Optimised Fractionation of Brewer’s Spent Grain for a Biorefinery Producing Sugars, Oligosaccharides, and Bioethanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Lignocellulosic Raw Material
2.2. Compositional Analysis
2.3. First Acidic Hydrolysis
2.4. Second Acidic Hydrolysis
2.5. Enzymatic Hydrolysis
2.6. Inoculum Preparation
2.7. Ethanol Fermentation
2.8. Analytical Methods
2.8.1. Determination of Sugars, Ethanol and Inhibitor Compounds
2.8.2. Determination of Total Sugar Content
2.8.3. Determination of Cell Concentration
2.9. Calculation of Yields of Sugars, GOS and AXOS, A/X Ratio and Ethanol Yield
2.10. Statistical Evaluation
3. Results and Discussion
3.1. Optimisation of the First Acidic Hydrolysis of BSG to Produce an Arabinose-Rich Hydrolysate
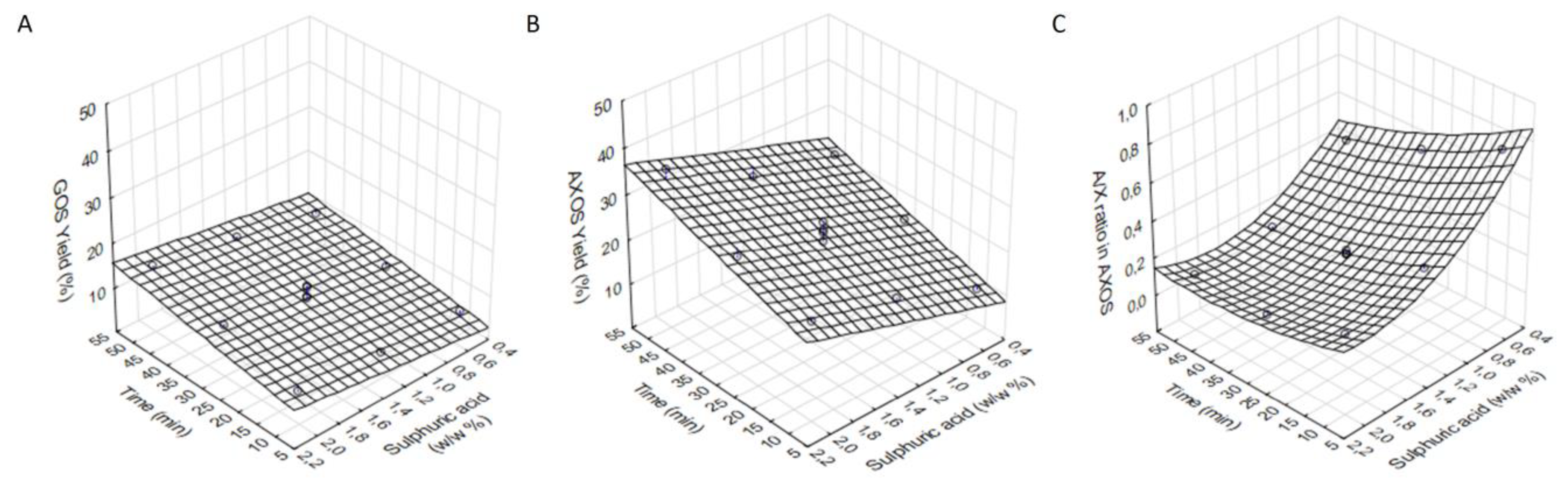
3.2. Solubilisation of the Hemicellulose Fraction from the Solid Residue of the First Acidic Hydrolysis of BSG
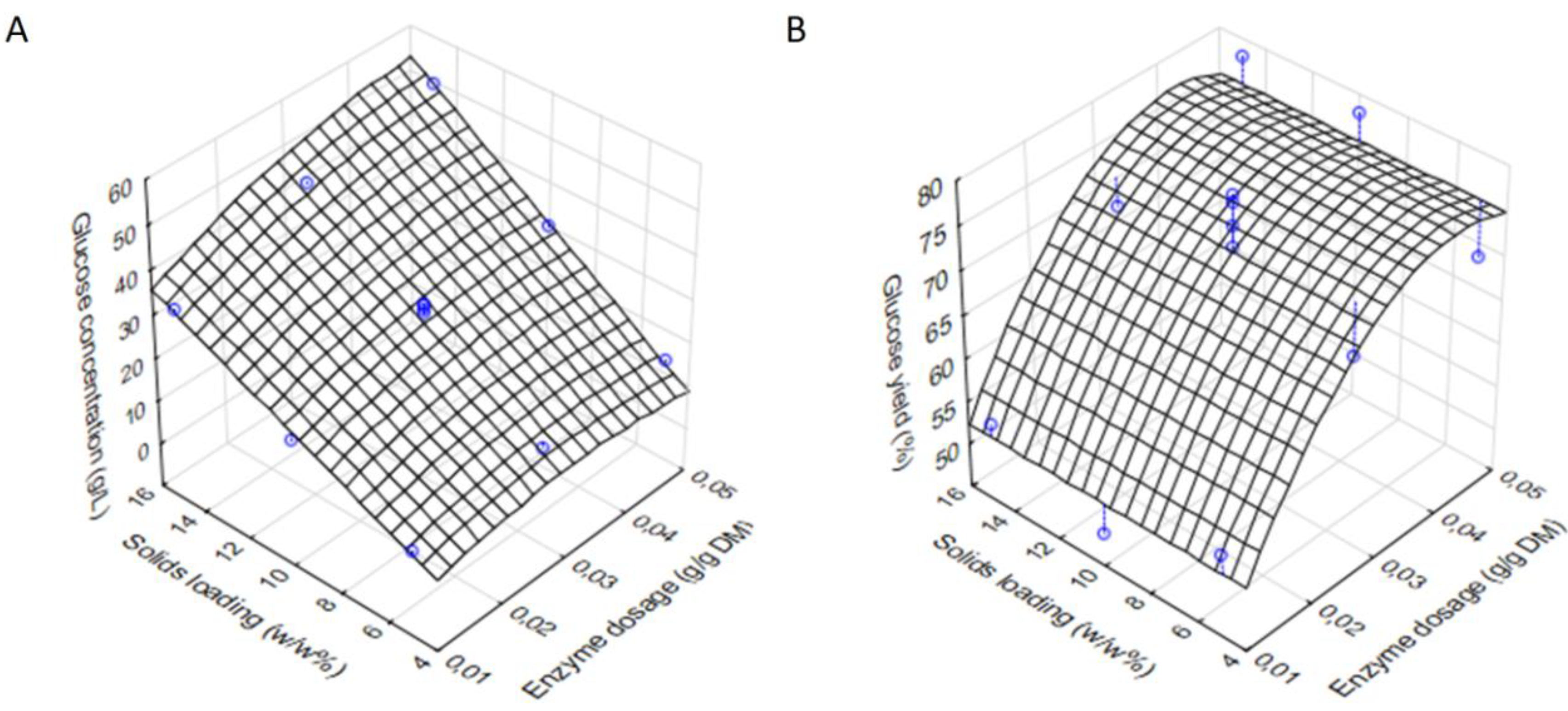
3.3. Optimisation of the Enzymatic Hydrolysis of the Solid Resiude Obtained in the Second Acidic Hydrolysis of BSG
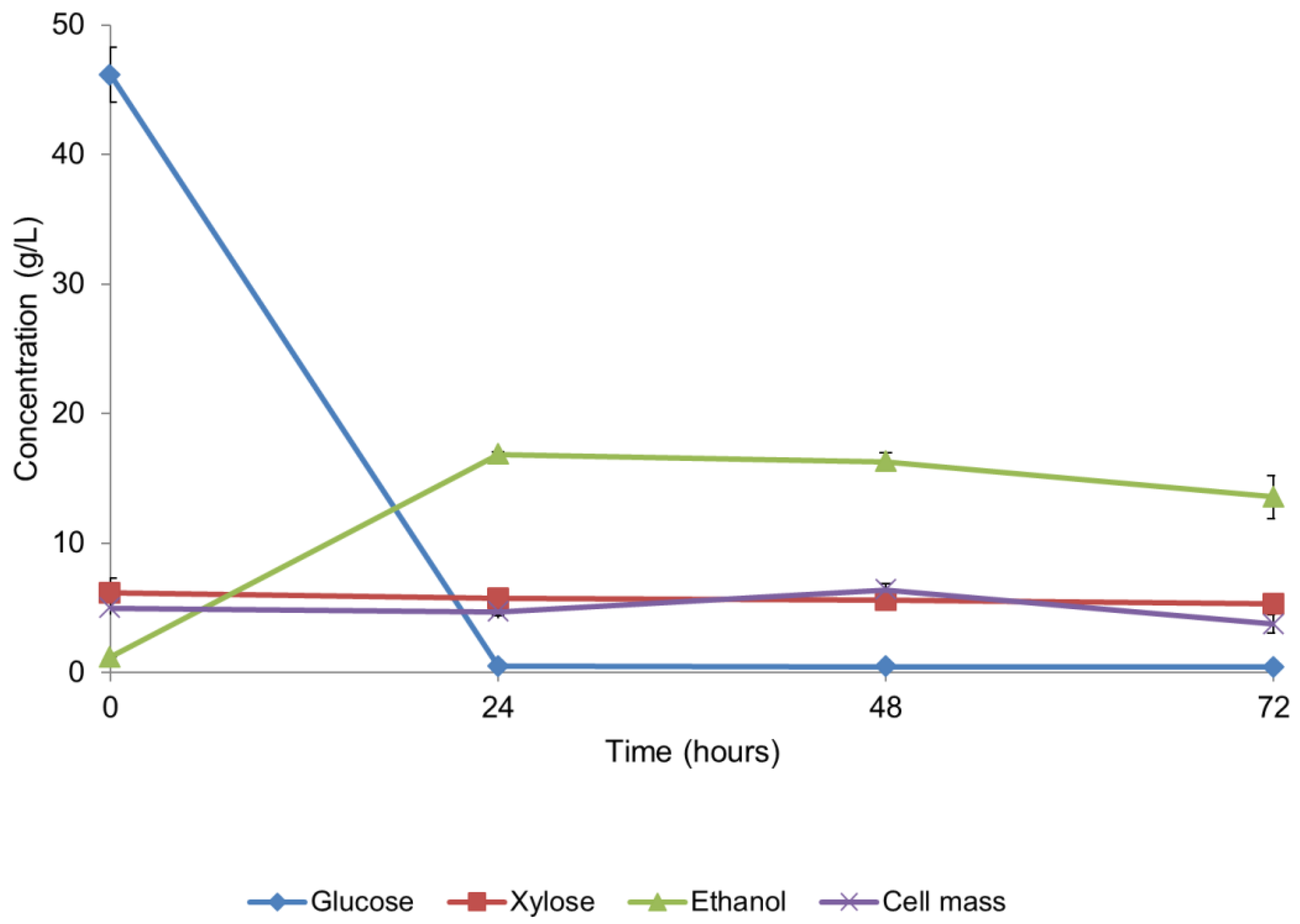
3.4. Bioethanol Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Biotechnological potential of brewers spent grain and its recent applications. Waste Biomass Valoriz. 2012, 3, 213–232. [Google Scholar] [CrossRef]
- Stroem, L.K.; Desai, D.K.; Hoadley, A.F.A. Superheated steam drying of Brewer’s spent grain in a rotary drum. Adv. Powder Technol. 2009, 20, 240–244. [Google Scholar] [CrossRef]
- Westendorf, M.L.; Wohlt, J.E. Brewing by-products: Their use as animal feeds. Vet. Clin. N. Am. Food Anim. Pract. 2002, 18, 233–252. [Google Scholar] [CrossRef]
- Weger, A.; Binder, S.; Franke, M.; Hornung, A.; Ru, W.; Mayer, W. Solid biofuel production by mechanical pre-treatment of brewers’ spent grain. Chem. Eng. Trans. 2014, 37, 661–666. [Google Scholar]
- Azevedo Borges, C.; Géczi, G.; Kovács, K.; Horváth, M.; Bácskai, I.; Korzenszky, P. Examination of energy recovery of brewers’ spent grain I.—Chemical process. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 116–119. [Google Scholar] [CrossRef][Green Version]
- Bochmann, G.; Drosg, B.; Fuchs, W. Anaerobic digestion of thermal pretreated brewers’ spent grains. Environ. Prog. Sustain. Energy 2015, 34, 1092–1096. [Google Scholar] [CrossRef]
- Waters, D.M.; Jacob, F.; Titze, J.; Arendt, E.K.; Zannini, E. Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Eur. Food Res. Technol. 2012, 235, 767–778. [Google Scholar] [CrossRef]
- D’Appolonia, B.L.; Prentice, N. High-Fiber Bread Containing Brewer’s Spent Grain. Cereal Chem. 1977, 54, 1084–1095. [Google Scholar]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Shen, Y.; Abeynayake, R.; Sun, X.; Ran, T.; Li, J.; Chen, L.; Yang, W. Feed nutritional value of brewers’ spent grain residue resulting from protease aided protein removal. J. Anim. Sci. Biotechnol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ spent grain; Bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: A review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef]
- Leite, P.; Silva, C.; Salgado, J.M.; Belo, I. Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Ind. Crops Prod. 2019, 137, 315–322. [Google Scholar] [CrossRef]
- Tan, Y.X.; Mok, W.K.; Lee, J.; Kim, J.; Chen, W.N. Solid state fermentation of Brewers’ spent grains for improved nutritional profile using Bacillus subtilis WX-17. Fermentation 2019, 5, 52. [Google Scholar] [CrossRef]
- Terrasan, C.R.F.; Carmona, E.C. Solid-state fermentation of brewer’s spent grain for xylanolytic enzymes production by Penicillium janczewskii and analyses of the fermented substrate. Biosci. J. 2015, 31, 1826–1836. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Chemical characterization and liberation of pentose sugars from brewer’s spent grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Paz, A.; Outeiriño, D.; Pérez Guerra, N.; Domínguez, J.M. Enzymatic hydrolysis of brewer’s spent grain to obtain fermentable sugars. Bioresour. Technol. 2019, 275, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.; Silvério, S.C.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Prather, K.L.J.; Rodrigues, L.R. Single-step production of arabino-xylooligosaccharides by recombinant Bacillus subtilis 3610 cultivated in brewers’ spent grain. Carbohydr. Polym. 2018, 199, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.; Silvério, S.C.; Rodrigues, L.R. One-step process for producing prebiotic arabino-xylooligosaccharides from brewer’s spent grain employing Trichoderma species. Food Chem. 2019, 270, 86–94. [Google Scholar] [CrossRef]
- Liguori, R.; Soccol, C.R.; de Souza Vandenberghe, L.P.; Woiciechowski, A.L.; Faraco, V. Second generation ethanol production from brewers’ spent grain. Energies 2015, 8, 2575–2586. [Google Scholar] [CrossRef]
- Brethauer, S.; Wyman, C.E. Review: Continuous hydrolysis and fermentation for cellulosic ethanol production. Bioresour. Technol. 2010, 101, 4862–4874. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Cardona, C.A. A biorefinery approach for the production of xylitol, ethanol and polyhydroxybutyrate from brewer’s spent grain. AIMS Agric. Food 2016, 1, 52–66. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Sajib, M.; Falck, P.; Sardari, R.R.R.; Mathew, S.; Grey, C.; Karlsson, E.N.; Adlercreutz, P. Valorization of Brewer’s spent grain to prebiotic oligosaccharide: Production, xylanase catalyzed hydrolysis, in-vitro evaluation with probiotic strains and in a batch human fecal fermentation model. J. Biotechnol. 2018, 268, 61–70. [Google Scholar] [CrossRef] [PubMed]
- González-García, S.; Morales, P.C.; Gullón, B. Estimating the environmental impacts of a brewery waste–based biorefinery: Bio-ethanol and xylooligosaccharides joint production case study. Ind. Crops Prod. 2018, 123, 331–340. [Google Scholar] [CrossRef]
- Lorente, A.; Remón, J.; Budarin, V.L.; Sánchez-Verdú, P.; Moreno, A.; Clark, J.H. Analysis and optimisation of a novel “bio-brewery” approach: Production of bio-fuels and bio-chemicals by microwave-assisted, hydrothermal liquefaction of brewers’ spent grains. Energy Convers. Manag. 2019, 185, 410–430. [Google Scholar] [CrossRef]
- Dehnavi, G.Z.; Laucerica, J.L.; Rodriguez, D.; Beatón, M.; Taherzadeh, M.J.; Martín, C. Fractionation of the main components of barley spent grains from a microbrewery. Cellul. Chem. Technol. 2011, 45, 339–345. [Google Scholar]
- Vieira, E.; Rocha, M.A.M.; Coelho, E.; Pinho, O.; Saraiva, J.A.; Ferreira, I.M.P.L.V.O.; Coimbra, M.A. Valuation of brewer’s spent grain using a fully recyclable integrated process for extraction of proteins and arabinoxylans. Ind. Crops Prod. 2014, 52, 136–143. [Google Scholar] [CrossRef]
- Ravindran, R.; Sarangapani, C.; Jaiswal, S.; Lu, P.; Cullen, P.J.; Bourke, P.; Jaiswal, A.K. Improving enzymatic hydrolysis of brewer spent grain with nonthermal plasma. Bioresour. Technol. 2019, 282, 520–524. [Google Scholar] [CrossRef]
- Tang, D.S.; Tian, Y.J.; He, Y.Z.; Li, L.; Hu, S.Q.; Li, B. Optimisation of ultrasonic-assisted protein extraction from brewer’s spent grain. Czech. J. Food Sci. 2010, 28, 9–17. [Google Scholar] [CrossRef]
- Rommi, K.; Niemi, P.; Kemppainen, K.; Kruus, K. Impact of thermochemical pre-treatment and carbohydrate and protein hydrolyzing enzyme treatment on fractionation of protein and lignin from brewer’s spent grain. J. Cereal Sci. 2018, 79, 168–173. [Google Scholar] [CrossRef]
- Treimo, J.; Westereng, B.; Horn, S.J.; Forssell, P.; Robertson, J.A.; Faulds, C.B.; Waldron, K.W.; Buchert, J.; Eijsink, V.G.H. Enzymatic solubilization of brewers’ spent grain by combined action of carbohydrases and peptidases. J. Agric. Food Chem. 2009, 57, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Giacobbe, S.; Piscitelli, A.; Raganati, F.; Lettera, V.; Sannia, G.; Marzocchella, A.; Pezzella, C. Butanol production from laccase-pretreated brewer’s spent grain. Biotechnol. Biofuels 2019, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Kuhn, D.D.; Ogejo, J.A.; O’Keefe, S.F.; Fraguas, C.F.; Wiersema, B.D.; Jin, Q.; Yu, D.; Huang, H. Wet fractionation process to produce high protein and high fiber products from brewer’s spent grain. Food Bioprod. Process. 2019, 117, 266–274. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of brewers’ spent grain for the production of lipids by oleaginous yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Moncada, J.; Roberto, I.C.; Cardona, C.A. Techno-economic analysis for brewer’s spent grains use on a biorefinery concept: The Brazilian case. Bioresour. Technol. 2013, 148, 302–310. [Google Scholar] [CrossRef]
- Bušić, A.; Mardetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Šantek, M.I.; Komes, D.; Novak, S.; Šantek, B. Bioethanol production from renewable raw materials and its separation and purification: A review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef] [PubMed]
- Solarte-Toro, J.C.; Romero-García, J.M.; Susmozas, A.; Ruiz, E.; Castro, E.; Cardona-Alzate, C.A. Techno-economic feasibility of bioethanol production via biorefinery of olive tree prunings (OTP): Optimization of the pretreatment stage. Holzforschung 2019, 73, 3–13. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Arantes, V. A review on commercial-scale high-value products that can be produced alongside cellulosic ethanol. Biotechnol Biofuels 2019, 12, 240. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Structural Carbohydrates and Lignin in Biomass; Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012; pp. 1–15. [Google Scholar]
- Fehér, C.; Gazsó, Z.; Tatijarern, P.; Molnár, M.; Barta, Z.; Réczey, K. Investigation of selective arabinose release from corn fibre by acid hydrolysis under mild conditions. J. Chem. Technol. Biotechnol. 2015, 90, 896–906. [Google Scholar] [CrossRef]
- Bedő, S.; Antal, B.; Rozbach, M.; Fehér, A.; Fehér, C. Optimised fractionation of wheat bran for arabinose biopurification and xylitol fermentation by Ogataea zsoltii within a biorefinery process. Ind. Crops Prod. 2019, 139, 111504. [Google Scholar] [CrossRef]
- Hames, B.; Scarlata, C.; Sluiter, A. Determination of Protein Content in Biomass; Technical Report NREL/TP-510-42625; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–5. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; Technical Report NREL/TP-510-42622; National Renewable Energy Laboratory: Golden, CO, USA, 2008; pp. 1–5. [Google Scholar]
- Ontañon, O.M.; Ghio, S.; Marrero Díaz de Villegas, R.; Garrido, M.M.; Talia, P.M.; Fehér, C.; Campos, E. A thermostable GH8 endoglucanase of Enterobacter sp. R1 is suitable for β-glucan deconstruction. Food Chem. 2019, 298, 124999. [Google Scholar] [CrossRef]
- Guo, X.; Cavka, A.; Jönsson, L.J.; Hong, F. Comparison of methods for detoxification of spruce hydrolysate for bacterial cellulose production. Microb. Cell Fact. 2013, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, I.K.; Kargi, F.; Oztekin, R. Effects of operating parameters on acid hydrolysis of ground wheat starch: Maximization of the sugar yield by statistical experiment design. Starch Staerke 2011, 63, 311–318. [Google Scholar] [CrossRef]
- González, G.; López-Santín, J.; Caminal, G.; Solà, C. Dilute acid hydrolysis of wheat straw hemicellulose at moderate temperature: A simplified kinetic model. Biotechnol. Bioeng. 1986, 28, 288–293. [Google Scholar] [CrossRef]
- Fehér, C. Novel approaches for biotechnological production and application of L-arabinose. J. Carbohydr. Chem. 2018, 37, 251–284. [Google Scholar] [CrossRef]
- Xu, J.; Thomsen, M.H.; Thomsen, A.B. Recovery of arabinan in acetic acid-catalyzed hydrothermal pretreatment on corn stover. Biomass Bioenergy 2009, 33, 1660–1663. [Google Scholar] [CrossRef]
- Shibanuma, K.; Nordisk, N. Partial Acid Hydrolysis of Corn Fiber for the Production of L-Arabinose. J. Appl. Glycosci. 1999, 46, 249–256. [Google Scholar] [CrossRef]
- Tian, Z.; Chen, J.; Ji, X.; Wang, Q.; Yang, G.; Fatehi, P. Dilute sulfuric acid hydrolysis of Pennisetum (sp.) Hemicellulose. BioResources 2017, 12, 2609–2617. [Google Scholar] [CrossRef]
- Martin, C.; Alriksson, B.; Sjöde, A.; Nilvebrant, N.-O.; Jönsson, L.J. Dilute Sulfuric Acid Pretreatment of Agricultural and Agro-Industrial Residues for Ethanol Production. Appl. Biochem. Biotecnol. 2007, 136, 339–352. [Google Scholar]
- Lee, H.-J.; Lee, W.-K.; Ryu, Y.-W. L-Arabinose Production from Diluted Sulfuric Acid Hydrolysis of Corn-fiber. KSBB J. 2007, 22, 201–206. [Google Scholar]
- Mäki-Arvela, P.; Salmi, T.; Holmbom, B.; Willför, S.; Murzin, D.Y. Synthesis of sugars by hydrolysis of hemicelluloses—A review. Chem. Rev. 2011, 111, 5638–5666. [Google Scholar] [CrossRef]
- Qing, Q.; Li, H.; Kumar, R.; Wyman, C. Xylooligosaccharides Production, Quantification, and Characterization in Context of Lignocellulosic Biomass Pretreatment. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; John Wiley & Sons Ltd.: Chichester, UK, 2013; pp. 391–415. [Google Scholar]
- Wijaya, H.; Sasaki, K.; Kahar, P.; Rahmani, N.; Hermiati, E.; Yopi, Y.; Ogino, C.; Prasetya, B.; Kondo, A. High enzymatic recovery and purification of xylooligosaccharides from empty fruit bunch via nanofiltration. Processes 2020, 8, 619. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. 8—Arabinoxylans from cereal by-products: Insights into structural features, recovery, and applications. In Sustainable Recovery and Reutilization of Cereal Processing By-Products; Galanakis, C.M., Ed.; Elsevier Ltd.: London, UK, 2018; pp. 227–251. [Google Scholar]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylan-oligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Enhanced ethanol production from brewer’s spent grain by a Fusarium oxysporum consolidated system. Biotechnol. Biofuels 2009, 2, 4. [Google Scholar] [CrossRef]
- Fehér, C.; Gazsó, Z.; Gál, B.; Kontra, A.; Barta, Z.; Réczey, K. Integrated Process of Arabinose Biopurification and Xylitol Fermentation Based on the Diverse Action of Candida boidinii. Chem. Biochem. Eng. Q. 2015, 29, 587–597. [Google Scholar] [CrossRef]
- Rafiqul, I.S.M.; Sakinah, A.M.M. Processes for the Production of Xylitol-A Review. Food Rev. Int. 2013, 29, 127–156. [Google Scholar] [CrossRef]
- Ioelovich, M.; Morag, E. Study of enzymatic hydrolysis of pretreated biomass at increased solids loading. BioResources 2012, 7, 4672–4682. [Google Scholar] [CrossRef]
- Caspeta, L.; Caro-Bermúdez, M.A.; Ponce-Noyola, T.; Martinez, A. Enzymatic hydrolysis at high-solids loadings for the conversion of agave bagasse to fuel ethanol. Appl. Energy 2014, 113, 277–286. [Google Scholar] [CrossRef]
- Cheng, M.H.; Kadhum, H.J.; Murthy, G.S.; Dien, B.S.; Singh, V. High solids loading biorefinery for the production of cellulosic sugars from bioenergy sorghum. Bioresour. Technol. 2020, 318, 124051. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, H.; Vibe-Pedersen, J.; Larsen, J.; Felby, C. Liquefaction of lignocellulose at high-solids concentrations. Biotechnol. Bioeng. 2007, 96, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Ramachandriya, K.D.; Wilkins, M.; Atiyeh, H.K.; Dunford, N.T.; Hiziroglu, S. Effect of high dry solids loading on enzymatic hydrolysis of acid bisulfite pretreated Eastern redcedar. Bioresour. Technol. 2013, 147, 168–176. [Google Scholar] [CrossRef] [PubMed]
- López-Linares, J.C.; Romero, I.; Cara, C.; Ruiz, E.; Moya, M.; Castro, E. Bioethanol production from rapeseed straw at high solids loading with different process configurations. Fuel 2014, 122, 112–118. [Google Scholar] [CrossRef]
- Manzanares, P.; Negro, M.J.; Oliva, J.M.; Saéz, F.; Ballesteros, I.; Ballesteros, M.; Cara, C.; Castro, E.; Ruiz, E. Different process configurations for bioethanol production from pretreated olive pruning biomass. J. Chem. Technol. Biotechnol. 2011, 86, 881–887. [Google Scholar] [CrossRef]
- Wilkinson, S.; Smart, K.A.; James, S.; Cook, D.J. Maximising high solid loading enzymatic saccharification yield from acid-catalysed hydrothermally-pretreated brewers spent grain. Biofuel Res. J. 2016, 3, 417–429. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Romero, I.; Ruiz, E.; Cara, C.; Castro, E. Comparison of fermentation strategies for ethanol production from pretreated brewers spent grain. Chem. Eng. Trans. 2017, 61, 637–642. [Google Scholar]
- Runquist, D.; Hahn-Hägerdal, B.; Rådström, P. Comparison of heterologous xylose transporters in recombinant Saccharomyces cerevisiae. Biotechnol. Biofuels 2010, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tesfaw, A.; Assefa, F. Current Trends in Bioethanol Production by Saccharomyces cerevisiae: Substrate, Inhibitor Reduction, Growth Variables, Coculture, and Immobilization. Int. Sch. Res. Not. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Smart, K.A.; Cook, D.J. Optimisation of alkaline reagent based chemical pre-treatment of Brewers spent grains for bioethanol production. Ind. Crops Prod. 2014, 62, 219–227. [Google Scholar] [CrossRef]
- Rojas-Chamorro, J.A.; Romero-García, J.M.; Cara, C.; Romero, I.; Castro, E. Improved ethanol production from the slurry of pretreated brewers’ spent grain through different co-fermentation strategies. Bioresour. Technol. 2019, 296, 122367. [Google Scholar] [CrossRef] [PubMed]
- White, J.S.; Yohannan, B.K.; Walker, G.M. Bioconversion of brewer’s spent grains to bioethanol. FEMS Yeast Res. 2008, 8, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
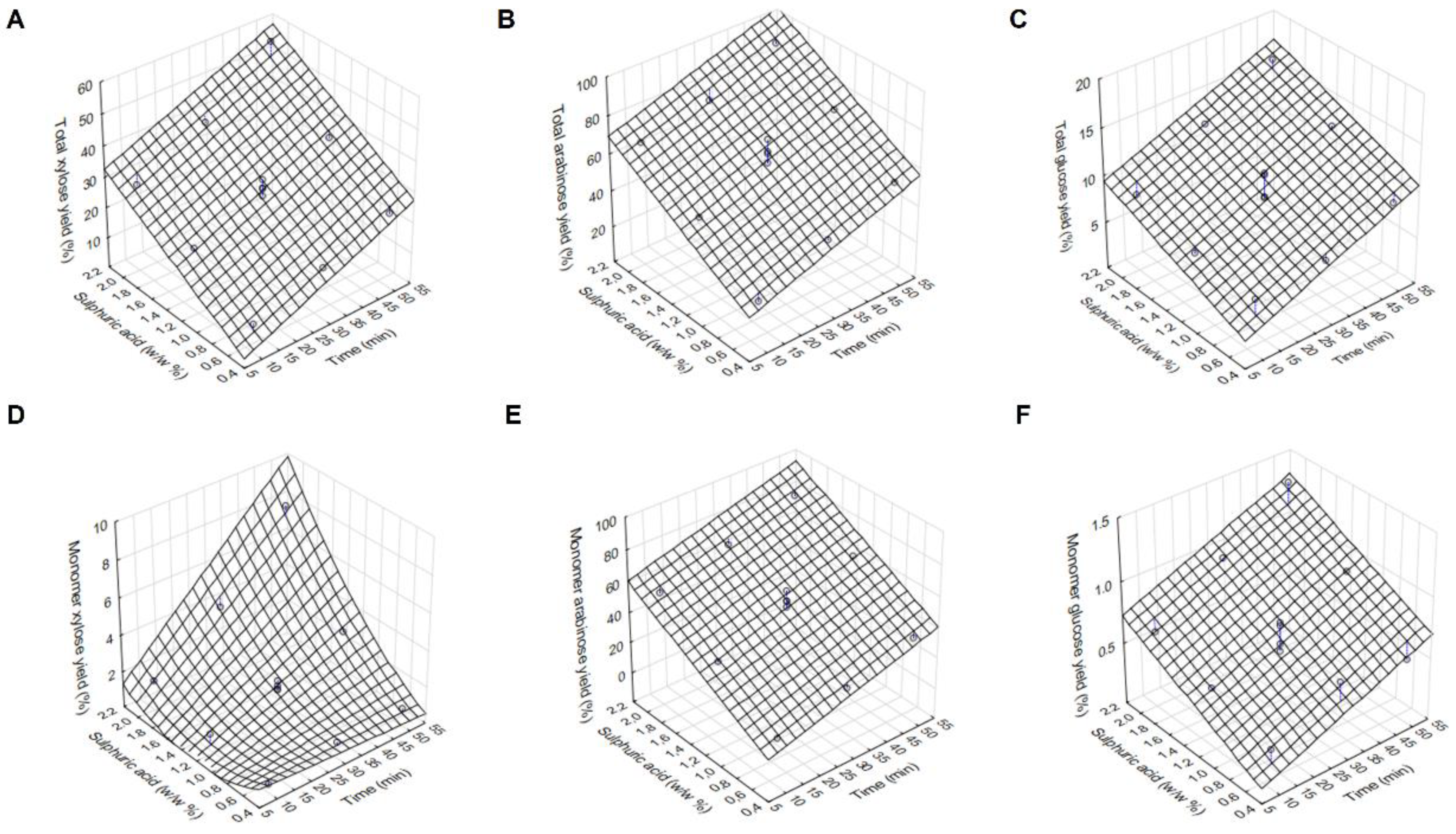
| Runs | Reaction Time (T, Min) | Sulphuric Acid Concentration (S, w/w%) | Total Xylose Yield (%) | Total Arabinose Yield (%) | Total Glucose Yield (%) | Monomer Xylose Yield (%) | Monomer Arabinose Yield (%) | Monomer Glucose Yield (%) |
|---|---|---|---|---|---|---|---|---|
| 1. | 10 | 0.50 | 9.3 | 25.2 | 5.9 | 0.5 | 11.9 | 0.3 |
| 2. | 10 | 1.25 | 20.4 | 48.0 | 6.3 | 0.9 | 34.6 | 0.5 |
| 3. | 10 | 2.00 | 28.5 | 67.1 | 8.2 | 1.6 | 54.2 | 0.6 |
| 4. | 30 | 0.50 | 16.6 | 40.1 | 6.4 | 0.9 | 22.5 | 0.6 |
| 5. (C) | 30 | 1.25 | 29.4 | 65.3 | 11.4 | 1.5 | 53.4 | 0.6 |
| 6. | 30 | 2.00 | 38.9 | 74.1 | 12.6 | 4.0 | 66.2 | 1.0 |
| 7. | 50 | 0.50 | 24.1 | 53.8 | 9.1 | 0.9 | 34.9 | 0.5 |
| 8. | 50 | 1.25 | 36.4 | 73.3 | 13.1 | 3.1 | 64.0 | 0.9 |
| 9. | 50 | 2.00 | 56.2 | 90.5 | 16.5 | 8.1 | 80.3 | 1.4 |
| 10. (C) | 30 | 1.25 | 29.7 | 66.0 | 8.7 | 1.7 | 49.6 | 0.7 |
| 11. (C) | 30 | 1.25 | 27.1 | 60.0 | 11.1 | 2.0 | 59.9 | 0.8 |
| 12. (C) | 30 | 1.25 | 32.6 | 72.5 | 8.9 | 1.6 | 53.8 | 0.5 |
| Runs | GOS Yield (%) | AXOS Yield (%) | A/X Ratio in AXOS |
|---|---|---|---|
| 1. | 5.6 | 10.4 | 0.74 |
| 2. | 5.8 | 17.6 | 0.34 |
| 3. | 7.6 | 22.3 | 0.23 |
| 4. | 5.8 | 16.3 | 0.55 |
| 5. (C) | 10.8 | 23.1 | 0.22 |
| 6. | 11.6 | 26.0 | 0.11 |
| 7. | 8.5 | 21.7 | 0.40 |
| 8. | 12.2 | 25.4 | 0.14 |
| 9. | 15.1 | 35.7 | 0.10 |
| 10. (C) | 8.0 | 20.5 | 0.20 |
| 11. (C) | 10.4 | 24.6 | 0.20 |
| 12. (C) | 8.4 | 22.4 | 0.20 |
| Total Xylose Yield (%) | Total Arabinose Yield (%) | Total Glucose Yield (%) | Monomer Xylose Yield (%) | Monomer Arabinose Yield (%) | Monomer Glucose Yield (%) | AXOS Yield (%) | A/X Ratio in AXOS | |
|---|---|---|---|---|---|---|---|---|
| Predicted | 32.3 | 69.8 | 9.8 | 2.4 | 58.6 | 0.7 | 23.8 | 0.17 |
| −95.% Conf. | 28.4 | 60.6 | 7.3 | 2.0 | 51.0 | 0.5 | 20.7 | 0.15 |
| +95.% Conf. | 36.3 | 78.9 | 12.3 | 2.8 | 66.2 | 0.9 | 26.9 | 0.18 |
| −95.% Pred. | 24.2 | 51.1 | 4.7 | 1.5 | 43.1 | 0.3 | 17.5 | 0.13 |
| +95.% Pred. | 40.5 | 88.4 | 14.9 | 3.2 | 74.1 | 1.2 | 30.1 | 0.20 |
| Measured average | 36.1 | 75.7 | 13.0 | 2.2 | 65.5 | 0.5 | 26.1 | 0.15 |
| Standard deviation | 2.2 | 5.0 | 1.0 | 0.3 | 4.4 | 0.2 | 1.6 | 0.01 |
| Runs | Enzyme Dosage (E, g/g DM) | Solids Loading (w/w%) | Glucose Concentration (g/L) | Glucose Yield (%) |
|---|---|---|---|---|
| 1. | 0.01 | 5 | 10.3 | 54.5 |
| 2. | 0.01 | 10 | 19.4 | 48.9 |
| 3. | 0.01 | 15 | 33.9 | 53.7 |
| 4. | 0.03 | 5 | 12.6 | 66.8 |
| 5. (C) | 0.03 | 10 | 30.8 | 77.6 |
| 6. | 0.03 | 15 | 43.9 | 69.6 |
| 7. | 0.05 | 5 | 12.9 | 68.4 |
| 8. | 0.05 | 10 | 30.0 | 78.0 |
| 9. | 0.05 | 15 | 49.2 | 77.9 |
| 10. (C) | 0.03 | 10 | 30.5 | 76.7 |
| 11. (C) | 0.03 | 10 | 29.5 | 74.2 |
| 12. (C) | 0.03 | 10 | 28.6 | 71.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bedő, S.; Rozbach, M.; Nagy, L.; Fehér, A.; Fehér, C. Optimised Fractionation of Brewer’s Spent Grain for a Biorefinery Producing Sugars, Oligosaccharides, and Bioethanol. Processes 2021, 9, 366. https://doi.org/10.3390/pr9020366
Bedő S, Rozbach M, Nagy L, Fehér A, Fehér C. Optimised Fractionation of Brewer’s Spent Grain for a Biorefinery Producing Sugars, Oligosaccharides, and Bioethanol. Processes. 2021; 9(2):366. https://doi.org/10.3390/pr9020366
Chicago/Turabian StyleBedő, Soma, Margaréta Rozbach, Leonóra Nagy, Anikó Fehér, and Csaba Fehér. 2021. "Optimised Fractionation of Brewer’s Spent Grain for a Biorefinery Producing Sugars, Oligosaccharides, and Bioethanol" Processes 9, no. 2: 366. https://doi.org/10.3390/pr9020366
APA StyleBedő, S., Rozbach, M., Nagy, L., Fehér, A., & Fehér, C. (2021). Optimised Fractionation of Brewer’s Spent Grain for a Biorefinery Producing Sugars, Oligosaccharides, and Bioethanol. Processes, 9(2), 366. https://doi.org/10.3390/pr9020366





