterase activities of methanolic and aqueous extracts of three endemic Centaurea L. species. Food Chem. Toxicol. 2013, 55, 290–296. [Google Scholar] [CrossRef]
- Zengin, G.; Sarikurkcu, C.; Aktumsek, A.; Ceylan, R.; Ceylan, O. A comprehensive study on phytochemical characterization of Haplophyllum myrtifolium Boiss. endemic to Turkey and its inhibitory potential against key enzymes involved in Alzheimer, skin diseases and type II diabetes. Ind. Crops Prod. 2014, 53, 244–251. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef] [PubMed]
- Maestro; Version 12.2; Schrodinger LLC: New York, NY, USA, 2015.
- Shelley, J.C.; Cholleti, A.; Frye, L.L.; Greenwood, J.R.; Timlin, M.R.; Uchimaya, M. Epik: A software program for pK a prediction and protonation state generation for drug-like molecules. J. Comput.-Aided Mol. Des. 2007, 21, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Mocan, A.; Zengin, G.; Simirgiotis, M.; Schafberg, M.; Mollica, A.; Vodnar, D.C.; Crişan, G.; Rohn, S. Functional constituents of wild and cultivated Goji (L. barbarum L.) leaves: Phytochemical characterization, biological profile, and computational studies. J. Enzym. Inhib. Med. Chem. 2017, 32, 153–168. [Google Scholar] [CrossRef]
- Uysal, A.; Zengin, G.; Mollica, A.; Gunes, E.; Locatelli, M.; Yilmaz, T.; Aktumsek, A. Chemical and biological insights on Cotoneaster integerrimus: A new (-)-epicatechin source for food and medicinal applications. Phytomedicine 2016, 23, 979–988. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Aktumsek, A.; Mocan, A.; Mollica, A.; Locatelli, M.; Custodio, L.; Neng, N.R.; Nogueira, J.M.; Aumeeruddy-Elalfi, Z. Euphorbia denticulata Lam.: A promising source of phyto-pharmaceuticals for the development of novel functional formulations. Biomed. Pharmacother. 2017, 87, 27–36. [Google Scholar] [CrossRef]
- Ligprep; VERSION 2.3; Schrödinger, LLC: New York, NY, USA, 2009; pp. 1–116.
- Epik. Schrödinger, LLC: New York, NY, USA. Available online: https://www.schrodinger.com/epik (accessed on 21 March 2018).
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein— ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef]
- Zengin, G.; Aumeeruddy-Elalfi, Z.; Mollica, A.; Yilmaz, M.A.; Mahomoodally, M.F. In vitro and in silico perspectives on biological and phytochemical profile of three halophyte species—A source of innovative phytopharmaceuticals from nature. Phytomedicine 2018, 38, 35–44. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach; John Wiley & Sons: Hoboken, NJ, USA, 2002. [Google Scholar]
- Taghizadeh, S.F.; Azizi, M.; Rezaee, R.; Madarshahi, F.S.; Mehmandoust, M.; Karimi, G.; Asili, J. Cytotoxic activity of cis-(E)-and trans-(Z)-spiroethers isolated from various Arnebia species. S. Afr. J. Bot. 2021, 142, 114–123. [Google Scholar] [CrossRef]
- Yoshinari, T.; Yaguchi, A.; Takahashi-Ando, N.; Kimura, M.; Takahashi, H.; Nakajima, T.; Sugita-Konishi, Y.; Nagasawa, H.; Sakuda, S. Spiroethers of German chamomile inhibit production of aflatoxin G1 and trichothecene mycotoxin by inhibiting cytochrome P450 monooxygenases involved in their biosynthesis. FEMS Microbiol. Lett. 2008, 284, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Franke, R.; Schilcher, H. Chamomile: Industrial Profiles; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Tomić, M.; Popović, V.; Petrović, S.; Stepanović-Petrović, R.; Micov, A.; Pavlović-Drobac, M.; Couladis, M. Antihyperalgesic and antiedematous activities of bisabolol-oxides-rich matricaria oil in a rat model of inflammation. Phytother. Res. 2014, 28, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. European Pharmacopoeia; Council of Europe: Strasbourg, France, 2019; version 10.0.1836 (01/2008). [Google Scholar]
- Kotnik, P.; Škerget, M.; Knez, Ž. Supercritical fluid extraction of chamomile flower heads: Comparison with conventional extraction, kinetics and scale-up. J. Supercrit. Fluids 2007, 43, 192–198. [Google Scholar] [CrossRef]
- Čekan, Z.; Herout, V.; Šorm, F. Über terpene LXXX. Die struktur von matricin, ein guajanolid aus der kamille (Matricaria chamomilla L.). Collect. Czechoslov. Chem. Commun. 1957, 22, 1921–1929. [Google Scholar] [CrossRef]
- Čekan, Z.; Procházka, V.; Herout, V.; Šorm, F. On terpenes. CI. Isolation and constitution of matricarin, another guaianolide from camomile (Matricaria chamomilla L.). Collect. Czechoslov. Chem. Commun. 1959, 24, 1554–1557. [Google Scholar] [CrossRef]
- Kaiser, C.; Römpp, H.; Schmidt, P. Supercritical carbon dioxide extraction of chamomile flowers: Extraction efficiency, stability, and in-line inclusion of chamomile-carbon dioxide extract in β-cyclodextrin. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 249–256. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crops Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303. [Google Scholar] [CrossRef]
- Association, A.D. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef]
- Bailey, C.J.; Day, C. Antidiabetic drugs. Br. J. Cardiol. 2003, 10, 128–136. [Google Scholar]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of two α-glucosidase inhibitors, voglibose and acarbose, on postprandial hyperglycemia correlates with subjective abdominal symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Francini, F.; Schinella, G.R. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef] [PubMed]
- Capetti, F.; Cagliero, C.; Marengo, A.; Bicchi, C.; Rubiolo, P.; Sgorbini, B. Bio-guided fractionation driven by in vitro α-amylase inhibition assays of essential oils bearing specialized metabolites with potential hypoglycemic activity. Plants 2020, 9, 1242. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Loizzo, M.R.; Statti, G.A.; Menichini, F. Comparative Radical Scavenging and Antidiabetic Activities of Methanolic Extract and Fractions from Achillea ligustica ALL. Biol. Pharm. Bull. 2005, 28, 1791–1794. [Google Scholar] [CrossRef]
- Sadiq, A.; Rashid, U.; Ahmad, S.; Zahoor, M.; AlAjmi, M.F.; Ullah, R.; Noman, O.M.; Ullah, F.; Ayaz, M.; Khan, I. Treating hyperglycemia from Eryngium caeruleum M. Bieb: In-vitro α-glucosidase, antioxidant, in-vivo antidiabetic and molecular docking-based approaches. Front. Chem. 2020, 8, 558641. [Google Scholar] [CrossRef]
- Fraihat, A.; Alatrash, L.; Abbasi, R.; Abu-Irmaileh, B.; Hamed, S.; Mohammad, M.; Abu-Rish, E.; Bustanji, Y. Inhibitory effects of methanol extracts of selected plants on the proliferation of two human melanoma cell lines. Trop. J. Pharm. Res. 2018, 17, 1081–1086. [Google Scholar] [CrossRef]
- Salem, M.A.; Radwan, R.A.; Mostafa, E.S.; Alseekh, S.; Fernie, A.R.; Ezzat, S.M. Using an UPLC/MS-based untargeted metabolomics approach for assessing the antioxidant capacity and anti-aging potential of selected herbs. RSC Adv. 2020, 10, 31511–31524. [Google Scholar] [CrossRef]
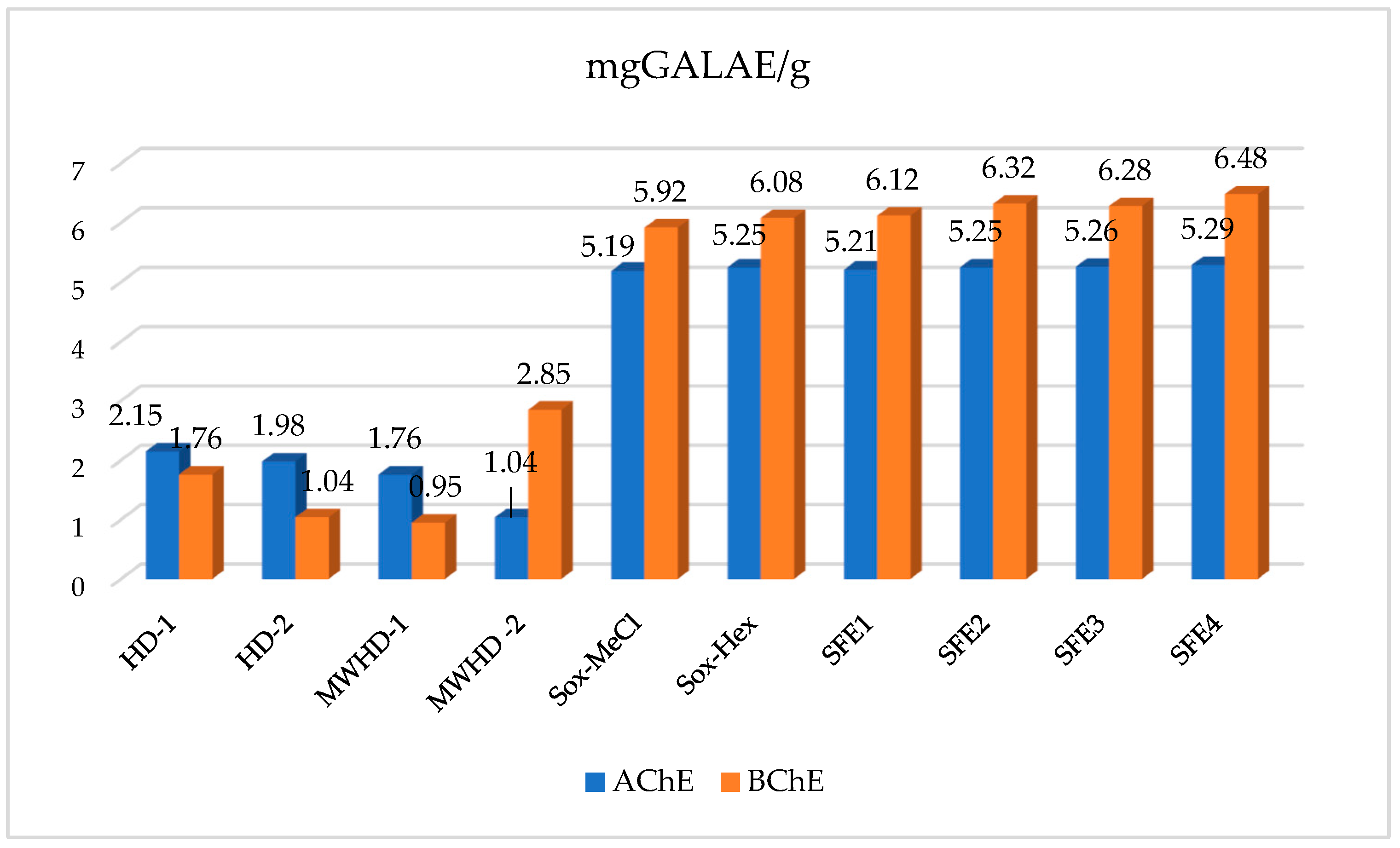
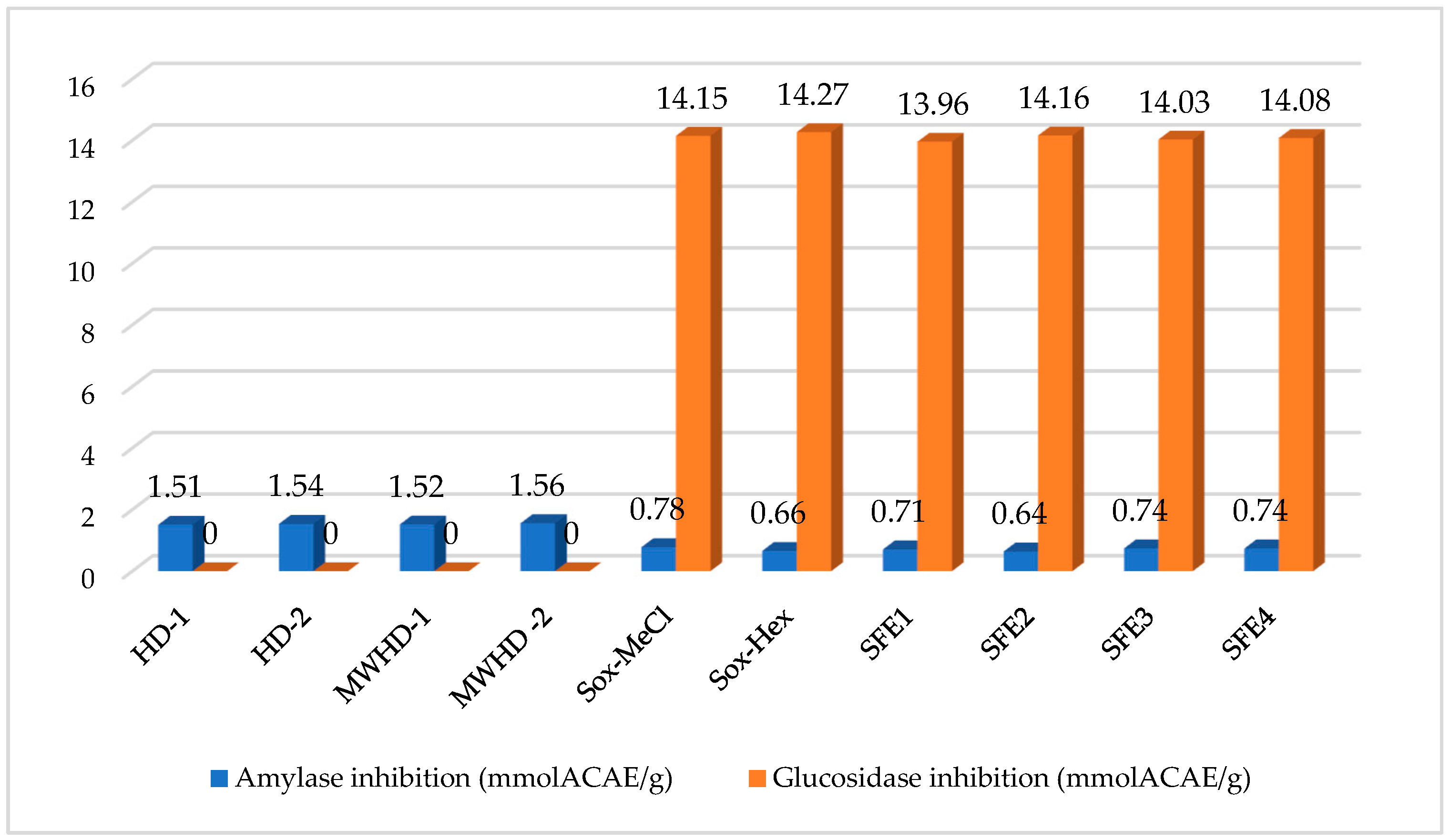

| Samples | TP (mgGAE/g) | TF (mgRE/g) | TAC (mmol TE/g) | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | Metal Chelating (mg EDTA/g) |
|---|---|---|---|---|---|---|---|---|
| HD-1 | / | / | 28.95 ± 1.61 | 30.69 ± 0.59 | 149.65 ± 1.81 | 455.67 ± 6.59 | 711.03 ± 6.48 | 16.86 ± 0.95 |
| HD-2 | / | / | 25.06 ± 1.68 | 32.44 ± 1.02 | 160.61 ± 2.94 | 485.84 ± 9.50 | 595.28 ± 5.03 | 41.29 ± 1.01 |
| MWHD-1 | / | / | 27.77 ± 0.54 | 28.41 ± 0.45 | 156.09 ± 3.07 | 478.36 ± 6.14 | 651.05 ± 6.54 | 35.60 ± 3.71 |
| MWHD-2 | / | / | 21.35 ± 4.04 | 26.28 ± 0.63 | 184.18 ± 0.77 | 409.05 ± 2.42 | 520.31 ± 9.46 | 21.70 ± 4.96 |
| Sox-MeCl | 22.65 ± 0.60 | 4.59 ± 0.11 | 2.58 ± 0.04 | 3.39 ± 0.65 | 6.53 ± 0.90 | 63.07 ± 2.45 | 37.28 ± 0.61 | 28.80 ± 1.64 |
| Sox-Hex | 19.08 ± 0.14 | 4.62 ± 0.13 | 1.95 ± 0.11 | 4.91 ± 0.90 | 13.04 ± 2.92 | 60.17 ± 1.57 | 32.89 ± 0.41 | 32.36 ± 1.51 |
| SFE1 | 22.12 ± 0.55 | 9.23 ± 0.10 | 2.72 ± 0.09 | 3.89 ± 0.59 | 11.53 ± 1.45 | 70.95 ± 2.76 | 36.25 ± 0.47 | 44.68 ± 1.79 |
| SFE2 | 21.92 ± 0.72 | 10.61 ± 0.33 | 2.51 ± 0.11 | 5.85 ± 0.53 | 17.59 ± 3.69 | 71.45 ± 1.96 | 36.79 ± 0.61 | 39.02 ± 2.90 |
| SFE3 | 32.49 ± 0.77 | 29.43 ± 0.42 | 4.16 ± 0.15 | 10.55 ± 0.64 | 33.85 ± 0.90 | 106.64 ± 2.84 | 58.76 ± 0.33 | 53.50 ± 1.76 |
| SFE4 | 15.56 ± 0.24 | 3.97 ± 0.26 | 1.91 ± 0.17 | 1.49 ± 0.67 | 5.10 ± 0.42 | 72.94 ± 1.21 | 26.03 ± 2.27 | 63.98 ± 1.17 |
| HD-1 (mgKAE/g) | HD-2 (mgKAE/g) | MWHD-1 (mgKAE/g) | MWHD-2 (mgKAE/g) | Sox-MeCl (mgKAE/g) | Sox-Hex (mgKAE/g) | SFE-1 (mgKAE/g) | SFE-2 (mgKAE/g) | SFE-3 (mgKAE/g) | SFE-4 (mgKAE/g) |
|---|---|---|---|---|---|---|---|---|---|
| 61.26 ± 0.39 | 66.59 ± 3.56 | 97.96 ± 0.80 | 62.40 ± 2.83 | 132.13 ± 0.94 | 133.01 ± 0.58 | 130.42 ± 0.96 | 130.96 ± 0.84 | 133.49 ± 1.92 | 132.28 ± 0.64 |
| Compound | ChemScore Fitness |
|---|---|
| CP1 | 23.76 |
| CP2 | 18.81 |
| CP3 | 26.71 |
| CP4 | 20.10 |
| CP5 | 21.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Mollica, A.; Arsenijević, J.; Pavlić, B.; Zeković, Z.; Sinan, K.I.; Yan, L.; Cvetanović Kljakić, A.; Ražić, S. A Comparative Study of Chamomile Essential Oils and Lipophilic Extracts Obtained by Conventional and Greener Extraction Techniques: Chemometric Approach to Chemical Composition and Biological Activity. Separations 2023, 10, 18. https://doi.org/10.3390/separations10010018
Zengin G, Mollica A, Arsenijević J, Pavlić B, Zeković Z, Sinan KI, Yan L, Cvetanović Kljakić A, Ražić S. A Comparative Study of Chamomile Essential Oils and Lipophilic Extracts Obtained by Conventional and Greener Extraction Techniques: Chemometric Approach to Chemical Composition and Biological Activity. Separations. 2023; 10(1):18. https://doi.org/10.3390/separations10010018
Chicago/Turabian StyleZengin, Gökhan, Adriano Mollica, Jelena Arsenijević, Branimir Pavlić, Zoran Zeković, Kouadio Ibrahime Sinan, Linlin Yan, Aleksandra Cvetanović Kljakić, and Slavica Ražić. 2023. "A Comparative Study of Chamomile Essential Oils and Lipophilic Extracts Obtained by Conventional and Greener Extraction Techniques: Chemometric Approach to Chemical Composition and Biological Activity" Separations 10, no. 1: 18. https://doi.org/10.3390/separations10010018
APA StyleZengin, G., Mollica, A., Arsenijević, J., Pavlić, B., Zeković, Z., Sinan, K. I., Yan, L., Cvetanović Kljakić, A., & Ražić, S. (2023). A Comparative Study of Chamomile Essential Oils and Lipophilic Extracts Obtained by Conventional and Greener Extraction Techniques: Chemometric Approach to Chemical Composition and Biological Activity. Separations, 10(1), 18. https://doi.org/10.3390/separations10010018














