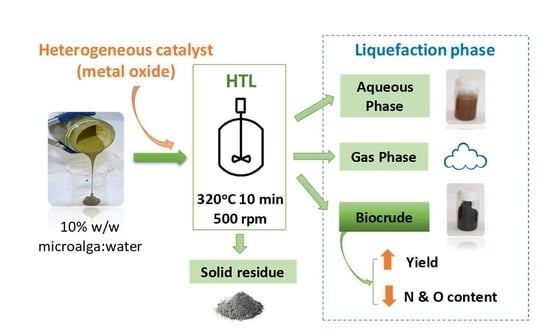
Hydrothermal Liquefaction of Microalga Using Metal Oxide Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga
2.2. Catalysts
2.3. Catalytic Liquefaction
2.4. Products Analysis
2.4.1. Biocrude Analysis
2.4.2. Analysis of Aqueous Phase
2.4.3. Analysis of Gas Phase
2.4.4. Statistical Analysis
3. Results and Discussion
3.1. Effects of Catalysts on the Yields of Hydrothermal Liquefaction (HTL) Processes
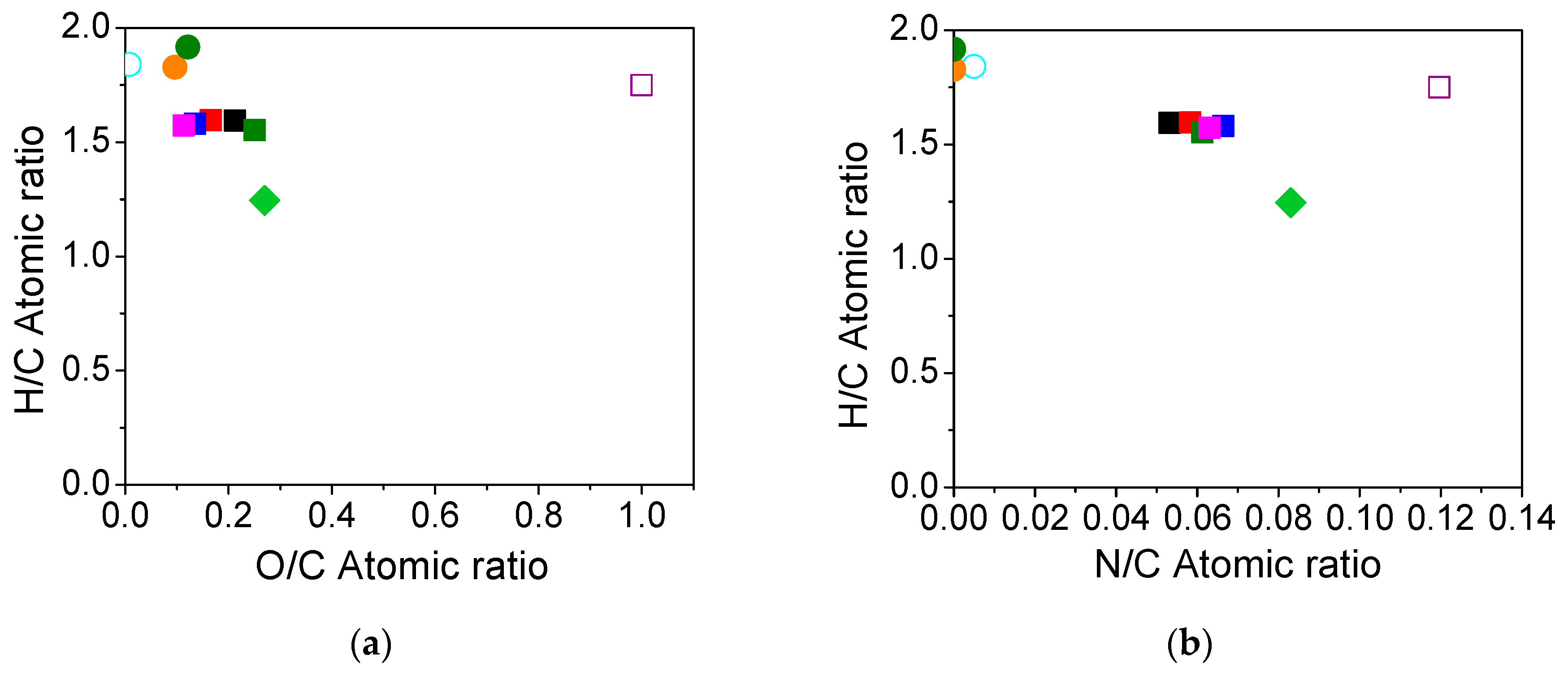
3.2. Element Content, Higher Heating Value (HHV) and Energy Recovery (ER) of Obtained Biocrude
3.3. Analysis of Aqueous Phase
3.4. Analysis of Gas Phase
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Biller, P.; Riley, R.; Ross, A.B. Catalytic hydrothermal processing of microalgae: Decomposition and upgrading of lipids. Bioresour. Technol. 2011, 102, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.; Xie, J.; Liu, H.; Yin, X.; Wu, C. Bio-oil production from hydrothermal liquefaction of high-protein high-ash microalgae including wild Cyanobacteria sp. and cultivated Bacillariophyta sp. Fuel 2016, 183, 9–19. [Google Scholar] [CrossRef]
- López Barreiro, D.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- López Barreiro, D.; Zamalloa, C.; Boon, N.; Vyverman, W.; Ronsse, F.; Brilman, W.; Prins, W. Influence of strain-specific parameters on hydrothermal liquefaction of microalgae. Bioresour. Technol. 2013, 146, 463–471. [Google Scholar] [CrossRef]
- Tian, C.; Li, B.; Liu, Z.; Zhang, Y.; Lu, H. Hydrothermal liquefaction for algal biorefinery: A critical review. Renew. Sustain. Energy Rev. 2014, 38, 933–950. [Google Scholar] [CrossRef]
- Jazrawi, C.; Biller, P.; He, Y.; Montoya, A.; Ross, A.B.; Maschmeyer, T.; Haynes, B.S. Two-stage hydrothermal liquefaction of a high-protein microalga. Algal Res. 2015, 8, 15–22. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C.C.; Kastner, J.R.R. Effect of operating conditions of thermochemical liquefaction on biocrude production from Spirulina platensis. Bioresour. Technol. 2011, 102, 6221–6229. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, C.; Li, Z.; Yang, X. Element and chemical compounds transfer in bio-crude from hydrothermal liquefaction of microalgae. Bioresour. Technol. 2015, 202, 8–14. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Hydrothermal liquefaction of algae and bio-oil upgrading into liquid fuels: Role of heterogeneous catalysts. Renew. Sustain. Energy Rev. 2018, 81, 1037–1048. [Google Scholar] [CrossRef]
- Jarvis, J.M.; Billing, J.M.; Hallen, R.T.; Schmidt, A.J.; Schaub, T.M. Hydrothermal Liquefaction Biocrude Compositions Compared to Petroleum Crude and Shale Oil. Energy Fuels 2017, 31, 2896–2906. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Mathimani, T.; Mallick, N. A review on the hydrothermal processing of microalgal biomass to bio-oil - Knowledge gaps and recent advances. J. Clean. Prod. 2019, 217, 69–84. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2014, 184, 314–327. [Google Scholar] [CrossRef]
- Patel, B.; Arcelus-Arrillaga, P.; Izadpanah, A.; Hellgardt, K. Catalytic Hydrotreatment of algal biocrude from fast Hydrothermal Liquefaction. Renew. Energy 2017, 101, 1094–1101. [Google Scholar] [CrossRef]
- Montero-Hidalgo, M.; Espada, J.J.; Rodríguez, R.; Morales, V.; Bautista, L.F.; Vicente, G. Mild Hydrothermal Pretreatment of Microalgae for the Production of Biocrude with a Low N and O Content. Processes 2019, 7, 630. [Google Scholar] [CrossRef]
- Prapaiwatcharapan, K.; Sunphorka, S.; Kuchonthara, P.; Kangvansaichol, K.; Hinchiranan, N. Single- and two-step hydrothermal liquefaction of microalgae in a semi-continuous reactor: Effect of the operating parameters. Bioresour. Technol. 2015, 191, 426–432. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Zhang, P.; Hua, D.; Yang, M.; Li, C.; Chen, Z.; Liu, J. Direct liquefaction of Dunaliella tertiolecta for bio-oil in sub/supercritical ethanol–water. Bioresour. Technol. 2012, 124, 190–198. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Savage, P.E. Near- and supercritical ethanol treatment of biocrude from hydrothermal liquefaction of microalgae. Bioresour. Technol. 2016, 211, 779–782. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Guo, S.; Wang, S.; Guo, Y.; Jing, Z. Catalytic hydrothermal liquefaction of algae and upgrading of biocrude: A critical review. Renew. Sustain. Energy Rev. 2018, 97, 103–118. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C.; Kastner, J.R. Comparison of the effects of Na2CO3, Ca3(PO4)2, and NiO catalysts on the thermochemical liquefaction of microalga Spirulina platensis. Appl. Energy 2012, 98, 368–375. [Google Scholar] [CrossRef]
- Yu, G.; Zhang, Y.; Guo, B.; Funk, T.; Schideman, L. Nutrient Flows and Quality of Bio-crude Oil Produced via Catalytic Hydrothermal Liquefaction of Low-Lipid Microalgae. Bioenergy Res. 2014, 7, 1317–1328. [Google Scholar] [CrossRef]
- Ross, A.B.; Biller, P.; Kubacki, M.L.; Li, H.; Lea-Langton, A.; Jones, J.M. Hydrothermal processing of microalgae using alkali and organic acids. Fuel 2010, 89, 2234–2243. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Liu, S.; Feng, L. Direct hydrothermal liquefaction of undried macroalgae Enteromorpha prolifera using acid catalysts. Energy Convers. Manag. 2014, 87, 938–945. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, R.; Yang, M.; Fang, L.; Wu, Y.; Wu, K.; Liu, Y.; Gong, J. Catalytic hydrothermal liquefaction for bio-oil production over CNTs supported metal catalysts. Chem. Eng. Sci. 2017, 161, 299–307. [Google Scholar] [CrossRef]
- Robin, T.; Jones, J.M.; Ross, A.B. Catalytic hydrothermal processing of lipids using metal doped zeolites. Biomass Bioenergy 2017, 98, 26–36. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2011, 50, 52–61. [Google Scholar] [CrossRef]
- Chang, F.; Zhou, Q.; Pan, H.; Liu, X.-F.; Zhang, H.; Xue, W.; Yang, S. Solid Mixed-Metal-Oxide Catalysts for Biodiesel Production: A Review. Energy Technol. 2014, 2, 865–873. [Google Scholar] [CrossRef]
- Gryglewicz, S. Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour. Technol. 1999, 70, 249–253. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.S. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Dossin, T.F.; Reyniers, M.-F.; Marin, G.B. Kinetics of heterogeneously MgO-catalyzed transesterification. Appl. Catal. B Environ. 2006, 62, 35–45. [Google Scholar] [CrossRef]
- Refaat, A.A. Biodiesel production using solid metal oxide catalysts. Int. J. Environ. Sci. Technol. 2011, 8, 203–221. [Google Scholar] [CrossRef]
- Mendoza, A.; Vicente, G.; Bautista, L.F.; Morales, V. Opportunities for Nannochloropsis gaditana biomass through the isolation of its components and biodiesel production. Green Process. Synth. 2015, 4, 97–102. [Google Scholar] [CrossRef]
- Li, H.; Liu, Z.; Zhang, Y.; Li, B.; Lu, H.; Duan, N.; Liu, M.; Zhu, Z.; Si, B. Conversion efficiency and oil quality of low-lipid high-protein and high-lipid low-protein microalgae via hydrothermal liquefaction. Bioresour. Technol. 2014, 154, 322–329. [Google Scholar] [CrossRef]
- López Barreiro, D.; Samorì, C.; Terranella, G.; Hornung, U.; Kruse, A.; Prins, W. Assessing microalgae biorefinery routes for the production of biofuels via hydrothermal liquefaction. Bioresour. Technol. 2014, 174, 256–265. [Google Scholar] [CrossRef]
- Shi, W.; Li, S.; Jin, H.; Zhao, Y.; Yu, W. The hydrothermal liquefaction of rice husk to bio-crude using metallic oxide catalysts. Energy Sources Part A Recover. Util. Environ. Eff. 2013, 35, 2149–2155. [Google Scholar] [CrossRef]
- Yim, S.C.; Quitain, A.T.; Yusup, S.; Sasaki, M.; Uemura, Y.; Kida, T. Metal oxide-catalyzed hydrothermal liquefaction of Malaysian oil palm biomass to bio-oil under supercritical condition. J. Supercrit. Fluids 2016, 120, 384–394. [Google Scholar] [CrossRef]
- Valdez, P.J.; Tocco, V.J.; Savage, P.E. A general kinetic model for the hydrothermal liquefaction of microalgae. Bioresour. Technol. 2014, 163, 123–127. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Wang, X.; Zhang, B.; Tian, W.; Zhang, J. Hydrothermal liquefaction of microalgae over transition metal supported TiO2 catalyst. Bioresour. Technol. 2018, 250, 474–480. [Google Scholar] [CrossRef]
- Pedersen, T.H.; Jensen, C.U.; Sandström, L.; Rosendahl, L.A. Full characterization of compounds obtained from fractional distillation and upgrading of a HTL biocrude. Appl. Energy 2017, 202, 408–419. [Google Scholar] [CrossRef]
- Karmakar, R.; Kundu, K.; Rajor, A. Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet. Sci. 2018, 15, 385–395. [Google Scholar] [CrossRef]
- Ahmed, I. Oxygenated Diesel: Emissions and Performance Characteristics of Ethanol-Diesel Blends in CI Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Kurtz, E.M.; Kuhel, D.; Anderson, J.E.; Mueller, S.A. A Comparison of Combustion and Emissions of Diesel Fuels and Oxygenated Fuels in a Modern DI Diesel Engine. SAE Int. J. Fuels Lubr. 2012, 5, 1199–1215. [Google Scholar] [CrossRef]
- Duan, P.; Wang, B.; Xu, Y. Catalytic hydrothermal upgrading of crude bio-oils produced from different thermo-chemical conversion routes of microalgae. Bioresour. Technol. 2015, 186, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Garcia Alba, L.; Torri, C.; Samorì, C.; Van Der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal treatment (HTT) of microalgae: Evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
| Biochemical Composition (wt%) | Elemental Composition (wt%) | Metals (mg/g) | |||
|---|---|---|---|---|---|
| Lipids | 35.52 ± 1.23 | H | 7.10 ± 0.18 | Na | 21.04 ± 2.01 |
| Proteins | 43.81 ± 3.50 | C | 48.7 ± 0.14 | K | 3.53 ± 0.09 |
| Carbohydrates | 15.70 ± 3.59 | N | 6.80 ± 0.04 | Mg | 0.90 ± 0.03 |
| Ashes | 4.50 ± 0.79 | S | 0.90 ± 0.04 | Fe | 0.16 ± 0.01 |
| O | 36.5 ± 0.20 | Ca | 0.08 ± 0.01 | ||
| P | 6.43 ± 0.02 | ||||
| Biocrude (wt%) | WSP 1 (wt%) | Gas (wt%) | SR 2 (wt%) | LP 3 (wt%) | ||
|---|---|---|---|---|---|---|
| Thermal | 42.60 b 4 | 31.98 c | 17.81 a | 7.60 d | 92.69 d | |
| Catalysts | CaO | 49.73 a | 37.43 b | 7.57 b | 5.25 c | 94.74 cd |
| CeO2 | 43.80 b | 44.12 a | 9.82 b | 2.25 ab | 97.74 ab | |
| MnO2 | 44.11 b | 37.61 b | 18.26 a | 0.50 a | 99.49 a | |
| La2O3 | 42.66 b | 38.98 b | 12.50 ab | 5.85 cd | 94.14 cd | |
| Al2O3 | 44.22 b | 34.02 c | 17.44 a | 4.30 bc | 95.69 bc |
| N (wt%) | C (wt%) | H (wt%) | S (wt%) | O (wt%) | HHV (MJ/kg) | ER (%) | ||
|---|---|---|---|---|---|---|---|---|
| Thermal | 6.11 c | 73.84 a 1 | 9.17 c | 0.31 c | 10.54 a | 33.18 a | 58.25 a | |
| Catalyst | CaO | 4.76 a | 69.98 b | 9.31 c | 0.40 d | 15.59 b | 31.47 b | 55.00 b |
| CeO2 | 4.62 a | 73.04 a | 9.83 a | 0.16 a | 12.32 a | 33.40 a | 58.50 a | |
| MnO2 | 5.45 b | 70.17 b | 9.17 c | 0.16 a | 14.94 b | 31.49 b | 55.25 b | |
| La2O3 | 4.63 a | 64.88 c | 8.39 d | 0.39 d | 21.68 c | 30.12 c | 53.00 c | |
| Al2O3 | 5.39 b | 73.36 a | 9.64 b | 0.20 b | 11.39 a | 33.43 a | 58.75 a | |
| Composition (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acid | Alcohol | Aldehyde | Amine | Amide | Ketone | HC | AHC | Ether | Nitrile | ||
| Thermal | 43.25 | 6.49 | 0.46 | 9.74 | 31.26 | 1.99 | 3.71 | 2.31 | 0.55 | 0.23 | |
| Catalyst | CaO | 28.09 | 18.48 | 0.00 | 16.51 | 10.10 | 7.31 | 11.26 | 4.10 | n.d | 3.11 |
| CeO2 | 31.18 | 9.46 | 0.00 | 18.84 | 16.30 | 9.44 | 12.51 | 0.33 | n.d | 1.71 | |
| MnO2 | 60.96 | 0.00 | 0.00 | 10.71 | 6.08 | 9.41 | 10.68 | 2.03 | n.d | 0.12 | |
| La2O3 | 24.99 | 17.32 | 0.00 | 15.12 | 9.36 | 16.53 | 13.26 | 0.00 | n.d | 3.13 | |
| Al2O3 | 17.82 | 17.27 | 0.00 | 12.02 | 34.78 | 8.31 | 7.10 | 1.81 | n.d | 0.14 | |
| Boiling Point (°C) | ||||||
|---|---|---|---|---|---|---|
| Distillation (%) | 20 | 40 | 60 | 80 | 100 | |
| Reference diesel | 223.40 | 290.10 | 332.90 | 379.70 | 476.10 | |
| Thermal | 417.80 | 422.50 | 438.90 | 476.30 | 545.60 | |
| Catalyst | CaO | 330.90 | 395.20 | 439.30 | 486.00 | 539.50 |
| CeO2 | 348.70 | 393.50 | 420.10 | 467.10 | 529.20 | |
| MnO2 | 319.80 | 388.50 | 418.10 | 467.90 | 539.20 | |
| La2O3 | 301.90 | 383.00 | 418.30 | 480.60 | 546.20 | |
| Al2O3 | 347.70 | 395.20 | 436.00 | 488.00 | 551.80 | |
| Water Recovery (wt%) | pH | TOC (mg/L) | ||
|---|---|---|---|---|
| Thermal | 90 ± 1 | 8.40 | 1283 ± 4 | |
| Catalysts | CaO | 93 ± 1 | 10.80 | 745 ± 1 |
| CeO2 | 94 ± 2 | 8.53 | 698 ± 2 | |
| MnO2 | 92 ± 1 | 8.70 | 694 ± 1 | |
| La2O3 | 94 ± 1 | 8.90 | 681 ± 2 | |
| Al2O3 | 95 ± 2 | 8.20 | 741 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bayo, A.; Rodríguez, R.; Morales, V.; Nasirian, N.; Bautista, L.F.; Vicente, G. Hydrothermal Liquefaction of Microalga Using Metal Oxide Catalyst. Processes 2020, 8, 15. https://doi.org/10.3390/pr8010015
Sánchez-Bayo A, Rodríguez R, Morales V, Nasirian N, Bautista LF, Vicente G. Hydrothermal Liquefaction of Microalga Using Metal Oxide Catalyst. Processes. 2020; 8(1):15. https://doi.org/10.3390/pr8010015
Chicago/Turabian StyleSánchez-Bayo, Alejandra, Rosalía Rodríguez, Victoria Morales, Nima Nasirian, Luis Fernando Bautista, and Gemma Vicente. 2020. "Hydrothermal Liquefaction of Microalga Using Metal Oxide Catalyst" Processes 8, no. 1: 15. https://doi.org/10.3390/pr8010015
APA StyleSánchez-Bayo, A., Rodríguez, R., Morales, V., Nasirian, N., Bautista, L. F., & Vicente, G. (2020). Hydrothermal Liquefaction of Microalga Using Metal Oxide Catalyst. Processes, 8(1), 15. https://doi.org/10.3390/pr8010015