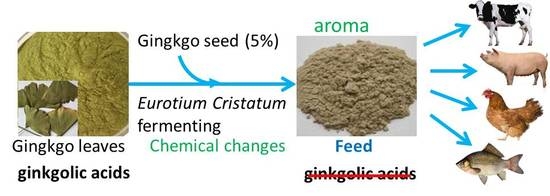
Improvement of the Quality of Ginkgo biloba Leaves Fermented by Eurotium cristatum as High Value-Added Feed
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spore Suspension of Eurotium cristatum
2.3. Prefermentation
2.4. Optimization of Fermentation Medium Components
2.5. Optimization of Fermentation Conditions
2.6. Determination of Spore Number in the Solid-State Culture
2.7. Determination of Physicochemical Components and Enzyme Activity
2.8. Statistical Analysis
3. Results and Discussion
3.1. Prefermentation of Pure G. biloba Leaves with E. cristatum
3.2. Fermentation of G. biloba Leaves with Addition of Excipients
3.3. Effects of Fermentation Conditions
3.4. Component Analysis of the Fermented and Unfermented G. biloba Leaves
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.B.; Cork, S.C.; Ronksley, P.E.; Barkema, H.W.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.D. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, 316–327. [Google Scholar] [CrossRef]
- Maron, D.F.; Smith, T.J.; Nachman, K.E. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Kho, W.L.; You, S.H.; Yeh, R.H.; Tang, S.W.; Hsieh, C.W. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae mixed fermented feed on the enhanced growth performance of broilers. Poult. Sci. 2009, 88, 309–315. [Google Scholar] [CrossRef] [PubMed]
- van Winsen, R.L.; Urlings, B.A.; Lipman, L.J.; Snijders, J.M.; Keuzenkamp, D.; Verheijden, J.H.; van Knapen, F. Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Appl. Environ. Microb. 2001, 67, 3071–3076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Cao, F.L.; Sun, Z.Y.; Yu, W.W.; Zhao, L.G.; Wang, G.B.; Wang, T. Effect of feeding Aspergillus niger-fermented G. biloba-leaves on growth, small intestinal structure and function of broiler chicks. Livestock Sci. 2012, 147, 170–180. [Google Scholar] [CrossRef]
- Santoso, U.; Tanaka, K.; Ohtani, S.; Sakaida, M. Effect of fermented product from Bacillus subtilis on feed conversion efficiency, lipid accumulation and ammonia production in broiler chicks. Asian Austral. J. Anim. 2001, 14, 333–337. [Google Scholar] [CrossRef]
- Wang, C.; Shi, C.; Zhang, Y.; Song, D.; Lu, Z.; Wang, Y. Microbiota in fermented feed and swine gut. Appl. Microbiol. Biotechnol. 2018, 102, 2941–2948. [Google Scholar] [CrossRef]
- Bleichner, G.; Blehaut, H.; Mentec, H.; Moyse, D. Saccharomyces boulardii prevents diarrhea in critically ill tube-fed patients. Intens. Care Med. 1997, 23, 517–523. [Google Scholar] [CrossRef]
- Okano, K.; Fukui, S.; Kitao, R.; Usagawa, T. Effects of culture length of Pleurotus eryngii grown on sugarcane bagasse on in vitro digestibility and chemical composition. Anim. Feed Sci. Technol. 2007, 136, 240–247. [Google Scholar] [CrossRef]
- Veerabhadrappa, M.B.; Shivakumar, S.B.; Devappa, S. Solid-state fermentation of Jatropha seed cake for optimization of lipase, protease and detoxification of anti-nutrients in Jatropha seed cake using Aspergillus versicolor CJS-98. J. Biosci. Bioeng. 2014, 117, 208–214. [Google Scholar] [CrossRef]
- Du, F.Y.; Li, X.M.; Li, C.S.; Shang, Z.; Wang, B.G. Cristatumins A–D, new indole alkaloids from the marine-derived endophytic fungus E. cristatum EN-220. Bioorg. Med. Chem. Lett. 2012, 22, 4650–4653. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, Y.; Zhang, X.; Li, Q.; Liu, X.; Huang, Y.; Tang, T.; Zheng, S.; Wang, W.; Tang, J. A new prenylated indole diketopiperazine alkaloid from E. cristatum. Molecules 2014, 19, 17839–17847. [Google Scholar] [CrossRef] [PubMed]
- Du, F.Y.; Li, X.; Li, X.M.; Zhu, L.W.; Wang, B.G. Indolediketopiperazine alkaloids from E. cristatum EN-220, an endophytic fungus isolated from the marine alga Sargassum thunbergii. Mar. Drugs 2017, 15, 24. [Google Scholar] [CrossRef]
- van Beek, T.A.; Montoro, P. Chemical analysis and quality control of G. biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kaur, P.; Singh, R.; Ahuja, P. Biology and chemistry of G. biloba. Fitoterapia 2008, 79, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.Z.; Ye, J.Z.; Chen, H.X.; Tao, R.; Zhang, Y.S. Solid-state fermentation of G. biloba L. residue for optimal production of cellulase, protease and the simultaneous detoxification of G. biloba L. residue using Candida tropicalis and Aspergillus oryzae. Eur. Food Res. Technol. 2015, 240, 379–388. [Google Scholar] [CrossRef]
- Ndjoko, K.; Wolfender, J.L.; Hostettmann, K. Determination of trace amounts of ginkgolic acids in G. biloba L. leaf extracts and phytopharmaceuticals by liquid chromatography–electrospray mass spectrometry. J. Chromatogr. B 2000, 744, 249–255. [Google Scholar] [CrossRef]
- Cao, F.L.; Zhang, X.H.; Yu, W.W.; Zhao, L.G.; Wang, T. Effect of feeding fermented G. biloba leaves on growth performance, meat quality, and lipid metabolism in broilers. Poult. Sci. 2012, 91, 1210–1221. [Google Scholar] [CrossRef]
- Yang, X.Y.; Lin, Y.X.; Li, Y. Effect of G. biloba extract on growth performance, slaughter performance and immune index in broilers. J. Fujian Agric. For. Univ. 2008, 37, 295–298. [Google Scholar]
- Su, E.Z.; Yang, M.; Cao, J.; Lu, C.; Wang, J.H.; Cao, F.L. Deep eutectic solvents as green media for efficient extraction of terpene trilactones from G. biloba leaves. J. Liq. Chromatogr. R. Technol. 2017, 40, 385–391. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.Y.; Li, M.H.; Cao, F.L.; Zhao, L.G.; Su, E.Z. Two-phase systems developed with hydrophilic and hydrophobic deep eutectic solvents for simultaneously extracting various bioactive compounds with different polarities. Green Chem. 2018, 20, 1879–1886. [Google Scholar] [CrossRef]
- Li, Q.; Sun, W.; Jiang, Y.; Cao, F.L.; Wang, G.B.; Zhao, L.G. Extraction and biodegradation of ginkgolic acids from G. biloba sarcotestae. Front. Agric. Sci. Eng. 2017, 4, 465–472. [Google Scholar] [CrossRef]
- National Standard of the People’s Republic of China. Determination of the Total Amount of Free Amino Acids in Tea (GB/T8314-2013); China Standard Press: Beijing, China, 2013; pp. 478–496. [Google Scholar]
- Yan, Z.; Fan, R.; Yin, S.; Zhao, X.; Liu, J.; Li, L.; Zhang, W.; Ge, L. Protective effects of G. biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int. J. Biol. Macromol. 2015, 80, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.L.; Li, F.; Li, J.W. Study on Relationship between Sugar Content and Cold–Hot Nature of 20 Kinds of Herbs by Fisher Analysis. World Sci. Technol. 2010, 12, 558–561. [Google Scholar]
- Zhao, C.L. Use Sugarcane LEAVES to Produce Protein Feed by Fermentation. Master’s Thesis, Guangxi University, Guiling, China, 2013. [Google Scholar]
- Wang, J.H.; Cao, F.L.; Zhu, Z.L.; Zhang, X.H.; Sheng, Q.Q.; Qin, W.S. Improvement of Quality and Digestibility of Moringa Oleifera Leaves Feed via Solid-State Fermentation by Aspergillus Niger. Int. J. Chem. React. Eng. 2018, 16. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.Y.; Li, M.H.; Cao, F.L.; Zhao, L.G.; Su, E.Z. Efficient extraction of proanthocyanidin from G. biloba leaves employing rationally designed deep eutectic solvent-water mixture and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2018, 158, 317–326. [Google Scholar] [CrossRef] [PubMed]
- National Standard of the People’s Republic of China. Determination of Enzymes Activity of Food Industry (GB 1886.174-2016); China Standard Press: Beijing, China, 2016. [Google Scholar]
- Qa’dan, F.; Nahrstedt, A.; Schmidt, M.; Mansoor, K. Polyphenols from G. biloba. Sci. Pharm. 2010, 78, 897–908. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2000; pp. 443–461. [Google Scholar]
- Liu, S.X.; Liu, S.C.; Li, Z.G. Effect of E. cristatum on flavones content in oat. China Brew. 2019, 38, 93–97. [Google Scholar]
- Han, J.; Yan, Y.C.; Chang, H.Y.; Wang, H.; Yu, L.T. Isolation and purification of terpene lactones from G. biloba. Chin. Herbal. Med. 2002, 33, 99–102. [Google Scholar]
- Wang, J.H.; Cao, F.L.; Su, E.Z.; Wu, C.E.; Zhao, L.G.; Ying, R.F. Improving flavonoid extraction from G. biloba leaves by prefermentation processing. J. Agric. Food Chem. 2013, 61, 5783–5791. [Google Scholar] [CrossRef]
- Zhao, L.G.; Zhang, X.H.; Cao, F.L.; Sun, D.F.; Wang, T.; Wang, G.B. Effect of dietary supplementation with fermented Ginkgo-leaves on performance, egg quality, lipid metabolism and egg-yolk fatty acids composition in laying hens. Lives Sci. 2013, 155, 77–85. [Google Scholar] [CrossRef]
- Moon, S.H.; Parulekar, S.J. A parametric study ot protease production in batch and fed-batch cultures of Bacillus firmus. Biotechnol. Bioeng. 1991, 37, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Qin, J. Study on growth conditions of golden fungus of Fu tea. Guizhou Sci. 1991, 1, 20–24. [Google Scholar]
- Yalemtesfa, B.; Alemu, T.; Santhanam, A. Solid substrate fermentation and conversion of orange waste in to fungal biomass using Aspergillus niger KA-06 and Chaetomium Spp KC-06. Afr. J. Microbiol. Res. 2010, 4, 1275–1281. [Google Scholar]
- De Loecker, R.; Goossens, W.; Van Duppen, V.; Verwilghen, R.; De Loecker, W. Osmotic effects of dilution on erythrocytes after freezing and thawing in glycerol-containing buffer. Cryobiology 1993, 30, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Anto, H.; Trivedi, U.; Patel, K. Glucoamylase production by solid-state fermentation using rice flake manufacturing waste products as substrate. Bioresour. Technol. 2006, 97, 1161–1166. [Google Scholar] [CrossRef]
- Zhao, L.G.; Cao, F.L.; Yu, T.; Li, T.J.; Wang, G.B. Conditions of Solid Fermentation of G. biloba L. Leaves to Produce Forage Multienzyme and Analysis of the Product. Chem. Ind. For. Prod. 2007, 2, 76–80. [Google Scholar]
- Zhao, G.H.; Chen, Z.D.; Wang, G.C.; Kan, J.Q. Effects of Modification on Functionalities and Structure of Corn Gluten Protein (II) Acylation. J. Chin. Cereal Oils Assoc. 2001, 3, 42–44. [Google Scholar]
- Wang, C.Z.; Shen, Z.B.; Tan, W.H.; Yu, Q.; Daugavietis, M. The studies on the chemistry, purification and pharmacology of polyprenols from G. biloba L. Nat. Prod. Res. Dev. 2001, 2, 43–45. [Google Scholar]
- Sharma, R.K.; Arora, D.S. Fungal degradation of lignocellulosic residues: An aspect of improved nutritive quality. Crit. Rev. Microbiol. 2015, 41, 52–60. [Google Scholar] [CrossRef]
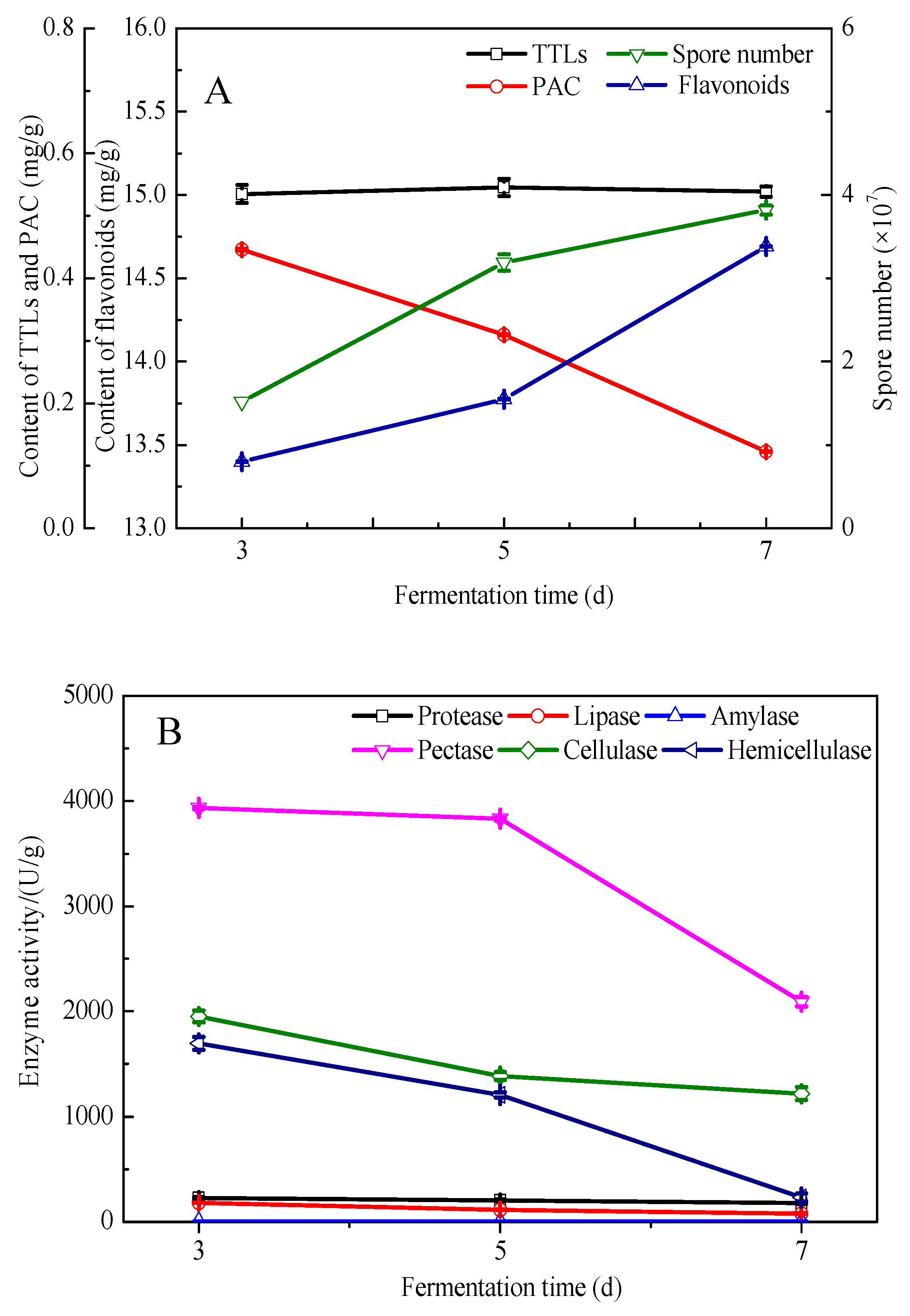
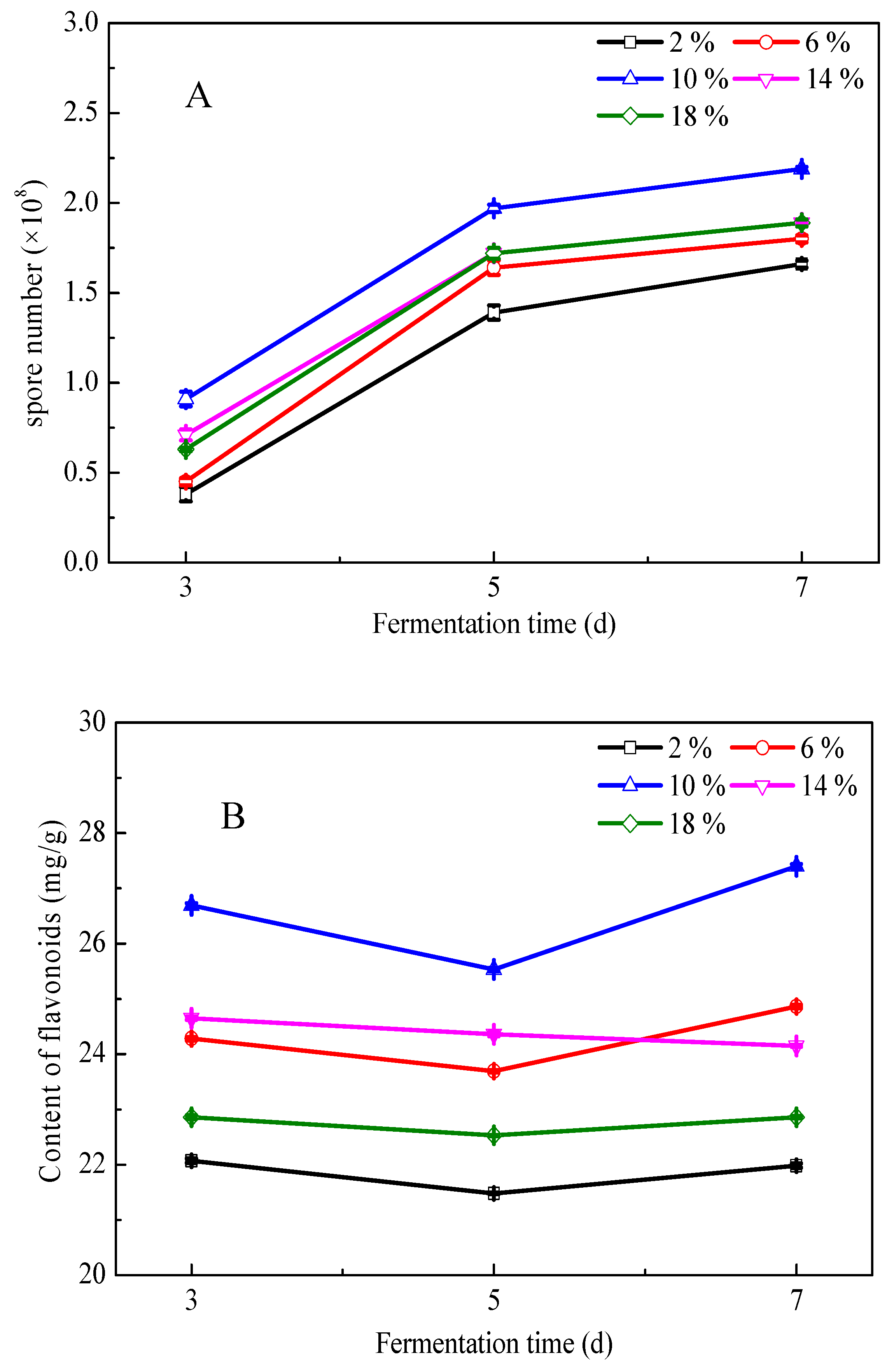
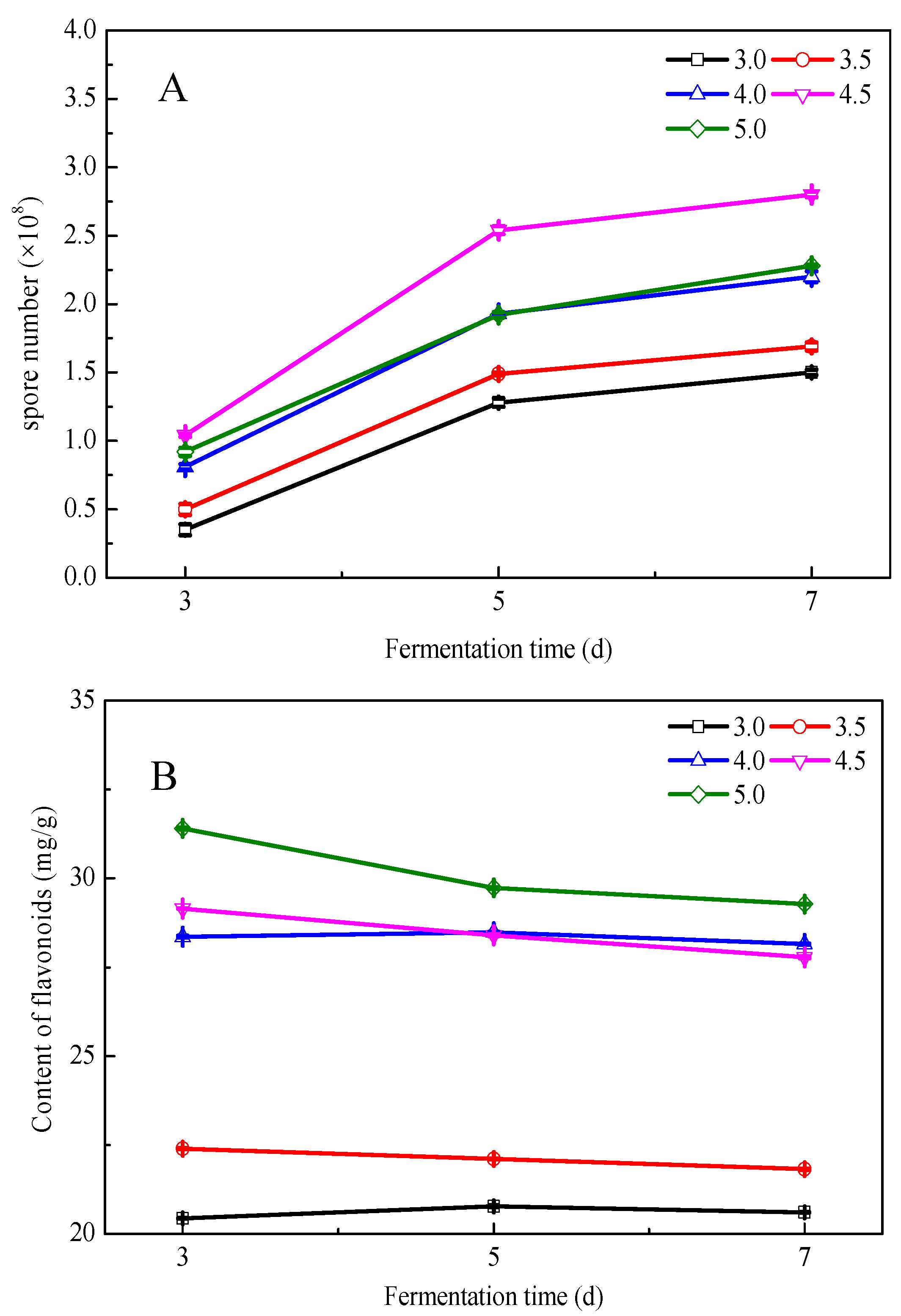
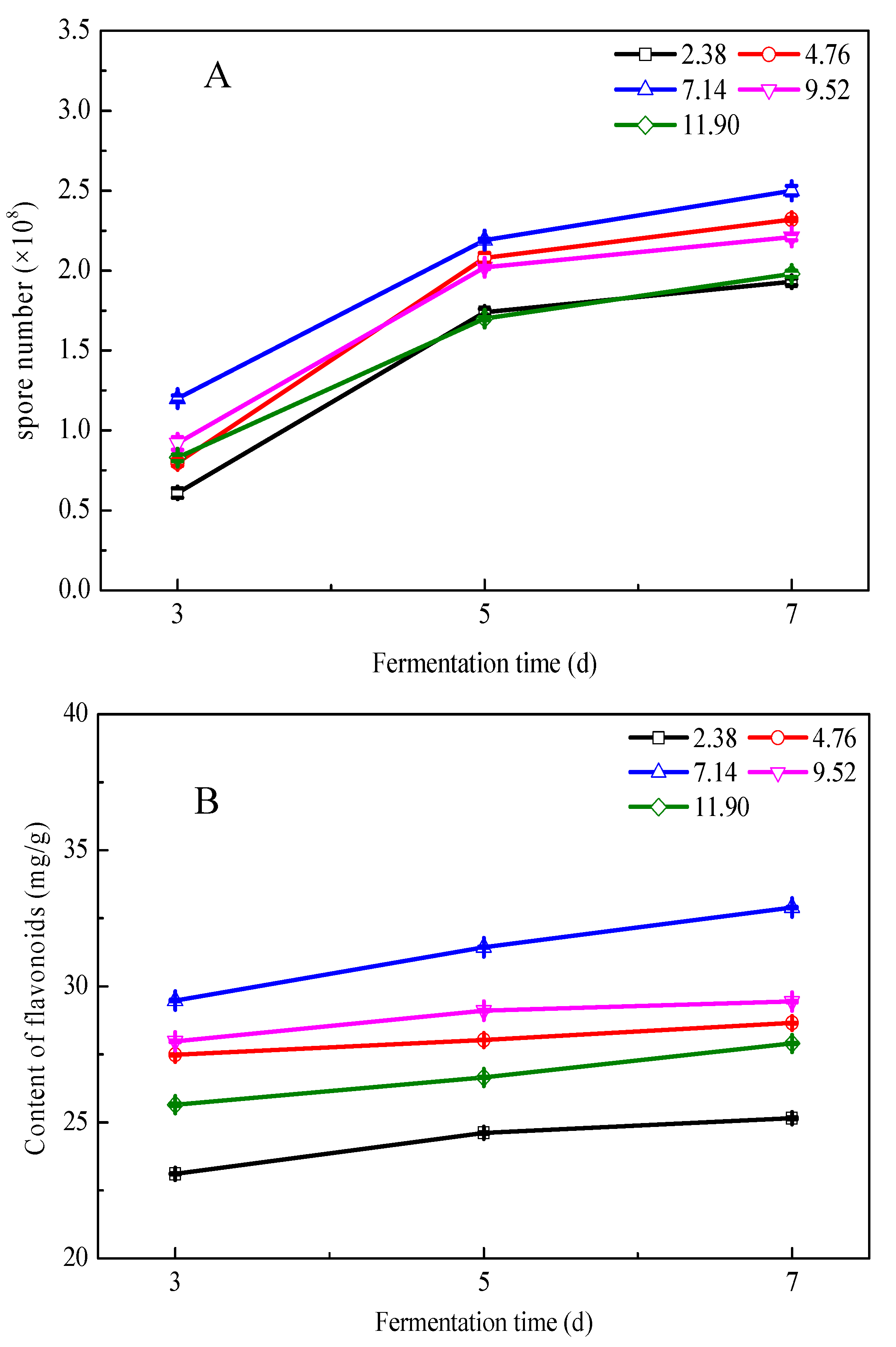
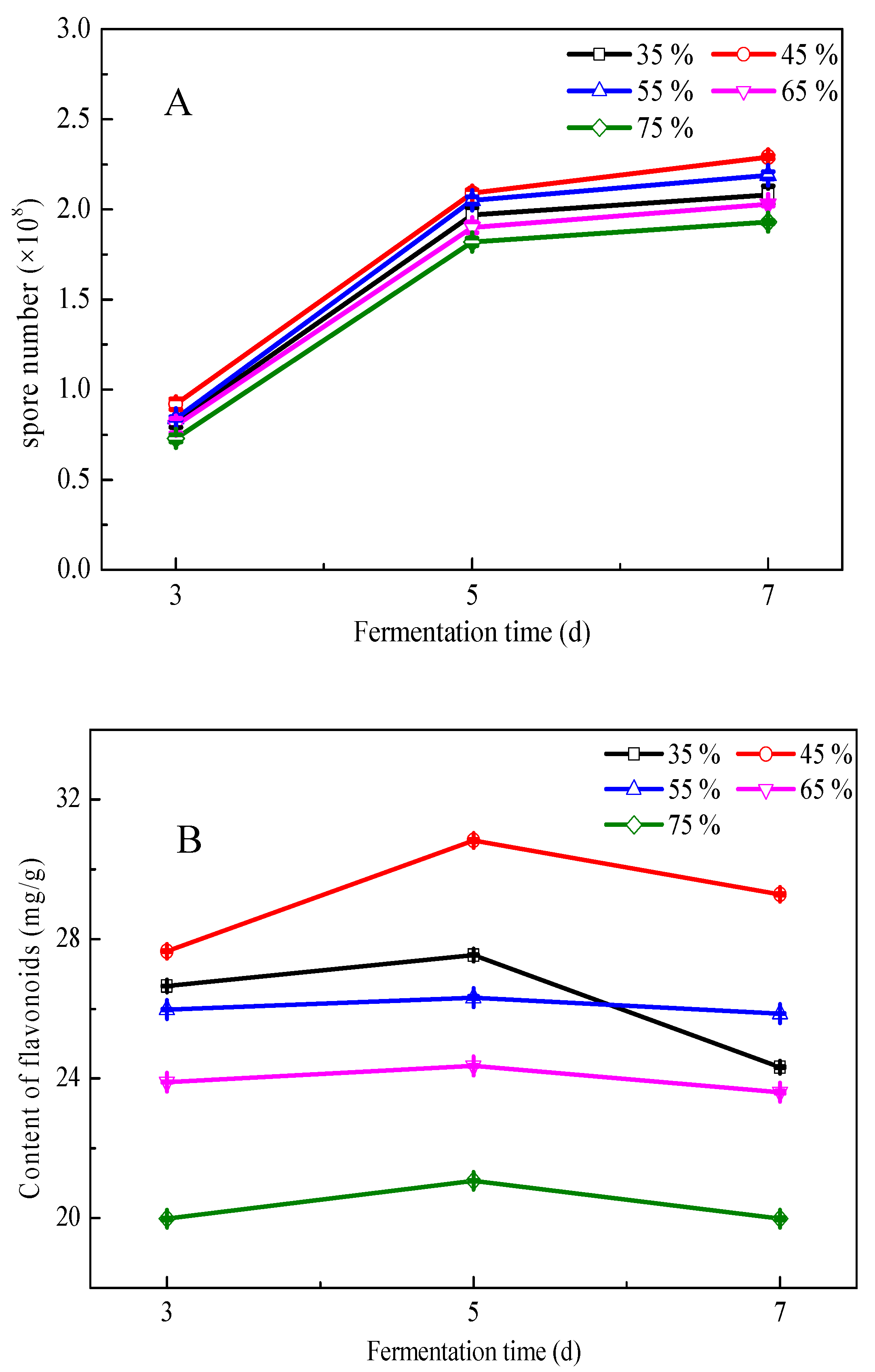
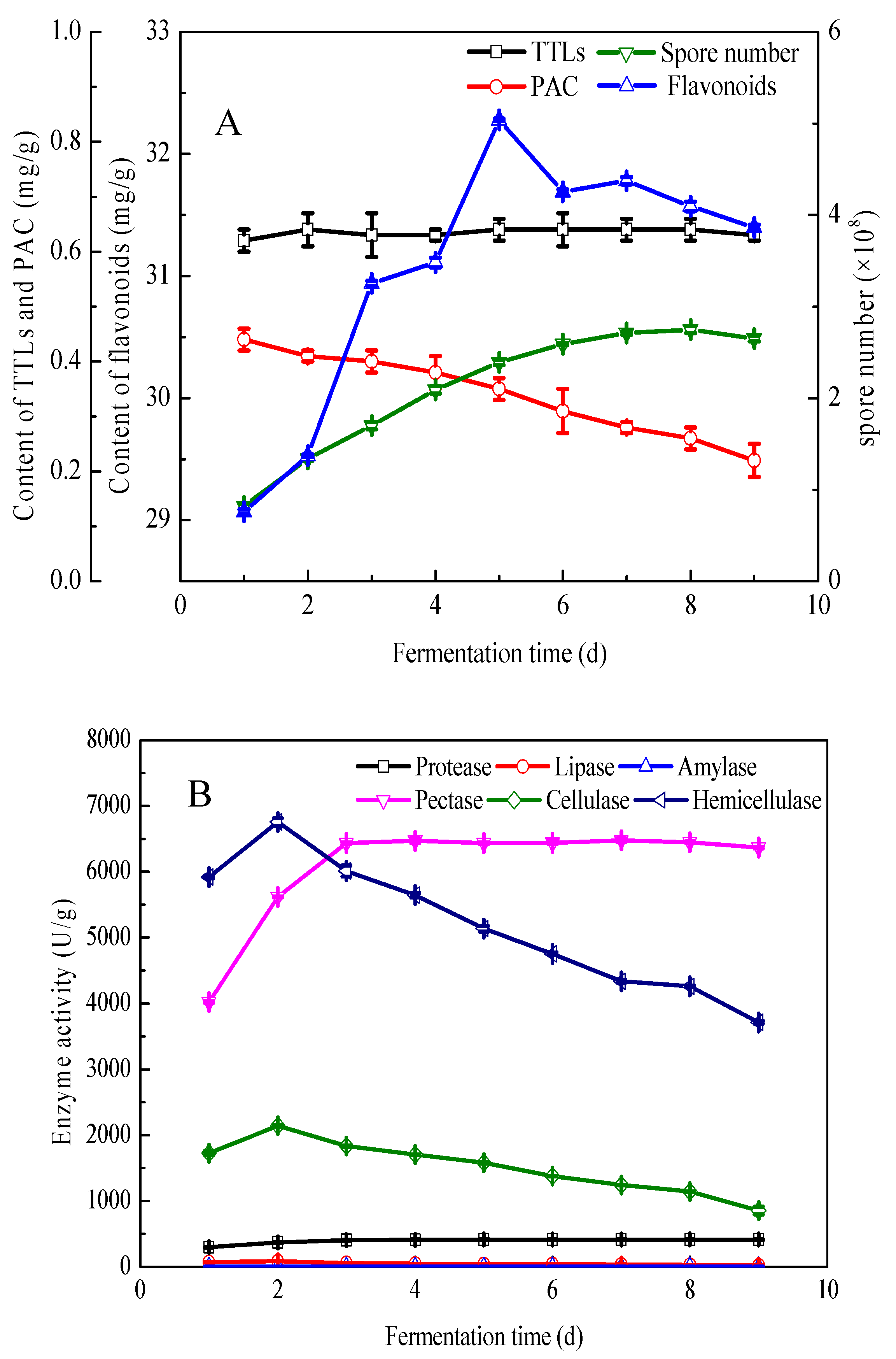
| Excipient | Time (d) | Spore Number (×108 CFU) | TTLs (mg/g) | PAC (mg/g) | Flavonoids (mg/g) |
|---|---|---|---|---|---|
| Maize | 3 | 1.05 ± 0.04n | 0.62 ± 0.02c | 0.05 ± 0.01g | 24.98 ± 0.03e |
| 5 | 2.44 ± 0.03e | 0.63 ± 0.02bc | 0.09 ± 0.02f | 23.44 ± 0.01g | |
| 7 | 2.75 ± 0.03c | 0.62 ± 0.03bc | 0.04 ± 0.02g | 25.32 ± 0.03d | |
| Rice bran | 3 | 1.25 ± 0.02m | 0.66 ± 0.01b | 0.24 ± 0.03c | 18.11 ± 0.03j |
| 5 | 2.22 ± 0.04f | 0.65 ± 0.02bc | 0.24 ± 0.02c | 16.15 ± 0.01k | |
| 7 | 2.47 ± 0.01de | 0.65 ± 0.01bc | 0.14 ± 0.01de | 19.48 ± 0.01hi | |
| Soybean meal | 3 | 0.91 ± 0.01o | 0.62 ± 0.01c | 0.09 ± 0.04f | 25.78 ± 0.01d |
| 5 | 1.73 ± 0.03k | 0.61 ± 0.01c | 0.12 ± 0.02de | 24.69 ± 0.01e | |
| 7 | 1.93 ± 0.02i | 0.62 ± 0.01c | 0.12 ± 0.01de | 26.65 ± 0.04c | |
| Barely | 3 | 0.61 ± 0.01q | 0.65 ± 0.02bc | 0.16 ± 0.01d | 24.28 ± 0.05f |
| 5 | 1.58 ± 0.04l | 0.64 ± 0.01bc | 0.16 ± 0.02d | 23.69 ± 0.01g | |
| 7 | 1.85 ± 0.01j | 0.65 ± 0.01bc | 0.04 ± 0.04h | 24.82 ± 0.01d | |
| Wheat | 3 | 0.93 ± 0.02o | 0.65 ± 0.01bc | 0.39 ± 0.03a | 29.32 ± 0.09a |
| 5 | 2.03 ± 0.02g | 0.65 ± 0.01bc | 0.28 ± 0.01b | 26.61 ± 0.05c | |
| 7 | 2.52 ± 0.04d | 0.66 ± 0.01b | 0.29 ± 0.08ab | 27.98 ± 0.02b | |
| Whole G. biloba seeds | 3 | 0.80 ± 0.01p | 0.60 ± 0.01c | 0.21 ± 0.03c | 25.86 ± 0.03d |
| 5 | 1.90 ± 0.03i | 0.62 ± 0.01c | 0.11 ± 0.02e | 24.15 ± 0.01f | |
| 7 | 2.02 ± 0.03g | 0.61 ± 0.01c | 0.04 ± 0.01g | 27.65 ± 0.01b | |
| G. biloba seeds | 3 | 1.87 ± 0.02j | 0.72 ± 0.01a | 0.34 ± 0.04a | 19.19 ± 0.01i |
| 5 | 2.90 ± 0.01b | 0.73 ± 0.01a | 0.34 ± 0.01a | 18.11 ± 0.04j | |
| 7 | 3.10 ± 0.04a | 0.72 ± 0.01a | 0.36 ± 0.02a | 19.78 ± 0.01h |
| Excipient | Time (d) | Enzyme Activity (U/g) | |||||
|---|---|---|---|---|---|---|---|
| Amylase | Lipase | Protease | Pectinase | Cellulase | Hemicellulase | ||
| Maize | 3 | 4.67 ± 0.01a | 142.22 ± 2.06e | 336.69 ± 9.93fg | 5586.39 ± 4.81d | 2900.75 ± 74.39b | 4136.11 ± 31.82c |
| 5 | 4.65 ± 0.01ab | 98.52 ± 1.22h | 314.07 ± 9.49h | 5572.50 ± 30.05d | 2133.14 ± 25.05f | 2920.83 ± 41.67h | |
| 7 | 4.65 ± 0.02ab | 73.33 ± 0.89j | 190.06 ± 6.62l | 4900.28 ± 76.98g | 845.38 ± 66.17l | 927.78 ± 52.43m | |
| Rice bran | 3 | 4.68 ± 0.01a | 124.44 ± 1.82f | 317.64 ± 10.96h | 5094.72 ± 41.94f | 1804.89 ± 91.42gh | 3663.89 ± 63.65e |
| 5 | 4.65 ± 0.01ab | 103.70 ± 1.88 | 307.12 ± 6.18hi | 4641.94 ± 19.25h | 1219.08 ± 58.07j | 2233.33 ± 55.12j | |
| 7 | 4.64 ± 0.01bc | 93.33 ± 0.35i | 199.78 ± 9.49l | 3203.06 ± 4.81k | 582.77 ± 74.39m | 573.61 ± 31.82n | |
| Soybean meal | 3 | 4.67 ± 0.02a | 182.22 ± 0.97b | 327.86 ± 12.10gh | 5697.50 ± 41.67d | 1850.34 ± 118.45g | 4962.50 ± 55.12b |
| 5 | 4.64 ± 0.03abc | 93.33 ± 2.05i | 321.81 ± 6.18gh | 5711.39 ± 24.06d | 1567.53 ± 43.05j | 3761.11 ± 12.03d | |
| 7 | 4.64 ± 0.04abc | 53.33 ± 1.22j | 258.51 ± 9.99k | 4594.72 ± 12.73h | 719.13 ± 97.53m | 1177.78 ± 52.43l | |
| Barely | 3 | 4.63 ± 0.02bc | 195.55 ± 2.12a | 377.56 ± 8.09bc | 4111.39 ± 20.97i | 2436.14 ± 133.32e | 3268.06 ± 52.43g |
| 5 | 4.60 ± 0.01c | 127.41 ± 1.94f | 371.81 ± 5.63c | 3994.72 ± 31.55i | 1804.89 ± 62.57g | 2316.67 ± 72.17j | |
| 7 | 4.59 ± 0.06c | 94.81 ± 2.06hi | 359.90 ± 9.75de | 3122.50 ± 8.33l | 1264.53 ± 64.46j | 184.72 ± 12.03o | |
| Wheat | 3 | 4.64 ± 0.03abc | 148.15 ± 2.91d | 360.50 ± 8.33de | 5108.61 ± 9.62f | 2678.54 ± 42.15c | 3400.00 ± 20.83f |
| 5 | 4.63 ± 0.01bc | 120.00 ± 1.22g | 291.25 ± 9.11i | 4833.61 ± 75.61g | 1703.89 ± 35.03h | 1420.83 ± 20.83k | |
| 7 | 4.62 ± 0.01bc | 56.30 ± 0.46k | 262.38 ± 8.73j | 3786.39 ± 79.20j | 633.28 ± 72.72m | 858.33 ± 20.83m | |
| Whole G. biloba seeds | 3 | 4.68 ± 0.02a | 184.44 ± 5.08b | 384.11 ± 5.78b | 6066.94 ± 41.11c | 2592.69 ± 4.96d | 5191.67 ± 83.33b |
| 5 | 4.64 ± 0.05ac | 139.26 ± 2.05e | 361.29 ± 7.03d | 5644.72 ± 127.29d | 1284.73 ± 21.90j | 2747.22 ± 86.74i | |
| 7 | 4.64 ± 0.01bc | 97.78 ± 1.22hi | 343.63 ± 8.84ef | 4830.83 ± 8.33g | 890.83 ± 8.06l | 1163.89 ± 43.37l | |
| G. biloba seeds | 3 | 4.60 ± 0.02c | 186.67 ± 5.56ab | 398.99 ± 6.62a | 6789.17 ± 22.05a | 3183.55 ± 4.96a | 5629.17 ± 75.12a |
| 5 | 4.59 ± 0.01c | 164.44 ± 2.05c | 373.39 ± 12.52bc | 6578.06 ± 45.90b | 1658.44 ± 4.96i | 2920.83 ± 90.81h | |
| 7 | 4.57 ± 0.03c | 166.68 ± 3.22c | 346.61 ± 7.54ef | 5214.17 ± 41.67e | 951.43 ± 12.12k | 1462.50 ± 126.72k | |
| Whole G. biloba Seeds (%, w/w) | Time (d) | Enzyme Activity (U/g) | |||||
|---|---|---|---|---|---|---|---|
| Amylase | Lipase | Protease | Pectinase | Cellulase | Hemicellulase | ||
| 2 | 3 | 4.40 ± 0.01f | 124.44 ± 4.15d | 287.88 ± 6.76d | 4091.94 ± 5.24k | 832.25 ± 31.54l | 1504.17 ± 36.08k |
| 5 | 4.40 ± 0.01f | 108.89 ± 2.90e | 292.04 ± 5.85d | 4169.72 ± 19.25j | 640.35 ± 8.75m | 1344.44 ± 12.03l | |
| 7 | 4.39 ± 0.01f | 83.71 ± 0.98i | 293.43 ± 5.85d | 4189.17 ± 11.02j | 539.35 ± 15.15n | 1212.50 ± 19.82m | |
| 6 | 3 | 4.51 ± 0.02c | 148.15 ± 5.34c | 315.85 ± 5.28c | 4880.83 ± 2.08g | 1700.86 ± 23.14g | 2295.83 ± 21.11g |
| 5 | 4.52 ± 0.01c | 120.00 ± 2.36d | 314.46 ± 6.52c | 4872.50 ± 2.08g | 1599.85 ± 15.15h | 2156.94 ± 18.13i | |
| 7 | 4.52 ± 0.02c | 100.00 ± 1.04f | 314.46 ± 5.97c | 4922.50 ± 3.61f | 1296.85 ± 15.15k | 1990.28 ± 31.82j | |
| 10 | 3 | 4.62 ± 0.02a | 192.59 ± 1.78a | 378.35 ± 5.63a | 6014.17 ± 2.08c | 2933.07 ± 15.15a | 4997.22 ± 25.89a |
| 5 | 4.60 ± 0.03ab | 140.00 ± 2.93c | 374.78 ± 6.76a | 6080.83 ± 5.51b | 2549.26 ± 38.13b | 4281.94 ± 45.91b | |
| 7 | 4.61 ± 0.04ab | 102.22 ± 2.36f | 377.96 ± 5.63a | 6166.94 ± 1.20a | 2347.26 ± 31.54c | 3677.78 ± 104.17d | |
| 14 | 3 | 4.58 ± 0.03ab | 174.07 ± 2.36b | 343.83 ± 6.32b | 5272.50 ± 4.17e | 2306.86 ± 8.75c | 4316.67 ± 73.49b |
| 5 | 4.56 ± 0.01b | 128.89 ± 1.04c | 336.09 ± 6.18b | 5250.28 ± 7.89e | 2024.06 ± 30.30e | 3941.67 ± 67.90c | |
| 7 | 4.57 ± 0.01b | 95.56 ± 5.34g | 339.27 ± 6.85b | 5341.94 ± 1.20d | 1912.96 ± 8.75f | 3525.00 ± 43.37e | |
| 18 | 3 | 4.46 ± 0.04d | 142.96 ± 5.34c | 299.19 ± 8.22d | 4608.61 ± 3.18h | 1700.86 ± 8.75g | 3677.78 ± 20.83d |
| 5 | 4.46 ± 0.02d | 120.74 ± 2.36d | 297.40 ± 6.76d | 4525.28 ± 4.81i | 1620.05 ± 8.75h | 2983.33 ± 31.93f | |
| 7 | 4.45 ± 0.01e | 88.15 ± 1.21h | 298.99 ± 6.13d | 4555.83 ± 3.61i | 1559.45 ± 8.75j | 2288.89 ± 21.34g | |
| pH | Time (d) | Enzymes Activity (U/g) | |||||
|---|---|---|---|---|---|---|---|
| Amylase | Lipase | Protease | Pectinase | Cellulase | Hemicellulase | ||
| 3 | 3 | 4.36 ± 0.03c | 144.44 ± 2.22d | 316.65 ± 5.28d | 3800.28 ± 29.27h | 956.48 ± 25.43f | 3865.28 ± 3.18h |
| 5 | 4.35 ± 0.01d | 115.56 ± 2.22f | 317.24 ± 6.18d | 3755.83 ± 8.33i | 774.68 ± 50.87g | 3573.61 ± 5.24k | |
| 7 | 4.37 ± 0.02c | 57.78 ± 2.22i | 314.46 ± 5.54d | 3741.94 ± 25.46i | 491.87 ± 25.43h | 3156.94 ± 5.24m | |
| 3.5 | 3 | 4.41 ± 0.02c | 160.75 ± 3.39c | 354.94 ± 5.54c | 4236.39 ± 12.73g | 1148.38 ± 16.65d | 4212.50 ± 2.08f |
| 5 | 4.41 ± 0.04c | 123.70 ± 3.39e | 352.36 ± 5.63c | 4214.17 ± 22.05g | 961.53 ± 25.43f | 3816.67 ± 3.61i | |
| 7 | 4.41 ± 0.01c | 76.30 ± 3.39h | 352.76 ± 7.03c | 4222.50 ± 22.05g | 799.93 ± 16.65g | 3115.28 ± 7.89n | |
| 4 | 3 | 4.52 ± 0.01b | 186.67 ± 2.22b | 371.41 ± 6.85ab | 5375.28 ± 17.35e | 1365.53 ± 34.66c | 4754.17 ± 2.08b |
| 5 | 4.53 ± 0.03b | 146.67 ± 2.22d | 370.81 ± 7.03ab | 5325.28 ± 41.11e | 1289.78 ± 25.43d | 4358.33 ± 4.17e | |
| 7 | 4.53 ± 0.02b | 95.56 ± 2.22g | 371.61 ± 5.54ab | 5291.94 ± 37.58f | 981.73 ± 16.65f | 3740.28 ± 9.39j | |
| 4.5 | 3 | 4.61 ± 0.03a | 212.59 ± 7.80a | 388.08 ± 5.85a | 6361.39 ± 12.73a | 1643.28 ± 34.66a | 5191.67 ± 4.17a |
| 5 | 4.60 ± 0.01a | 182.22 ± 2.22b | 385.50 ± 5.85a | 6378.06 ± 34.69a | 1451.38 ± 16.65b | 4594.44 ± 3.18d | |
| 7 | 4.60 ± 0.01a | 146.67 ± 2.22d | 387.68 ± 7.32a | 6330.83 ± 8.33b | 1128.18 ± 25.43e | 3865.28 ± 3.18h | |
| 5 | 3 | 4.51 ± 0.02b | 182.22 ± 2.22b | 363.67 ± 7.62b | 5608.61 ± 17.35c | 1340.28 ± 34.66c | 4670.83 ± 10.42c |
| 5 | 4.51 ± 0.02b | 147.41 ± 4.63d | 369.23 ± 5.54ab | 5561.39 ± 17.35d | 1128.18 ± 25.43e | 4073.61 ± 3.18g | |
| 7 | 4.51 ± 0.02b | 98.52 ± 3.39g | 367.64 ± 6.44b | 5555.83 ± 14.43d | 961.53 ± 25.43f | 3538.89 ± 4.34l | |
| Inoculum Size (×106 CFU) | Time (d) | Enzymes Activity (U/g) | |||||
|---|---|---|---|---|---|---|---|
| Amylase | Lipase | Protease | Pectinase | Cellulase | Hemicellulase | ||
| 2.38 | 3 | 4.55 ± 0.02cd | 151.11 ± 2.22d | 321.01 ± 6.52g | 4819.72 ± 20.97g | 1148.38 ± 16.65g | 4302.78 ± 4.34h |
| 5 | 4.56 ± 0.01cd | 115.56 ± 2.22f | 318.43 ± 5.85g | 4730.83 ± 16.67h | 966.58 ± 16.65j | 4031.94 ± 3.18i | |
| 7 | 4.53 ± 0.02d | 101.48 ± 3.39g | 304.35 ± 7.32h | 4655.83 ± 16.67i | 744.38 ± 34.66k | 3413.89 ± 3.18m | |
| 4.76 | 3 | 4.61 ± 0.02ab | 195.56 ± 2.22b | 378.55 ± 6.73c | 6080.83 ± 30.05b | 1400.88 ± 25.43c | 5059.72 ± 3.18c |
| 5 | 4.60 ± 0.01b | 149.63 ± 3.39de | 373.59 ± 6.85cd | 6094.72 ± 12.73b | 1299.88 ± 16.65d | 4344.44 ± 8.42g | |
| 7 | 4.60 ± 0.01b | 100.00 ± 2.22g | 362.88 ± 7.88de | 6041.94 ± 17.35b | 1072.63 ± 16.65g | 3747.22 ± 3.18k | |
| 7.14 | 3 | 4.66 ± 0.03a | 223.70 ± 3.39a | 395.62 ± 5.63a | 6305.83 ± 30.05a | 1587.73 ± 76.30a | 5434.72 ± 4.34a |
| 5 | 4.66 ± 0.01a | 191.85 ± 3.39bc | 351.57 ± 39.33e | 6316.94 ± 17.35a | 1304.93 ± 25.43d | 4552.78 ± 3.18e | |
| 7 | 4.65 ± 0.02a | 154.07 ± 3.39d | 383.31 ± 6.85b | 6316.94 ± 19.25a | 1118.08 ± 33.30g | 3809.72 ± 3.18j | |
| 9.52 | 3 | 4.58 ± 0.01bc | 199.26 ± 4.63b | 355.54 ± 6.52e | 5233.50 ± 16.67d | 1451.38 ± 16.65b | 5143.06 ± 3.18b |
| 5 | 4.58 ± 0.01bc | 183.70 ± 3.39c | 343.63 ± 9.23f | 5333.61 ± 25.46c | 1264.53 ± 25.43ef | 4504.17 ± 2.08f | |
| 7 | 4.58 ± 0.02bc | 135.56 ± 4.44e | 336.09 ± 6.18f | 5297.50 ± 41.67c | 991.83 ± 25.43j | 3650.00 ± 5.51l | |
| 11.90 | 3 | 4.51 ± 0.01e | 188.15 ± 3.39c | 312.48 ± 7.62g | 4925.28 ± 33.68e | 1229.18 ± 50.87f | 4608.33 ± 2.08d |
| 5 | 4.52 ± 0.01e | 143.70 ± 3.39e | 302.56 ± 6.52h | 4861.39 ± 17.35f | 1052.43 ± 41.90h | 4025.00 ± 4.17i | |
| 7 | 4.51 ± 0.02e | 100.74 ± 3.39g | 297.20 ± 6.52h | 4700.28 ± 4.81h | 774.68 ± 50.87k | 3663.89 ± 4.34l | |
| Water Content (%, w/w) | Time (d) | Enzymes Activity (U/g) | |||||
|---|---|---|---|---|---|---|---|
| Amylase | Lipase | Protease | Pectinase | Cellulase | Hemicellulase | ||
| 35 | 3 | 4.51 ± 0.02c | 173.18 ± 2.22b | 375.43 ± 6.13a | 5804.44 ± 17.35c | 1273.27 ± 9.61b | 4486.47 ± 2.08c |
| 5 | 4.51 ± 0.02c | 145.23 ± 2.22d | 373.88 ± 5.85a | 5712.28 ± 8.33d | 877.88 ± 16.65e | 3989.29 ± 3.18h | |
| 7 | 4.52 ± 0.01c | 92.77 ± 3.39g | 372.19 ± 5.85a | 5688.93 ± 12.73e | 812.65 ± 16.65f | 3398.34 ± 3.18k | |
| 45 | 3 | 4.60 ± 0.02a | 194.07 ± 3.39a | 378.55 ± 6.76a | 6111.39 ± 20.97a | 1431.18 ± 9.61a | 4934.72 ± 5.24a |
| 5 | 4.60 ± 0.02a | 147.41 ± 3.39d | 378.15 ± 8.25a | 6116.94 ± 12.73a | 1289.78 ± 25.43b | 4275.00 ± 4.17e | |
| 7 | 4.60 ± 0.01a | 102.22 ± 2.22f | 374.98 ± 6.44a | 6072.50 ± 16.67b | 1077.68 ± 34.66c | 3691.67 ± 4.17i | |
| 55 | 3 | 4.59 ± 0.03ab | 176.30 ± 3.39b | 378.35 ± 5.28a | 5808.61 ± 120.57c | 1284.73 ± 16.65b | 4518.06 ± 5.24b |
| 5 | 4.57 ± 0.02ab | 128.15 ± 3.39e | 375.77 ± 6.13a | 5730.83 ± 22.05d | 900.93 ± 25.43e | 4031.94 ± 3.18f | |
| 7 | 4.57 ± 0.01ab | 82.96 ± 3.39h | 373.59 ± 5.85a | 5708.61 ± 17.35d | 830.23 ± 16.65f | 3608.33 ± 2.08j | |
| 65 | 3 | 4.51 ± 0.01c | 162.96 ± 3.39c | 360.30 ± 7.32b | 5553.06 ± 133.94f | 1269.58 ± 16.65b | 4483.33 ± 4.17c |
| 5 | 4.52 ± 0.05bc | 128.89 ± 2.22e | 357.12 ± 6.44b | 5355.83 ± 8.33h | 1102.93 ± 16.65c | 3920.83 ± 2.08g | |
| 7 | 4.52 ± 0.02c | 80.00 ± 2.22h | 341.05 ± 5.85c | 5403.06 ± 12.73g | 850.43 ± 9.61f | 3406.94 ± 4.34 | |
| 75 | 3 | 4.50 ± 0.02c | 142.96 ± 3.39d | 354.74 ± 5.85b | 4886.39 ± 4.81j | 1077.68 ± 50.87c | 4330.56 ± 6.36d |
| 5 | 4.51 ± 0.04c | 105.19 ± 4.63f | 341.65 ± 5.85c | 4847.50 ± 44.10jk | 966.58 ± 16.65d | 3615.28 ± 4.34j | |
| 7 | 4.50 ± 0.03c | 74.07 ± 3.39j | 328.15 ± 6.13d | 4836.39 ± 29.27k | 825.18 ± 34.66f | 2955.56 ± 3.18l | |
| Proteins (mg/g) | FAAs (ug/g) | RS * (ug/g) | PPAs (mg/g) | Gas * (%) | Cellulose (%) | Lignin (%) | |
|---|---|---|---|---|---|---|---|
| Before | 69.75 ± 0.001b | 2708.33 ± 0.02a | 6214.29 ± 0.01a | 40.08 ± 0.02b | 2.33 ± 0.01a | 60.92 ± 0.00a | 1.82 ± 0.03a |
| After | 115.03 ± 0.001a | 236.11 ± 0.07b | 3285.71 ± 0.01b | 44.34 ± 0.01a | 1.11 ± 0.02b | 48.70 ± 0.03b | 1.12 ± 0.05b |
| Extraction Solvent | ABTS Free Radical Scavenging Capability (%) | DPPH Free Radical Scavenging Capability (%) | Total Reduction Capacity | |
|---|---|---|---|---|
| Water | Before | 1.540 ± 0.002 | 0.739 ± 0.001 | 0.282 ± 0.004 |
| After | 2.269 ± 0.001 | 0.898 ± 0.001 | 0.360 ± 0.002 | |
| 80% Methanol | Before | 3.943 ± 0.001 | 2.217 ± 0.001 | 0.263 ± 0.004 |
| After | 4.592 ± 0.001 | 2.652 ± 0.001 | 0.332 ± 0.001 | |
| 80% Ethanol | Before | 3.241 ± 0.001 | 2.304 ± 0.001 | 0.292 ± 0.007 |
| After | 4.322 ± 0.001 | 2.579 ± 0.001 | 0.340 ± 0.002 | |
| 80% Acetone | Before | 2.809 ± 0.001 | 2.449 ± 0.001 | 0.275 ± 0.001 |
| After | 3.566 ± 0.001 | 2.782 ± 0.001 | 0.373 ± 0.003 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, M.; Guo, X.; Huang, Y.; Cao, F.; Su, E.; Wang, J. Improvement of the Quality of Ginkgo biloba Leaves Fermented by Eurotium cristatum as High Value-Added Feed. Processes 2019, 7, 627. https://doi.org/10.3390/pr7090627
Zou M, Guo X, Huang Y, Cao F, Su E, Wang J. Improvement of the Quality of Ginkgo biloba Leaves Fermented by Eurotium cristatum as High Value-Added Feed. Processes. 2019; 7(9):627. https://doi.org/10.3390/pr7090627
Chicago/Turabian StyleZou, Minmin, Xiaohan Guo, Yan Huang, Fuliang Cao, Erzheng Su, and Jiahong Wang. 2019. "Improvement of the Quality of Ginkgo biloba Leaves Fermented by Eurotium cristatum as High Value-Added Feed" Processes 7, no. 9: 627. https://doi.org/10.3390/pr7090627
APA StyleZou, M., Guo, X., Huang, Y., Cao, F., Su, E., & Wang, J. (2019). Improvement of the Quality of Ginkgo biloba Leaves Fermented by Eurotium cristatum as High Value-Added Feed. Processes, 7(9), 627. https://doi.org/10.3390/pr7090627