Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas
Abstract
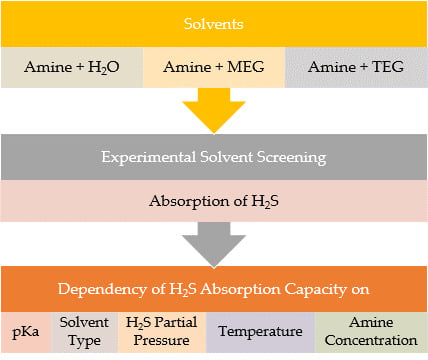
:1. Introduction
2. Material and Methodology
2.1. Materials
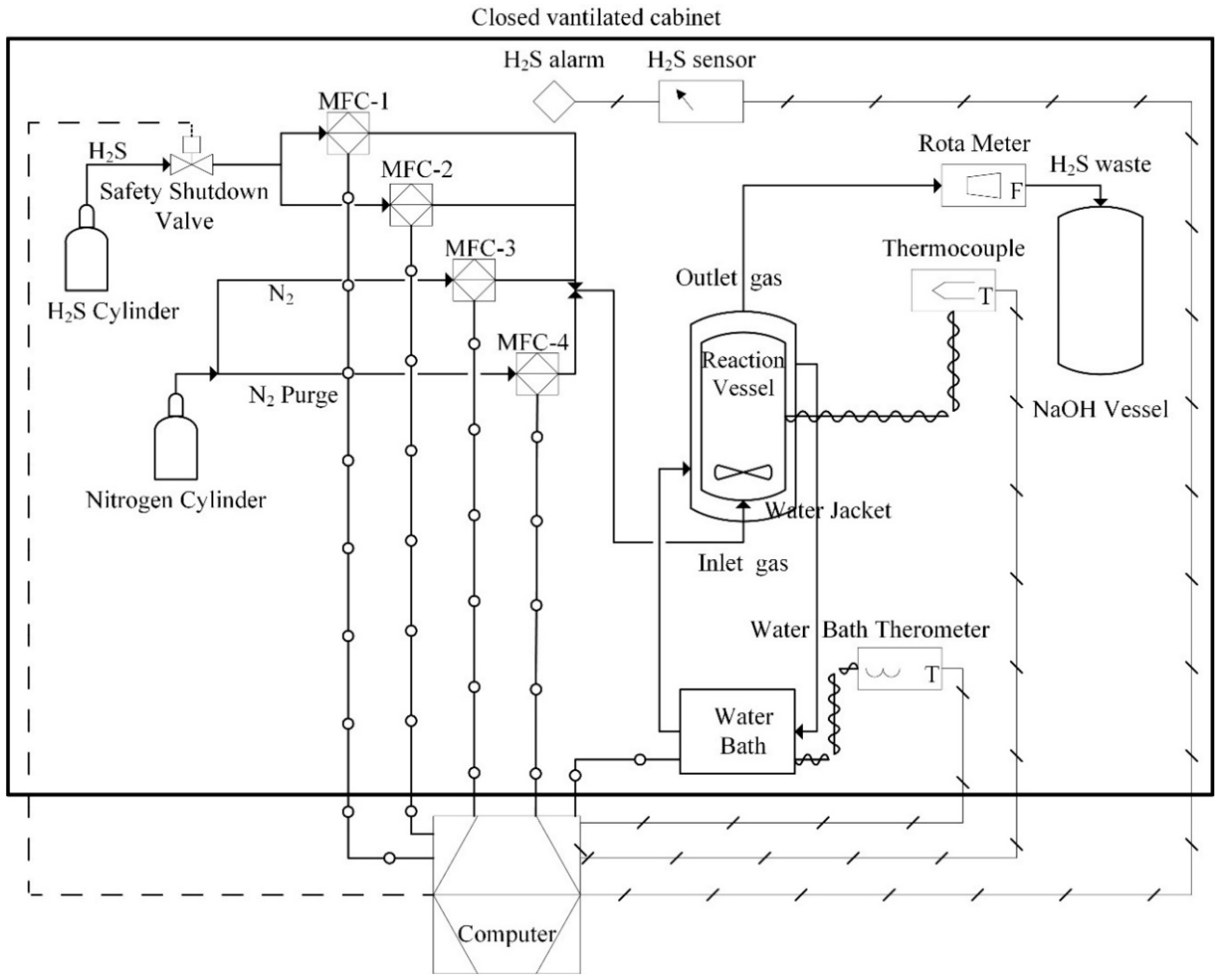
2.2. Methodology and Equipment
3. Results
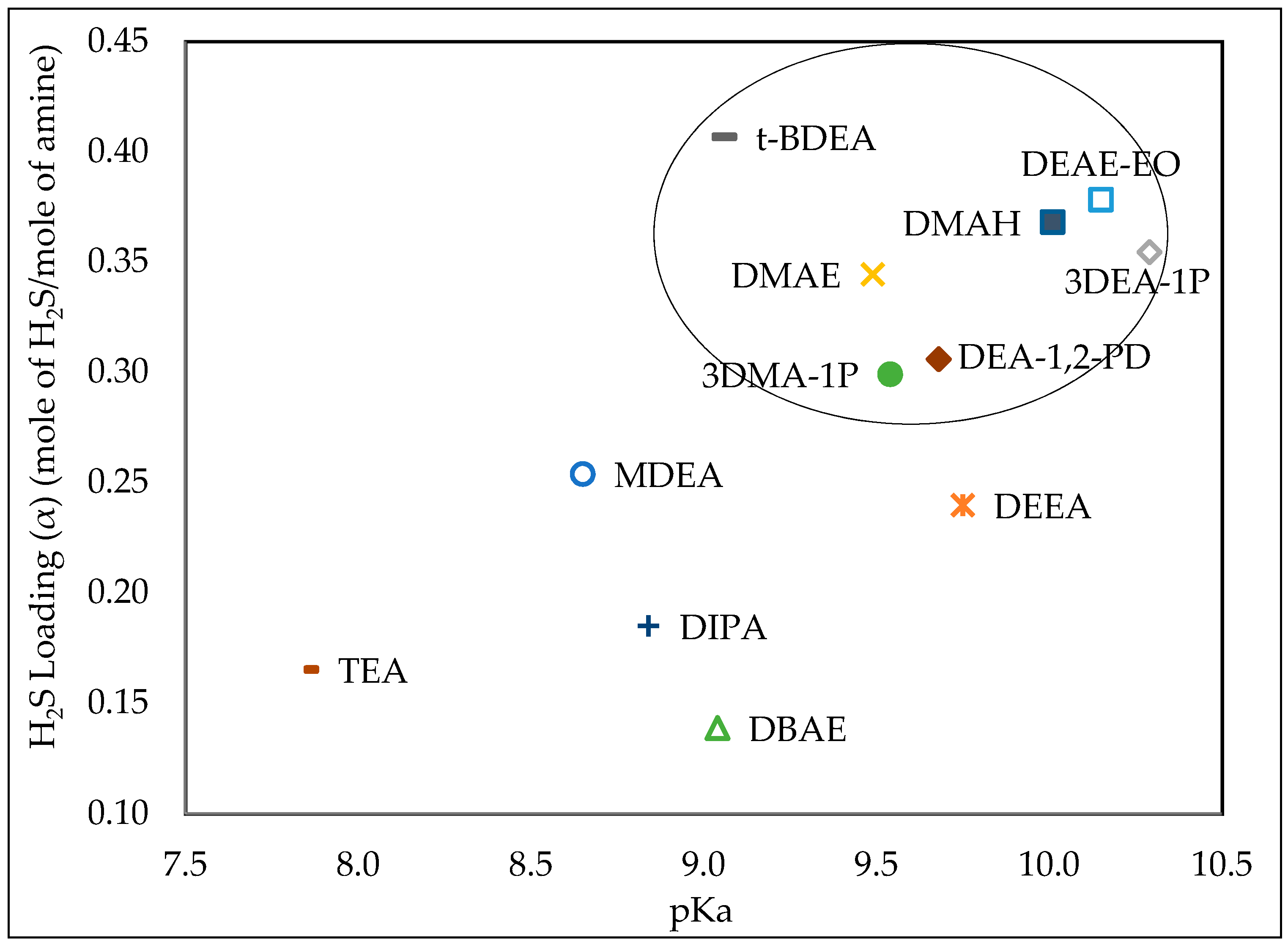
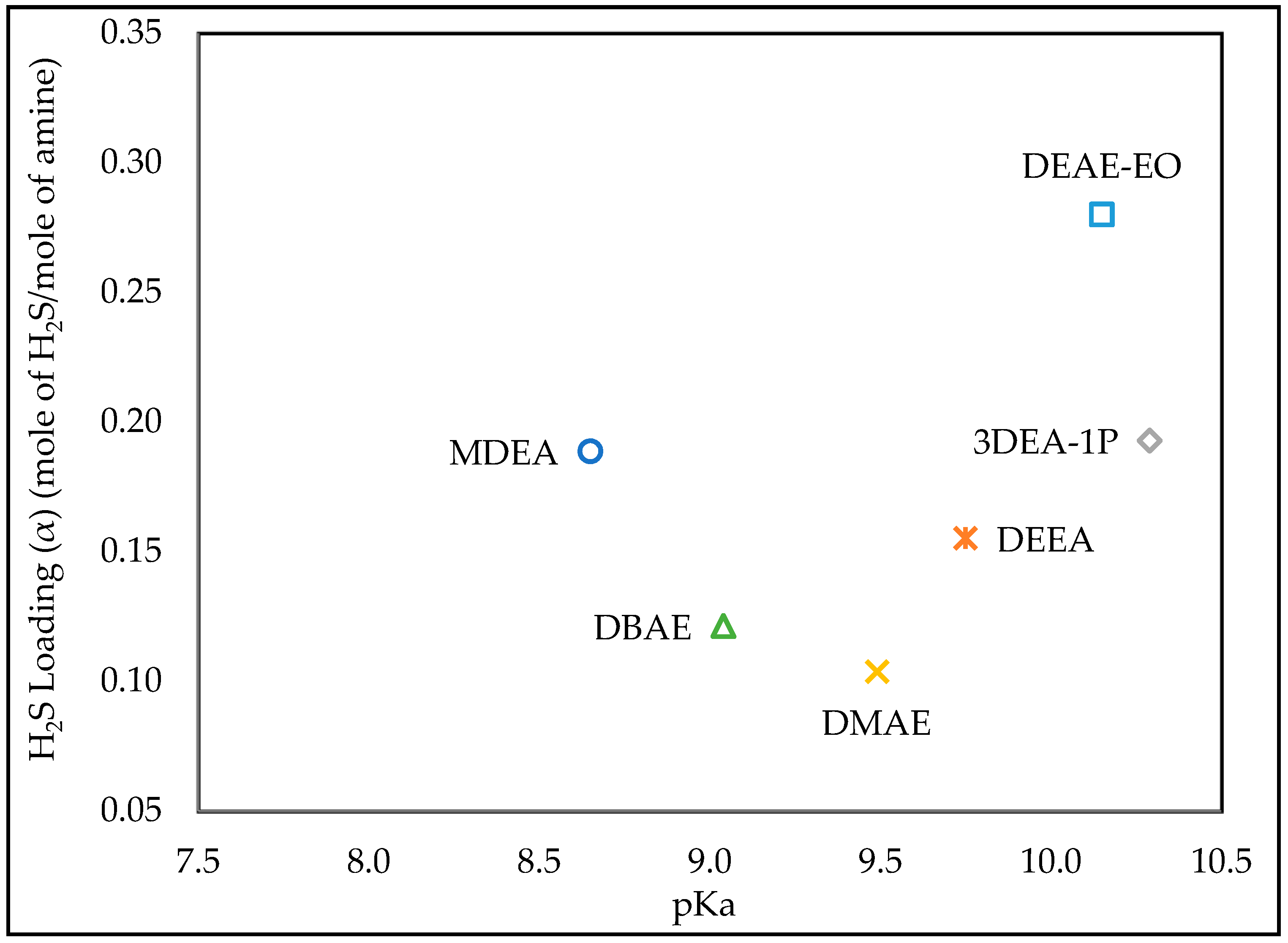
3.1. Effect of pKa
3.2. Effect of Solvent
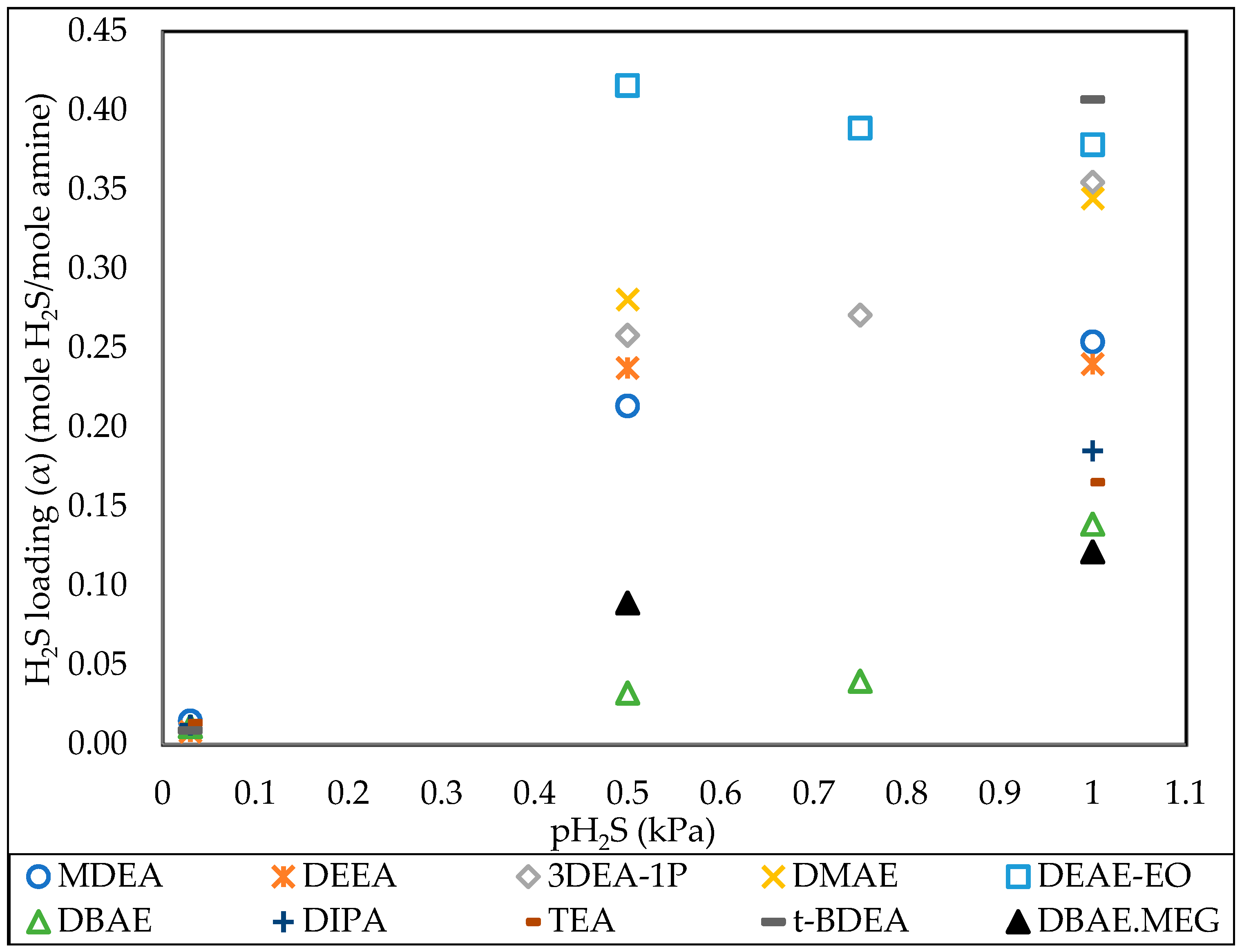
3.3. Effect of H2S Partial Pressure
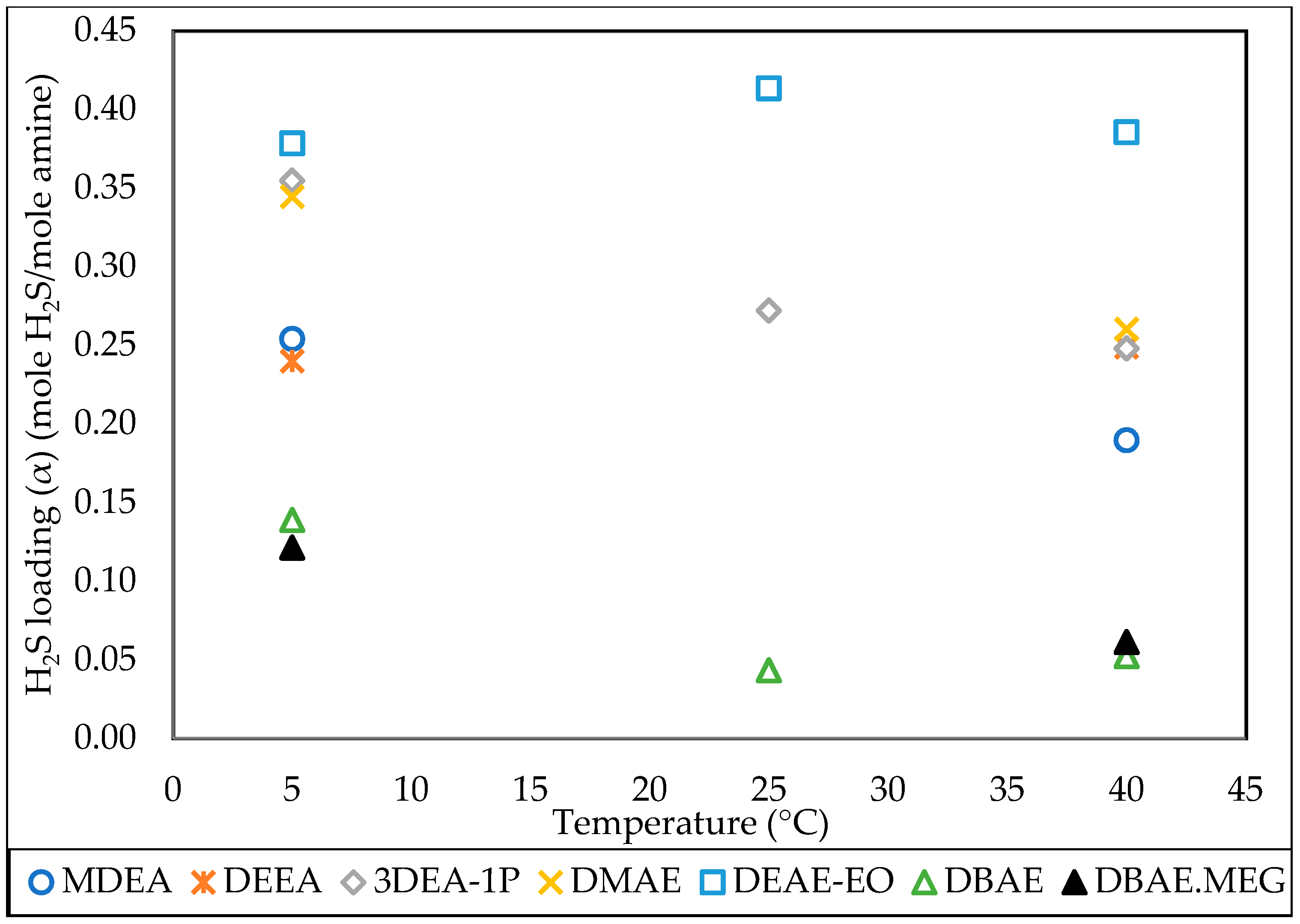
3.4. Effect of Temperature
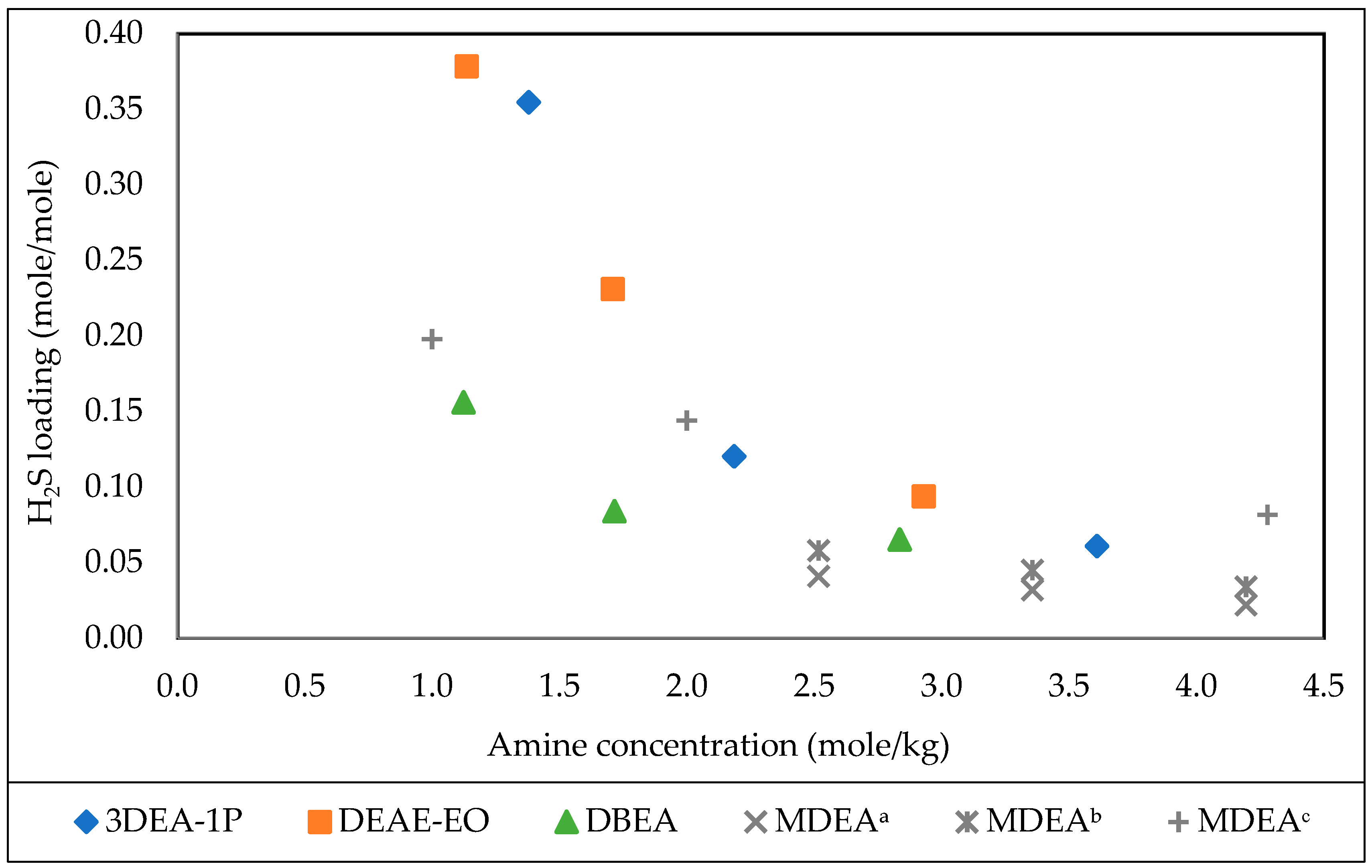
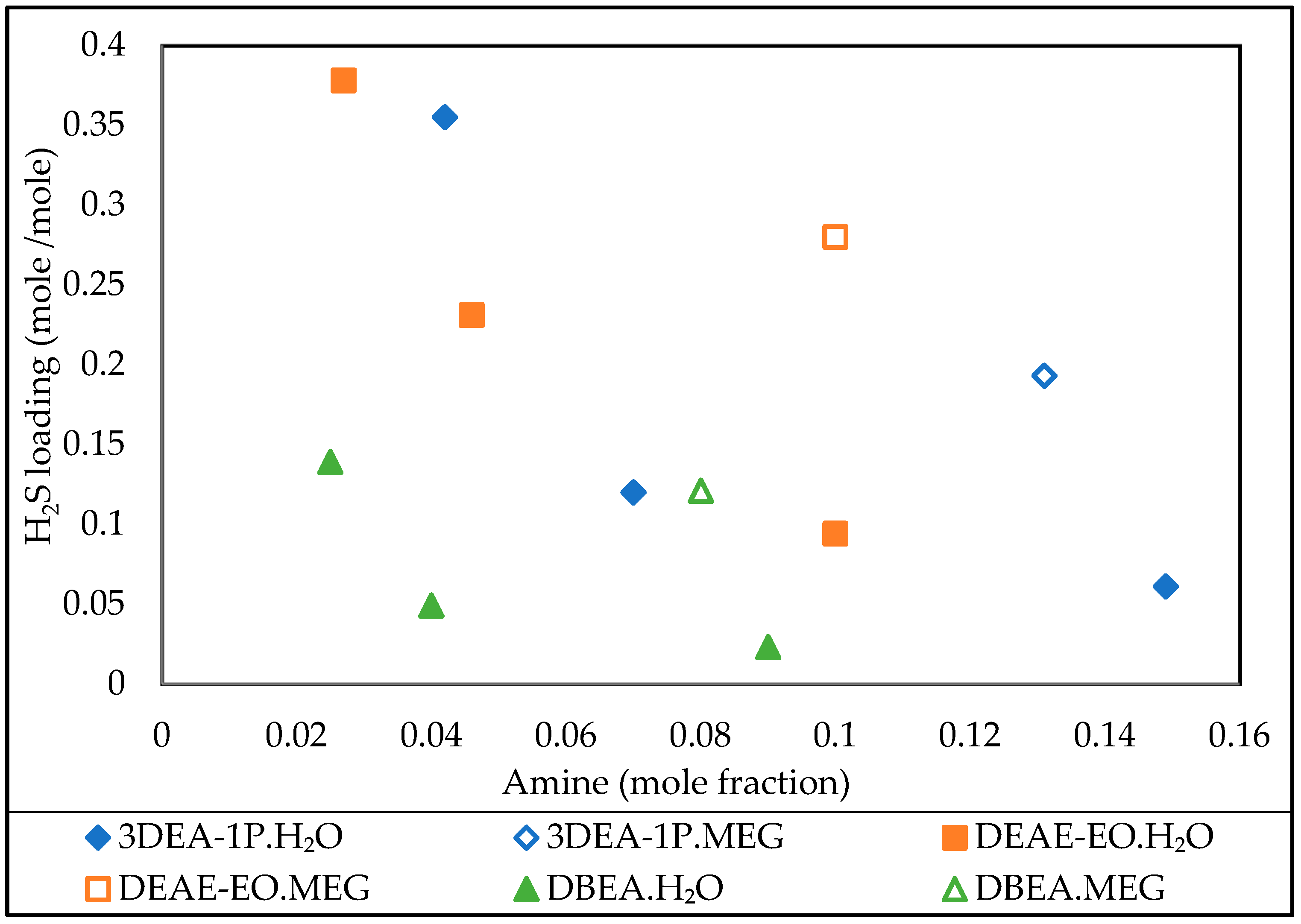
3.5. Effect of Amine Concentration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- BP Statistical Review of World Energy. 67th Edition. 2018. Available online: https://www.bp.com/content/dam/bp/en/corporate/pdf/energy-economics/statistical-review/bp-stats-review-2018-full-report.pdf (accessed on 19 October 2018).
- Subramaniam, R.; Yasa, S.; Bertrand, T.; Fontenot, B.; Dupuis, T.F.; Hernandez, R. Advanced simulation of H2S scavenging process with triazine at different depths of gas well. J. Nat. Gas Sci. Eng. 2018, 49, 417–427. [Google Scholar] [CrossRef]
- El-Gendy, N.S.; Speight, J.G. Handbook of Refinery Desulfurization; Taylor & Francis: Boca Raton, FL, USA, 2015. [Google Scholar]
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Amosa, M.; Mohammed, I.; Yaro, S. Sulphide scavengers in oil and gas industry—A review. Nafta 2010, 61, 85–92. [Google Scholar]
- Grunnlag for Fastsettelse av Administrativ Norm for for Hydrogensulfid (H2S); Arbeidstilsynet Statens Hus: Trondheim, Norway, 2011; Volume 7468.
- Kohl, A.L.; Nielsen, R.B. Chapter 2—Alkanolamines for Hydrogen Sulfide and Carbon Dioxide Removal. In Gas Purification, 5th ed.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 40–186. [Google Scholar]
- Kohl, A.L.; Nielsen, R.B. Chapter 11—Absorption of Water Vapor by Dehydrating Solutions. In Gas Purification, 5th ed.; Kohl, A.L., Nielsen, R.B., Eds.; Gulf Professional Publishing: Houston, TX, USA, 1997; pp. 946–1021. [Google Scholar]
- Hutchinson, A.J. Process for Treating Gases. U.S. Patent Application No. 2177068 A, 24 October 1939. [Google Scholar]
- McCartney, E.R. Gas Purification and Dehydration Process. U.S. Patent Application No. 2435089, 27 January 1948. [Google Scholar]
- McCartney, E.R. Extraction of acidic impurities and moisture from gases. U.S. Patent Application No. 2547278, 3 April 1951. [Google Scholar]
- Chapin, W.F. Purification and Dehydration of Gases. U.S. Patent Application No. 2518752, 2 August 1950. [Google Scholar]
- Shoukat, U.; Fytianos, G.; Knuutila, H.K. Thermal Stability and Corrosion Studies of Amines for Combined Acid Gas Removal and Hydrate Control for Subsea Gas Treatment Systems. In Proceedings of the 2016 Techno-Ocean (Techno-Ocean), Kobe, Japan, 6–8 October 2016; pp. 176–180. [Google Scholar]
- Eimer, D. Simultaneous Removal of Water and Hydrogen Sulphide from Natural Gas; Department of Chemical Engineering, Norwegian University of Science and Technology: Trondheim, Norway, 1994. [Google Scholar]
- Mathias, P.M.; Jasperson, L.V.; VonNiederhausern, D.; Bearden, M.D.; Koech, P.K.; Freeman, C.J.; Heldebrant, D.J. Assessing anhydrous tertiary alkanolamines for high-pressure gas purifications. Ind. Eng. Chem. Res. 2013, 52, 17562–17572. [Google Scholar] [CrossRef]
- Jou, F.Y.; Mather, A.E.; Otto, F.D. Solubility of hydrogen sulfide and carbon dioxide in aqueous methyldiethanolamine solutions. Ind. Eng. Chem. Process Des. Dev. 1982, 21, 539–544. [Google Scholar] [CrossRef]
- Macgregor, R.J.; Mather, A.E. Equilibrium solubility of H2S and CO2 and their mixtures in a mixed solvent. Can. J. Chem. Eng. 1991, 69, 1357–1366. [Google Scholar] [CrossRef]
- Li, M.H.; Shen, K.P. Solubility of hydrogen sulfide in aqueous mixtures of monoethanolamine with N-methyldiethanolamine. J. Chem. Eng. Data 1993, 38, 105–108. [Google Scholar] [CrossRef]
- Kuranov, G.; Rumpf, B.; Smirnova, N.; Maurer, G. Solubility of single gases carbon dioxide and hydrogen sulfide in aqueous solutions of N-methyldiethanolamine in the temperature range 313–413 K at pressures up to 5 MPa. Ind. Eng. Chem. Res. 1996, 35, 1959–1966. [Google Scholar] [CrossRef]
- Lemoine, B.; Li, Y.G.; Cadours, R.; Bouallou, C.; Richon, D. Partial vapor pressure of CO2 and H2S over aqueous methyldiethanolamine solutions. Fluid Phase Equilib. 2000, 172, 261–277. [Google Scholar] [CrossRef]
- Pérez-Salado Kamps, Á.; Balaban, A.; Jödecke, M.; Kuranov, G.; Smirnova, N.A.; Maurer, G. Solubility of single gases carbon dioxide and hydrogen sulfide in aqueous solutions of N-methyldiethanolamine at temperatures from 313 to 393 K and pressures up to 7.6 MPa: New experimental data and model extension. Ind. Eng. Chem. Res. 2001, 40, 696–706. [Google Scholar]
- Sidi-Boumedine, R.; Horstmann, S.; Fischer, K.; Provost, E.; Fürst, W.; Gmehling, J. Experimental determination of hydrogen sulfide solubility data in aqueous alkanolamine solutions. Fluid Phase Equilib. 2004, 218, 149–155. [Google Scholar] [CrossRef]
- Huttenhuis, P.J.G.; Agrawal, N.J.; Hogendoorn, J.A.; Versteeg, G.F. Gas solubility of H2S and CO2 in aqueous solutions of N-methyldiethanolamine. J. Pet. Sci. Eng. 2007, 55, 122–134. [Google Scholar] [CrossRef]
- Xu, H.J.; Zhang, C.F.; Zheng, Z.S. Solubility of hydrogen sulfide and carbon dioxide in a solution of methyldiethanolamine mixed with ethylene glycol. Ind. Eng. Chem. Res. 2002, 41, 6175–6180. [Google Scholar] [CrossRef]
- Sadegh, N.; Thomsen, K.; Solbraa, E.; Johannessen, E.; Rudolfsen, G.I.; Berg, O.J. Solubility of hydrogen sulfide in aqueous solutions of N-methyldiethanolamine at high pressures. Fluid Phase Equilib. 2015, 393, 33–39. [Google Scholar] [CrossRef]
- Maddox, R.N.; Bhairi, A.H.; Diers, J.R.; Thomas, P.A. Equilibrium solubility of carbon dioxide or hydrogen sulfide in aqueous solutions of monoethanolamine, diglycolamine, diethanoiamine and methyldiethanolamine. In GPA Research Report: Project 104; GPA: Tulsa, OK, USA, 1987. [Google Scholar]
- Tian, X.; Wang, L.; Fu, D.; Li, C. Absorption and Removal Efficiency of Low-Partial-Pressure H2S in a Monoethanolamine-Activated N-Methyldiethanolamine Aqueous Solution. Energy Fuels 2019, 33, 629–635. [Google Scholar] [CrossRef]
- Jagushte, M.V.; Mahajani, V.V. Low pressure equilibrium between H2S and alkanolamine revisited. Ind. J. Chem. Technol. 1999, 6, 125–133. [Google Scholar]
- Mazloumi, S.H.; Haghtalab, A.; Jalili, A.H.; Shokouhi, M. Solubility of H2S in aqueous diisopropanolamine + piperazine solutions: New experimental data and modeling with the electrolyte cubic square-well equation of state. J. Chem. Eng. Data 2012, 57, 2625–2631. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Yamada, H.; Higashii, T.; Goto, K.; Onoda, M. CO2 capture by tertiary amine absorbents: A performance comparison study. Ind. Eng. Chem. Res. 2013, 52, 8323–8331. [Google Scholar] [CrossRef]
- Hartono, A.; Vevelstad, S.J.; Ciftja, A.; Knuutila, H.K. Screening of strong bicarbonate forming solvents for CO2 capture. Int. J. Greenh. Gas Control 2017, 58, 201–211. [Google Scholar] [CrossRef]
- ScolarTM, S. Advanced Chemistry Development (ACD/Labs) Software V11.02 2016; ACD/Labs: Toronto, ON, Canada, 2016. [Google Scholar]
- Hamborg, E.S.; Versteeg, G.F. Dissociation Constants and Thermodynamic Properties of Amines and Alkanolamines from (293 to 353) K. J. Chem. Eng. Data 2009, 54, 1318–1328. [Google Scholar] [CrossRef]
- Woolley, E.M.; Tomkins, J.; Hepler, L.G. Ionization constants for very weak organic acids in aqueous solution and apparent ionization constants for water in aqueous organic mixtures. J. Solut. Chem. 1972, 1, 341–351. [Google Scholar] [CrossRef]
- Jacob, P. Potential Membrane Based Treatment of Triethylene Glycol Wastewater from Gas Separation Plant. J. Water Sustain. 2014, 4, 123–136. [Google Scholar]
- Ma’mun, S.; Jakobsen, J.P.; Svendsen, H.F.; Juliussen, O. Experimental and Modeling Study of the Solubility of Carbon Dioxide in Aqueous 30 Mass% 2-((2-Aminoethyl)amino)ethanol Solution. Ind. Eng. Chem. Res. 2006, 45, 2505–2512. [Google Scholar] [CrossRef]
- Shoukat, U.; Baumeister, E.; Pinto, D.D.D.; Knuutila, H.K. Thermal stability and corrosion of tertiary amines in aqueous amine and amine-glycol-water solutions for combined acid gas and water removal. J. Nat. Gas Sci. Eng. 2019, 62, 26–37. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Krokvik, I.R.T.; Jens, K.-J.; Knuutila, H.K. Performance of MAPA Promoted Tertiary Amine Systems for CO2 Absorption: Influence of Alkyl Chain Length and Hydroxyl Groups. Energy Procedia 2017, 114, 1682–1688. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.V.; Zahra, M.R.M.A. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Eimer, D. Gas Treating: Absorption Theory and Practice; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Fu, D.; Chen, L.; Qin, L. Experiment and model for the viscosity of carbonated MDEA–MEA aqueous solutions. Fluid Phase Equilib. 2012, 319, 42–47. [Google Scholar] [CrossRef]
- Fu, D.; Wang, L.; Zhang, P.; Mi, C. Solubility and viscosity for CO2 capture process using MEA promoted DEAE aqueous solution. J. Chem. Thermodyn. 2016, 95, 136–141. [Google Scholar] [CrossRef]
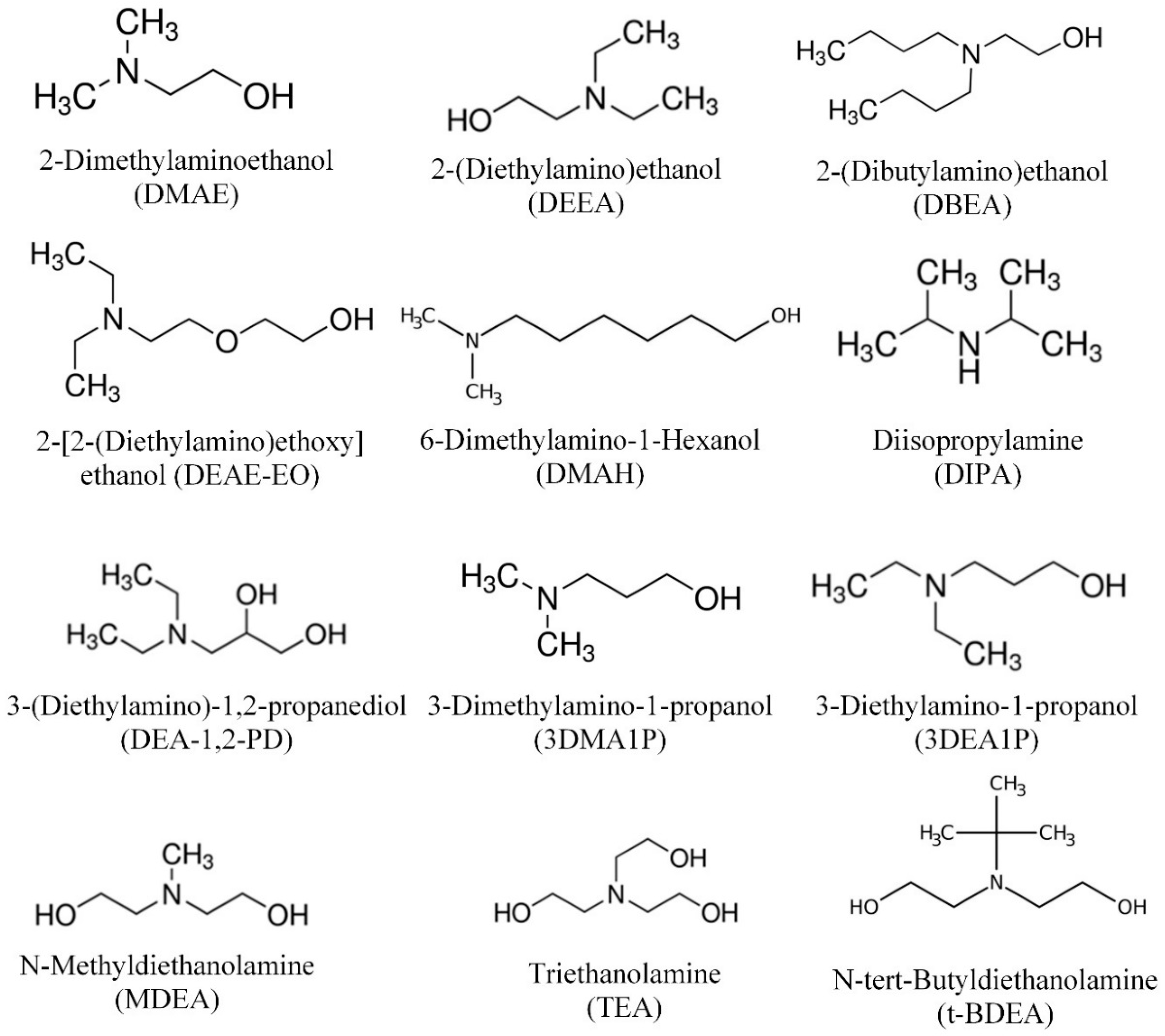


| Chemical | CAS | Purity (wt.%) | Molecular Weight (g/mol) | pKa |
|---|---|---|---|---|
| 2-Dimethylaminoethanol (DMAE) | 108-01-0 | ≥99.5 | 89.14 | 9.49 [30] |
| 2-(Diethylamino)ethanol (DEEA) | 100-37-8 | ≥99.5 | 117.19 | 9.75 [31] |
| 2-(Dibutylamino)ethanol (DBAE) | 102-81-8 | ≥99.0 | 173.30 | 9.04 [32] |
| 2-[2-(Diethylamino)ethoxy] ethanol (DEAE-EO) | 140-82-9 | >98.0 | 161.25 | 10.15 [31] |
| 6-Dimethylamino-1-Hexanol (DMAH) | 1862-07-3 | >97.0 | 145.24 | 10.01 [31] |
| Diisopropylamine (DIPA) | 108-18-9 | ≥99.0 | 101.19 | 8.84 [33] |
| 3-(Diethylamino)-1,2-propanediol (DEA-1,2-PD) | 621-56-7 | >98.0 | 147.22 | 9.68 [31] |
| 3-Dimethylamino-1-propanol (3DMA-1P) | 3179-63-3 | ≥99.0 | 103.16 | 9.54 [30] |
| 3-Diethylamino-1-propanol (3DEA-1P) | 622-93-5 | ≥95.0 | 131.22 | 10.29 [30] |
| N-Methyldiethanolamine (MDEA) | 105-59-9 | ≥99.0 | 119.16 | 8.65 [30] |
| Triethanolamine (TEA) | 102-71-6 | ≥99.0 | 146.19 | 7.85 [30] |
| N-tert-Butyldiethanolamine (t-BDEA) | 2160-93-2 | ≥97.0 | 161.24 | 9.06 [30] |
| Ethylene glycol (MEG) | 107-21-1 | ≥99.5 | 62.07 | 14.44 [34] |
| Triethylene glycol (TEG) | 112-27-6 | ≥99.8 | 150.17 | 14.50 [35] |
| Amine | Initial Amine (wt.%) | Solvent | H2S Loading (α) | Inlet pH2S (kPa) | Temperature (°C) |
|---|---|---|---|---|---|
| MDEA | 20% | Water | 0.015 | 0.03 | 5 |
| DEEA | 20% | Water | 0.008 | 0.03 | 5 |
| DBEA | 20% | Water | 0.011 | 0.03 | 5 |
| DIPA | 20% | Water | 0.012 | 0.03 | 5 |
| TEA | 20% | Water | 0.013 | 0.03 | 5 |
| t-BDEA | 20% | Water | 0.009 | 0.03 | 5 |
| MDEA | 20% | MEG | 0.010 | 0.03 | 5 |
| MDEA | 20% | TEG | 0.006 | 0.03 | 5 |
| MDEA | 20% | Water | 0.213 | 0.5 | 5 |
| DEEA | 20% | Water | 0.237 | 0.5 | 5 |
| 3DEA-1P | 20% | Water | 0.258 | 0.5 | 5 |
| DMAE | 20% | Water | 0.281 | 0.5 | 5 |
| DEAE-EO | 20% | Water | 0.416 | 0.5 | 5 |
| DBAE | 20% | Water | 0.032 | 0.5 | 5 |
| DBAE | 20% | MEG | 0.089 | 0.5 | 5 |
| 3DEA-1P | 20% | Water | 0.271 | 0.75 | 5 |
| DEAE-EO | 20% | Water | 0.389 | 0.75 | 5 |
| DBAE | 20% | Water | 0.040 | 0.75 | 5 |
| MDEA | 20% | Water | 0.254 | 1 | 5 |
| MDEA | 20% | Water | 0.189 | 1 | 40 |
| DEEA | 20% | Water | 0.240 | 1 | 5 |
| DEEA | 20% | Water | 0.249 | 1 | 40 |
| 3DEA-1P | 20% | Water | 0.355 | 1 | 5 |
| 3DEA-1P | 20% | Water | 0.272 | 1 | 25 |
| 3DEA-1P | 20% | Water | 0.248 | 1 | 40 |
| 3DEA-1P | 30% | Water | 0.120 | 1 | 5 |
| 3DEA-1P | 50% | Water | 0.061 | 1 | 5 |
| DMAE | 20% | Water | 0.344 | 1 | 5 |
| DMAE | 20% | Water | 0.260 | 1 | 40 |
| DEAE-EO | 20% | Water | 0.378 | 1 | 5 |
| DEAE-EO | 20% | Water | 0.413 | 1 | 25 |
| DEAE-EO | 20% | Water | 0.385 | 1 | 40 |
| DEAE-EO | 50% | Water | 0.094 | 1 | 5 |
| DEAE-EO | 30% | Water | 0.231 | 1 | 5 |
| DBAE | 20% | Water | 0.139 | 1 | 5 |
| DBAE | 20% | Water | 0.043 | 1 | 25 |
| DBAE | 20% | Water | 0.052 | 1 | 40 |
| DBAE | 30% | Water | 0.049 | 1 | 5 |
| DBAE | 50% | Water | 0.023 | 1 | 5 |
| DIPA | 20% | Water | 0.185 | 1 | 5 |
| TEA | 20% | Water | 0.165 | 1 | 5 |
| t-BDEA | 20% | Water | 0.407 | 1 | 5 |
| DEA-1,2-PD | 20% | Water | 0.306 | 1 | 5 |
| DMAH | 20% | Water | 0.368 | 1 | 5 |
| 3DMA1P | 20% | Water | 0.299 | 1 | 5 |
| MDEA | 20% | MEG | 0.189 | 1 | 5 |
| DEEA | 20% | MEG | 0.155 | 1 | 5 |
| 3DEA-1P | 20% | MEG | 0.193 | 1 | 5 |
| DMAE | 20% | MEG | 0.104 | 1 | 5 |
| DEAE-EO | 20% | MEG | 0.280 | 1 | 5 |
| DBAE | 20% | MEG | 0.121 | 1 | 5 |
| DBAE | 20% | MEG | 0.061 | 1 | 40 |
| MDEA | 20% | TEG | 0.040 | 1 | 5 |
| DEEA | 20% | TEG | 0.035 | 1 | 5 |
| 3DEA-1P | 20% | TEG | 0.080 | 1 | 5 |
| DMAE | 20% | TEG | 0.025 | 1 | 5 |
| DEAE-EO | 20% | TEG | 0.073 | 1 | 5 |
| DBAE | 20% | TEG | 0.049 | 1 | 5 |
| u(pH2S) = ±2%; u(T) = ±0.1 °C; u(CAmine) = ±1.5%; u(CH2S) = ±2.5% | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoukat, U.; Pinto, D.D.D.; Knuutila, H.K. Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas. Processes 2019, 7, 160. https://doi.org/10.3390/pr7030160
Shoukat U, Pinto DDD, Knuutila HK. Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas. Processes. 2019; 7(3):160. https://doi.org/10.3390/pr7030160
Chicago/Turabian StyleShoukat, Usman, Diego D. D. Pinto, and Hanna K. Knuutila. 2019. "Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas" Processes 7, no. 3: 160. https://doi.org/10.3390/pr7030160
APA StyleShoukat, U., Pinto, D. D. D., & Knuutila, H. K. (2019). Study of Various Aqueous and Non-Aqueous Amine Blends for Hydrogen Sulfide Removal from Natural Gas. Processes, 7(3), 160. https://doi.org/10.3390/pr7030160