Acid and Enzymatic Fractionation of Olive Stones for Ethanol Production Using Pachysolen tannophilus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Dilute Acid Pretreatment
2.3. Response Surface Methodology (RSM)
2.4. Detoxification and Fermentation of Acid Prehydrolysates
2.5. Enzymatic Hydrolysis
2.6. Fermentation of Enzymatic Hydrolysates
2.7. Analytical Methods
3. Results
3.1. Dilute Acid Pretreatment: Experimental Results
3.2. Dilute Acid Pretreatment: Modelling and Optimization
3.3. Fermentation of Acid Prehydrolysates
3.4. Enzymatic Hydrolysis of Pretreated Solids
3.5. Fermentation of Enzymatic Hydrolysates
3.6. Mass Macroscopic Balance for Complete Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhillon, R.S.; Wuehlisch, G.V. Mitigation of global warming through renewable biomass. Biomass Bioenergy 2013, 48, 75–89. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manage. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Prasad, R.K.; Chatterjee, S.; Mazumder, P.B.; Gupta, S.K.; Sharma, S.; Vairale, M.G.; Datta, S.; Dwivedi, S.K.; Gupta, D.K. Bioethanol production from waste lignocelluloses: A review on microbial degradation potential. Chemosphere 2019, 231, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Banu, R.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent development in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol 2020, 121936. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barret, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Cuevas, M.; García, J.F.; Sánchez, S. Enhanced enzymatic hydrolysis of pretreated almond-tree prunings for sugar production. Carbohydr. Polym. 2014, 99, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, H.; Tsang, D.C.W.; Luo, G.; Hao, S.; Zhang, S.; Chen, J. Optimizing xylose production from pinewood sawdust through dilute-phosphoric-acid hydrolysis by response surface methodology. J. Clean. Prod. 2018, 178, 572–579. [Google Scholar] [CrossRef]
- Jang, S.-K.; Kim, J.-H.; Jeong, H.; Choi, J.-H.; Lee, S.-M.; Choi, I.-G. Investigation of conditions for dilute acid pretreatment for improving xylose solubilization and glucose production by supercritical water hydrolysis from Quercus mongolica. Renew. Energy 2018, 117, 150–156. [Google Scholar] [CrossRef]
- García-Martín, J.F.; Sánchez, S.; Cuevas, M. Evaluation of the effect of the dilute acid hydrolysis on sugars release from olive prunings. Renew. Energy 2013, 51, 382–387. [Google Scholar] [CrossRef]
- Sánchez, S.; Bravo, V.; Moya, A.J.; Castro, E.; Camacho, F. Influence of temperature on the fermentation of D-xylose by Pachysolen tannophilus to produce ethanol and xylitol. Process Biochem. 2004, 39, 673–679. [Google Scholar] [CrossRef]
- Cuevas, M.; Sánchez, S.; García, J.F. Thermochemical and biochemical conversion of olives stones. In Agricultural Wastes: Characteristics, Types and Management; Foster, C.N., Ed.; Nova Science Publishers: New York, NY, USA, 2015; pp. 61–86. [Google Scholar]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, M.; Sánchez, S.; Bravo, V.; Cruz, N.; García, J.F. Fermentation of enzymatic hydrolysates from olives stones by Pachysolen tannophilus. J. Chem. Technol. Biotechnol. 2009, 84, 461–467. [Google Scholar] [CrossRef]
- Cuevas, M.; García, J.F.; Hodaifa, G.; Sánchez, S. Oligosaccharides and sugars production from olive stones by autohydrolysis and enzymatic hydrolysis. Ind. Crops Prod. 2015, 70, 100–106. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Martínez-Patiño, C.; Ruiz, E.; Romero, I.; Castro, E. Ethanol production from olive stone hydrolysates by xylose fermenting microorganisms. Bioethanol 2016, 2, 51–65. [Google Scholar] [CrossRef]
- Saleh, M.; Cuevas, M.; García, J.F.; Sánchez, S. Valorization of olive stones for xylitol and ethanol production from dilute acid pretreatment via enzymatic hydrolysis and fermentation by Pachysolen tannophilus. Biochem. Eng. J. 2014, 90, 286–293. [Google Scholar] [CrossRef]
- Sánchez, S.; Bravo, V.; Castro, E.; Moya, A.J.; Camacho, F. The fermentation of mixtures of D-glucose and D-xylose by Candida shehatae, Pichia stipitis or Pachysolen tannophilus to produce ethanol. J. Chem. Technol. Biotechnol. 2002, 77, 641–648. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Wine, R.H. Use of detergent in the analysis of fibrous feeds. IV. Determination of plant cell-wall constituents. J. Assoc. Off. Anal. Chem. 1967, 50, 50–55. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. In Laboratory Analytical Procedure (LAP); NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Puls, J.; Poutanen, K.; Körner, H.; Viikari, L. Biotechnical utilization of wood carbohydrates after steaming pretreatment. Appl. Microbiol. Biotechnol. 1985, 22, 416–423. [Google Scholar] [CrossRef]
- Beutler, H.O. Ethanol. In Methods of Enzymatic Analysis; Bergmeyer, H., Ed.; Academic Press: New York, NY, USA, 1974; pp. 594–606. [Google Scholar]
- Beutler, H.O.; Becker, J. Enzymatische bestimmung von D-sorbit und xylit in lebensmitteln. Dtsch. Lebensmitt. Rundsch. 1977, 6, 182–187. [Google Scholar]
- Bergmeyer, H.; Möllering, H. Acetic acid. In Methods of Enzymatic Analysis; Bergmeyer, H., Ed.; Academic Press: New York, NY, USA, 1974; pp. 1520–1528. [Google Scholar]
- Karimi, K.; Kheradmandinia, S.; Taherzadeh, M.J. Conversion of rice straw to sugars by dilute-acid hydrolysis. Biomass Bioenergy 2006, 30, 247–253. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Brás, T.S.; Lourenço, P.M.L.; Duarte, L.C.; Carvalheiro, F.; Bernardo, P.; Marques, S.; Neves, L.A. The effect of autohydrolysis pretreatment on the production of second generation bioethanol from extracted olive pomace. In Proceedings of the 4th International Conference on Engineering for Waste and Biomass Valorisation, Porto, Portugal, 10–13 September 2012. [Google Scholar]
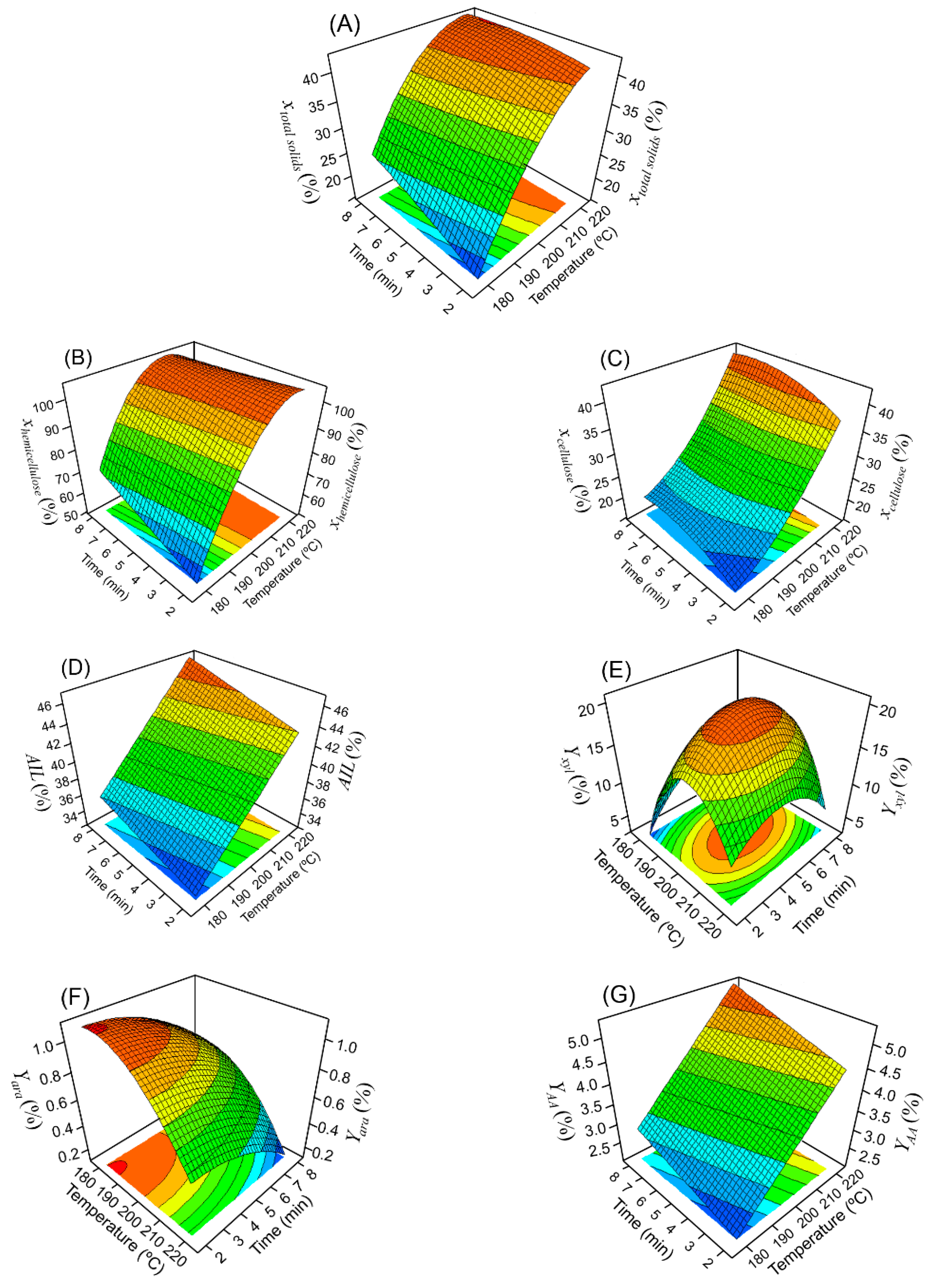


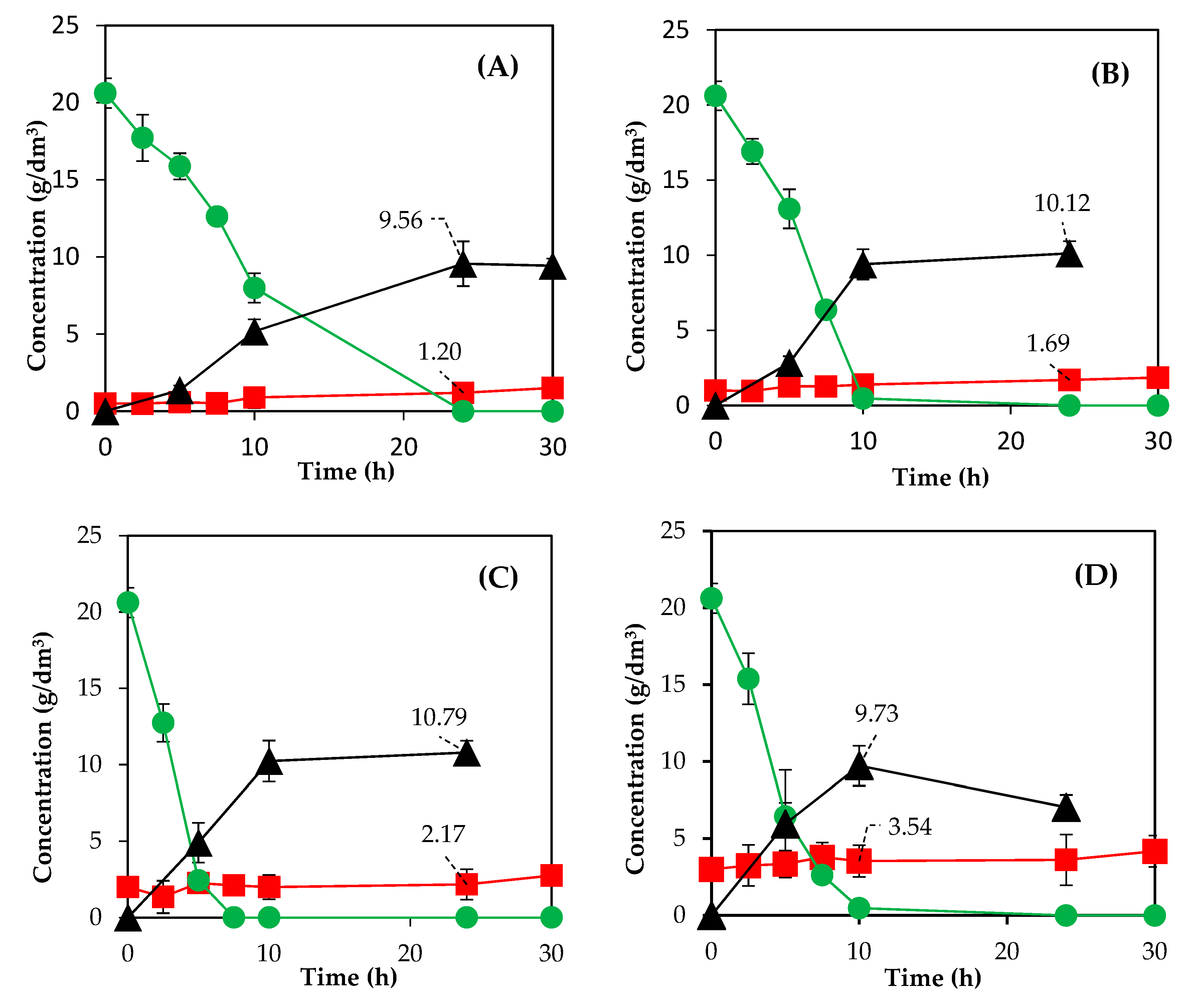
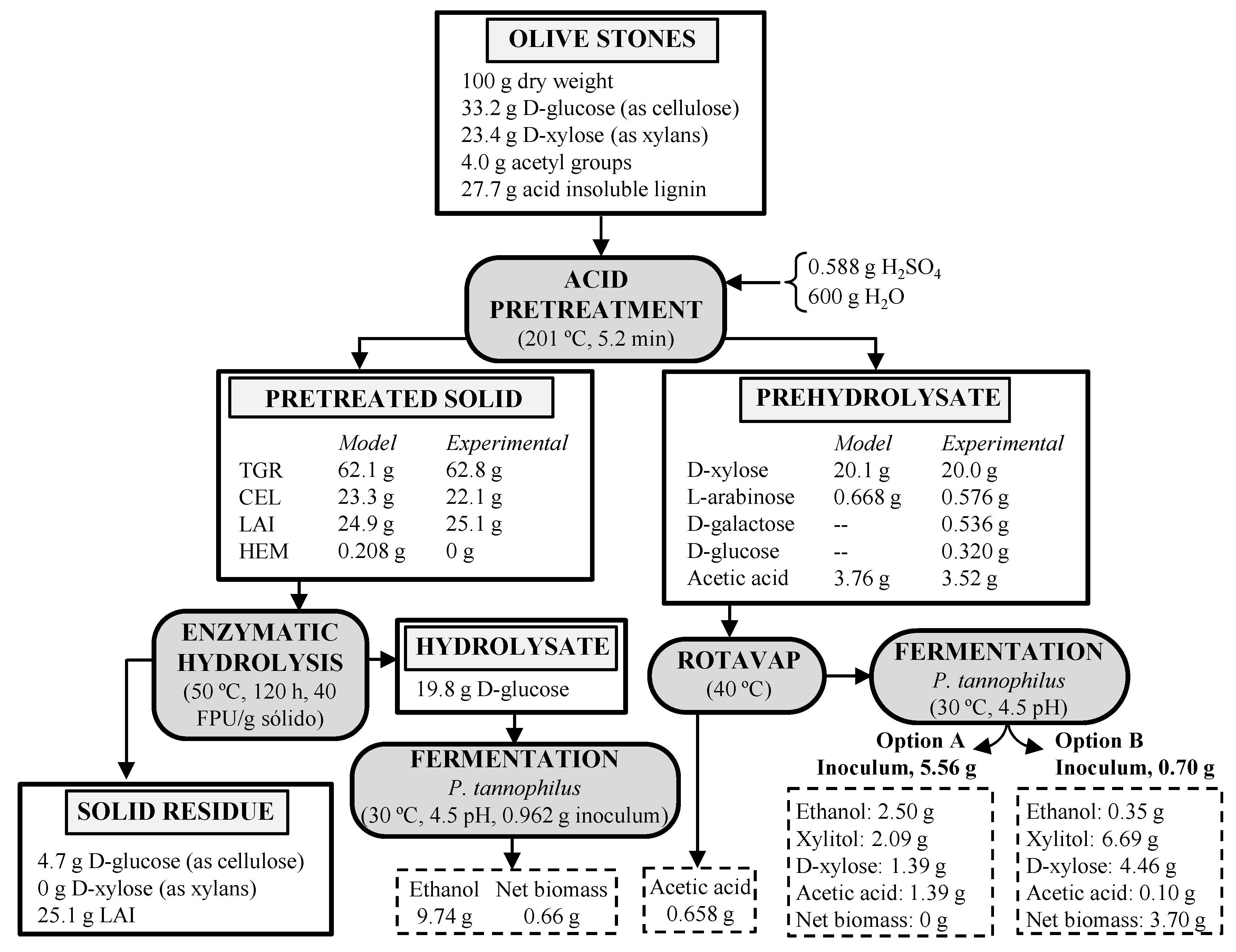
| Run | Temperature (°C) | Time (min) | ||
|---|---|---|---|---|
| Actual Values (T) | Coded Values (X1) | Actual Values (t) | Coded Values (X2) | |
| 1 | 172 | 0 | 5.00 | −1.414 |
| 2 | 180 | −1 | 2.00 | −1 |
| 3 | 180 | 1 | 8.00 | −1 |
| 4 | 200 | −1.414 | 0.76 | 0 |
| 5 | 200 | 0 | 5.00 | 0 |
| 6 | 200 | 0 | 5.00 | 0 |
| 7 | 200 | 0 | 5.00 | 0 |
| 8 | 200 | 1.414 | 9.24 | 0 |
| 9 | 220 | −1 | 2.00 | 1 |
| 10 | 220 | 1 | 8.00 | 1 |
| 11 | 228 | 0 | 5.00 | 1.414 |
| Acid-Treated Solids a | Prehydrolysates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run Number | T | t | TGR | Hem | Cel | AIL | Product Yield (as g/100 g Dry Raw Material) | |||||
| (°C) | (min) | (%) | (%) | (%) | (%) | D-Xylose | L-Arabinose | D-Galactose | D-Glucose | AA | 5-HMF | |
| 1 | 172 | 5 | 86.53 | 23.05 | 30.38 | 32.01 | 1.07 | 0.92 | 0.11 | <0.01 | 2.47 | <0.01 |
| 2 | 180 | 2 | 82.96 | 16.72 | 33.35 | 32.87 | 1.45 | 1.11 | 0.14 | <0.01 | 1.45 | <0.01 |
| 3 | 180 | 8 | 73.55 | 9.42 | 35.56 | 35.44 | 5.53 | 0.77 | 0.25 | <0.01 | 2.71 | <0.01 |
| 4 | 200 | 0.76 | 67.56 | 2.31 | 37.72 | 35.97 | 10.38 | 1.04 | 0.34 | <0.01 | 3.43 | <0.01 |
| 5 | 200 | 5 | 62.47 | 0.00 | 37.76 | 40.04 | 18.99 | 0.87 | 0.49 | <0.01 | 3.44 | <0.01 |
| 6 | 200 | 5 | 63.55 | 1.03 | 35.97 | 38.96 | 21.83 | 0.97 | 0.62 | <0.01 | 3.34 | <0.01 |
| 7 | 200 | 5 | 62.32 | 0.00 | 36.93 | 40.98 | 19.31 | 0.85 | 0.50 | <0.01 | 3.77 | <0.01 |
| 8 | 200 | 9.24 | 60.52 | 0.00 | 38.62 | 42.07 | 13.90 | 0.32 | 0.42 | <0.01 | 4.12 | 0.03 |
| 9 | 220 | 2 | 58.45 | 0.00 | 33.20 | 44.20 | 8.17 | 0.43 | 0.24 | 0.24 | 4.59 | 0.12 |
| 10 | 220 | 8 | 56.78 | 0.00 | 32.02 | 45.87 | 4.57 | 0.21 | 0.23 | 1.46 | 5.77 | 0.80 |
| 11 | 228 | 5 | 56.54 | 0.00 | 25.10 | 48.64 | 2.81 | 0.12 | 0.17 | 1.63 | 5.29 | 1.07 |
| Response Variable | Coefficient | EE | SD | p-Value (Prob > F) | R2 | Radjust2 |
|---|---|---|---|---|---|---|
| xtotal solids, % | Constant | 37.220 | ±0.290 | 5.460 × 10−10 | 0.999 | 0.996 |
| X1 | 10.462 | ±0.178 | 2.674 × 10−8 | |||
| X2 | 2.630 | ±0.178 | 2.547 × 10−5 | |||
| X1 · X1 | −4.415 | ±0.212 | 4.673 × 10−6 | |||
| X2 · X2 | −0.667 | ±0.212 | 2.532 × 10−2 | |||
| X1 · X2 | −1.935 | ±0.251 | 5.891 × 10−4 | |||
| xhemicellulose, % | Constant | 98.430 | ±1.964 | 4.238 × 10−9 | 0.978 | 0.964 |
| X1 | 21.829 | ±1.653 | 1.166 × 10−5 | |||
| X2 | 4.075 | ±1.653 | 4.882 × 10−2 | |||
| X1 · X1 | −16.978 | ±1.881 | 1.036 × 10−4 | |||
| X1 · X2 | −6.183 | ±2.338 | 3.831 × 10−2 | |||
| xcellulose, % | Constant | 22.557 | ±0.770 | 1.051 × 10−7 | 0.994 | 0.990 |
| X1 | 13.938 | ±0.472 | 9.964 × 10−8 | |||
| X2 | 2.391 | ±0.472 | 2.289 × 10−3 | |||
| X1 · X1 | 4.445 | ±0.562 | 2.159 × 10−4 | |||
| X2 · X2 | −2.565 | ±0.562 | 3.819 × 10−3 | |||
| AIL, % | Constant | 39.732 | ±0.283 | 7.366 × 10−15 | 0.975 | 0.969 |
| X1 | 5.660 | ±0.332 | 1.411 × 10−7 | |||
| X2 | 1.608 | ±0.332 | 1.272 × 10−3 |
| Factors | Regression | ||||||
|---|---|---|---|---|---|---|---|
| Responses | EE | SD | p-Value (Prob > F) | R2 | Radjust2 | p-Value | |
| Yxyl, % | Constant | 20.044 | ±1.083 | 8.478 × 10−6 | 0.970 | 0.939 | 0.001 |
| X1 | 1.028 | ±0.663 | 1.820 × 10−1 | ||||
| X2 | 0.682 | ±0.663 | 3.509 × 10−1 | ||||
| X1 · X1 | −9.581 | ±0.790 | 6.717 × 10−5 | ||||
| X2 · X2 | −4.480 | ±0.790 | 2.368 × 10−3 | ||||
| X1 · X2 | −1.920 | ±0.938 | 9.602 × 10−2 | ||||
| Yara, % | Constant | 0.897 | ±0.048 | 1.512 × 10−6 | 0.967 | 0.945 | 0.000 |
| X1 | −0.296 | ±0.029 | 5.496 × 10−5 | ||||
| X2 | −0.197 | ±0.029 | 5.294 × 10−4 | ||||
| X1 · X1 | −0.181 | ±0.035 | 2.069 × 10−3 | ||||
| X2 · X2 | −0.101 | ±0.035 | 2.791 × 10−2 | ||||
| YAA, % | Constant | 3.671 | ±0.115 | 1.029 × 10−9 | 0.925 | 0.906 | 0.000 |
| X1 | 1.274 | ±0.135 | 1.321 × 10−5 | ||||
| X2 | 0.427 | ±0.135 | 1.341 × 10−2 | ||||
| Starting Inoculum Concentration (g/dm3) | 0.5 | 1.0 | 2.0 | 4.0 |
|---|---|---|---|---|
| Net biomass concentration (g/dm3) | 4.8 ± 0.2 (120 h) 1 | 4.6 ± 0.7 (72 h) | 3.2 ± 0.1 (72 h) | 1.9 ± 0.2 (72 h) |
| Ethanol concentration (g/dm3) | 0.25 ± 0.00 (10 h) | 0.41 ± 0.04 (10 h) | 0.97 ± 0.10 (10 h) | 1.8 ± 0.1 (10 h) |
| Xylitol concentration (g/dm3) | 4.81 ± 1.63 (72 h) | 3.25 ± 0.59 (48 h) | 2.43 ± 0.30 (48 h) | 1.50 ± 0.05 (48 h) |
| Xylitol yield 2 (g/g) | 0.42 ± 0.08 (72 h) | 0.26 ± 0.08 (48 h) | 0.18 ± 0.01 (48 h) | 0.14 ± 0.02 (48 h) |
| Xylitol volumetric productivity (g/dm3·h) | 0.07 ± 0.01 (72 h) | 0.07 ± 0.02 (48 h) | 0.05 ± 0.01 (48 h) | 0.04 ± 0.00 (48 h) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas, M.; Saleh, M.; García-Martín, J.F.; Sánchez, S. Acid and Enzymatic Fractionation of Olive Stones for Ethanol Production Using Pachysolen tannophilus. Processes 2020, 8, 195. https://doi.org/10.3390/pr8020195
Cuevas M, Saleh M, García-Martín JF, Sánchez S. Acid and Enzymatic Fractionation of Olive Stones for Ethanol Production Using Pachysolen tannophilus. Processes. 2020; 8(2):195. https://doi.org/10.3390/pr8020195
Chicago/Turabian StyleCuevas, Manuel, Marwa Saleh, Juan F. García-Martín, and Sebastián Sánchez. 2020. "Acid and Enzymatic Fractionation of Olive Stones for Ethanol Production Using Pachysolen tannophilus" Processes 8, no. 2: 195. https://doi.org/10.3390/pr8020195
APA StyleCuevas, M., Saleh, M., García-Martín, J. F., & Sánchez, S. (2020). Acid and Enzymatic Fractionation of Olive Stones for Ethanol Production Using Pachysolen tannophilus. Processes, 8(2), 195. https://doi.org/10.3390/pr8020195







