1. Introduction
Aerobic composting is an effective and beneficial means of treating organic waste. Overproduction of livestock and their waste can create serious environmental problems [
1]. Excess amounts of manure waste cause nutrient surplus in agricultural areas when applied in excess amounts. Proper management of manure should include volume reduction and state-of-the-art design and operation. The composting process significantly reduces the manure volume through aerobic biochemical reactions, producing a good-quality end product called “compost” [
2,
3]. Using compost as a fertilizer in agriculture reduces the potential danger of pathogens and improves the soil quality [
4]. The process of composting is selected and designed based on the C:N ratio of the input materials. The suggested C:N ratio in the literature varies between 25 and 35, in which C and N are used as an energy source and a nutrient source, respectively. If the C:N ratio is not at the desired value during composting, it will hinder the compost process and result in N losses. According to Pardo, Moral [
3] and Bernal, Alburquerque [
2], gas emissions in manure pile include approximately 60 and 70% of N and C, respectively. The loss of nitrogen is usually from the volatilization of ammonia, which occurs during the first weeks of the process [
5,
6,
7].
Microorganisms play an important role in transportation and transformation of nutrients during the process of composting. Numerous bacteria are responsible for N conversion, and some fungi and bacteria also help the solubilization of P and K. Consequently, availability of these nutrients in the soil will increase [
8]. Examples of compost bacteria are:
Pseudomonas, Zymomonas, Xanthomonas Nitrosomonas, and
Nitrospira. Some chemolithotrophs can also be found in compost such as heterotrophic nitrifiers [
9,
10,
11]. Fungi are also found in compost piles, especially in the first and final composting stages. Examples of fungi in the composting process are:
Aspergillus, Chrysosporium, Fusarium, Penicillium, and
Trichoderma [
11]. There are a number of studies suggesting that inoculation of microorganisms can increase the efficiency and promote maturity in cattle manure composting [
12]. One study investigated the effects of moisture and C:N ratio on gaseous emissions and maturity during composting of cornstalks and found that filtered cornstalks became more compostable when their moisture decreased from 76% to 60% or C:N ratio increased from 12 to 24 [
13]. It was also shown that the population of phylum
Bacteroidetes, Chloroflexi, Proteobacteria, and
Actinobacteria was increased as composting progressed.
Similar to other waste management processes, composting can also generate greenhouse gas (GHG) emissions during waste transportation and treatment practices [
14]. GHG emissions generated from waste management activities were reported as 1.3 GtCO
2-e, which accounts for approximately 2.8% of the global GHG emissions [
15]. Alternative technologies to conventional waste disposal methods such as composting, waste minimization, recycling, and reuse have been recognized and implemented in many countries to reduce the GHG emissions from waste management activities. Whether during transport or treatment, composting always generates GHG emissions. Besides GHGs, other gaseous emissions such as H
2S, N
2O, and NH
3 from manure composting can cause serious environmental problems. For instance, it was reported that low total carbon (TC) and total nitrogen (TN) content can simultaneously reduce CH
4, CO
2, and N
2O emissions [
16]. Xu, Zhao [
17] investigated the effect of different air moisture conditions on the performance and odor emission in the composting process. It was shown that in the areas with low air humidity, a similar effectiveness was achieved on the degradation of organics and values of germination index in windrow and trough composting. Windrow composting displaced the lower H
2S emission, but with a higher NH
3 release compared to trough composting. In another study, it was reported that nitrogen (N) lost as NH
3 and N
2O emissions represented 26.4 and 3.8% of the initial N in windrow composting [
18].
Although there are a great number of studies on bioaugmentation and manure composting, not many are available of the methodology for the quantification of greenhouse gas emissions during composting. Therefore, the hypothesis of this study was that although microbial inoculation would reduce the time required for composting, the process itself would produce greenhouse gas emissions, contributing to global warming. The aims of this study were: (i) To investigate the effects of microbial inoculum addition on the acceleration and maturation of the compost process; (ii) to assess windrow composting as a management strategy for cattle manure by performing microbial inoculation; (iii) to examine the physico-chemical properties of the compost; (iv) to determine the quality of cured compost by using agronomic tests, and (iv) to investigate the GHG emissions generated during the composting process.
2. Materials and Methods
The majority of the organics can be composted under natural conditions, which, however, can result in an inconsistent and poor product. For the best results, C:N ratio, total nitrogen (Ntot), moisture, electrical conductivity (EC), pile temperatures, microbial density, and pH were monitored during this study. Each experiment included 2 replicates and every experiment was repeated at least 2 times.
2.1. Preparation of Manure
Manure, in general, is an excellent nutrient source for plants; however, its properties can vary depending on animal diet, animal age, housing, and environment. For instance, the solid content of cattle manure is an important parameter in the composting process. The nutrient content of manure is also very important as it will affect the rates of land application. Thus, cattle manure, which is rich in these nutrients, was selected for the windrow composting in this study. Cattle manure of 50 tons for each pile was placed on an impermeable concrete floor and was mixed by using a tractor for homogenization.
2.2. Installation of Windrows
The outdoor windrow composting was performed on a concrete and impermeable ground. The cattle manure was passed through a solid–liquid separator before its application to the piles. Moisture content of the manure was reduced to approximately 80% after the separation process. Pile 1 was used for microbial inoculation and pile 2 was used as the control. The bottom length, height, upper length, and longitudinal length of the piles were selected as 2.1, 1.7, 0.6, and 54 m, respectively.
2.3. Application of Microbial Inoculum
A microbial inoculum was purchased in powder form (Compost Activator), which contained compost bacteria of the genera Bacillus, Pseudomonas, and Lactobacillus species. The total number of bacteria (microbial inoculum) in the purchased product was determined by the plate counting method. The colonies on the plates were counted and defined as the total colony forming units (CFU). The plate counting method showed that the microbial inoculum contained CFU of 1.E+07 (1 × 107) per gram of dry product. The amount of microbial inoculum to be added was selected as 454 g for each 8 m3 of manure (provided by the manufacturer). The volume of each pile was determined to be 125 m3. Then, the amount of microbial inoculum required was calculated as 7 kg. Microbial inoculum was manufactured as powder and needed to be dissolved in water before use. The amount of water required to dissolve the product depended on the initial moisture level in pile 1, which was determined as 71% in this study. First, it is important to hydrate the product to ensure that the bacteria are ready to work when applied to the pile. As the hydrated inoculum solution was sprayed over pile 1 with a hose, a windrow tractor mixed the material to disperse the solution equally in the pile.
2.4. Aeration
The rate of decomposition of organics is higher for aerobic processes; however, movement of air in a pile can vary significantly depending on pore size and water content. Porosity is the property of soil that expresses the total volume of pore space in a given material, which is usually occupied with air. Conversely, when the moisture is very high, the pores will be occupied with water, which would then decrease porosity. In this study, complete turning and tilling of the compost material were performed every 7 days by using a windrow turner, along with the addition of water.
2.5. Moisture Content
The optimal moisture content of piles without any limitation on aeration can vary between 40 and 60%. If the moisture content is less than 40%, microbial activities can be reduced, whereas if the pile contains excessive moisture, nutrients will be lost due to leaching. In this study, the solid–liquid-separated manure was exposed to the sun to reduce the water content to about 71%. As the study was done in the summer, frequent watering was required to maintain the desired moisture content in the piles. As for the method of watering, hand watering with a hose was applied typically once a day. In the warmer days where temperatures reach over 30 °C, watering was applied twice a day. The water volumes added to the piles varied depending on the initial water content of the piles, as the intention was to maintain a water content of between 40% and 60%.
2.6. Sampling and Analysis
Samples were collected every day at 6 sampling points (20, 60, 100, 140, 180, and 200 cm down from the pile surface). The samples were handled according to the U.S. Composting Council’s Test Methods for the Examination of Composting and Compost (TMECC) protocols [
19]. About 100 g of sample collected from each point was placed in a container for analysis. A portion of 50 g was used for the determination of moisture content. The remaining 50 g portion was screened through a 2 mm mesh and stored at 20 °C for analytical measurements and microbial analysis. Samples were evaluated for moisture content, pH, EC, organic matter (%), N
tot %, total potassium TK (%), C/N ratio, and microbial analysis. The contents of NO
3– and NH
4+ were determined after extraction of the fresh compost with 0.0125 molar CaCl
2. In the extract, the contents of NO
3– and NH
4+ were measured with a spectrophotometer. Total phosphorus and total potassium were determined through the Mo–Sb colorimetric method and the flame photometer method, respectively. EC value of the compost samples was determined using a conductivity meter (LF91, Wiss. Techn. Workstation, Lindenstruth, Germany).
Compost temperature monitoring is a key factor within the process. The temperature of sampling points was determined by using a thermometer (REOTEMP) and averaged to obtain the pile temperature. The moisture content was determined by drying the samples at 105 °C for 24 h in an electro-thermal blast dryer (TOPTION Blast Drying Oven). The pH of compost samples was measured with a pH meter (Kelway PHD Soil PH Meter). Determinations of organic matter (OM) were performed by weighing the samples and burning in a furnace (550 °C). Both C and Ntot analysis were performed by using a LECO® CN628 instrument.
For the microbial analysis, 10 g of compost sample was added to distilled water of 95 mL and mixed at 15,000 rpm for 15 min with a homogenizer. The total number of microbial populations in pile 1 (inoculated) and pile 2 (not inoculated) was determined by the plate-counting method. The microbial density of bacteria was determined as explained by Nakasaki and Hirai [
20]. Amphotericin B solution at a concentration of 100 μL/L was added to TS agar medium and incubated for 3 days to measure the growth of bacteria. The density measurement was repeated three times for each sample and the averaged value was used. For the determination of compost bacteria, genomic DNA was extracted from the samples by using the Fecal Genomic DNA Kit. For the pathogens, copy numbers of
Salmonella, Shigella, and
Escherichia coli genes were determined by quantitative polymerase chain reaction (qPCR) using a Bio-Rad CFX96 thermocycler.
2.7. Compost Quality Testing
Upon completion of the active composting process, composite samples from different pile locations (pile 1) were collected for analysis. Each compost sample was screened and analyzed for N, P, K, maturity, and agronomic parameters. All samples were transported and handled according to the U.S. Composting Council’s Test Methods for the Examination of Composting and Compost protocols [
19]. Compost samples from pile 1 were tested for maturity by using the respirometry test [
21].
2.8. Greenhouse Gasses (GHG) Generated during Windrow Composting
The composting process includes five stages that can either generate GHG emissions or reduce GHG emissions, which are: Transportation, process, decomposition, product handling, and application [
22]. In the first stage, transportation of waste usually consumes fossil fuels, which consequently results in GHG emissions. In the second stage, GHG emissions are generated as a result of fossil fuel use during pile building and compost pile turning. The third stage is the biodegradation of organics in which the organic materials are aerobically decomposed, producing biogenic CO
2. Composting can also produce fugitive CH
4 and N
2O emissions during the composting and the curing stages [
23]. The fourth stage is the fuel consumption during the handling and transportation of compost products. The last stage is the application of compost as a fertilizer. Data used for the determination of GHG emissions in this study were obtained through the literature [
3,
24,
25].
The generated and avoided GHG emissions of CH
4, CO
2, and N
2O were calculated by using a numeric model [
24]. This model internally uses data from the Intergovernmental Panel on Climate Change (IPCC) and United State Environmental Protection Agency (USEPA). To determine the direct GHG emissions of CH
4, CO
2, and N
2O, emission factors of 5,111 g CH
4·t
−1, 247,000 g CO
2·t
−1, and 221 g N
2O·t
−1 were selected, respectively [
24]. Besides direct emissions, GHG emissions resulting from fuel and electricity consumptions were also considered in the model.
As shown below, Equation (1) through Equation (6) were used to calculate the generated and avoided GHGs [
24]. Biogenic carbon dioxide emissions generated during the windrow composting process were calculated by using Equation (1).
where: GHG
Carbon dioxide = total CO
2 emissions (ton CO
2-e), EF
Carbon dioxide = emission factor for composting (247,000 g CO
2-e·t
−1 manure), and W
Manure = manure (ton).
Direct methane emissions generated during the windrow composting process were calculated by using Equation (2).
where: GHG
Methane = total methane emissions (ton CH
4·yr
−1), EF
Methane = emission factor for composting (5111 g CH
4·t
−1 manure), and W
Manure = manure (ton).
Direct N
2O emissions generated during the windrow composting process were calculated by using Equation (3).
where: GHG
Nitrous oxide = total nitrous oxide emissions (ton N
2O), EF
Nitrous oxide = emission factor for composting (221 g N
2O·t
−1 manure), and W
Manure = manure (ton).
GHG emissions from consumptions of diesel fuel and electricity were calculated by using Equations (4) and (5), respectively.
where: GHG
Diesel = total GHG emissions from diesel use (ton CO
2-e), EF
Diesel = emission factor for diesel use (2.68 tCO
2-e·m
−3 diesel), V
Diesel = volume of diesel fuel consumed (m
3), GHG
Electricity = total GHG emissions from electricity use (ton CO
2-e), E
Electricity = emission factor for electricity use (0.48 kg CO
2-e·MWh
−1 electricity consumed), and E
Electricity = electricity consumed (MWh).
Avoided GHG emissions through chemical fertilizers substitution of compost were calculated by using Equation (6).
where: GHG
Avoided = total GHG emissions avoided from chemical fertilizers substitution (ton CO
2-e), EF
Substitution = emission factor for chemical fertilizers substitution (40.20 tCO
2-e·t
−1 compost land spread), and W
Compost = compost land spread (ton).
2.9. Statistical Analysis
As all the experiments were replicated, statistical analysis using excel was performed to determine whether the observed differences for the experiments were significant. Excel-based ANOVA variance analyses were performed on the data.
3. Results
Physical and chemical characteristics of the manure used in this study are presented in
Table 1.
3.1. Physical, Chemical, and Biological Characteristics of the Compost Samples
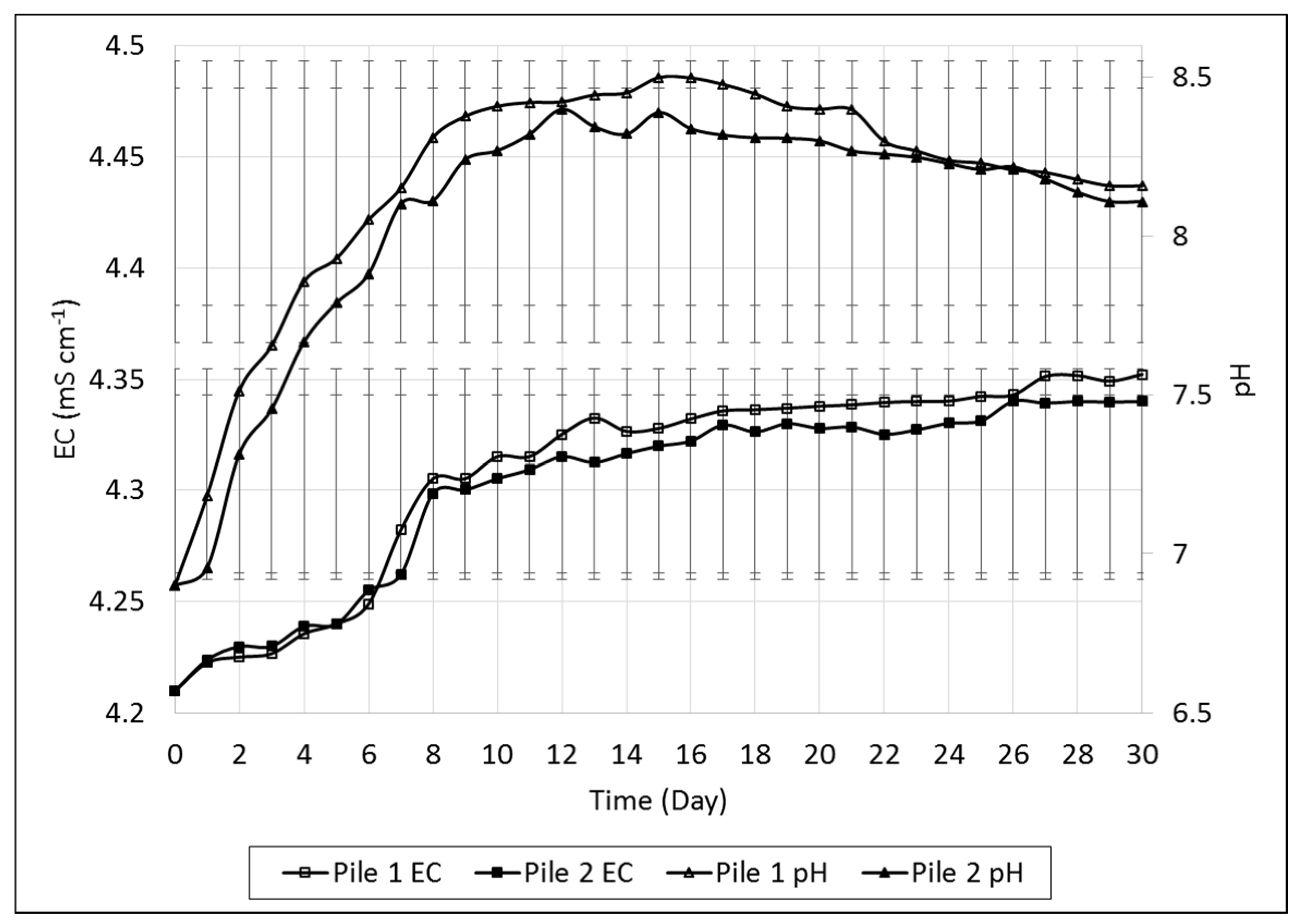
In this study, temperatures of both piles were kept above 50 °C for a minimum of 2 weeks to destroy the pathogens. Temperature variations in pile 1 monitored over the study varied from 30 °C on day 1 to a maximum of 66 °C on day 6, and the thermophilic stage was reached on day 5 for pile 1 and on day 10 for pile 2. The pH value of the compost piles ranged between 6.9 and 8.5.
The EC values for both piles varied between 4.21 and 4.35 mS·cm
−1 throughout the study. This slight increase in EC in both piles was as a result of increases in the concentrations of some ions, such as phosphate, ammonium, and potassium, which are produced as a result of biodegradation of organic matter [
26]. The variations in pH and EC throughout the study are shown in
Figure 1.
In the inoculated pile (pile 1), the initial organic matter (OM), Ntot, and P2O5 concentrations for both piles were 84%, 1.89%, and 1.95%, respectively (based on dry weight). At the end of the study, OM decreased from 84% to 59.35%, which is equal to 24.65% OM loss. Finally, P2O5 was increased from 1.95% to 2.24%, which is equal to a 0.29% increase. It was clearly seen that OM loss was greater during the bio-oxidation phase in the inoculated pile, which indicates higher microbial activity.
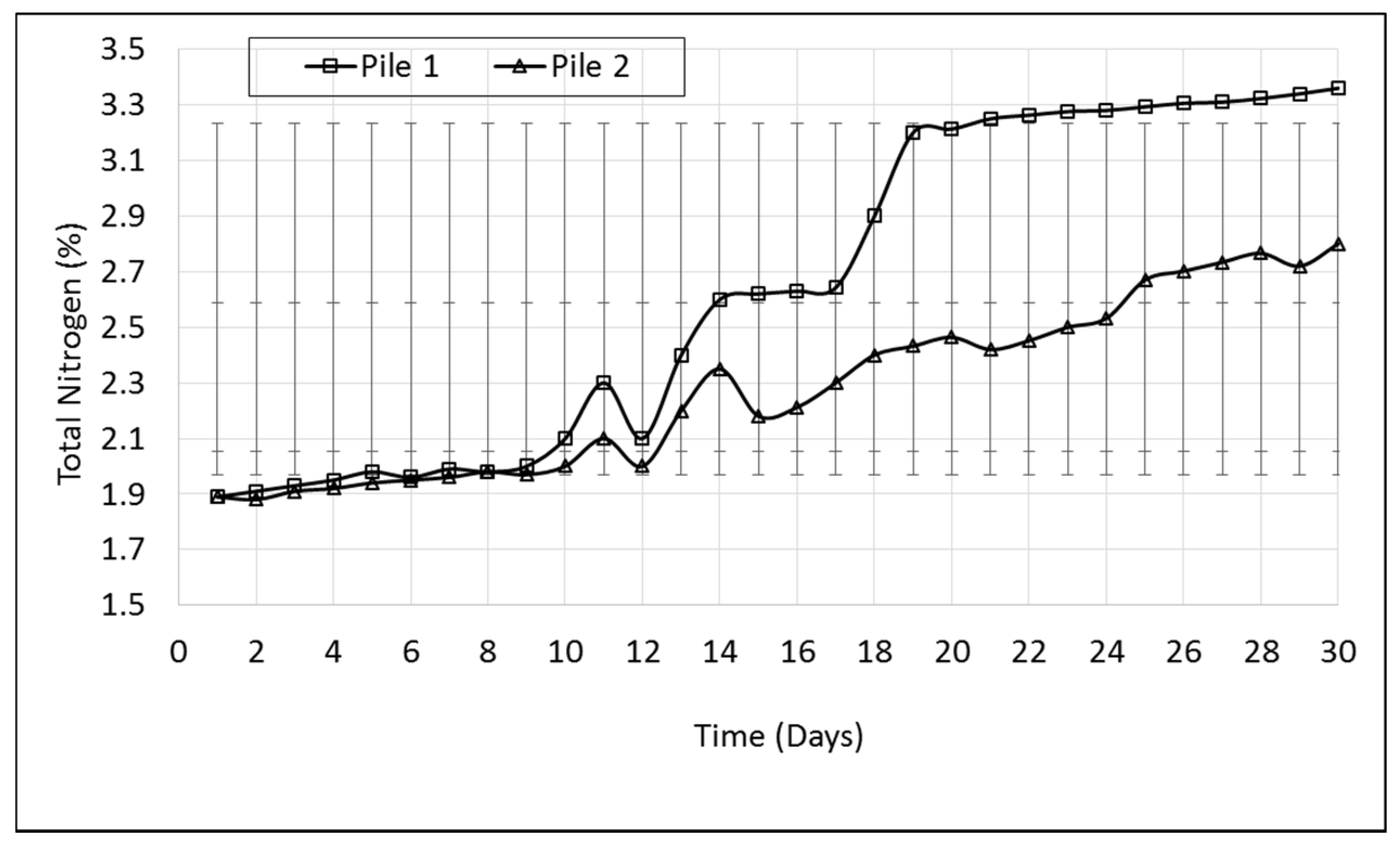
In order to produce a good-quality compost, the C:N ratio should be monitored. In this study, the C:N ratio decreased from 25.6 to 13.6 (Pile 1) and 20.1 (Pile 2), with a significant reduction from day 4 to day 12 for pile 1 (
Figure 2). The concentration of N
tot increased from 1.89% to 2.80%, which is equal to a 0.91% increase. The variations in N
tot throughout this study are presented in
Figure 3. N
tot in pile 1 increased from 1.89% to 3.2% on day 19, followed by a small change to the end of the study. However, N
tot gradually increased from 1.89% to 2.67% on day 25 in pile 2. N
tot in pile 1 and pile 2 started to increase as a percentage especially after day 10 as a result of reduced carbon content. Nevertheless, the rate of N
tot increase was more significant in pile 1.
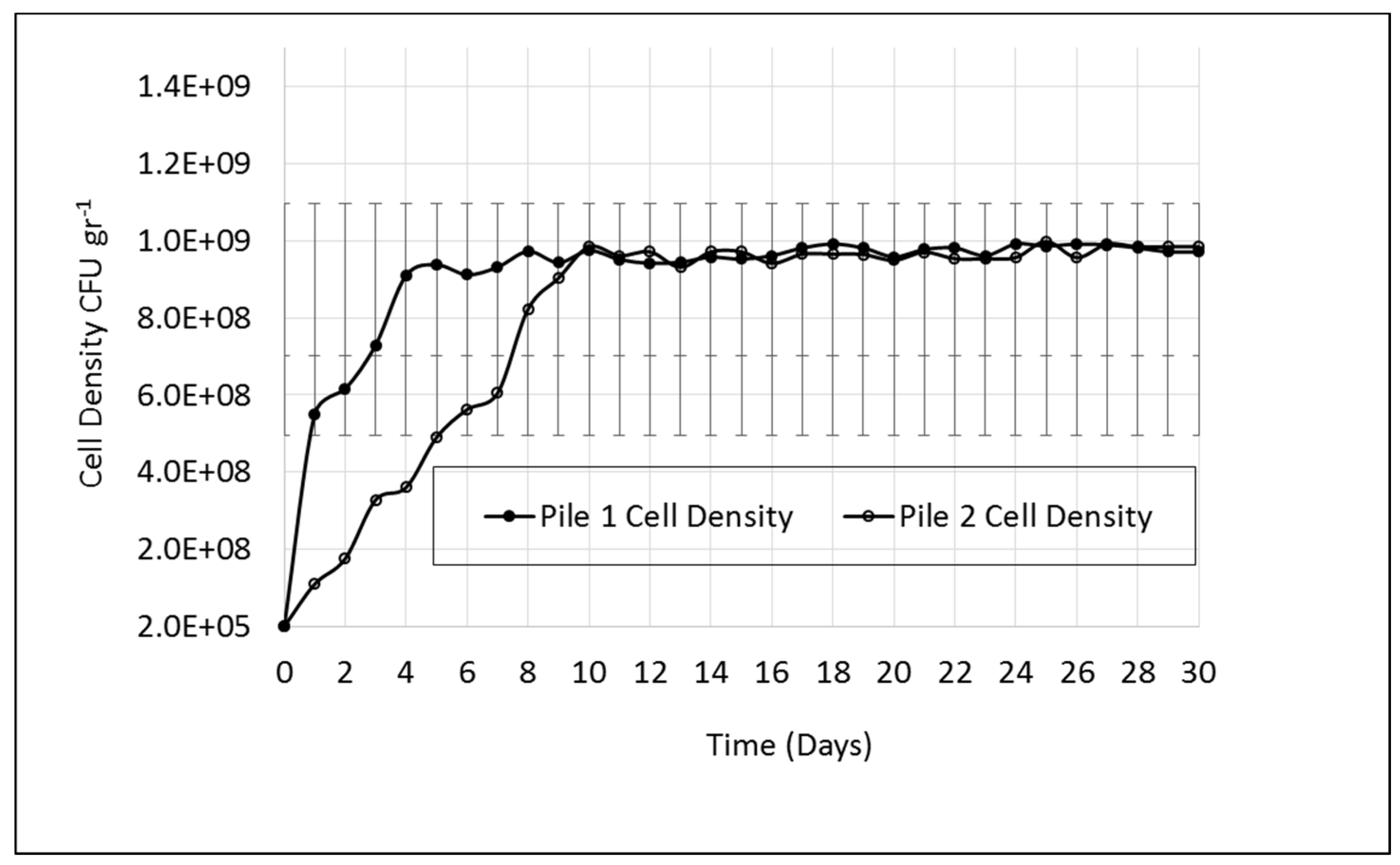
3.2. Microbial Analysis
As a result of microbial analysis in both piles, bacteria belonging to Bacillus, Pseudomonas, Lactobacillus, Psychrobacter pulmonis, and Trichococcus sp. were isolated from the samples. The initial microbial population in both piles was about 2.E+07 (2 × 10
5) CFU·g
−1 dry sample.
Figure 4 shows the microbial population changes in the piles throughout the study.
3.3. Pathogen Testing
Pathogens in the compost product indicate the applicability of the product as a fertilizer. Samples from days 0, 5, 20, and 30 corresponded to the raw manure, mesophilic stage, thermophilic stage, and the final stage for pathogen detection, respectively. The pathogens obtained from these samples contained Salmonella, Shigella, and Escherichia coli, which are commonly present in manure (
Table 2). Pathogenic bacteria such as Shigella, Salmonella, and E. coli were not detected in the final product, indicating that the product can be used as a fertilizer after curing.
3.4. Maturity Testing
Upon finalizing the composting process, various microorganisms start to mineralize nutrients and organic compounds, which increases the quality of compost. This period is called curing and it improves the quality of the compost before land application. In this study, 30 days of time were given for the maturation of the compost, without moisture maintenance and aeration.
The respirometry test identifies the organo-chemical condition of the compost product by determining the amount of CO
2 generated by microorganisms [
27]. Maturity analysis showed that the compost was mature and stable, with no phytotoxicity in the final product and a very low CO
2 respiration rate (0.25 mg CO
2·g
−1 OM·day
−1) (
Table 3).
3.5. Agronomic Testing for Cured Compost
Following the curing stage, 25 matured compost samples from pile 1 were tested for the agronomic value and are presented in
Table 4. The agronomic testing measured several parameters such as N
tot, organic matter, C:N ratio, NO
3−-N, etc. The agronomic test results showed that the final product indicated high-quality compost.
3.6. Greenhouse Gas Analysis
As a result of model outputs, net production of GHG emissions was calculated in this study. During the composting study, the waste truck and front-end loader consumed 8.58 L of diesel for loading and emptying the wet material over the composting area, which is equal to 0.0858 L of diesel per ton of wet material. The windrow turner consumed 3.04 L of diesel for turning the piles, which is equal to 0.03048 L of fuel per ton of waste turned. The last activity was the transportation and land spreading of the finished compost. The distance from the compost area to the land application site was about 20 km. Thus, the total diesel fuel consumed for the transport and land spreading of 50 tons of finished compost was 20 L. The finished compost was land-spread over 2 ha of land. The electricity consumed in the office located near the composting area was calculated as 168 MWh during the study. By using the model mentioned before, GHG emissions from diesel and electricity consumed were calculated. To calculate the GHG emissions generated during the composting process, the amounts of diesel fuel consumed and the electricity used were entered into the model. Then, the amount of finished compost land spread was used in the model to calculate the GHG emissions avoided.
Total methane emission from the composting process in two piles was calculated as 13 tCO2-e. Biogenic CO2 emission was determined to be 25 tCO2-e; however, it is not considered as GHG emission, as it is not produced from a combustion process. Direct N2O emission from the compost piles was calculated as 6.59 tCO2-e. GHG emissions from onsite equipment diesel use (31.62 L) were calculated as 85 tCO2-e. GHG emission from onsite electricity consumption in the office space was calculated as 8 × 10−5 tCO2-e. Finally, the avoided GHG emission through the chemical fertilizer substitution was calculated as 0.4 tCO2-e. Furthermore, the model calculated the direct GHG emissions as 103.9 tCO2-e. Then, the net GHG emission was calculated by subtracting the avoided emission (0.4 tCO2-e) from the generated emission (103.9 tCO2-e) as 103.5 tCO2-e. Then, the global warming potential (GWP) was calculated as 1.04 tCO2-e per ton of cattle manure composted.
4. Discussion
This study showed that the time in days to reach the thermophilic stage was similar to the times reported by Magrí and Teira-Esmatges [
28], and the total composting time was significantly reduced. The results indicated that rapid transition to the thermophilic phase can significantly reduce the composting time. The compost mass in the piles were maintained at temperatures above 65 °C for at least 3 days as recommended by the US composting council and other studies [
19,
29,
30]. Maintaining optimum moisture is very important, as it can increase or reduce microbial activity [
31]. The water content in both piles decreased throughout the composting period, from 71% to 59%. Variations in moisture content during composting are strongly affected by the characteristics of the input materials [
32].
Results of this study indicated that the pH value of the compost piles varied from 6.9 to 8.5. A pH value between 6.7 and 9.0 enhances the microbial activity during composting [
2]. The most suitable pH range for manure composting is reported to be between 5.5 and 8 [
33,
34]. As suggested by the US Composting Council, all final compost products should have pH values above 5.9 [
19]. This study showed that OM loss was much higher during the bio-oxidation phase in the inoculated pile, which indicates higher microbial activity. Gavilanes-Terán, Jara-Samaniego [
30] also observed similar OM losses in their compost studies with broccoli, tomato waste, laying hen manure, and sawdust.
Good-quality compost can be produced by selecting the most appropriate C:N ratio. As shown in this study, the C:N ratio value in pile 1 decreased to the desired value in as little as 4 days. Diaz, Bertoldi [
35] recommends a suitable C:N ratio of the input material of between 25 and 30. Kumar, Ou [
36] suggests even smaller C:N ratios such as less than 20. It is reported that a C:N ratio indicates the degree of compost maturity [
37,
38]. Nitrogen loss during composting occurs primarily via NH
3 volatilization at high temperatures, and it is desired that the loss is minimized, because less nitrogen loss means higher nitrogen concentration in the fertilizer [
39,
40].
It is clearly seen in
Figure 2 and
Figure 3 that the inoculated pile (pile 1) performed better than the control pile (pile 2) in terms of temperature rise, C:N ratio, and N
tot. It can be pointed out that the addition of microbial inoculum to pile 1 reduced the time to complete the composting time. The C:N ratio in pile 1 decreased to less than 20 in the first week of the study and was below 15 on day 18, whereas the C:N ratio in pile 2 stayed above 20 throughout the study.
As a result of microbial analysis in both piles,
Bacillus, Pseudomonas, Lactobacillus, Psychrobacter pulmonis, and
Trichococcus sp. were isolated in this study. Several types of
Bacillus sp. can be isolated from the samples collected during both mesophilic and thermophilic stages. The optimum temperature and pH range for the growth of
Bacillus species is 50 °C and 5.4 to 8.5, respectively [
41]. The dominant species isolated from samples collected during the thermophilic stage were mostly from Flavobacterium thermophilic bacteria. These bacteria can tolerate high temperatures and accelerate the composting process. It can be concluded that with decreasing ambient temperature in the compost piles, microbial metabolism was reduced, affecting the composting process. Adding compost bacteria such as
Bacillus, as performed in this study, can increase the population of compost bacteria and improve metabolism to finalize the process.
The colonies on the plates were counted and defined as the total colony forming units (CFU) g−1 dry sample. Compared to pile 2, a rapid increase in microbial population was observed within 5 days in samples collected from pile 1, which indicates that inoculating the pile has accelerated the growth of composting bacteria. A similar microbial population was observed on day 10 in pile 2. The inoculation of bacteria combined with optimum temperature, pH, and moisture content was effective in maintaining a high activity of microorganisms. Pathogenic bacteria were not detected in the pathogen testing experiments, indicating that the product can be used as a fertilizer after curing. In addition to pathogen testing experiments, maturity testing showed that the compost was mature and stable, indicating very low CO2 respiration rates.
The agronomic test results are important for the quality of the final product. As a reference rule, a C:N ratio less than 15 usually indicates maturity and greater than 15 indicates immaturity. The other guiding principle is that the NH
4+-N:NO
3−-N ratio can also be used as an indicator of compost maturity if this ratio is less than 1 [
42]. The results of this study satisfied both a C:N ratio of less than 15 and NH
4+-N:NO
3−-N ratio of less than 1.
This study predicted that the whole composting process generated 1.04 tCO
2-e per ton of cattle manure composted. Greenhouse gas emissions were generated from the energy consumed during the compost process. Komilis and Ham [
43] recommended a required energy for the front-end loader of 0.33 kWh·t
−1 waste, which was similar to the value measured in this study. Levis and Barlaz [
44] suggested that compost turner trucks need an energy of 0.24 kWh·t
−1. Komilis and Ham [
43] recommended an office space of 0.007 m
2 based on the daily amount of waste handled. An office space electricity use of 290 kWh·m
−2·yr
−1 was suggested by Levis and Barlaz [
44]. In addition to loading and transporting the compost, land application requires 10 L·ha
−1 diesel fuel. For land application by a tractor, a rate of 25 t·ha
−1 is recommended [
44]. The avoided GHG emissions were calculated based on the chemical fertilizer’s substitution. It is recommended that 8 kg CO
2-e is avoided per ton of organic waste composted [
24]. Kong, Shan [
22] reported that windrow composting of organic materials resulted in net reductions of GHG emissions.