Nanotechnology in Enhanced Oil Recovery
Abstract
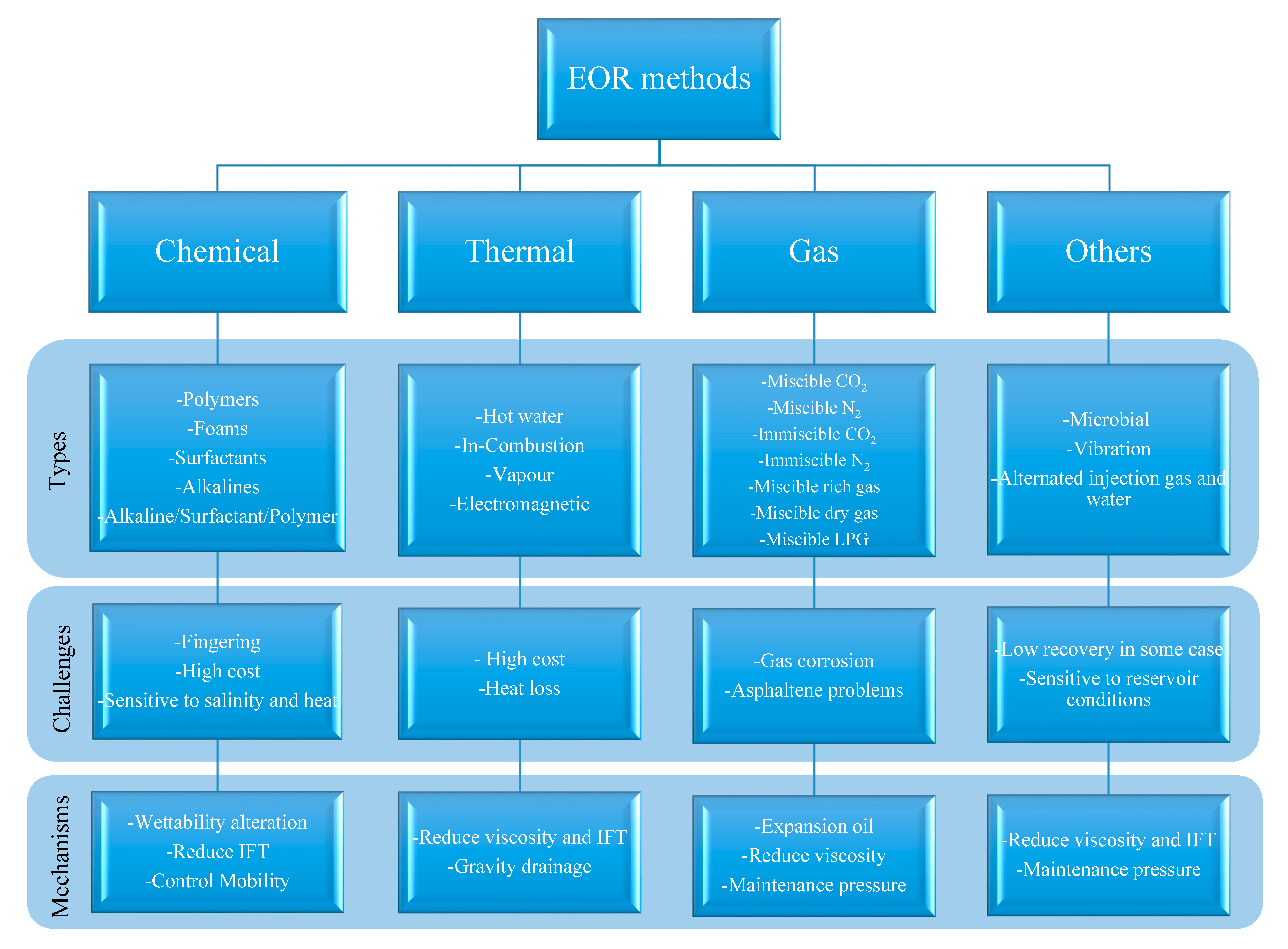
1. Introduction
2. Effects of Nanoparticles on Oil Recovery
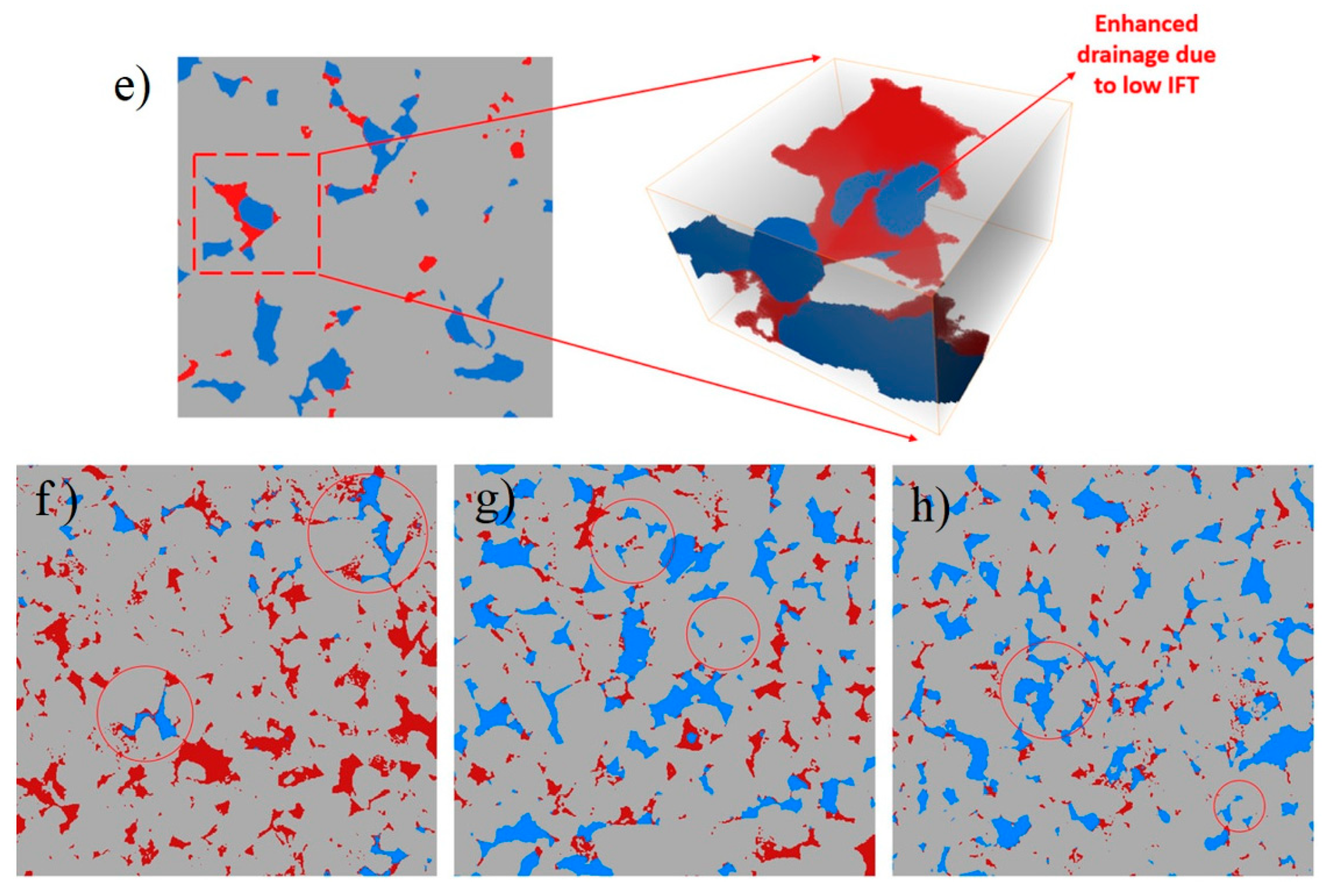
2.1. Effects of Nanoparticles on IFT
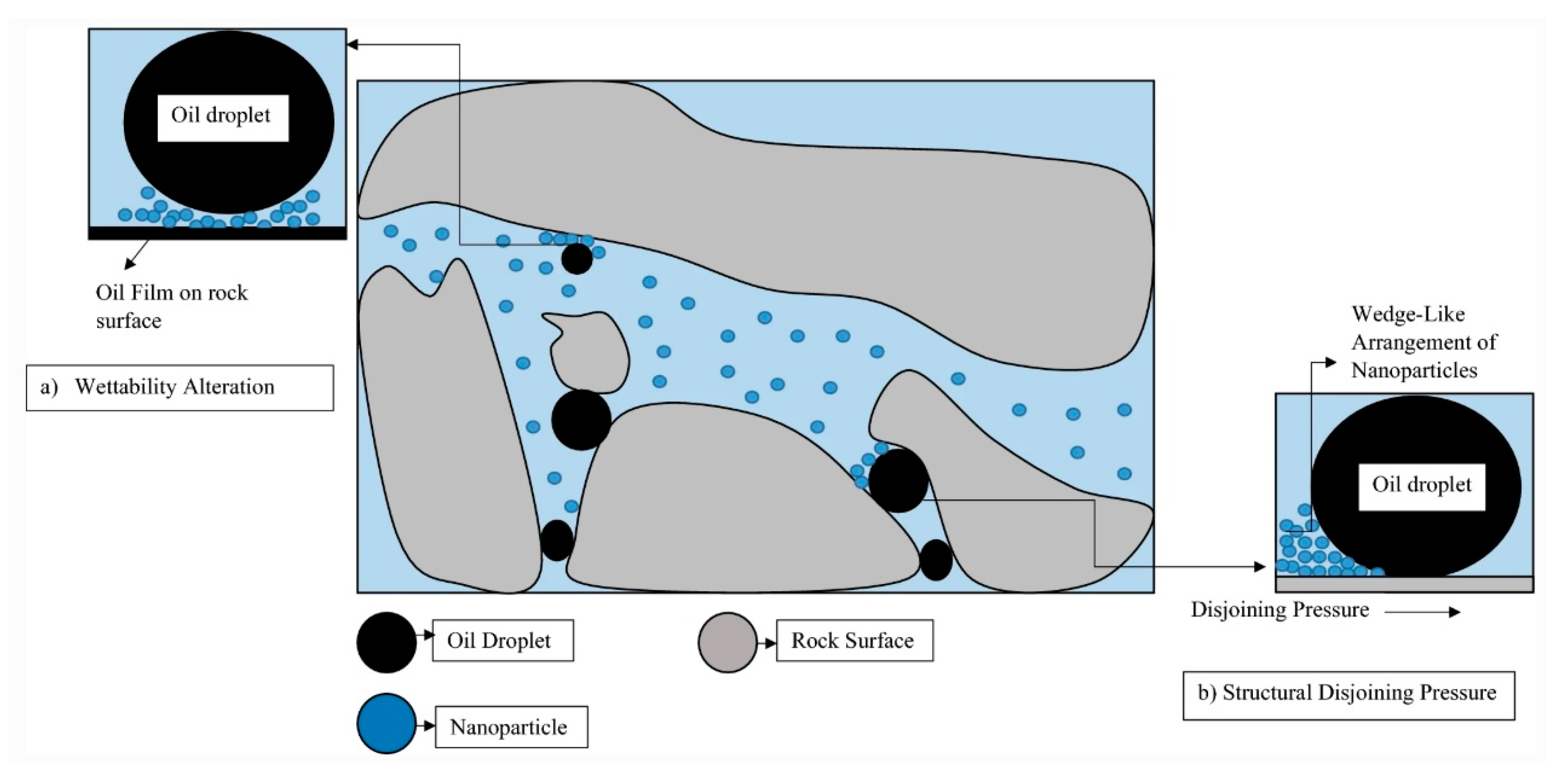
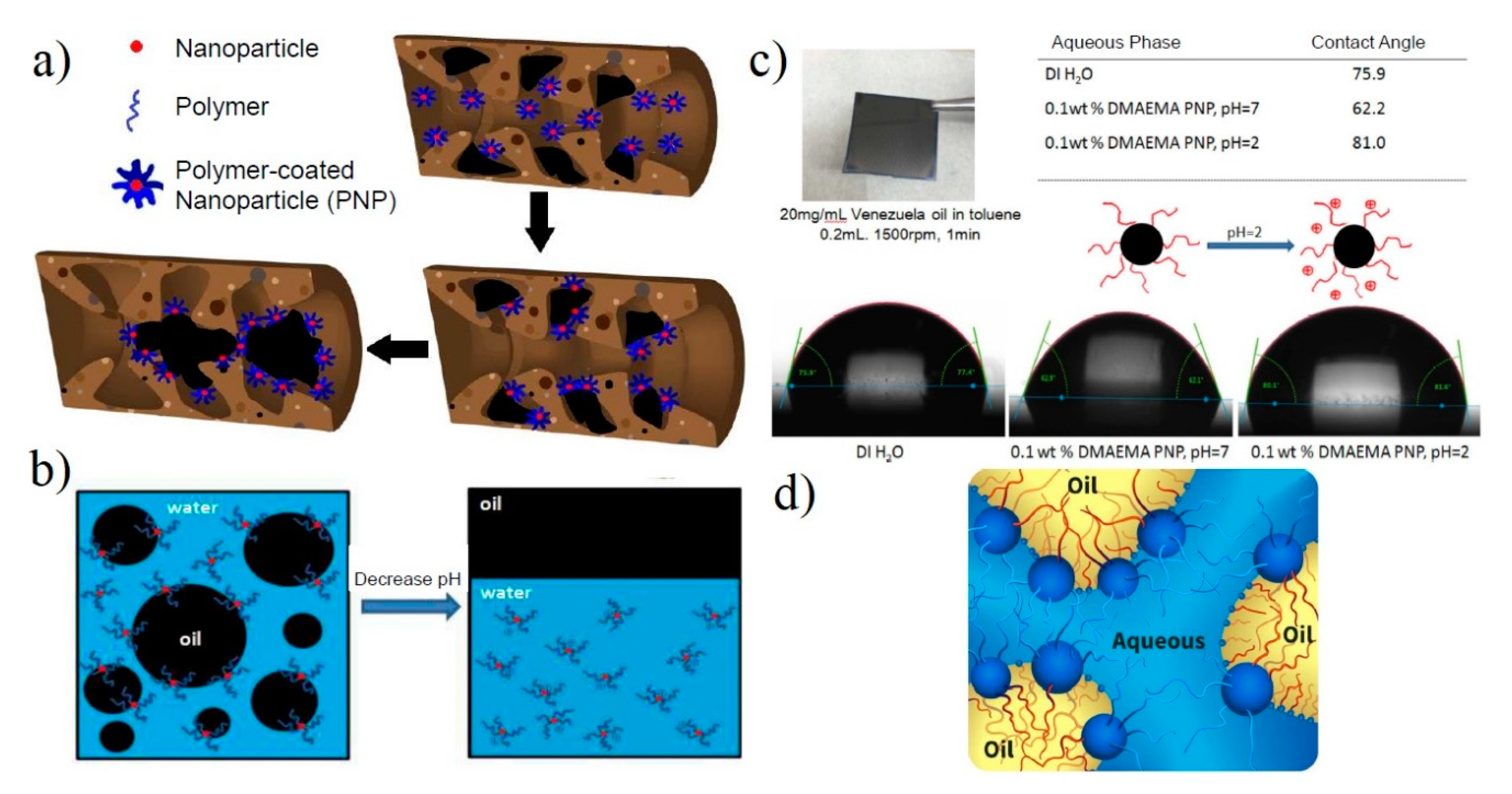
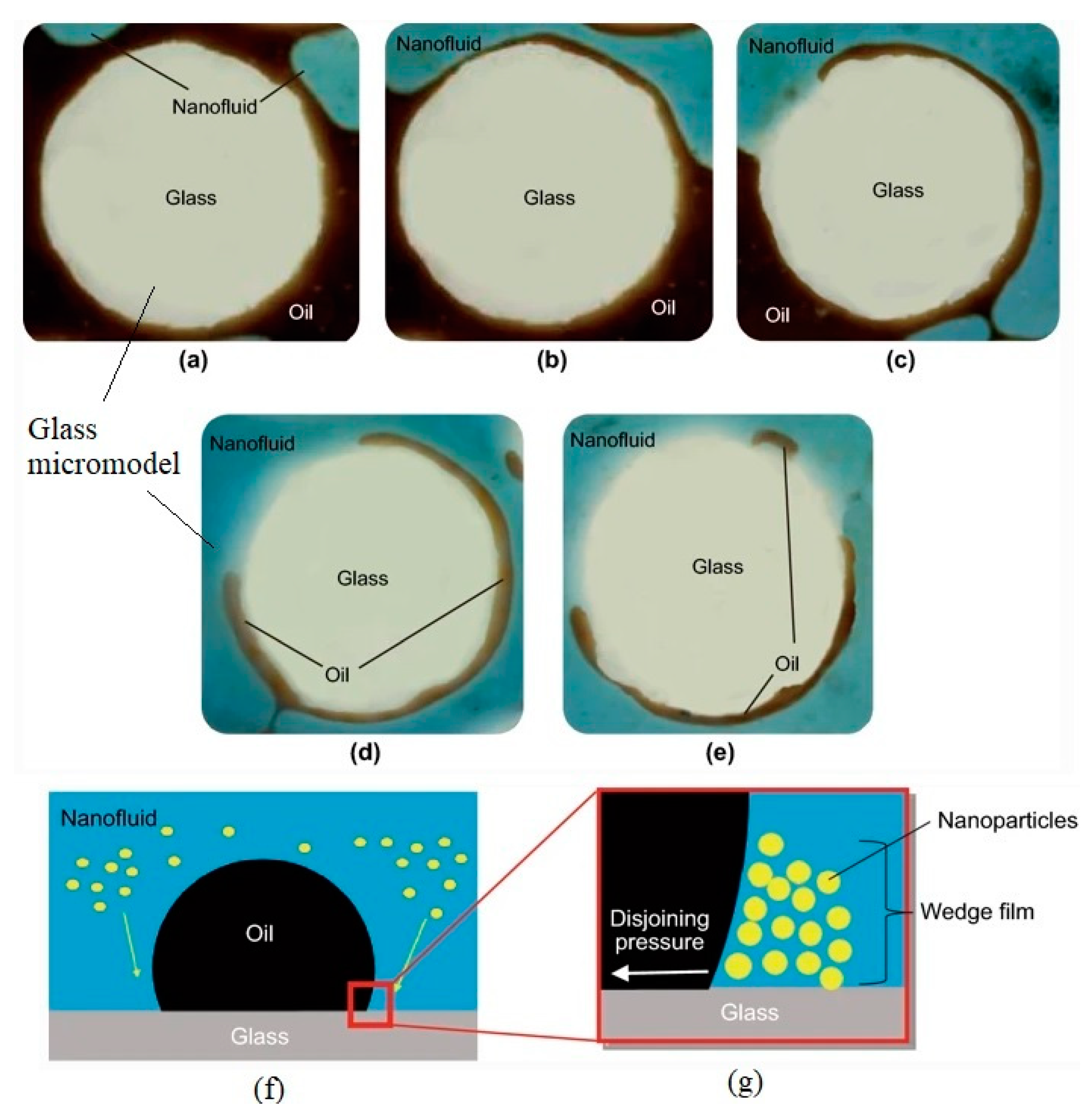
2.2. Effects of Nanoparticles on Wettability
3. Challenges and Future Research
4. Summary
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Abbreviation | Full Form |
| NPs | Nanoparticles |
| IFT | Interfacial tension |
| EOR | Enhanced oil recovery |
| LPG | Liquid petroleum gas |
| PSNP | Polysilicon NPs |
| HLPN | Hydrophobic and lipophilic polysilicon nanoparticles |
| Na-Mt | Na-montmorillonite |
| HPAM | Hydrolyzed polyacrylamide |
| SEM | Scanning electron microscope |
| SDS | Sodium dodecyl sulfate |
| CMC | Critical micelle concentration |
References
- Cheraghian, G.; Wistuba, M.P. Ultraviolet aging study on bitumen modified by a composite of clay and fumed silica nanoparticles. Sci. Rep. 2020, 10, 11216. [Google Scholar] [CrossRef] [PubMed]
- Cheraghian, G.; Nezhad, S.S.K.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Effect of nanoclay on improved rheology properties of polyacrylamide solutions used in enhanced oil recovery. J. Pet. Explor. Prod. Technol. 2014, 5, 189–196. [Google Scholar] [CrossRef]
- Cheraghian, G.; Nezhad, S.S.K.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Adsorption polymer on reservoir rock and role of the nanoparticles, clay and SiO2. Int. Nano Lett. 2014, 4, 114. [Google Scholar] [CrossRef]
- Aliabadian, E.; Sadeghi, S.; Moghaddam, A.R.; Maini, B.B.; Chen, Z.; Sundararaj, U. Application of graphene oxide nanosheets and HPAM aqueous dispersion for improving heavy oil recovery: Effect of localized functionalization. Fuel 2020, 265, 116918. [Google Scholar] [CrossRef]
- Miranda, C.R.; De Lara, L.S.; Tonetto, B.C. Stability and Mobility of Functionalized Silica Nanoparticles for Enhanced Oil Recovery Applications, SPE 157033-MS. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Roustaei, A.; Saffarzadeh, S.; Mohammadi, M. An evaluation of modified silica nanoparticles’ effciency in enhancing oil recovery of light and intermediate oil reservoirs. Egypt. J. Pet. 2013, 22, 427–433. [Google Scholar] [CrossRef]
- Ju, B.; Fan, T.; Ma, M. Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuol. 2006, 4, 41–46. [Google Scholar] [CrossRef]
- Nezhad, S.S.K.; Cheraghian, G.; Roayaei, E.; Tabatabaee, H.; Karambeigi, M.S. Improving heavy oil recovery in the polymer flooding process by utilizing hydrophilic silica nanoparticles. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Huang, B.; Wang, C.; Zhang, W.; Fu, C.; Liu, H.; Wang, H. Study on the Stability of Produced Water from Alkali/Surfactant/Polymer Flooding under the Synergetic Effect of Quartz Sand Particles and Oil Displacement Agents. Processes 2020, 8, 315. [Google Scholar] [CrossRef]
- Cheraghian, G.; Nezhad, S.S.K. Improvement of heavy oil recovery and role of nanoparticles of clay in the surfactant flooding process. Pet. Sci. Technol. 2016, 34, 1397–1405. [Google Scholar] [CrossRef]
- Torsater, O.; Engeset, B.; Hendraningrat, L.; Suwarno, S. Improved Oil Recovery by Nanofluids Flooding: An Experimental Study. In Proceedings of the SPE Kuwait International Petroleum Conference and Exhibition, Kuwait City, Kuwait, 10–12 December 2012. [Google Scholar]
- Hendraningrat, L.; Li, S.; Torsæter, O. Enhancing oil recovery of low-permeability berea sandstone through optimized nanoûuids concentration. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 2–4 July 2013. [Google Scholar]
- Cheraghian, G.; Wu, Q.; Mostofi, M.; Li, M.-C.; Afrand, M.; Sangwai, J.S. Effect of a novel clay/silica nanocomposite on water-based drilling fluids: Improvements in rheological and filtration properties. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 339–350. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hemmati, M.; Bazgir, S. Application of TiO2 and fumed silica nanoparticles and improve the performance of drilling fluids. AIP Conf. Proc. 2014, 1590, 266–270. [Google Scholar] [CrossRef]
- Cheraghian, G. Application of Nano-Particles of Clay to Improve Drilling Fluid. Int. J. Nanosci. Nanotechnol. 2017, 13, 177–186. [Google Scholar]
- Engeset, B. The Potential of Hydrophilic Silica Nanoparticles for EOR Purposes: A Literature Review and an Experimental Study. Master’s Thesis, Norwegian University of Science and Technology, Department of Petroleum Engineering and Applied Geophysics, Trondheim, Norway, 2012. [Google Scholar]
- Cheraghian, G.; Tardasti, S. Improved Oil Recovery by the Efficiency of Nano-particle in Imbibition Mechanism. In Proceedings of the 74th EAGE Conference and Exhibition Incorporating (EUROPEC 2012), Copenhagen, Denmark, 4–7 July 2012. [Google Scholar]
- Cheraghian, G. A New Thermal Method Concept for IOR from Oil Reservoir Using Optimized In-situ Combustion. In Proceedings of the 78th EAGE Conference and Exhibition, Vienna, Austria, 30 May–2 June 2016. [Google Scholar] [CrossRef]
- Kanj, M.; Funk, J.J.; Al-Yousif, Z. Nanofluid Coreflood Experiments in the ARAB-D. In Proceedings of the SPE Saudi Arabia Section Technical Symposium, Al-Khobar, Saudi Arabia, 9–11 May 2009. [Google Scholar]
- Yuan, W.; Liu, X.; Wei, H.; Liu, J.; Yang, H.; Hu, S.; Li, Y.; Wang, D. Research and application effect of polymeric microsphere in Wen-10 of Sinopec Zhongyuan Oil ûeld. Inner Mong. Petrochem. 2010, 12, 122–126. [Google Scholar]
- Li, X.; Ying, Z.; Jia, Y.; Liu, X.; Yang, T.; Ma, L. Application of nanosphere deep proûle control and displacement technology in Chanqing oilûeld. Oil Field Chem. 2012, 29, 13–16. [Google Scholar]
- Tian, Q.Y.; Wang, L.; Tang, Y.; Liu, C.; Ma, C.; Wang, T. Research and Application of Nano Polymer Microspheres Diversion Technique of Deep Fluid. In Proceedings of the SPE International Oilfield Nanotechnology Conference and Exhibition, SPE 156999-MS, Noordwijk, The Netherlands, 12–14 June 2012. [Google Scholar]
- Shokrlu, Y.H.; Babadagli, T. Effects of Nano-Sized Metals on Viscosity Reduction of Heavy Oil/Bitumen During Thermal Applications. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AB, Canada, 19–21 October 2010. [Google Scholar]
- Skauge, T.; Hetland, S.; Spildo, K.; Skauge, A. Nano- Sized Particles for EOR, SPE 129933. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010. [Google Scholar]
- Cheraghian, G. Thermal Resistance and Application of Nanoclay on Polymer Flooding in Heavy Oil Recovery. Pet. Sci. Technol. 2015, 33, 1580–1586. [Google Scholar] [CrossRef]
- Cheraghian, G. Nanokomposit stabilisiert Bohrflüssigkeiten. Keram. Z. 2019, 71, 25. [Google Scholar] [CrossRef]
- Cheraghian, G. Improved Heavy Oil Recovery by Nanofluid Surfactant Flooding—An Experimental Study. In Proceedings of the 78th EAGE Conference and Exhibition, Vienna, Austria, 30 May–2 June 2016. [Google Scholar] [CrossRef]
- Nezhad, S.S.K.; Cheraghian, G. Mechanisms behind injecting the combination of nano-clay particles and polymer solution for enhanced oil recovery. Appl. Nanosci. 2015, 6, 923–931. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hemmati, M.; Masihi, M.; Bazgir, S. An experimental investigation of the enhanced oil recovery and improved performance of drilling fluids using titanium dioxide and fumed silica nanoparticles. J. Nanostruct. Chem. 2013, 3, 78. [Google Scholar] [CrossRef]
- Le, N.Y.T.; Pham, D.K.; Le, K.H.; Nguyen, P.T. Design and screening of synergist ic blends of SiO2 nanoparticles and surfactants for enhanced oil recovery in high-temperature reservoirs. J. Adv. Nat. Sci. 2011, 2, 35013–35019. [Google Scholar]
- Afolabi, R.O. Enhanced oil recovery for emergent energy demand: Challenges and prospects for a nanotechnology paradigm shift. Int. Nano Lett. 2019, 9, 1–15. [Google Scholar] [CrossRef]
- Mohajeri, M.; Hemmati, M.; Shekarabi, A.S. An experimental study on using a nanosurfactant in an EOR process of heavy oil in a fractured micromodel. J. Pet. Sci. Eng. 2015, 126, 162–173. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part A: Effects of nanoparticles on interfacial tension. Int. Nano Lett. 2016, 6, 129–138. [Google Scholar] [CrossRef]
- Cheraghian, G.; Nezhad, S.K. Experimental Investigation of Polymer Solutions Used in Enhanced Oil Recovery-Thermal properties Improved by Nanoclay. In Proceedings of the 77th EAGE Conference and Exhibition, Madrid, Spain, 1–4 June 2015. [Google Scholar]
- Cheraghian, G. Synthesis and properties of polyacrylamide by nanoparticles, effect nanoclay on stability polyacrylamide solution. Micro Nano Lett. 2017, 12, 40–44. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Ahadian, M.M.; Taghikhani, V. Enhanced Heavy Oil Recovery Using TiO2 Nanoparticles: Investigation of Deposition during Transport in Core Plug. Energy Fuels 2015, 29, 1–8. [Google Scholar] [CrossRef]
- Shah, R.D. Application of Nanoparticle Saturated Injectant Gases for EOR of Heavy Oils. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 4–7 October 2009; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2009. [Google Scholar]
- Kanj, M.; Rashid, H.; Giannelis, E. Industry First Field Trial of Reservoir Nanoagents. In Proceedings of the SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September 2011; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2011. [Google Scholar]
- Wang, Y.; Lu, F.; Li, Y.; Wu, T.; Sun, D.; Zhang, G.; Huang, X.; Wang, G. Effects of Na–montmorillonite particles on the emulsification stability of polymer flooding produced water. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 125–129. [Google Scholar] [CrossRef]
- Qi, L.; Song, C.; Wang, T.; Li, Q.; Hirasaki, G.J.; Verduzco, R. Polymer-Coated Nanoparticles for Reversible Emulsification and Recovery of Heavy Oil. Langmuir 2018, 34, 6522–6528. [Google Scholar] [CrossRef]
- Qi, L.; ShamsiJazeyi, H.; Ruan, G.; Mann, J.A.; Lin, Y.-H.; Song, C.; Ma, Y.; Wang, L.; Tour, J.M.; Hirasaki, G.J.; et al. Segregation of Amphiphilic Polymer-Coated Nanoparticles to Bicontinuous Oil/Water Microemulsion Phases. Energy Fuels 2017, 31, 1339–1346. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Torsæter, O. Metal oxide-based nanoparticles: Revealing their potential to enhance oil recovery in different wettability systems. Appl. Nanosci. 2015, 5, 181–199. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Kharrat, R.; Barati, N. Enhancement of surfactant flooding performance by the use of silica nanoparticles. Fuel 2015, 143, 21–27. [Google Scholar] [CrossRef]
- Kim, B.-J.; Kang, K.-S. Fabrication of a Crack-Free Large Area Photonic Crystal with Colloidal Silica Spheres Modified with Vinyltriethoxysilane. Cryst. Growth Des. 2012, 12, 4039–4042. [Google Scholar] [CrossRef]
- Ye, Z.; Qin, X.; Lai, N.; Peng, Q.; Li, X.; Li, C. Synthesis and Performance of an Acrylamide Copolymer Containing Nano-SiO2 as Enhanced Oil Recovery Chemical. J. Chem. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Hendraningrat, L.; Zhang, J. Polymeric nanospheres as a displacement fluid in enhanced oil recovery. Appl. Nanosci. 2015, 5, 1009–1016. [Google Scholar] [CrossRef]
- Cheraghian, G.; Nezhad, S.S.K. Effect of Nanoclay on Heavy Oil Recovery During Polymer Flooding. Pet. Sci. Technol. 2015, 33, 999–1007. [Google Scholar] [CrossRef]
- Saha, R.; Uppaluri, R.V.S.; Tiwari, P. Impact of Natural Surfactant (Reetha), Polymer (Xanthan Gum), and Silica Nanoparticles to Enhance Heavy Crude Oil Recovery. Energy Fuels 2019, 33, 4225–4236. [Google Scholar] [CrossRef]
- Joonaki, E.; Ghanaatian, S. The Application of Nanofluids for Enhanced Oil Recovery: Effects on Interfacial Tension and Coreflooding Process. Pet. Sci. Technol. 2014, 32, 2599–2607. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. An Experimental Investigation of Silica Nanoparticles Effect on the Rheological Behavior of Polyacrylamide Solution to Enhance Heavy Oil Recovery. Pet. Sci. Technol. 2013, 31, 500–508. [Google Scholar] [CrossRef]
- Rahimi, K.; Adibifard, M. Experimental Study of the Nanoparticles Effect on Surfactant Absorption and Oil Recovery in One of the Iranian Oil Reservoirs. Pet. Sci. Technol. 2014, 33, 79–85. [Google Scholar] [CrossRef]
- Sharma, T.; Velmurugan, N.; Patel, P.; Chon, B.H.; Sangwai, J.S. Use of oil-inwater pickering emulsion stabilized by nanoparticles in combination with polymer flood for enhanced oil recovery. Pet. Sci. Technol. 2015, 33, 1595–1604. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Effect of Hydrophobic and Hydrophilic Metal Oxide Nanoparticles on the Performance of Xanthan Gum Solutions for Heavy Oil Recovery. Nanomaterials 2019, 9, 94. [Google Scholar] [CrossRef]
- Giraldo, J.; Benjumea, P.; Lopera, S.; Cortés, F.B.; Ruiz, M.A. Wettability Alteration of Sandstone Cores by Alumina-Based Nanofluids. Energy Fuels 2013, 27, 3659–3665. [Google Scholar] [CrossRef]
- El-Diasty, A.I.; Aly, A.M. Understanding the Mechanism of Nanoparticles Applications in Enhanced Oil Recovery. In Proceedings of the SPE North Africa Technical Conference and Exhibition, Cairo, Egypt, 14–16 September 2015; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2015. [Google Scholar]
- Cheraghian, G. An Experimental Study of Surfactant Polymer for Enhanced Heavy Oil Recovery Using a Glass Micromodel by Adding Nanoclay. Pet. Sci. Technol. 2015, 33, 1410–1417. [Google Scholar] [CrossRef]
- Karimi, A.; Fakhroueian, Z.; Bahramian, A.; Pour Khiabani, N.; Darabad, J.B.; Azin, R.; Arya, S. Wettability Alteration in Carbonates using Zirconium Oxide Nanofluids: EOR Implications. Energy Fuels 2012, 26, 1028–1036. [Google Scholar] [CrossRef]
- Sagala, F.; Montoya, T.; Hethnawi, A.; Vitale, G.; Nassar, N.N. Nanopyroxene-Based Nanofluids for Enhanced Oil Recovery in Sandstone Cores at Reservoir Temperature. Energy Fuels 2019, 33, 877–890. [Google Scholar] [CrossRef]
- Chandran, K. Multiwall Carbon Nanotubes (MWNT) Fluid in EOR Using Core Flooding Method under the Presence of Electromagnetic Waves; Universiti Teknologi PETRONAS: Perak, Malaysia, 2013. [Google Scholar]
- Cheraghian, G.; Kiani, S.; Nassar, N.N.; Alexander, S.; Barron, A.R. Silica Nanoparticle Enhancement in the Efficiency of Surfactant Flooding of Heavy Oil in a Glass Micromodel. Ind. Eng. Chem. Res. 2017, 56, 8528–8534. [Google Scholar] [CrossRef]
- Moghaddam, R.N.; Bahramian, A.; Fakhroueian, Z.; Karimi, A.; Arya, S. Comparative Study of Using Nanoparticles for Enhanced Oil Recovery: Wettability Alteration of Carbonate Rocks. Energy Fuels 2015, 29, 2111–2119. [Google Scholar] [CrossRef]
- Cheraghian, G. Effect of nano titanium dioxide on heavy oil recovery during polymer flooding. Pet. Sci. Technol. 2016, 34, 633–641. [Google Scholar] [CrossRef]
- Jafarnezhad, M.; Giri, M.S.; Alizadeh, M. Impact of SnO2 nanoparticles on enhanced oil recovery from carbonate media. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 39, 121–128. [Google Scholar] [CrossRef]
- Ogolo, N.A.; Olafuyi, O.A.; Onyekonwu, M.O. Enhanced oil recovery using nanoparticles. In Proceedings of the SPE 160847-MS, SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 8–11 April 2012. [Google Scholar]
- Alnarabiji, M.S.; Yahya, N.; Shafie, A.; Solemani, H.; Chandran, K.; Hamid, S.B.A.; Azizi, K. The Influence of Hydrophobic Multiwall Carbon Nanotubes Concentration on Enhanced Oil Recovery. Procedia Eng. 2016, 148, 1137–1140. [Google Scholar] [CrossRef]
- Li, Y.; Dai, C.; Zhou, H.; Wang, X.; Lv, W.; Wu, Y.; Zhao, M. A Novel Nanofluid Based on Fluorescent Carbon Nanoparticles for Enhanced Oil Recovery. Ind. Eng. Chem. Res. 2017, 56, 12464–12470. [Google Scholar] [CrossRef]
- Corredor, L.M.; Husein, M.M.; Maini, B.B. Impact of PAM-Grafted Nanoparticles on the Performance of Hydrolyzed Polyacrylamide Solutions for Heavy Oil Recovery at Different Salinities. Ind. Eng. Chem. Res. 2019, 58, 9888–9899. [Google Scholar] [CrossRef]
- Rellegadla, S.; Bairwa, H.K.; Kumari, M.R.; Prajapat, G.; Nimesh, S.; Pareek, N.; Jain, S.; Agarwal, A. An Effective Approach for Enhanced Oil Recovery Using Nickel Nanoparticles Assisted Polymer Flooding. Energy Fuels 2018, 32, 11212–11221. [Google Scholar] [CrossRef]
- Behzadi, A.; Mohammadi, A. Environmentally responsive surface-modified silica nanoparticles for enhanced oil recovery. J. Nanopart. Res. 2016, 18, 266. [Google Scholar] [CrossRef]
- Rosen, M.J.; Wang, H.; Shen, P.; Zhu, Y. Ultralow Interfacial Tension for Enhanced Oil Recovery at Very Low Surfactant Concentrations. Langmuir 2005, 21, 3749–3756. [Google Scholar] [CrossRef]
- Qiu, F. The Potential Applications in Heavy Oil EOR With the Nanoparticle and Surfactant Stabilized Solvent-Based Emulsion. In Proceedings of the Canadian Unconventional Resources and International Petroleum Conference, Calgary, AL, Canada, 19–21 October 2010; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2010. [Google Scholar]
- Suleimanov, B.; Ismailov, F.; Veliyev, E.F. Nanofluid for enhanced oil recovery. J. Pet. Sci. Eng. 2011, 78, 431–437. [Google Scholar] [CrossRef]
- Parsa, S.M.; Rahbar, A.; Koleini, M.; Aberoumand, S.; Afrand, M.; Amidpour, M. A renewable energy-driven thermoelectric-utilized solar still with external condenser loaded by silver/nanofluid for simultaneously water disinfection and desalination. Desalination 2020, 480, 114354. [Google Scholar] [CrossRef]
- Cheraghian, G. Evaluation of Clay and Fumed Silica Nanoparticles on Adsorption of Surfactant Polymer during Enhanced Oil Recovery. J. Jpn. Pet. Inst. 2017, 60, 85–94. [Google Scholar] [CrossRef]
- Cheraghian, G. Effects of nanoparticles on wettability: A review on applications of nanotechnology in the enhanced Oil recovery. Int. J. Nano Dimens. 2015, 6, 443–452. [Google Scholar]
- Khalilinezhad, S.S.; Mobaraki, S.; Zakavi, M.; Sorkhabadi, M.O.; Cheraghian, G.; Jarrahian, K. Mechanistic Modeling of Nanoparticles-Assisted Surfactant Flood. Arab. J. Sci. Eng. 2018, 43, 6609–6625. [Google Scholar] [CrossRef]
- Munshi, A.M.; Singh, V.; Kumar, M.; Singh, J.P. Effect of nanoparticle size on sessile droplet contact angle. J. Appl. Phys. 2008, 103, 84315. [Google Scholar] [CrossRef]
- Kumar, G.; Kakati, A.; Mani, E.; Sangwai, J.S. Nanoparticle Stabilized Solvent-Based Emulsion for Enhanced Heavy Oil Recovery. In Proceedings of the SPE Canada Heavy Oil Conference, Calgary, AL, Canada, 13–14 March 2018; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2018. [Google Scholar]
- Ravera, F.; Santini, E.; Loglio, G.; Ferrari, A.M.; Liggieri, L. Effect of Nanoparticles on the Interfacial Properties of Liquid/Liquid and Liquid/Air Surface Layers. J. Phys. Chem. B 2006, 110, 19543–19551. [Google Scholar] [CrossRef]
- Xu, D.; Bai, B.; Wu, H.; Hou, J.; Meng, Z.; Sun, R.; Li, Z.; Lu, Y.; Kang, W. Mechanisms of imbibition enhanced oil recovery in low permeability reservoirs: Effect of IFT reduction and wettability alteration. Fuel 2019, 244, 110–119. [Google Scholar] [CrossRef]
- Zargartalebi, M.; Barati, N.; Kharrat, R. Influences of hydrophilic and hydrophobic silica nanoparticles on anionic surfactant properties: Interfacial and adsorption behaviors. J. Pet. Sci. Eng. 2014, 119, 36–43. [Google Scholar] [CrossRef]
- Betancur, S.; Olmos, C.M.; Pérez, M.S.; Lerner, B.; Franco, C.A.; Riazi, M.; Gallego, J.; Carrasco-Marín, F.; Cortés, F.B. A microfluidic study to investigate the effect of magnetic iron core-carbon shell nanoparticles on displacement mechanisms of crude oil for chemical enhanced oil recovery. J. Pet. Sci. Eng. 2020, 184, 106589. [Google Scholar] [CrossRef]
- Moslana, M.S.; Sulaiman, W.R.W.; Ismaila, A.R.; Jaafara, M.Z. Applications of Aluminium Oxide and Zirconium Oxide Nanoparticles in Altering Dolomite Rock Wettability using Different Dispersing Medium. Chem. Eng. 2017, 56, 1339–1344. [Google Scholar]
- Sagala, F.; Hethnawi, A.; Nassar, N.N. Hydroxyl-functionalized silicate-based nanofluids for enhanced oil recovery. Fuel 2020, 269, 117462. [Google Scholar] [CrossRef]
- Esmaeilzadeh, P.; Hosseinpour, N.; Bahramian, A.; Fakhroueian, Z.; Arya, S. Effect of ZrO2 nanoparticles on the interfacial behavior of surfactant solutions at air–water and n-heptane–water interfaces. Fluid Phase Equilibria 2014, 361, 289–295. [Google Scholar] [CrossRef]
- Ali, J.A.; Kolo, K.; Manshad, A.K.; Stephen, K.D. Potential application of low-salinity polymeric-nanofluid in carbonate oil reservoirs: IFT reduction, wettability alteration, rheology and emulsification characteristics. J. Mol. Liq. 2019, 284, 735–747. [Google Scholar] [CrossRef]
- Manshad, A.; Ali, J.; Imani, I.; Sajadi, S.-M.; Tayeb, N.; Keshavarz, A. Green Synthesize of CuO@Fe3O4@Xantan Nanocomposites and Its Application in Enhanced Oil Recovery by considering IFT and Wettability Behaviors. Micro Nano Lett. 2020. [Google Scholar] [CrossRef]
- Rostami, S.; Aghakhani, S.; Hajatzadeh Pordanjani, A.; Afrand, M.; Cheraghian, G.; Oztop, H.F.; Shadloo, M.S. A Review on the Control Parameters of Natural Convection in Different Shaped Cavities with and without Nanofluid. Processes 2020, 8, 1011. [Google Scholar] [CrossRef]
- Kuang, W.; Saraji, S.; Piri, M. Pore-Scale Sweep Efficiency Enhancement by Silica-Based Nanofluids in Oil-Wet Sandstone. Energy Fuels 2019, 34, 1297–1308. [Google Scholar] [CrossRef]
- Ju, B.; Fan, T. Experimental study and mathematical model of nanoparticle transport in porous media. Powder Technol. 2009, 192, 195–202. [Google Scholar] [CrossRef]
- Onyekonwu, M.O.; Ogolo, N.A. Investigating the Use of Nanoparticles in Enhancing Oil Recovery. Paper No. 140744-MS. In Proceedings of the Nigerian Annual International Conference and Exhibition, Calabar, Nigeria, 31 July–7 August 2010; Society of Petroleum Engineers (SPE): Richardson, TX, USA, 2010. [Google Scholar]
- Roustaei, A.; Bagherzadeh, H. Experimental investigation of SiO2 nanoparticles on enhanced oil recovery of carbonate reservoirs. J. Pet. Explor. Prod. Technol. 2015, 5, 27–33. [Google Scholar] [CrossRef]
- Safari, M. Variations in Wettability Caused by Nanoparticles. Pet. Sci. Technol. 2014, 32, 1505–1511. [Google Scholar] [CrossRef]
- Bishan, J.; Tailang, F.; Mingxua, M. Enhanced oil recovery by flooding with hydrophilic nanoparticles. China Particuol. 2005, 4, 41–46. [Google Scholar]
- Wu, S.; Firoozabadi, A. Permanent Alteration of Porous Media Wettability from Liquid-Wetting to Intermediate Gas-Wetting. Transp. Porous Media 2010, 85, 189–213. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Giese, R.F. The hydrophilicity and hydrophobilicity of clay minerals. Clays Clay Miner. 1995, 43, 474–477. [Google Scholar] [CrossRef]
- Maghzi, A.; Mohebbi, A.; Kharrat, R.; Ghazanfari, M.H. Pore-Scale Monitoring of Wettability Alteration by Silica Nanoparticles During Polymer Flooding to Heavy Oil in a Five-Spot Glass Micromodel. Transp. Porous Media 2011, 87, 653–664. [Google Scholar] [CrossRef]
- Rostami, P.; Sharifi, M.; Aminshahidy, B.; Fahimpour, J. The effect of nanoparticles on wettability alteration for enhanced oil recovery: Micromodel experimental studies and CFD simulation. Pet. Sci. 2019, 16, 859–873. [Google Scholar] [CrossRef]
- Ershadi, M.; Alaei, M.; Rashidi, A.; Ramazani, A.; Khosravani, S. Carbonate and sandstone reservoirs wettability improvement without using surfactants for Chemical Enhanced Oil Recovery (C-EOR). Fuel 2015, 153, 408–415. [Google Scholar] [CrossRef]
- Parsa, S.M.; Rahbar, A.; Koleini, M.H. First approach on nanofluid-based solar still in high altitude for water desalination and solar water disinfection (SODIS). Desalination 2020, 491, 114592. [Google Scholar] [CrossRef]
- Cheraghian, G. Application of nano-fumed silica in heavy oil recovery. Pet. Sci. Technol. 2016, 34, 12–18. [Google Scholar] [CrossRef]
- Cheraghian, G. Effects of titanium dioxide nanoparticles on the efficiency of surfactant flooding of heavy oil in a glass micromodel. Pet. Sci. Technol. 2016, 34, 260–267. [Google Scholar] [CrossRef]
- Cheraghian, G.; Hendraningrat, L. A review on applications of nanotechnology in the enhanced oil recovery part B: Effects of nanoparticles on flooding. Int. Nano Lett. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Cheraghian, G.; Khalili Nezhad, S.S.; Bazgir, S. Improvement of thermal stability of polyacryl amide solution used as a nano-fluid in enhanced oil recovery process by nanoclay. Int. J. Nanosci. Nanotechnol. 2015, 11, 201–208. [Google Scholar]
- Mohammadi, M.; Moghadasi, J.; Naseri, S. An Experimental Investigation of Wettability Alteration in Carbonate Reservoir Using γ-Al2O3 Nanoparticles. Iran. J. Oil Gas Sci. Technol. 2014, 3, 18–26. [Google Scholar]
- Hendraningrat, L.; Torsæter, O. Experimental Investigations of Wettability Alteration Due to Various Nanoparticles: An EOR Implication with Nanofluids. In Proceedings of the Paper SCA2014-082 Presented at the International Symposium of the Society of Core Analysts, Avignon, France, 8–11 September 2014. [Google Scholar]
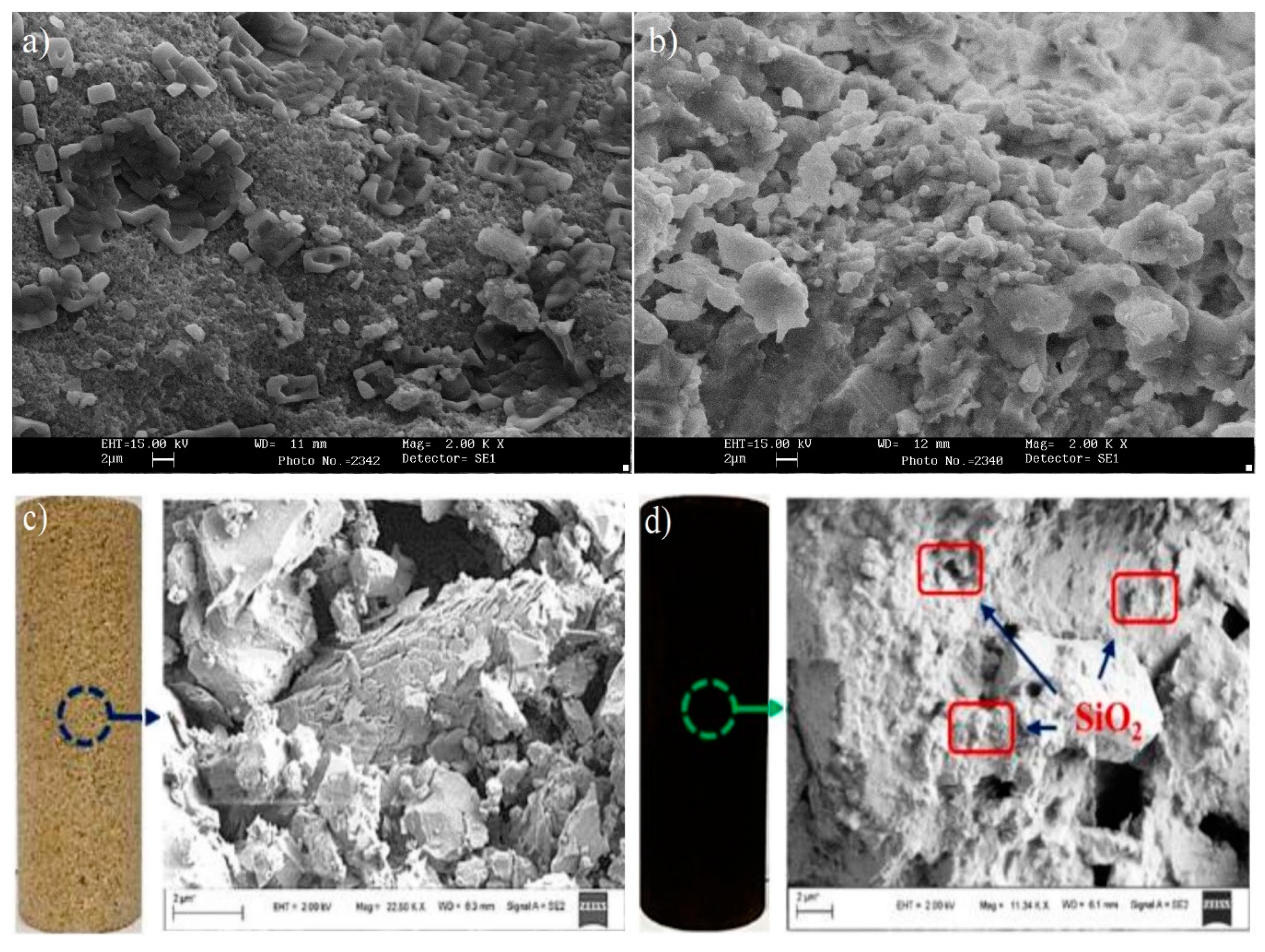
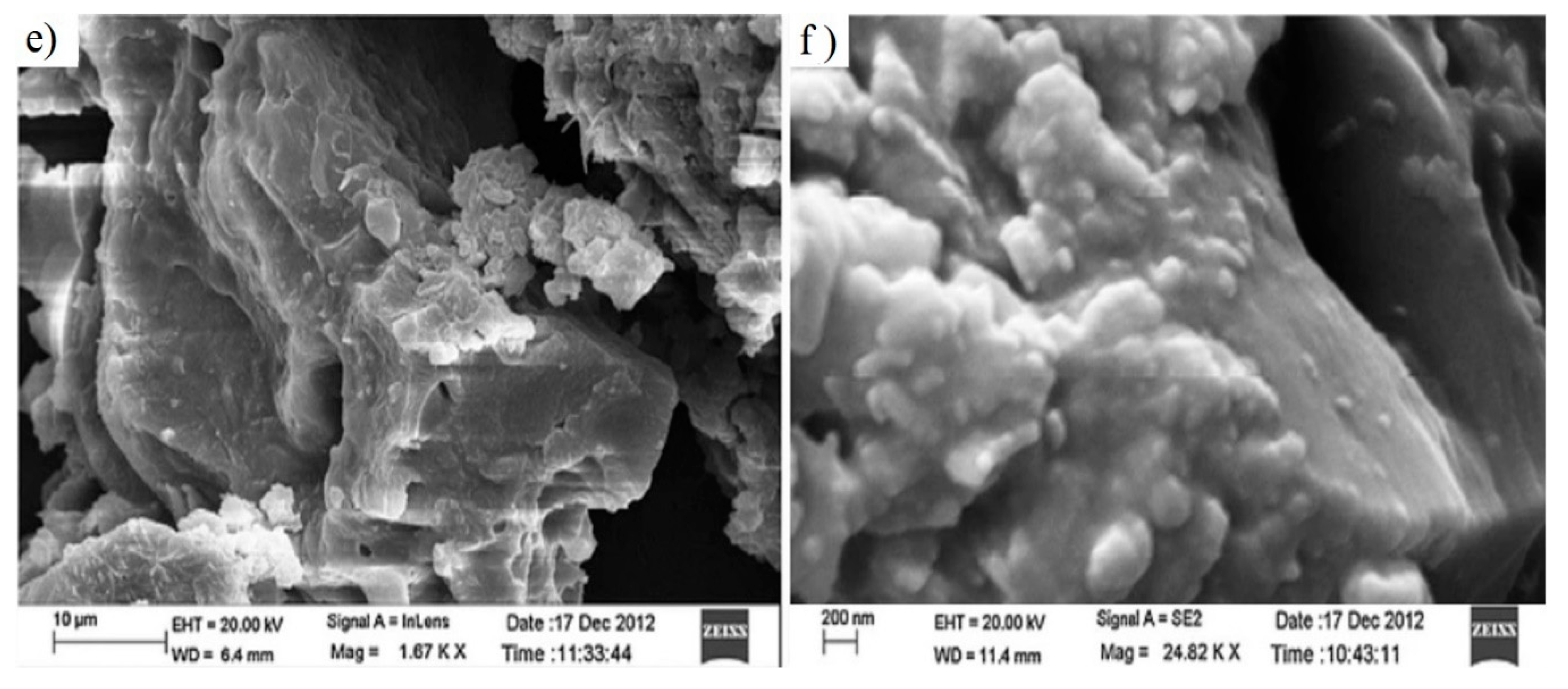
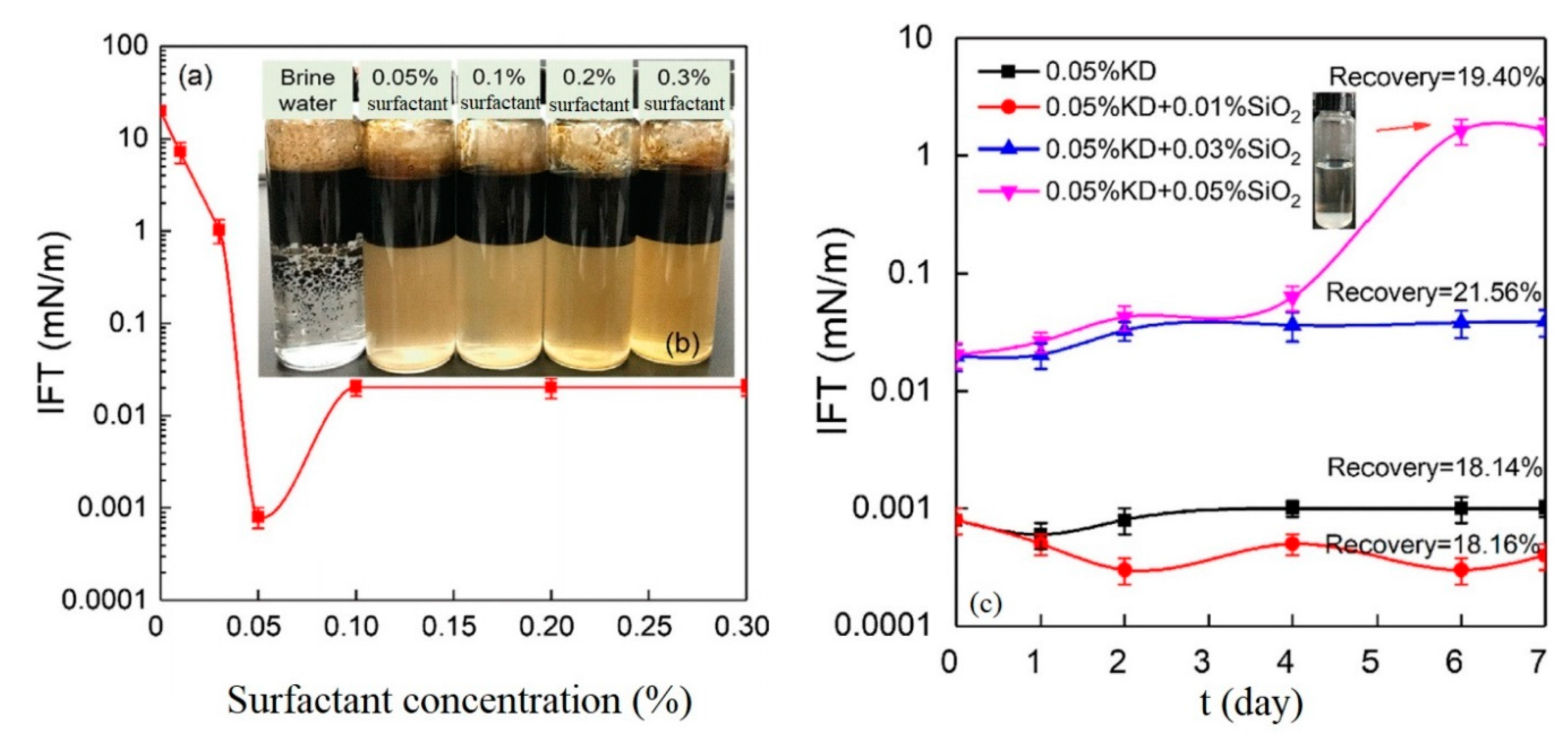
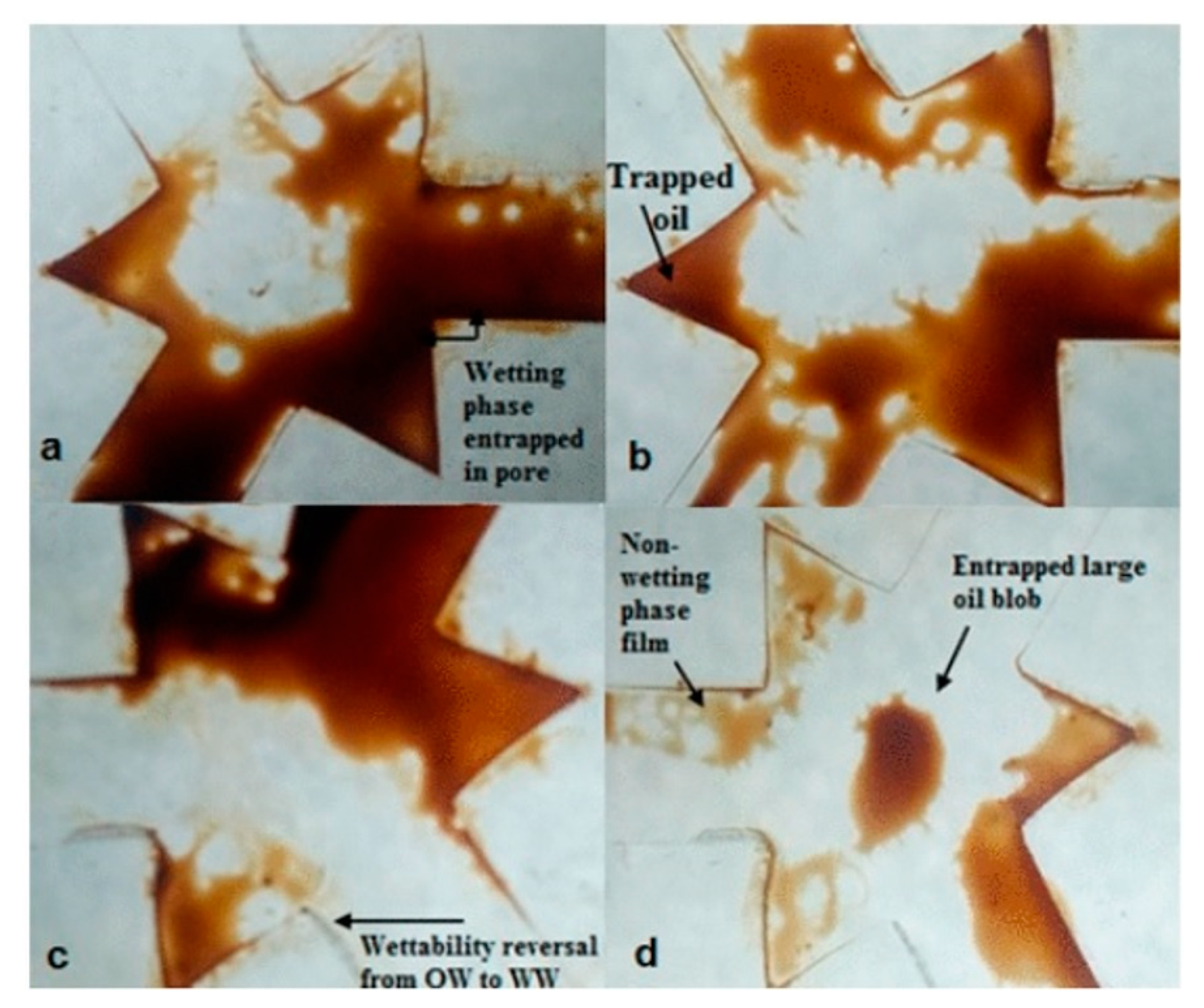
| Type of NP | NP Concentration | Additive | Incremental Oil Recovery | Remarkable Findings | Ref. |
|---|---|---|---|---|---|
| SiO2 | 1 wt.% | Polyacrylamide | 10% | Increased solution viscosity and thermal stability of polymer solution | [52] |
| SiO2, Fe(OH)3, TiO2, Al2O3 | 0.2 wt.% | Xanthan gum | 1–9% | Improved solution viscosity | [53] |
| Al2O3 | 0.05 wt.% | Anionic surfactant | 12.5% | Wettability alteration | [54] |
| SiO2 | 0.01–3 wt.% | - | 9–19% | Improved oil displacement in porous media | [55] |
| Clay | 0.9 wt.% | Polyacrylamide | 5–6% | Improved rheological properties | [56] |
| ZrO2 | 0.1 g/cc | Cetrimonium bromide surfactant | 40% | Wettability alteration | [57] |
| Pyroxene | 1 wt.% | - | 10.5% | Reduced IFT and altered contact angle | [58] |
| Carbon | 1 wt.% | - | 24.5% | Reduced oil viscosity | [59] |
| Fumed silica | 2 wt.% | Sodium dodecyl sulfate surfactant | 13% | Increased solution viscosity | [60] |
| CeO2, ZrO2, Al2O3, TiO2, CNT, MgO, CaCO3, SiO2 | 5 wt.% | - | 8–9% | Increased solution viscosity | [61] |
| TiO2 | 1.9–2.5 wt.% | Sodium dodecyl sulfate surfactant | 4% | Improved rheological properties | [62] |
| SnO2 | 2 wt.% | - | 22% | Reduced IFT | [63] |
| ZnO | 1.5 wt.% | - | 11% | Change in rock wettability, reduced IFT, oil viscosity, mobility ratio and permeability alterations | [64] |
| Carbon | 0.1 wt.% | - | 32% | Wettability alteration | [65] |
| Fluorescent carbon | 0.05 wt.% | - | 22% | Reduced IFT | [66] |
| SiO2 | 0.3 wt.% | Xanthan gum polymer-surfactant | 27% | Increased solution viscosity | [48] |
| SiO2, TiO2, Al2O3 | 0.2–0.4 wt.% | HPAM | 7% | Improved rheological properties | [67] |
| Nickel | 0.005–0.02 wt.% | Xanthan gum | 6% | Increased solution viscosity | [68] |
| SiO2 | 1 wt.% | Polyethylene glycol | 8% | Improved rheological properties | [69] |
| Type of NPs | NPs Concentration | IFT (mN/m) | Reference |
|---|---|---|---|
| SiO2 | 0.05 wt.% | 17.5 | [42] |
| Al2O3 | 0.5–3 g/L | 3.5 | [49] |
| Fe2O3 | 3 g/L | 2.7 | [49] |
| CNP | 0.1 wt.% | 13.4 | [66] |
| SiO2 | 1 wt.% | 15 | [81] |
| Iron core–carbon shell | 100 mg L−1 | 2.7 | [82] |
| Al2O3 | 0.05 wt.% | 23.1 | [82] |
| ZrO2 | 0.05 wt.% | 6.6 | [83] |
| Hydroxylated nanopyroxene | 50 ppm | 10 | [84] |
| ZrO2 | 10–500 mg/L | 2.5 | [85] |
| ZnO/SiO2 | 2000 ppm | 29.5 | [86] |
| CuO/Fe3O4 | 2000 ppm | 4.5 | [87] |
| Type of NP | NP Concentration | Initial Contact Angle (°) | Difference in Contact Angle (°) | Reference |
|---|---|---|---|---|
| SiO2 | 0.05 wt.% | 29 | 28 | [42] |
| Al2O3 | 0.5–3 g/L | 134 | 39 | [49] |
| Fe2O3 | 3 g/L | 134 | 31 | [49] |
| Al2O3 | 1000 ppm | 141 | 32 | [54] |
| Fumed silica | 2.2 wt.% | 102 | 92 | [60] |
| MgO | 0.005 g/ml | 39 | 42 | [61] |
| CNP | 0.1 wt.% | 35 | 85 | [66] |
| Al2O3 | 0.05 wt.% | 110 | 70 | [82] |
| Iron core–carbon shell | 100 mg L−1 | 108 | 76 | [82] |
| ZrO2 | 0.05 wt.% | 70 | 10 | [83] |
| Hydroxylated nanopyroxene | 50 ppm | 76 | 52 | [84] |
| ZnO/SiO2 | 2000 ppm | 132 | 98 | [86] |
| CuO/Fe3O4 | 2000 ppm | 135 | 61 | [87] |
| γ-Al2O3 | 0.5 wt.% | 80 | 11 | [105] |
| SiO2 | 0.05 wt.% | 39 | 28 | [106] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheraghian, G.; Rostami, S.; Afrand, M. Nanotechnology in Enhanced Oil Recovery. Processes 2020, 8, 1073. https://doi.org/10.3390/pr8091073
Cheraghian G, Rostami S, Afrand M. Nanotechnology in Enhanced Oil Recovery. Processes. 2020; 8(9):1073. https://doi.org/10.3390/pr8091073
Chicago/Turabian StyleCheraghian, Goshtasp, Sara Rostami, and Masoud Afrand. 2020. "Nanotechnology in Enhanced Oil Recovery" Processes 8, no. 9: 1073. https://doi.org/10.3390/pr8091073
APA StyleCheraghian, G., Rostami, S., & Afrand, M. (2020). Nanotechnology in Enhanced Oil Recovery. Processes, 8(9), 1073. https://doi.org/10.3390/pr8091073






