Optimization of Microwave-Assisted Extraction of Polyphenols from Lemon Myrtle: Comparison of Modern and Conventional Extraction Techniques Based on Bioactivity and Total Polyphenols in Dry Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical and Reagents
2.3. Experimental Design
2.3.1. Optimization of MAE Conditions Using Response Surface Methodology (RSM)
2.3.2. Ultrasound-Assisted Extraction (UAE)
2.3.3. Conventional Extraction Using Shaking Water Bath Extraction (SWB)
2.4. UV Spectrophotometric Measurement of Total Phenolics (TPC), Flavonoids (TFC), and Proanthocyanidins (Pro. A)
2.5. Measurement of Antioxidant Properties
2.6. Total Chlorophyll Content
2.7. Antimicrobial Activity Assay
2.7.1. Bacterial Culture and Preparation of Inoculum
2.7.2. Disc Diffusion Bioassay
2.8. Statistical Analysis
3. Result and Discussion
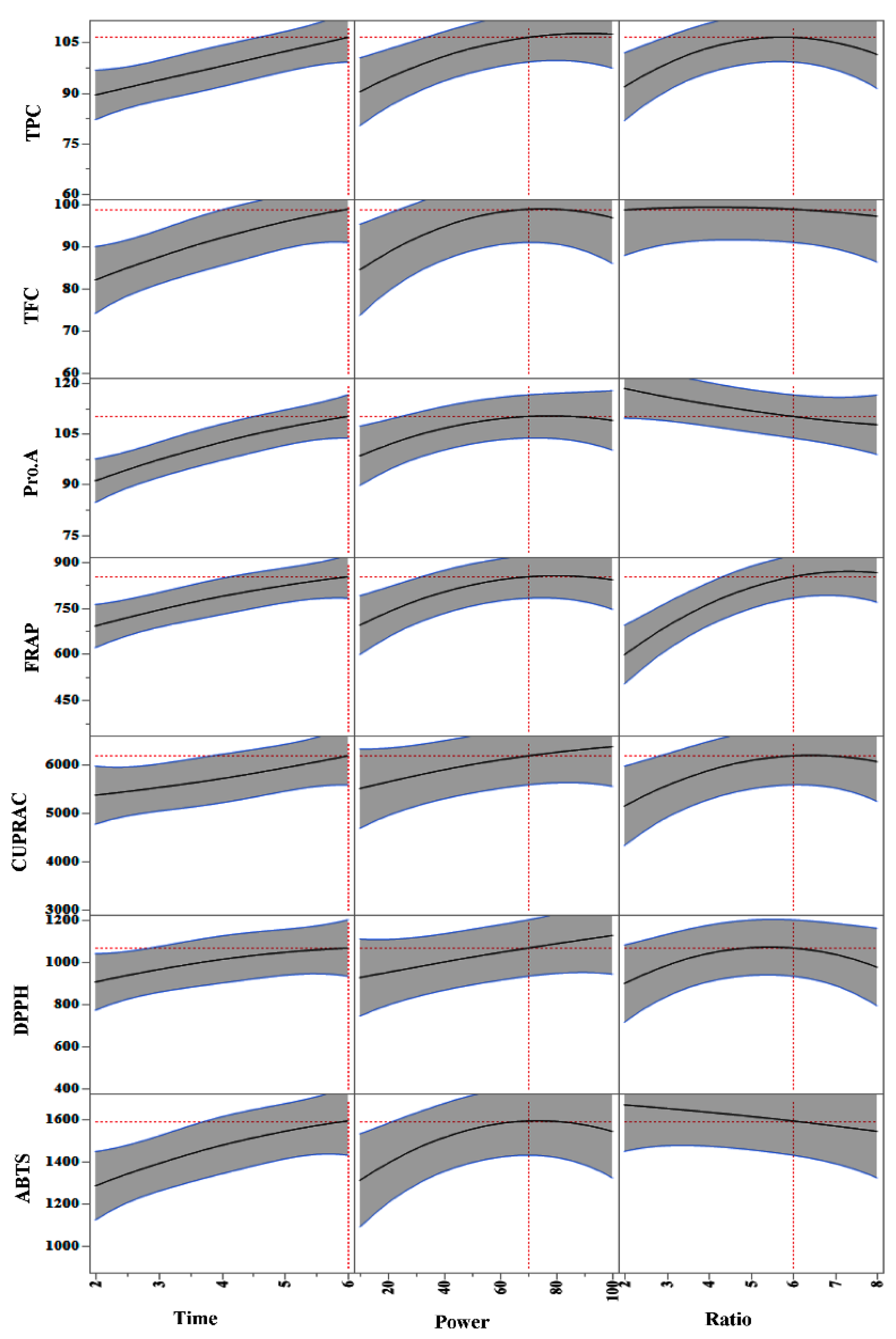
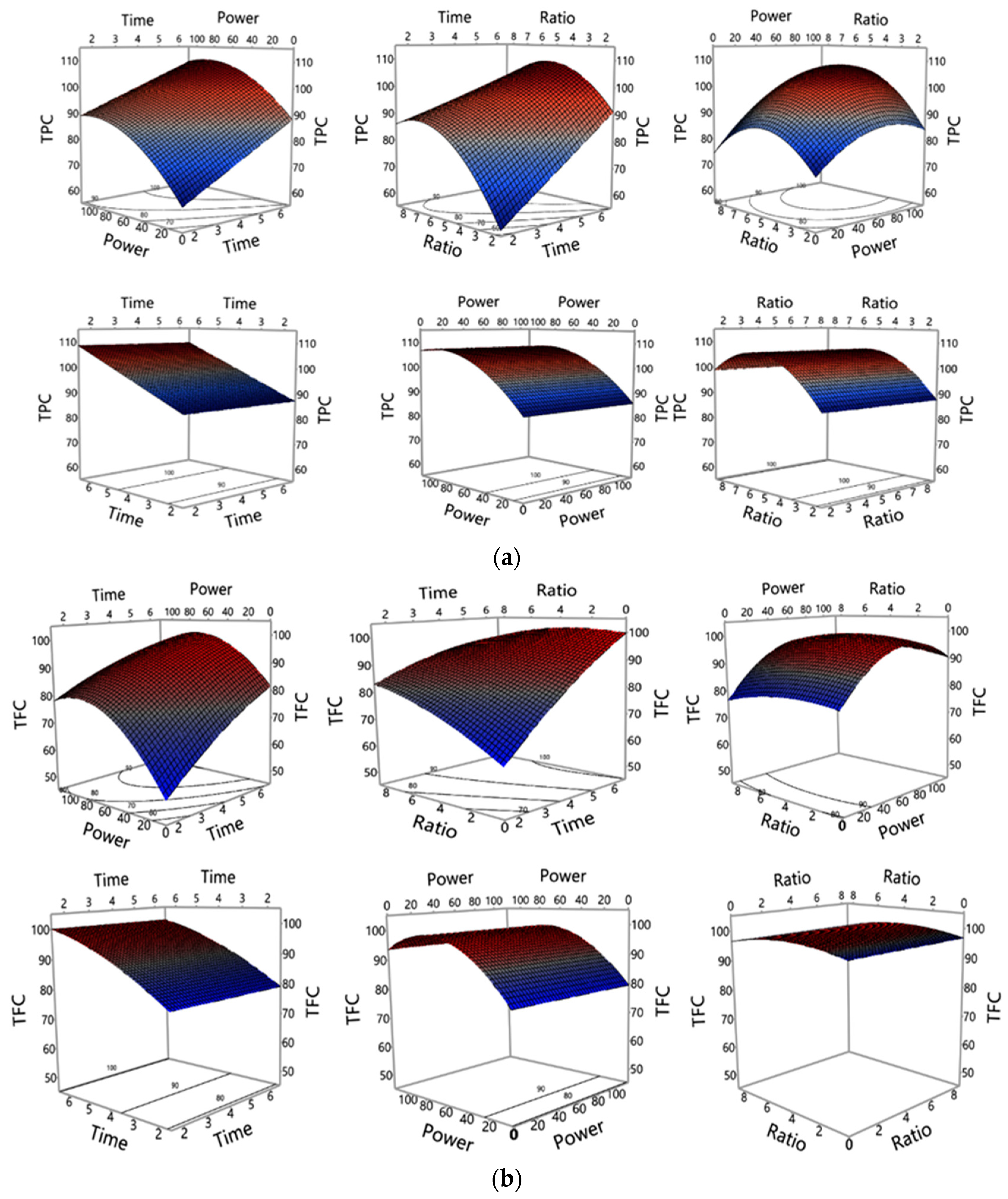
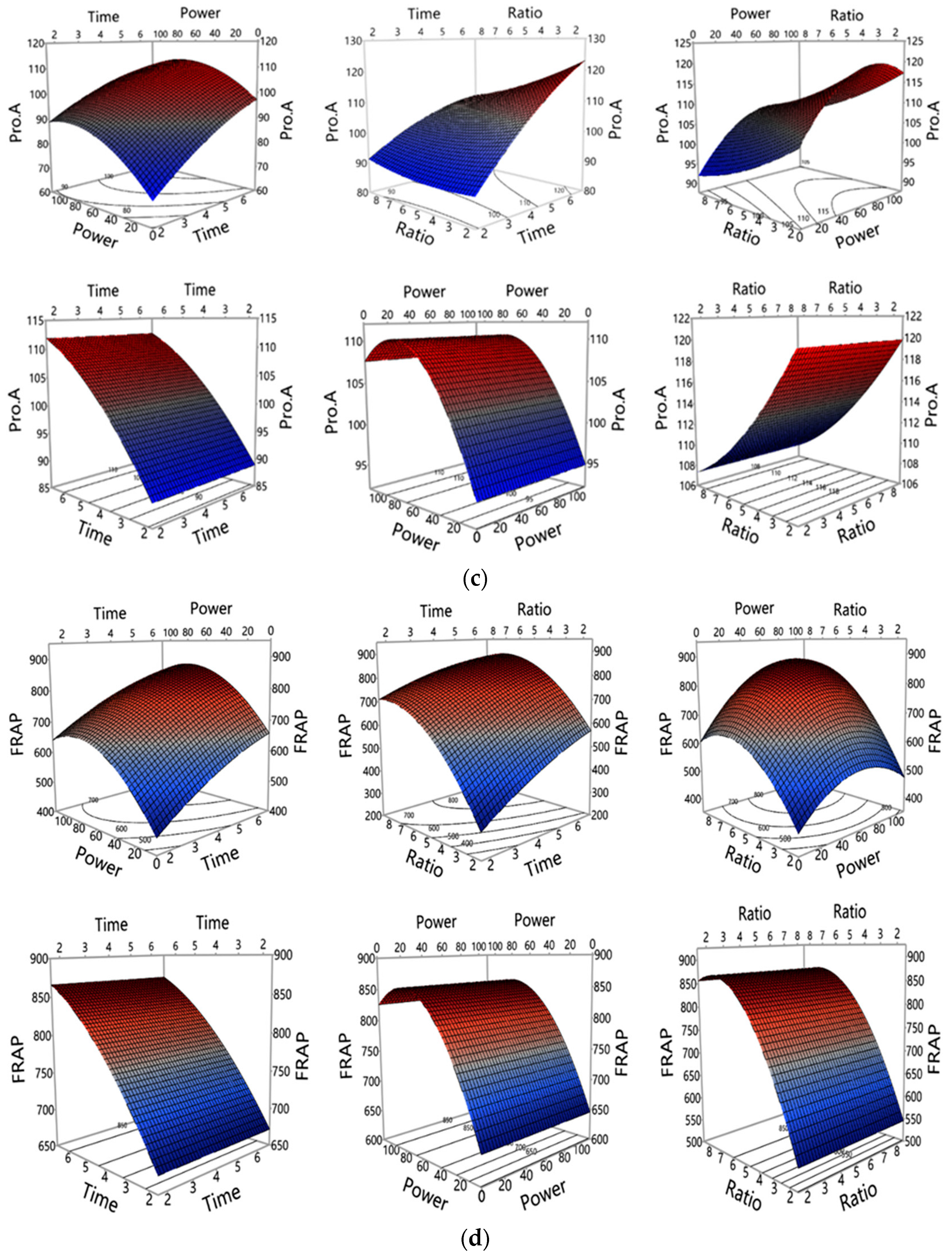
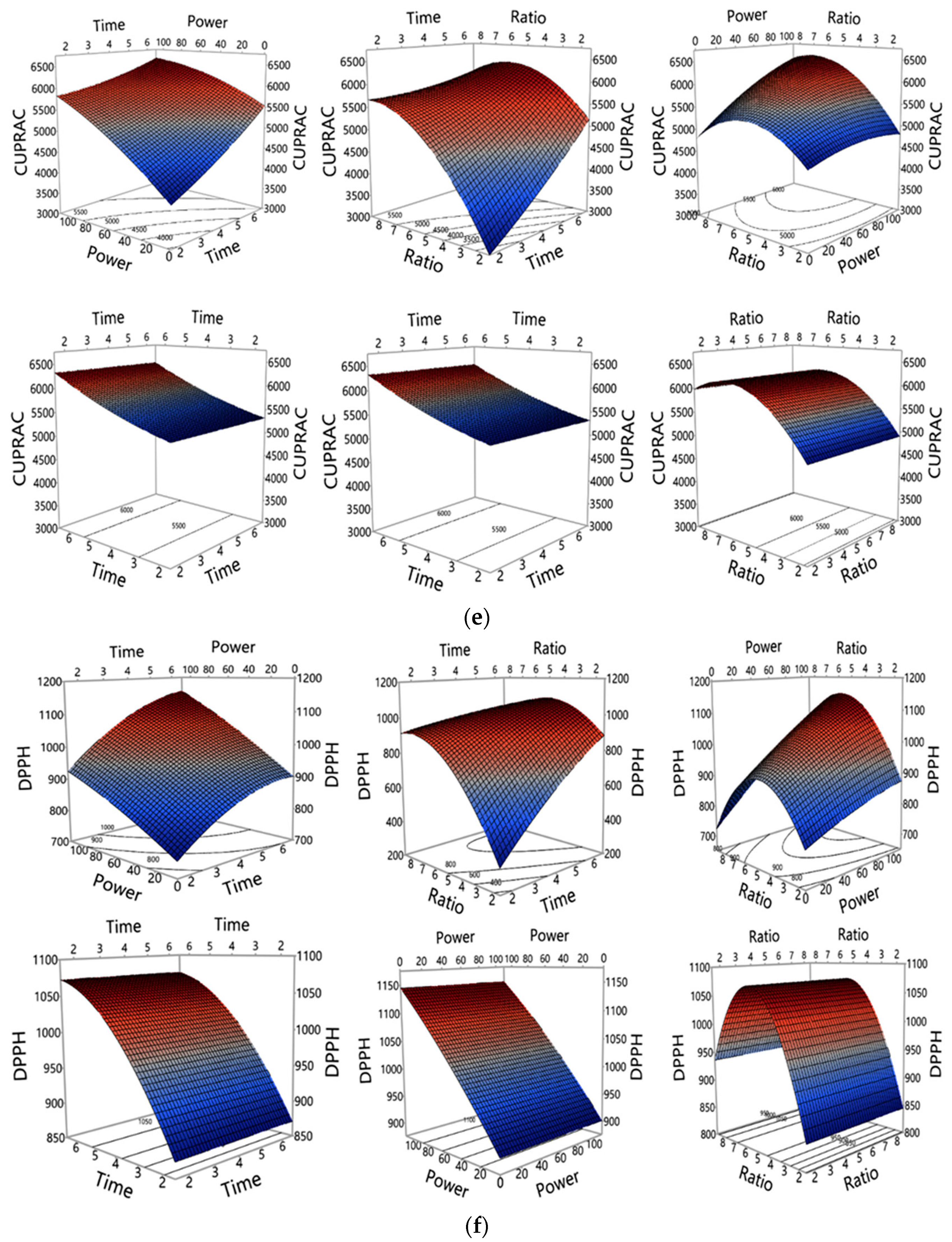
3.1. Investigation of MAE Influencing Factors and Optimisation of MAE Conditions
3.1.1. Accuracy of the Polynomial Prediction Models
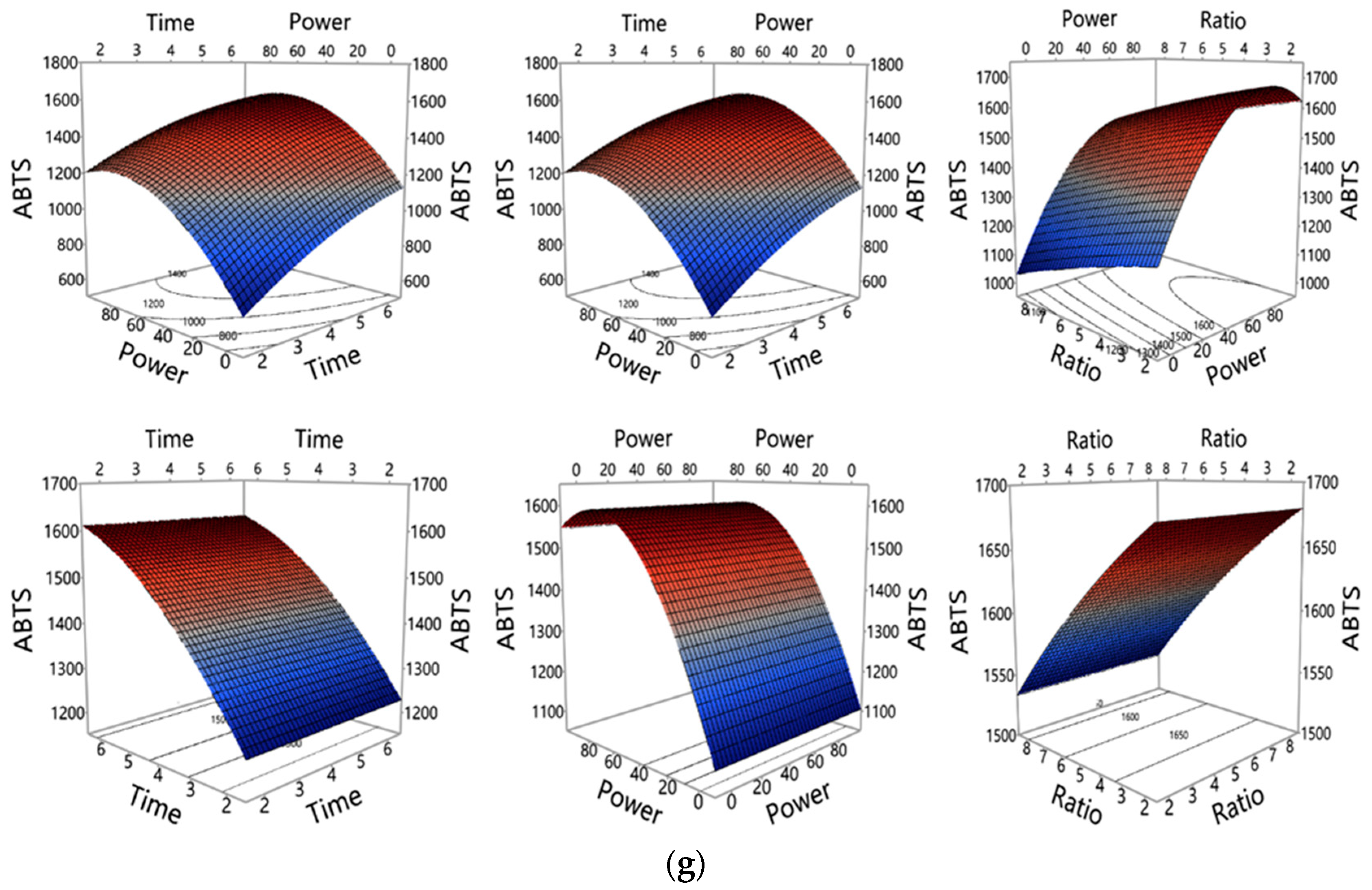
3.1.2. Influence of MAE Parameters on Polyphenols and Antioxidant Properties Yield
3.1.3. Optimization and Validation
3.2. Comparison of the Effects of Conventional and Novel Extraction Techniques on Phenolics, Chlorophyll, Antioxidants, and Antimicrobial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. Phenolic Compounds Biological Activity, 1st ed.; Marcos, S.H., Mariana, P.T., Rosario, G.M., Eds.; IntechOpen: London, UK, 2017; pp. 39–58. [Google Scholar]
- Bhuyan, D.J.; Basu, A. Phenolic compounds: Potential Health Benefits and Toxicity. In Utilization of Bioactive Compounds from Agricultural and Food Waste; Vuong, Q.V., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 27–59. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Chapter 59—Polyphenols in Health and Disease: Practice and Mechanisms of Benefits. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 757–778. [Google Scholar]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Effects of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 2019, 5, e03044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and phenolic compounds in commercially grown native Australian herbs and spices. Food Chem. 2010, 122, 260–266. [Google Scholar] [CrossRef]
- Guo, Y.; Sakulnarmrat, K.; Konczak, I. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicol. Rep. 2014, 1, 385–390. [Google Scholar] [CrossRef] [Green Version]
- Kang, E.J.; Lee, J.K.; Park, H.R.; Kim, H.; Kim, H.S.; Park, J. Antioxidant and anti-inflammatory activities of phenolic compounds extracted from lemon myrtle (Backhousia citriodora) leaves at various extraction conditions. Food Sci. Biotechnol. 2020, 29, 1425–1432. [Google Scholar] [CrossRef]
- Dupont, S.; Caffin, N.; Bhandari, B.; Dykes, G.A. In vitro antibacterial activity of Australian native herb extracts against food-related bacteria. Food Control 2006, 17, 929–932. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Hipwell, M.; Ryan, T.; Cavanagh, H.M.A. Bioactivity of Backhousia citriodora: Antibacterial and Antifungal Activity. J. Agric. Food Chem. 2003, 51, 76–81. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Fenech, M.; Thomas, P.; Konczak, I. Cytoprotective and pro-apoptotic activities of native Australian herbs polyphenolic-rich extracts. Food Chem. 2013, 136, 9–17. [Google Scholar] [CrossRef]
- Mazzorana, G.; Mazzorana, M. Cultivation of Lemon Myrtle (Backhousia citriodora), 1st ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 113–126. [Google Scholar]
- Brophy, J.J.; Goldsack, R.J.; Fookes, C.J.R.; Forster, P.I. Leaf Oils of the Genus Backhousia (Myrtaceae). J. Essent. Oil Res. 1995, 7, 237–254. [Google Scholar] [CrossRef]
- Lassak, V.E. Revision of Backhousia Citriodora Essential Oil Standard; R. I. R. D. C. Publication No. 11/37; Union Offset Printing: Canberra, Australia, 2012. [Google Scholar]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Optimisation and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem. 2015, 169, 141–149. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Carrillo, N.; Aguilar-Santamaría, M.d.l.Á.; Vernon-Carter, E.J.; Jiménez-Alvarado, R.; Cruz-Sosa, F.; Román-Guerrero, A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crop. Prod. 2017, 103, 213–221. [Google Scholar] [CrossRef]
- Sharmila, G.; Nikitha, V.S.; Ilaiyarasi, S.; Dhivya, K.; Rajasekar, V.; Kumar, N.M.; Muthukumaran, K.; Muthukumaran, C. Ultrasound assisted extraction of total phenolics from Cassia auriculata leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2016, 84, 13–21. [Google Scholar] [CrossRef]
- Odabaş, H.İ.; Koca, I. Application of response surface methodology for optimizing the recovery of phenolic compounds from hazelnut skin using different extraction methods. Ind. Crop. Prod. 2016, 91, 114–124. [Google Scholar] [CrossRef]
- De Monte, C.; Carradori, S.; Granese, A.; Di Pierro, G.B.; Leonardo, C.; De Nunzio, C. Modern extraction techniques and their impact on the pharmacological profile of Serenoa repens extracts for the treatment of lower urinary tract symptoms. BMC Urol. 2014, 14, 63. [Google Scholar] [CrossRef] [Green Version]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahmoune, F.; Nayak, B.; Moussi, K.; Remini, H.; Madani, K. Optimization of microwave-assisted extraction of polyphenols from Myrtus communis L. leaves. Food Chem. 2015, 166, 585–595. [Google Scholar] [CrossRef]
- Ince, A.E.; Sahin, S.; Sumnu, G. Comparison of microwave and ultrasound-assisted extraction techniques for leaching of phenolic compounds from nettle. J. Food Sci. Technol. 2014, 51, 2776–2782. [Google Scholar] [CrossRef]
- Belwal, T.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Microwave-assisted extraction (MAE) conditions using polynomial design for improving antioxidant phytochemicals in Berberis asiatica Roxb. ex DC. leaves. Ind. Crop. Prod. 2017, 95, 393–403. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; Vuong, Q.V. Development of Ultrasound-assisted Extraction Conditions for the Optimal Yield of Phenolic Compounds and Antioxidant Properties from Lemon Myrtle (Backhousia citriodora) Leaves. Curr. Nutraceuticals 2021, 2, 1–14. [Google Scholar] [CrossRef]
- Nana, O.; Momeni, J.; Boyom, F.F.; Njintang, N.Y.; Ngassoum, M.B. Microwave-assisted extraction as an advanced technique for optimisation of limonoid yields and antioxidant potential from Trichilia roka (Meliaceae). Curr. Res. Green Sustain. Chem. 2021, 4, 100–147. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Vuong, Q. Comparison of conventional extraction technique with ultrasound assisted extraction on recovery of phenolic compounds from lemon scented tea tree (Leptospermum petersonii) leaves. Heliyon 2020, 6, e03666. [Google Scholar] [CrossRef]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Özyürek, M.; Karademir, S.E. Novel Total Antioxidant Capacity Index for Dietary Polyphenols and Vitamins C and E, Using Their Cupric Ion Reducing Capability in the Presence of Neocuproine: CUPRAC Method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Sudhakar, P.; Latha, P.; Reddy, P.V. Chapter 15—Plant pigments. In Phenotyping Crop Plants for Physiological and Biochemical Traits; Sudhakar, P., Latha, P., Reddy, P.V., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 121–127. [Google Scholar]
- Bhuyan, D.J.; Vuong, Q.V.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Phytochemical, antibacterial and antifungal properties of an aqueous extract of Eucalyptus microcorys leaves. S. Afr. J. Bot. 2017, 112, 180–185. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Venkatesalu, V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 2004, 91, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Nwabueze, T.U. Review article: Basic steps in adapting response surface methodology as mathematical modelling for bioprocess optimisation in the food systems. Int. J. Food Sci. Technol. 2010, 45, 1768–1776. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; Vuong, Q. Maximising extraction yields of gallic acid and hesperetin from lemon myrtle (Backhousia citriodora) leaf using microwave assisted extraction. Results Chem. 2020, 2, 100080. [Google Scholar] [CrossRef]
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345. [Google Scholar] [CrossRef]
- Harel, O. The estimation of R2 and adjusted R2 in incomplete data sets using multiple imputation. J. Appl. Stat. 2009, 36, 1109–1118. [Google Scholar] [CrossRef]
- Yan, M.-M.; Liu, W.; Fu, Y.-J.; Zu, Y.-G.; Chen, C.-Y.; Luo, M. Optimisation of the microwave-assisted extraction process for four main astragalosides in Radix Astragali. Food Chem. 2010, 119, 1663–1670. [Google Scholar] [CrossRef]
- Dahmoune, F.; Boulekbache, L.; Moussi, K.; Aoun, O.; Spigno, G.; Madani, K. Valorization of Citrus limon residues for the recovery of antioxidants: Evaluation and optimization of microwave and ultrasound application to solvent extraction. Ind. Crop. Prod. 2013, 50, 77–87. [Google Scholar] [CrossRef]
- Xu, D.-P.; Zheng, J.; Zhou, Y.; Li, Y.; Li, S.; Li, H.-B. Ultrasound-assisted extraction of natural antioxidants from the flower of Limonium sinuatum: Optimization and comparison with conventional methods. Food Chem. 2017, 217, 552–559. [Google Scholar] [CrossRef]
- Jianming, W.; Yuan, G.; Ping, L.; Feng, H.; Liying, L. Optimization of Ultrasound-Assisted Extraction Procedure to Determine Total Isoflavones in Chinese Soybean Cheese by Box–Behnken Design. Food Anal. Methods 2013, 6, 221–226. [Google Scholar] [CrossRef]
- Doulabi, M.; Golmakani, M.-T.; Ansari, S. Evaluation and optimization of microwave-assisted extraction of bioactive compounds from eggplant peel by-product. J. Food Process. Preserv. 2020, 44, e14853. [Google Scholar] [CrossRef]
- Ali, A.; Lim, X.Y.; Chong, C.H.; Mah, S.H.; Chua, B.L. Optimization of ultrasound-assisted extraction of natural antioxidants from Piper betle using response surface methodology. LWT 2018, 89, 681–688. [Google Scholar] [CrossRef]
- Tomšik, A.; Pavlić, B.; Vladić, J.; Ramić, M.; Brindza, J.; Vidović, S. Optimization of ultrasound-assisted extraction of bioactive compounds from wild garlic (Allium ursinum L.). Ultrason. Sonochem. 2016, 29, 502–511. [Google Scholar] [CrossRef]
- Milutinović, M.; Radovanović, N.; Rajilić-Stojanović, M.; Šiler-Marinković, S.; Dimitrijević, S.; Dimitrijević-Branković, S. Microwave-assisted extraction for the recovery of antioxidants from waste Equisetum arvense. Ind. Crop. Prod. 2014, 61, 388–397. [Google Scholar] [CrossRef]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimum conditions of microwave-assisted extraction for phenolic compounds and antioxidant capacity of the brown alga Sargassum vestitum. Sep. Sci. Technol. 2018, 53, 1711–1723. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Van Vuong, Q.; Chalmers, A.C.; van Altena, I.A.; Bowyer, M.C.; Scarlett, C.J. Microwave-assisted extraction of Eucalyptus robusta leaf for the optimal yield of total phenolic compounds. Ind. Crop. Prod. 2015, 69, 290–299. [Google Scholar] [CrossRef]
- Grigonis, D.; Venskutonis, R.; Sivik, B.; Sandahl, M.; Eskilsson, C.S. Comparison of different extraction techniques for isolation of antioxidants from sweet grass (Hierochloë odorata). J. Supercrit. Fluids 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hamid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process. 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Gaur, S.; Shivhare, U.S.; Sarkar, B.C.; Ahmed, J. Thermal Chlorophyll Degradation Kinetics of Mint Leaves Puree. Int. J. Food Prop. 2007, 10, 853–865. [Google Scholar] [CrossRef]
- Pham HN, T.; Sakoff, J.A.; Van Vuong, Q.; Bowyer, M.C.; Scarlett, C.J. Screening phytochemical content, antioxidant, antimicrobial and cytotoxic activities of Catharanthus roseus (L. ) G. Don stem extract and its fractions. Biocatal. Agric. Biotechnol. 2018, 16, 405–411. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Wall-Medrano, A.; Goñi, M.G.; Ramos-Clamont-Montfort, G.; Ayala-Zavala, J.F.; González-Aguilar, G.A. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett Appl. Microbiol. 2018, 66, 25–31. [Google Scholar] [CrossRef]
- Rempe, C.S.; Burris, K.P.; Lenaghan, S.C.; Stewart, C.N., Jr. The Potential of Systems Biology to Discover Antibacterial Mechanisms of Plant Phenolics. Front. Microbiol. 2017, 8, 422. [Google Scholar] [CrossRef] [Green Version]
- Zamuz, S.; Munekata, P.E.; Dzuvor, C.K.; Zhang, W.; Sant’Ana, A.S.; Lorenzo, J.M. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021, 110, 385–392. [Google Scholar] [CrossRef]
- Cock, I. Antimicrobial activity of Backhousia citriodora (lemon myrtle) methanolic extracts. Pharmacogn. Commun. 2013, 3, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Alderees, F.; Mereddy, R.; Webber, D.; Nirmal, N.; Sultanbawa, Y. Mechanism of Action against Food Spoilage Yeasts and Bioactivity of Tasmannia lanceolata, Backhousia citriodora and Syzygium anisatum Plant Solvent Extracts. Foods 2018, 7, 179. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, A.; Murtaza, G. Phenolic contents, antimicrobial and antioxidant activity of Olea ferruginea Royle (Oleaceae). BMC Complementary Altern. Med. 2018, 18, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.J.; Chang, S.K.C. A Comparative Study on Phenolic Profiles and Antioxidant Activities of Legumes as Affected by Extraction Solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef]
- El Mannoubi, I. Effect of extraction solvent on phenolic composition, antioxidant and antibacterial activities of skin and pulp of Tunisian red and yellow–orange Opuntia Ficus Indica fruits. J. Food Meas. Charact. 2021, 15, 643–651. [Google Scholar] [CrossRef]

| Run | Experimental Conditions (Independent Variables) | Observed Responses (Dependent Variables) (n = 3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattern | X1 | X2 | X3 | Phytochemicals | Antioxidant Capacity | ||||||
| TPC | TFC | Pro. A | FRAP | CUPRAC | DPPH | ABTS | |||||
| 1 | +−0 | 6 | 10 | 5 | 93.67247 | 88.02825 | 104.0927 | 683.4416 | 5827.626 | 985.7716 | 1371.562 |
| 2 | −0+ | 2 | 55 | 8 | 89.31072 | 85.19821 | 92.55855 | 737.6218 | 5723.661 | 991.6801 | 1296.063 |
| 3 | 000 | 4 | 55 | 5 | 94.77291 | 86.74644 | 99.4709 | 695.8874 | 5338.929 | 997.0779 | 1322.186 |
| 4 | 0++ | 4 | 100 | 8 | 96.58725 | 91.91176 | 103.6901 | 793.6621 | 6163.616 | 1007.508 | 1407.265 |
| 5 | −+0 | 2 | 100 | 5 | 85.43417 | 77.76763 | 87.44164 | 621.3235 | 5105.042 | 839.3812 | 1221.161 |
| 6 | −−0 | 2 | 10 | 5 | 69.86795 | 62.6842 | 74.89885 | 484.4347 | 4070.798 | 718.2009 | 990.0688 |
| 7 | ++0 | 6 | 100 | 5 | 105.8023 | 95.40555 | 111.5702 | 811.5801 | 6260.000 | 1155.195 | 1549.675 |
| 8 | 0−+ | 4 | 10 | 8 | 71.02841 | 70.43143 | 87.62903 | 599.0081 | 4384.782 | 777.6093 | 1087.066 |
| 9 | 000 | 4 | 55 | 5 | 95.1325 | 92.49178 | 103.0034 | 730.4654 | 5451.429 | 951.2987 | 1561.580 |
| 10 | 0−− | 4 | 10 | 2 | 67.76044 | 71.67215 | 91.63295 | 436.1154 | 3884.874 | 703.0176 | 1239.114 |
| 11 | 0+− | 4 | 100 | 2 | 82.07033 | 91.03641 | 107.7913 | 493.9362 | 4659.139 | 789.9637 | 1520.961 |
| 12 | −0− | 2 | 55 | 2 | 62.37495 | 68.82536 | 92.46291 | 370.5118 | 3378.939 | 471.9729 | 1216.816 |
| 13 | +0− | 6 | 55 | 2 | 86.92227 | 94.55304 | 115.2998 | 561.0199 | 4783.613 | 796.4094 | 1609.769 |
| 14 | 000 | 4 | 55 | 5 | 92.35194 | 89.33138 | 102.3861 | 772.8705 | 5309.874 | 927.3300 | 1505.634 |
| 15 | +0+ | 6 | 55 | 8 | 100.2345 | 97.62057 | 103.7533 | 854.7754 | 5900.446 | 945.2110 | 1548.363 |
| Model Parameters | Phytochemicals | Antioxidant Capacity Measures | |||||
|---|---|---|---|---|---|---|---|
| TPC | TFC | Pro. A | FRAP | CUPRAC | DPPH | ABTS | |
| Intercept β0 | 94.08 *** | 89.52 *** | 101.62 *** | 733.07 *** | 5366.74 *** | 958.67 *** | 1463.13 *** |
| Linear term | |||||||
| β1 | 9.96 *** | 10.14 ** | 10.92 *** | 87.12 ** | 561.66 ** | 107.67 * | 169.41 ** |
| β2 | 8.45 ** | 7.91 ** | 6.53 ** | 64.68 ** | 502.46 * | 75.93 * | 126.41 * |
| β3 | 7.25 ** | 2.38 | −2.44 | 140.44 *** | 683.24 ** | 120.08 ** | −30.99 |
| Interactions | |||||||
| β12 | −0.86 | −1.93 | −1.27 | −2.19 | −150.47 | 12.06 | −13.24 |
| β13 | −3.41 | −3.33 | −2.91 | −18.34 | −306.97 | −92.73 | −35.16 |
| β23 | 2.81 | 0.53 | −0.02 | 34.21 | 251.14 | 35.74 | 9.59 |
| Quadratic | |||||||
| β11 | −0.02 | −1.63 | −1.89 | −16.29 | 61.34 | −26.07 | −37.93 |
| β22 | −5.37 | −6.9190 * | −5.23 * | −66.59 * | −112.22 | −7.86 | −142.08 |
| β33 | −9.35 ** | −1.34 | 1.29 | −85.81 * | −481.42 * | −131.1815 * | −7.45 |
| Model fitting indicators | |||||||
| R2 | 0.96 | 0.94 | 0.95 | 0.97 | 0.94 | 0.92 | 0.91 |
| Adjusted R2 | 0.89 | 0.82 | 0.87 | 0.92 | 0.83 | 0.77 | 0.74 |
| RMSE | 4.337 | 4.7065 | 3.8377 | 41.805 | 359.27 | 79.956 | 96.53 |
| Lack of fit | 0.0709 | 0.2157 | 0.1415 | 0.4642 | 0.0259 | 0.116 | 0.8132 |
| F ratio of model | 13.2033 | 8.0893 | 11.34 | 18.99 | 8.53 | 6.24 | 5.34 |
| P of model > F | 0.006 | 0.017 | 0.0078 | 0.0024 | 0.0147 | 0.0289 | 0.0397 |
| Values (n = 3) | ||
|---|---|---|
| Predicted | Experimental | |
| TPC (mg GAE/g DW) | 106.51 ± 7.32 a | 108.06 ± 3.73 a |
| TFC (mg CE/g DW) | 98.85 ± 7.88 a | 101.41 ± 3.82 a |
| Proanthocyanidins (mg CE/g DW) | 110.18 ± 6.43 a | 114.23 ± 2.39 a |
| FRAP (mM TE/g DW) | 852.30 ± 70.03 a | 947.15 ± 36.37 a |
| CUPRAC (mMTE/g DW) | 6194.44 ± 601.82 a | 6395.53 ± 157.82 a |
| DPPH (mMTE/g DW) | 1067.14 ± 133.93 a | 1188.311 ± 39.17 a |
| ABTS (mMTE/g DW) | 1594.73 ± 161.7 a | 1797.17 ± 91.20 a |
| MAE | UAE | SWB | |
|---|---|---|---|
| Phenolic compounds | |||
| TPC (mg GAE/g DW) | 406.67 ± 8.57 a | 399.03 ± 7.65 a | 398.10 ± 2.76 a |
| TFC (mg CE/g DW) | 384.57 ± 2.74 a | 381.57 ± 5.29 a | 379.13 ± 7.77 a |
| Pro. A (mg CE/g DW) | 336.54 ± 7.09 a | 347.58 ± 6.85 a | 347.39 ± 5.76 a |
| Antioxidant capacities | |||
| FRAP (mM TE/g DW) | 3175.56 ± 79.39 a | 3221.73 ± 158.54 a | 3272.45 ± 101.46 a |
| CUPRAC (mM TE/g DW) | 22,479.77 ± 87.89 c | 23,270.17 ± 146.33 b | 24,520.28 ± 154.34 a |
| ABTS (mM TE/g DW) | 7023.99 ± 83.49 b | 7040.03 ± 103.22 b | 7241.42 ± 38.75 a |
| DPPH (mM TE/g DW) | 4531.04 ± 33.02 ab | 4404.50 ± 53.14 b | 4645.36 ± 72.92 a |
| Total chlorophyll content mg/g dry extract | 1.14 ± 0.49 b | 1.29 ± 0.09 a | 1.33 ± 0.05 a |
| Bacteria | Inhibition Zone (mm) (n = 9) | ||||||
|---|---|---|---|---|---|---|---|
| MAE | UAE | SWB | Ciprofloxacin (5 µg/disc) | ||||
| 15 mg/mL | 20 mg/mL | 15 mg/mL | 20 mg/mL | 15 mg/mL | 20 mg/mL | ||
| E. coli | ND | ND | ND | ND | ND | ND | |
| E. aerogenes | ND | ND | ND | ND | ND | ND | |
| S. lugdunensis | 9.42 ± 0.49 b | 10.42 ± 0.38 b | 9.25 ± 0.61 b | 10.58 ± 0.38 b | 8.83 ± 0.81 b | 10.17 ± 0.26 b | 34.92 ± 0.20 a |
| B. cereus | 8.58 ± 0.38 b | 9.33 ± 0.26 b | 8.75 ± 0.61 b | 9.33 ± 0.26 b | 8.67 ± 0.26 b | 9.83 ± 0.26 b | 26.08 ± 0.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saifullah, M.; McCullum, R.; Vuong, Q.V. Optimization of Microwave-Assisted Extraction of Polyphenols from Lemon Myrtle: Comparison of Modern and Conventional Extraction Techniques Based on Bioactivity and Total Polyphenols in Dry Extracts. Processes 2021, 9, 2212. https://doi.org/10.3390/pr9122212
Saifullah M, McCullum R, Vuong QV. Optimization of Microwave-Assisted Extraction of Polyphenols from Lemon Myrtle: Comparison of Modern and Conventional Extraction Techniques Based on Bioactivity and Total Polyphenols in Dry Extracts. Processes. 2021; 9(12):2212. https://doi.org/10.3390/pr9122212
Chicago/Turabian StyleSaifullah, Md, Rebecca McCullum, and Quan Van Vuong. 2021. "Optimization of Microwave-Assisted Extraction of Polyphenols from Lemon Myrtle: Comparison of Modern and Conventional Extraction Techniques Based on Bioactivity and Total Polyphenols in Dry Extracts" Processes 9, no. 12: 2212. https://doi.org/10.3390/pr9122212
APA StyleSaifullah, M., McCullum, R., & Vuong, Q. V. (2021). Optimization of Microwave-Assisted Extraction of Polyphenols from Lemon Myrtle: Comparison of Modern and Conventional Extraction Techniques Based on Bioactivity and Total Polyphenols in Dry Extracts. Processes, 9(12), 2212. https://doi.org/10.3390/pr9122212







