The Efficiency of Bimodal Silica as a Carbon Dioxide Adsorbent for Natural Gas Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Adsorbent
2.2. Characterization
2.3. Obtaining the Adsorption Isotherms
3. Results and Discussions
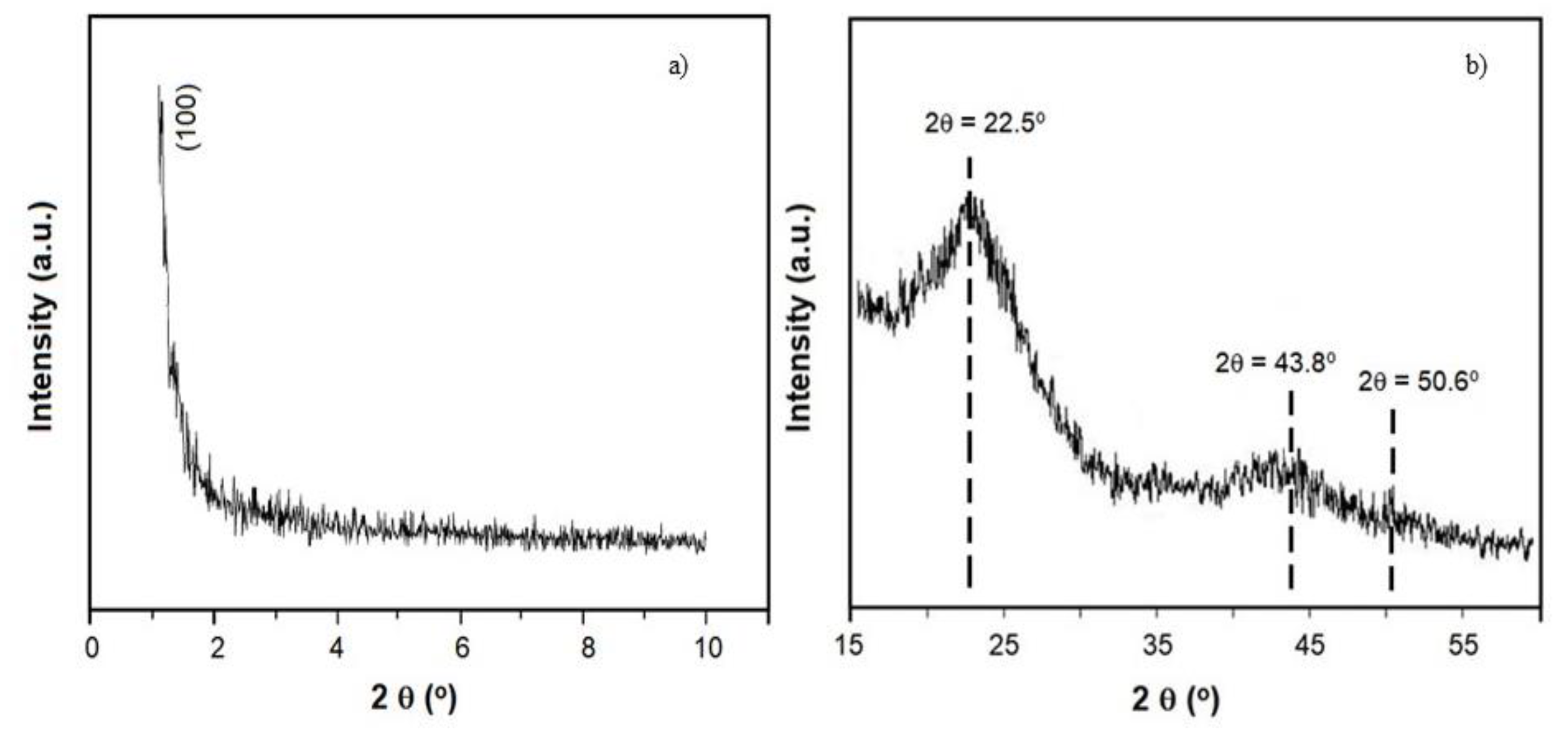
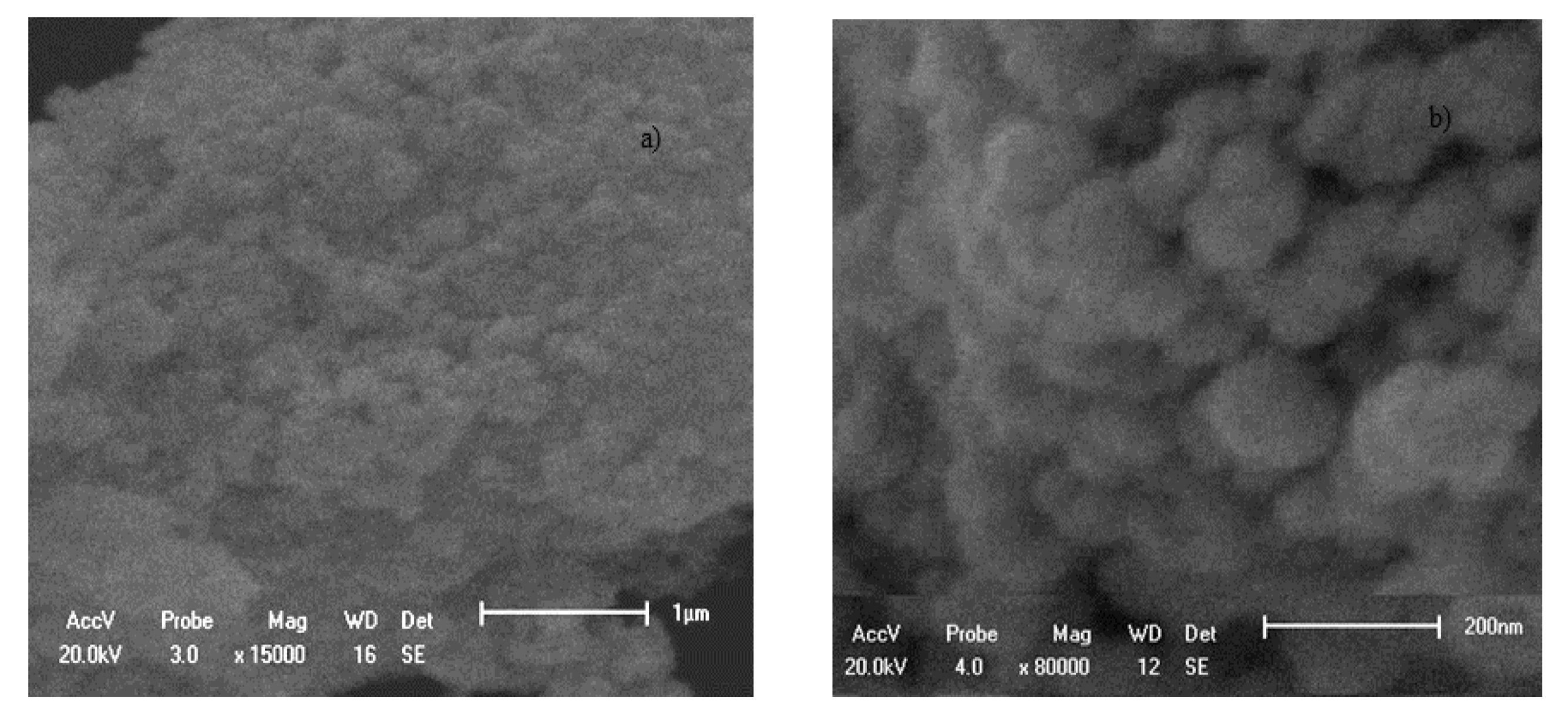
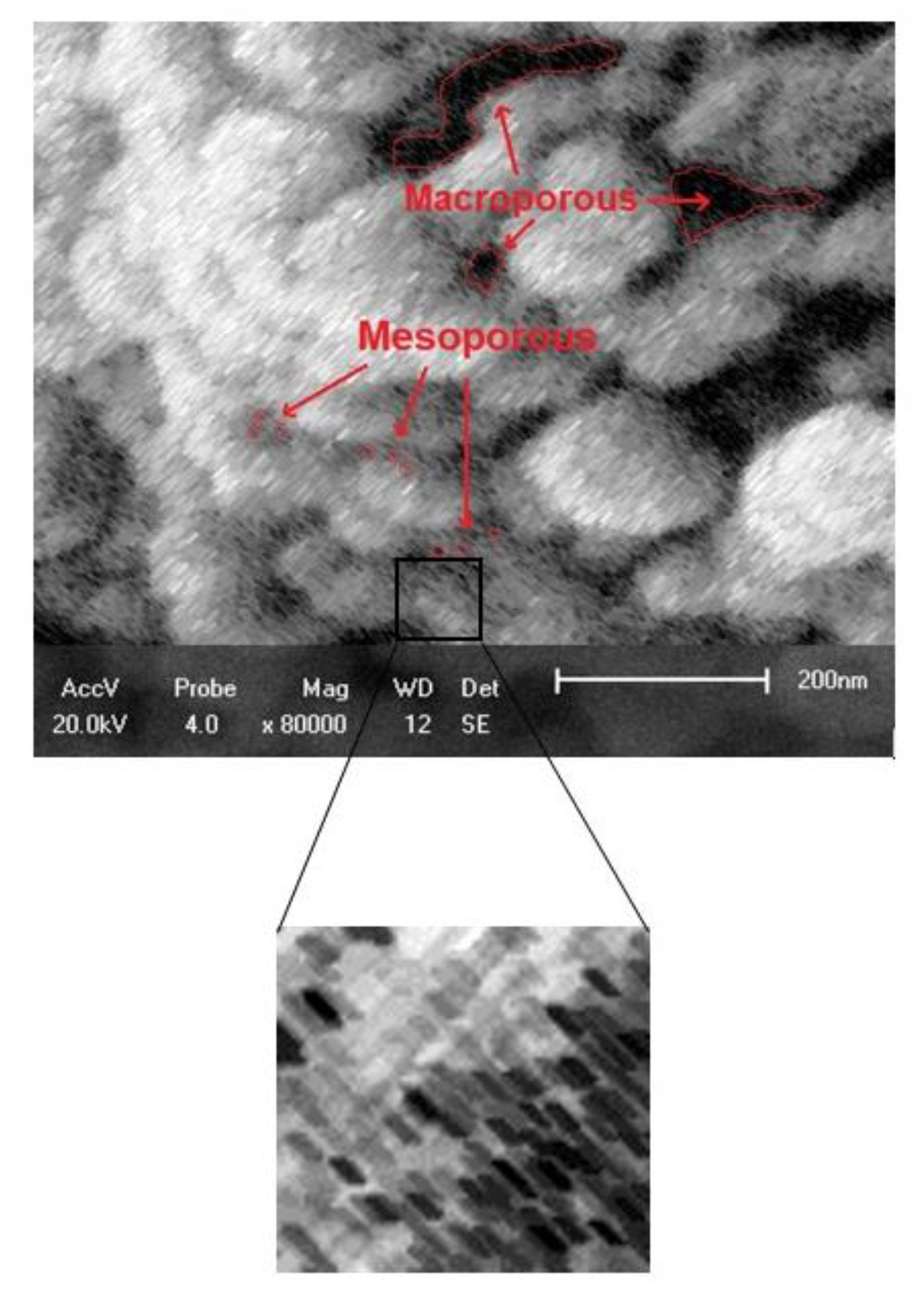
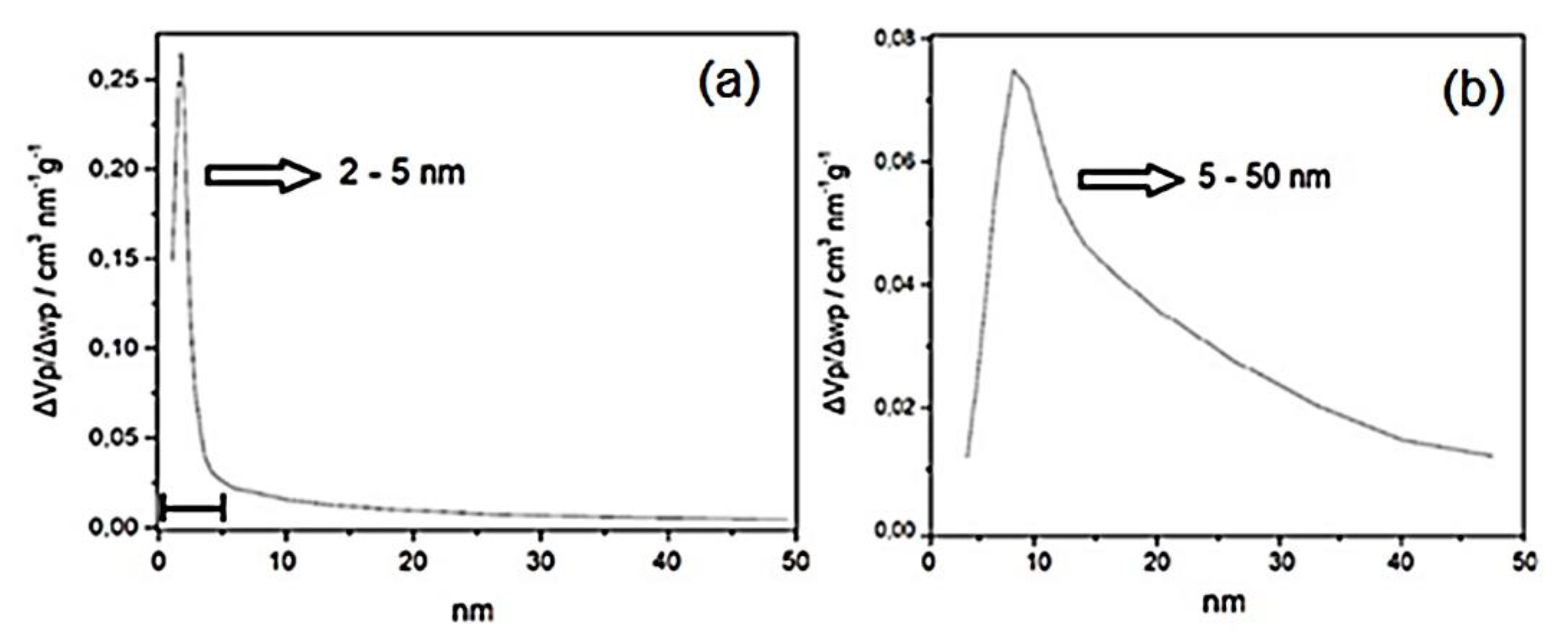
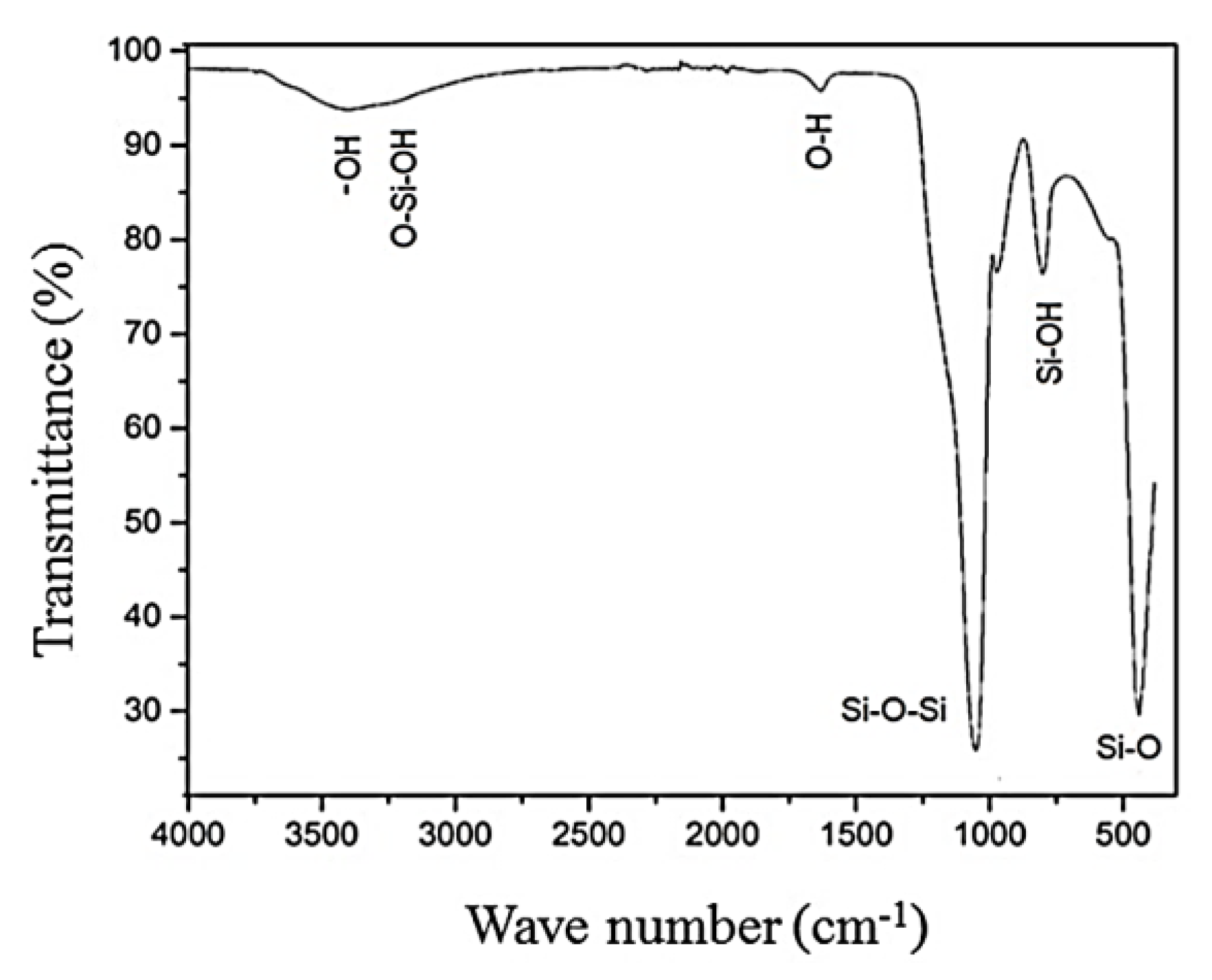
3.1. Characterization of the Adsorbents Obtained
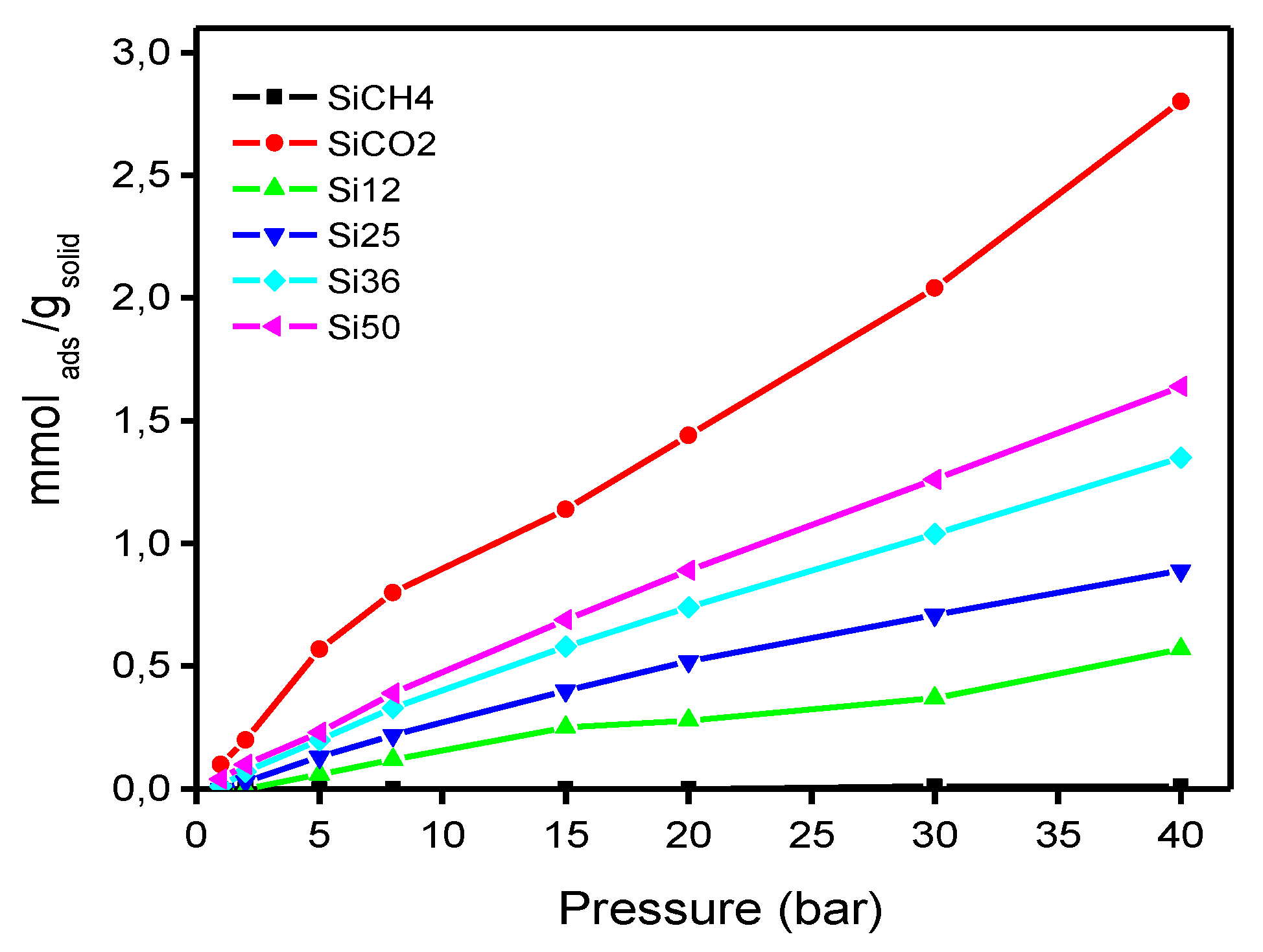
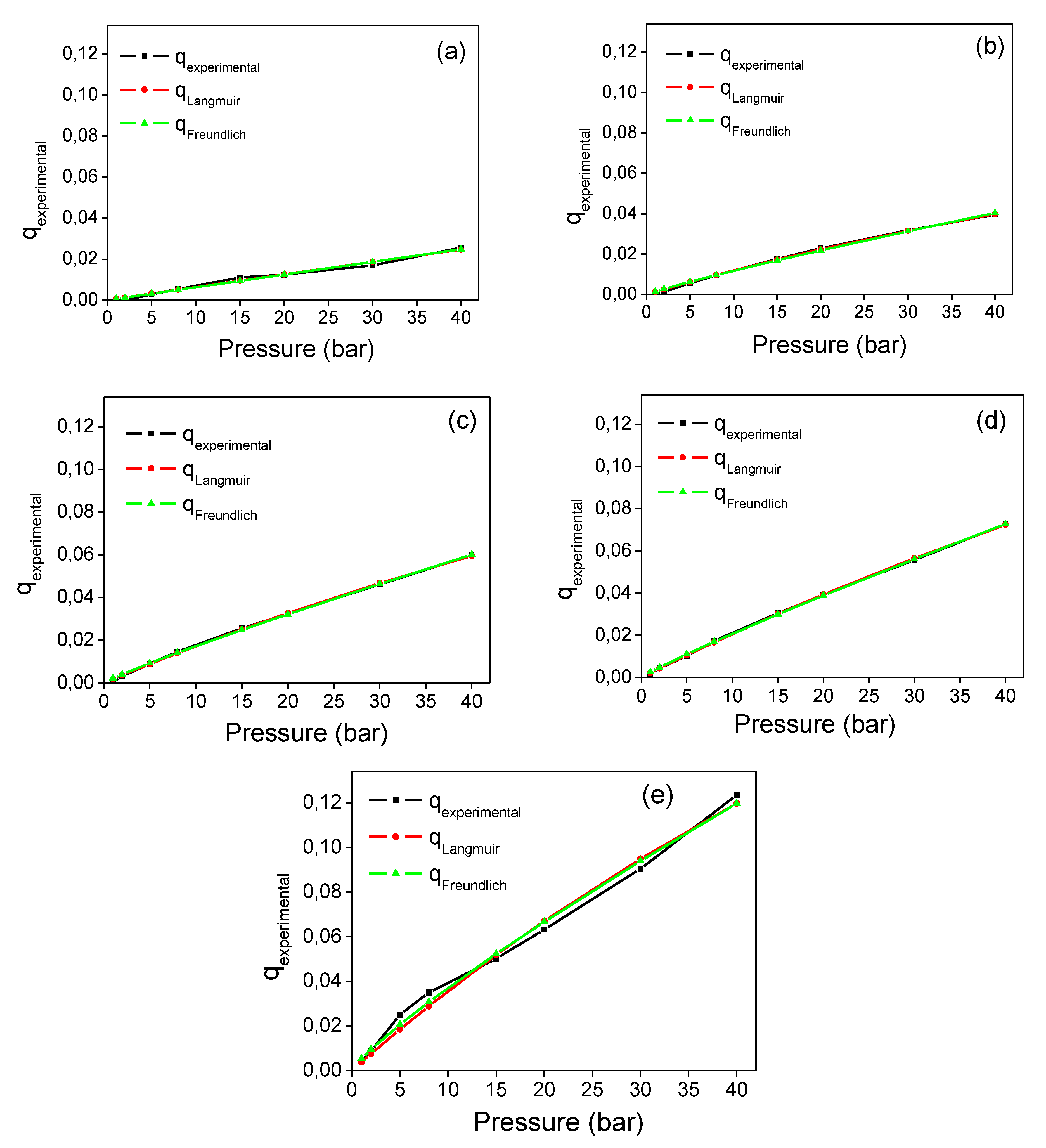
3.2. Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Empresa de Pesquisa Energética. Brazilian Energy Balance 2017 Year 2016; Empresa de Pesquisa Energética: Rio de Janeiro, Brazil, 2017.
- Araújo, O.R. Comparative analysis of separation technologies for processing carbon dioxide rich natural gas in ultra-deepwater oil fields. J. Clean. Prod. 2017, 155, 12–22. [Google Scholar] [CrossRef]
- Petrobras Annual Report 2018. Available online: https://www.investidorpetrobras.com.br/ptb/206/Relatorio_anual_27_03.pdf (accessed on 8 November 2019).
- Brazil Ministry of Science, Technology, Innovation and Communication. Fugitive Emissions of Greenhouse Gases in the Oil and Natural Gas Industry. Reference Reports: Energy Sector. 2nd Brazilian Inventory of Anthropogenic Emissions and Removal of Greenhouse Gases; DF MCTI: Brasília, Brazil, 2010.
- Machado, E. Low carbon economy: Assessment of impacts of restrictions and technological perspectives: Petroleum and petrochemistry. Available online: https://www.researchgate.net/publication/303693117_economia_de_baixo_carbono_avaliacao_de_impactos_de_restricoes_e_perspectivas_tecnologicas_petroleo_e_petroquimica (accessed on 20 October 2019).
- Empresa de Pesquisa Energética. Occurrence of CO2 in Oil Fields on the Brazilian East Bank. Available online: http://www.epe.gov.br/sites-pt/publicacoes-dados-abertos/publicacoes/PublicacoesArquivos/publicacao-322/OCORR%C3%8ANCIA%20DE%20CO2%20EM%20CAMPOS%20PETROL%C3%8DFEROS%20NA%20MARGEM%20LESTE%20BRASILEIRA.PDF (accessed on 20 December 2019).
- Sugimoto, L. Research Measures Gas Emissions in Offshore Platforms. Available online: http://www.unicamp.br/unicamp/ju/661/pesquisa-mensura-emissao-de-gases-emplatforms-no-mar (accessed on 11 December 2019).
- Gaffney, Cline & Associates. Review and Evaluation of Ten Selected Discoveries and Prospects in the Pre-Salt Play of the Deepwater Santos Basin, Brazil. 2010. Available online: http://www.anp.gov.br/?dw=39137 (accessed on 3 December 2019).
- Cruz-Navarro, D.S.; Torres-Rodríguez, M.; Mugica-Álvarez, V.; Gutiérrez-Arzaluz, M. Separation and Capture of CO2 through A Zeolitic Membrane. Proceedings 2018, 2, 1436. [Google Scholar] [CrossRef]
- Santos, T.C.; Ronconi, C.M. CO2 Capture in Hybrid Materials. Rev. Virtual Quim. 2014, 6, 112–130. [Google Scholar] [CrossRef]
- Leal, O.; Bolivar, C.; Ovalles, C.; Garcia, J.J.; Espidel, Y. Reversible adsorption of carbon dioxide on amine surface-bonded silica gel. Inorg. Chim. Acta 1995, 240, 183–189. [Google Scholar] [CrossRef]
- Takahashi, R.; Sato, S.; Sodesawa, T.; Azuma, T. Silica with Bimodal Pores for Solid Catalysts Prepared from Water Glass. J. Sol Gel Sci. Technol. 2004, 31, 373–376. [Google Scholar] [CrossRef]
- Benvenutti, E.V.; Moro, C.C.; Costa, T.M. Silica-based hybrid materials obtained by sol-gel method. Quim. Nova 2009, 32, 1926–1933. [Google Scholar] [CrossRef]
- Bacsik, Z.; Ahlsten, N.; Ziadi, A.; Zhao, G.; Garcia-Bennett, A.E.; Martín-Matute, B.; Hedin, N. Mechanisms and kinetics for sorption of CO2 on bicontinuous mesoporous silica modified with n-propylamine. Langmuir 2011, 27, 11118–11128. [Google Scholar] [CrossRef] [PubMed]
- Lessa, M.D. Evaluation of CO2 Adsorption Capacity in 13X Zeolite with Synthetic Gases Originated from Sewage Sludge Pyrolysis. Master’s Thesis, Technology Center, Department of Chemical Engineering, Federal University of Rio Grande do Norte, Natal, Brazil, 2012. [Google Scholar]
- Sanz-Pérez, E.; Dantas, T.; Arencibia, A.; Calleja, G.; Guedes, A.; Araujo, A.S.; Sanz, R. Reuse and recycling of amine-functionalized silica materials for CO2 adsorption. Chem. Eng. J. 2017, 308, 1021–1033. [Google Scholar] [CrossRef]
- Dantas, T.C. Synthesis and Characterization of Inorganic-Organic Mesoporous Materials for Application in Carbon Dioxide Adsorption. Ph.D. Thesis, Institute of Chemistry, Federal University of Rio Grande do Norte, Natal, Brazil, 2016. [Google Scholar]
- Yu, J.; Le, Y.; Cheng, B. CO2 performance and adsorption of porous bimodal silica hollow spheres with amine modified surfaces. RSC Adv. 2012, 2, 6784–6791. [Google Scholar] [CrossRef]
- Barbosa, M.N. Study of Mesoporous Materials Functionalized with Different Amines to Capture Carbon Dioxide Through the Adsorption Process. Ph.D. Thesis, Center for Exact and Earth Sciences, Institute of Chemistry, Federal University of Rio Grande do Norte, Natal, Brazil, 2013. [Google Scholar]
- Oliveira, T.G.; Machado, S.W.; Santos, S.C.; Souza, M.J.; Pedrosa, A.M. CO2 adsorption in micro and mesoporous molecular sieves. Quím. Nova 2014, 37, 610–617. [Google Scholar] [CrossRef]
- Silva, E.G. CO2 Adsorption Using Amine-Impregnated Mesoporous Material. Final Course Work. Available online: http://monografias.ufrn.br/handle/123456789/2043 (accessed on 11 December 2019).
- Nascimento, P.F. Use of Metal-Containing Solid Waste from the Biomass Pyrolysis Process for CO2 Adsorption. Master’s Thesis, Technology Center, Department of Chemical Engineering, Federal University of Rio Grande do Norte, Natal, Brazil, 2015. [Google Scholar]
- Santamaría, E.; Maestro, A.; Porras, M.; Gutiérrez, J.M.; González, C. Preparation of structured meso–macroporous silica materials: Influence of composition variables on material characteristics. J. Porous Mater. 2014, 21, 263–274. [Google Scholar]
- Nascimento, P.F.P.; Sousa, J.F.; Oliveira, J.A.; Possa, R.D.; Santos, L.S.; Carvalho, F.C.; Ruiz, J.A.C.; Pedroza, M.M.; Bezerra, M.B.D. Wood Sawdust and Sewage Sludge Pyrolysis Chars for CO2. Can. J. Chem. Eng. 2017, 95, 2148–2156. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Xu, D.; Wang, D.; Xue, Y.; Su, W. Pressure-induced crystallization of amorphous SiO2 with silicon–hydroxy group and the quick synthesis of coesite under lower temperature. High Press. Res. 2008, 28, 641–650. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Oliveira, R.S.; Vieira, R.S.; Cavalcante, C.L.; Azevedo, D.C. Adsorption of CO2 on nitrogen-enriched activated carbon and zeolite 13X. Adsorption 2011, 17, 235–246. [Google Scholar] [CrossRef]
- Neimark, A.V.; Sing, K.S.W. Characterization of Solid Catalysts. In Handbook of Heterogeneous Catalysts; Wiley-VCH Verlag: Weinheim, Germany, 2008. [Google Scholar]
- Nascimento, A.R.; Figueredo, G.P.; Rodrigues, G.; Melo, M.A.; Souza, M.J.; Melo, D.M. Synthesis and characterization of nickel modified mesoporous materials for CO2 capture. Ceramics 2014, 60, 482–489. [Google Scholar] [CrossRef][Green Version]
- Heidari, A.; Younesi, H.; Rashidi, A.; Ghoreyshi, A.A. Evaluation of CO2 adsorption with eucalyptus wood based activated carbon modified by ammonia solution through heat treatment. Chem. Eng. J. 2014, 254, 503–513. [Google Scholar] [CrossRef]

| GAS | NAME |
|---|---|
| Pure Gas CO2 | SiCO2 |
| Mixture 12% CO2 + CH4 (Balance) | Si12 |
| Mixture 25% CO2 + CH4 (Balance) | Si25 |
| Mixture 36% CO2 + CH4 (Balance) | Si36 |
| Mixture 50% CO2 + CH4 (Balance) | Si50 |
| Pure Gas CH4 | SiCH4 |
| Presssure (Bar) | Adsorption of CO2 (mmol/g) | ||||
|---|---|---|---|---|---|
| SiCO2 | Si12 | Si25 | Si36 | Si50 | |
| 1 | 0.10 | 0.00 | 0.00 | 0.01 | 0.04 |
| 2 | 0.20 | 0.00 | 0.03 | 0.07 | 0.10 |
| 5 | 0.57 | 0.06 | 0.13 | 0.20 | 0.23 |
| 8 | 0.80 | 0.12 | 0.22 | 0.33 | 0.39 |
| 15 | 1.14 | 0.25 | 0.40 | 0.58 | 0.69 |
| 20 | 1.44 | 0.28 | 0.52 | 0.74 | 0.89 |
| 30 | 2.04 | 0.37 | 0.71 | 1.04 | 1.26 |
| 40 | 2.80 | 0.57 | 0.89 | 1.35 | 1.64 |
| TOTAL | 9.08 | 1.66 | 2.90 | 4.32 | 5.25 |
| Adsorbents | Adsorption of CO2 (mmol·g−1) | Reference |
|---|---|---|
| SiCO2 | 0.1 | Author |
| MCM-41 | 0.18 | Barbosa (2013) |
| SBA-15 | 0.64 | Barbosa (2013) |
| 10% E-SBA16 | 1.07 | Dantas (2016) |
| 30% E-SBA16 | 0.82 | Dantas (2016) |
| 50% E-SBA16 | 0.77 | Dantas (2016) |
| Adsorbents | Adsorption Capacity (mmol·g−1) | Reference |
|---|---|---|
| 100CO2 | 9.10 | Author |
| MCM-48 | 14.89 | Nascimento et al. (2014) |
| SBA-15 | 9.97 | Nascimento et al. (2014) |
| Model | Parameters | Si12 | Si25 | Si36 | Si50 | SiCO2 |
|---|---|---|---|---|---|---|
| Langmuir | qm (g) | 0.626 | 0.177 | 0.334 | 0.4419 | 0.560 |
| b (L/g) | 0.001 | 0.007 | 0.005 | 0.005 | 0.007 | |
| R2 | 0.98 | 0.99 | 0.99 | 0.99 | 0.98 | |
| Freundlich | KF(L/g) | 0.0006 | 0.00152 | 0.00216 | 0.00257 | 0.0053 |
| 1/n | - | - | - | - | - | |
| R2 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia de Carvalho, F.; do Nascimento, P.F.; Oliveira de Souza, M.R.; Souza Araujo, A. The Efficiency of Bimodal Silica as a Carbon Dioxide Adsorbent for Natural Gas Treatment. Processes 2020, 8, 289. https://doi.org/10.3390/pr8030289
Correia de Carvalho F, do Nascimento PF, Oliveira de Souza MR, Souza Araujo A. The Efficiency of Bimodal Silica as a Carbon Dioxide Adsorbent for Natural Gas Treatment. Processes. 2020; 8(3):289. https://doi.org/10.3390/pr8030289
Chicago/Turabian StyleCorreia de Carvalho, Fabíola, Paula Fabiane do Nascimento, Márcio Rodrigo Oliveira de Souza, and Antonio Souza Araujo. 2020. "The Efficiency of Bimodal Silica as a Carbon Dioxide Adsorbent for Natural Gas Treatment" Processes 8, no. 3: 289. https://doi.org/10.3390/pr8030289
APA StyleCorreia de Carvalho, F., do Nascimento, P. F., Oliveira de Souza, M. R., & Souza Araujo, A. (2020). The Efficiency of Bimodal Silica as a Carbon Dioxide Adsorbent for Natural Gas Treatment. Processes, 8(3), 289. https://doi.org/10.3390/pr8030289