Thermal and Kinetic Behaviors during Co-Pyrolysis of Microcrystalline Cellulose and Styrene–Butadiene–Styrene Triblock Copolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments
2.3. Kinetic Method
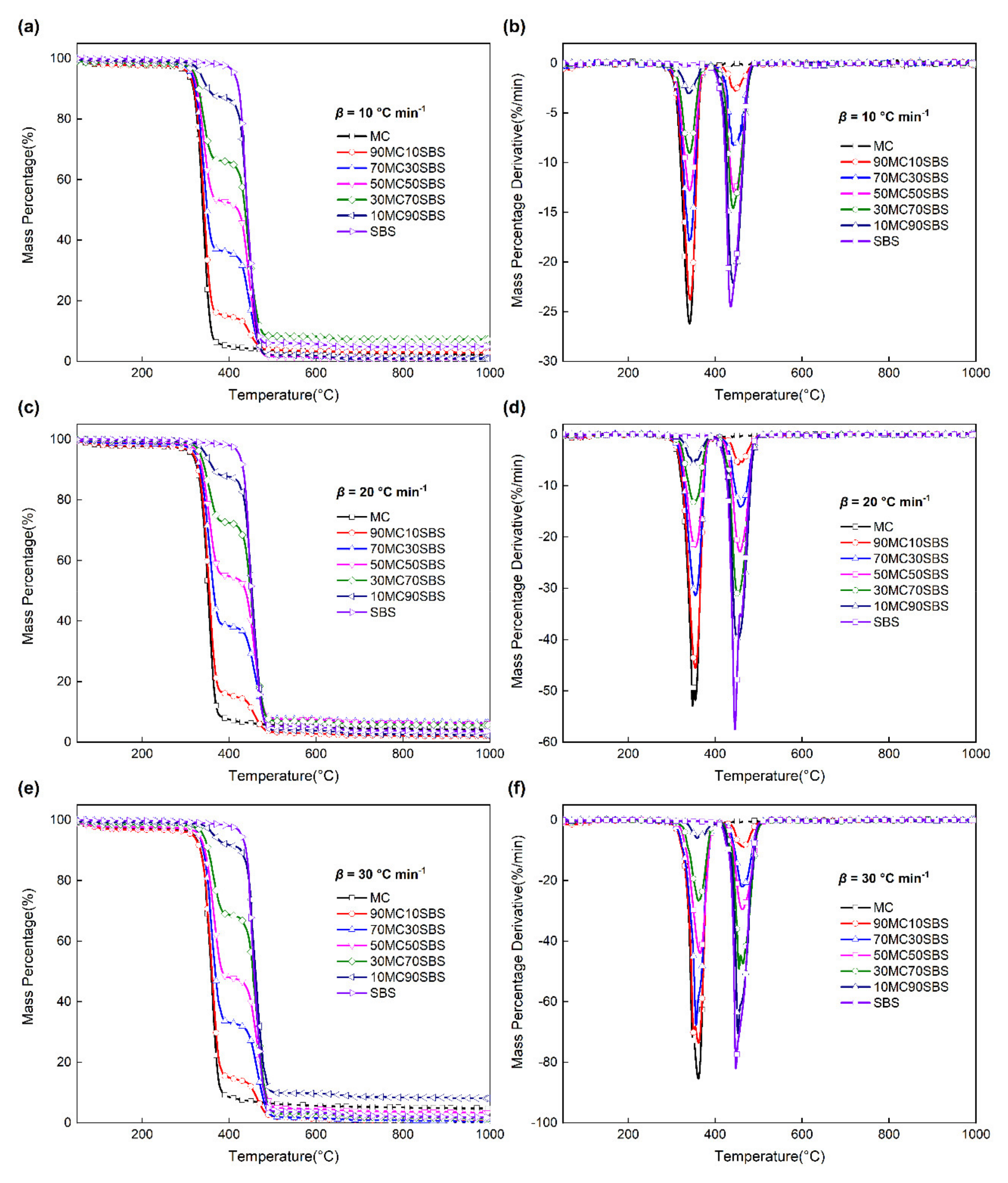
3. Results and Discussion
3.1. Pyrolysis of Single Component
3.2. Pyrolysis of Mixed Components
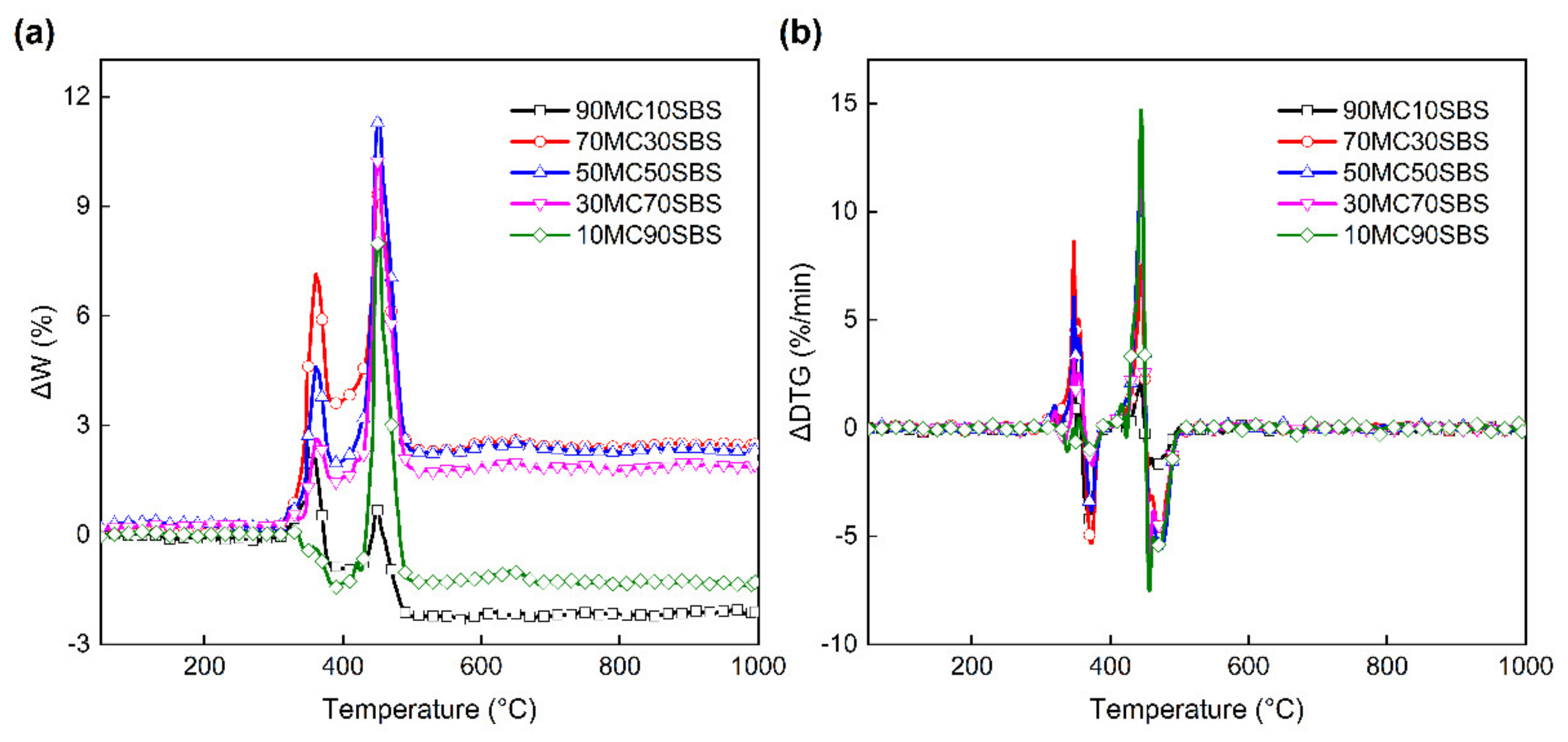
3.3. Interaction between MC and SBS
3.3.1. Interaction on the TG Curves
3.3.2. Interaction on the DTG Curves
3.3.3. Interaction Indices
3.4. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Wu, X.W. Factors influencing municipal solid waste generation in China: A multiple statistical analysis study. Waste Manag. Res. 2011, 29, 371–378. [Google Scholar] [PubMed]
- Ma, S.; Lu, J.; Gao, J. Study of the low temperature pyrolysis of PVC. Energy Fuels 2002, 16, 338–342. [Google Scholar] [CrossRef]
- Huai, X.L.; Xu, W.L.; Qu, Z.Y.; Li, Z.G.; Zhang, F.P.; Xiang, G.M.; Zhu, S.Y.; Chen, G. Numerical simulation of municipal solid waste combustion in a novel two-stage reciprocating incinerator. Waste Manag. 2008, 28, 15–29. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y. Novel incineration technology integrated with drying, pyrolysis, gasification, and combustion of MSW and ashes vitrification. Environ. Sci. Technol. 2005, 39, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Paradela, F.; Pinto, F.; Ramos, A.M.; Gulyurtlu, I.; Cabrita, I. Study of the slow batch pyrolysis of mixtures of plastics, tyres and forestry biomass wastes. J. Anal. Appl. Pyrolysis 2009, 85, 392–398. [Google Scholar] [CrossRef]
- Lee, K.-H.; Shin, D.-H. Characteristics of liquid product from the pyrolysis of waste plastic mixture at low and high temperatures: Influence of lapse time of reaction. Waste Manag. 2007, 27, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Aboulkas, A.; El Harfi, K.; El Bouadili, A. Thermal degradation behaviors of polyethylene and polypropylene. Part I: Pyrolysis kinetics and mechanisms. Energy Convers. Manag. 2010, 51, 1363–1369. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A.; Lopez-Cuesta, J.M. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- Fang, S.; Lin, Y.; Huang, Z.; Huang, H.; Chen, S.; Ding, L. Investigation of co-pyrolysis characteristics and kinetics of municipal solid waste and paper sludge through TG-FTIR and DAEM. Thermochim. Acta 2021, 700, 178889. [Google Scholar] [CrossRef]
- Genieva, S.; Gonsalvesh, L.; Georgieva, V.; Tavlieva, M.; Vlaev, L. Kinetic analysis and pyrolysis mechanism of raw and impregnated almond shells. Thermochim. Acta 2021, 698, 178877. [Google Scholar] [CrossRef]
- Hu, D.H.; Chen, M.Q.; Huang, Y.W.; Wei, S.H.; Zhong, X.B. Evaluation on isothermal pyrolysis characteristics of typical technical solid wastes. Thermochim. Acta 2020, 688, 178604. [Google Scholar] [CrossRef]
- Radojević, M.; Janković, B.; Stojiljković, D.; Jovanović, V.; Čeković, I.; Manić, N. Improved TGA-MS measurements for evolved gas analysis (EGA) during pyrolysis process of various biomass feedstocks. Syngas energy balance determination. Thermochim. Acta 2021, 699, 178912. [Google Scholar] [CrossRef]
- Sfakiotakis, S.; Vamvuka, D. Study of co-pyrolysis of olive kernel with waste biomass using TGA/DTG/MS. Thermochim. Acta 2018, 670, 44–54. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.H.; Long, Y.Q.; Li, Q.H.; Zhang, Y.G. An overview of characteristics of municipal solid waste fuel in China: Physical, chemical composition and heating value. Renew. Sustain. Energy Rev. 2014, 36, 107–122. [Google Scholar] [CrossRef]
- Jia, C.; Chen, J.; Liang, J.; Song, S.; Liu, K.; Jiang, A.; Wang, Q. Pyrolysis characteristics and kinetic analysis of rice husk. J. Therm. Anal. Calorim. 2020, 139, 577–587. [Google Scholar] [CrossRef]
- Fan, F.; Yang, Z.; Xing, X. Study on the pyrolysis properties of corn straw by TG–FTIR and TG–GC/MS. J. Therm. Anal. Calorim. 2021, 143, 3783–3791. [Google Scholar] [CrossRef]
- Ji, M.X.; Chen, L.; Que, J.J.; Zheng, L.L.; Chen, Z.Z.; Wu, Z.S. Effects of transition metal oxides on pyrolysis properties of PVC. Process Saf. Environ. Prot. 2020, 140, 211–220. [Google Scholar] [CrossRef]
- Wang, S.; Guo, X.; Wang, K.; Luo, Z. Influence of the interaction of components on the pyrolysis behavior of biomass. J. Anal. Appl. Pyrolysis 2011, 91, 183–189. [Google Scholar] [CrossRef]
- Çepelioğullar, Ö.; Pütün, A.E. Thermal and kinetic behaviors of biomass and plastic wastes in co-pyrolysis. Energy Convers. Manag. 2013, 75, 263–270. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Carrier, M.; Meyer, E.L.; Knoetze, H.; Görgens, J.F. Slow and pressurized co-pyrolysis of coal and agricultural residues. Energy Convers. Manag. 2013, 65, 198–207. [Google Scholar] [CrossRef]
- Ateş, F.; Pütün, A.E.; Pütün, E. Fixed bed pyrolysis of Euphorbia rigida with different catalysts. Energy Convers. Manag. 2005, 46, 421–432. [Google Scholar] [CrossRef]
- Park, D.K.; Kim, S.D.; Lee, S.H.; Lee, J.G. Co-pyrolysis characteristics of sawdust and coal blend in TGA and a fixed bed reactor. Bioresour. Technol. 2010, 101, 6151–6156. [Google Scholar] [CrossRef]
- Lin, X.N.; Zhang, D.H.; Ren, X.J.; Zhang, Q.F.; Cai, H.Z.; Yi, W.M.; Lei, H.W. Catalytic co-pyrolysis of waste corn stover and high-density polyethylene for hydrocarbon production: The coupling effect of potassium and HZSM-5 zeolite. J. Anal. Appl. Pyrolysis 2020, 150, 104895. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Chen, W.; Hu, J.H.; Li, S.Q.; Chen, Y.Q.; Yang, H.P.; Chen, H.P. Co-pyrolysis of microalgae with low-density polyethylene (LDPE) for deoxygenation and denitrification. Bioresour. Technol. 2020, 311, 123502. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.N.; Cao, B.; Uzoejinwa, B.B.; Odey, E.A.; Wang, S.; Shang, H.; Li, C.H.; Hu, Y.M.; Wang, Q.; Nwakaire, J.N. Synergistic effects of catalytic co-pyrolysis of macroalgae with waste plastics. Process Saf. Environ. Prot. 2020, 137, 34–48. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishaq, M.; Ahmad, I.; Shakirullah, M.; Khan, M.A.; ur Rehman, H.; Bahader, A. Pyrolysis of some whole plastics and plastics–coal mixtures. Energy Convers. Manag. 2006, 47, 3216–3223. [Google Scholar] [CrossRef]
- Shen, B.; Qin, L. Study on MSW catalytic combustion by TGA. Energy Convers. Manag. 2006, 47, 1429–1437. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, X.; Wei, L.; Wang, H. Research on pyrolysis characteristics and kinetics of super fine and conventional pulverized coal. Energy Convers. Manag. 2007, 48, 797–802. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, H.; Zhou, Q.; Zhu, S.; Chen, G. Effect of inorganic matter on reactivity and kinetics of coal pyrolysis. Fuel 2004, 83, 713–718. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Kantarelis, E.K.; Theodoropoulos, C.D. Sunflower shells utilization for energetic purposes in an integrated approach of energy crops: Laboratory study pyrolysis and kinetics. Bioresour. Technol. 2008, 99, 3174–3181. [Google Scholar] [CrossRef]
- Zhou, H. Combustible Solid Waste Thermochemical Conversion: A Study of Interactions and Influence Factors; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Varma, A.K.; Mondal, P. Physicochemical characterization and kinetic study of pine needle for pyrolysis process. J. Therm. Anal. Calorim. 2016, 124, 487–497. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Burnham, A.K. Computational aspects of kinetic analysis: Part D: The ICTAC kinetics project—Multi-thermal–history model-fitting methods and their relation to isoconversional methods. Thermochim. Acta 2000, 355, 165–170. [Google Scholar] [CrossRef]
- Yan, L.; He, B.; Hao, T.; Pei, X.; Li, X.; Wang, C.; Duan, Z. Thermogravimetric study on the pressurized hydropyrolysis kinetics of a lignite coal. Int. J. Hydrogen Energy 2014, 39, 7826–7833. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 5, 285–292. [Google Scholar] [CrossRef]
- Cong, K.; Zhang, Y.U.; Gan, Y.; Li, Q.H. Experimental study of the ignition temperatures of low-rank coals using TGA under oxygen-deficient conditions. J. Therm. Anal. Calorim. 2018, 133, 1597–1607. [Google Scholar] [CrossRef]
- Müsellim, E.; Tahir, M.H.; Ahmad, M.S.; Ceylan, S. Thermokinetic and TG/DSC-FTIR study of pea waste biomass pyrolysis. Appl. Therm. Eng. 2018, 137, 54–61. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.A.; Ismail, K.; Alias, A.B.; Rashid, Z.A.; Aris, M.J. Investigation on thermochemical behaviour of low rank Malaysian coal, oil palm biomass and their blends during pyrolysis via thermogravimetric analysis (TGA). Bioresour. Technol. 2010, 101, 4584–4592. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.Q.; Meng, A.H.; Chen, S.; Li, Q.H.; Zhang, Y.G. A novel method for kinetics analysis of pyrolysis of hemicellulose, cellulose, and lignin in TGA and macro-TGA. RSC Adv. 2015, 5, 26509–26516. [Google Scholar] [CrossRef]
- Zhou, H.; Long, Y.Q.; Meng, A.H.; Li, Q.H.; Zhang, Y.G. The pyrolysis simulation of five biomass species by hemi-cellulose, cellulose and lignin based on thermogravimetric curves. Thermochim. Acta 2013, 566, 36–43. [Google Scholar] [CrossRef]
- Zhou, H.; Meng, A.H.; Long, Y.Q.; Li, Q.H.; Zhang, Y.G. Interactions of municipal solid waste components during pyrolysis: A TG-FTIR study. J. Anal. Appl. Pyrolysis 2014, 108, 19–25. [Google Scholar] [CrossRef]
- Chen, R.J.; Zhang, S.Y.; Cong, K.L.; Li, Q.H.; Zhang, Y.G. Insightinto synergistic effects of biomass -polypropylene co -pyrolysis using representative biomass constituents. Bioresour. Technol. 2020, 307, 8. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, Y.; Wu, K.; Chen, Y.; Hu, H.; Yang, M. Study on pyrolytic kinetics and behavior: The co-pyrolysis of microalgae and polypropylene. Bioresour. Technol. 2015, 192, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Cong, K.L.; Han, F.; Zhang, Y.G.; Li, Q.H. The investigation of co-combustion characteristics of tobacco stalk and low rank coal using a macro-TGA. Fuel 2019, 237, 126–132. [Google Scholar] [CrossRef]
- Önal, E.; Uzun, B.B.; Pütün, A.E. Bio-oil production via co-pyrolysis of almond shell as biomass and high density polyethylene. Energy Convers. Manag. 2014, 78, 704–710. [Google Scholar] [CrossRef]



| Samples | MC | SBS |
|---|---|---|
| Proximate analysis/wt% | ||
| Ash, db | 0.00 | 5.97 |
| Volatile matter, daf | 85.66 | 94.03 |
| Fixed carbon, db | 14.34 | 0.00 |
| Ultimate analysis/wt%, db | ||
| C | 42.21 | 81.92 |
| H | 6.25 | 11.42 |
| O (by difference) | 51.29 | 6.37 |
| N | 0.21 | 0.25 |
| S | 0.04 | 0.05 |
| Material | Conversion (%) | T (°C) | E (kJ/mol) | (kJ/mol) | R2 |
|---|---|---|---|---|---|
| MC | 20 | 336.2 | 174.8 | 183.3 | 0.9980 |
| 40 | 345.5 | 183.9 | 0.9999 | ||
| 60 | 353.0 | 183.8 | 0.9999 | ||
| 80 | 360.9 | 190.9 | 0.9996 | ||
| 90MC10SBS | 20 | 337.6 | 185.6 | 184.6 | 0.9990 |
| 40 | 347.9 | 189.2 | 0.9982 | ||
| 60 | 356.5 | 184.0 | 0.9997 | ||
| 80 | 367.9 | 179.7 | 0.9992 | ||
| 70MC30SBS | 20 | 341.9 | 171.2 | 212.0 | 0.9995 |
| 40 | 353.8 | 181.3 | 0.9960 | ||
| 60 | 368.0 | 202.5 | 0.9925 | ||
| 80 | 454.1 | 292.9 | 0.9952 | ||
| 50MC50SBS | 20 | 346.8 | 170.0 | 232.6 | 0.9980 |
| 40 | 363.5 | 188.2 | 0.9990 | ||
| 60 | 443.7 | 303.3 | 0.9080 | ||
| 80 | 461.7 | 268.8 | 1.0000 | ||
| 30MC70SBS | 20 | 356.0 | 135.7 | 224.4 | 0.9936 |
| 40 | 438.4 | 210.7 | 0.9842 | ||
| 60 | 452.7 | 254.8 | 0.9999 | ||
| 80 | 466.5 | 296.5 | 0.9990 | ||
| 10MC90SBS | 20 | 435.0 | 197.0 | 219.5 | 0.9741 |
| 40 | 446.7 | 239.2 | 0.9989 | ||
| 60 | 456.2 | 232.0 | 1.0000 | ||
| 80 | 467.4 | 210.0 | 0.9990 | ||
| SBS | 20 | 438.1 | 263.4 | 281.2 | 0.9999 |
| 40 | 445.6 | 280.0 | 0.9980 | ||
| 60 | 454.0 | 284.6 | 0.9956 | ||
| 80 | 464.5 | 296.8 | 1.0000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, S.; Yang, X.; Zhou, H.; Tan, Z.; Cong, K.; Zhang, Y.; Li, Q. Thermal and Kinetic Behaviors during Co-Pyrolysis of Microcrystalline Cellulose and Styrene–Butadiene–Styrene Triblock Copolymer. Processes 2021, 9, 1335. https://doi.org/10.3390/pr9081335
Yu S, Yang X, Zhou H, Tan Z, Cong K, Zhang Y, Li Q. Thermal and Kinetic Behaviors during Co-Pyrolysis of Microcrystalline Cellulose and Styrene–Butadiene–Styrene Triblock Copolymer. Processes. 2021; 9(8):1335. https://doi.org/10.3390/pr9081335
Chicago/Turabian StyleYu, Shijie, Xiaoxiao Yang, Hui Zhou, Zhongchao Tan, Kunlin Cong, Yanguo Zhang, and Qinghai Li. 2021. "Thermal and Kinetic Behaviors during Co-Pyrolysis of Microcrystalline Cellulose and Styrene–Butadiene–Styrene Triblock Copolymer" Processes 9, no. 8: 1335. https://doi.org/10.3390/pr9081335
APA StyleYu, S., Yang, X., Zhou, H., Tan, Z., Cong, K., Zhang, Y., & Li, Q. (2021). Thermal and Kinetic Behaviors during Co-Pyrolysis of Microcrystalline Cellulose and Styrene–Butadiene–Styrene Triblock Copolymer. Processes, 9(8), 1335. https://doi.org/10.3390/pr9081335









