Carbon Mineralization by Reaction with Steel-Making Waste: A Review
Abstract
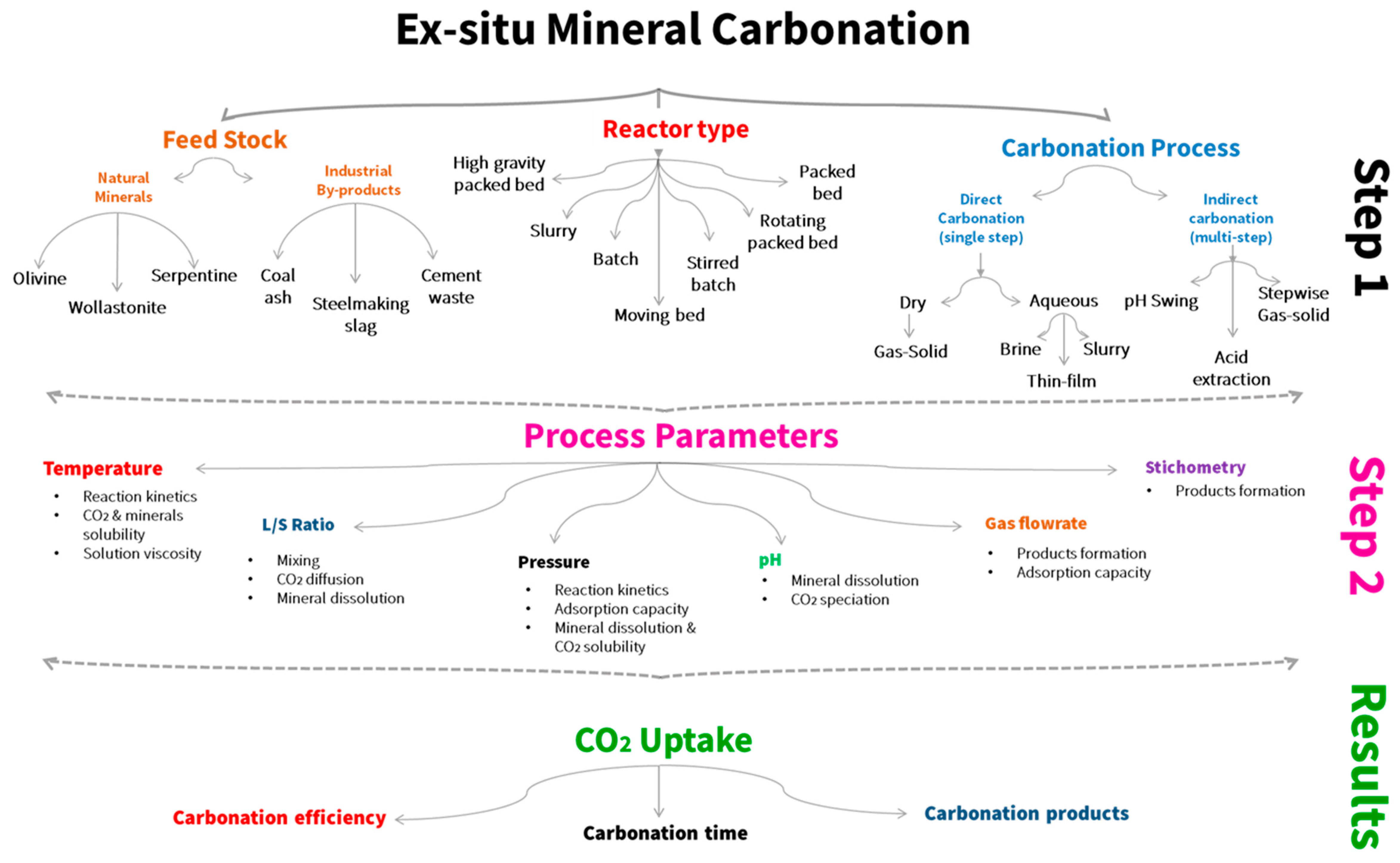
1. Introduction
1.1. CO2 Storage
1.2. Mineral Carbon Sequestration
1.3. Indirect Carbonation
2. Direct Carbonation
2.1. Gas-Solid Carbonation
2.2. Direct Aqueous Carbonation
- (1)
- Dissolution of alkali earth element into the solution (leaching step).
- (2)
- Formation of mineral carbonate (carbonation step).
3. Steelmaking Waste Mineral Carbonation
3.1. Temperature and Particle Size
3.2. Liquid to Solid Ratio
3.3. Pressure
4. Summary and Future Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Gale, J.; Abanades, J.C.; Bachu, S.; Jenkins, C. Special Issue commemorating the 10th year anniversary of the publication of the Intergovernmental Panel on Climate Change Special Report on CO2 Capture and Storage. Int. J. Greenh. Gas Control 2015, 40, 1–5. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Chunbao Charles, X.U.; Cang, D.Q. A brief overview of low CO2 emission technologies for iron and steel making. J. Iron Steel Res. Int. 2010, 17, 1–7. [Google Scholar]
- White, C.M.; Strazisar, B.R.; Granite, E.J.; Hoffman, J.S.; Pennline, H.W. Separation and capture of CO2 from large stationary sources and sequestration in geological formations—Coalbeds and deep saline aquifers. J. Air Waste Manag. Assoc. 2003, 53, 645–715. [Google Scholar] [CrossRef] [PubMed]
- Torp, T.A.; Gale, J. Demonstrating storage of CO2 in geological reservoirs: The Sleipner and SACS projects. Energy 2004, 29, 1361–1369. [Google Scholar] [CrossRef]
- Maldal, T.; Tappel, I.M. CO2 underground storage for Snøhvit gas field development. Energy 2004, 29, 1403–1411. [Google Scholar] [CrossRef]
- Matter, J.M.; Kelemen, P.B. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Michael, K.; Golab, A.; Shulakova, V.; Ennis-King, J.; Allinson, G.; Sharma, S.; Aiken, T. Geological storage of CO2 in saline aquifers-A review of the experience from existing storage operations. Int. J. Greenh. Gas Control 2010, 4, 659–667. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Mohammadian, E.; Hamidi, H.; Junin, R.; Karaei, M.A. A review on carbon dioxide mineral carbonation through pH-swing process. Chem. Eng. J. 2015, 279, 615–630. [Google Scholar] [CrossRef]
- Metz, B.; Davidson, O.; de Coninck, H.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Kunzler, C.; Alves, N.; Pereira, E.; Nienczewski, J.; Ligabue, R.; Einloft, S.; Dullius, J. CO2 storage with indirect carbonation using industrial waste. Energy Procedia 2011, 4, 1010–1017. [Google Scholar] [CrossRef]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Harrould-kolieb, E.R. A governing framework for international ocean acidification policy. Mar. Policy 2019, 102, 10–20. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon Mineralization: From Natural Analogues to Engineered Systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Mun, M.; Cho, H. Mineral carbonation for carbon sequestration with industrial waste. Energy Procedia 2013, 37, 6999–7005. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Seifritz, W. CO2 Disposal by Means of Silicates; Nature Publishing Group: London, UK, 1990. [Google Scholar]
- Gunning, P.J.; Hills, C.D.; Carey, P.J. Accelerated carbonation treatment of industrial wastes. Waste Manag. 2010, 30, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mineral CO2 sequestration by steel slag carbonation. Environ. Sci. Technol. 2005, 39, 9676–9682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, W.; Hu, J.; Wang, L.; Gao, J.; Liang, B.; Yue, H.; Zhang, G.; Luo, D.; Li, C. Energy-efficient mineral carbonation of blast furnace slag with high value-added products. J. Clean. Prod. 2018, 197, 242–252. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.W.; Chae, S.; Bang, J.H.; Lee, S.W. CO2 sequestration technology through mineral carbonation: An extraction and carbonation of blast slag. J. CO2 Util. 2016, 16, 336–345. [Google Scholar] [CrossRef]
- Dri, M.; Sanna, A.; Maroto-Valer, M.M. Mineral carbonation from metal wastes: Effect of solid to liquid ratio on the efficiency and characterization of carbonated products. Appl. Energy 2014, 113, 515–523. [Google Scholar] [CrossRef]
- Sanna, A.; Dri, M.; Hall, M.R.; Maroto-Valer, M. Waste materials for carbon capture and storage by mineralisation (CCSM)—A UK perspective. Appl. Energy 2012, 99, 545–554. [Google Scholar] [CrossRef]
- Dananjayan, R.R.T.; Kandasamy, P.; Andimuthu, R. Direct mineral carbonation of coal fly ash for CO2 sequestration. J. Clean. Prod. 2016, 112, 4173–4182. [Google Scholar] [CrossRef]
- Nyambura, M.G.; Mugera, G.W.; Felicia, P.L.; Gathura, N.P. Carbonation of brine impacted fractionated coal fly ash: Implications for CO2 sequestration. J. Environ. Manag. 2011, 92, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, M.C.; Andrés, J.M.; Gimeno, M.P. Optimization of mineral carbonation process for CO2 sequestration by lime-rich coal ashes. Fuel 2013, 106, 448–454. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Wang, X.; Grigore, M.; French, D.; Gözükara, Y.M.; Yu, J.; Zeng, M. CO2 sequestration by direct mineralisation using fly ash from Chinese Shenfu coal. Fuel Process. Technol. 2017, 156, 429–437. [Google Scholar] [CrossRef]
- Mazzella, A.; Errico, M.; Spiga, D. CO2 uptake capacity of coal fly ash: Influence of pressure and temperature on direct gas-solid carbonation. J. Environ. Chem. Eng. 2016, 4, 4120–4128. [Google Scholar] [CrossRef]
- Wee, J.H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Reddy, K.J.; John, S.; Weber, H.; Argyle, M.D.; Bhattacharyya, P.; Taylor, D.T.; Christensen, M.; Foulke, T.; Fahlsing, P. Simultaneous capture and mineralization of coal combustion flue gas carbon dioxide (CO2). Energy Procedia 2011, 4, 1574–1583. [Google Scholar] [CrossRef]
- Jo, H.Y.; Kim, J.H.; Lee, Y.J.; Lee, M.; Choh, S.J. Evaluation of factors affecting mineral carbonation of CO2 using coal fly ash in aqueous solutions under ambient conditions. Chem. Eng. J. 2012, 183, 77–87. [Google Scholar] [CrossRef]
- Uibu, M.; Velts, O.; Kuusik, R. Developments in CO2 mineral carbonation of oil shale ash. J. Hazard. Mater. 2010, 174, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Araizi, P.K.; Hills, C.D.; Maries, A.; Gunning, P.J.; Wray, D.S. Enhancement of accelerated carbonation of alkaline waste residues by ultrasound. Waste Manag. 2016, 50, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R.; Prigiobbe, V. Comparison of different reaction routes for carbonation of APC residues. Energy Procedia 2009, 1, 4851–4858. [Google Scholar] [CrossRef]
- Gadikota, G.; Fricker, K.J.; Jang, S.-H.; Park, A.-H.A. Carbonation of silicate minerals and industrial wastes and their potential use as sustainable construction materials. In Advances in CO2 Capture, Sequestration, and Conversion; American Chemical Society: Washington, DC, USA, 2015; pp. 115–137. [Google Scholar]
- Gomes, H.I.; Mayes, W.M.; Rogerson, M.; Stewart, D.I.; Burked, I.T. Alkaline residues and the environment: A review of impacts, management practices and opportunities. J. Clean. Prod. 2016, 112, 3571–3582. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef]
- Damiani, D.; Litynski, J.T.; McIlvried, H.G.; Vikara, D.M.; Srivastava, R.D. The US Department of Energy’s R&D program to reduce greenhouse gas emissions through beneficial uses of carbon dioxide. Greenh. Gases Sci. Technol. 2012, 2, 9–19. [Google Scholar]
- Pan, S.-Y. CO2 Capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Said, A.; Laukkanen, T.; Järvinen, M. Pilot-scale experimental work on carbon dioxide sequestration using steelmaking slag. Appl. Energy 2016, 177, 602–611. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R. Influence of particle size on the carbonation of stainless steel slag for CO2 storage. Energy Procedia 2009, 1, 4859–4866. [Google Scholar] [CrossRef]
- Jung, W.M.; Kang, S.H.; Kim, W.S.; Choi, C.K. Particle morphology of calcium carbonate precipitated by gas-liquid reaction in a Couette-Taylor reactor. Chem. Eng. Sci. 2000, 55, 733–747. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Chizmeshya, A.V.G.; Diefenbacher, J.; Béarat, H.; Wolf, G. Exploration of the role of heat activation in enhancing serpentine carbon sequestration reactions. Environ. Sci. Technol. 2004, 38, 6897–6903. [Google Scholar] [CrossRef] [PubMed]
- Maroto-Valer, M.M.; Fauth, D.J.; Kuchta, M.E.; Zhang, Y.; Andrésen, J.M. Activation of magnesium rich minerals as carbonation feedstock materials for CO2 sequestration. Fuel Process. Technol. 2005, 86, 1627–1645. [Google Scholar] [CrossRef]
- Kleiv, R.A.; Thornhill, M. Mechanical activation of olivine. Miner. Eng. 2006, 19, 340–347. [Google Scholar] [CrossRef]
- Veetil, S.P.; Mercier, G.; Blais, J.F.; Cecchi, E.; Kentish, S. Magnetic separation of serpentinite mining residue as a precursor to mineral carbonation. Int. J. Miner. Process. 2015, 140, 19–25. [Google Scholar] [CrossRef]
- Said, A.; Mattila, O.; Eloneva, S.; Järvinen, M. Enhancement of calcium dissolution from steel slag by ultrasound. Chem. Eng. Process. Process Intensif. 2015, 89, 1–8. [Google Scholar] [CrossRef]
- Eloneva, S.; Teir, S.; Salminen, J.; Fogelholm, C.J.; Zevenhoven, R. Fixation of CO2 by carbonating calcium derived from blast furnace slag. Energy 2008, 33, 1461–1467. [Google Scholar] [CrossRef]
- Park, A.H.A.; Fan, L.S. CO2 mineral sequestration: Physically activated dissolution of serpentine and pH swing process. Chem. Eng. Sci. 2004, 59, 5241–5247. [Google Scholar] [CrossRef]
- Lackner, K.S.; Butt, D.P.; Wendt, C.H. Progress on binding CO2 in mineral substrates. Energy Convers. Manag. 1997, 38, 259–264. [Google Scholar] [CrossRef]
- O’Connor, W.; Dahlin, D.; Nilsen, D. Research status on the sequestration of carbon dioxide by direct aqueous mineral carbonation. In Proceedings of the 18th Annual International Pittsburgh Coal Conference, Newcastle, Australia, 3–7 December 2001; p. 12. [Google Scholar]
- Huijgen, W.J.J.; Ruijg, G.J.; Comans, R.N.J.; Witkamp, G.J. Energy consumption and net CO2 sequestration of aqueous mineral carbonation. Ind. Eng. Chem. Res. 2006, 45, 9184–9194. [Google Scholar] [CrossRef]
- Fagerlund, J.; Nduagu, E.; Zevenhoven, R. Recent developments in the carbonation of serpentinite derived Mg(OH)2 using a pressurized fluidized bed. Energy Procedia 2011, 4, 4993–5000. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Zhao, Y.; Liu, R.; Zheng, C. CO2 sequestration by direct aqueous mineral carbonation under low-medium pressure conditions. J. Chem. Eng. Jpn. 2015, 48, 937–946. [Google Scholar] [CrossRef]
- Bhardwaj, R.; van Ommen, J.R.; Nugteren, H.W.; Geerlings, H. Accelerating natural CO2 mineralization in a fluidized bed. Ind. Eng. Chem. Res. 2016, 55, 2946–2951. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Southam, G. Bioleaching of ultramafic tailings by Acidithiobacillus spp. for CO2 sequestration. Environ. Sci. Technol. 2010, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Kwon, S.; Fan, M.; Dacosta, H.F.M.; Russell, A.G. Factors affecting the direct mineralization of CO2 with olivine. J. Environ. Sci. 2011, 23, 1233–1239. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Ling, T.-C.; Park, A.-H.A.; Chiang, P.-C. An overview: Reaction mechanisms and modelling of CO2 utilization via mineralization. Aerosol Air Qual. Res. 2018, 18, 829–848. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.J.; Comans, R.N.J. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem. Eng. Sci. 2006, 61, 4242–4251. [Google Scholar] [CrossRef]
- Yadav, S.; Mehra, A. Dissolution of steel slags in aqueous media. Environ. Sci. Pollut. Res. 2017, 24, 16305–16315. [Google Scholar] [CrossRef] [PubMed]
- De Windt, L.; Chaurand, P.; Rose, J. Kinetics of steel slag leaching: Batch tests and modeling. Waste Manag. 2011, 31, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Han, D.R.; Namkung, H.; Lee, H.M.; Huh, D.G.; Kim, H.T. CO2 sequestration by aqueous mineral carbonation of limestone in a supercritical reactor. J. Ind. Eng. Chem. 2015, 21, 792–796. [Google Scholar] [CrossRef]
- Tu, M.; Zhao, H.; Lei, Z.; Wang, L.; Chen, D.; Yu, H.; Qi, T. Aqueous carbonation of steel slag: A kinetics study. ISIJ Int. 2015, 55, 2509–2514. [Google Scholar] [CrossRef]
- Revathy, T.D.R.; Palanivelu, K.; Ramachandran, A. Direct mineral carbonation of steelmaking slag for CO2 sequestration at room temperature. Environ. Sci. Pollut. Res. 2016, 23, 7349–7359. [Google Scholar] [CrossRef] [PubMed]
- Ghacham, A.B.; Pasquier, L.C.; Cecchi, E.; Blais, J.F.; Mercier, G. CO2 sequestration by mineral carbonation of steel slags under ambient temperature: Parameters influence, and optimization. Environ. Sci. Pollut. Res. 2016, 23, 17635–17646. [Google Scholar] [CrossRef] [PubMed]
- Ukwattage, N.L.; Ranjith, P.G.; Li, X. Steel-making slag for mineral sequestration of carbon dioxide by accelerated carbonation. Measurement 2017, 97, 15–22. [Google Scholar] [CrossRef]
- Lekakh, S.N.; Rawlins, C.H.; Robertson, D.G.C.; Richards, V.L.; Peaslee, K.D. Kinetics of aqueous leaching and carbonization of steelmaking slag. Metall. Mater. Trans. B 2008, 39, 125–134. [Google Scholar] [CrossRef]
- Bonenfant, D.; Kharoune, L.; Sauve, S.; Hausler, R.; Niquette, P.; Mimeault, M.; Kharoune, M. CO2 sequestration potential of steel slags at ambient pressure and temperature. Ind. Eng. Chem. Res. 2008, 47, 7610–7616. [Google Scholar] [CrossRef]
- Kodama, S.; Nishimoto, T.; Yamamoto, N.; Yogo, K.; Yamada, K. Development of a new pH-swing CO2 mineralization process with a recyclable reaction solution. Energy 2008, 33, 776–784. [Google Scholar] [CrossRef]
- Doucet, F.J. Effective CO2-specific sequestration capacity of steel slags and variability in their leaching behaviour in view of industrial mineral carbonation. Miner. Eng. 2010, 23, 262–269. [Google Scholar] [CrossRef]
- Morales-Flórez, V.; Santos, A.; Lemus, A.; Esquivias, L. Artificial weathering pools of calcium-rich industrial waste for CO2 sequestration. Chem. Eng. J. 2011, 166, 132–137. [Google Scholar] [CrossRef]
- Chang, E.E.; Chen, C.H.; Chen, Y.H.; Pan, S.Y.; Chiang, P.C. Performance evaluation for carbonation of steel-making slags in a slurry reactor. J. Hazard. Mater. 2011, 186, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Accelerated carbonation of steelmaking slags in a high-gravity rotating packed bed. J. Hazard. Mater. 2012, 227–228, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Chiang, P.C.; Chen, Y.H.; Tan, C.S.; Chang, E.E. Ex Situ CO2 capture by carbonation of steelmaking slag coupled with metalworking wastewater in a rotating packed bed. Environ. Sci. Technol. 2013, 47, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.E.; Chiu, A.C.; Pan, S.Y.; Chen, Y.H.; Tan, C.S.; Chiang, P.C. Carbonation of basic oxygen furnace slag with metalworking wastewater in a slurry reactor. Int. J. Greenh. Gas Control 2013, 12, 382–389. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J.; Li, K.; Yan, F.; Chen, X. Performance of steel slag in carbonation–calcination looping for CO2 capture from industrial flue gas. RSC Adv. 2014, 4, 6858–6862. [Google Scholar] [CrossRef]
- Pan, S.Y.; Chiang, P.C.; Chen, Y.H.; Chang, E.E.; da Chen, C.; Shen, A.L. Process intensification of steel slag carbonation via a rotating packed Bed: Reaction kinetics and mass transfer. Energy Procedia 2014, 63, 2255–2260. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; di Gianfilippo, M.; Polettini, A.; Pomi, R.; Stramazzo, A. Thin-film versus slurry-phase carbonation of steel slag: CO2 uptake and effects on mineralogy. J. Hazard. Mater. 2015, 283, 302–313. [Google Scholar] [CrossRef] [PubMed]
- El-Naas, M.H.; el Gamal, M.; Hameedi, S.; Mohamed, A.M.O. CO2 sequestration using accelerated gas-solid carbonation of pre-treated EAF steel-making bag house dust. J. Environ. Manag. 2015, 156, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Liu, H.L.; Chang, E.E.; Kim, H.; Chen, Y.H.; Chiang, P.C. Multiple model approach to evaluation of accelerated carbonation for steelmaking slag in a slurry reactor. Chemosphere 2016, 154, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Polettini, A.; Pomi, R.; Stramazzo, A. CO2 sequestration through aqueous accelerated carbonation of BOF slag: A factorial study of parameters effects. J. Environ. Manag. 2016, 167, 185–195. [Google Scholar] [CrossRef] [PubMed]
| Reservoir Type | Estimated Range of Storage Capacity (GtCO2) |
|---|---|
| Mineral carbonation | Very large (>10,000) a |
| Saline aquifers | 1000–10,000 |
| Oil and gas fields | 675–900 b |
| Coal beds | 3–200 |
| Mineral/Formula | U (mass CO2/mass mineral) |
|---|---|
| Serpentine (Mg3Si2O5(OH)4) | 0.40 |
| Serpentine ((Mg,Fe)3Si2O5(OH)4) | 0.48 |
| Wollastonite (CaSiO3) | 0.36 |
| Olivine (Fe2SiO4) | 0.36 |
| Olivine (Mg2SiO4) | 0.56 |
| Alkaline Solid Waste | Production Per Year (t) | CO2 Emissions Per Year (t) a | Examples | Composition (wt.%) | Ref. |
|---|---|---|---|---|---|
| Steel slags | 315–420 a | 171 a | Basic Oxygen Furnace (BOF) Electric Arc Furnace (EAF) Blast Furnace Slag (BFS) | Ca: 45–55, Mg: 2–5 Ca: 40–46, Mg: 1–6.5 Ca: 35–43, Mg: 4–7 | [17,22,23,24] |
| Waste cement | 1100 a | 62 a | Cement kiln dust | Ca: 35–50, Mg: 0–2 | [25] |
| Fly ash | 600 a | 12,000 a | Coal fly ash Oil shale ash | Ca: 35–53, Mg: 0–3 Ca: 10–25 | [26,27,28,29,30,31,32,33,34] |
| Air pollution control (APC) residues | 1.2 b | N/A | Waste incineration plant residues | Ca: 35–38, Mg: 0–1 | [35,36] |
| Red mud | 1.25 a | 3.6 a | Red gypsum | Ca: 1–6, Mg: 1–5 | [37] |
| Pre-Treatment Method | Description | Advantages/Disadvantages | Ref. |
|---|---|---|---|
| Grinding (reduction in size) | Rock minerals undergo grinding process to reduce particle size of the minerals to less than 63 μm (smaller particles size, more surface area available) | Advantages:
Disadvantages:
| [43,44] |
| Heat (thermal) activation | Naturally occurring minerals contains water molecules bounded to its chemical structure i.e., up to 13% of serpentine is water. By heating the mineral up to 600 °C water is removed and more mineral is available for carbonation | Advantages:
Disadvantages:
| [45,46] |
| Surface activation | Increasing the mineral surface area by treating it with steam or extraction acids | Advantages:
Disadvantages:
| [47] |
| Magnetic separation | The presence of iron element in mineral rocks can decrease the carbonation efficiency due to the formation iron oxides layers on the surface of the mineral. Separating iron compounds magnetically before carbonation can solve this issue | Advantages:
Disadvantages:
| [48] |
| Sonication (ultrasound) | Ultrasound waves are used with extraction acid in conjunction to enhance the rate of mineral dissolution in the acid. The waves forms bubbles in the liquid that can enhance the mass transfer and the mineral dissolution rate. | Advantages:
Disadvantages:
| [49] |
| Material | Carbonation Method | Composition (wt.%) | Maximum CO2 Uptake % | Reactor Type | Conditions/Remarks | Ref. |
|---|---|---|---|---|---|---|
| Serpentine | Direct aqueous carbonation | MgO: 40 | 30% | Batch | Temperature: 300 °C CO2 Partial pressure: 335.4 atm | [52] |
| Olivine | Direct aqueous carbonation | CaO: 0.07 SiO2: 41.4 MgO: 49.7 Al2O3: 0.21 Fe2O3: 2.7 | 91% | CSTR | Temperature: 155 °C Process pressure: 185 atm | [53] |
| Serpentine | Indirect using pH swing | - | 42% | Batch | Temperature: 70 °C Process pressure: 1 atm | [51] |
| Serpentine | Direct aqueous carbonation | CaO: 0.15 SiO2: 39.5 MgO: 38.7 Al2O3: 0.35 Fe2O3: 4.86 | 7% | Batch | Temperature: 25 °C Process pressure: 125 atm | [46] |
| Wollastonite CaSiO3 | Direct aqueous carbonation | - | 69% | Batch | Temperature: 200 °C Process pressure: 20 bar Particle size: <38 μm L/S: 2 | [54] |
| Serpentinite | Dry carbonation | - | 50% | Fluidized bed | Temperature: 500 °C Process pressure: 20 bar | [55] |
| Wollastonite CaSiO3 | Direct aqueous carbonation | - | 83.5% | Batch | Temperature: 150 °C Process pressure: 40 bar Particle size: <30 μm | [56] |
| Serpentinite | Dry carbonation | MgO: 35.3 | 0.0075 gCO2/g serpentinite | Fluidized bed | Temperature: 90 °C Moist CO2 Process pressure: 1 bar | [57] |
| Indirect Carbonation | Description/Reactions | Ref. |
|---|---|---|
| Acid extraction | Numerous acids are investigated in the literature as an extraction agent. Examples of these acids are: acetic acid, nitric acid, formic acid and hydrochloric acid. Acid extraction is achieved through multiple routes. The most straightforward extraction method includes mixing the mineral and the extracting agent in a stirred reactor or vessel at a certain temperature and pressure to extract the minerals, followed by carbonation process of the extracted mineral according to the following reactions: | [46] |
| Molten salt | The molten salt process is aimed to reduce energy requirements resulting from HCl extraction. It shares many similarities with HCl extraction except the molten salt, , is being used as an extracting agent. | [12] |
| Ammonia | Using as extraction agent according to the following reaction: | [51] |
| Caustic soda | Using sodium hydroxide as an extracting agent: | [12] |
| Bioleaching | Bioleaching is defined as the process of using bacteria to extract minerals from natural rocks, can be applied for the extraction of Ca & Mg oxides from silicates. | [58] |
| Mineral | Maximum Carbonation Temperature (°C) |
|---|---|
| Olivine (Mg2SiO4) | 241 |
| Wollastonite (CaSiO3) | 280 |
| Calcium oxide (CaO)/Calcium hydroxide (Ca(OH)2) | 887 |
| Magnesium oxide (MgO)/Magnesium hydroxide (Mg(OH)2) | 406 |
| Material | Carbonation Method | Composition (wt.%) | Maximum CO2 Uptake | Reactor Type | Conditions/Remarks | Year | Ref |
|---|---|---|---|---|---|---|---|
| Steel Slag | Direct aqueous carbonation | Fe2O3: 35.5 CaO: 31.7 SiO2: 9.1 MgO: 6.0 | 74% of Ca content | Batch | CO2 pressure: 19 bar Temperature:100 °C Particle size: <38 Reaction time: 30 min | 2005 | [21] |
| BSF | Indirect aqueous carbonation: (extraction) using acetic acid | CaO: 40.6 SiO2: 34.1 MgO: 10.7 Al2O3: 9.4 | 0.23 g CO2/g CaO | Stirred batch | 3.6 liters of Acetic acid was used to produce carbonates by leaching | 2008 | [50] |
| Steel slag | Indirect carbonation | CaO: 32.1 SiO2: 19.4 MgO: 9.4 Al2O3: 8.6 Fe2O3: 26.4 | 30% | Batch | Slag was leached in deionized water Ambient temperature and pressure. Increasing the leachate temperature from 60 °C enhanced the Ca-leaching | 2008 | [70] |
| LFS | Indirect aqueous carbonation | CaO: 58.1 SiO2: 26.4 MgO: 6.2 Al2O3: 4.6 FeO: 4.30 | 0.247 g CO2/g CaO | Stirred batch | L/S: 10 Temperature: 20 °C Process pressure: 1 bar CO2: 15 vol.% CO2 flowrate: 5 mL/min Rotational speed: 200 rpm | 2008 | [71] |
| Steel slag | Indirect aqueous using pH swing using NH4Cl | CaO: 44.5 SiO2: 9.28 MgO:7.6 Fe2O3: 19.1 Al2O3: 2.3 | 70% of Ca content | Stirred batch | CO2: 13 vol.% Temperature: 80 °C Pressure: 1 atm Rotational speed: 300 rpm | 2008 | [72] |
| APC | Direct aqueous carbonation | CaO: 35 SiO2: 1.01 Al2O3: 0.21 MgO: 0.84 | 0.25 g CO2/g CaO | Batch | CO2: 100 vol.% L/S: 0.2 Temperature: 30 °C Process pressure: 3 bar | 2009 | [36] |
| EAF Slag | Indirect aqueous carbonation (extraction) using nitric acid | CaO: 41.6 SiO2: 18.8 MgO: 8.0 Al2O3: 3.4 | 0.359 g CO2/g CaO & MgO | Batch | L/S: 0.2 Temperature: 22 °C | 2010 | [73] |
| Industrial wastes from acetylene production | Carbonation by atmospheric CO2 | CaO: 41.6 SiO2: 18.8 | 0.476 g CO2/g waste | N/A | L/S: 0.33 | 2010 | [74] |
| BFS | Indirect carbonation (extraction) using nitric acid | CaO: 51.1 SiO2: 11.5 MgO: 4.2 Al2O3: 1.5 Fe2O3: 24.1 | 0.27 g CO/g CaO | Slurry | L/S: 10 Temperature: 70 °C CO2 Partial pressure: 101.3 kPa CO2 flowrate: 0.1 L/min Particles size: <44 | 2011 | [75] |
| Steel slag | Direct aqueous carbonation | CaO: 38.84 MgO: 10.36 Al2O3: 3.91 Fe2O3: 32.8 | 93% based on CaO content | high-gravity rotating packed bed | Rotational speed: 750 rpm Temperature: 65 °C Process pressure: 1 bar L/S: 20 | 2012 | [76] |
| BOFS | Direct aqueous carbonation | CaO: 36.37 MgO: 7 Al2O3: 1.89 Fe2O3: 10.36 | 99% based on CaO content | Rotating packed bed | Rotational speed: 1000 rpm Temperature: 25 °C L/S: 20 mg/L Process pressure: 1 bar CO2: 30 vol.% CO2 flowrate: 1.8 L/min | 2013 | [77] |
| BOFS | Direct aqueous carbonation | CaO: 41.15 SiO2: 10.59 MgO: 9.21 Al2O3: 2.24 Fe2O3: 24.41 MnO: 2.75 | 89.4% | Slurry reactor | Temperature: 25 °C L/S: 20 CO2 pressure: 1 bar CO2 flowrate: 1 L/min Slurry volume: 350 mL | 2013 | [78] |
| BSF | Indirect aqueous carbonation (extraction) using EDTA | CaO: 47.15 SiO2: 31.08 MgO: 3.34 Al2O3: 13.81 Fe2O3: 0.378 MnO: 0.71 | 0.09 g CO2/g slag | Batch | Temperature: 25 °C Process pressure: 1 bar CO2 flowrate: 1.5 L/min | 2013 | [17] |
| Steel slag | Dry carbonation | - | 0.0449 g CO2/g slag | Batch | Temperature: 600 °C CO2%: 10 vol.% CO2 flowrate: 1.5 L/min | 2014 | [79] |
| BOFS | Direct aqueous carbonation | CaO: 43 SiO2: 12.9 Fe2O3: 28.7 | 0.16 g CO2/g CaO | rotating packed bed | Rotational speed: 541 rpm Temperature: 25 °C L/S: 10 Process pressure: 1 bar | 2014 | [80] |
| Steel slag | Indirect aqueous carbonation (extraction) using NH4SO4 | CaO: 38.98 SiO2: 12.13 MgO: 8.96 Al2O3: 2.74 Fe2O3: 22.53 MnO: 3.58 | 74% | Batch | Temperature: 65 °C L/S: 15 g/L Process pressure: 1 bar | 2014 | [24] |
| Steel slag | Direct aqueous carbonation | CaO: 41.3 SiO2: 20.9 MgO: 6.2 Al2O3: 2.3 Fe2O3: 20.7 | 0.264 g CO2/g CaO | Batch | Temperature: 60 °C L/S: 10 CO2 flowrate: 0.6 L/min Process pressure: 10 bar CO2%: 100 vol.% | 2015 | [66] |
| BOFS | Direct aqueous carbonation | CaO: 23 SiO2: 6 MgO: 3.8 Al2O3: 1.1 Fe2O3: 25 | 0.403 g CO2/g CaO | Batch | Temperature: 100 °C L/S: 5 L/kg CO2: 100 vol.% Process pressure: 10 bar Particle size: <150 μm | 2015 | [81] |
| EAFS | Dry carbonation | CaO: 42.8 SiO2: 4.49 MgO: 4.96 Al2O3: 0.28 Fe2O3: 42.8 | 0.657 g/g CaO | Slurry | 2015 | [82] | |
| BOFS | Direct aqueous carbonation | CaO: 51.1 SiO2: 11.2 MgO: 4.2 Al2O3: 1.2 Fe2O3: 24 | 57% | Batch | Temperature: 50 °C L/S: 20 mL/g CO2 flowrate: 0.1 L/min Process pressure: 1 bar | 2016 | [83] |
| Steel slag | Dry carbonation | CaO: 28.27 SiO2: 15.4 MgO: 7.88 Al2O3: 1.01 Fe2O3: 24.25 | 0.011 g CO2/g slag | Batch | Temperature: 50 °C CO2: 100 vol.% | 2016 | [67] |
| EAF | - | CaO: 33.19 SiO2: 16.71 MgO: 9.43 Al2O3: 6.73 Fe2O3: 38.19 | 0.052 g CO2/g slag | Batch | Temperature: 25 °C Process pressure: 10.68 bar L/S: 10 | 2016 | [68] |
| BOF | Direct aqueous carbonation | CaO: 31 SiO2: 5.1 MgO: 7.5 Fe2O3: 27 | 0.536 g CO2/g slag | Batch | Temperature: 83.7 °C Process pressure: 5.9 bar L/S: 5 L/kg CO2: 60.6 vol.% | 2016 | [84] |
| BFS | Direct aqueous carbonation | CaO: 42.5 SiO2: 31.9 MgO: 4.81 Al2O3: 13 Fe2O3: 0.34 | 0.0295 g CO2/g slag | Batch | Temperature: 50 °C Process pressure: 5 bar L/S: 3 CO2: 100 vol.% | 2017 | [69] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.H.; El-Naas, M.H.; Benamor, A.; Al-Sobhi, S.S.; Zhang, Z. Carbon Mineralization by Reaction with Steel-Making Waste: A Review. Processes 2019, 7, 115. https://doi.org/10.3390/pr7020115
Ibrahim MH, El-Naas MH, Benamor A, Al-Sobhi SS, Zhang Z. Carbon Mineralization by Reaction with Steel-Making Waste: A Review. Processes. 2019; 7(2):115. https://doi.org/10.3390/pr7020115
Chicago/Turabian StyleIbrahim, Mohamed H., Muftah H. El-Naas, Abdelbaki Benamor, Saad S. Al-Sobhi, and Zhien Zhang. 2019. "Carbon Mineralization by Reaction with Steel-Making Waste: A Review" Processes 7, no. 2: 115. https://doi.org/10.3390/pr7020115
APA StyleIbrahim, M. H., El-Naas, M. H., Benamor, A., Al-Sobhi, S. S., & Zhang, Z. (2019). Carbon Mineralization by Reaction with Steel-Making Waste: A Review. Processes, 7(2), 115. https://doi.org/10.3390/pr7020115









