Abstract
Cell-free protein synthesis (CFPS) is an emerging tool for the rapid production of difficult-to-express proteins as well as for identifying protein synthesis bottlenecks. In CFPS, the biotic phase is substituted by extracts of living cells devoid of any of their own genetic material. The main advantage is that these systems delineate cell growth from recombinant protein production, enabling the expression of targets that would otherwise place too big a burden on living cells. We have conducted a techno-economic analysis of a CFPS system to produce monoclonal antibodies (mAbs) using extracts of Chinese hamster ovary (CHO) cells. We compare the performance of the CFPS system with two alternative production strategies: stable and transient gene expression in CHO cells. Our assessment shows that the viability of CFPS for mAb production requires a significant increase in the product yield and the recycling of high-cost components such as DNA. Nevertheless, CFPS shows significant promise for personalized medicine applications, providing a platform for on-demand production and simplified supply chains.
1. Introduction
Mammalian cells have been the dominant expression system for various types of recombinant proteins including monoclonal antibodies (mAbs), fusion proteins, and other high value proteins with complex folding and post-translational modification requirements [1,2,3]. More than 70% of commercial mAbs are industrially produced in Chinese hamster ovary (CHO) cells [4,5]. The large-scale production of mAbs is currently achieved by stable gene expression (SGE) of recombinant DNA, whereas transient gene expression (TGE) is also used to produce material for early-stage pre-clinical studies [6,7,8]. SGE involves the stable integration of the gene encoding for the recombinant protein in the DNA of the host cell, in this case CHO cells, by the application of a selective pressure, whereas in TGE the same gene is temporarily expressed for rapid protein production. TGE typically yields low product titres; although Backliwal et al. have reported a transient mAb yield > 1 g/L in human embryonic kidney HEK-293 cells in 14 day-cultures [8], milligram quantities of recombinant mAb have mostly been reported with CHO cells [9,10]. This contrasts with SGE, in which a 1–3 g mAb/L yield can be readily achieved [3,5], increasing to 5–10 g mAb/L in optimized fed-batch and perfusion systems [11,12].
Although CHO cell-based processes are successfully used for mAb production, they involve time-consuming cell line development and cloning steps for each new product [13]. Additionally, the energy trade-off between cell growth and recombinant protein production limits the product yield. Cell-free protein synthesis (CFPS) has emerged as an alternative, agnostic platform for the rapid production of small amounts of proteins that is particularly well-suited for difficult-to-express proteins [14,15]. Mammalian CFPS systems have been developed using Chinese hamster ovary (CHO) cell lysates, which have already been reported to produce antibodies and erythropoietin [16]. These systems offer direct access to the synthesis reaction components and environment, with ease in control and automation, while maintaining manufacturing consistency [17]. Additionally, in CFPS the generation of cell extracts is separate from the protein production steps, which simplifies the supply chain management for the manufacturing process [18]. However, the techno-economic assessment of CFPS and benchmarking against cell-based production routes are essential to ensure cost-effectiveness. The economics of cell-based production, particularly under SGE, have been widely evaluated previously [19,20,21,22,23].
In this work, we conduct a comparative techno-economic assessment of the manufacture of antibodies using intact CHO cells and CHO cell extracts. The CFPS process model is designed based on the experimental data reported by Thoring et al. for mAb synthesis using CHO cell lysates, essential amino acids, T7-RNA polymerase (T7-RNAP), and plasmid DNA [15,24]. This is compared with cell-based production at an industrial scale for stable antibody expression and at a small scale for transient antibody expression. All process models were developed and simulated in SuperPro Designer V10.0, which evaluates the overall process economics, including the total capital investment, annual operating cost, and cost of goods. We further -analyse the potential of the CFPS route by performing a rigorous sensitivity analysis considering uncertainties in various important economic factors. Finally, we consider the suitability of CFPS-based antibody expression for on-demand manufacture in personalized medicine applications and discuss opportunities for simplifying the supply chain management by improving the mAb manufacturing process.
2. Materials and Methods
SGE and TGE in CHO cells and CFPS production routes were considered to develop the process model for mAb manufacture. The simulation tool SuperPro Designer v10.0 was used to model all three production routes. Here, two production capacities were considered for the mAb manufacturing process: 200 kg/yr for industrial scale with SGE and CFPS and 25 kg/yr for mAb use in pre-clinical or early stage antibody drug development with TGE and CFPS.
The mAb production process model was divided into two main areas: upstream and downstream unit operations. The common upstream operations are inoculum preparation, fermentation, and harvest material clarification. Downstream operations mainly include Protein-A affinity chromatography, viral inactivation, ion exchange chromatography (IEX) and hydrophobic interaction chromatography (HIC), viral filtration, and polishing filtration for the final mAb recovery. A block diagram for each of the three production processes is presented in Figure S1.
2.1. Upstream Processing (USP)
2.1.1. Stable Gene Expression
SGE in CHO cells was considered to yield an average mAb titre of 3 g/L in 12 days [25,26]. The process was designed to produce 200 kg/yr of mAb as the final product. In the inoculum preparation section, seed train bioreactors were sized at 10% of the next bioreactor volume with 4 days of batch fermentation to reach a cell density of 4 × 106 cells/mL. The main production fermenter was operated in a fed-batch mode at 37 °C, and cells were grown in serum-free media to reach a final density of 16 × 106 cells/mL. A fermenter volume of 5800 L was used for the mAb production to achieve a 3 g/L mAb titre yield in 12 days. The total cycle time was 15 days, including cleaning and sterilization. The secreted mAb was isolated by disk stack centrifugation and stored in a harvest tank after passing through a polishing filter.
2.1.2. Transient Gene Expression
The TGE process model is based on the conservative literature value of the mAb yield of 250 mg/L in 14 days [27], although values of up to 1 g/L have been verbally reported by leading manufacturers. The model was designed to produce 25 kg of mAb/yr as the final product. In the inoculum preparation section, seed train bioreactors were sized at 10% of the next bioreactor volume with 3 days of batch fermentation to reach a cell density of 8 × 105 cells/mL. The main mAb production fermenter was operated in a fed-batch mode. The transfection condition was assumed as mild hypothermic with a temperature of 32 °C. It was assumed that transfection is performed at a cell density of 2 × 106 cells/mL with DNA addition as 1 mg/L cell culture volume and with the transfecting agent PEI at a mass ratio of PEI:DNA as 5:1. It was further assumed that the culture commenced at 37 °C, with mild hypothermic conditions (32 °C) introduced 24 h post transfection, according to Sou et al. [10].
2.1.3. Cell-Free Protein Synthesis
The upstream operations of CFPS consist of inoculum preparation, fermentation, cell extract preparation, and CFPS reaction for mAb synthesis. Like the cell-based process, the inoculum preparation was designed to reach a cell density of 4 × 106 cells/mL in 96 hr. CHO cells were collected after 5 days of fed-batch fermentation (at a cell density of 8 × 106 cells/mL) by centrifugation, when they were assumed to possess a higher translational activity. Cell extract preparation: Collected cells were re-suspended in an equal amount of binding buffer and lysed by the high-pressure homogenizer at 800 bar. The lysate solution was recovered after centrifugation and then fed to the CFPS reactor. CFPS reaction: The CFPS reaction was designed based on experimental data reported by Thoring et al. for a continuous exchange CHO cell-free reaction (CHO-CECF) at 33 °C with a titre of 1 g mAb/L in 2 days [15,24]. The CFPS reaction mixture comprised CHO cell-extract, CFPS medium, T7-RNA polymerase, and the DNA template. Here, CFPS medium is defined as a mixture of amino acids and energy components (Supplementary Table S1). The cell-free reaction mixture contained 50% (v/v) of v/v medium and 50% (v/v) of CHO cell-extract solution with the addition of 40 nM DNA and T7-RNA polymerase at 1 UNIT/µL of the reaction volume.
2.2. Downstream Processing (DSP)
DSP consisted of Protein-A chromatography, viral inactivation, two chromatographic separation steps, followed by viral filtration and then a diafiltration step for mAb recovery. Protein-A chromatography: The USP-harvested cell culture fluid (HCCF) was loaded onto the Protein-A chromatography column, and the number of chromatography cycles was estimated based on an assumed mAb binding capacity of 15 g/L [28]. Viral inactivation and polishing: Viral inactivation was performed using the detergent agent polysorbate-80 for 1.5 h. Subsequently, two additional chromatographic steps were included in DSP for the removal of mAb aggregates, residual DNA, host cell proteins (HCPs), leached protein-A, and viral particles: ion exchange chromatography (IEX) and Hydrophobic interaction chromatography (HIC). Viral filtration: Virus removal was achieved by passing the product solution through the ultrafiltration membrane. Diafiltration: Diafiltration followed by a polishing filter was the final step considered in DSP. The purified final product mAb was considered to be stored at −80 °C. It was assumed that mAb recovery in DSP was 75% for all three production routes at all scales [19,25].
3. Results
The mAb manufacturing process models were designed in SuperPro Designer (v10.0) for each production route, as presented in Supplementary Figures S1–S3, which include the equipment sizes. The bulk price of all raw materials was assumed to be 10% of the advertised retail price, as specified in the Supplementary Table S1.
3.1. Process Economics
3.1.1. Large-Scale Production
The economic analysis was performed on two different production capacities. First, we considered mAb production on an industrial scale, i.e., 200 kg mAb per year by SGE in cells and CFPS. Under SGE, 20 batches of an estimated cycle time of 15 days (363 hr) each are required to achieve the production target. The batch cycle time includes a 12-day production reactor step and 3 days of cleaning and sterilization. A bioreactor with a 5800 L capacity is estimated to produce 13.33 kg of mAb per batch, designed based on the reported literature value on the mAb yield of 3 g/L in 12 days achieved in the fed-batch CHO cell culture process [25]. The associated capital and operating costs are summarized in Table 1. The equipment cost was calculated in SuperPro Designer v10.0. With a 3 g/L mAb titre yield in USP and a 75% mAb recovery in DSP, the total capital investment for SGE was estimated to be $47.6 M, which involves direct fixed capital ($45 M), start-up and validation costs ($2.2 M), and working capital ($0.46 M). The annual operating cost was estimated to be $17.04 M, which includes $11.02 M USP operating costs and $6.02 M DSP costs. A detailed analysis is provided in Supplementary Table S2. Based on the operating cost, the overall production cost for the SGE route was estimated at $85/g mAb for a 200 kg/yr mAb production capacity facility. The annual production scale (kg/year) and mAb yield (g/L) affects the mAb cost per gram. In this paper, we further considered the variation of the annual production scale (kg/year) and mAb yield in the sensitivity analysis below to determine the mAb production cost. Werner reported COG/g in the range of $260–$1500/g for different mammalian systems producing mAb with a titre of 1 to 0.1 g/L, respectively, at an annual production scale of 250 kg/year [20,23]. Bunnak et al. provides a mAb COG ($/g) of $494/g and $504/g from a fed-batch with a titre of 5 g mAb/L and perfusion (with a titre of 2 g mAb/L) based processes, respectively, at a production scale of 28 kg/year [21]. Pollock et al. provides mAb COG for different process strategies of fed batches, alternating a tangential flow (ATF) in the range of $49/gm to $31/gm for a 500 kg/year production scale with an equivalent fed-batch titre of 5 g/L [22].

Table 1.
Process economics for mAb production for large-scale (SGE, CFPS) and small-scale manufacturing (TGE, CFPS).
The CFPS process model was designed based on the experimental studies by Thoring et al. which reported a cell-free reaction mAb yield of 1.0 g/L in 2 days [15,24]. The CHO cell cultivation and cell extract preparation steps were also included in the process model. In this CFPS route, the batch cycle time was estimated to be 160 hr based on a CHO cell culture duration of 120 hr in order to reach a cell density of 8 × 106 cells/mL, including 40 hr of cleaning and sterilization of the bioreactor. The CFPS reaction was performed in a 7300 L reactor capacity, which contained 50% (v/v) cell lysates and 50% CFPS medium with 1 UNIT/µL of T7-RNA polymerase (T7-RNAP) and 40 nM plasmid DNA. In the USP section, 5.82 kg mAb was produced per batch. Assuming a 75% mAb recovery in DSP, a total of 4.365 kg mAb are produced per batch, i.e., 45 batches per year are required to achieve the production target. As given in Table 1, the capital cost for the CFPS production route was estimated to be $113.0 M/yr with a $540 M/yr operating cost. Major contributors to the high operating cost are the raw materials of T7-RNAP (65%) and plasmid DNA (28%).The mAb production cost was estimated to be $2700/g mAb, including raw material (95.88%), facility dependency (2.13%), labour dependency (1.14%), consumables (0.72%), and utilities costs (0.02%). The equipment purchase cost was estimated to be 46% higher than the SGE production route due to the extensive fermentation for the extract preparation and the additional step of the cell-free reaction.
3.1.2. Small-Scale Production
In addition to large-scale production, we also evaluated the potential for using CFPS for the rapid provision of material for early-stage evaluation. An equivalent cell-based method is TGE, which rapidly produces material for pre-clinical studies. Commonly, TGE protocols involve a 7–14 day fermentation step (in addition to the time required to grow the cells from frozen stocks to the production reactor), whereas scaled-up CFPS could, in theory, provide the same amount of material in a timeframe of up to 2 days. Specifically, we chose to compare the two production methods for a production capacity of 25 kg/yr. The TGE process model was designed with a 250 mg/L mAb yield in 14 days post transfection [9]. The production reactor has a working volume of 7420 L, to which 7.4 g of DNA is added per batch (1 mg of DNA per one liter of cell culture volume). A total of 16 batches are required to produce 25 kg of mAb per year with a batch cycle time of 460 hr. After centrifugation and cell harvest, the supernatant is transferred to the DSP section, where four cycles of Protein-A chromatography, three cycles of ion-exchange, and three cycles of hydrophobic chromatography are required for purification. For the CFPS route, the mAb synthesis yield was assumed to be 1.0 g/L in 2 days, as before [15,24]. The production target can be achieved in 45 batches with a batch cycle time of 160 hr. For both production routes, the DNA cost was assumed to be $100/mg. The cost breakdown for the total capital investment and annual operating cost is given in Table 1. In the case of CFPS, the capital investment was calculated to be $53 M, while the annual operating cost was $81 M/yr. For TGE, the capital investment was estimated to be $50 M/yr with an annual operating cost estimated at $24 M/yr. The mAb production cost was estimated at $3230/g and $958/g in CFPS and TGE, respectively. DNA constitutes a significant cost component in both routes, contributing to 93% of the raw material cost in TGE and 28% in CFPS.
3.2. Sensitivity Analysis
A sensitivity analysis on the mAb production cost was performed by considering the variations of the most important process parameters that drive the economic performance of each production route. The base case values for all three routes are given in Table 2. The results are summarized in Figure 1 and Figure 2. Under SGE, we evaluated the impact of an increase in the mAb yield from 3 to 5 g/L: this would reduce the production cost by 10%, i.e., from $85/g to $76/g. In contrast, a 5% variation in raw material costs, such as less expensive serum-free cell culture media, resulted in a negligible (0.05%) reduction in the production cost.

Table 2.
The base case values for the process parameters in mAb production via SGE, CFPS, and TGE.
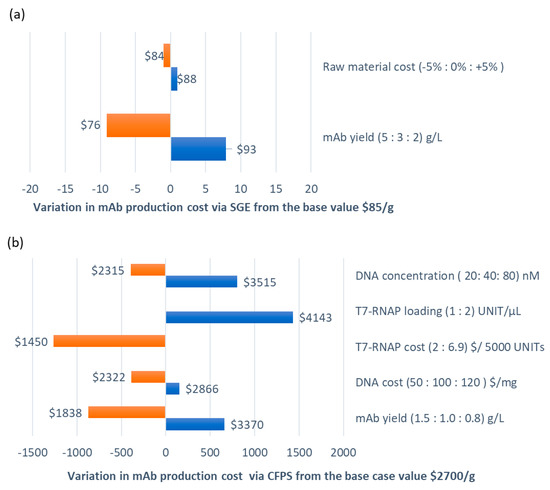
Figure 1.
Sensitivity analysis of the mAb production cost for large-scale manufacturing, 200 kg mAb/year via (a) SGE, base case mAb production cost = $85/g and (b) CFPS, base case mAb production cost = $2700/g.
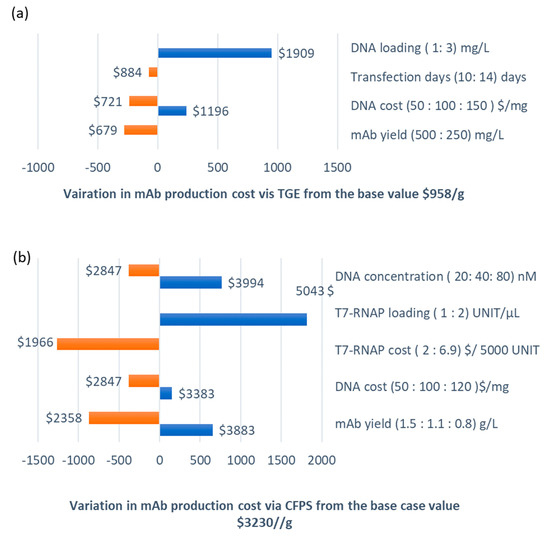
Figure 2.
Sensitivity analysis of the mAb production cost for small scale manufacturing, 25 kg mAb/year via (a) TGE, base case mAb production cost = $958/g and (b) CFPS, base case mAb production cost = $3230/g.
Under large-scale CFPS, the raw material cost has a significant impact on process economics as it contributes to 95% of the annual operating cost. Additionally, there is a high level of uncertainty in raw material costs as prices vary significantly between different suppliers. For instance as shown in Figure 1b, a change to the cost of T7-RNA polymerase from the base case value of $6.9/(5000 UNIT) (New England Biolabs, NEB, UK, cited on December 2019) to $2.0/(5000 UNIT) (Molecular Cloning Laboratories, McLab, USA, December 2019) would reduce costs by 46%, i.e., from $2700/g to $1450/g. Furthermore, lowering the DNA cost to $50/g from $100/g, would also bring the cost down to $2322/g. Variations in the CFPS reaction yield were also considered in the sensitivity analysis. An increase from 1.0 g/L to 1.5 g/L would reduce the unit cost by 32%, to $1838/g.
Figure 2a shows the sensitivity analysis results for the TGE route. The production cost was found to be dependent on the DNA cost, DNA loading per cell culture volume, mAb transfection titre yield, and cell culture duration. A reduction in the DNA cost to $50/mg would reduce the production cost by 25%, i.e., to $721.41/g. An increase in the mAb yield to 500 mg/L (base case value is 250 mg/L) after 14 days of culture would reduce the production cost by 30%. Similarly, if the base value for the yield (250 mg/L) could be achieved in 10 days instead of 14 days, the mAb production cost would be reduced by 7% to $884/g. However, the production cost doubles with an increase in DNA loading from 1 to 3 mg/L.
Figure 2b presents the sensitivity analysis results for the CFPS route at a 25 kg/yr capacity. Herein, we evaluated the impact of the same process parameters as in the large-scale case, and the results were broadly similar in terms of the direction and magnitude of the impact on the production cost.
Finally, Figure 3 presents the impact of the annual production scale on the mAb production cost: with an increase in the production scale, the mAb production cost decreases. However, this is dependent on the monoclonal antibodies demand for the treatment of diseases types. For large-scale manufacturing via the CFPS route, experimental validation at a demonstrated scale is important, as this could affect the process performance such as the mAb yield.
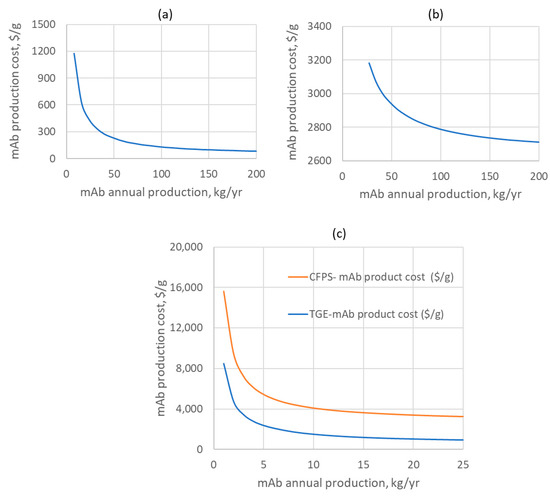
Figure 3.
The mAb production cost versus the annual throughput capacity for (a) SGE from 8 to 200 kg/yr; (b) large-scale CFPS from 27 to 200 kg/yr; and (c) small-scale TGE and CFPS from 1 to 25 kg/yr.
3.3. CFPS for Personalized Medicines Manufacture
Owing to the short reaction duration and system agility, CFPS carries a lot of promise for the on-demand production of personalized medicines. The system itself is agnostic to the protein of interest, and the DNA can be readily altered to encode for the protein of interest. CFPS can therefore serve as a production platform for a wide range of proteins on demand. Hence, we can envisage CFPS being deployed for producing proteins with patient-specific characteristics or for the treatment of orphan diseases for which large batch sizes are not required. Recently, Ogonah et al. reviewed the strengths and weaknesses of microbial and mammalian CFPS systems in terms of the sustainability of the supply chain, process scalability, and manufacturing potential for stratified medicines [18]. Since stratified medicines are targeted to a specific patient or to a group of patients, the production of a small-dosage amount can be achieved on-site using CFPS. Timm et al. have described a microfluidic bioreactor system where the concentration of active components was maintained with the continuous removal of inhibitors from the reaction system to achieve a high yield in the production of single-dose proteins [29]. The stratified medicine demand is dependent on the type of disease, population affected, and location where the direct supply of monoclonal antibodies or other therapeutic proteins is required. As an example, the production capacity of the Avastin monoclonal antibody could be ~28 kg/yr. This was estimated based on a dose size of 15.5 g of Avastin mAb per person over a year for lung cancer disease. Therefore, for the treatment of lung cancer patients in the United Kingdom, 28 kg of mAb per year will be required. The Supplementary Material section S2 specifies the detailed calculation for the amount of mAb required over the year and the number of lung cancer patients in the United Kingdom. With the currently available data on the fixed dosing of mAb for the treatment of various types of cancer, the production scale could range from 1 to 28 kg-mAb/yr [21]. In this paper, we designed the process model for personalized medicine by integrating the protein synthesis step with downstream purification, as shown in Supplementary Figure S3. To develop a model for a cell-free reaction, cell-lysate, T7-RNAP, plasmid DNA, and energy components were utilized, as described in the Materials and Methods, using the same assumptions for the downstream process development as before.
Although CFPS is typically employed for the production of small amount of proteins, Zawada et al. have demonstrated a process scale-up from 1 mL to 100 L for the consistent production of a cytokine using an Escherichia coli CFPS system [14]. Considering the scale-up consistency of CFPS, we estimated the cost of production of mAb for a 100 L reaction volume. The operating cost breakdown for the mAb synthesis process, including the purification step, is presented in Figure 4. Employing 100 L of CFPS reaction volume, ~78 g of purified mAb can be produced in a batch reaction within 48 hr. The mAb production cost was estimated at $3585/g. However, the production cost decreased by 22% to $2806/g of mAb when DNA was re-used, for example using immobilization on beads [30,31]. The cost of equipment and beads for this purpose has not been included in the calculations presented herein.
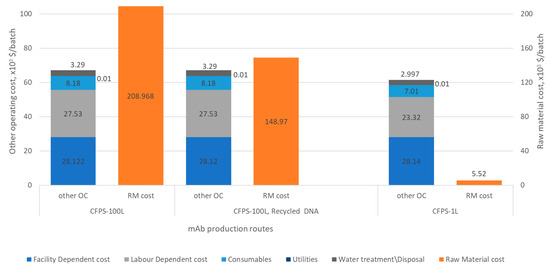
Figure 4.
Total annual operating cost (in thousand $) for 100 L and 1 L CFPS reaction volumes (other OC—Other Operating Cost = Facility-dependent, Labour-dependent, Consumables, Utilities, Water treatment/Disposal, Raw material).
With the currently available knowledge based on clinical studies on fixed dosing for lung cancer patients, 600 mg of mAb are required every 2 weeks [32]. Therefore, we also considered a process with a 1 L CFPS reaction volume. Figure 4 shows the operating cost for such a small-scale reactor. In this case, the operating cost is redefined by eliminating the facility-dependent cost (FDC) related to the maintenance and depreciation of equipment used in the process flowsheet, as at this scale it would be preferable to use single-use bioreactors. The redefined operating cost per batch was estimated as $38,854. If we consider DNA recycling, the mAb production cost was estimated to be $49/mg. This production cost decreases to $48/mg when the cost of T7-RNAP is taken to be $2/5000 UNITs. A 20% loss in DNA recovery results in the estimated mAb production cost varying between $42.38/mg and $41.12/mg for different T7-RNAP costs ($6.9 to $2/5000 UNITs, respectively, as in Supplementary Table S3). A detailed analysis of the effect that the extent of DNA recovery has on COG is presented in Supplementary Figure S4.
Decreasing the production scale from 100 L to 1 L increases the mAb production cost substantially from $2.8/mg to $48/mg (Figure 4). Therefore, for the assumed mAb demand of 20 g/person/year, the estimated COG (i.e., raw materials cost, as shown in Figure 4) is in the range of $54,277/person/year (100 L batch) to $127,714/person/year (1 L batch). This shows the potential of CFPS for cost-effective mAb manufacturing and a possible alternative for the production of personalized medicine, but it is highly dependent on the production scale. The production of therapeutic proteins using CFPS could be particularly well-suited for the production of small amounts of mAb in short timeframes, as short as 2 days. Some commercial in vitro translation kits reported a high yield of protein in 7–8 hr; for instance the Thermo Scientific 1-Step Human High-Yield In Vitro Translation (IVT) Kits enables the expression of up to 750 µg/mL functional proteins with a continuous-exchange cell-free (CECF) system in 7–8 hr (Thermo Fisher, Catalog number: 88890, Waltham, MA, U.S.A.). However, previous research has demonstrated only 100 mg/L of mAb production using a commercially available CHO extract [33]. Further development is necessary to increase the yield of CHO-based CFPS platforms to achieve the level of protein yields attainable with microbial systems, currently reported to be in the order of 1 g/L [34].
4. Discussion
In this work, we explored the economics of cell-free antibody synthesis and compared these with established cell-based production routes using stable gene expression at a large scale and transient gene expression at a small scale. Commercially, Sutro biopharma utilizes the cell-free route for the production of cytokine rhGM-CSF at a 200 L scale [14] and antibody fragments at a 5 L scale using bacterial extracts [35]. In our process model, we considered larger-scale production, at 200 kg/yr. Under SGE, this target could be achieved in 6000 L fermenters, whereas CFPS would require two 20,000 L fermenters for cell extract preparation. The CFPS reaction itself requires a 5000 L reactor. Similarly, at the 25 kg of mAb production capacity plant, 2 kg of mAb can be produced per batch in a 10,000 L fermenter under TGE. In contrast, the CFPS route would require a 5000 L for cell extract preparation and a ~1000 L reactor for the CFPS reaction. This inverse trend in reactor volumes can be explained by the fact that the mAb productivity is higher in CFPS at 0.55 g mAb/L/day in comparison to 0.25 g/L/day in SGE and 0.017 g/L/day in TGE.
Process economics are considerably more favorable, however, when considering the production of small amounts of product for personalized applications or for the production of orphan drugs. Limited patient numbers do not justify the construction of manufacturing facilities for the latter, which paves the way for CFPS to become the preferred production platform thanks to the limited capital expenditure that is required. The treatment costs for orphan drugs can amount to $300,000–$500,000/patient/year, including the recovery of Research and Development costs not accounted for herein [36]. Our estimates for COG for the low-scale CFPS platform (100 L synthesis reaction) are in line with such figures.
An advantage of the CFPS route is that the cell extract preparation steps require less monitoring than the production reactor under SGE/TGE, while the cell-free reaction step itself takes place in a fully controlled environment. Decoupling the fermentation step from the synthesis reaction also means that the components of the CFPS platform can be pre-prepared and deployed rapidly to express any product on demand, without the need to revive frozen cell stocks, which adds to time savings.
Overall, the mAb production cost was found to be higher for the CFPS route at both production scales. A cost-effective energy provision is essential when demonstrating commercial applicability. In one of the leading publications by Sutro Biopharma on immunoglobulin expression in E. coli cell-free protein synthesis [37], the authors demonstrated the use of in-house technology for the preparation of expensive reagents to reduce the reagent cost for CFPS reactions, particularly for T7-RNAP and plasmid DNA reagents. In our CFPS process model, we observed that T7-RNAP and DNA contribute to 98% of the total raw material cost. This is due to the fact that our process model takes into account the addition of fresh T7-RNAP and DNA to each batch. Recently, Levine et al. reported DNA recycling in E. coli CFPS by maintaining the magnesium levels required for DNA replication, transcription, and translation [38]. In our CFPS process model, re-using plasmid DNA could reduce operating costs by up to 29%, and thus mAb production costs are estimated to be $2465/g at a smaller scale and $1933/g for large-scale production.
5. Conclusions
We performed a comprehensive techno-economic analysis on the use of a CHO cell-derived CFPS system for large- and medium-scale productions of mAbs, as well as for the production of personalized medicines. Our results show that its cost effectiveness highly depends on scale and intended application, with large-scale production being less favorable to it when compared to traditional stable expression in CHO cells. In contrast, CFPS offers a higher flexibility and controllability, which is necessary for distributed manufacturing, e.g., for the on-demand provision of personalized medicines. Additionally, CFPS facilitates a considerably faster production in the order of hours/days when compared to weeks when using cell-based systems, while the associated process economics are also favorable. The detailed information presented in this work can be used for the selection of the most suitable production route based on either the processing time or the protein cost. Furthermore, the in-house preparation of expensive components would pave the way for more economical and sustainable CFPS systems.
Supplementary Materials
The following are available online at https://www.mdpi.com/2227-9717/8/4/454/s1, Figure S1: Schematic process block diagrams for large-scale mAb production (200 kg/year) by: (a) SGE and (b) CFPS; Figure S2: Schematic process block diagrams for small-scale mAb production (25 kg/year) by (a) TGE and (b) CFPS; Figure S3: Process flow for personalized medicine manufacturing process by CFPS; Figure S4: The effect of DNA recovery on mAb COG, $/person/year for the assumed mAb demand as 20 g mAb/person/year. (mAb COG = Raw material cost); Table S1: CFPS medium components; Table S2: Process Economic data for large-scale manufacturing via SGE and CFPS production route (200 kg mAb/year); Table S3: The operating cost per batch of 1 L CFPS reaction volume.
Author Contributions
Conceptualization, V.T., N.S. and C.K.; methodology, V.T., D.T. and C.K.; analysis, V.T. and D.T.; writing—Original draft preparation, V.T.; writing—Review and editing, V.T., D.T., N.S. and C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was performed with financial support from the UK Engineering and Physical Sciences Research Council under grant EP/K038648/1.
Acknowledgments
The authors thank Karen Polizzi and Chiara Heide for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trill, J.J.; Shatzman, A.R.; Subinay, G. Production of monoclonal antibodies in COS and CHO cells. Curr. Opin. Biotechnol. 1995, 6, 553–560. [Google Scholar] [CrossRef]
- Oberbek, A.; Matasci, M.; Hacker, D.L.; Wurm, F.M. Generation of stable, high-producing cho cell lines by lentiviral vector-mediated gene transfer in serum-free suspension culture. Biotechnol. Bioeng. 2010, 108, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Kunert, R.; Reinhart, D. Advances in recombinant antibody manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.-G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2011, 93, 917–930. [Google Scholar] [CrossRef]
- Jayapal, K.; Wlaschin, K.F.; Hu, W.S.; Yap, M.G.S. Recombinant protein therapeutics from CHO Cells—20 years and counting. Chem. Eng. Prog. 2007, 103, 40–47. [Google Scholar]
- Zhu, J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol. Adv. 2012, 30, 1158–1170. [Google Scholar] [CrossRef]
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393–1398. [Google Scholar] [CrossRef]
- Backliwal, G.; Hildinger, M.; Hasija, V.; Wurm, F.M. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol. Bioeng. 2007, 99, 721–727. [Google Scholar] [CrossRef]
- Rajendra, Y.; Kiseljak, D.; Baldi, L.; Hacker, D.L.; Wurm, F.M. A simple high-yielding process for transient gene expression in CHO cells. J. Biotechnol. 2011, 153, 22–26. [Google Scholar] [CrossRef]
- Sou, S.N.; Lee, K.; Nayyar, K.; Polizzi, K.M.; Sellick, C.; Kontoravdi, C. Exploring cellular behavior under transient gene expression and its impact on mAb productivity and Fc-glycosylation. Biotechnol. Bioeng. 2017, 115, 512–518. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Hu, W.; Rustandi, E.; Chang, K.; Ryll, T.; Yusuf-Makagiansar, H. Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol. Prog. 2010, 26, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Lee, K.; Rucker-Pezzini, J.; Lee, J.H. Implementation of Fully Integrated Continuous Antibody Processing: Effects on Productivity and COGm. Biotechnol. J. 2018, 14. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.D.; Gan, R.; Hodgman, C.E.; Jewett, M.C. Cell-free protein synthesis: Applications come of age. Biotechnol. Adv. 2011, 30, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Zawada, J.F.; Yin, G.; Steiner, A.R.; Yang, J.; Naresh, A.; Roy, S.M.; Gold, D.S.; Heinsohn, H.G.; Murray, C.J. Microscale to manufacturing scale-up of cell-free cytokine production-a new approach for shortening protein production development timelines. Biotechnol. Bioeng. 2011, 108, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Thoring, L.; Dondapati, S.K.; Stech, M.; Wüstenhagen, D.A.; Kubick, S. High-yield production of “difficult-to-express” proteins in a continuous exchange cell-free system based on CHO cell lysates. Sci. Rep. 2017, 7, 11710. [Google Scholar] [CrossRef] [PubMed]
- Brödel, A.K.; Wüstenhagen, D.A.; Kubick, S. Cell-Free Protein Synthesis Systems Derived from Cultured Mammalian Cells. Adv. Struct. Saf. Stud. 2014, 1261, 129–140. [Google Scholar]
- Heide, C.; Ces, O.; Polizzi, K.; Kontoravdi, C. Creating cell-free protein synthesis factories. Pharm. Bioprocess. 2018, 6, 3–6. [Google Scholar]
- Ogonah, O.; Polizzi, K.M.; Bracewell, D. Cell free protein synthesis: A viable option for stratified medicines manufacturing? Curr. Opin. Chem. Eng. 2017, 18, 77–83. [Google Scholar] [CrossRef]
- Farid, S. Process economic drivers in industrial monoclonal antibody manufacture. Process. Scale Purif. Antib. 2009, 12, 445–466. [Google Scholar]
- Farid, S.S. Process economics of industrial monoclonal antibody manufacture. J. Chromatogr. B 2007, 848, 8–18. [Google Scholar] [CrossRef]
- Bunnak, P.; Allmendinger, R.; Ramasamy, S.V.; Lettieri, P.; Titchener-Hooker, N.J. Life-cycle and cost of goods assessment of fed-batch and perfusion-based manufacturing processes for mAbs. Biotechnol. Prog. 2016, 32, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Pollock, J.; Ho, S.V.; Farid, S.S. Fed-batch and perfusion culture processes: Economic, environmental, and operational feasibility under uncertainty. Biotechnol. Bioeng. 2012, 110, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Werner, R.G. Economic aspects of commercial manufacture of biopharmaceuticals. J. Biotechnol. 2004, 113, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Thoring, L.; Wüstenhagen, D.A.; Borowiak, M.; Stech, M.; Sonnabend, A.; Kubick, S. Cell-Free Systems Based on CHO Cell Lysates: Optimization Strategies, Synthesis of “Difficult-to-Express” Proteins and Future Perspectives. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B. Industrialization of mAb production technology: The bioprocessing industry at a crossroads. mAbs 2009, 1, 443–452. [Google Scholar] [CrossRef]
- Jiang, Z.; Droms, K.; Geng, Z.; Casnocha, S.; Xiao, Z.; Gorfien, S.; Jacobia, S.J. Fed-Batch Cell Culture Process Optimization. Bioprocess. Int. 2012, 10, 40–45. [Google Scholar]
- Pereira, J.; Rajendra, Y.; Baldi, L.; Hacker, D.L.; Wurm, F.M. Transient gene expression with CHO cells in conditioned medium: A study using TubeSpin® bioreactors. BMC Proc. 2011, 5, P38. [Google Scholar] [CrossRef]
- Liu, H.F.; Ma, J.; Winter, C.; Bayer, R. Recovery and purification process development for monoclonal antibody production. mAbs 2010, 2, 480–499. [Google Scholar] [CrossRef]
- Timm, A.; Shankles, P.G.; Foster, C.M.; Doktycz, M.; Retterer, S.T. Microreactors: Toward Microfluidic Reactors for Cell-Free Protein Synthesis at the Point-of-Care (Small 6/2016). Small 2016, 12, 690. [Google Scholar] [CrossRef]
- Finkler, M.; Ott, A. Bead-based assay for spatiotemporal gene expression control in cell-free transcription–translation systems. Biotechnology 2019, 66, 29–33. [Google Scholar] [CrossRef]
- Nord, O.; Uhlén, M.; Nygren, P.-Å. Microbead display of proteins by cell-free expression of anchored DNA. J. Biotechnol. 2003, 106, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, J.J.; Haanen, J.B.; Voest, E.E.; Schellens, J.H.; Huitema, A.D.; Beijnen, J.H. Fixed Dosing of Monoclonal Antibodies in Oncology. Oncology 2017, 22, 1212–1221. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.W.; Majewska, N.; Chen, C.X.; Albanetti, T.E.; Jimenez, R.B.C.; Schmelzer, A.E.; Jewett, M.C.; Roy, V. Development of a CHO-Based Cell-Free Platform for Synthesis of Active Monoclonal Antibodies. Acs Synth. Biol. 2017, 6, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Sutro Biopharma Inc. Xpress CFTM: A Rapid Platform for Drug Development From Antibody Discovery to Manufacturing; Sutro Biopharma Inc.: San Francisco, CA, USA, 2014. [Google Scholar]
- Yin, G.; Garces, E.D.; Yang, J.; Zhang, J.; Tran, C.; Steiner, A.R.; Roos, C.; Bajad, S.; Hudak, S.; Penta, K.; et al. Aglycosylated antibodies and antibody fragments produced in a scalable in vitro transcription-translation system. mAbs 2012, 4, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Jayasundara, K.; Hollis, A.; Krahn, M.D.; Mamdani, M.; Hoch, J.S.; Grootendorst, P. Estimating the clinical cost of drug development for orphan versus non-orphan drugs. Orphanet J. Rare Dis. 2019, 14, 12. [Google Scholar] [CrossRef]
- Cai, Q.; Hanson, J.A.; Steiner, A.R.; Tran, C.; Masikat, M.R.; Chen, R.; Zawada, J.F.; Sato, A.K.; Hallam, T.J.; Yin, G. A simplified and robust protocol for immunoglobulin expression inEscherichia colicell-free protein synthesis systems. Biotechnol. Prog. 2015, 31, 823–831. [Google Scholar] [CrossRef]
- Levine, M.; Gregorio, N.E.; Jewett, M.C.; Watts, K.R.; Oza, J.P. Escherichia coli-Based Cell-Free Protein Synthesis: Protocols for a robust, flexible, and accessible platform technology. J. Vis. Exp. 2019, e58882. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).