Abstract
The latest class of engineered nanomaterials, viz., carbon quantum dots (CQDs), has attracted attention because they are synthesized through green chemical procedures and from organic waste matter. The synthesis of these nano-sized particles synthesized from biomass such as fruit peel and other organic matter results in mixtures of CQD species that differ in chemical identity, activity and photo-physical properties. Generally used collectively as chemically heterogeneous ensemble, they have already had an impact on multiple sectors of our environment by use as wastewater sensors, switches, model agro-fertilizers, and in biomedicine. The transitioning of their applications to crops is an important crossover point that calls for an accurate and detailed assessment of their genomic, proteomic, and metabolomics impact on agriculturally important crops and produce. We review the current status of CQDs vis-à-vis their impact on the biosphere via recent model studies and comment on the knowledge gaps that need to be bridged to ensure their safe use in agronomy. A detailed knowledge of their impact on aquatic systems and the food-chain is critical for human and environmental safety and sustainability.
1. Introduction
Carbon quantum dots (CQDs) have emerged as a viable and relatively biofriendly successor to inorganic nanoparticles, such as CdS, CeO2, gold nanoparticles, and paramagnetic lanthanide ions. CQDs have drawn attention due to their enticing chemical, physical, optical, and surface properties. Restricted in size from 2–10 nm, naturally fluorescent CQDs have found widespread application in environmental sensing, water purification, biosensing, imaging, as switches, theranostics, prophylactics, and therapeutics [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37].
The fabrication of CQDs from neat carbon-containing sources such as citric acid, resorcinol, urea, sugars, as well as from a variety of benign agro-based waste products including lemon peel, leaves, water melon shell, bio-waste lignin, and paper has made them particularly attractive in terms of reducing the environmental impact and the carbon footprint [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
The mode of synthesis is also relatively eco-friendly. Compared to other reported techniques including laser ablation, electric arc, and microwave-assisted protocols, the hydrothermal method affords the greatest control, is relatively inexpensive, and is environmentally friendly [13,18,37]. Through this method, CQDs are routinely engineered from the aforementioned natural carbon precursors without the involvement of any solvents that may be toxic or hazardous.
2. CQDs in Plants
It is of particular interest to note that CQDs, as a consequence of their innately high hydrophilicity and high cell permeability, have frequently come to be used in water-based and biologically relevant applications [12,19].
For example, in a relatively recent and landmark study, the individual impact of five oxygen-containing graphene-derived CQDs was measured on rice plants [12]. Using microscopy imaging, the CQDs were found to penetrate all parts of the plant, albeit to different extents. “CQD-1”, which possessed the highest oxygen content among those tested, infiltrated the nucleus and elicited the greatest number of physiological responses. By interfering with the DNA structure CQD-1 increased the Os06g32600 gene expression (thionin), which in turn enhanced the rice-plant disease-resistance ability. CQD-1 addition also was also correlated with a 42% increase in RuBisCO activity. The metabolic fate of CQDs being structurally similar to plant-hormones is perhaps a cause for their promotion of plant growth. Additionally, the CO2 liberated during CQD catabolism is likely to fuel the anabolic photosynthetic pathways. A net increase in rice yield and enhanced disease-resistance were the favorable phenotypic end-results [12].
The focus in CQDs as engineered nanomaterials (ENMs) have to date centered largely on green synthetic routes and applications. However, there is a lack of necessary and rigorous understanding regarding the environmental effects of CQDs, such as its transport, fate, and impact in the biome. Nevertheless, as discussed subsequently, the mere notion that carbon-based QDs are less toxic than their inorganic counterparts is not a sufficient or valid reason to disregard issues related to their effects on the ecosphere.
While the model rice study appears to suggest that CQD infiltration in a plant species is relatively innocuous, it is to be noted that the synthesis of CQDs produces an ensemble of CQD and non-CQD species as evidenced from a recent study describing the preparation of CQDs from resorcinol, which revealed the presence of 21 chemically distinct species using reversed-phase HPLC. [11]. Furthermore, based on their fluorescence signatures, only 10 out of the 21 compounds generated through the hydrothermal treatment of resorcinol could be classified as CQDs. At the time of writing, there is no toxicological information or exact chemical classification available on any of the 21 compounds [14]. CQDs 1–5 from the rice study are precisely termed as such for the same underlying cause.
3. CQD Chemical Footprint and the Biome
Of late, CQDs have been synthesized from cigarette waste [1,2,9] and have also been sourced from diesel exhaust [25]. Yet, studies concerning the environmental risks of CQDs to both plant and aquatic lifeforms appear to be largely inadequate and have been ignored.
To compound this status, even the mildest technique used for fabricating CQDs, viz., hydrothermal synthesis, produces byproducts beyond the desired sp2 and sp3 hybridized carbon frameworks [16]. These include reactive carbonyls such as keto-aldehydes, dicarbonyls, and reductones, among others that act as precursors to toxic products including glyoxal, methylglyoxal, 3-deoxyglucosone (3-DG), hetero-cyclic amines, and acryl amides. Most of these chemical entities could potentially constitute byproducts that might not possess fluorescence and hence may not be easily tracked or followed via fluorescence-based tracking protocols. This might result in the entry of toxic agents into the biosphere without being getting detected and/or further examined [26,30].
By way of example, citric acid and ethylenediamine-condensed carbonized CQDs were found to induce mortality and immobility in D. magna with a 48-h EC50 value and LC50 value of 97.5 and 160.3 mg/L, respectively [33]. These results suggest that the CQDs themselves, or their byproducts, were toxic. In green algae (S. obliquus), the same CQDs inhibited photosynthesis and nutrition absorption in a dose- and time-dependent manner [33]. Furthermore, algal growth was also inhibited with a 96-h EC50 value of 74.8 mg/L. The citric acid and ethylenediamine-derived CQDs induced an increase in oxidative stress in algae cells and a decrease in pH value of an algae medium, indicating that oxidative stress and water acidification may be the mechanisms underlying the toxicity of CQDs to S. obliquus [33].
Even considering a scenario wherein these products are non-toxic to plants, their entry into the food chain could result in a health hazard (Scheme 1). It therefore behooves the community to turn to the lessons learned from the presence of nanoparticles present in the environment. Initially ignored, nanoparticle impact on plant growth, bioaccumulation, and their impact on the aquatic environment have now become studies in their own right, with the emerging results demonstrating the impact they have on agricultural produce and leading to the shaping of global policies. Similarly, it becomes important to fractionate CQD ensembles into their individual components and establish their chemical identity prior to the testing of chemically singular species on the genomics, proteomics, or metabolomics of plants.
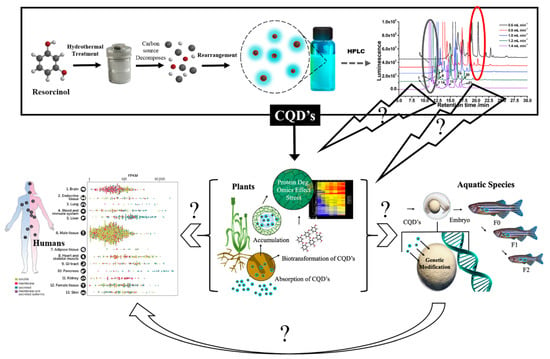
Scheme 1.
Synthesis of CQDs (top) and their yet-to-be-determined impact on the biosphere including plants (through agri-use; bottom center); other elements of the food-chain (bottom-left) and on the apex consumers (bottom-right). CQDs are synthesized as a mixture of species (top-right) whose individual effects on the biosphere are also yet to be established.
The terminal sink for all pollutants, the aquatic ecosystem, plays important roles in the full life cycle of ENMs due to their intentional and unintentional release [22]. The behavior of ENMs in the aquatic ecosystem has thus become an urgent issue in view of their risk assessment. The varied sources of CQDs, and the ease with which they are prepared, suggests that these recently discovered nanosized engineered carbon-based materials will enter the environment faster than the metal-based counterparts [1,14].
4. Conclusions
In closing, only a few papers have shown the effects of CQDs in plants. Zheng et al., for example, reported that a 25-day exposure of 10–30 mg/L of CQDs, obtained from rapeseed (Brassica napus) pollen, to hydroponically grown Rome lettuce (Lactuca sativa) improved plant growth. The mechanism was suggested to be through an increase in leaf number and foliar area [35]. They also reported that the 20 mg/L CQD treatment reduced nitrate content in leaves; suggesting that the CQDs interfere with physiological pathways. Although the data is of interest and hints at agricultural uses, the lettuce plants were exposed to a family of CQDs, which makes it difficult to link the plant response to a specific biochemical mechanism altered by a singular chemical stimulant. In another study, 400 and 800 mg/L of 4 nm citric acid-derived CQDs were exposed for two weeks to pumpkin (Cucurbita pepo) seedlings. The treatments consisted of pristine, polyacrylic acid (PAA), and polyethylenimine (PEI) surface modified CQDs. The authors reported that the CQDs-PEI significantly decreased total fresh weight and altered stress enzymes [2]. However, the exposure period does not permit the prediction of these effects in a life-cycle plant growth. This includes the unfeasibility of evaluating the effects on the final product (the fruit). In addition, as in the previously mentioned study, the article does not show a full characterization of the pristine CQDs or proofs of an effective surface coating. Finally, the study by Li et al. depicts the first sign of the genotoxic impact of CQDs [12]. These emerging papers have shown significant impacts of CQDs at physiological and molecular levels in higher plants.
However, as depicted in Scheme 1, data on the environmental fate, bioaccumulation in plants and aquatic organisms, compartmentalization, biotransformation, persistence in the environment, mutagenic or transgenerational effects are yet to be gathered. In summary, while CQD production could reach massive scales in a short time period, our knowledge about their environmental impacts is practically in its infancy. Moreover, the data from Li et al. reveal signs of genotoxicity in rice plants, which could cause serious impacts in living organisms [12]. If CQDs accumulate in plants and move in the trophic chain, they will finally reach the apex consumers generating the million dollar question: will the human health be affected?
The National Organic Standards Board made recommendations in 2010 that ENM’s be barred from qualifying for the USDA’s much sought-after “Organic” moniker. A similar fate may await CQDs which may likely be cast in the same category as “GMO” as far as organic foods are concerned. Worse, a lack of adequate knowledge of CQDs may trigger outcries for a total ban on their consumer use, resulting in a premature discarding of this organic ENM without realizing its true potential.
The scientific community invested in securing and preserving the ecological and agricultural wealth must apply the same rigor to understanding CQD impact on the environment, as has been done for other inorganic ENMs. The footprint exercised by this confined nanostructure is likely to be ginormous. Whether “biobeneficial” or “hazardous” is yet to-be-determined. To establish an accurate footprint of CQD impact on any aspect of the biome, resolution of the following variables is necessary: (1) Determine whether the CQD ensemble is purely made of CQDs or whether byproducts are also present; (2) Fractionate the CQD ensemble (Scheme 1) into its constituents and collect the individual components separately; (3) Test whether the individual species are truly CQDs (using fluorescence and size assays); (4) If needed (particularly for biomedical applications), determine the chemical structure of the individual components including molar mass, chemical constitution and spatial orientation; (5) Establish the cytotoxic profile of each constituent using cell line, aquatic, terrestrial and agricultural models; and (6) Finally, test the biobeneficial aspect of the CQD. This will depend upon its application in agriculture, in animal husbandry or in biomedicine (Scheme 1).
It is possible to first test for (6) and then, depending upon the results, assay for 1–5. This insulates against wasting time and effort on 1–5, if the CQD fails in (6).
The challenges (1–5) with respect to effort and cost spent in robustly characterizing CQDs from their “as-is” mixture are worthwhile in mitigating the short- and long-term potential risks of their use toward the environment and human population.
Author Contributions
J.P.-V., S.T.S., and M.N., contributed equally to the conceptualization of the paper. M.N. and J.P.-V. wrote the paper and M.N., S.T.S., and J.P.-V. edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank Angela Lopez for generating Scheme 1.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anmei, S.; Qingmei, Z.; Yuye, C.; Yilin, W. Preparation of carbon quantum dots from cigarette filters and its application for fluorescence detection of Sudan I. Anal. Chim. Acta 2018, 1023, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bandi, R.; Devulapalli, N.P.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Facile Conversion of Toxic Cigarette Butts to N,S-Codoped Carbon Dots and Their Application in Fluorescent Film, Security Ink, Bioimaging, Sensing and Logic Gate Operation. ACS Omega 2018, 3, 13454–13466. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.W.; Adeleye, A.; Ji, Z.; Keller, A.A. Stability, metal leaching, photoactivity and toxicity in freshwater systems of commercial single wall carbon nanotubes. Water Res. 2013, 47, 4074–4085. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, X.; Meziani, M.J.; Lu, F.; Wang, H.; Luo, P.G.; Lin, Y.; Harruff, B.A.; Veca, L.M.; Murray, D. Carbon dots for multiphoton bioimaging. J. Am. Chem. Soc. 2007, 129, 11318–11319. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, S.-T.; Wang, X.; Luo, P.G.; Liu, J.-H.; Sahu, S.; Liu, Y.; Sun, Y.-P. Competitive performance of carbon “quantum” dots in optical bioimaging. Theranostics 2012, 2, 295–301. [Google Scholar] [CrossRef]
- De, B.; Karak, N. A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice. RSC Adv. 2013, 3, 8286–8290. [Google Scholar] [CrossRef]
- Frigerio, C.; Ribeiro, D.S.; Rodrigues, S.S.M.; Abreu, V.L.; Barbosa, J.A.; Prior, J.A.; Marques, K.L.; Santos, J.L. Application of quantum dots as analytical tools in automated chemical analysis: A review. Anal. Chim. Acta 2012, 735, 9–22. [Google Scholar] [CrossRef]
- Hurt, R.H.; Monthioux, M.; Kane, A. Toxicology of carbon nanomaterials: Status, trends, and perspectives on the special issue. Carbon 2006, 44, 1028–1033. [Google Scholar] [CrossRef]
- Kandasamy, G. Recent Advancements in Doped/Co-Doped Carbon Quantum Dots for Multi-Potential Applications. C—J. Carbon Res. 2019, 5, 24. [Google Scholar] [CrossRef]
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.; Choi, S.; Kwon, S.H.; et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 2018, 13, 812–818. [Google Scholar] [CrossRef]
- Li, C.-L.; Ou, C.-M.; Huang, C.-C.; Wu, W.-C.; Chen, Y.-P.; Lin, T.-E.; Ho, L.-C.; Wang, C.-W.; Shih, C.-C.; Zhou, H.-C.; et al. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J. Mater. Chem. B 2014, 2, 4564–4571. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Lu, F.; Liu, Y.; Song, Y.; Sun, Y.; Zhong, J.; Huang, H.; Wang, Y.; Li, S.; et al. Impacts of Carbon Dots on Rice Plants: Boosting the Growth and Improving the Disease Resistance. ACS Appl. Bio Mater. 2018, 1, 663–672. [Google Scholar] [CrossRef]
- Li, X.; Rui, M.; Song, J.; Shen, Z.; Zeng, H. Carbon and graphene quantum dots for optoelectronic and energy devices: A review. Adv. Funct. Mater. 2015, 25, 4929–4947. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, J.; Yuan, H.; Xiao, D. Separation of carbon quantum dots on a C18 column by binary gradient elution via HPLC. Anal. Methods 2014, 6, 8124–8128. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental applications of carbon-based nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Molaei, M.J. Carbon quantum dots and their biomedical and therapeutic applications: A review. RSC Adv. 2019, 9, 6460–6481. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon nanomaterials in agriculture: A critical review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Namdari, P.; Negahdari, B.; Eatemadi, A. Synthesis, properties and biomedical applications of carbon-based quantum dots: An updated review. Biomed. Pharmacother. 2017, 87, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Guo, H.; Chen, G.; Ma, C.; Xing, B. Distribution of different surface modified carbon dots in pumpkin seedlings. Sci. Rep. 2018, 8, 7991. [Google Scholar] [CrossRef]
- Qu, S.; Wang, X.; Lu, Q.; Liu, X.; Wang, L. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew. Chem. Int. Ed. 2012, 51, 12215–12218. [Google Scholar] [CrossRef]
- Sachdev, A.; Gopinath, P. Green synthesis of multifunctional carbon dots from coriander leaves and their potential application as antioxidants, sensors and bioimaging agents. Analyst 2015, 140, 4260–4269. [Google Scholar] [CrossRef] [PubMed]
- Selck, H.; Handy, R.D.; Fernandes, T.F.; Klaine, S.J.; Petersen, E.J. Nanomaterials in the aquatic environment: A European Union-United States perspective on the status of ecotoxicity testing, research priorities, and challenges ahead. Environ. Toxicol. Chem. 2016, 35, 1055–1067. [Google Scholar] [CrossRef]
- Servin, A.; Elmer, W.; Mukherjee, A.; De la Torre-Roche, R.; Hamdi, H.; White, J.C.; Bindraban, P.; Dimkpa, C. A review of the use of engineered nanomaterials to suppress plant disease and enhance crop yield. J. Nanopart. Res. 2015, 17, 92. [Google Scholar] [CrossRef]
- Sharon, M.; Sharon, M. Carbon Nanoforms and Applications; McGraw-Hill: New York, NY, USA, 2010. [Google Scholar]
- Tripathi, K.M.; Sonker, A.K.; Sonkar, S.K.; Sarkar, S. Pollutant soot of diesel engine exhaust transformed to carbon dots for multicoloured imaging of E. coli and sensing cholesterol. RSC Adv. 2014, 4, 30100–30107. [Google Scholar] [CrossRef]
- Wei, W.; Xu, C.; Wu, L.; Wang, J.; Ren, J.; Qu, X. Non-enzymatic-browning-reaction: A versatile route for production of nitrogen-doped carbon dots with tunable multicolor luminescent display. Sci. Rep. 2014, 4, 3564. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Li, L.; Liu, J.; Yu, L.; Li, H.; Cheng, F.; Yi, X.; He, J.; Li, B. Green Synthesis of Fluorescent Carbon Dots from Gynostemma for Bioimaging and Antioxidant in Zebrafish. ACS Appl. Mater. Interfaces 2019, 11, 9832–9840. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Cai, W.; Li, W.; Sreeprasad, T.S.; He, Z.; Ong, W.-J.; Li, N. Two-dimensional quantum dots: Fundamentals, photoluminescence mechanism and their energy and environmental applications. Mater. Today Energy 2018, 10, 222–240. [Google Scholar] [CrossRef]
- Xu, Q.; Kuang, T.; Liu, Y.; Cai, L.; Peng, X.; Sreenivasan Sreeprasad, T.; Zhao, P.; Yu, Z.; Li, N. Heteroatom-doped carbon dots: Synthesis, characterization, properties, photoluminescence mechanism and biological applications. J. Mater. Chem. B 2016, 4, 7204–7219. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.; Gao, C.; Wei, J.; Zhou, H.; Chen, Y.; Dong, C.; Sreeprasad, T.S.; Li, N.; Xia, Z. Synthesis, mechanistic investigation, and application of photoluminescent sulfur and nitrogen co-doped carbon dots. J. Mater. Chem. C 2015, 3, 9885–9893. [Google Scholar] [CrossRef]
- Yang, S.-T.; Cao, L.; Luo, P.G.; Lu, F.; Wang, X.; Wang, H.; Meziani, M.J.; Liu, Y.; Qi, G.; Sun, Y.-P. Carbon dots for optical imaging in vivo. J. Am. Chem. Soc. 2009, 131, 11308–11309. [Google Scholar] [CrossRef]
- Yang, S.-T.; Wang, X.; Wang, H.; Lu, F.; Luo, P.G.; Cao, L.; Meziani, M.J.; Liu, J.-H.; Liu, Y.; Chen, M. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J. Phys. Chem. C 2009, 113, 18110–18114. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Lv, X.; Zheng, G.; Chen, Z.; Jiang, Y.; Zhu, X.; Wang, Z.; Cai, Z. Effects of Carbon Quantum Dots on Aquatic Environments: Comparison of Toxicity to Organisms at Different Trophic Levels. Environ. Sci. Technol. 2018, 52, 14445–14451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.-W.; Meng, X.; Lee, C.-S.; Wang, P.; Zhang, W. Green Synthesis of Bifunctional Fluorescent Carbon Dots from Garlic for Cellular Imaging and Free Radical Scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, G.; Zhang, X.; Chen, Z.; Cai, Y.; Yu, W.; Liu, H.; Shan, J.; Li, R.; Liu, Y.; et al. Bioimaging Application and Growth-Promoting Behavior of Carbon Dots from Pollen on Hydroponically Cultivated Rome Lettuce. ACS Omega 2017, 2, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sheng, Z.; Han, H.; Zou, M.; Li, C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater. Lett. 2012, 66, 222–224. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).