Plasma Agriculture from Laboratory to Farm: A Review
Abstract
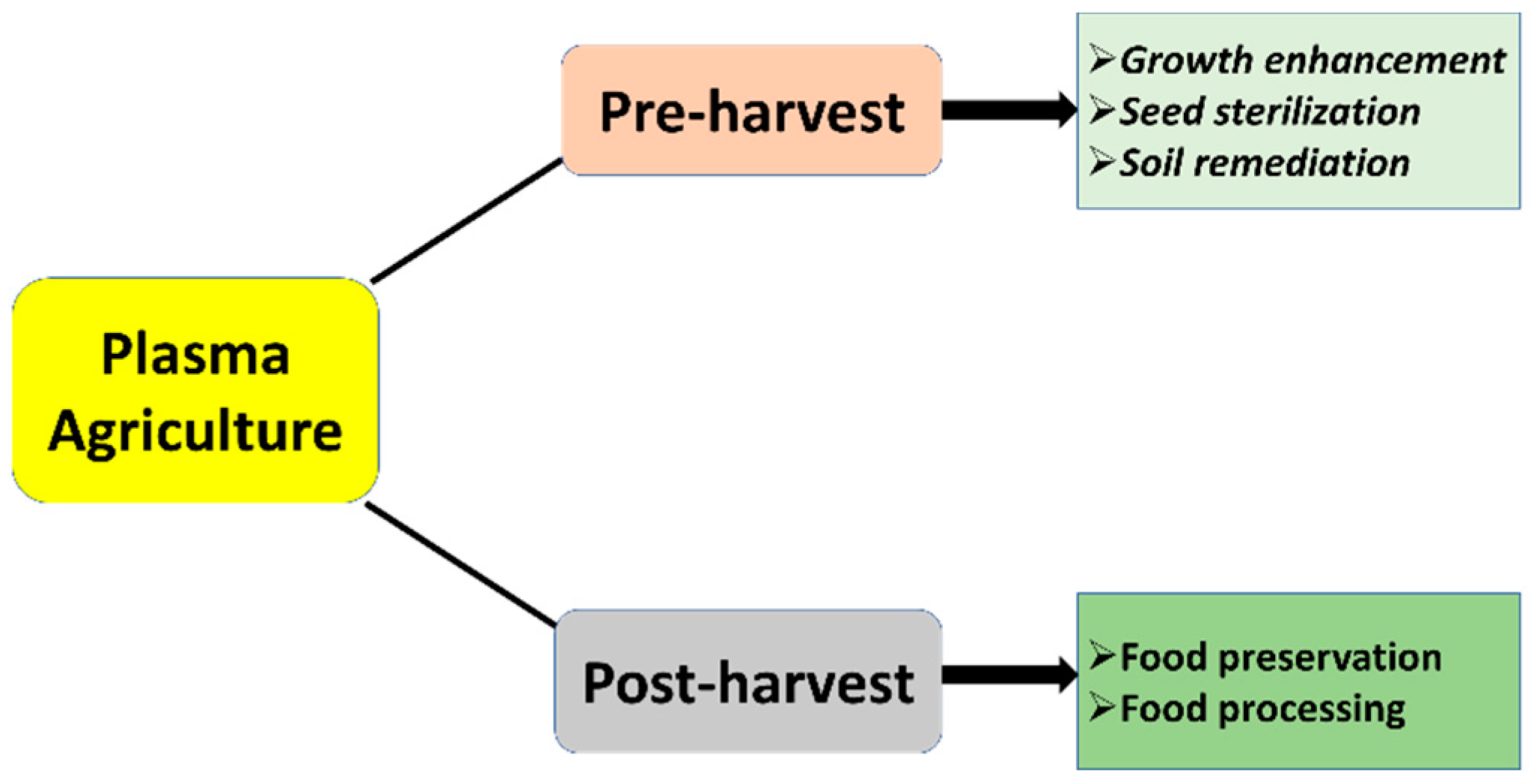
1. Introduction
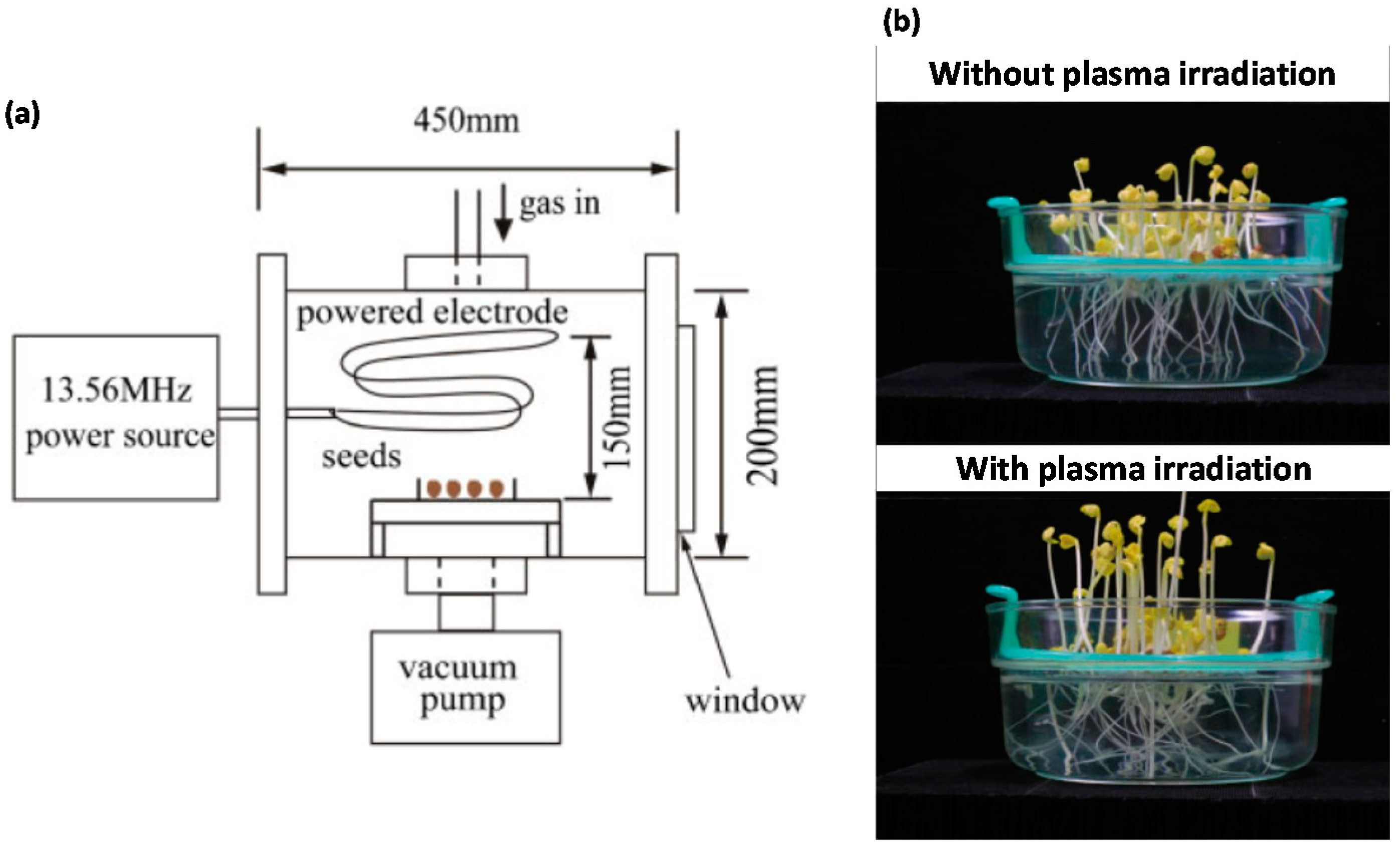
2. Effect of NTP at Low/Medium (≈6.7 × 10−2 to 53,328 Pa) Pressure on Seed Germination and Growth Enhancement
3. Effect of NTP at Atmospheric Pressure on Seed Germination and Growth Enhancement
4. Effect of Plasma Treated Water on Seed Germination and Growth Enhancement
5. Patents Related to Seed Germination and Seed Growth Using Low-Pressure/Medium-Pressure/Atmospheric-Pressure Plasma
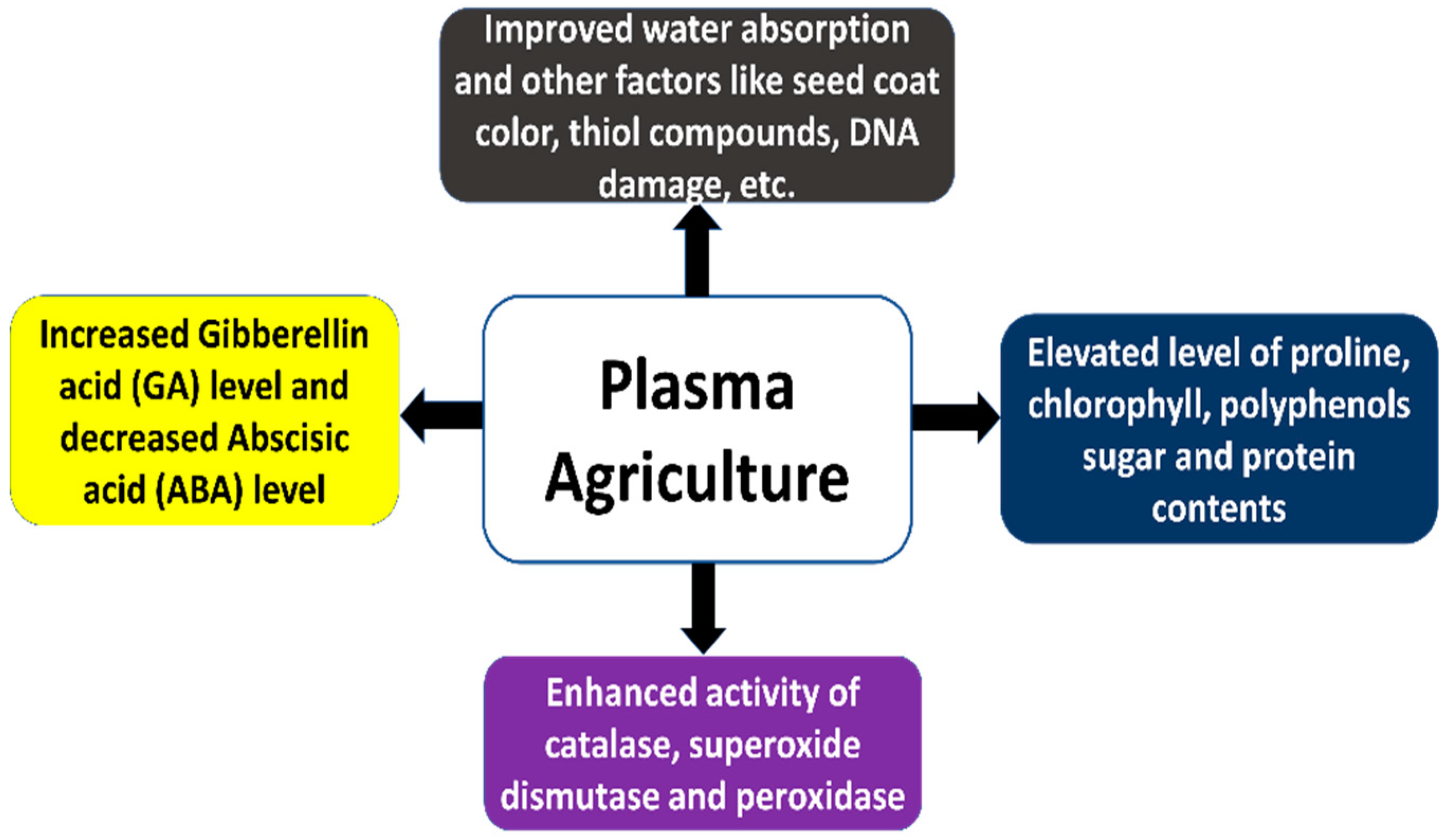
6. Probable Mechanism and Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Heydecker, W.; Coolbear, P. Seed treatments for improved performance survey and attempted prognosis. Seed Sci. Technol. 1977, 5, 353–426. [Google Scholar]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Halmer, P. Seed Technology and Seed Enhancement. Acta Hortic. 2008, 771, 17–26. [Google Scholar] [CrossRef]
- Elsayed, B.B.; Hassan, M.M.; El Ramady, H.R. Phylogenetic and characterization of salt-tolerant rhizobial strain nodulating faba bean plants. Afr. J. Biotechnol. 2013, 12, 4324–4337. [Google Scholar] [CrossRef]
- Araújo, S.D.; Paparella, S.; Dondi, D.; Bentivoglio, A.; Carbonera, D.; Balestrazzi, A. Physical methods for seed invigoration: Advantages and challenges in seed technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef]
- Šerá, B.; Špatenka, P.; Šerý, M.; Vrchotová, N.; Hrušková, I. Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Dubinov, A.E.; Lazarenko, E.M.; Selemir, V.D. Effect of glow discharge air plasma on grain crops seed. IEEE Trans. Plasma Sci. 2000, 28, 180–183. [Google Scholar] [CrossRef]
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M.T. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Straňák, V.; Tichý, M.; Kříha, V.; Scholtz, V.; Šerá, B.; Špatenka, P. Technological applications of surfatron produced discharge. J. Optoelectron. Adv. Mater. 2007, 9, 852–857. [Google Scholar]
- Attri, P.; Razzokov, J.; Yusupov, M.; Koga, K.; Shiratani, M.; Bogaerts, A. Influence of osmolytes and ionic liquids on the Bacteriorhodopsin structure in the absence and presence of oxidative stress: A combined experimental and computational study. Int. J. Biol. Macromol. 2020, 148, 657–665. [Google Scholar] [CrossRef]
- Attri, P.; Kim, M.; Choi, E.H.; Cho, A.E.; Koga, K.; Shiratani, M. Impact of an ionic liquid on protein thermodynamics in the presence of cold atmospheric plasma and gamma rays. Phys. Chem. Chem. Phys. 2017, 19, 25277–25288. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Kim, M.; Sarinont, T.; Ha Choi, E.; Seo, H.; Cho, A.E.; Koga, K.; Shiratani, M. The protective action of osmolytes on the deleterious effects of gamma rays and atmospheric pressure plasma on protein conformational changes. Sci. Rep. 2017, 7, 8698. [Google Scholar] [CrossRef] [PubMed]
- Hoon Park, J.; Kumar, N.; Hoon Park, D.; Yusupov, M.; Neyts, E.C.; Verlackt, C.C.W.; Bogaerts, A.; Ho Kang, M.; Sup Uhm, H.; Ha Choi, E.; et al. A comparative study for the inactivation of multidrug resistance bacteria using dielectric barrier discharge and nano-second pulsed plasma. Sci. Rep. 2015, 5, 13849. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Kim, Y.H.; Park, D.H.; Park, J.H.; Hong, Y.J.; Uhm, H.S.; Kim, K.-N.; Fridman, A.; Choi, E.H. Generation mechanism of hydroxyl radical species and its lifetime prediction during the plasma-initiated ultraviolet (UV) photolysis. Sci. Rep. 2015, 5, 9332. [Google Scholar] [CrossRef]
- Attri, P.; Han, J.; Choi, S.; Choi, E.H.; Bogaerts, A.; Lee, W. CAP modifies the structure of a model protein from thermophilic bacteria: Mechanisms of CAP-mediated inactivation. Sci. Rep. 2018, 8, 10218. [Google Scholar] [CrossRef]
- Choi, S.; Attri, P.; Lee, I.; Oh, J.; Yun, J.-H.; Park, J.H.; Choi, E.H.; Lee, W. Structural and functional analysis of lysozyme after treatment with dielectric barrier discharge plasma and atmospheric pressure plasma jet. Sci. Rep. 2017, 7, 1027. [Google Scholar] [CrossRef]
- Attri, P.; Bogaerts, A. Perspectives of Plasma-treated Solutions as Anticancer Drugs. Anticancer Agents Med. Chem. 2019, 19, 436–438. [Google Scholar] [CrossRef]
- Attri, P. Cold Atmospheric Plasma Activated Solution: A New Approach for Cancer Treatment. Anticancer Agents Med. Chem. 2018, 18, 768. [Google Scholar] [CrossRef]
- Attri, P.; Choi, E.H. Influence of Reactive Oxygen Species on the Enzyme Stability and Activity in the Presence of Ionic Liquids. PLoS ONE 2013, 8, e75096. [Google Scholar] [CrossRef]
- Attri, P.; Sarinont, T.; Kim, M.; Amano, T.; Koga, K.; Cho, A.E.; Choi, E.H.; Shiratani, M. Influence of ionic liquid and ionic salt on protein against the reactive species generated using dielectric barrier discharge plasma. Sci. Rep. 2015, 5, 17781. [Google Scholar] [CrossRef]
- Attri, P.; Park, J.H.; Ali, A.; Choi, E.H. How Does Plasma Activated Media Treatment Differ From Direct Cold Plasma Treatment? Anticancer Agents Med. Chem. 2018, 18, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Attri, P.; Tochikubo, F.; Park, J.H.; Choi, E.H.; Koga, K.; Shiratani, M. Impact of Gamma rays and DBD plasma treatments on wastewater treatment. Sci. Rep. 2018, 8, 2926. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Kumar, N.; Kwak, H.S.; Park, J.H.; Uhm, H.S.; Bogaerts, A.; Choi, E.H.; Attri, P. Bacterial inactivation by plasma treated water enhanced by reactive nitrogen species. Sci. Rep. 2018, 8, 11268. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, M.; Shiratani, M.; Cho, A.E.; Choi, E.H.; Attri, P. Variation in structure of proteins by adjusting reactive oxygen and nitrogen species generated from dielectric barrier discharge jet. Sci. Rep. 2016, 6, 35883. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Attri, P.; Choi, E.H.; Sup Uhm, H. Influence of water vapour with non-thermal plasma jet on the apoptosis of SK-BR-3 breast cancer cells. RSC Adv. 2015, 5, 14670–14677. [Google Scholar] [CrossRef]
- Attri, P.; Yusupov, M.; Park, J.H.; Lingamdinne, L.P.; Koduru, J.R.; Shiratani, M.; Choi, E.H.; Bogaerts, A. Mechanism and comparison of needle-type non-thermal direct and indirect atmospheric pressure plasma jets on the degradation of dyes. Sci. Rep. 2016, 6, 34419. [Google Scholar] [CrossRef]
- Sarangapani, C.; Patange, A.; Bourke, P.; Keener, K.; Cullen, P.J. Recent Advances in the Application of Cold Plasma Technology in Foods. Annu. Rev. Food Sci. Technol. 2018, 9, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Keener, K.M. Cold plasma: Background, applications and current trends. Curr. Opin. Food Sci. 2017, 16, 49–52. [Google Scholar] [CrossRef]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold Plasma: A novel Non-Thermal Technology for Food Processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A review on non-thermal atmospheric plasma for food preservation: Mode of action, determinants of effectiveness, and applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Randeniya, L.K.; de Groot, G.J.J.B. Non-Thermal Plasma Treatment of Agricultural Seeds for Stimulation of Germination, Removal of Surface Contamination and Other Benefits: A Review. Plasma Process. Polym. 2015, 12, 608–623. [Google Scholar] [CrossRef]
- Ito, M.; Ohta, T.; Hori, M. Plasma agriculture. J. Korean Phys. Soc. 2012, 60, 937–943. [Google Scholar] [CrossRef]
- Ito, M.; Oh, J.-S.; Ohta, T.; Shiratani, M.; Hori, M. Current status and future prospects of agricultural applications using atmospheric-pressure plasma technologies. Plasma Process. Polym. 2018, 15, 1700073. [Google Scholar] [CrossRef]
- Puač, N.; Gherardi, M.; Shiratani, M. Plasma agriculture: A rapidly emerging field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Kitazaki, S.; Koga, K.; Shiratani, M.; Hayashi, N. Growth Enhancement of Radish Sprouts Induced by Low Pressure O2 Radio Frequency Discharge Plasma Irradiation. Jpn. J. Appl. Phys. 2012, 51, 01AE01. [Google Scholar] [CrossRef]
- Hayashi, N.; Ono, R.; Uchida, S. Growth Enhancement of Plant by Plasma and UV Light Irradiation to Seeds. J. Photopolym. Sci. Technol. 2015, 28, 445–448. [Google Scholar] [CrossRef][Green Version]
- Sarinont, T.; Amano, T.; Kitazaki, S.; Koga, K.; Uchida, G.; Shiratani, M.; Hayashi, N. Growth enhancement effects of radish sprouts: Atmospheric pressure plasma irradiation vs. heat shock. J. Phys. Conf. Ser. 2014, 518, 012017. [Google Scholar] [CrossRef]
- Kitazaki, S.; Sarinont, T.; Koga, K.; Hayashi, N.; Shiratani, M. Plasma induced long-term growth enhancement of Raphanus sativus L. using combinatorial atmospheric air dielectric barrier discharge plasmas. Curr. Appl. Phys. 2014, 14, S149–S153. [Google Scholar] [CrossRef]
- Sarinont, T.; Amano, T.; Attri, P.; Koga, K.; Hayashi, N.; Shiratani, M. Effects of plasma irradiation using various feeding gases on growth of Raphanus sativus L. Arch. Biochem. Biophys. 2016, 605, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Matra, K. Non-thermal Plasma for Germination Enhancement of Radish Seeds. Procedia Comput. Sci. 2016, 86, 132–135. [Google Scholar] [CrossRef]
- Mihai, A.L.; Dobrin, D.; Popa, M.E.; Mihai, A.L.; Dobrin, D.; Măgureanu, M.; Popa, M.E. Positive effect of non-thermal plasma treatment on radish. Rom. Rep. Phys. 2014, 66, 1110–1117. [Google Scholar]
- Thisawech, M.; Saritnum, O.; Sarapirom, S.; Prakrajang, K.; Phakham, W. Effects of plasma technique and gamma irradiation on seed germination and seedling growth of chili pepper. Chiang Mai J. Sci. 2020, 47, 73–82. [Google Scholar]
- Hayashi, N.; Ono, R.; Nakano, R.; Shiratani, M.; Tashiro, K.; Kuhara, S.; Yasuda, K.; Hagiwara, H. DNA microarray analysis of plant seeds irradiated by active oxygen species in oxygen plasma. Plasma Med. 2016, 6, 459–471. [Google Scholar] [CrossRef]
- Koga, K.; Thapanut, S.; Amano, T.; Seo, H.; Itagaki, N.; Hayashi, N.; Shiratani, M. Simple method of improving harvest by nonthermal air plasma irradiation of seeds of Arabidopsis thaliana (L.). Appl. Phys. Express 2016, 9, 016201. [Google Scholar] [CrossRef]
- Roy, N.C.; Hasan, M.M.; Talukder, M.R.; Hossain, M.D.; Chowdhury, A.N. Prospective Applications of Low Frequency Glow Discharge Plasmas on Enhanced Germination, Growth and Yield of Wheat. Plasma Chem. Plasma Process. 2018, 38, 13–28. [Google Scholar] [CrossRef]
- Saberi, M.; Modarres-Sanavy, S.A.M.; Zare, R.; Ghomi, H. Improvement of photosynthesis and photosynthetic productivity of winter wheat by cold plasma treatment under haze condition. J. Agric. Sci. Technol. 2020, 21, 1889–1904. [Google Scholar]
- Iqbal, T.; Farooq, M.; Afsheen, S.; Abrar, M.; Yousaf, M.; Ijaz, M. Cold plasma treatment and laser irradiation of Triticum spp. seeds for sterilization and germination. J. Laser Appl. 2019, 31, 042013. [Google Scholar] [CrossRef]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.-D. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of Germination and Seedling Growth of Wheat Seed Using Dielectric Barrier Discharge Plasma with Various Gas Sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Meng, Y.; Qu, G.; Sun, Q.; Liang, D.; Hu, S. Air Atmospheric Dielectric Barrier Discharge Plasma Induced Germination and Growth Enhancement of Wheat Seed. Plasma Chem. Plasma Process. 2017, 37, 1621–1634. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 16680. [Google Scholar] [CrossRef]
- Lotfy, K.; Al-Harbi, N.A.; Abd El-Raheem, H. Cold Atmospheric Pressure Nitrogen Plasma Jet for Enhancement Germination of Wheat Seeds. Plasma Chem. Plasma Process. 2019, 39, 897–912. [Google Scholar] [CrossRef]
- Zukiene, R.; Nauciene, Z.; Januskaitiene, I.; Pauzaite, G.; Mildaziene, V.; Koga, K.; Shiratani, M. Dielectric barrier discharge plasma treatment-induced changes in sunflower seed germination, phytohormone balance, and seedling growth. Appl. Phys. Express 2019, 12, 126003. [Google Scholar] [CrossRef]
- Matra, K. Atmospheric non-thermal argon–oxygen plasma for sunflower seedling growth improvement. Jpn. J. Appl. Phys. 2018, 57, 01AG03. [Google Scholar] [CrossRef]
- Yawirach, S.; Sarapirom, S.; Janpong, K. The effects of dielectric barrier discharge atmospheric air plasma treatment to germination and enhancement growth of sunflower seeds. J. Phys. Conf. Ser. 2019, 1380, 12148. [Google Scholar] [CrossRef]
- Li, L.; Jiang, J.; Li, J.; Shen, M.; He, X.; Shao, H.; Dong, Y. Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci. Rep. 2014, 4, 5859. [Google Scholar] [CrossRef]
- Pérez-Pizá, M.C.; Cejas, E.; Zilli, C.; Prevosto, L.; Mancinelli, B.; Santa-Cruz, D.; Yannarelli, G.; Balestrasse, K. Enhancement of soybean nodulation by seed treatment with non–thermal plasmas. Sci. Rep. 2020, 10, 4917. [Google Scholar] [CrossRef]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of Low-Temperature Plasma on the Structure of Seeds, Growth and Metabolism of Endogenous Phytohormones in Pea (Pisum sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Khatami, S.; Ahmadinia, A. Increased germination and growth rates of pea and Zucchini seed by FSG plasma. J. Theor. Appl. Phys. 2018, 12, 33–38. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K.K. Effects of Atmospheric-Pressure N2, He, Air, and O2 Microplasmas on Mung Bean Seed Germination and Seedling Growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.I.; Mohsenimehr, S.; Hadian, J.; Ghorbanpour, M.; Shokri, B. Physico-chemical induced modification of seed germination and early development in artichoke (Cynara scolymus L.) using low energy plasma technology. Phys. Plasmas 2018, 25, 013525. [Google Scholar] [CrossRef]
- Gholami, A.; Safa, N.N.; Khoram, M.; Hadian, J.; Ghomi, H. Effect of Low-Pressure Radio Frequency Plasma on Ajwain Seed Germination. Plasma Med. 2016, 6, 389–396. [Google Scholar] [CrossRef]
- Šerá, B.; Gajdová, I.; Šerý, M.; Špatenka, P. New Physicochemical Treatment Method of Poppy Seeds for Agriculture and Food Industries. Plasma Sci. Technol. 2013, 15, 935–938. [Google Scholar] [CrossRef]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef]
- Sera, B.; Sery, M.; Gavril, B.; Gajdova, I. Seed Germination and Early Growth Responses to Seed Pre-treatment by Non-thermal Plasma in Hemp Cultivars (Cannabis sativa L.). Plasma Chem. Plasma Process. 2017, 37, 207–221. [Google Scholar] [CrossRef]
- Holc, M.; Primc, G.; Iskra, J.; Titan, P.; Kovač, J.; Mozetič, M.; Junkar, I. Effect of Oxygen Plasma on Sprout and Root Growth, Surface Morphology and Yield of Garlic. Plants 2019, 8, 462. [Google Scholar] [CrossRef]
- Singh, R.; Prasad, P.; Mohan, R.; Verma, M.K.; Kumar, B. Radiofrequency cold plasma treatment enhances seed germination and seedling growth in variety CIM-Saumya of sweet basil (Ocimum basilicum L.). J. Appl. Res. Med. Aromat. Plants 2019, 12, 78–81. [Google Scholar] [CrossRef]
- Billah, M.; Sajib, S.A.; Roy, N.C.; Rashid, M.M.; Reza, M.A.; Hasan, M.M.; Talukder, M.R. Effects of DBD air plasma treatment on the enhancement of black gram (Vigna mungo L.) seed germination and growth. Arch. Biochem. Biophys. 2020, 681, 108253. [Google Scholar] [CrossRef] [PubMed]
- Măgureanu, M.; Sîrbu, R.; Dobrin, D.; Gîdea, M. Stimulation of the Germination and Early Growth of Tomato Seeds by Non-thermal Plasma. Plasma Chem. Plasma Process. 2018, 38, 989–1001. [Google Scholar] [CrossRef]
- Volkov, A.G.; Hairston, J.S.; Patel, D.; Gott, R.P.; Xu, K.G. Cold plasma poration and corrugation of pumpkin seed coats. Bioelectrochemistry 2019, 128, 175–185. [Google Scholar] [CrossRef]
- Schnabel, U.; Niquet, R.; Krohmann, U.; Winter, J.; Schlüter, O.; Weltmann, K.D.; Ehlbeck, J. Decontamination of microbiologically contaminated specimen by direct and indirect plasma treatment. Plasma Process. Polym. 2012, 9, 569–575. [Google Scholar] [CrossRef]
- Tong, J.; He, R.; Zhang, X.; Zhan, R.; Chen, W.; Yang, S. Effects of atmospheric pressure air plasma pretreatment on the seed germination and early growth of andrographis paniculata. Plasma Sci. Technol. 2014, 16, 260–266. [Google Scholar] [CrossRef]
- Fadhlalmawla, S.A.; Mohamed, A.A.H.; Almarashi, J.Q.M.; Boutraa, T. The impact of cold atmospheric pressure plasma jet on seed germination and seedlings growth of fenugreek (Trigonella foenum-graecum). Plasma Sci. Technol. 2019, 21, 105503. [Google Scholar] [CrossRef]
- Alves, C., Jr.; de Oliveira Vitoriano, J.; da Silva, D.L.; de Lima Farias, M.; de Lima Dantas, N.B. Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Sci. Rep. 2016, 6, 33722. [Google Scholar] [CrossRef]
- Silva, D.L.; Farias, M.D.; Vitoriano, J.D.; Alves, C., Jr.; Torres, S.B. Use of Atmospheric Plasma in Germination of Hybanthus calceolaria (L.) Schulze-Menz Seeds. Rev. Caatinga 2018, 31, 632–639. [Google Scholar] [CrossRef]
- Molina, R.; López-Santos, C.; Gómez-Ramírez, A.; Vílchez, A.; Espinós, J.P.; González-Elipe, A.R. Influence of irrigation conditions in the germination of plasma treated Nasturtium seeds. Sci. Rep. 2018, 8, 16442. [Google Scholar] [CrossRef]
- Pawlat, J.; Starek, A.; Sujak, A.; Kwiatkowski, M.; Terebun, P.; Budzeń, M. Effects of atmospheric pressure plasma generated in GlidArc reactor on Lavatera thuringiaca L. seeds’ germination. Plasma Process. Polym. 2018, 15, 1700064. [Google Scholar] [CrossRef]
- Štěpánová, V.; Slavíček, P.; Kelar, J.; Prášil, J.; Smékal, M.; Stupavská, M.; Jurmanová, J.; Černák, M. Atmospheric pressure plasma treatment of agricultural seeds of cucumber (Cucumis sativus L.) and pepper (Capsicum annuum L.) with effect on reduction of diseases and germination improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Ji, S.H.; Choi, K.H.; Pengkit, A.; Im, J.S.; Kim, J.S.; Kim, Y.H.; Park, Y.; Hong, E.J.; Jung, S.K.; Choi, E.H.; et al. Effects of high voltage nanosecond pulsed plasma and micro DBD plasma on seed germination, growth development and physiological activities in spinach. Arch. Biochem. Biophys. 2016, 605, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Lee, M.J.; Ra, J.E.; Lee, K.S.; Eom, S.; Ham, H.M.; Kim, H.Y.; Kim, S.B.; Lim, J. Growth and bioactive phytochemicals in barley (Hordeum vulgare L.) sprouts affected by atmospheric pressure plasma during seed germination. J. Phys. D Appl. Phys. 2020, 53, 314002. [Google Scholar] [CrossRef]
- Sery, M.; Zahoranova, A.; Kerdik, A.; Sera, B. Seed Germination of Black Pine (Pinus nigra Arnold) after Diffuse Coplanar Surface Barrier Discharge Plasma Treatment. IEEE Trans. Plasma Sci. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Ambrico, P.F.; Šimek, M.; Ambrico, M.; Morano, M.; Prukner, V.; Minafra, A.; Allegretta, I.; Porfido, C.; Senesi, G.S.; Terzano, R. On the air atmospheric pressure plasma treatment effect on the physiology, germination and seedlings of basil seeds. J. Phys. D Appl. Phys. 2019, 53, 104001. [Google Scholar] [CrossRef]
- Sajib, S.A.; Billah, M.; Mahmud, S.; Miah, M.; Hossain, F.; Omar, F.B.; Roy, N.C.; Hoque, K.M.F.; Talukder, M.R.; Kabir, A.H.; et al. Plasma activated water: The next generation eco-friendly stimulant for enhancing plant seed germination, vigor and increased enzyme activity, a study on black gram (Vigna mungo L.). Plasma Chem. Plasma Process. 2020, 40, 119–143. [Google Scholar] [CrossRef]
- Sarinont, T.; Katayama, R.; Wada, Y.; Koga, K.; Shiratani, M. Plant Growth Enhancement of Seeds Immersed in Plasma Activated Water. MRS Adv. 2017, 2, 995–1000. [Google Scholar] [CrossRef]
- Sivachandiran, L.; Khacef, A. Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: Combined effect of seed and water treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Lo Porto, C.; Ziuzina, D.; Los, A.; Boehm, D.; Palumbo, F.; Favia, P.; Tiwari, B.; Bourke, P.; Cullen, P.J. Plasma activated water and airborne ultrasound treatments for enhanced germination and growth of soybean. Innov. Food Sci. Emerg. Technol. 2018, 49, 13–19. [Google Scholar] [CrossRef]
- Darmanin, M.; Kozak, D.; de Oliveira Mallia, J.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Generation of plasma functionalized water: Antimicrobial assessment and impact on seed germination. Food Control 2020, 113, 107168. [Google Scholar] [CrossRef]
- Naumova, I.K.; Maksimov, A.I.; Khlyustova, A.V. Stimulation of the germinability of seeds and germ growth under treatment with plasma-activated water. Surf. Eng. Appl. Electrochem. 2011, 47, 263–265. [Google Scholar] [CrossRef]
- Takaki, K.; Takahata, J.; Watanabe, S.; Satta, N.; Yamada, O.; Fujio, T.; Sasaki, Y. Improvements in plant growth rate using underwater discharge. J. Phys. Conf. Ser. 2013, 418, 012140. [Google Scholar] [CrossRef]
- Zhang, S.; Rousseau, A.; Dufour, T. Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. RSC Adv. 2017, 7, 31244–31251. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene expression in Tomato seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef]
- Islam, S.; Omar, F.B.; Sajib, S.A.; Roy, N.C.; Reza, A.; Hasan, M.; Talukder, M.R.; Kabir, A.H. Effects of LPDBD Plasma and Plasma Activated Water on Germination and Growth in Rapeseed (Brassica napus). Gesunde Pflanz. 2019, 71, 175–185. [Google Scholar] [CrossRef]
- Sarinont, T.; Amano, T.; Koga, K.; Shiratani, M.; Hayashi, N. Multigeneration Effects of Plasma Irradiation to Seeds of Arabidopsis Thaliana and Zinnia on Their Growth. MRS Online Proc. Libr. Arch. 2015, 1723, mrsf14-1723-g03-04. [Google Scholar] [CrossRef]
- Hayashi, N.; Ono, R.; Shiratani, M.; Yonesu, A. Antioxidative activity and growth regulation of Brassicaceae induced by oxygen radical irradiation. Jpn. J. Appl. Phys. 2015, 54, 06GD01. [Google Scholar] [CrossRef]
- Zahoranová, A.; Hoppanová, L.; Šimončicová, J.; Tučeková, Z.; Medvecká, V.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on Maize Seeds: Enhancement of Seedlings Growth and Surface Microorganisms Inactivation. Plasma Chem. Plasma Process. 2018, 38, 969–988. [Google Scholar] [CrossRef]
- Khamsen, N.; Onwimol, D.; Teerakawanich, N.; Dechanupaprittha, S.; Kanokbannakorn, W.; Hongesombut, K.; Srisonphan, S. Rice (Oryza sativa L.) Seed Sterilization and Germination Enhancement via Atmospheric Hybrid Nonthermal Discharge Plasma. ACS Appl. Mater. Interfaces 2016, 8, 19268–19275. [Google Scholar] [CrossRef]
- Amnuaysin, N.; Korakotchakorn, H.; Chittapun, S.; Poolyarat, N. Seed germination and seedling growth of rice in response to atmospheric air dielectric-barrier discharge plasma. Songklanakarin J. Sci. Technol. 2018, 40, 819–823. [Google Scholar] [CrossRef]
- Mitra, A.; Li, Y.F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of Surface-Borne Microorganisms and Increased Germination of Seed Specimen by Cold Atmospheric Plasma. Food Bioprocess Technol. 2014, 7, 645–653. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M.; Maric, D.; Reid, J.P.; Ceriani, E.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, U.; Handorf, O.; Yarova, K.; Zessin, B.; Zechlin, S.; Sydow, D.; Zellmer, E.; Stachowiak, J.; Andrasch, M.; Below, H.; et al. Plasma-Treated Air and Water—Assessment of Synergistic Antimicrobial Effects for Sanitation of Food Processing Surfaces and Environment. Foods 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Yong, H.I.; Park, J.; Kim, H.-J.; Jung, S.; Park, S.; Lee, H.J.; Choe, W.; Jo, C. An innovative curing process with plasma-treated water for production of loin ham and for its quality and safety. Plasma Process. Polym. 2018, 15, 1700050. [Google Scholar] [CrossRef]
- WIPO—Search International and National Patent Collections. Available online: https://patentscope2.wipo.int/search/en/search.jsf (accessed on 25 June 2020).
- JP2019/030643 Rice Plant Production Method. Available online: http://www.freepatentsonline.com/WO2020027342A1.html (accessed on 25 June 2020).
- JP2016009066A Animal and Plant Growth Promotion Methods. Available online: http://www.freepatentsonline.com/JP2016152796A.html (accessed on 25 June 2020).
- RU02317668 Method for Treatment of Plant Seeds and Apparatus for Performing the Same. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=RU29606471&_cid=JP1-KBU5QY-51437-1 (accessed on 25 June 2020).
- CN108738474 Seed Treatment Method of Selenium-Rich Black Beans. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN233991167&_cid=JP1-KBU5TM-53840-1 (accessed on 25 June 2020).
- CN109041641 Seed Treatment Method for Improving Zinc and Selenium Content of Pumpkin. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN235612147&_cid=JP1-KBU5UW-55097-1 (accessed on 25 June 2020).
- CN106817954 Method for Culturing Seedling of Oily Peony Seeds. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN199374287&_cid=JP1-KBU5W2-56424-1 (accessed on 25 June 2020).
- CN103999593 Method for Breeding Wheat by Cold Plasma Treatment. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN107363409&_cid=JP1-KBU5WY-57195-1 (accessed on 25 June 2020).
- CN107960254 Seedling Growing Method of Dalbergia Odorifera. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN215523929&_cid=JP1-KBU5XU-57795-1 (accessed on 25 June 2020).
- CN104770103 Seedling Raising Method for Oil-Used Peony Seeds. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN151324684&_cid=JP1-KBU5YQ-58665-1 (accessed on 25 June 2020).
- CN108243662 Plasma Preparation Used for Increasing Corn Yield, and Preparation Method and Application Method Thereof. Available online: https://patentscope2.wipo.int/search/en/detail.jsf?docId=CN223835065&_cid=JP1-KBU5ZP-59644-1 (accessed on 25 June 2020).
- Sarinont, T.; Amano, T.; Koga, K.; Shiratani, M.; Hayashi, N. Effects of Atmospheric Air Plasma Irradiation to Seeds of Radish Sprouts on Chlorophyll and Carotenoids Concentrations in their Leaves. MRS Proc. 2015, 1723, mrsf14-1723-g02-04. [Google Scholar] [CrossRef]
- Degutytė-Fomins, L.; Paužaitė, G.; Žūkienė, R.; Mildažienė, V.; Koga, K.; Shiratani, M. Relationship between cold plasma treatment-induced changes in radish seed germination and phytohormone balance. Jpn. J. Appl. Phys. 2020, 59, SH1001. [Google Scholar] [CrossRef]
- Kyzek, S.; Holubová, Ľ.; Medvecká, V.; Tomeková, J.; Gálová, E.; Zahoranová, A. Cold Atmospheric Pressure Plasma Can Induce Adaptive Response in Pea Seeds. Plasma Chem. Plasma Process. 2019, 39, 475–486. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zhou, R.-W.; De Groot, G.; Bazaka, K.; Murphy, A.B.; Ostrikov, K. Spectral characteristics of cotton seeds treated by a dielectric barrier discharge plasma. Sci. Rep. 2017, 7, 5601. [Google Scholar] [CrossRef]
- Koga, K.; Attri, P.; Kamataki, K.; Itagaki, N.; Shiratani, M.; Mildaziene, V. Impact of radish sprouts seeds coat color on the electron paramagnetic resonance signals after plasma treatment. Jpn. J. Appl. Phys. 2020, 59, SHHF01. [Google Scholar] [CrossRef]
- Ahn, C.; Gill, J.; Ruzic, D.N. Growth of Plasma-Treated Corn Seeds under Realistic Conditions. Sci. Rep. 2019, 9, 4355. [Google Scholar] [CrossRef] [PubMed]
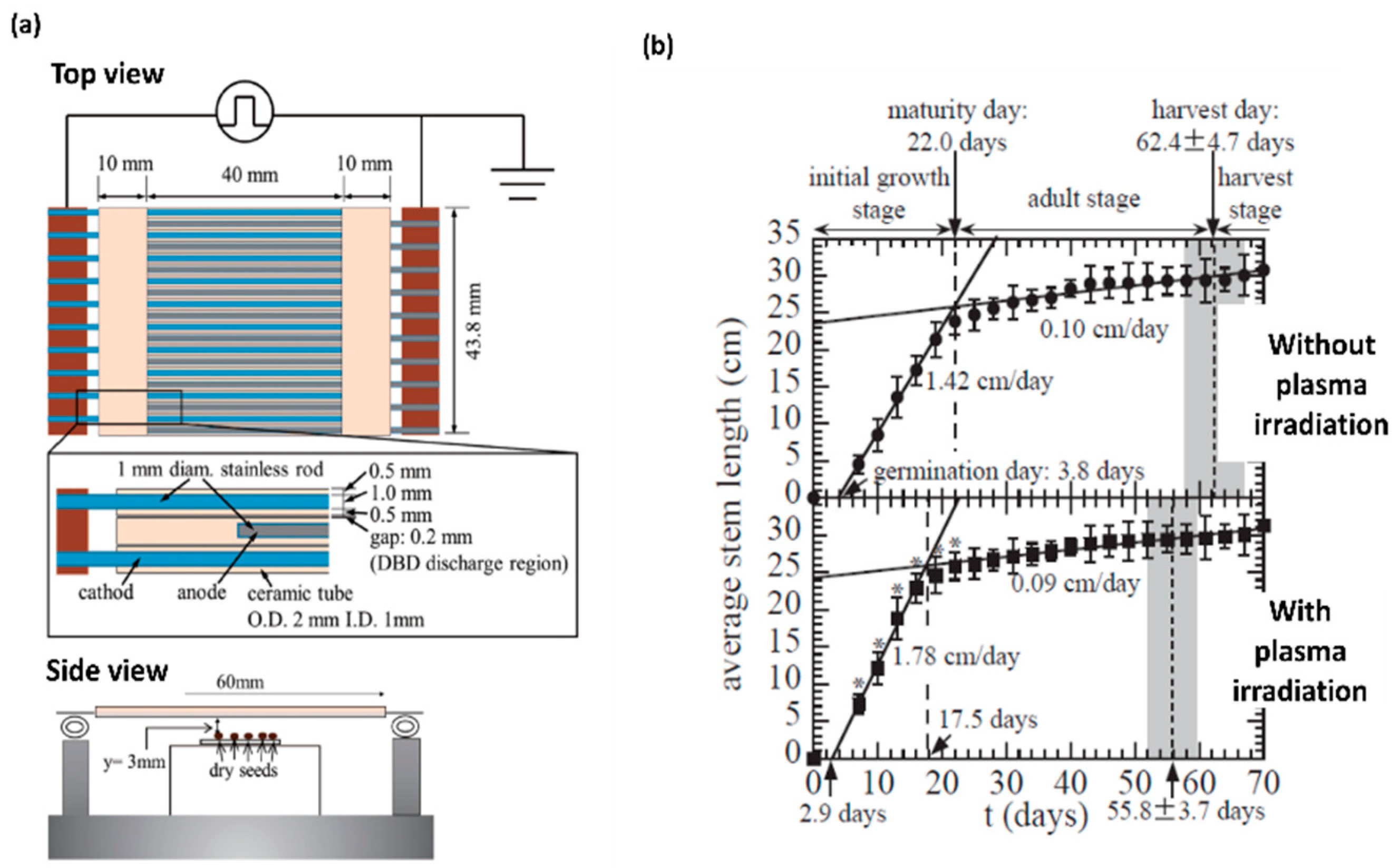
| Seeds | Plasma Type | Results | Reference |
|---|---|---|---|
| Radish sprouts | Low-pressure plasma (100 Pa) | Radish sprouts growth increases with O2 plasma treatment, while no effect was observed for seed germination. In contrast, no effect on the average length of sprouts for N2 Low-pressure plasma. | [37] |
| Radish sprouts | Low-pressure plasma (40 Pa) | Growth enhancement. | [38] |
| Radish sprouts | Scalar dielectric-barrier discharge (DBD) plasma | Growth enhancement. | [39] |
| Radish sprouts | Scalar DBD plasma | The average seedling length was 250% longer than the control samples. | [40] |
| Radish sprouts | Scalar DBD plasma | Enhanced plant growth for O2, Air and NO (10%) + N2 feeding gases plasma. While no significant growth enhancement for He, N2, and Ar gases plasma. | [41] |
| Radish sprouts | Plasma jet | The total mass and average lengths of radish sprouts increased. | [42] |
| Radish | Surface discharge plasma | No effect on the germination dynamics but the length of root and sprout increased. | [43] |
| Chili pepper | Plasma jet | Enhanced seed germination. | [44] |
| Arabidopsis thaliana | Low-pressure plasma (20–80 Pa) | Lengths of the leaves and stems of Arabidopsis increased ≈1.5 times over the control. | [45] |
| Arabidopsis thaliana | Scalar DBD plasma | Enhanced growth, shorter harvest, and increased total weight. | [46] |
| Wheat | Medium pressure glow discharge plasma (≈1333 Pa) | Increased growth activity and dry matter accumulation. | [47] |
| Wheat | Low-pressure plasma | Plasma treatment increased the grain and spike yield. | [48] |
| Wheat | Low-pressure plasma | Enhanced seed germination rate. | [49] |
| Wheat | Low-pressure plasma (150 Pa) | Improved germination potential, germination rate. | [50] |
| Wheat | Surface discharge plasma | Little effect on the germination rate while a substantial impact on growth parameters. | [51] |
| Wheat | DBD plasma | Improved the germination and seedling growth. | [52] |
| Wheat | DBD plasma | The germination rate, germination potential, germination index, and vigor index increased after plasma treatment. | [53] |
| Wheat | DBD plasma | The germination rate, germination potential, root length, and shoot length of the wheat seedlings increased. | [54] |
| Wheat | Plasma jet | Increased dry weight after plasma treatment. | [55] |
| Wheat | Low-pressure plasma (140 Pa) | Germination acceleration was inhibited on first day after plasma treatment. | [6] |
| Sunflower | Scalar DBD plasma | Adverse effects on germination kinetics. | [56] |
| Sunflower | Streamer like plasma | Growth enhancement and increased dry weight. | [57] |
| Sunflower | DBD plasma | The distribution of sprouts length and the dry weight increased after plasma treatment. | [58] |
| Soybean | Low-pressure plasma (150 Pa) | Germination and vigor indices significantly increased after plasma treatment. | [59] |
| Soybean | DBD plasma | Total fresh weight increased by 1.2-fold for DBD plasma | [60] |
| Pea | Diffuse coplanar surface barrier discharge (DCSBD) plasma | Increased in germination percentage and growth parameters. | [61] |
| Pea and Zucchini | FSG plasma (a semi-automatic device) system. | Germination of Pea and Zucchini increased after plasma treatment. | [62] |
| Mung bean | Microplasma array plasma. | Germination index increased for Air and O2 plasma, and no significant difference observed for He or N2 plasma compared to control. | [63] |
| Beans | Low-pressure plasma (6.7 × 10−2 Pa) | The final germination percentage of seeds was not affected by plasma treatment. However, the rate of germination was improved for the plasma-treated samples. | [64] |
| Artichoke | Low-pressure plasma (1.8 Pa) | Improved the germination rate and seedling growth. | [65] |
| Ajwain | Low-pressure plasma (9.9 Pa) | Improved seed germination percentage and germination index. | [66] |
| Poppy | Plasonic AR-550-M | Enhanced seed germination | [67] |
| Oilseed rape | Low-pressure plasma (150 Pa) | Improved germination rate and seedling growth. | [68] |
| Hemp | Gliding arc and downstream microwave devices (low-pressure, 140 Pa) | Gliding arc treatment increased the length of seedlings, seedling accretion, and weight of seedling, while downstream microwave plasma treatment had an inhibiting effect. | [69] |
| Garlic seed bulbs | Low-pressure plasma (15–60 Pa) | Increased dried bulb mass after plasma treatment. | [70] |
| Sweet basil | Low-pressure plasma (40 Pa) | Increased germination and seedling vigor after plasma treatment. | [71] |
| Black gram | Medium pressure DBD plasma (≈53,328 Pa) | Enhanced seed germination rate and seedling growth. | [72] |
| Tomato | Coaxial DBD reactor plasma | The root-to-shoot ratio (R/S) ratio increased significantly for plasma-treated samples. | [73] |
| Pumpkin | Plasma jet | Plasma jets accelerated the germination of pumpkin seeds. | [74] |
| Brassica napus | DBD plasma | No significant difference in seed germination. | [75] |
| Andrographis paniculata | DBD plasma | Increased seed germination. | [76] |
| Fenugreek | Plasma jet. | Enhanced seed germination rate. | [77] |
| Mulungu | Plasma jet | Enhanced seed germination rate. | [78] |
| Hybanthus calceolaria | Plasma jet | Enhanced seed germination rate. | [79] |
| Nasturtium | DBD plasma | Enhanced seed germination for short plasma treatment. | [80] |
| Thuringian Mallow | GlidArc reactor | Enhanced seed germination. | [81] |
| Cucumber and Pepper | DCSBD plasma | Improved germination observed for both seeds. | [82] |
| Spinach | High voltage nanosecond pulsed plasma and micro DBD plasma. | Germination and dry weight of seedlings increased after both plasma treatment. | [83] |
| Barley | Surface DBD plasma | Accelerated the early growth of sprouts and enhance bioactive phytochemicals in the sprouts. | [84] |
| Barley | Low-pressure plasma (≈26 Pa) | No effect of plasma treatment. | [7] |
| Oat | Low-pressure plasma (≈13 Pa) | Quantity of germination seeds increased by 27% after plasma treatment than control on 5th day. | [7] |
| Oat | Low-pressure plasma (140 Pa) | No significant difference in rate of germination. | [6] |
| Black Pine | DCSBD plasma | The germination index increased for short treatment time. | [85] |
| Basil | Volume DBD plasma | Increased overall germination rate. | [86] |
| Black Gram | PTW | Increased cumulative germination and vigor index. | [87] |
| Radish sprout | PTW | Increased growth of sprouts. | [88] |
| Radish | PTW | Enhanced seeds germination rate and the seedling growth. | [89] |
| Soybeans | PTW | Enhanced seeds germination. | [90] |
| Mung bean | PTW | No significant difference in growth rate. | [91] |
| Zinnia annual | PTW | Increased germinability and growth of flowers of Zinnia annual. | [92] |
| Chinese Cabbage | PTW | Increased dried weight of the plant. | [93] |
| Lentils | PTW | Enhanced seeds germination as compared with commercial fertilizer. | [94] |
| Tomato | PTW | Enhanced shoot and root length. | [95] |
| Rapeseed | PTW | Significant improvement in germination rate and seedling vigor. | [96] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. https://doi.org/10.3390/pr8081002
Attri P, Ishikawa K, Okumura T, Koga K, Shiratani M. Plasma Agriculture from Laboratory to Farm: A Review. Processes. 2020; 8(8):1002. https://doi.org/10.3390/pr8081002
Chicago/Turabian StyleAttri, Pankaj, Kenji Ishikawa, Takamasa Okumura, Kazunori Koga, and Masaharu Shiratani. 2020. "Plasma Agriculture from Laboratory to Farm: A Review" Processes 8, no. 8: 1002. https://doi.org/10.3390/pr8081002
APA StyleAttri, P., Ishikawa, K., Okumura, T., Koga, K., & Shiratani, M. (2020). Plasma Agriculture from Laboratory to Farm: A Review. Processes, 8(8), 1002. https://doi.org/10.3390/pr8081002






