Synthesis and Characterization of New Schiff Base/Thiol-Functionalized Mesoporous Silica: An Efficient Sorbent for the Removal of Pb(II) from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods (Experimental Section)
2.1. Materials
2.2. Instrumentation
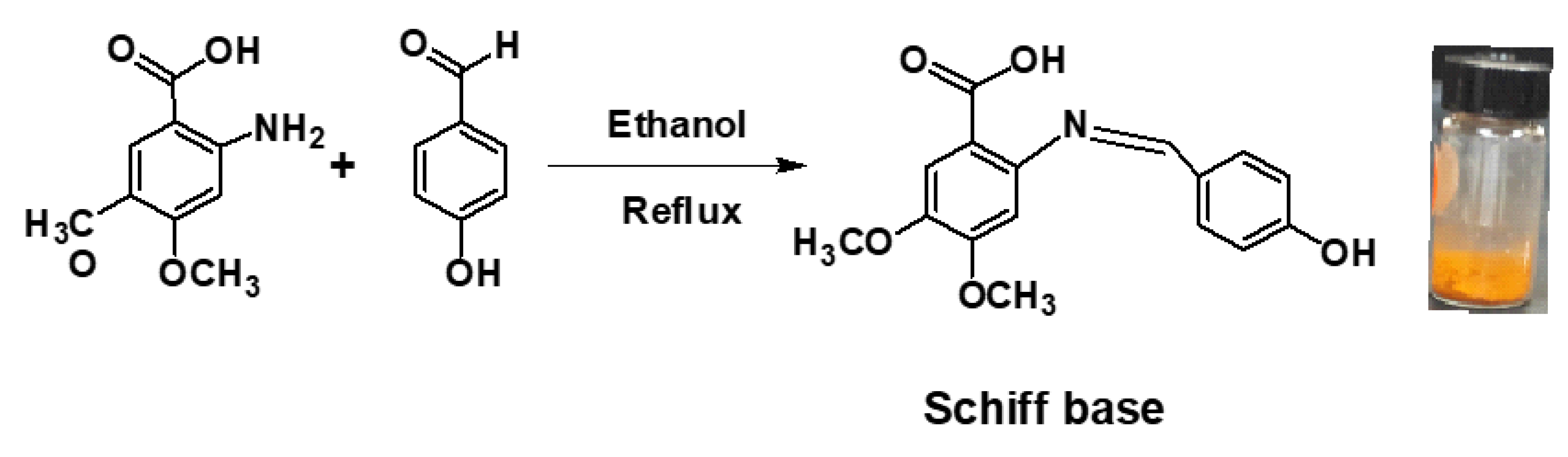
2.3. Preparation of the Schiff Base 2-(4-Hydroxybenzylideneamino)-4,5-Dimethoxybenzoic Acid
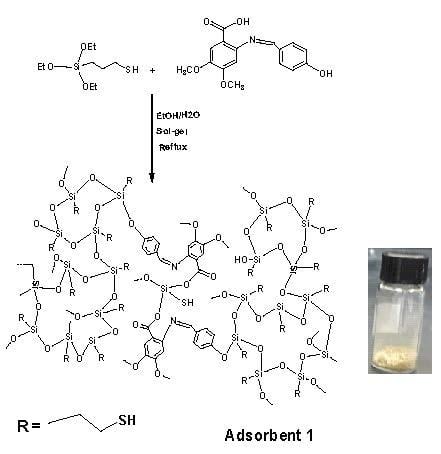
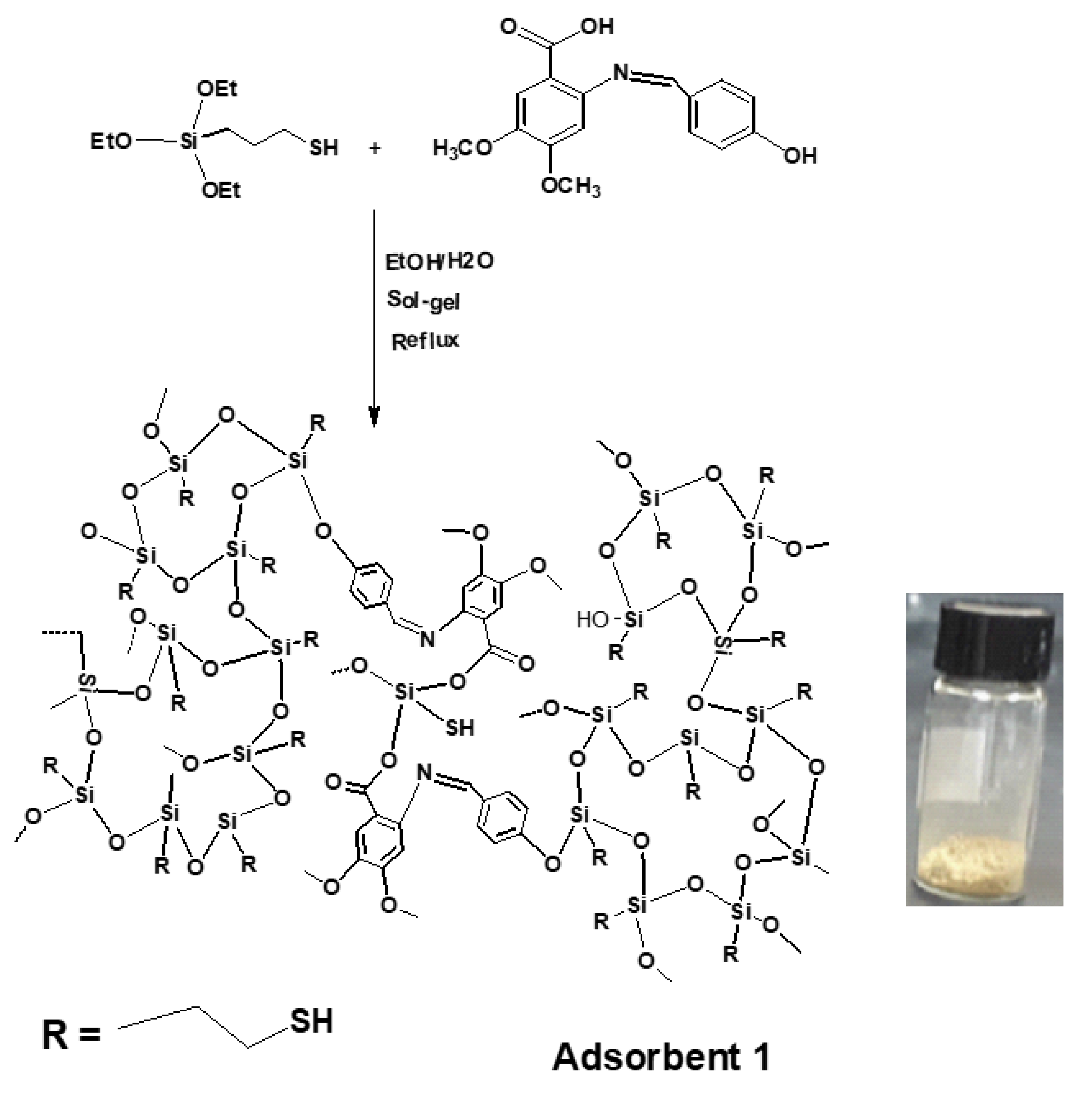
2.4. Preparation of Schiff Base-Thiol Silica (Adsorbent 1)
2.5. Sorption Experiment
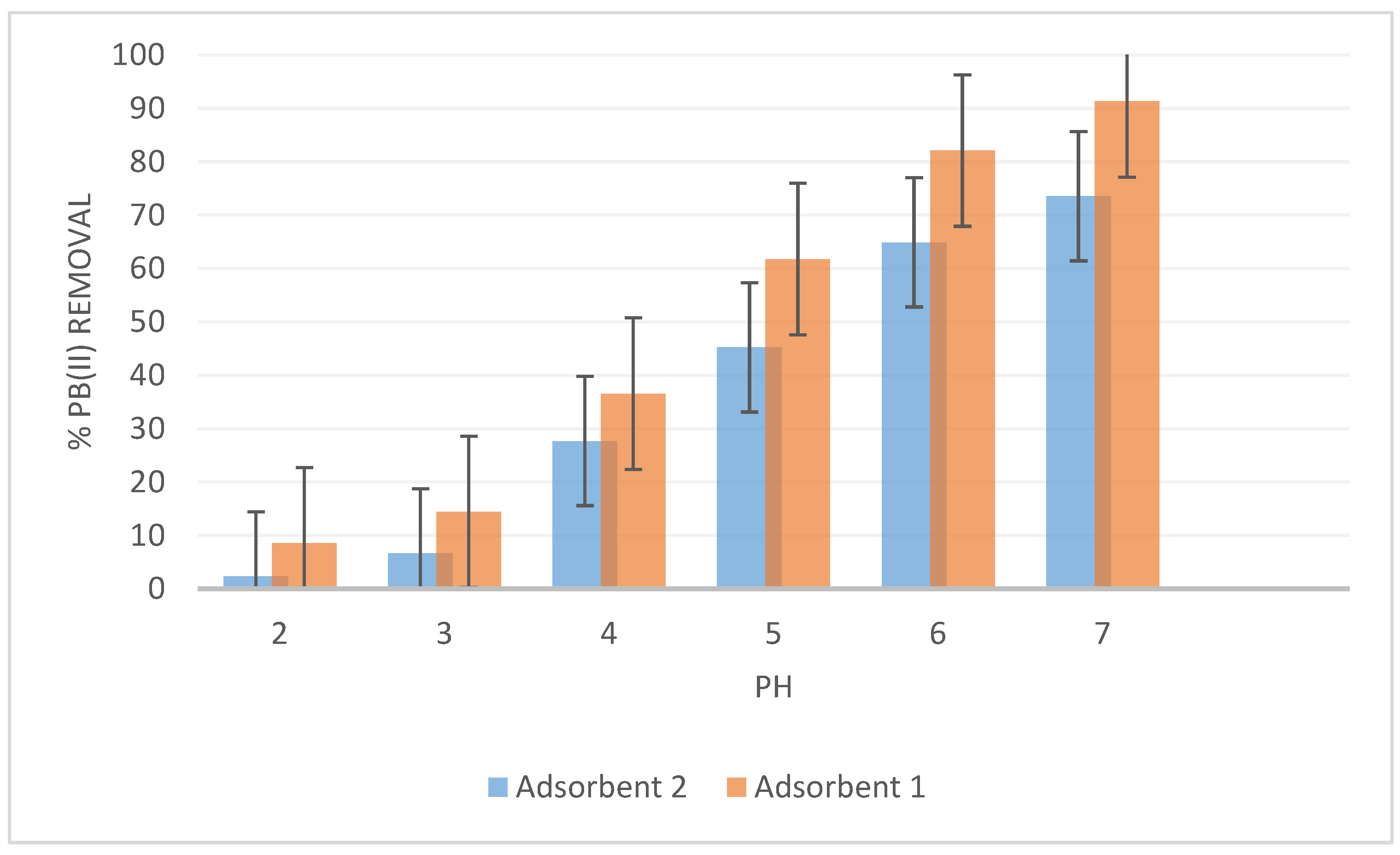
2.5.1. pH Optimization
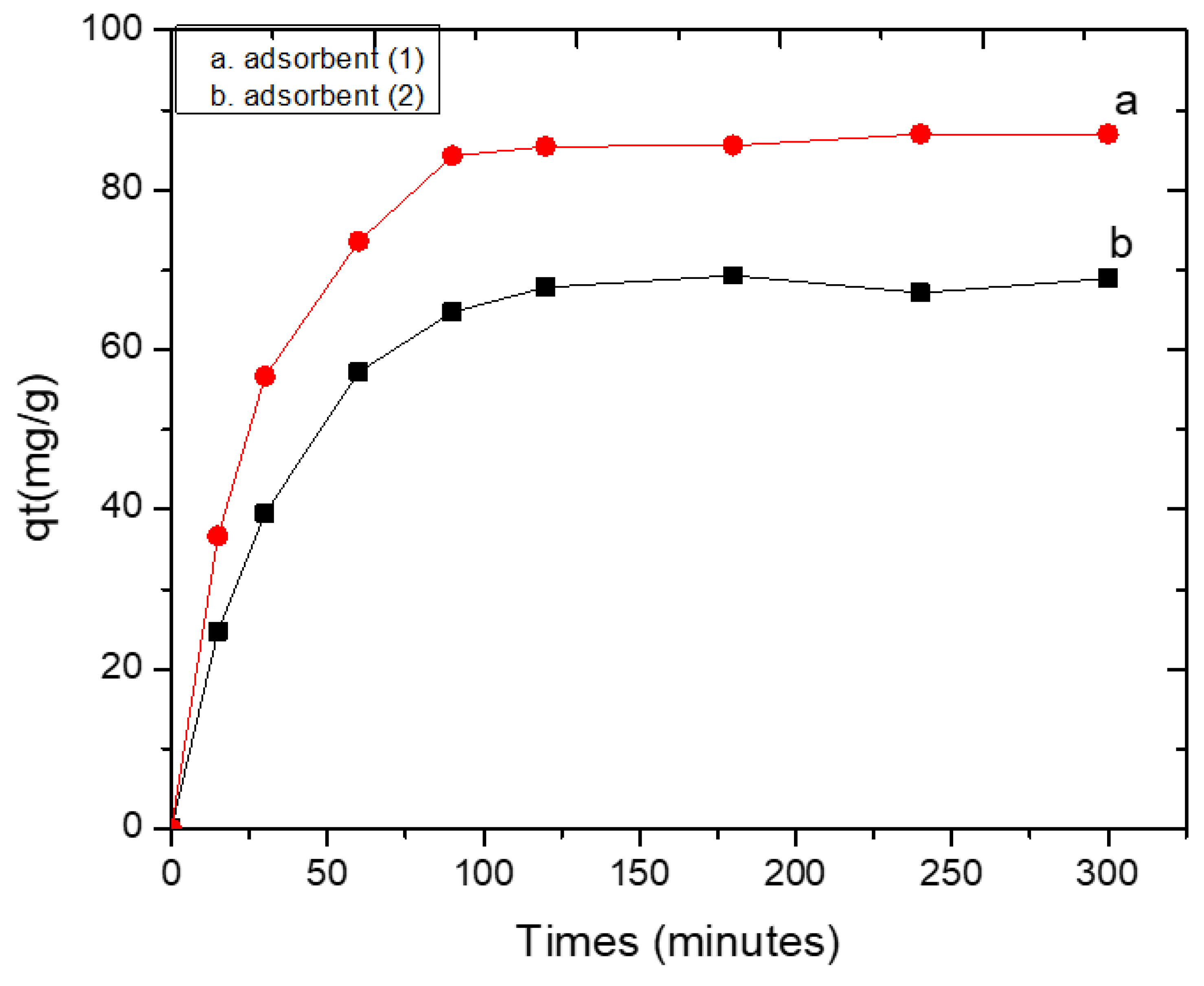
2.5.2. Effect of Contact Time for Pb(II)
3. Results and Discussion
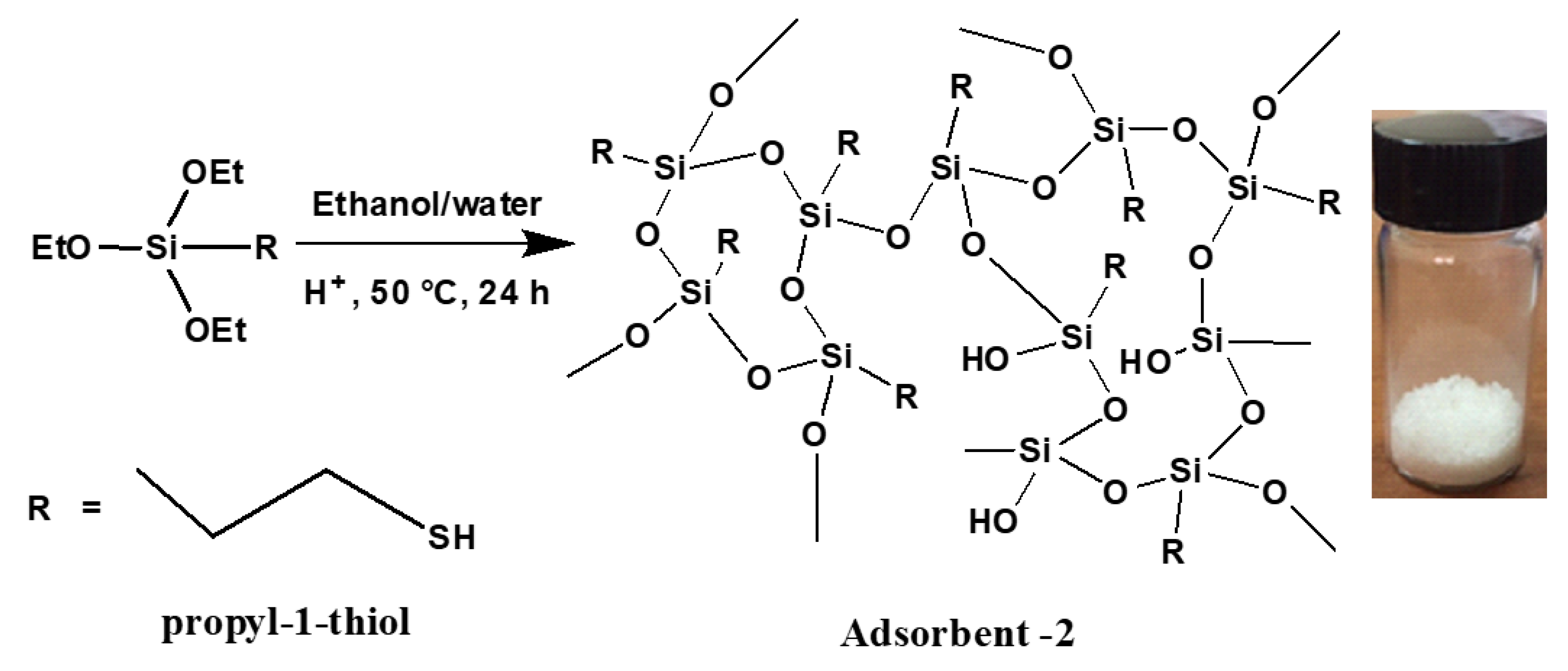
3.1. Synthesis of Adsorbents 1 and 2
3.2. Characterization
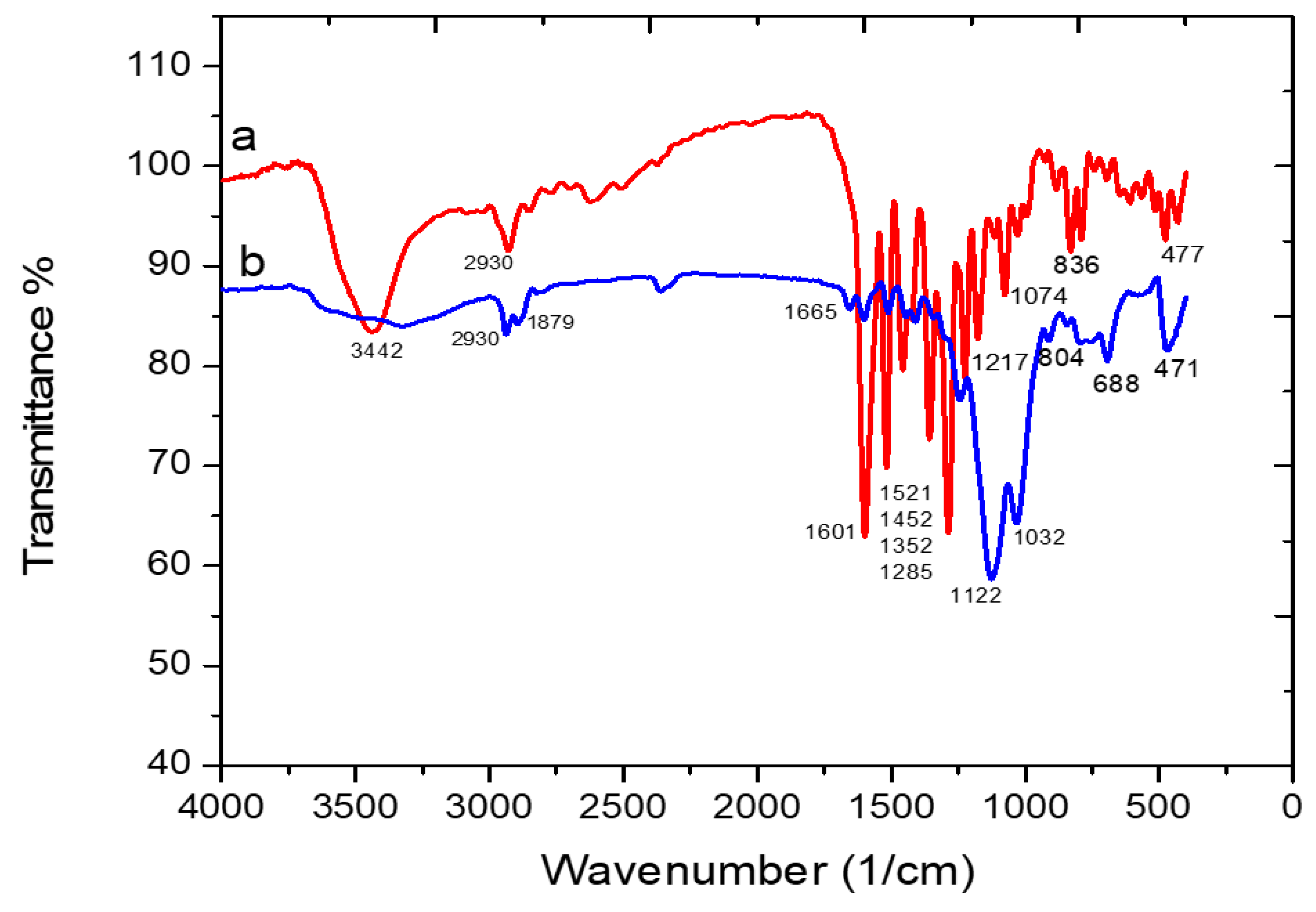
3.2.1. The FT-IR Spectra of Free Schiff Base and the Schiff Base Functionalized Silica (Adsorbent 1)
3.2.2. The FT-IR Spectra of Adsorbent 1 before and after Loading Pb(II) Ions
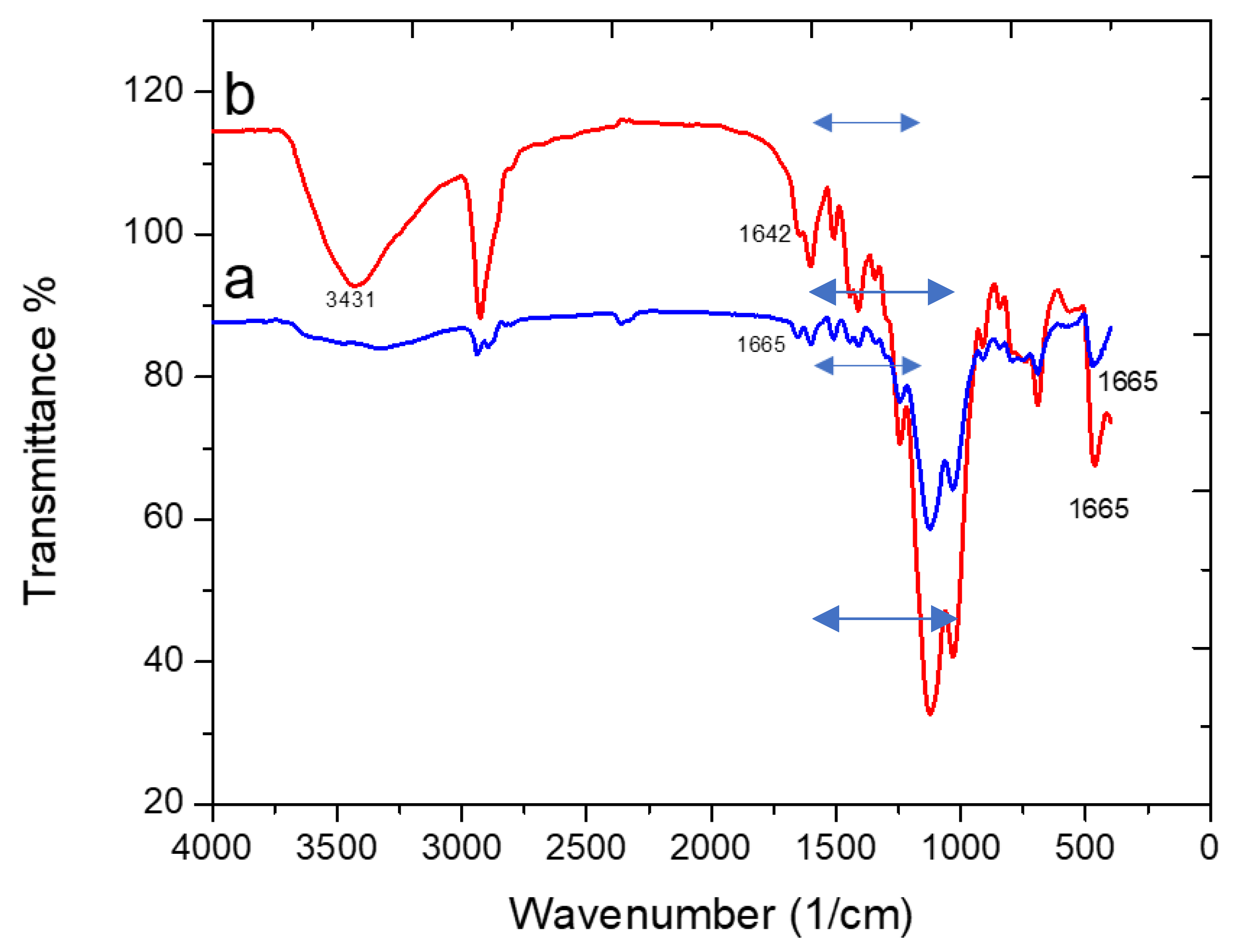
3.2.3. The FT-IR Spectra of Adsorbent 2 before and after Loading Pb(II) Ions
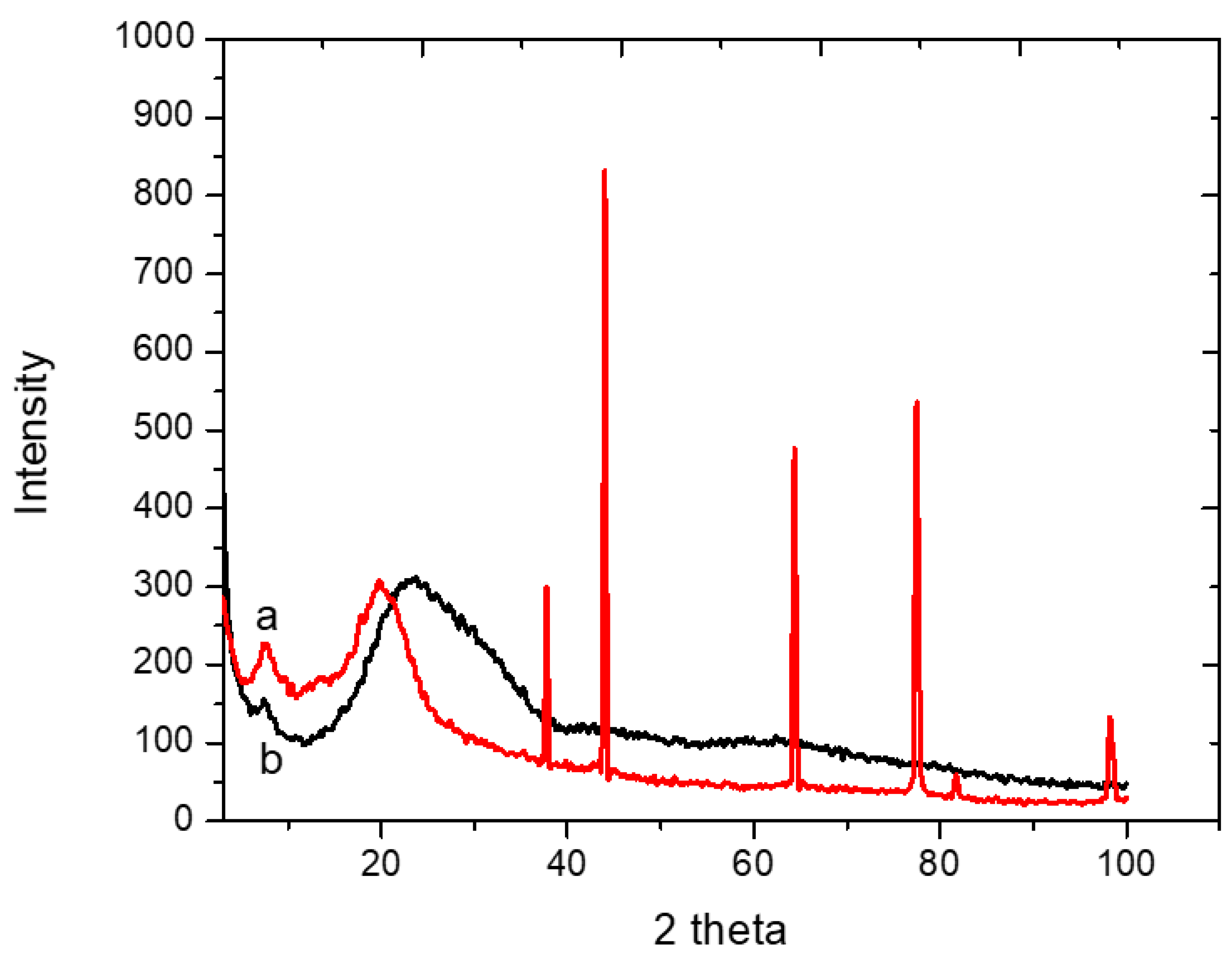
3.2.4. XRD of Adsorbents 1 and 2
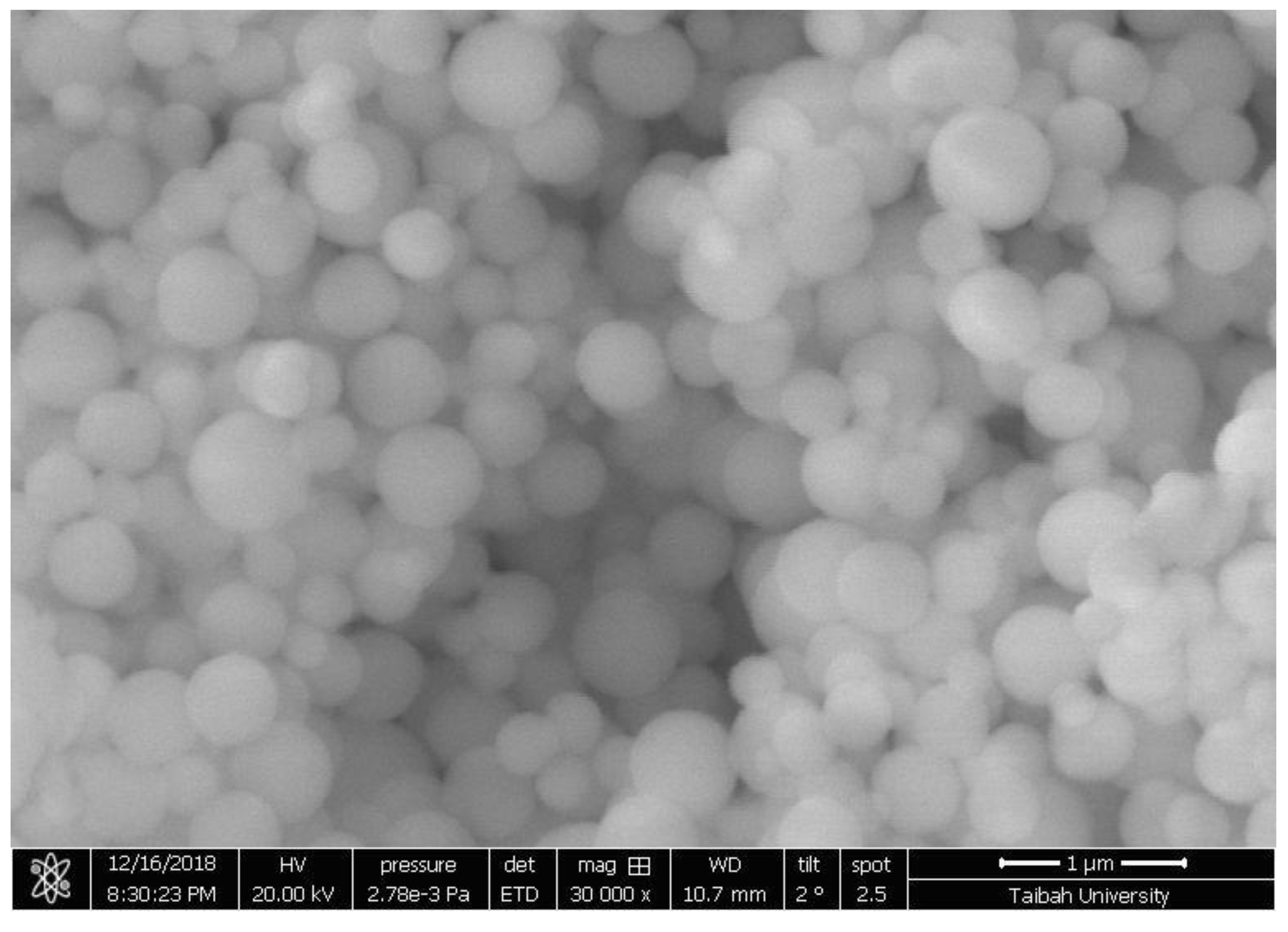
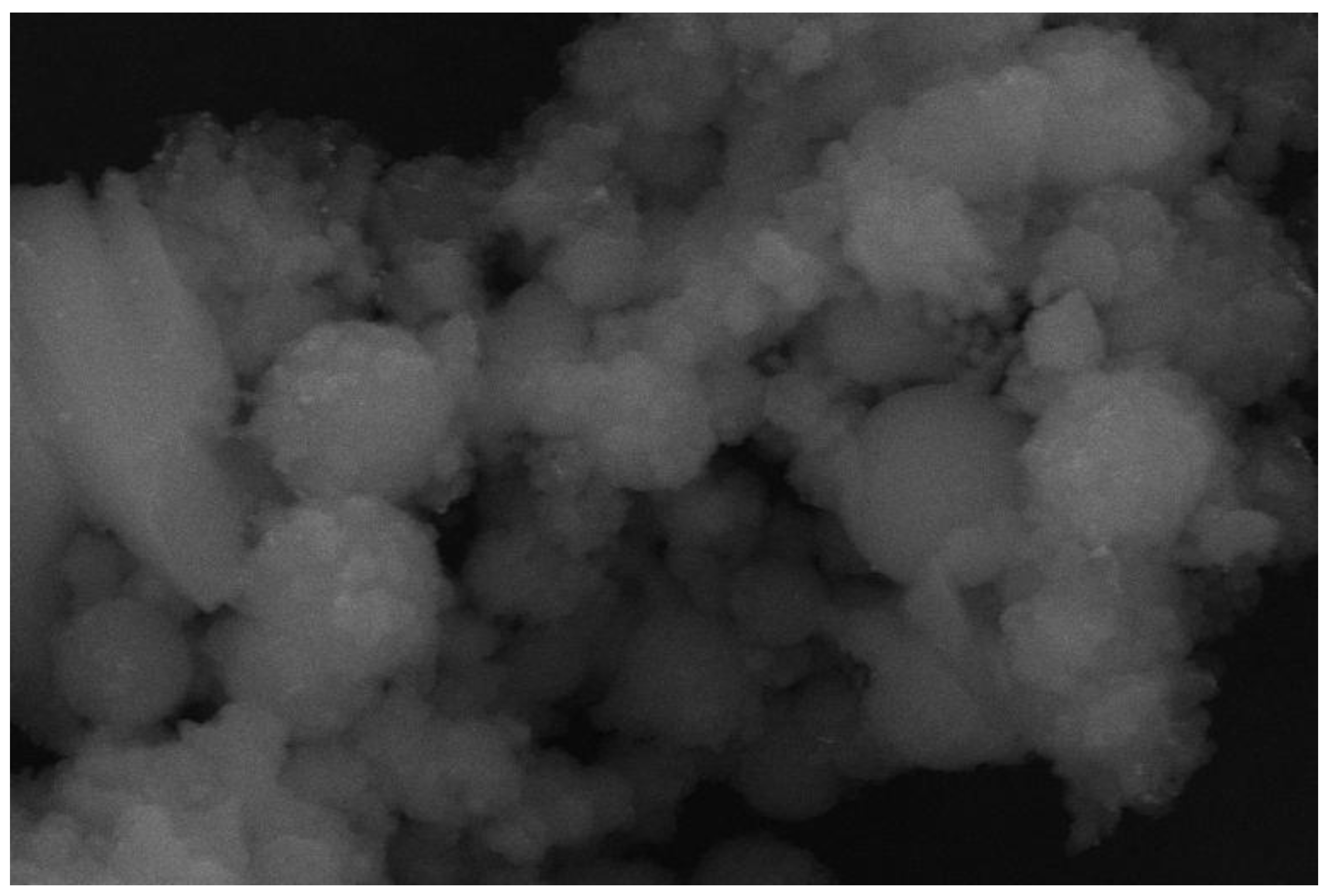
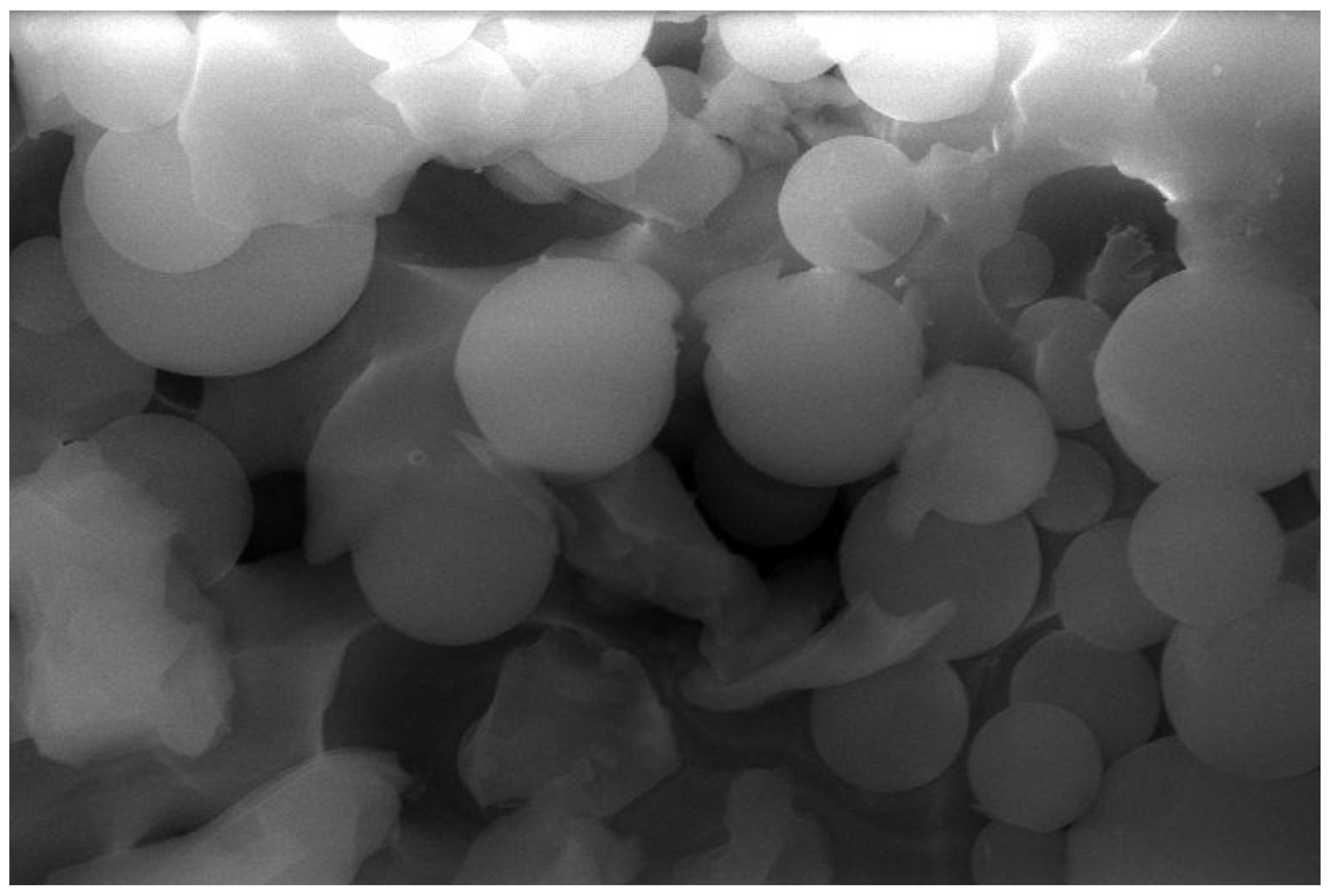
3.2.5. SEM of Adsorbents 1
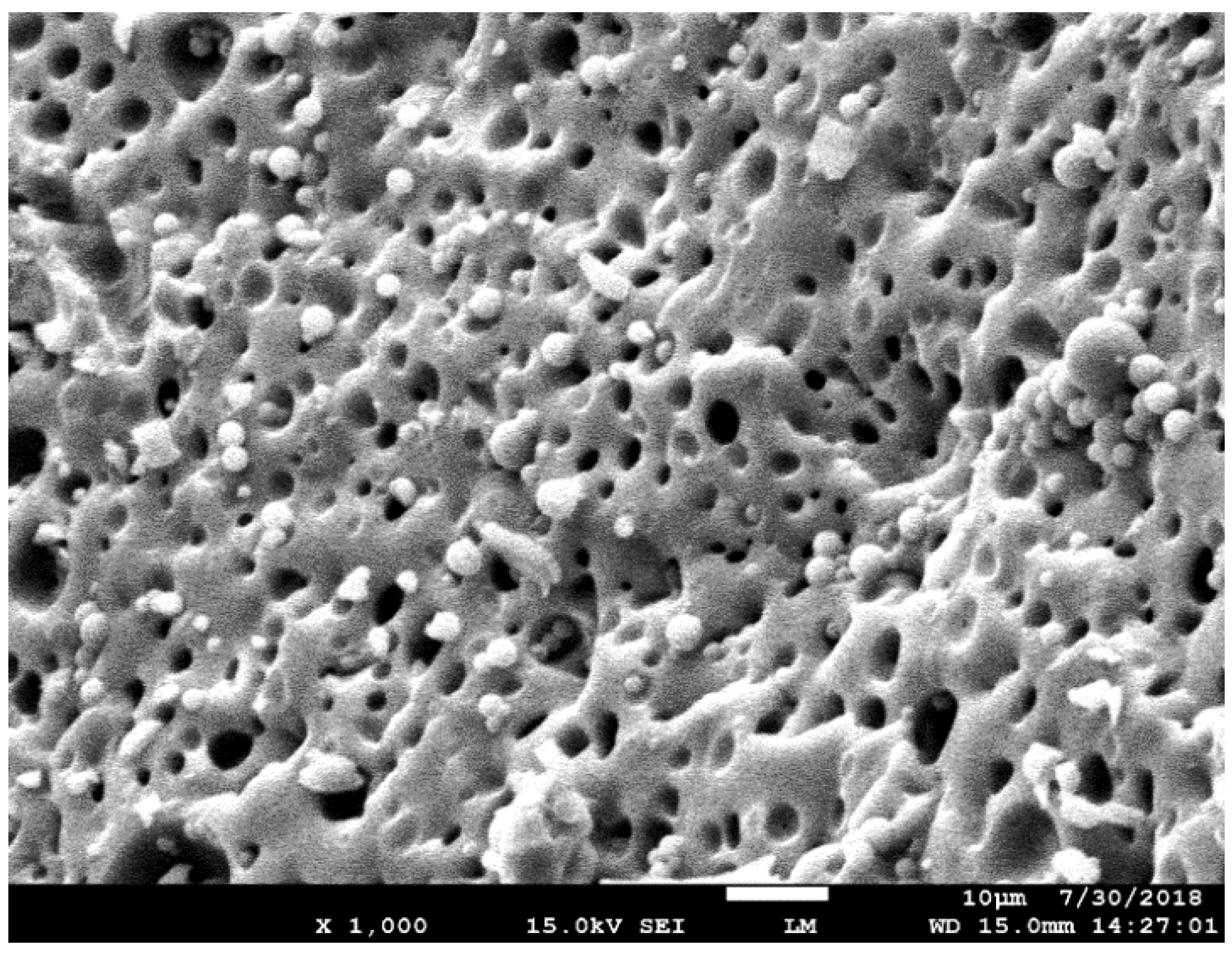
3.2.6. Field Emission Scanning Microscope (FESEM) of Adsorbent 2
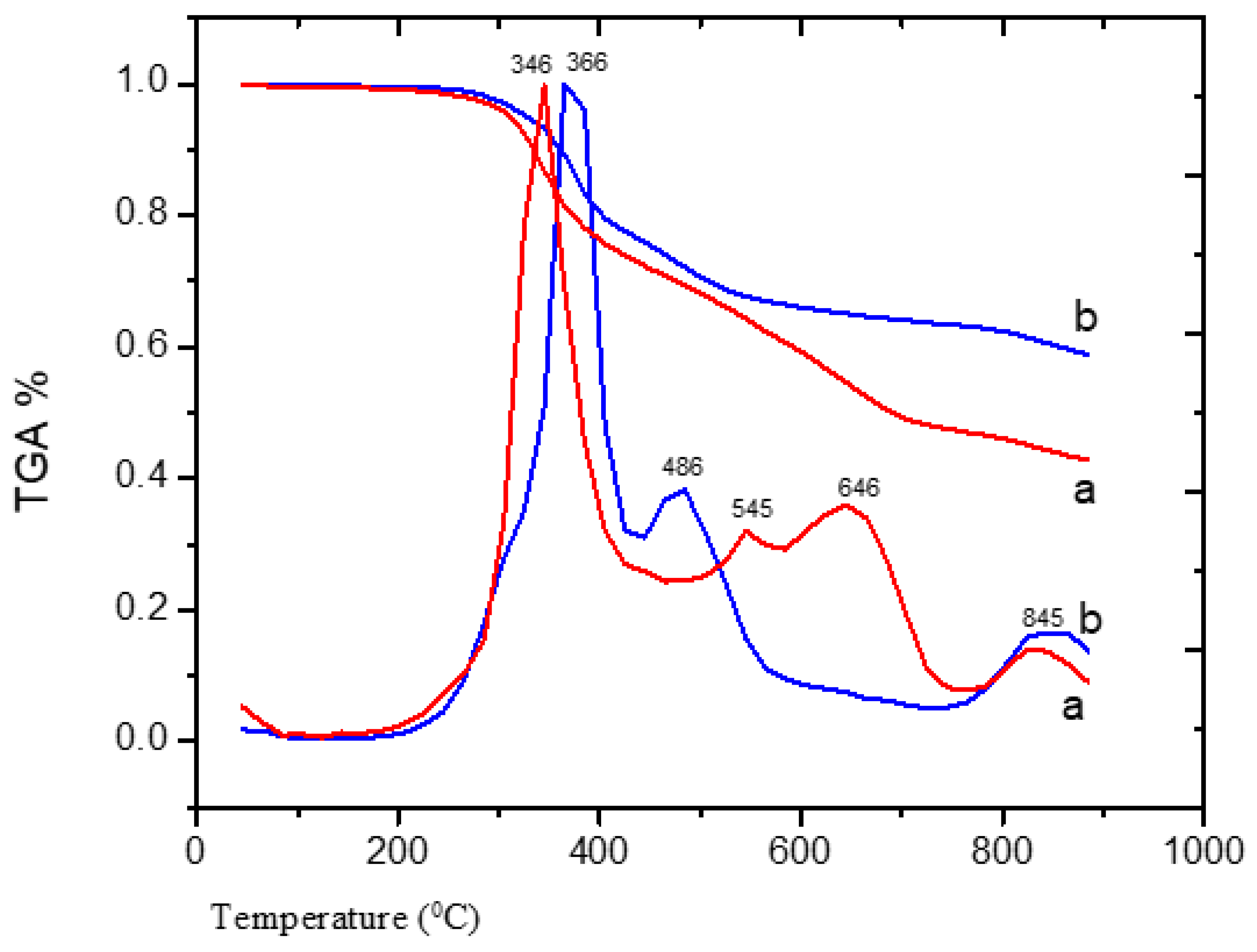
3.2.7. TGA of Adsorbent 1 and 2
3.2.8. Surface Properties of Adsorbents 1 and 2
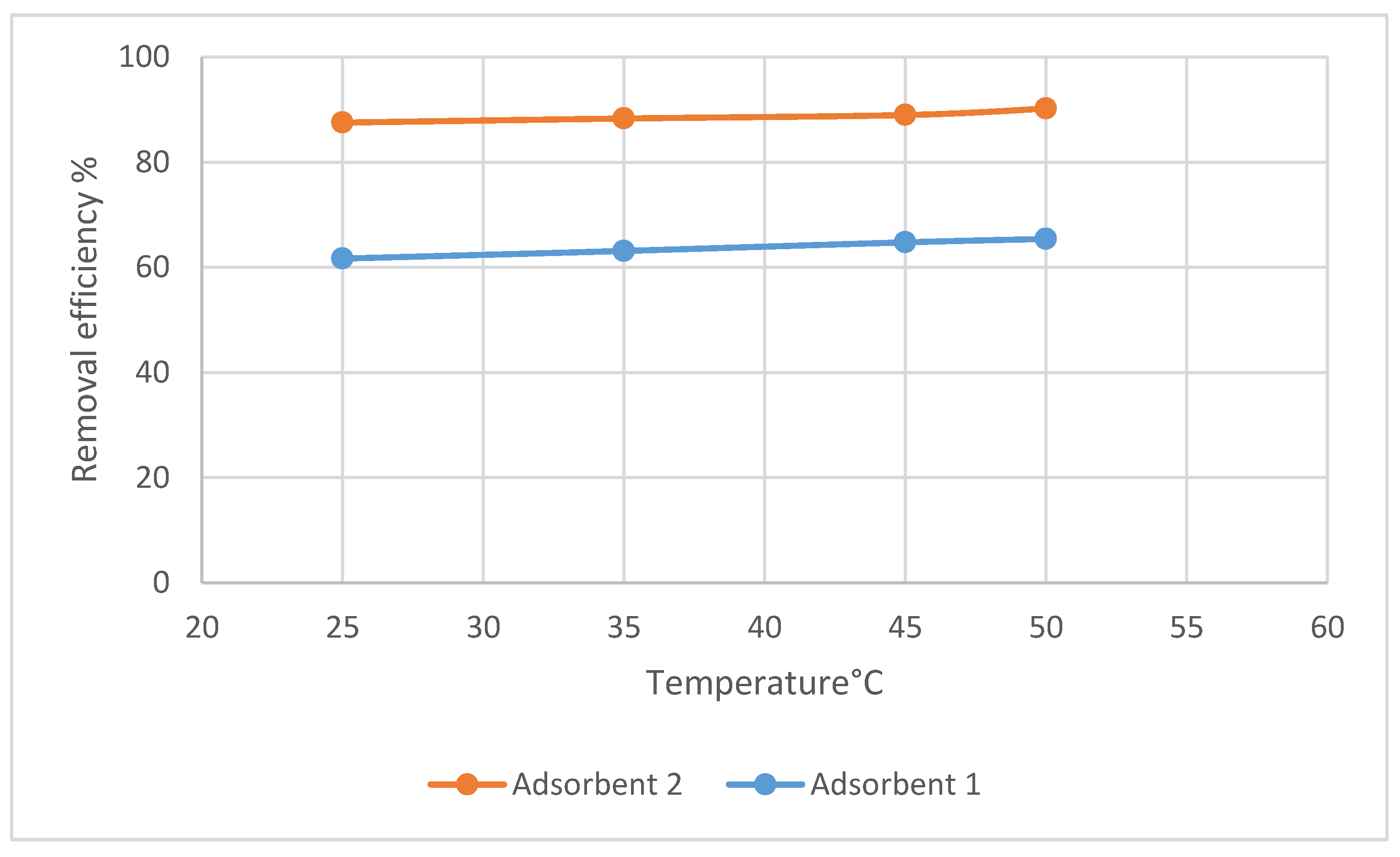
3.3. Adsorption Study
3.3.1. Effects of pH on the Adsorption of Pb(II) by Adsorbent 1
3.3.2. Effect of Contact Time and Kinetics for the Adsorption of Pb(II) by Adsorbents 1 and 2
3.3.3. Adsorption Kinetics for the Adsorption of Pb(II) by Adsorbents 1 and 2
3.3.4. Adsorption Isotherms
- The Langmuir Isotherm Model [43]where qe is the is the lead concentration adsorbed per specific amount of adsorbent (mg g−1), Ceis the equilibrium concentration of Pb(II) expressed in mg L−1, and qm is the maximum amount of lead ions required to form a monolayer (mg g−1). The Langmuir equation can be rearranged to a linear form (Equation (6)) for the convenience of plotting and determination of the Langmuir constant (KL, the first coefficient related to the energy of adsorption) as below. The values of qm and KL can be determined from the linear plot of 1/Ce versus 1/qe:qe = qm KL Ce/1 + KL CeThe dimensionless equilibrium parameter or separation factor (RL) could be expressed as in the following equation [44]:1/qe = 1/qm + 1/KL qm × 1/Cewhere C0 is the initial concentration of the lead ion, and KL is the Langmuir constant. The value of RL is between 0 and 1 for favourable adsorption, while RL > 1 represents unfavorable adsorption, and RL = 1 represents linear adsorption. The adsorption process is irreversible if RL = 0.RL = 1/1 + KLC0
- The Freundlich Isotherm Model [45]qe = KF Ce1/nwhere, qe is the equilibrium uptake capacity (mg g−1) and Ceis the equilibrium concentration of Pb(II)) expressed in mg L−1. kF is the first coefficient related to the energy of adsorption, and n is a coefficient. Both constants kF and n can be calculated from the plot of log qe vs. log Ce.Log qe = LogKF + (1/n) Log Ce
3.3.5. Linear Fitting of the Isotherm Models
- The Langmuir Isotherm Model
- The Freundlich Isotherm Model
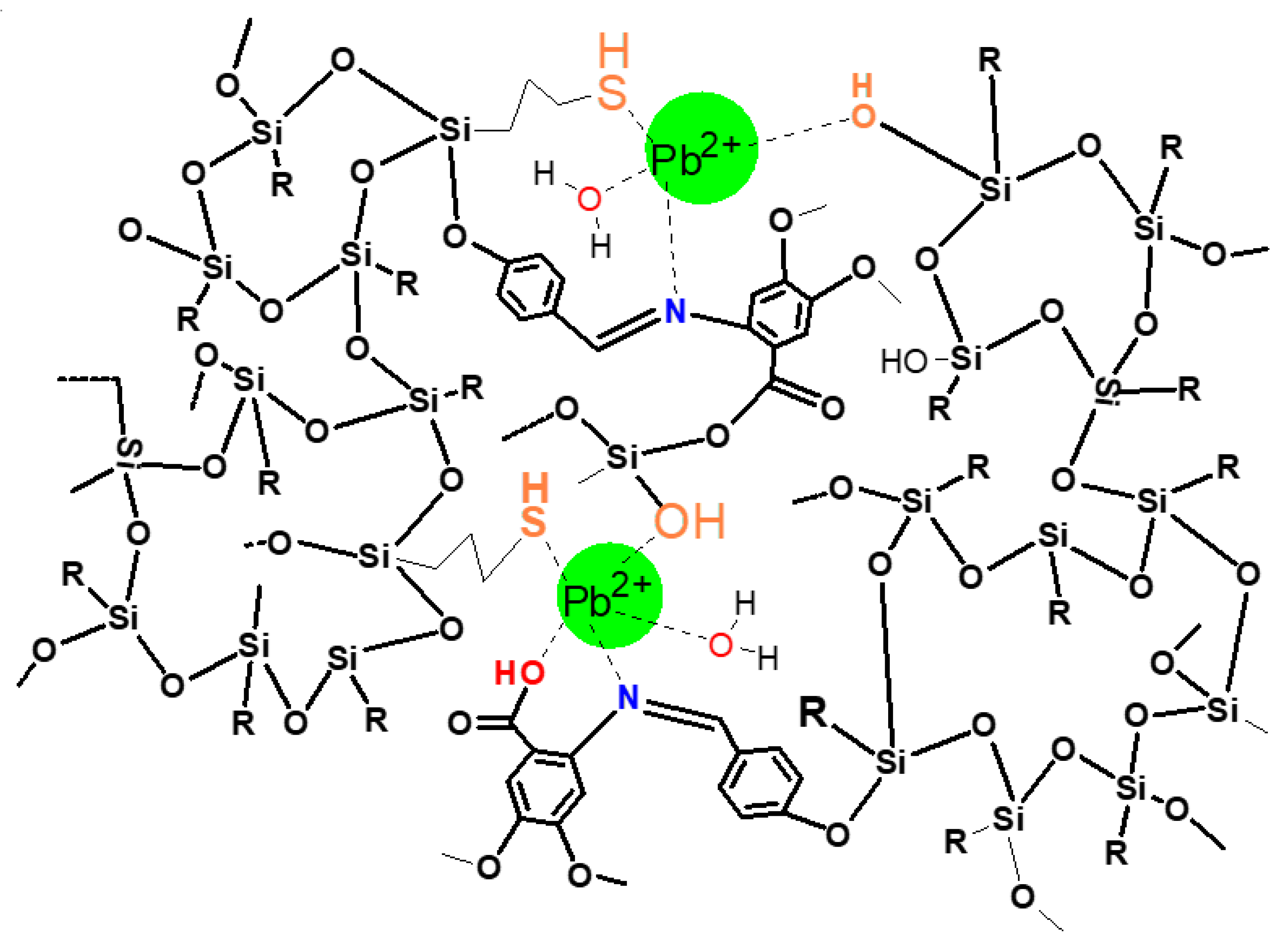
3.3.6. Complexation of Pb(II) by Adsorbent 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huuha, T.S.; Kurniawan, T.A.; Sillanpaa, M.E.T. Removal of silicon from pulping whitewater using integrated treatment of chemical precipitation and evaporation. Chem. Eng. J. 2010, 158, 584–592. [Google Scholar] [CrossRef]
- El-Nasser, A.; Parish, R. Solid polysiloxane ligands containing glycine- or iminodiacetate-groups: Synthesis and application to binding and separation of metal ions. J. Chem. Soc. Dalton Trans. 1999, 19, 3463–3466. [Google Scholar] [CrossRef]
- El-Nahhal, I.M.; El-Shetary, B.A.; Salib, K.A.R.; El-Ashgar, N.M.; El-Hashash, A.M. Uptake of divalent metal ions (Cu2+, Ni2+, and Co2+) by polysiloxane immobilized triamine-thiol- and thiocyanate ligand system. Anal. Lett. 2001, 34, 2189–2202. [Google Scholar] [CrossRef]
- Najafi, M.; Rostamian, R.; Rafati, A.A. Chemically modified silica gel with thiol group as an adsorbent for retention of some toxic soft metal ions from water and industrial effluent. Chem. Eng. J. 2011, 168, 426–432. [Google Scholar] [CrossRef]
- Esalah, O.; Weber, M.; Vera, J. Removal of lead, cadmium and zinc from aqueous solutions by precipitation with sodium Di-(n-octyl) phosphinate. Can. J. Chem. Eng. 2000, 78, 948–954. [Google Scholar] [CrossRef]
- Lertlapwasin, R.; Bhawawet, N.; Imyim, A.; Fuangswasdi, S. Ionic liquid extraction of heavy metal ions by 2-aminothiophenol in 1-butyl-3-methylimidazolium hexafluorophosphate and their association constants. Sep. Purif. Technol. 2010, 72, 70–76. [Google Scholar] [CrossRef]
- Emamjomeh, M.M.; Sivakumar, M. Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manag. 2009, 90, 1663–1679. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hoadley, A. An evaluation of a hybrid ion exchange electrodialysis process in the recovery of heavy metals from simulated dilute industrial wastewater. Water Res. 2012, 46, 3364–3376. [Google Scholar] [CrossRef]
- Yurloval, L.; Kryvoruchko, A.; Kornilovich, B. Removal of Ni (II) ions from wastewater by micellar-enhanced ultrafiltration. Desalination 2002, 144, 255–260. [Google Scholar] [CrossRef]
- Greenlee, L.; Lawler, D.; Freeman, B.; Marrot, B.; Moulin, P. Reverse Osmosis Desalination: Water Sources, Technology and Today’s Challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef]
- Benit, Y.; Ruiz, M. Reverse osmosis applied to metal finishing wastewater. Desalination 2002, 142, 229–234. [Google Scholar] [CrossRef]
- Rubio, J.; Souza, M.; Smith, R.W. Overview of flotation as a wastewater treatment technique. Miner. Eng. 2002, 15, 139–155. [Google Scholar] [CrossRef]
- Ghurye, G.; Clifford, D.; Tripp, A. Iron coagulation and direct microfiltration to remove arsenic from groundwater. Am. Water Work. Assoc. 2004, 96, 143–152. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Xu, W.; Huang, X.; Liu, J.H. Stable Organic–Inorganic Hybrid of Polyaniline/α-Zirconium Phosphate for Efficient Removal of Organic Pollutants in Water Environment. Appl. Mater. Interfaces 2012, 4, 2686–2692. [Google Scholar] [CrossRef]
- Gao, B.; Gao, Y.; Li, Y. Preparation and chelation adsorption property of composite chelating material poly (amidoxime)/SiO2 towards heavy metal ions. Chem. Eng. J. 2010, 158, 542–549. [Google Scholar] [CrossRef]
- Zaitseva, N.; Zaitsev, V.; Walcarius, A. Chromium (VI) removal via reduction–sorption on bi-functional silica adsorbents. J. Hazard. Mater. 2013, 250, 454–461. [Google Scholar] [CrossRef]
- Simsek, E.; Duranoglu, D.; Beker, U. Heavy Metal Adsorption by Magnetic Hybrid-Sorbent: An Experimental and Theoretical Approach. Sep. Sci. Technol. 2012, 47, 1334–1340. [Google Scholar] [CrossRef]
- Suchithra, P.; Vazhayal, L.; Mohamed, A.; Ananthakumar, S. Mesoporous organic–inorganic hybrid aerogels through ultrasonic assisted sol–gel intercalation of silica–PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012, 200, 589–600. [Google Scholar] [CrossRef]
- Repo, E.; Warchoł, J.; Bhatnagar, A.; Sillanpää, M. Heavy metals adsorption by novel EDTA-modified chitosan–silica hybrid materials. J. Colloid Interface Sci. 2011, 358, 261–267. [Google Scholar] [CrossRef]
- Ge, P.; Li, F.; Zhang, B. Synthesis of modified mesoporous materials and comparative studies of removal of heavy metal from aqueous solutions. Pollut. J. Environ. Stud. 2010, 19, 301–308. [Google Scholar]
- Pang, Y.; Zeng, G.; Tang, L.; Zhang, Y.; Liu, Y.; Lei, X.; Li, Z.; Zhang, J.; Xie, G. PEI-grafted magnetic porous powder for highly effective adsorption of heavy metal ions. Desalination 2011, 281, 278–284. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Wang, A. Fast removal of methylene blue from aqueous solution by adsorption onto chitosan-g-poly (acrylic acid)/attapulgite composite. Desalination 2011, 266, 33–39. [Google Scholar] [CrossRef]
- Mercier, L.; Pinnavaia, T. Heavy Metal Ion Adsorbents Formed by the Grafting of a Thiol Functionality to Mesoporous Silica Molecular Sieves: Factors Affecting Hg (II) Uptake. Environ. Sci. Technol. 1998, 32, 2749–2754. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Schhimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous material. Bull. Chem. Soc. 1990, 63, 988–992. [Google Scholar] [CrossRef]
- Kubota, Y.; Nishizaki, Y.; Sugi, Y. High Catalytic Activity of as-Synthesized, Ordered Porous Silicate–Quaternary Ammonium Composite for Knoevenagel Condensation. Chem. Lett. 2000, 29, 998–999. [Google Scholar] [CrossRef]
- Wu, J.; Liu, X.; Tolbert, S. High-Pressure Stability in Ordered Mesoporous Silicas: Rigidity and Elasticity through Nanometer Scale Arches. J. Phys. Chem. 2000, 104, 11837–11841. [Google Scholar] [CrossRef]
- Wu, J.; Abu-Omar, M.; Tolbert, S. Fluorescent Probes of the Molecular Environment within Mesostructured Silica/Surfactant Composites under High Pressure. Nanoletters 2001, 1, 27–31. [Google Scholar] [CrossRef]
- Diaz, J.; Balkus, J.; Bedioui, F.; Kurshev, V.; Kevan, L. Synthesis and Charact-erization of Cobalt-Complex Functionalized MCM-41. Chem. Mater. 1997, 9, 61–67. [Google Scholar] [CrossRef]
- Liu, J.; Song, L.; Shao, G. Novel Zwitterionic Inorganic—Organic Hybrids: Kinetic and Equilibrium Model Studies on Pb2+ Removal from Aqueous Solution. J. Chem. Eng. Data 2011, 56, 2119–2127. [Google Scholar] [CrossRef]
- Pavan, F.; Costa, T.; Benvenutti, E. Adsorption of CoCl2, ZnCl2 and CdCl2 on aniline/silica hybrid material obtained by sol-gel method. Colloids Surf. A Physicochem. Eng. Asp. 2003, 226, 95–100. [Google Scholar] [CrossRef]
- Radi, S.; Tighadouini, S.; Bacquet, M.; Degoutin, S.; Cazier, F.; Zaghrioui, M.; Mabkhot, Y.N. Organically Modified Silica with Pyrazole-3-carbaldehyde as a New Sorbent for Solid-Liquid Extraction of Heavy Metals. Molecules 2014, 19, 247–262. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, Y.; Qu, W.; Sun, C.; Chen, J.; Ping, Y.; Chen, H.; Niu, Y. Mercury adsorption by sulfur- and amidoxime-containing bifunctional silica gel based hybrid materials. Chem. Eng. J. 2013, 219, 51–61. [Google Scholar] [CrossRef]
- Anbarasu, G.; Malathy, M.; Karthikeyan, P.; Rajavel, R. Silica functionalized Cu (II) acetylacetonate Schiff base complex: An efficient catalyst for the oxidative condensation reaction of benzyl alcohol with amines. J. Solid State Chem. 2017, 253, 305–312. [Google Scholar] [CrossRef]
- Singh, H.; Singh, J.B. Synthesis and Characterization of New Lead (II) and Organotin (IV) Complexes of Schiff Bases Derived from Histidine and Methionine. Int. J. Inorg. Chem. 2012, 2012. [Google Scholar] [CrossRef]
- Sandhu, G.K.; Verma, S.P. Triorganotin (IV) derivatives of five membered heterocyclic 2-carboxylic acids. Polyhedron 1987, 6, 587–592. [Google Scholar] [CrossRef]
- Miao, J.; Qian, J.; Wang, X.; Zhang, Y.; Yang, H.; He, P. Synthesis and characterization of ordered mesoporous silica by using polystyrene microemulsion as templates. Mater. Lett. 2009, 63, 989–990. [Google Scholar] [CrossRef]
- Helloa, K.; Ibrahimb, A.; Jawad, K.; Shneineb, J. Simple method for functionalization of silica with alkyl silane and organic ligand. South Afr. J. Chem. Eng. 2018, 25, 159–168. [Google Scholar] [CrossRef]
- Naghmeh, D.; Niaz, M.; Mahmood, T. Preparation and Characterization of a Molybdenum (VI) Schiff Base Complex as Magnetic Nanocatalyst for Synthesis of 2-Amino-4H-benzo[h]chromenes. J. Nanostruct. 2016, 6, 312–321. [Google Scholar] [CrossRef]
- Xue, X.; Li, F. Removal of Cu (II) from aqueous solution by adsorption onto functionalized SBA-16 mesoporous silica. Microporous Mesoporous Mater. 2008, 116, 116–122. [Google Scholar] [CrossRef]
- Ramesh, A.; Hasegawa, H.; Maki, T.; Ueda, K. Adsorption of inorganic and organic arsenic from aqueous solutions by polymeric Al/Fe modified montmorillonite. Sep. Purif. Technol. 2007, 56, 90–100. [Google Scholar] [CrossRef]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plain surface of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1368. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen. Zeitschrift für Physikalische. Chemie 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Karthik, R.; Meenakshi, S. Removal of Pb (II) and Cd (II) ions from aqueous solution using polyaniline grafted chitosan. Chem. Eng. J. 2015, 263, 168–177. [Google Scholar] [CrossRef]

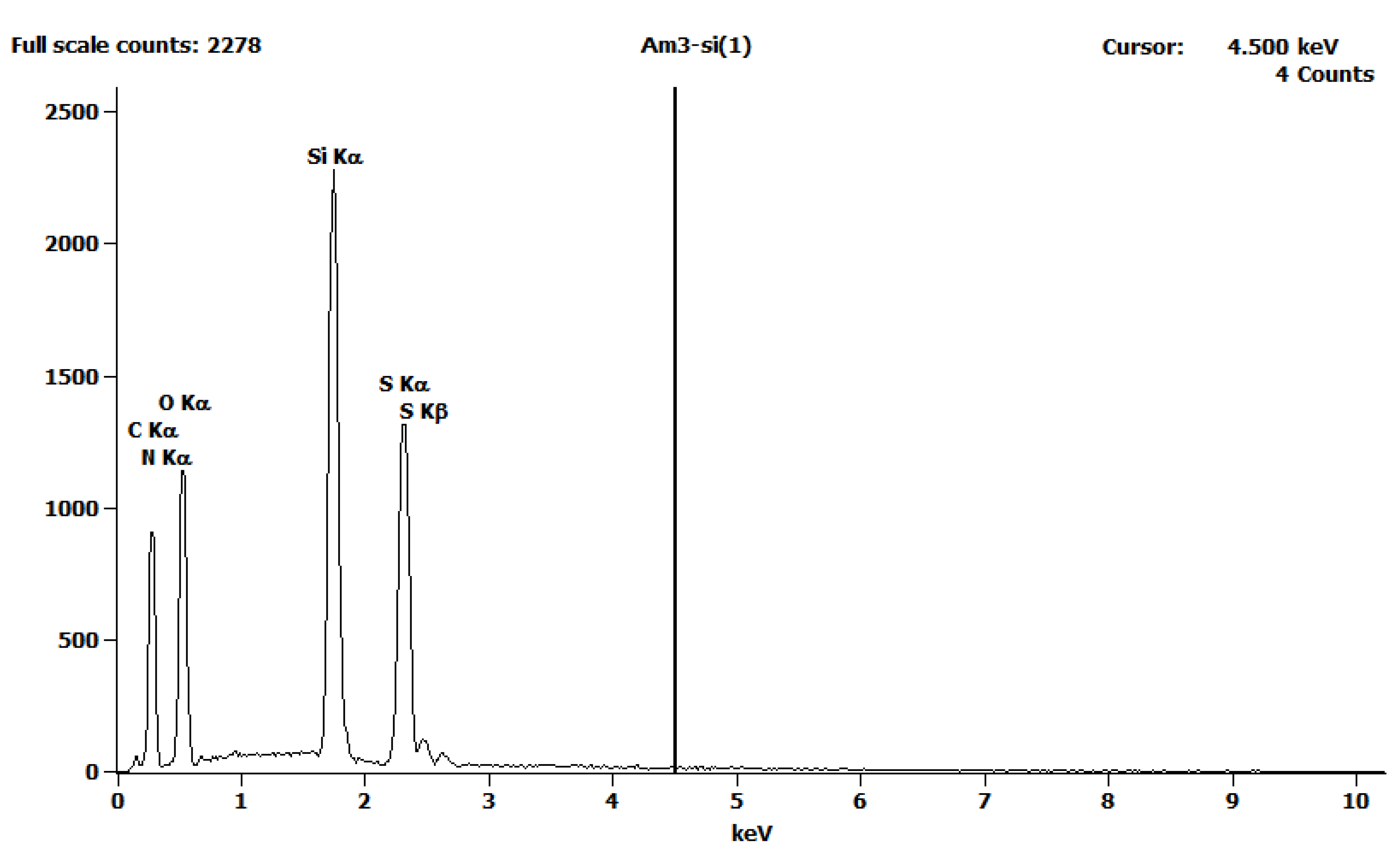

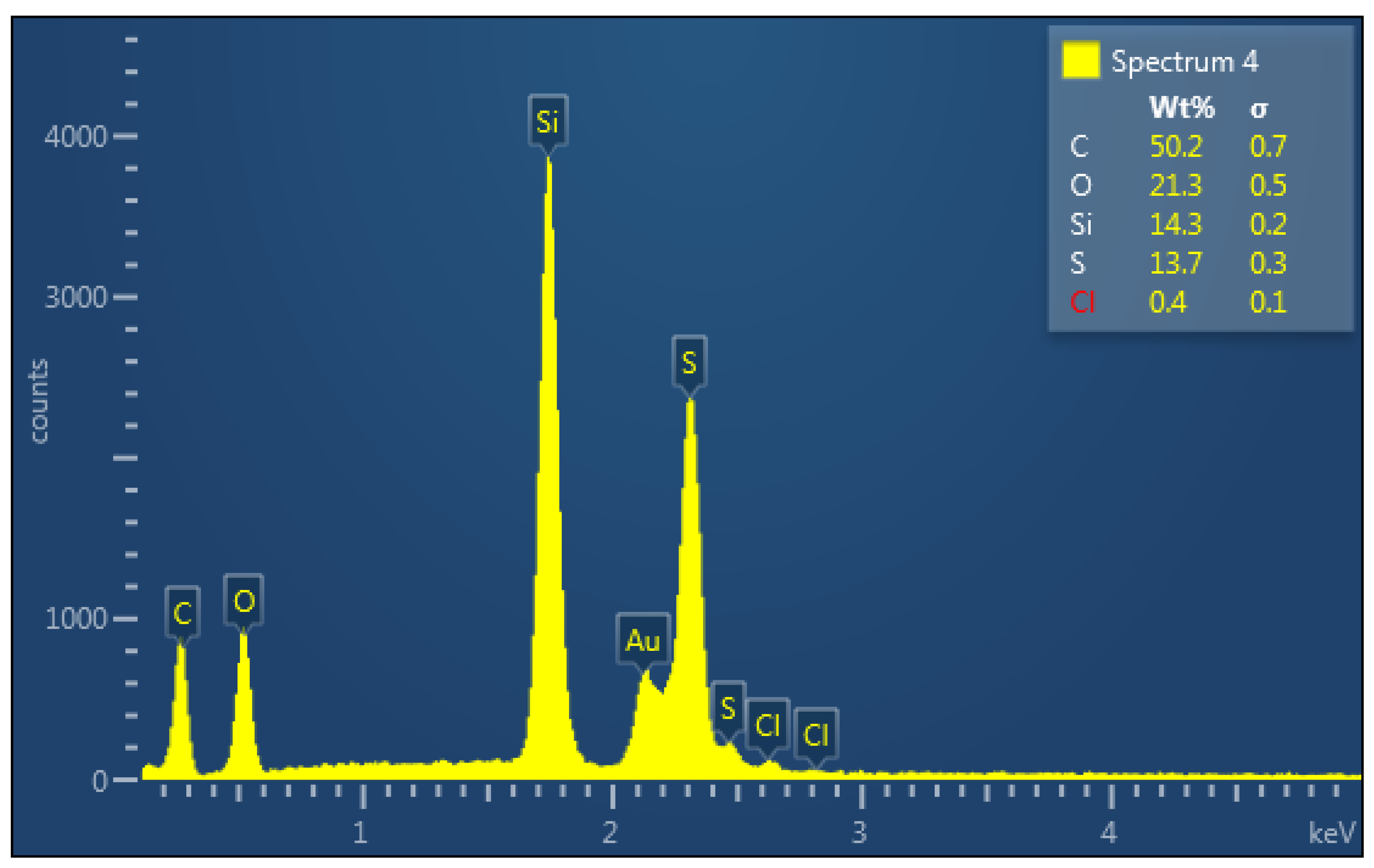




| Element | Weight % | Atom % |
|---|---|---|
| C | 20.99 | 34.36 |
| N | 1.19 | 1.66 |
| O | 22.46 | 27.59 |
| Si | 28.09 | 19.66 |
| S | 27.28 | 16.73 |
| Total | 100.00 | 100.00 |
| Element | Weight % | Atom % |
|---|---|---|
| C | 24.07 | 36.73 |
| N | 1.78 | 2.33 |
| O | 29.81 | 34.16 |
| Si | 21.60 | 14.10 |
| S | 22.08 | 12.63 |
| As | 0.00 | 0.00 |
| Pb | 0.66 | 0.06 |
| Total | 100.00 | 100.00 |
| Element | Weight % | Atom % |
|---|---|---|
| C | 30.19 | 46.20 |
| O | 24.74 | 28.42 |
| Si | 21.87 | 14.32 |
| S | 18.54 | 10.63 |
| As | 0.10 | 0.02 |
| Pb | 4.55 | 0.40 |
| Total | 100.00 | 100.00 |
| Parameters | Adsorbent 1 | Adsorbent 2 |
|---|---|---|
| Calculated equilb. uptake qe (mg/g) | 90.09 | 71.43 |
| K2 (g mg−1 min−1) | 1.23 × 10−4 | 1.03 × 10−3 |
| R2 | 0.9982 | 0.9969 |
| Adsorbent | Langmuir Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| Freundlich Isotherm | |||||||
| qm (mg/g) | K (L/mg) | RL | R2 | KF | 1/n | R2 | |
| 1 | 39.37 | 0.0333 | 0.601 | 0.956 | 2.032 | 1.54 | 0.902 |
| 2 | 1.134 | 0.079 | 0.717 | 0.983 | 0.051 | 0.470 | 0.871 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.O.; Shrpip, A.; Mansoor, M. Synthesis and Characterization of New Schiff Base/Thiol-Functionalized Mesoporous Silica: An Efficient Sorbent for the Removal of Pb(II) from Aqueous Solutions. Processes 2020, 8, 246. https://doi.org/10.3390/pr8020246
Ahmed MO, Shrpip A, Mansoor M. Synthesis and Characterization of New Schiff Base/Thiol-Functionalized Mesoporous Silica: An Efficient Sorbent for the Removal of Pb(II) from Aqueous Solutions. Processes. 2020; 8(2):246. https://doi.org/10.3390/pr8020246
Chicago/Turabian StyleAhmed, Moawia O., Ameen Shrpip, and Muhammad Mansoor. 2020. "Synthesis and Characterization of New Schiff Base/Thiol-Functionalized Mesoporous Silica: An Efficient Sorbent for the Removal of Pb(II) from Aqueous Solutions" Processes 8, no. 2: 246. https://doi.org/10.3390/pr8020246
APA StyleAhmed, M. O., Shrpip, A., & Mansoor, M. (2020). Synthesis and Characterization of New Schiff Base/Thiol-Functionalized Mesoporous Silica: An Efficient Sorbent for the Removal of Pb(II) from Aqueous Solutions. Processes, 8(2), 246. https://doi.org/10.3390/pr8020246