Studies on Influence of Cell Temperature in Direct Methanol Fuel Cell Operation
Abstract
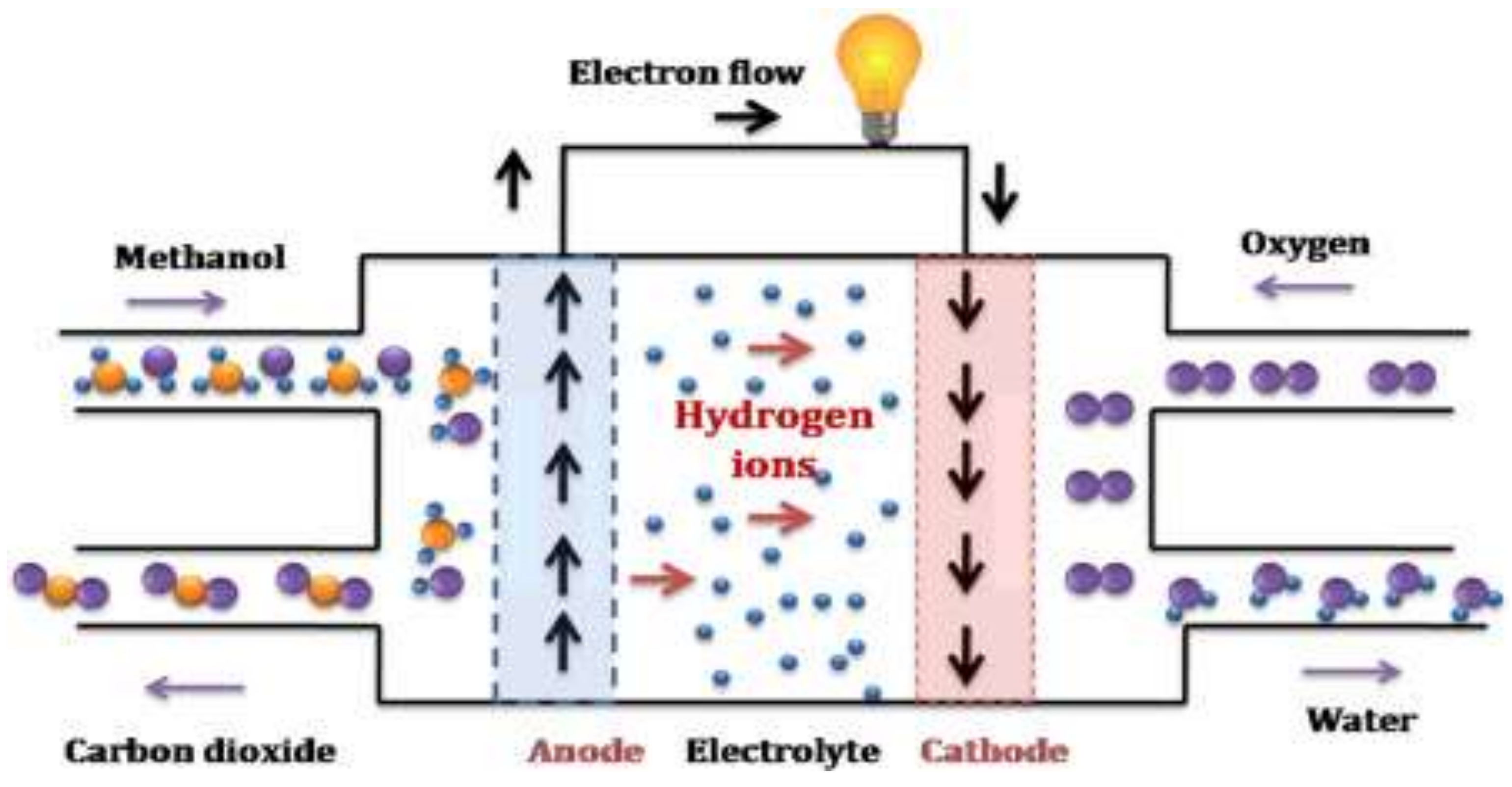
1. Introduction
2. Materials and Methods
2.1. Model-Based Simulation
2.2. Real-Time Experimentation
3. Results and Discussion
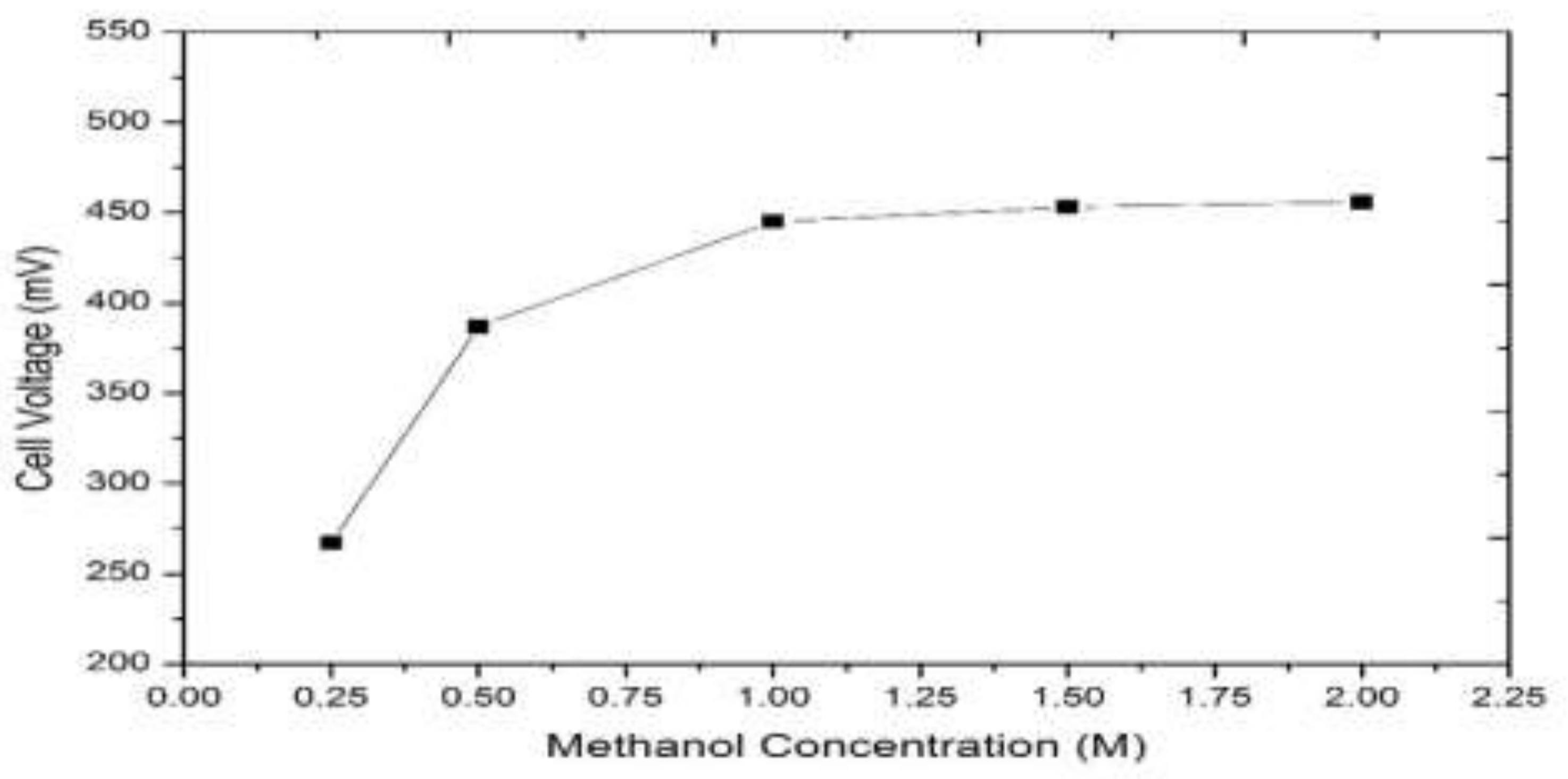
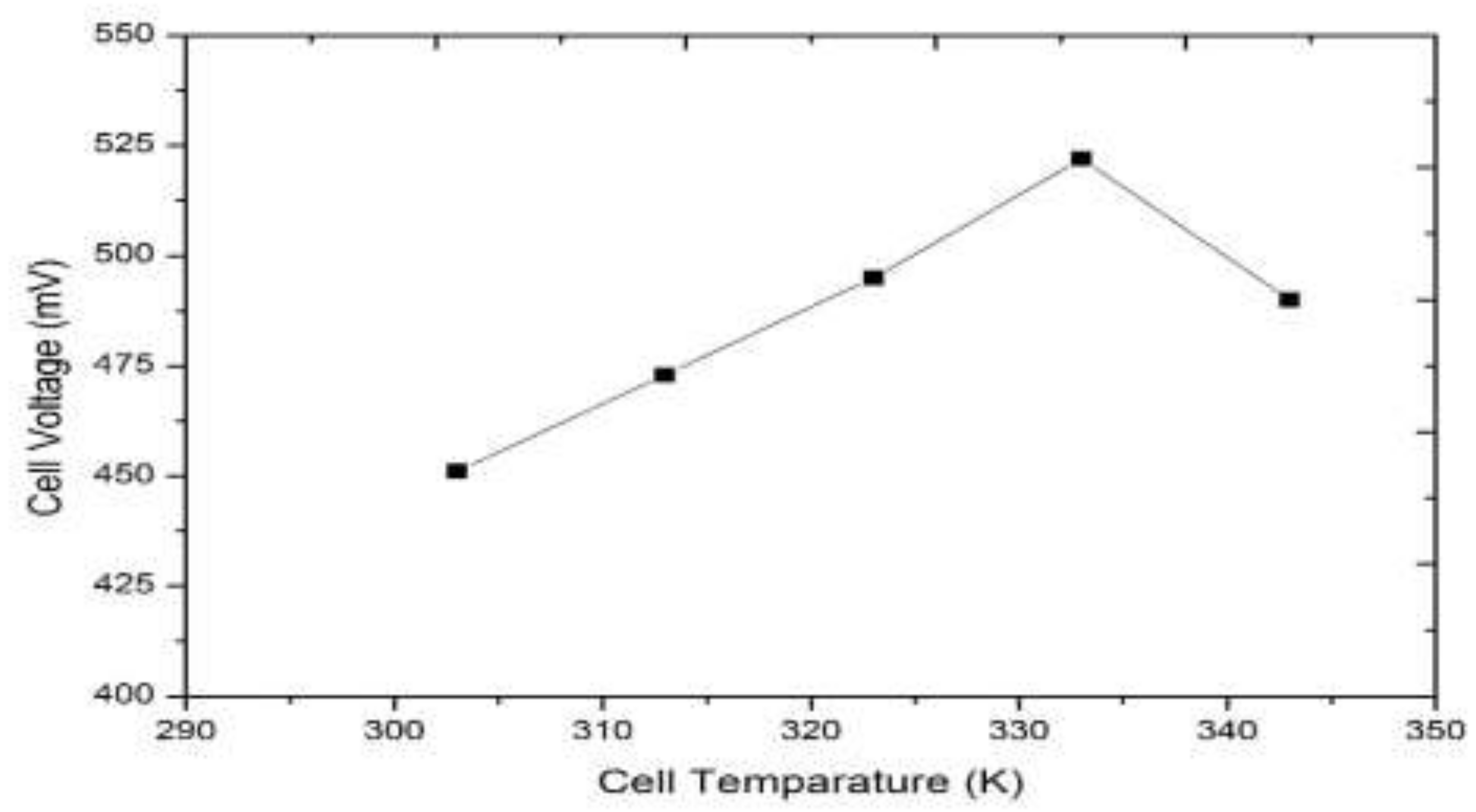
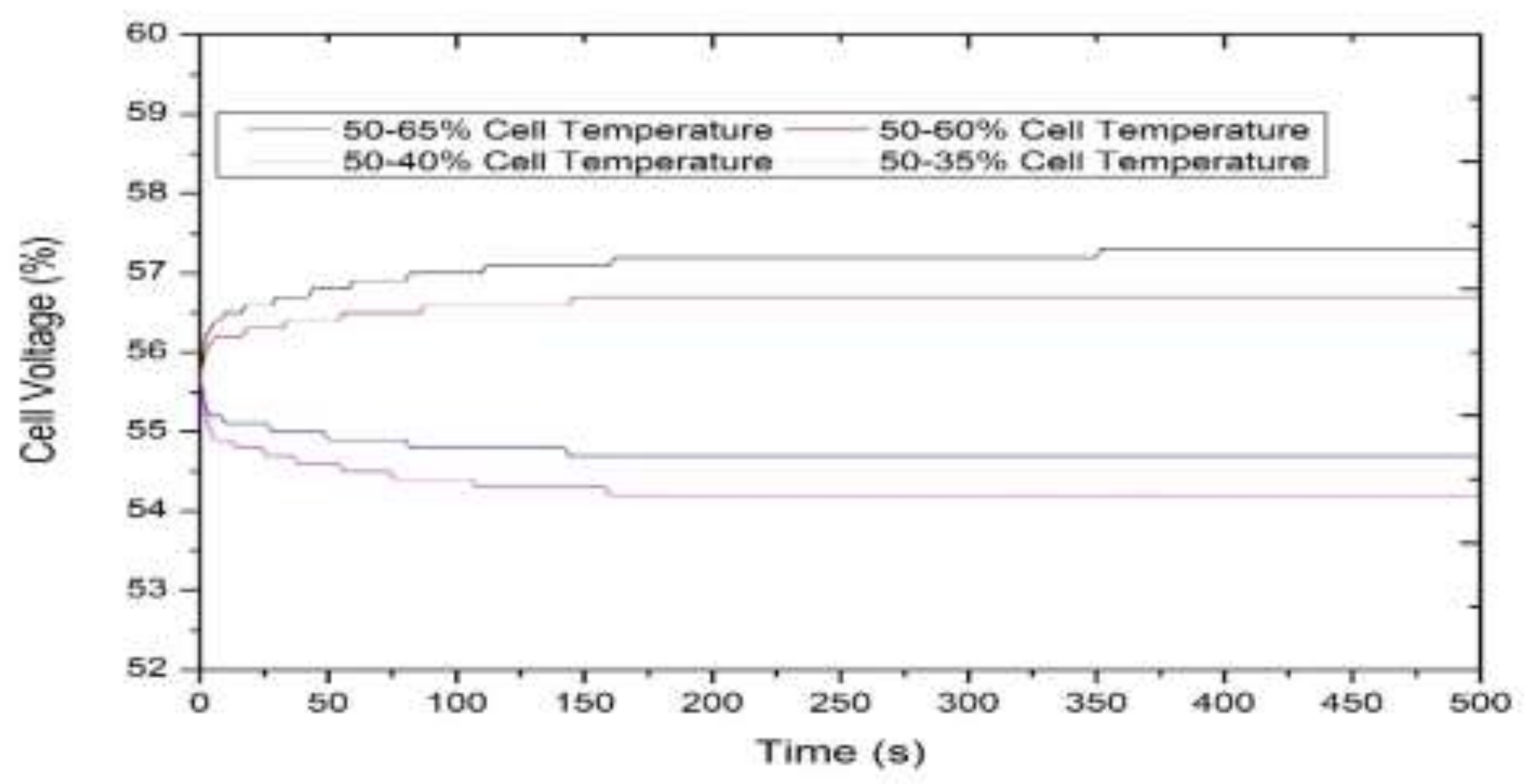
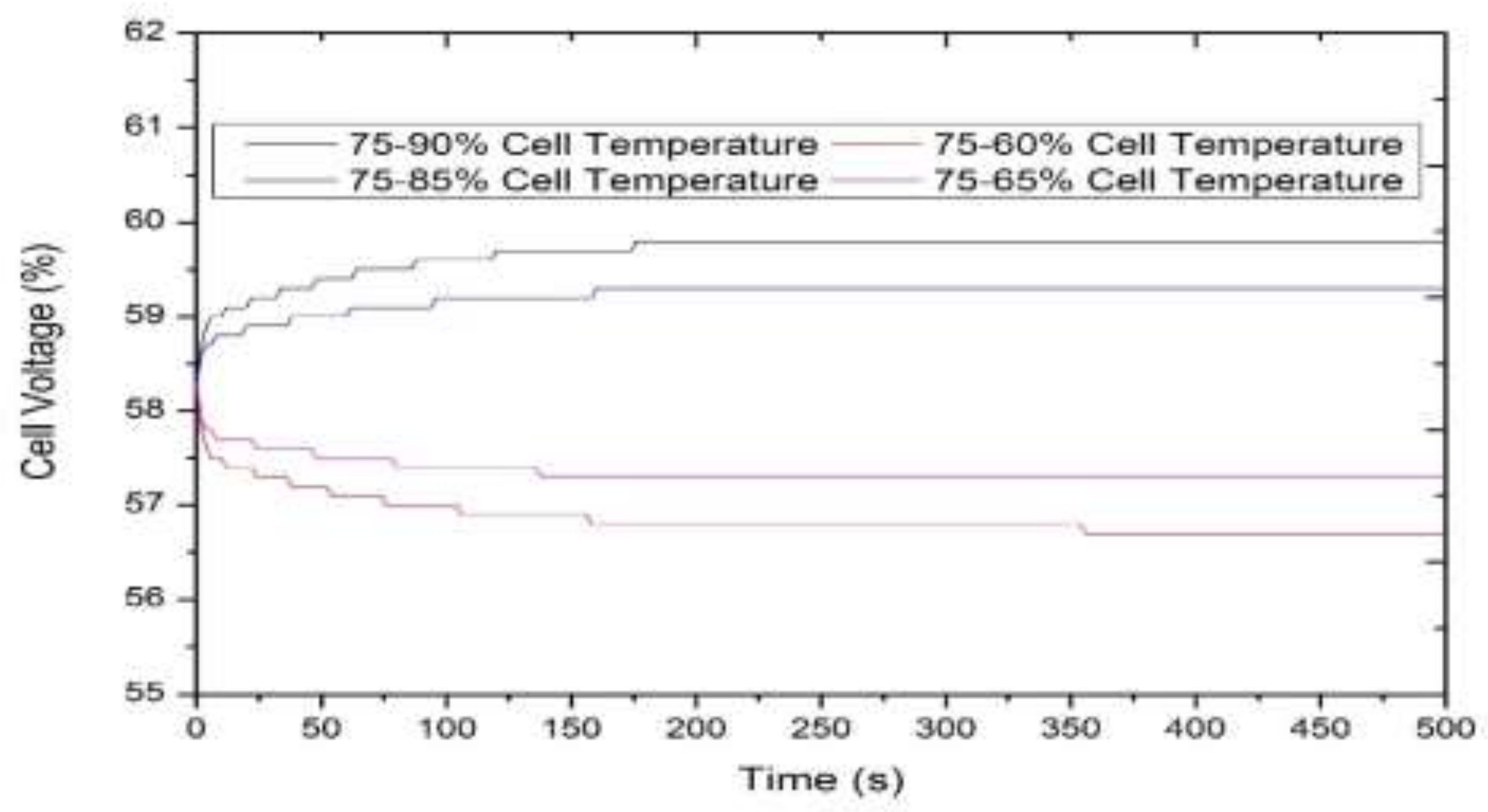
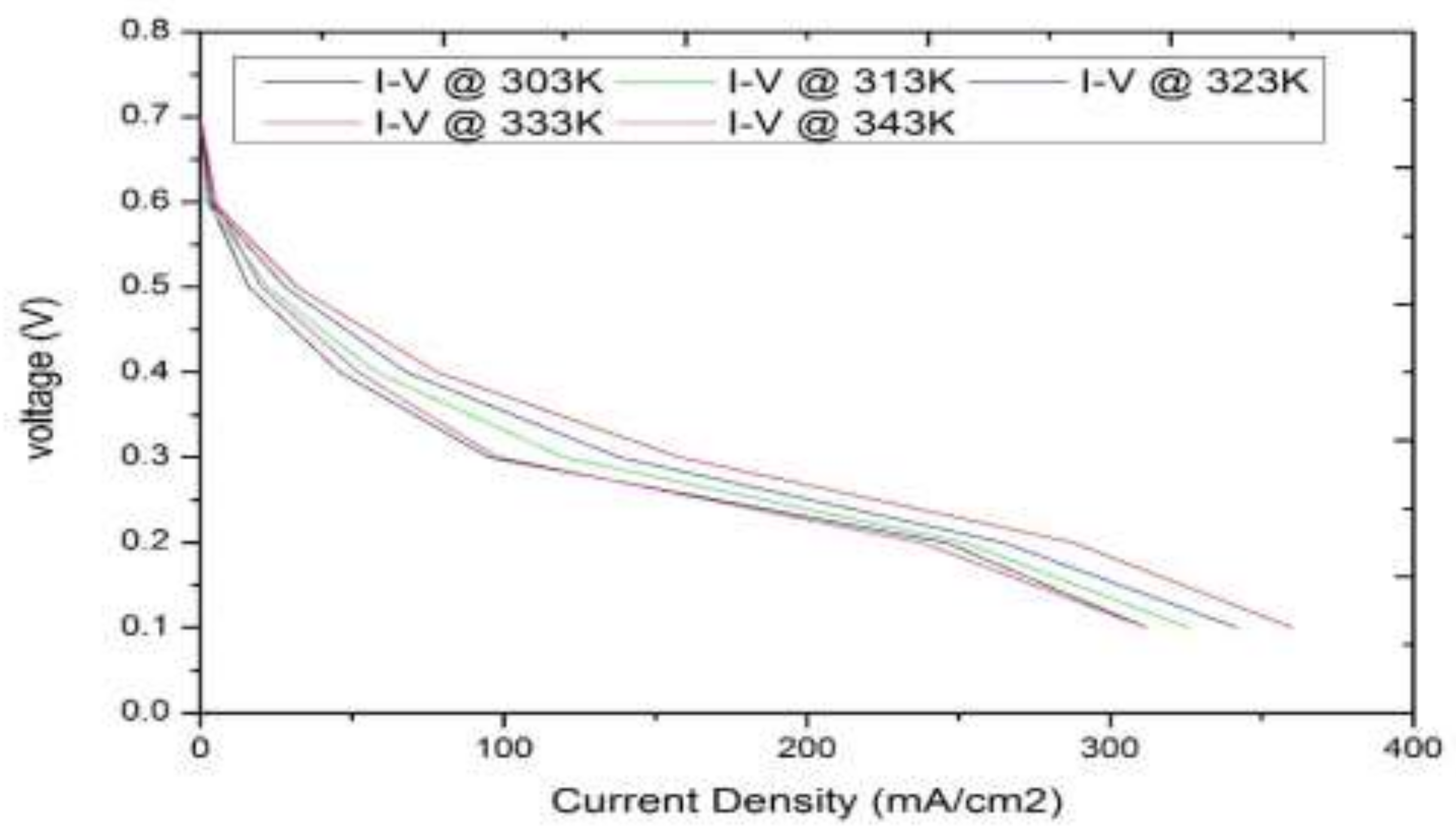
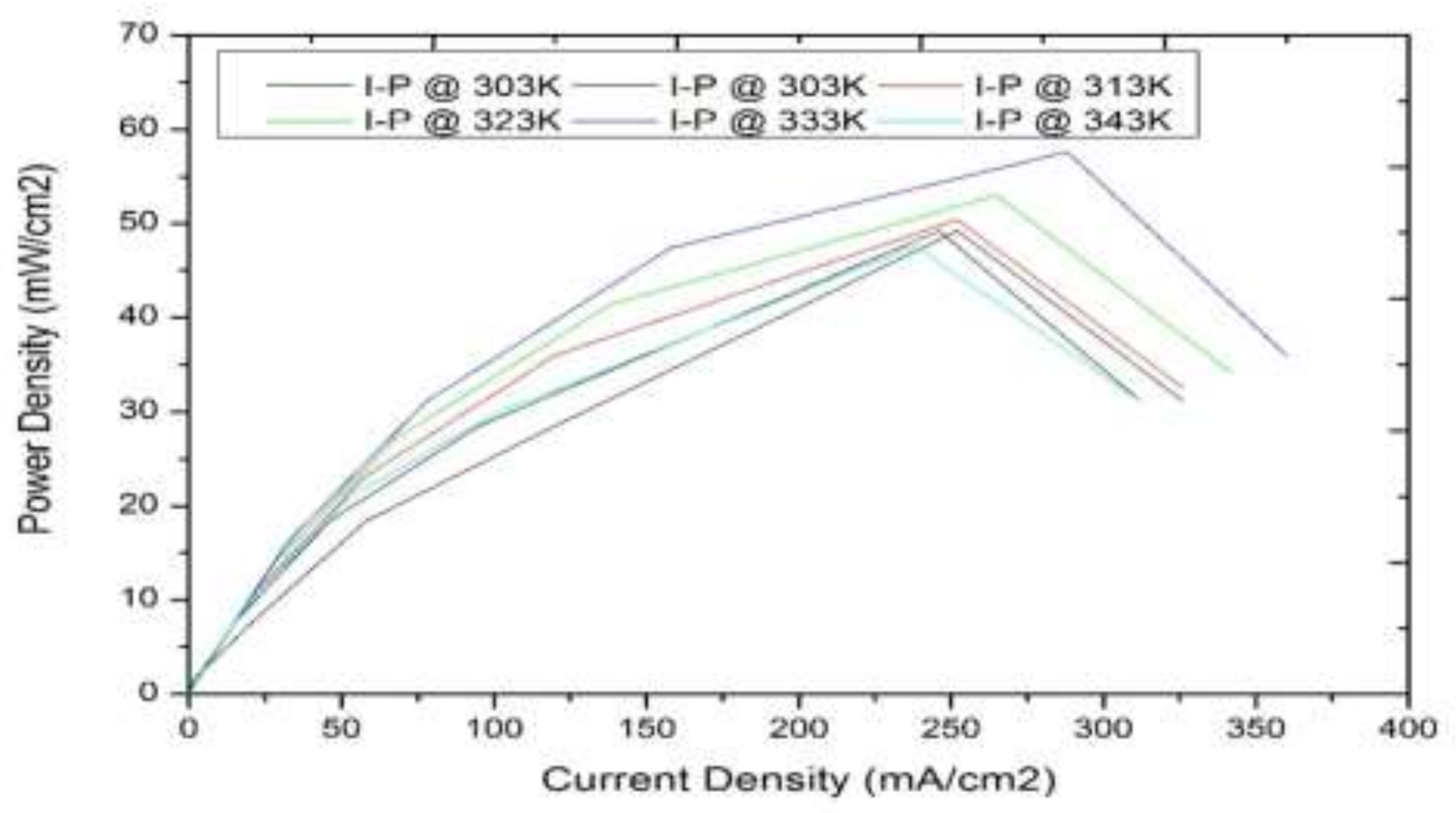
3.1. Model-Based Simulation
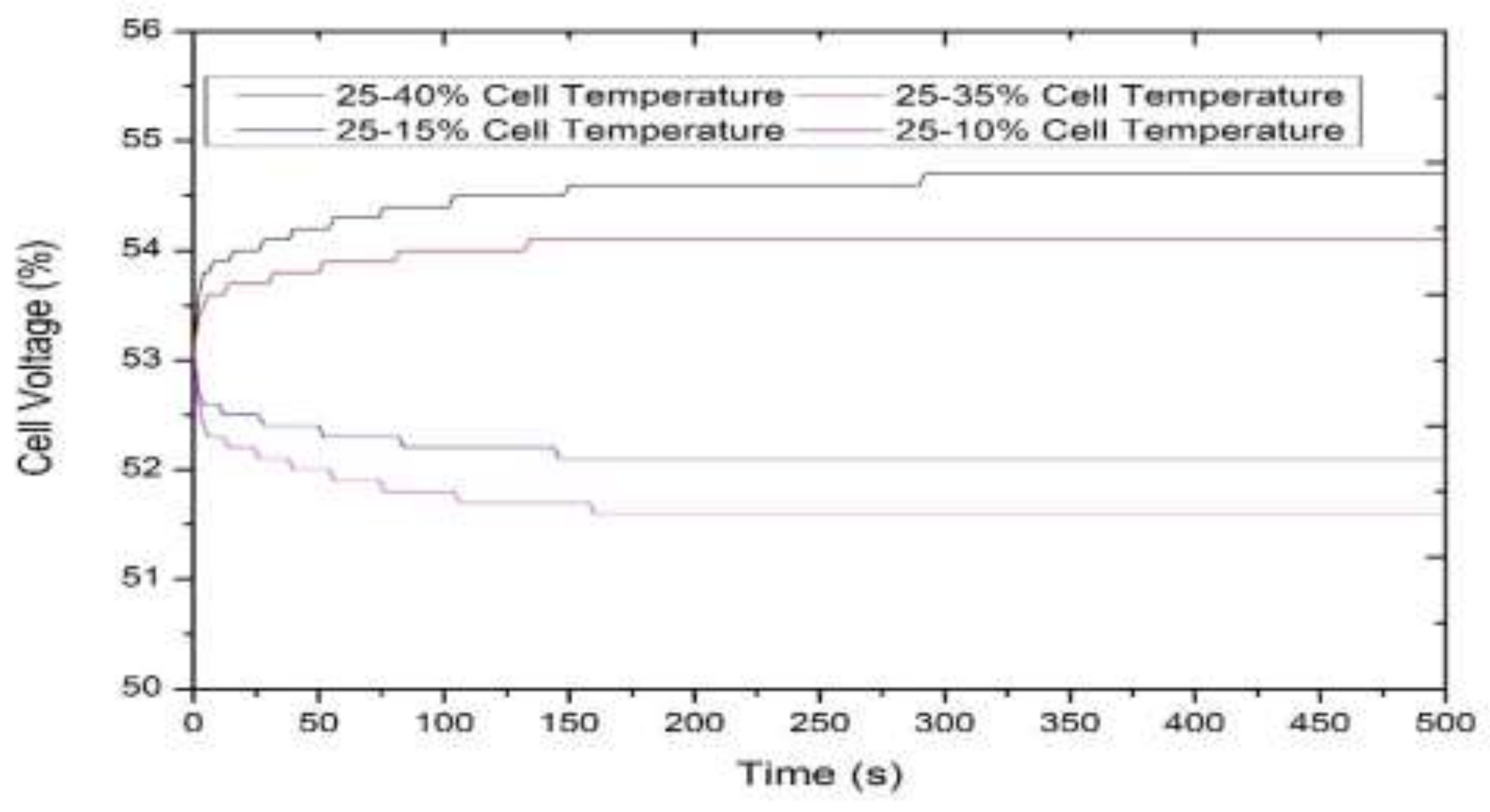
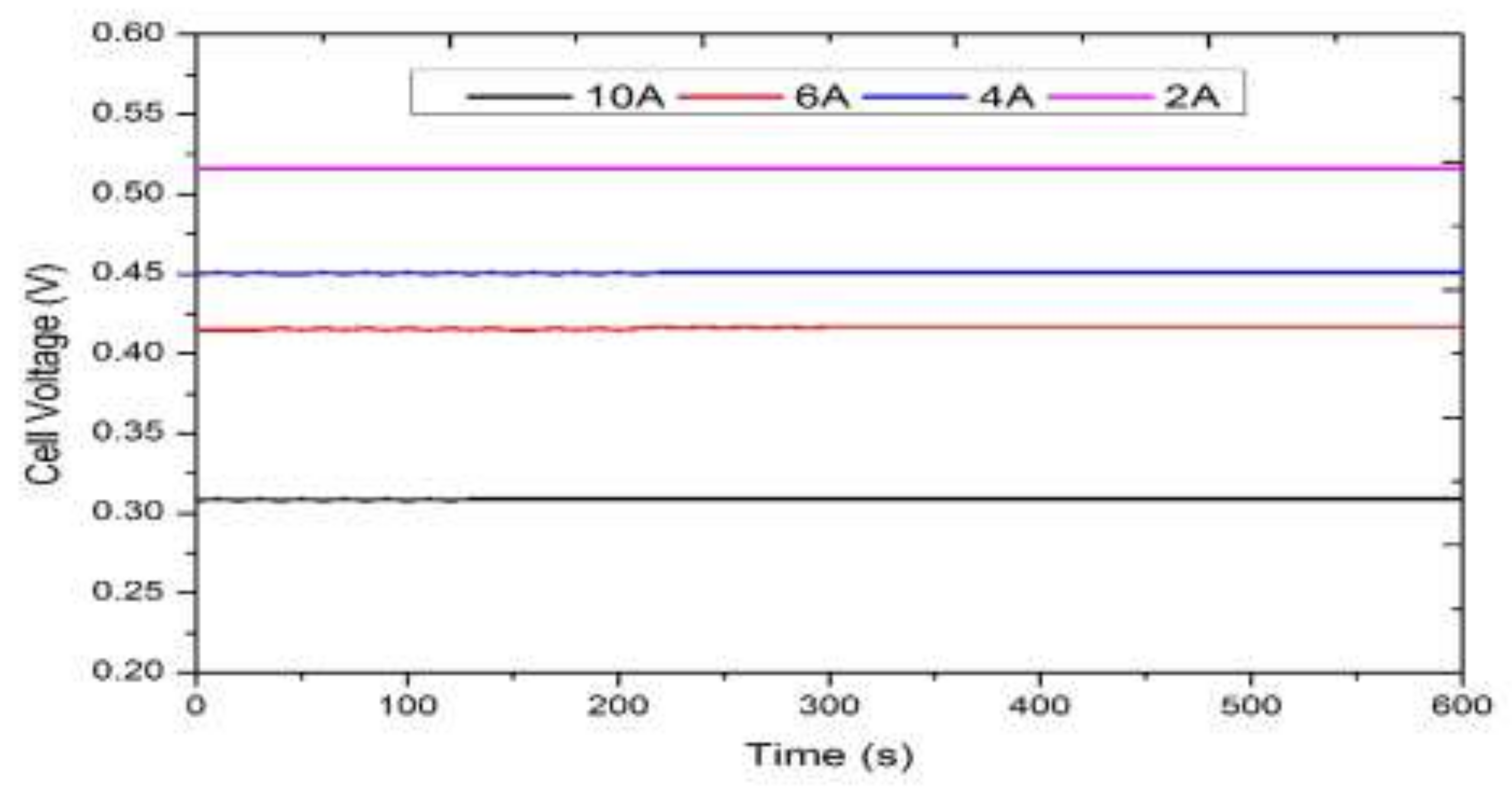
3.2. Real-Time Experimentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinesh, J.; Easwaramoorthi, M.; Muthukumar, M. State of Research Developments in Direct Methanol Fuel cell. Int. J. Eng. Trends Technol. 2017, 43, 284–296. [Google Scholar] [CrossRef]
- Dillon, R.; Srinivasan, S.; Arico, S.A.; Antonucci, V. International activities in DMFCR&D: Status of technologies and potential applications. J. Power Sources 2004, 127, 112–126. [Google Scholar]
- Vielstich, W.; Gasteiger, H.; Lamm, A. Handbook of Fuel Cell: Fundamentals, Technology, Applications; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Arico, S.; Srinivasan, S.; Antonucci, V. DMFCs: From Fundamental Aspects to Technology Development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Bostaph, J.; Xie, C.G.; Pavio, J.; Fisher, A.M.; Mylan, B.; Hallmark, J. Design of a 1-W direct methanol fuel cell system in Direct Methanol Fuel Cell. In Proceedings of the 40 th Power Sources Conference, Cherry Hill, NJ, USA, 10–13 June 2002; pp. 211–214. [Google Scholar]
- Venkatkarthick, R.; Elamathi, S.; Sangeetha, D.; Balaji, R.; Kannan, B.S.; Vasudevan, S.; Davidson, D.J.; Sozhan, G.; Ravichandran, S. Studies on polymer modified metaloxideanodeforoxy gen evolution reaction in saline water. J. Electroanal. Chem. 2013, 697, 1–4. [Google Scholar] [CrossRef]
- Balaji, R.; Kannana, B.S.; Lakshmi, J.; Senthil, N.; Vasudevan, S.; Sozhan, G.; Shukla, A.K.; Ravichandran, S. An alternative approach to selective seawater oxidation for hydrogen production. Electrochem. Commun. 2009, 11, 1700–1702. [Google Scholar] [CrossRef]
- Wang, C.Y. Principles of direct methanol fuel cells for portable and micropower. In Mini-Micro Fuel Cells; Springer: Ankara, Turkey, 2008; pp. 235–242. [Google Scholar]
- Chang, J.Y.; Kuan, Y.D.; Lee, S.M. Experimental Investigation of a Direct Methanol Fuel Cell with Hilbert Fractal Current Collectors. Hindawi-J. Chem. 2014, 2014, 371616. [Google Scholar] [CrossRef]
- Balasinga, S.K.; Nallathamb, K.S.; Jabbar, M.H.A.; Ramadoss, A.; Kamaraj, S.K.; Nanomaterials, M.K. Electrochemical Energy Conversion and Storage Technologies. Hindawi-J. Nanomater. 2019. [Google Scholar] [CrossRef]
- Yuan, W.; Fang, G.; Li, Z.; Chen, Y.; Tang, Y. Using Electrospinning-Based Carbon Nanofiber Webs for Methanol Crossover Control in Passive Direct Methanol Fuel Cells. Materials 2018, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, V.; Krishnamurthy, B. Modeling the transient temperature distribution in a Direct Methanol fuel cell. J. Electroanal. Chem. 2018, 809, 1–7. [Google Scholar] [CrossRef]
- Youngseung, N.; Zenith, F.; Krewer, U. Increasing Fuel Efficiency of Direct Methanol Fuel Cell Systems with Feed forward Control of the Operating Concentration. Energies 2015, 8, 10409–10429. [Google Scholar] [CrossRef]
- Ohenoj, M.; Ruusunen, M.; Leivisk, K. Hierarchical Control of an Integrated Fuel Processing and Fuel Cell System. Materials 2019, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Simoglou, A.; Argyropoulos, P.; Martin, E.B.; Scott, K.; Morris, A.J.; Taama, W.M. Dynamics modeling of the voltage response of direct methanol fuel cell sandstacks Part I: Mode development and validation. Chem. Eng. Sci. 2001, 56, 6761–6772. [Google Scholar] [CrossRef]
- Govindarasu, R.; Parthiban, R.; Bhaba, P.K. Recent evolutions in modelling of Direct Methanol Fuel Cell. Elixir Int. J. Chem. Eng. 2014, 72, 25428–25433. [Google Scholar]
- Maynard, H.L.; Meyers, J.P. Miniature fuel cells for portable power: Design considerations and challenges. J. Vac. Sci. Technol. 2002, 20, 1287–1297. [Google Scholar] [CrossRef]
- Govindarasu, R.; Parthiban, R.; Bhaba, P.K. Investigation of Flow Maldistribution in proton exchange membrane fuel cell. Int. J. Renew. Energy Res. 2012, 2, 653–656. [Google Scholar]
- Manokaran, A.; Vijayakumar, R.; Sridhar, P.; Pitchumani, S.; Shukla, K. A self-supported Direct methanol fuel cell system. J. Chem. Sci. 2011, 123, 343–347. [Google Scholar] [CrossRef]
- Premkumar, S.; Prabhakar, S.; Lingeswaran, K.; Ramnathan, P. Development of Direct Methanol Fuel Cell and Improving the efficiency. Middle-East J. Sci. Res. 2014, 20, 1277–1280. [Google Scholar]


| S.No. | Variation of CCH3OH and The Anode/Cathode Over Potential |
|---|---|
| 1 | |
| 2 | |
| 3 |
| S.No. | Coverage of Adsorbed Species at Anode |
|---|---|
| 1 | |
| 2 | ) |
| 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govindarasu, R.; Somasundaram, S. Studies on Influence of Cell Temperature in Direct Methanol Fuel Cell Operation. Processes 2020, 8, 353. https://doi.org/10.3390/pr8030353
Govindarasu R, Somasundaram S. Studies on Influence of Cell Temperature in Direct Methanol Fuel Cell Operation. Processes. 2020; 8(3):353. https://doi.org/10.3390/pr8030353
Chicago/Turabian StyleGovindarasu, R., and S. Somasundaram. 2020. "Studies on Influence of Cell Temperature in Direct Methanol Fuel Cell Operation" Processes 8, no. 3: 353. https://doi.org/10.3390/pr8030353
APA StyleGovindarasu, R., & Somasundaram, S. (2020). Studies on Influence of Cell Temperature in Direct Methanol Fuel Cell Operation. Processes, 8(3), 353. https://doi.org/10.3390/pr8030353




