Fluctuation and Re-Establishment of Aerobic Granules Properties during the Long-Term Operation Period with Low-Strength and Low C/N Ratio Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Setup and Operation
2.2. Media
2.3. Analytical Methods
3. Results and Discussion
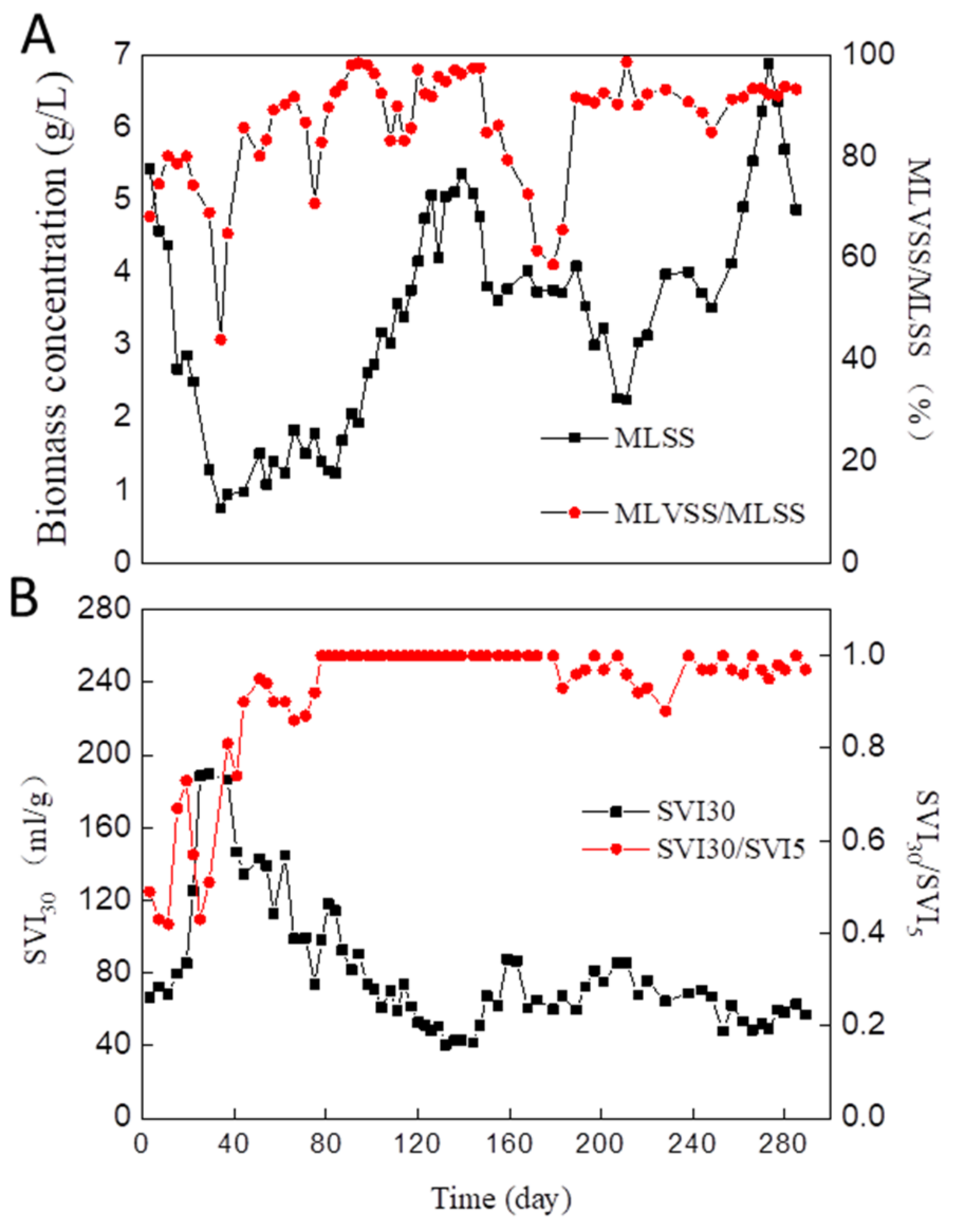
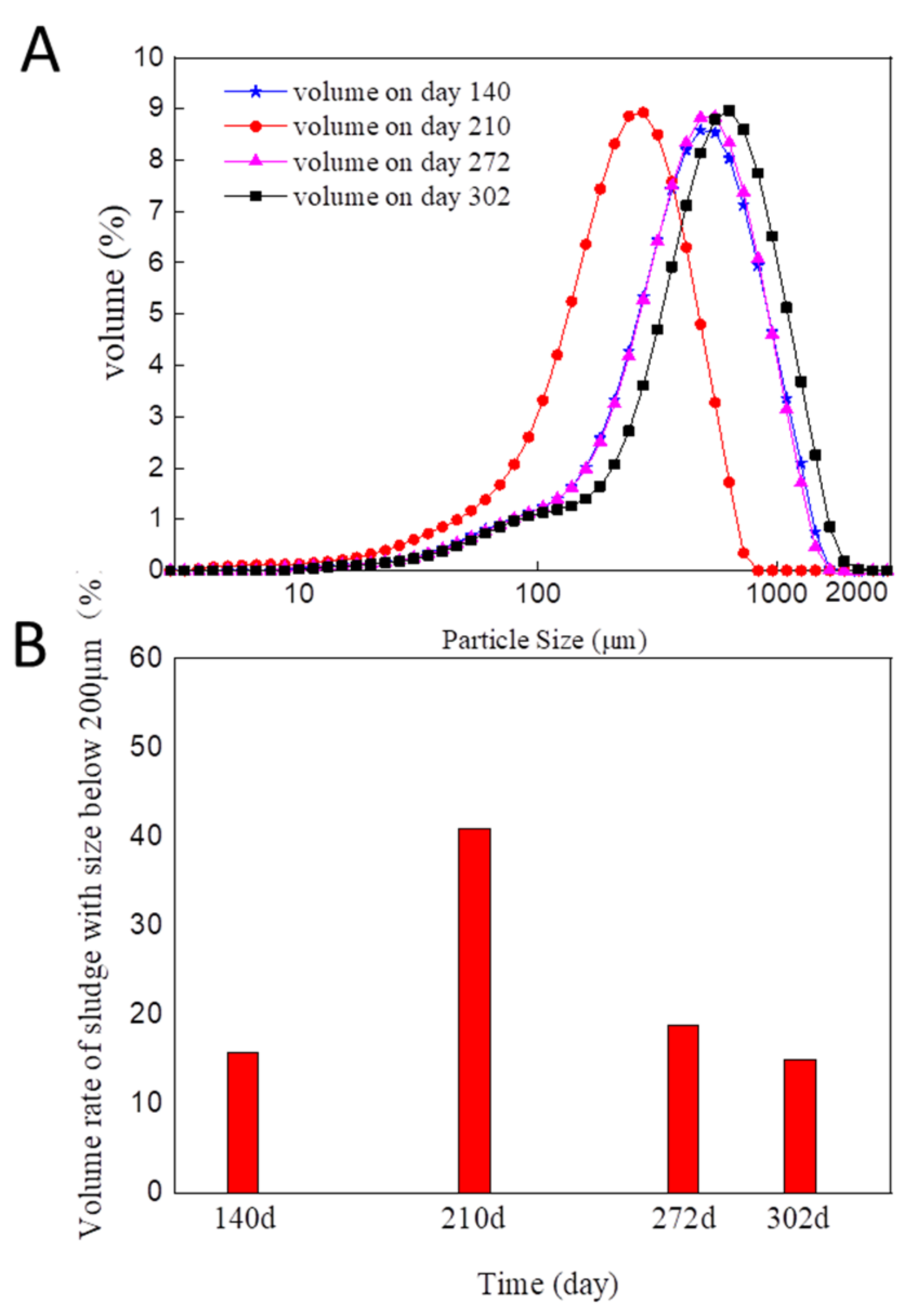
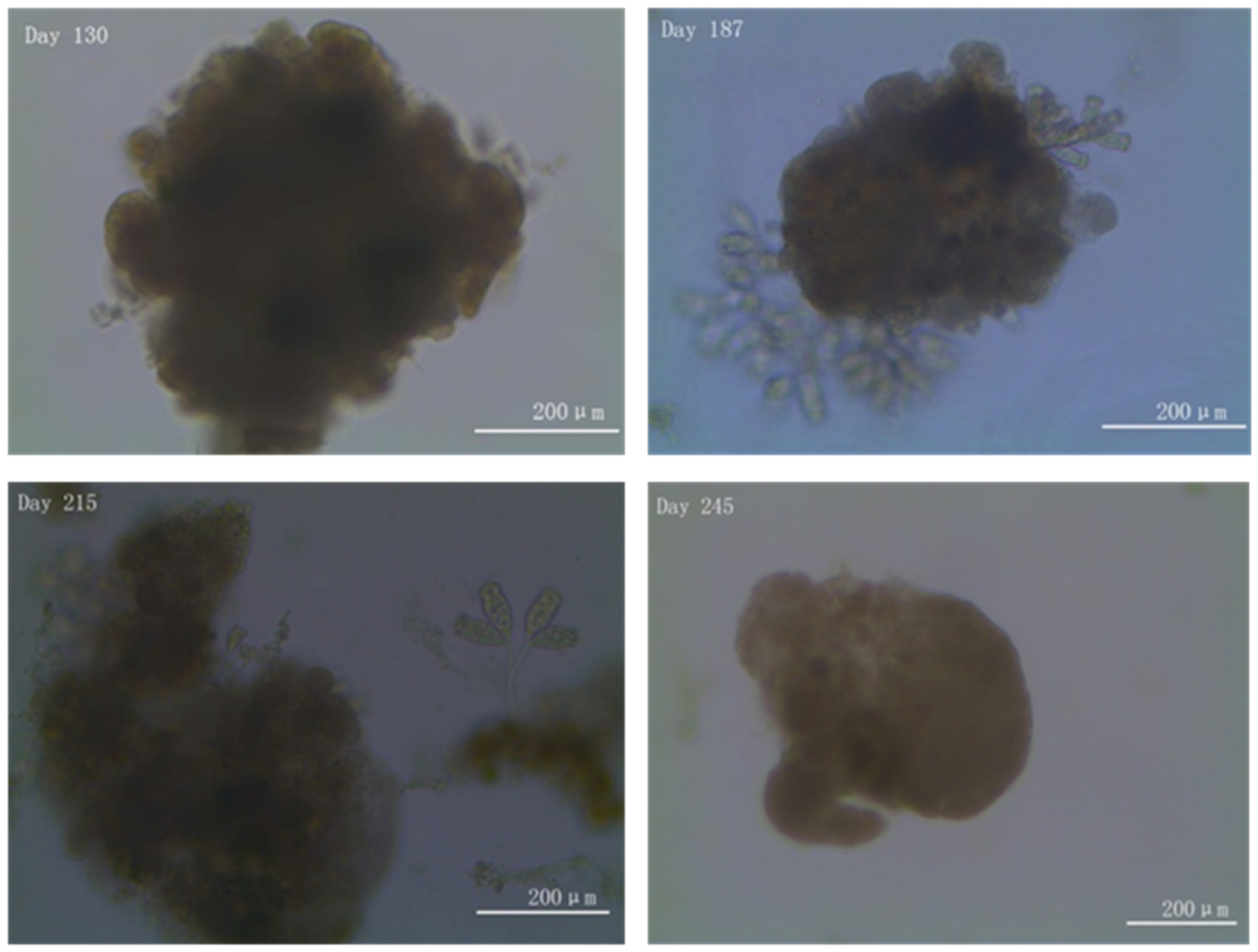
3.1. Granule Formation, Disintegration, and Re-Establishment during the Long-Term Operation Period
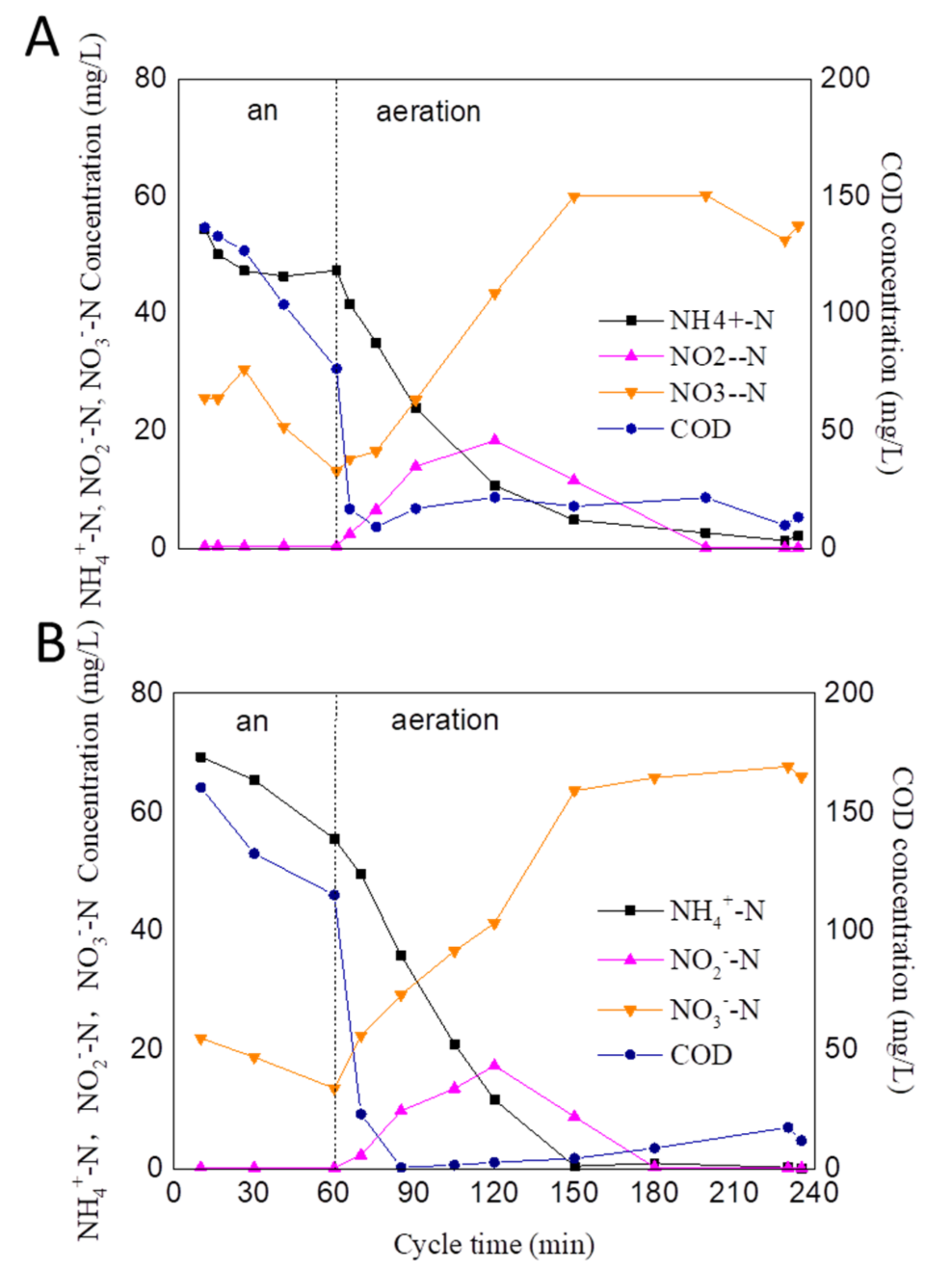
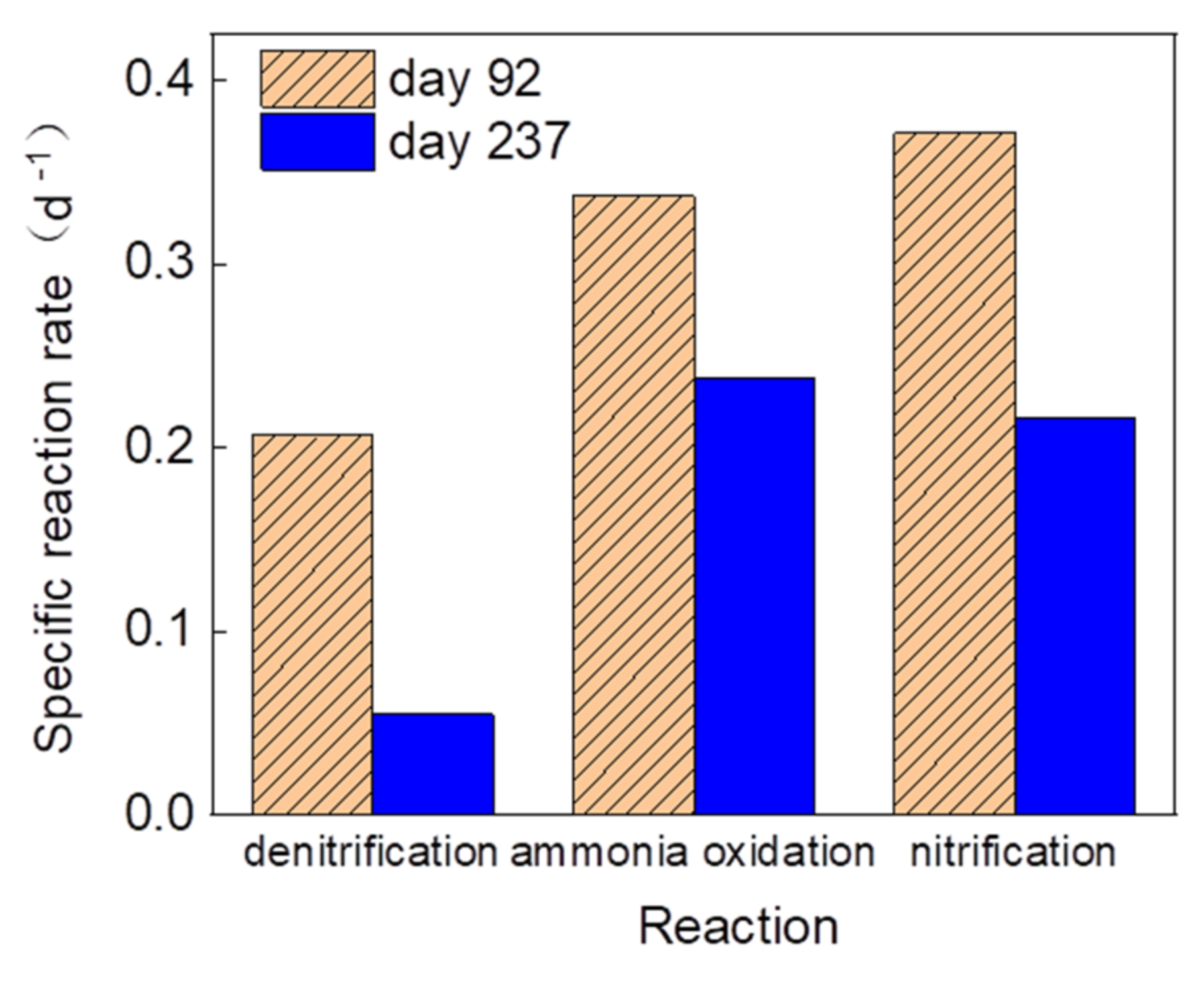
3.2. The Removal Performance of COD and Ammonium-Nitrogen during the Long-Term Operation Period with Varying Sludge Characteristics
3.3. Microbial Community Analysis of Sludge during the Periods with Granule Disintegration and Re-Establishment
3.4. The Disintegration and Re-Establishment of the Aerobic Granules under Identical Operation Conditions
4. Conclusions
- Aerobic granules were easily formed but experienced disintegration and re-granulation without any external intervention. The changes in granule size and biomass concentration during the operation period due to varying sludge-settling ability caused by the selective pressure and growing competition between flocs and granules altered environmental conditions sludge resided and, thus, granule stability.
- Although the sludge experienced a change in form from flocs to granules, granules to flocs, and flocs to granules again, the reactor performance in terms of COD removal and nitrification were almost stable.
- Aerobic granules formed from inoculated flocs (i.e., first granulation) and from flocs after granule disintegration had different dominant microbial populations. Moreover, Thauera, Sphingomonas, and other functional microorganisms were dominant in the re-granulated sludge. This indicates there was a dynamic microbial community structure of sludge in the reactor but with a relatively stable performance regarding wastewater treatment.
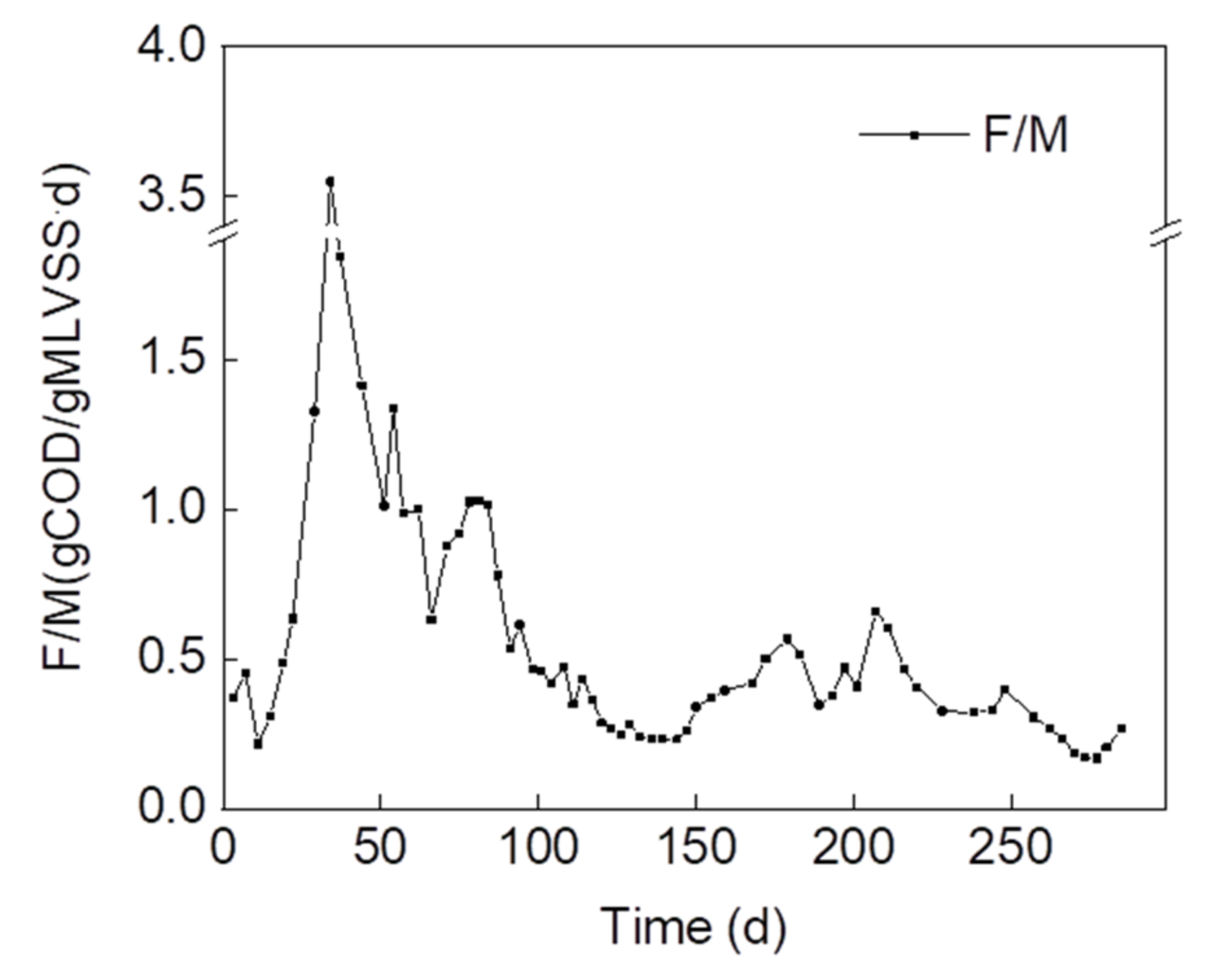
- F/M varied significantly due to the changes in biomass concentration caused by strong selective pressure and the change in sludge-settling ability in the reactor. F/M ratios should be controlled between 0.3 and 1.0 gCOD/gSS·d to maintain the stable structure of granules to minimize the fluctuation of sludge properties under the conditions used in this study.
Author Contributions
Funding
Conflicts of Interest
References
- Arrojo, B.; Mosquera-Corral, A.; Garrido, J.M.; Méndez, R. Aerobic granulation with industrial wastewater in sequencing batch reactors. Water Res. 2004, 38, 3389–3399. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-F.; Tay, J.-H.; Liu, Y. Respirometric Activities of Heterotrophic and Nitrifying Populations in Aerobic Granules Developed at Different Substrate N/COD Ratios. Curr. Microbiol. 2004, 49, 42–46. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Tay, J.-H.; Tay, S.-L. Aggregation of immobilized activated sludge cells into aerobically grown microbial granules for the aerobic biodegradation of phenol. Lett. Appl. Microbiol. 2002, 35, 439–445. [Google Scholar] [CrossRef]
- Beun, J.; Hendriks, A.; van Loosdrecht, M.; Morgenroth, E.; Wilderer, P.; Heijnen, J. Aerobic granulation in a sequencing batch reactor. Water Res. 1999, 33, 2283–2290. [Google Scholar] [CrossRef]
- Deng, S.; Ting, Y.-P. Characterization of PEI-modified biomass and biosorption of Cu(II), Pb(II) and Ni(II). Water Res. 2005, 39, 2167–2177. [Google Scholar] [CrossRef]
- Zhou, Y.; Pijuan, M.; Yuan, Z. Development of a 2-sludge, 3-stage system for nitrogen and phosphorous removal from nutrient-rich wastewater using granular sludge and biofilms. Water Res. 2008, 42, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Tay, J.-H.; Liu, Y. Selection pressure is a driving force of aerobic granulation in sequencing batch reactors. Process Biochem. 2004, 39, 579–584. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Tay, J.-H. Fast formation of aerobic granules by combining strong hydraulic selection pressure with overstressed organic loading rate. Water Res. 2015, 80, 256–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Liu, Q.-S. Causes and control of filamentous growth in aerobic granular sludge sequencing batch reactors. Biotechnol. Adv. 2006, 24, 115–127. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Tay, J.-H. The competition between flocculent sludge and aerobic granules during the long-term operation period of granular sludge sequencing batch reactor. Environ. Technol. 2012, 33, 2619–2626. [Google Scholar] [CrossRef]
- Luo, J.; Hao, T.; Wei, L.; Mackey, H.R.; Lin, Z.; Chen, G.-H. Impact of influent COD/N ratio on disintegration of aerobic granular sludge. Water Res. 2014, 62, 127–135. [Google Scholar] [CrossRef]
- Long, B.; Yang, C.-Z.; Pu, W.-H.; Yang, J.-K.; Liu, F.-B.; Zhang, L.; Zhang, J.; Cheng, K. Tolerance to organic loading rate by aerobic granular sludge in a cyclic aerobic granular reactor. Bioresour. Technol. 2015, 182, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tay, J.-H. State of the art of biogranulation technology for wastewater treatment. Biotechnol. Adv. 2004, 22, 533–563. [Google Scholar] [CrossRef]
- Lee, D.-J.; Chen, Y.-Y.; Show, K.-Y.; Whiteley, C.G.; Tay, J.-H. Advances in aerobic granule formation and granule stability in the course of storage and reactor operation. Biotechnol. Adv. 2010, 28, 919–934. [Google Scholar] [CrossRef] [PubMed]
- Alias, M.A.; Muda, K.; Affam, A.C.; Aris, A.; Hashim, N. The effect of divalent and trivalent cations on aggregation and surface hydrophobicity of selected microorganism. Environ. Eng. Res. 2016, 22, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Claude, V.; Mahy, J.G.; Tilkin, R.G.; Lambert, S.D. Enhancement of the catalytic performances and lifetime of Ni/γ-Al2O3 catalysts for the steam toluene reforming via the combination of dopants: Inspection of Cu, Co, Fe, Mn, and Mo species addition. Mater. Today Chem. 2020, 15, 100229. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, S.-F.; Tay, J.-H. Improved stability of aerobic granules by selecting slow-growing nitrifying bacteria. J. Biotechnol. 2004, 108, 161–169. [Google Scholar] [CrossRef] [PubMed]
- De Kreuk, M.; Heijnen, J.; van Loosdrecht, M. Simultaneous COD, nitrogen, and phosphate removal by aerobic granular sludge. Biotechnol. Bioeng. 2005, 90, 761–769. [Google Scholar] [CrossRef]
- Pronk, M.; de Kreuk, M.; de Bruin, B.; Kamminga, P.; Kleerebezem, R.; van Loosdrecht, M. Full scale performance of the aerobic granular sludge process for sewage treatment. Water Res. 2015, 84, 207–217. [Google Scholar] [CrossRef]
- Yang, S.-F.; Tay, J.-H.; Liu, Y. Effect of Substrate Nitrogen/Chemical Oxygen Demand Ratio on the Formation of Aerobic Granules. J. Environ. Eng. 2005, 131, 86–92. [Google Scholar] [CrossRef]
- De Kreuk, M.; Van Loosdrecht, M. Selection of slow growing organisms as a means for improving aerobic granular sludge stability. Water Sci. Technol. 2004, 49, 9–17. [Google Scholar] [CrossRef]
- Yang, S.-F.; Tay, J.-H.; Liu, Y. Inhibition of free ammonia to the formation of aerobic granules. Biochem. Eng. J. 2004, 17, 41–48. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Liu, Y.-Q.; Tay, J.-H.; Ning, P. Rapid formation of nitrifying granules treating high-strength ammonium wastewater in a sequencing batch reactor. Appl. Microbiol. Biotechnol. 2015, 99, 4445–4452. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Qiao, Z.; Zhang, Y.; Hao, L.; Si, W.; Du, B.; Wei, Q. Effect of COD/N ratio on cultivation of aerobic granular sludge in a pilot-scale sequencing batch reactor. Appl. Microbiol. Biotechnol. 2012, 97, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Peng, C.; Peng, Y.; Li, L.; Wang, S.; Ma, Y. Effect of wastewater COD/N ratio on aerobic nitrifying sludge granulation and microbial population shift. J. Environ. Sci. 2012, 24, 234–241. [Google Scholar] [CrossRef]
- Kocaturk, I.; Erguder, T.H. Influent COD/TAN ratio affects the carbon and nitrogen removal efficiency and stability of aerobic granules. Ecol. Eng. 2016, 90, 12–24. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Moy, B.; Kong, Y.-H.; Tay, J.-H. Formation, physical characteristics and microbial community structure of aerobic granules in a pilot-scale sequencing batch reactor for real wastewater treatment. Enzym. Microb. Technol. 2010, 46, 520–525. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Bernat, K.; Zielinska, M.; Wojnowska-Baryła, I. Cycle length and COD/N ratio determine properties of aerobic granules treating high-nitrogen wastewater. Bioprocess Biosyst. Eng. 2013, 37, 1305–1313. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Deng, S.; Wang, S.; Su, H. Analysis of aerobic granules under the toxic effect of ampicillin in sequencing batch reactors: Performance and microbial community. J. Environ. Manag. 2017, 204, 152–159. [Google Scholar] [CrossRef]
- Ma, J.; Quan, X.; Li, H. Application of high OLR-fed aerobic granules for the treatment of low-strength wastewater: Performance, granule morphology and microbial community. J. Environ. Sci. 2013, 25, 1549–1556. [Google Scholar] [CrossRef]
- Yun, L.; Yu, Z.; Li, Y.; Luo, P.; Jiang, X.; Tian, Y.; Ding, X. Ammonia nitrogen and nitrite removal by a heterotrophic Sphingomonas sp. strain LPN080 and its potential application in aquaculture. Aquaculture 2019, 500, 477–484. [Google Scholar] [CrossRef]
- Vijayalayan, P.; Thanh, B.X.; Visvanathan, C.; Bui, X.-T. Simultaneous nitrification denitrification in a Batch Granulation Membrane Airlift Bioreactor. Int. Biodeterior. Biodegrad. 2014, 95, 139–143. [Google Scholar] [CrossRef]
- Bankar, A.; Nagaraja, G. Chapter 18—Recent Trends in Biosorption of Heavy Metals by Actinobacteria. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, B.P., Gupta, V.K., Passari, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 257–275. [Google Scholar]
- Liu, Y.; Tay, J.-H. Relationship between size and mass transfer resistance in aerobic granules. Lett. Appl. Microbiol. 2005, 40, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.-H.; Ivanov, V.; Pan, S.; Tay, S.T.-L. Specific layers in aerobically grown microbial granules. Lett. Appl. Microbiol. 2002, 34, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Madoni, P. Protozoa in wastewater treatment processes: A minireview. Ital. J. Zool. 2011, 78, 3–11. [Google Scholar] [CrossRef]
- Peyong, Y.N.; Zhou, Y.; Abdullah, A.Z.; Vadivelu, V. The effect of organic loading rates and nitrogenous compounds on the aerobic granules developed using low strength wastewater. Biochem. Eng. J. 2012, 67, 52–59. [Google Scholar] [CrossRef]
- Corsino, S.F.; di Biase, A.; Devlin, T.R.; Munz, G.; Torregrossa, M.; Oleszkiewicz, J.A. Effect of extended famine conditions on aerobic granular sludge stability in the treatment of brewery wastewater. Bioresour. Technol. 2017, 226, 150–157. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Zhang, Z.; Yu, Z.; Zhu, L. Optimization of F/M ratio for stability of aerobic granular process via quantitative sludge discharge. Bioresour. Technol. 2018, 252, 150–156. [Google Scholar] [CrossRef]
- Franca, R.D.; Pinheiro, H.M.; van Loosdrecht, M.C.; Lourenço, N.D. Stability of aerobic granules during long-term bioreactor operation. Biotechnol. Adv. 2018, 36, 228–246. [Google Scholar] [CrossRef]

| Day 85 | Day 227 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bd | Closest Relatives in GenBank (Accession no.) | Similarity (%) | Classification | Bd | Closest Relatives in GenBank (Accession no.) | Similarity (%) | Classification | ||||
| Genus | Family | Phylum | Genus | Family | Phylum | ||||||
| 1 | Hydrogenophaga sp. KMM 6726 | 100 | Hydrogenophaga | Comamonadaceae | Proteobacteria | 1 | Acinetobacter sp. XJ127 | 100 | Acinetobacter | Moraxellaceae | Proteobacteria |
| 2 | Thauera sp. G3DM-88 | 100 | Thauera | Rhodocyclaceae | Proteobacteria | 2 | Nitrosomonas Ms1 | 100 | Nitrosomonas | Nitrosomonadaceae | Proteobacteria |
| 3 | Thauera sp. CJSOPY1 (T-IV) | 100 | Thauera | Rhodocyclaceae | Proteobacteria | 3 | Uncultured bacterium | 95.1 | Bdellovibrio | Bdellovibrionaceae | Proteobacteria |
| 4 | bacterium G14(AY345397) | 92 | Acidovorax | Comamonadaceae | Proteobacteria | 4 | Novosphingobium tardaugens (T) | 100 | Novosphingobium | Sphingomonadaceae | Proteobacteria |
| 5 | Thauera sp. CJSOPY1 (T-IV) | 96.3 | Thauera | Rhodocyclaceae | Proteobacteria | 5 | Uncultured bacterium | 100 | Unclassified_“Saprospiraceae” | Saprospiraceae | Bacteroidetes |
| 6 | Uncultured Leadbetterella sp. | 100 | Leadbetterella | Cytophagaceae | Bacteroidetes | 6 | Sphingopyxis baekryungensis | 84.0 | Sphingopyxis | Sphingomonadaceae | Bacteroidetes |
| 7 | Thauera sp. R-26885 | 100 | Thauera | Rhodocyclaceae | Proteobacteria | 7 | Thauera sp. CJSOPY1 (T-IV) | 100 | Thauera | Rhodocyclaceae | Proteobacteria |
| 8 | Uncultured Thauera sp. | 100 | Thauera | Rhodocyclaceae | Proteobacteria | 8 | Uncultured bacterium | 100 | Unclassified_Cytophagales | Cytophagaceae | Bacteroidetes |
| 9 | Thauera sp. CJSOPY1 (T-IV) | 100 | Thauera | Rhodocyclaceae | Proteobacteria | 9 | Thauera aromatica | 100 | Thauera | Rhodocyclaceae | Proteobacteria |
| 10 | Uncultured bacteria | 100 | Thauera | Rhodocyclaceae | Proteobacteria | 10 | Thauera aromatica | 96.3 | Thauera | Rhodocyclaceae | Proteobacteria |
| 11 | Hyphomicrobium sp. PMC | 92 | Hyphomicrobium | Hyphomicrobiaceae | Proteobacteria | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, L.; Liu, Y.-Q.; Duan, W.; Sternberg, C.E.W.; Yuan, Q.; Chen, F. Fluctuation and Re-Establishment of Aerobic Granules Properties during the Long-Term Operation Period with Low-Strength and Low C/N Ratio Wastewater. Processes 2021, 9, 1290. https://doi.org/10.3390/pr9081290
Cha L, Liu Y-Q, Duan W, Sternberg CEW, Yuan Q, Chen F. Fluctuation and Re-Establishment of Aerobic Granules Properties during the Long-Term Operation Period with Low-Strength and Low C/N Ratio Wastewater. Processes. 2021; 9(8):1290. https://doi.org/10.3390/pr9081290
Chicago/Turabian StyleCha, Lijuan, Yong-Qiang Liu, Wenyan Duan, Christain E. W. Sternberg, Qiangjun Yuan, and Fangyuan Chen. 2021. "Fluctuation and Re-Establishment of Aerobic Granules Properties during the Long-Term Operation Period with Low-Strength and Low C/N Ratio Wastewater" Processes 9, no. 8: 1290. https://doi.org/10.3390/pr9081290
APA StyleCha, L., Liu, Y.-Q., Duan, W., Sternberg, C. E. W., Yuan, Q., & Chen, F. (2021). Fluctuation and Re-Establishment of Aerobic Granules Properties during the Long-Term Operation Period with Low-Strength and Low C/N Ratio Wastewater. Processes, 9(8), 1290. https://doi.org/10.3390/pr9081290







