Efficient Removal of Ni(II) from Aqueous Solution by Date Seeds Powder Biosorbent: Adsorption Kinetics, Isotherm and Thermodynamics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Treatment of Adsorbent
2.2. Reagents
2.3. Batch Adsorption
2.4. Characterization of Biosorbent
3. Results and Discussion
3.1. Characterization of the DSP
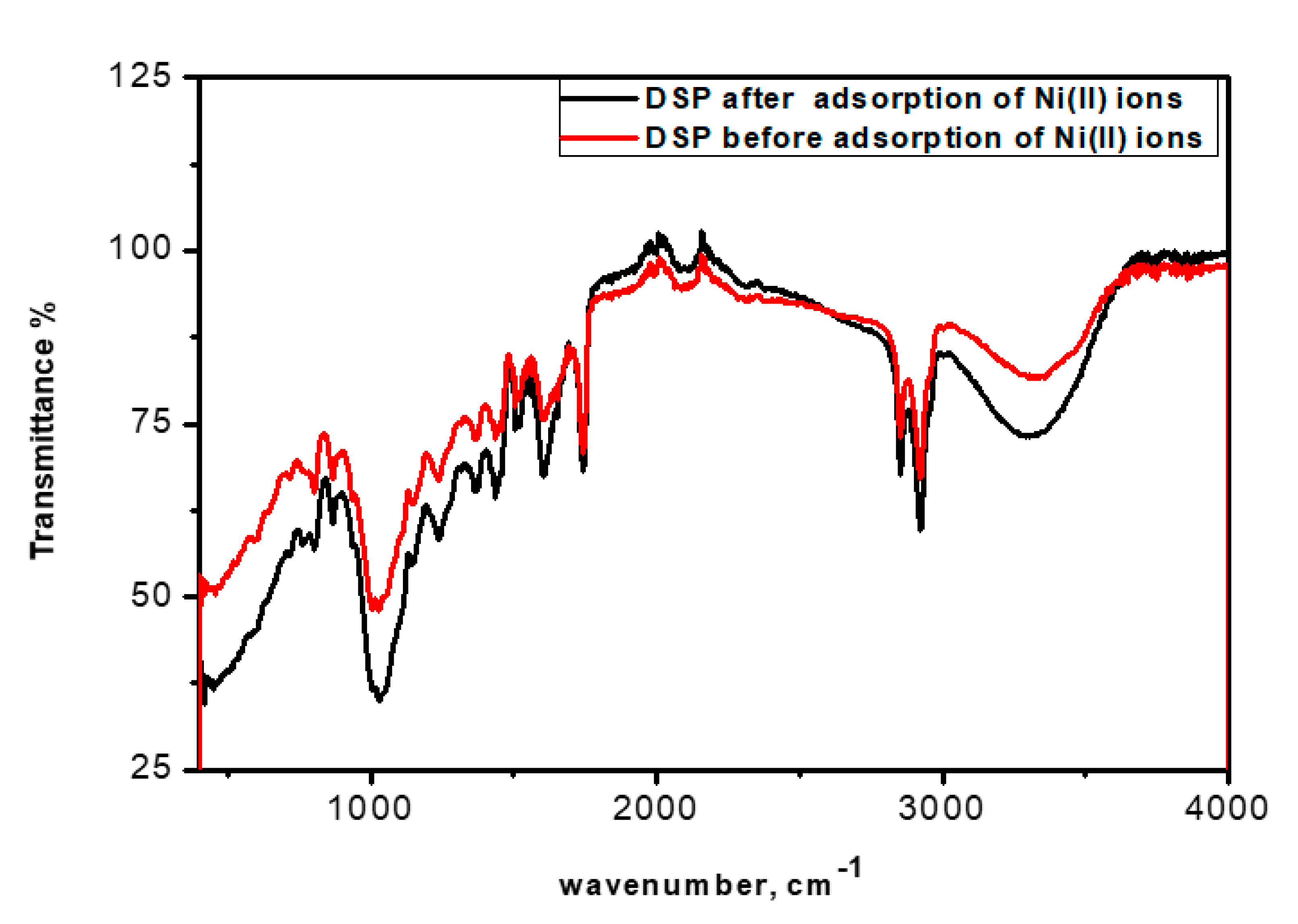
3.2. ATR—FTIR Spectrum
3.3. Scanning Electron Microscope Technique (SEM)
3.4. Effect of pH Values
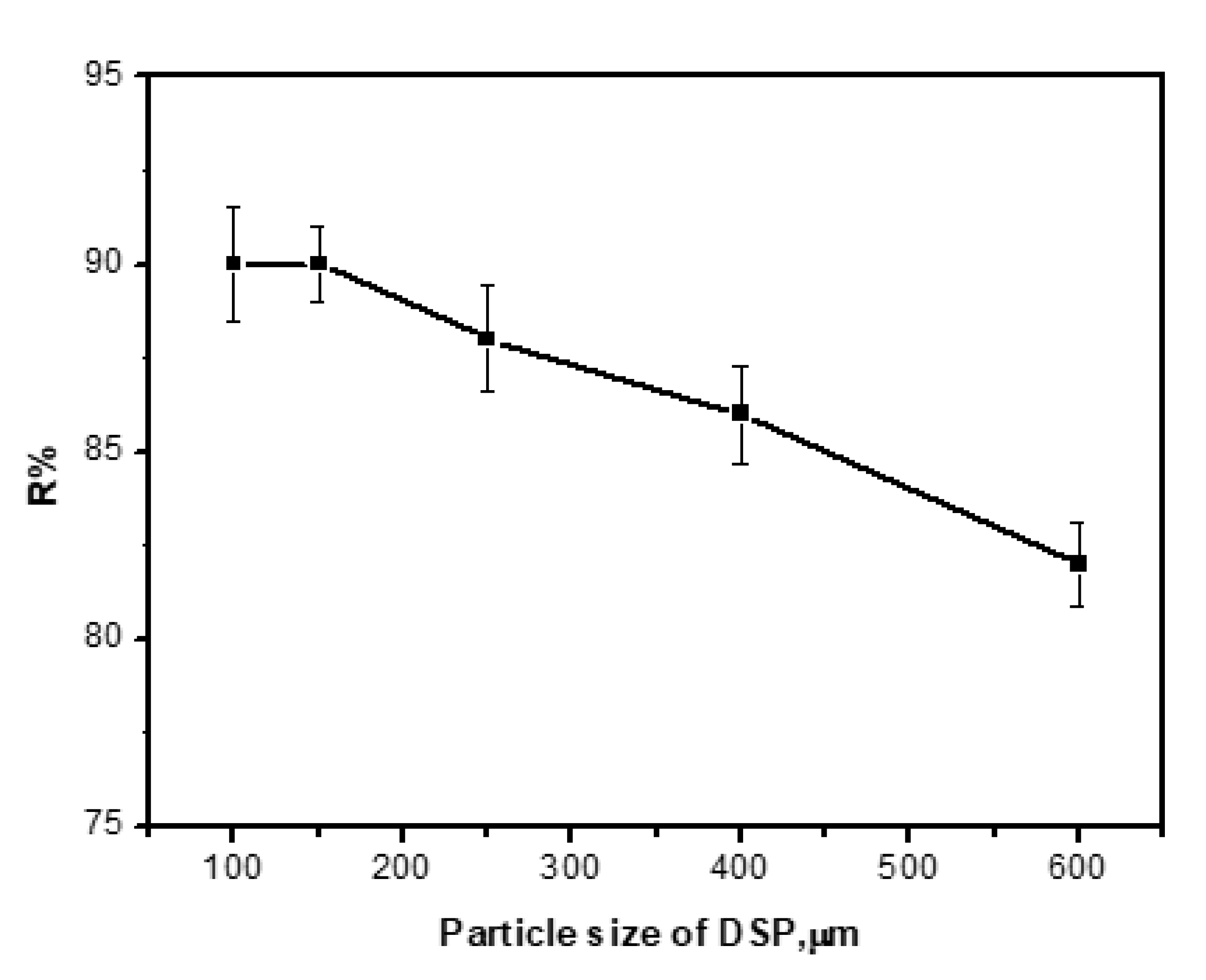
3.5. Effect of Adsorbent Particle Size
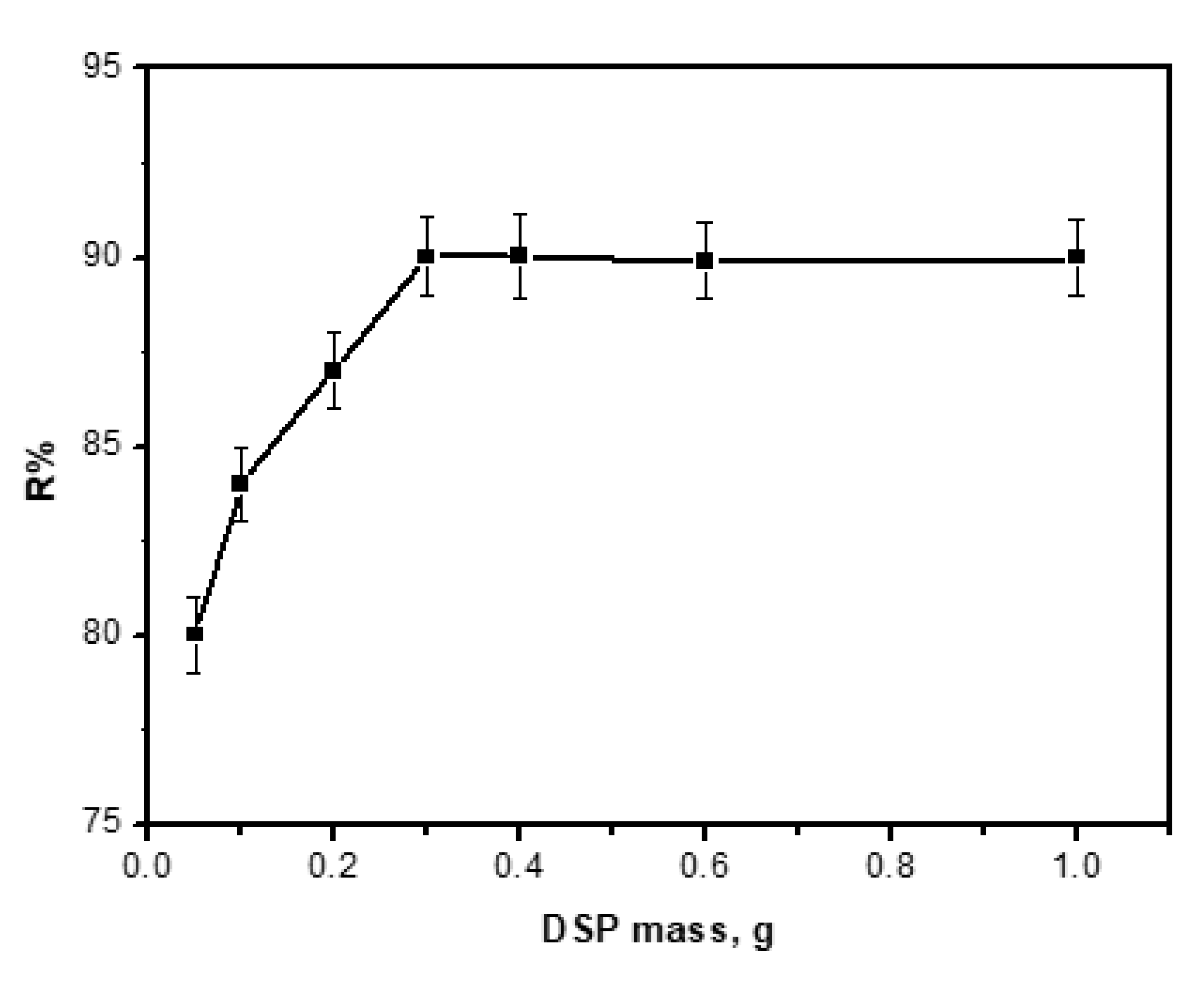
3.6. Effect of Adsorbent Mass
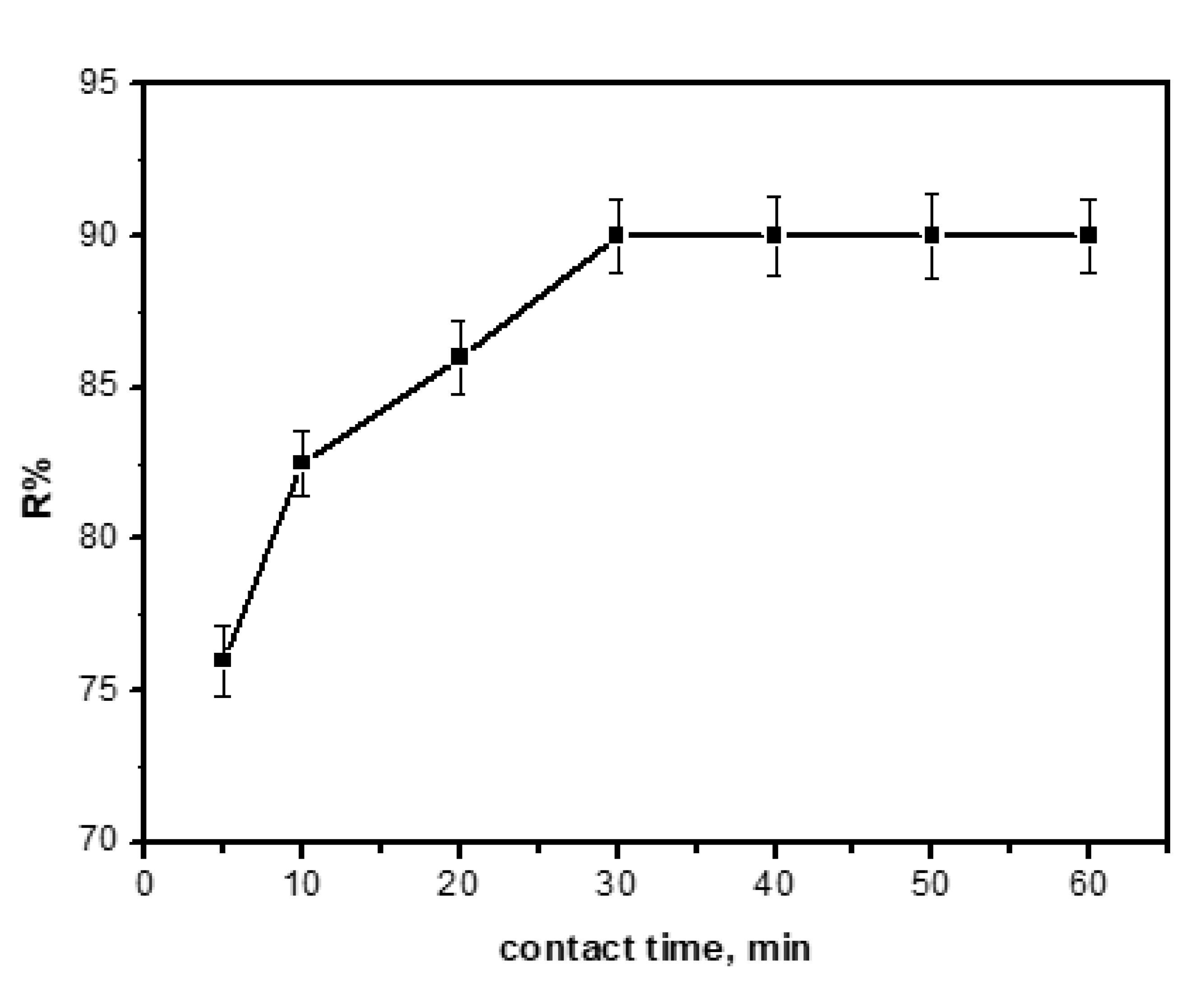
3.7. Effect of Contact Time
3.8. Adsorption Kinetics
3.8.1. Pseudo-First-Order Model
3.8.2. Intra-Particle Diffusion Kinetic Model
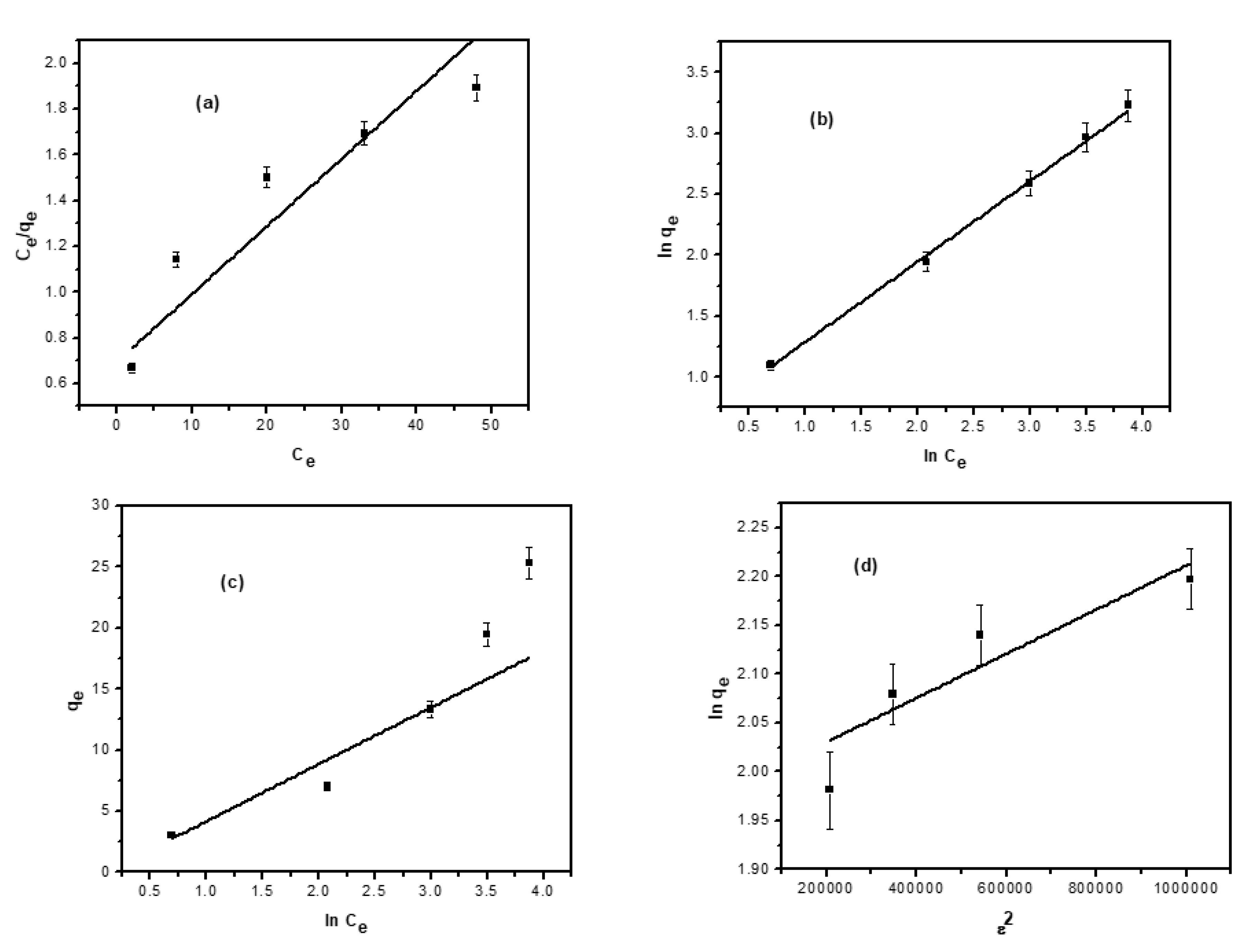
3.9. Adsorption Isotherm
3.9.1. Langmuir Model
3.9.2. Freundlich Model
3.9.3. Temkin Isotherm
3.9.4. Dubinin–Radushkevich (D–R) Isotherm Model
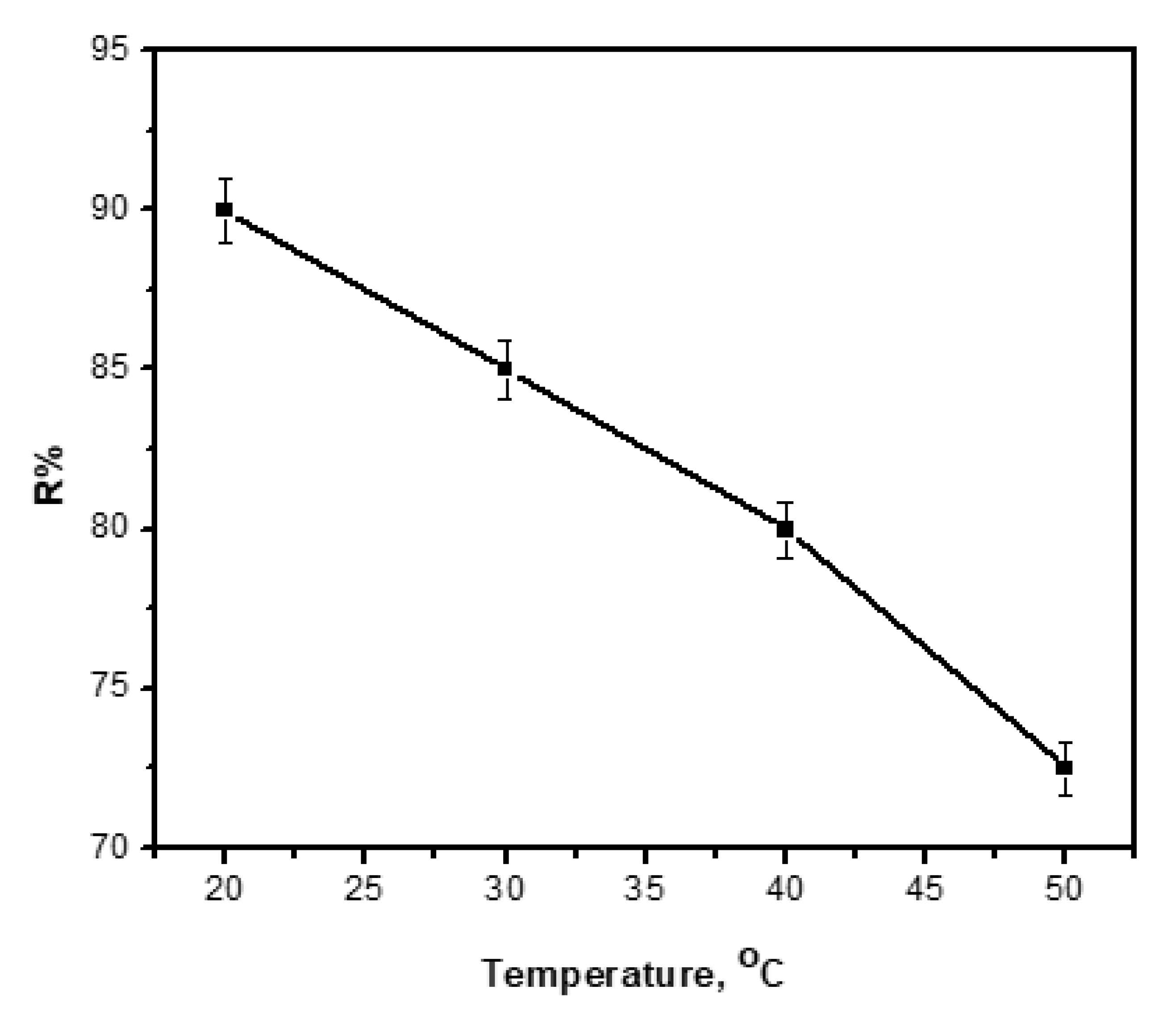
3.10. Effect of Temperature
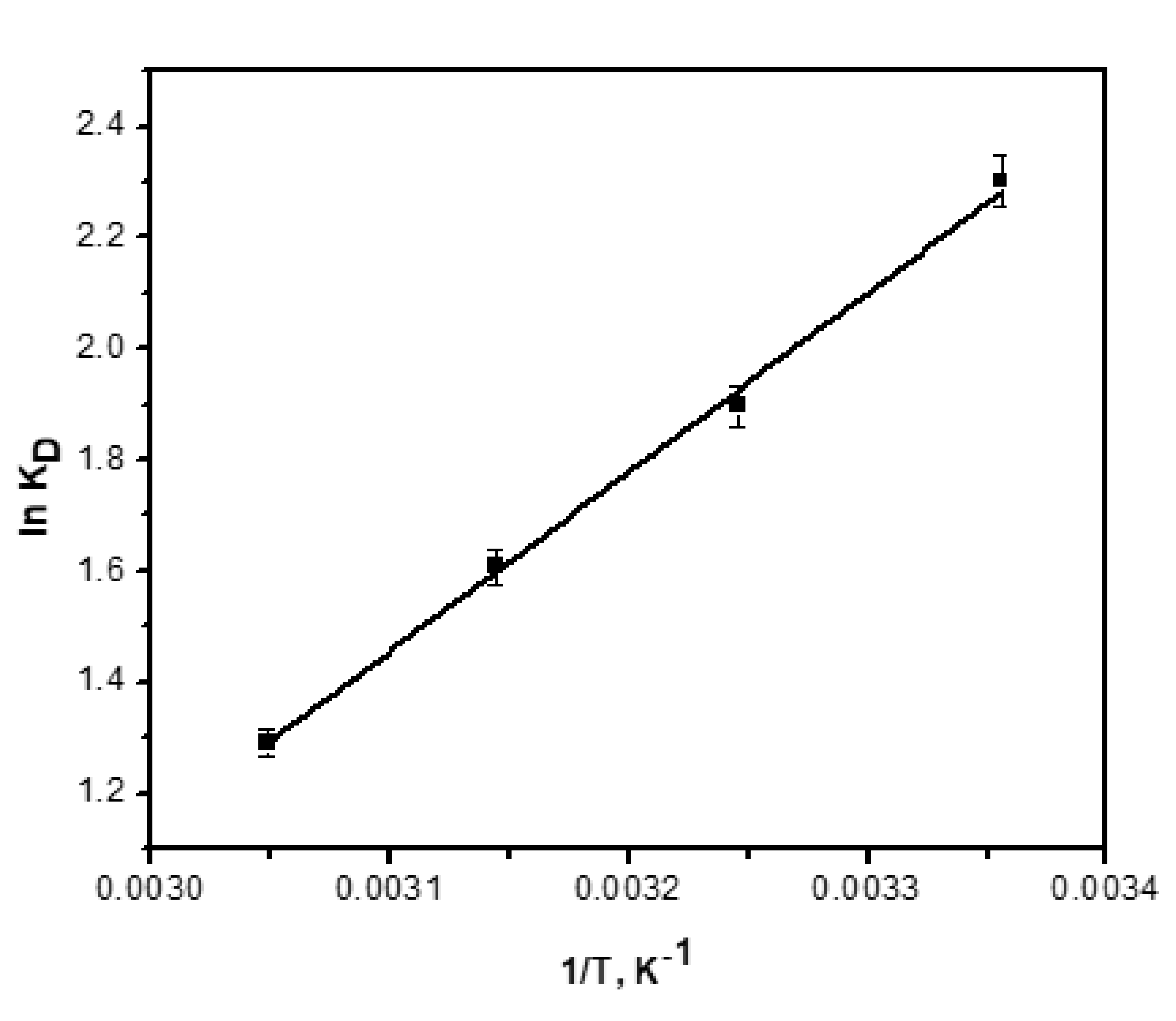
3.11. Adsorption Thermodynamics
3.12. Comparisons with Other Adsorbents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kulkarni, R.M.; Shetty, K.V.; Srinikethan, G. Cadmium (II) and nickel (II) biosorption by Bacillus laterosporus (MTCC 1628). J. Taiwan Inst. Chem. Eng. 2014, 45, 1628–1635. [Google Scholar] [CrossRef]
- Putra, W.P.; Kamari, A.; Yusoff, S.N.M.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M. Biosorption of Cu (II), Pb (II) and Zn (II) ions from aqueous solutions using selected waste materials: Adsorption and characterisation studies. J. Encapsul. Adsorpt. Sci. 2014, 4, 720–726. [Google Scholar]
- Karatas, M. Removal of Pb(II) from water by natural zeolitic tuff: Kinetics and thermodynamics. J. Hazard. Mater. 2012, 199, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Danish, M.; Rafatullah, M.; Ghazali, A.; Sulaiman, O.; Hashim, R.; Ibrahim, M.N.M. The use of date palm as a potential adsorbent for wastewater treatment: A review. Environ. Sci. Pollut. Res. 2012, 19, 1464–1468. [Google Scholar] [CrossRef]
- Lee, C.G.; Lee, S.; Park, J.A.; Park, C.; Lee, S.J.; Kim, S.B.; An, B.; Yun, S.T.; Lee, S.H.; Choi, J.W. Removal of copper, nickel and chromium mixtures from metal plating wastewater by adsorption with modified carbon foam. Chemosphere 2017, 166, 203–211. [Google Scholar] [CrossRef]
- Prithviraj, D.; Deboleena, K.; Neelu, N.; Noor, N.; Aminur, R.; Balasaheb, K.; Abul, M. Biosorption of nickel by Lysinibacillus sp. BA2 native to bauxite mine. Ecotoxicol. Environ. Saf. 2014, 107, 260–268. [Google Scholar] [CrossRef]
- Salem, N.M.; Awwad, A.M. Biosorption of Ni(II) from electroplating wastewater by modified (Eriobotrya japonica) loquat bark. J. Saudi Chem. Soc. 2014, 18, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Onundi, Y.B.; Mamun, A.A.; Al Khatib, M.F.; Ahmed, M. Adsorption of copper, nickel and lead ions from synthetic semiconductor industrial wastewater by palm shell activated carbon. Int. J. Environ. Sci. Technol. 2010, 7, 751–758. [Google Scholar] [CrossRef] [Green Version]
- Alomá, I.; Martín-Lara, M.A.; Rodríguez, I.L.; Blázquez, G.; Calero, M. Removal of nickel (II) ions from aqueous solutions by biosorption on sugarcane bagasse. J. Taiwan Inst. Chem. Eng. 2012, 43, 275–281. [Google Scholar] [CrossRef]
- El Marouani, M.; Azoulay, K.; Bencheikh, I.; Elfakir, L.; Rghioui, L.; El Hajji, A.; Sebbahi, S.; El Hajjaji, S.; Kifani-Sahban, F. Application of raw and roasted date seeds for dyes removal from aqueous solution. J. Mater. Environ. Sci. 2018, 9, 2387–2396. [Google Scholar]
- Chang, Y.S.; Au, P.I.; Mubarak, N.M.; Khalid, M.; Jagadish, P.; Walvekar, R.; Abdullah, E.C. Adsorption of Cu(II) and Ni(II) ions from wastewater onto bentonite and bentonite/GO composite. Environ. Sci. Pollut. Res. 2020, 27, 33270–33296. [Google Scholar] [CrossRef] [PubMed]
- Mehrmad, N.; Moravegi, M.; Parvareh, A. Adsorption of Pb(II), Cu(II) and Ni(II) ions from aqueous solutions by functionalised henna powder (Lawsonia Inermis); isotherm, kinetic and thermodynamic studies. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Khan, M.I.; Almesfer, M.K.; Danish, M.; Ali, I.H.; Shoukry, H.; Patel, R.; Gardy, J.; Nizami, A.S.; Rehan, M. Potential of Saudi natural clay as an effective adsorbent in heavy metals removal from wastewater. Desalin. Water Treat. 2019, 158, 140–151. [Google Scholar] [CrossRef]
- Bartczak, P.; Norman, M.; Klapiszewski, L.; Karwan’ska, N.; Kawalec, M.; Baczyn´ska, M.; Wysokowski, M.; Zdarta, J.; Ciesielczyk, F.; Jesionowski, T. Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: A kinetic and equilibrium study. Arab. J. Chem. 2018, 11, 1209–1222. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.H.; Al Mesfer, M.K.; Khan, M.I.; Danish, M.; Alghamdi, M.M. Exploring Adsorption Process of Lead (II) and Chromium (VI) Ions from Aqueous Solutions on Acid Activated Carbon Prepared from Juniperus procera Leaves. Processes 2019, 7, 217. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.F.; Chang, C.Y.; Chen, K.H.; Tsai, W.T.; Shie, J.L.; Chen, Y.H. Adsorption of naphthalene on zeolite from aqueous solution. J. Colloid. Interface Sci. 2004, 277, 29–34. [Google Scholar] [CrossRef]
- Ali, I.H.; Sulfab, Y. Kinetics and mechanism of oxidation of cisdiaquabis(glycinato)chromium(III) by periodate ion in aqueous solutions. Transit. Met. Chem. 2013, 38, 79–84. [Google Scholar] [CrossRef]
- Tempkin, M.I.; Pyzhev, V. Kinetic of Ammonia Synthesis on Promoted Iron Catalyst. Acta Phys. Chim. USSR 1940, 12, 327–356. [Google Scholar]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A. Removal of nickel (II) from aqueous solution by biosorption on A. barbadensis Miller waste leaves powder. Appl. Water Sci. 2019, 9, 96–107. [Google Scholar] [CrossRef] [Green Version]
- Priyantha, N.; Kotabewatta, P.A. Biosorption of heavy metal ions on peel of Artocarpus nobilis fruit: 1—Ni(II) sorption under static and dynamic conditions. Appl. Water Sci. 2019, 9, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Sharma, S.K.; Kumar, A. Biosorption of Ni(II) ions from aqueous solution using modified Aloe barbadensis Miller leaf powder. Water Sci. Eng. 2019, 12, 27–36. [Google Scholar] [CrossRef]
- Barquilha, C.E.R.; Cossich, E.S.; Tavares, C.R.; Silva, E.A. Biosorption of nickel(II) and copper(II) ions by Sargassum sp. in nature and alginate extraction products. Bioresour. Technol. Rep. 2019, 5, 43–50. [Google Scholar] [CrossRef]
- Pandey, P.K.; Choubey, S.; Verma, Y.; Pandey, M.; Kamal, S.S.; Chandrashekhar, K. Biosorptive removal of Ni(Ii) from wastewater and industrial effluent. Int. J. Environ. Res. Public Health 2007, 4, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thevannan, A.; Mungroo, R.; Niu, C. Biosorption of nickel with barley straw. Bioresour. Technol. 2010, 101, 1776–1780. [Google Scholar] [CrossRef] [Green Version]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J. Hazard. Mater 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Malkoc, E.; Nuhoglu, Y. Investigations of nickel(II) removal from aqueous solutions using tea factory waste. J. Hazard. Mater 2005, 127, 120–128. [Google Scholar] [CrossRef]



| Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (A°) |
|---|---|---|
| 124 | 46.9 × 10−2 | 98 |
| Kinetic Models | Parameters | |
|---|---|---|
| Pseudo-first-order model | qe (mg/g) | 12.2 |
| k1 (min−1) | 4.0 × 10−4 | |
| R2 | 0.6988 | |
| Pseudo-second-order model | qe (mg/g) | 3.1 |
| k2 (g/mg min) | 0.3 | |
| R2 | 0.9937 | |
| Intraparticle diffusion model | kid (mg/g. min(1/2)) | 0.056 |
| I | 2.6 | |
| R2 | 0.7978 | |
| Adsorption Model | Isotherm Constants | Value |
|---|---|---|
| Langmuir | qmax (mg/g) | 40.8 |
| KL (L/g) | 0.029 | |
| R2 | 0.887 | |
| Freundlich | N | 0.67 |
| Kf ((mg/g)/(mg/L) n) | 4.6 | |
| R2 | 0.9981 | |
| Temkin | A (L/g) | 2.1 |
| bt (kJ/mol) | 370.3 | |
| R2 | 0.899 | |
| D-R | β | 2.0 × 10−7 |
| qm (mg/g) | 7.18 | |
| R2 | 0.846 |
| T, K | KD | ∆G° (kJ/mol) | ∆S° (J/mol K) | ∆H° (kJ/mol) | Ea (kJ/mol) | S* (J K/mol) |
|---|---|---|---|---|---|---|
| 298 | 10.0 | −5.7 | 71.7 | −27.0 | −2.01 | 0.002 |
| 308 | 6.7 | −4.9 | ||||
| 318 | 5.0 | −4.2 | ||||
| 328 | 3.6 | −3.5 |
| Biosorbent | qm (mg/g) | Isotherm Model | Kinetic Model | Optimal pH | Reference |
|---|---|---|---|---|---|
| Date seeds powder | 41.0 | Freundlich | 2nd order | 7.00 | This study |
| Aloe barbadensis Miller leaves | 10.0 | Freundlich | 2nd order | 3.00 | [20] |
| peel of Artocarpus nobilis fruit | 12.1 | Langmuir | ** | 4.00 | [21] |
| Modified Aloe barbadensis leaves | 29.0 | Langmuir | 2nd order | 7.00 | [22] |
| brown algae Sargassum sp. | 1.3 | Langmuir | 2nd order | 6.50 | [23] |
| Calotropis procera roots | 0.6 | Langmuir | ** | 3.00 | [24] |
| Peat | 61.3 | Langmuir | 2nd order | 9.00 | [14] |
| Barely straw | 35.8 | Langmuir | ** | 4.85 | [25] |
| Orange peel | 162.6 | Langmuir | 2nd order | 5.50 | [26] |
| Tea factory waste | 18.4 | Langmuir | ** | 4.00 | [27] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkhaleefa, A.; Ali, I.H.; Brima, E.I.; Elhag, A.B.; Karama, B. Efficient Removal of Ni(II) from Aqueous Solution by Date Seeds Powder Biosorbent: Adsorption Kinetics, Isotherm and Thermodynamics. Processes 2020, 8, 1001. https://doi.org/10.3390/pr8081001
Elkhaleefa A, Ali IH, Brima EI, Elhag AB, Karama B. Efficient Removal of Ni(II) from Aqueous Solution by Date Seeds Powder Biosorbent: Adsorption Kinetics, Isotherm and Thermodynamics. Processes. 2020; 8(8):1001. https://doi.org/10.3390/pr8081001
Chicago/Turabian StyleElkhaleefa, Abubakr, Ismat H. Ali, Eid I. Brima, A. B. Elhag, and Babiker Karama. 2020. "Efficient Removal of Ni(II) from Aqueous Solution by Date Seeds Powder Biosorbent: Adsorption Kinetics, Isotherm and Thermodynamics" Processes 8, no. 8: 1001. https://doi.org/10.3390/pr8081001
APA StyleElkhaleefa, A., Ali, I. H., Brima, E. I., Elhag, A. B., & Karama, B. (2020). Efficient Removal of Ni(II) from Aqueous Solution by Date Seeds Powder Biosorbent: Adsorption Kinetics, Isotherm and Thermodynamics. Processes, 8(8), 1001. https://doi.org/10.3390/pr8081001





