Application of VES Acid System on Carbonate Rocks with Uninvaded Matrix for Acid Etching and Fracture Propagation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Apparatus
2.2. Experiment Design and Methods
2.2.1. Preparation of Core Samples and Acid Fluid
2.2.2. Rheological Investigation
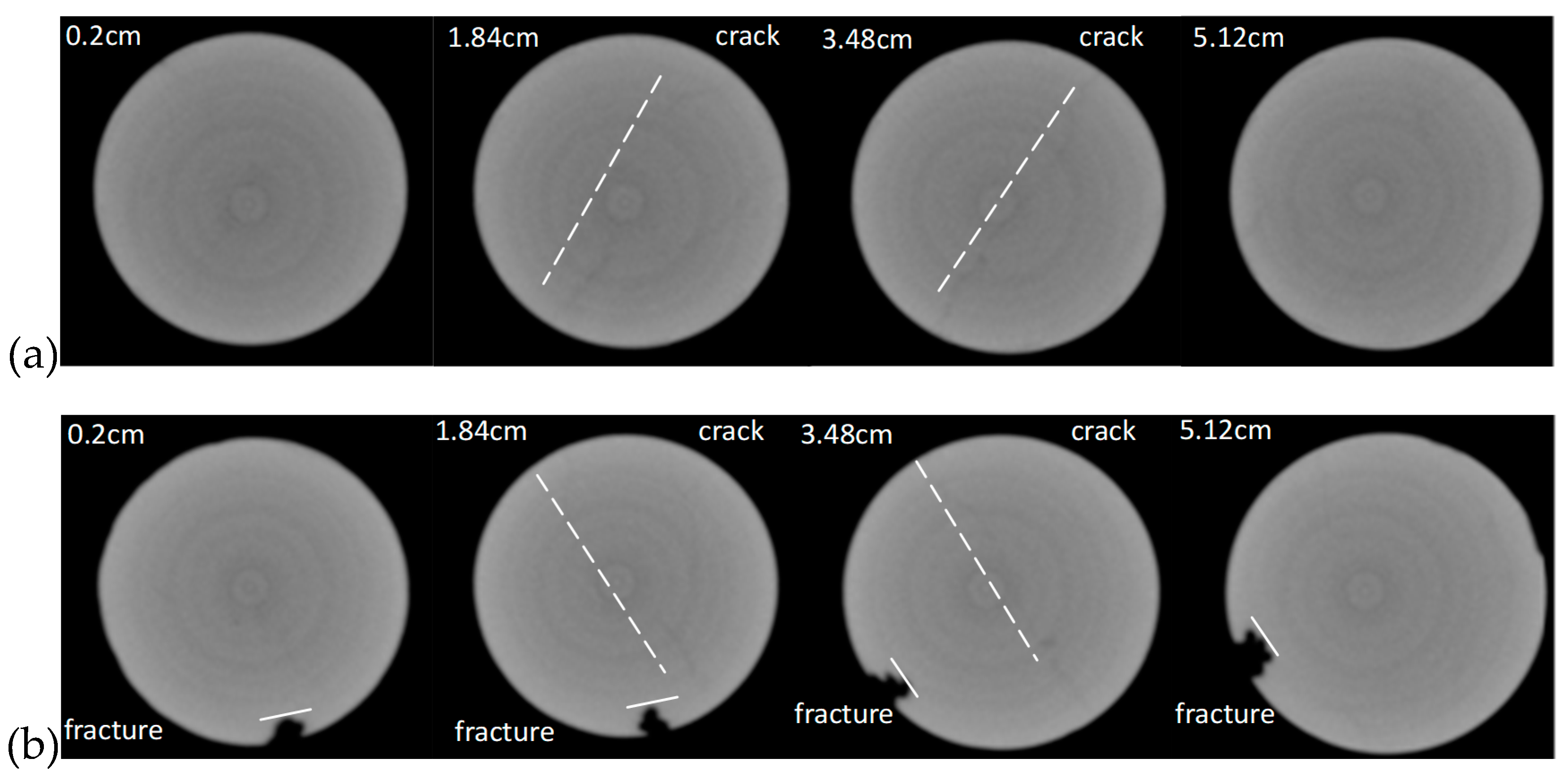
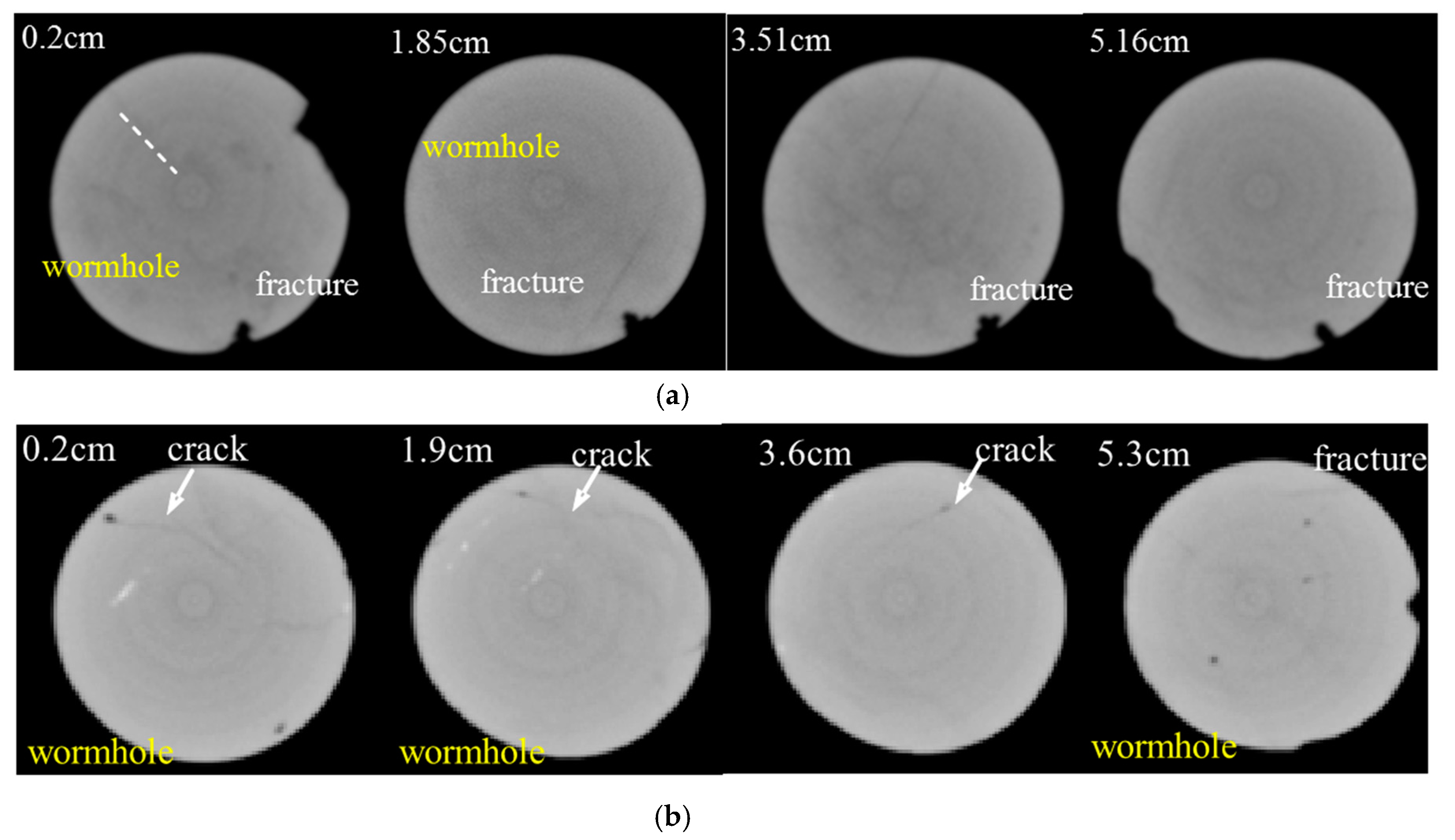
2.2.3. Core CT Analysis
2.2.4. Core Flooding Experiments
3. Results and Discussion
3.1. Effect of VES and Ca2+ Concentration on Fluid Viscosity
3.2. Effect of VES Concentration on the Dissolution Quality
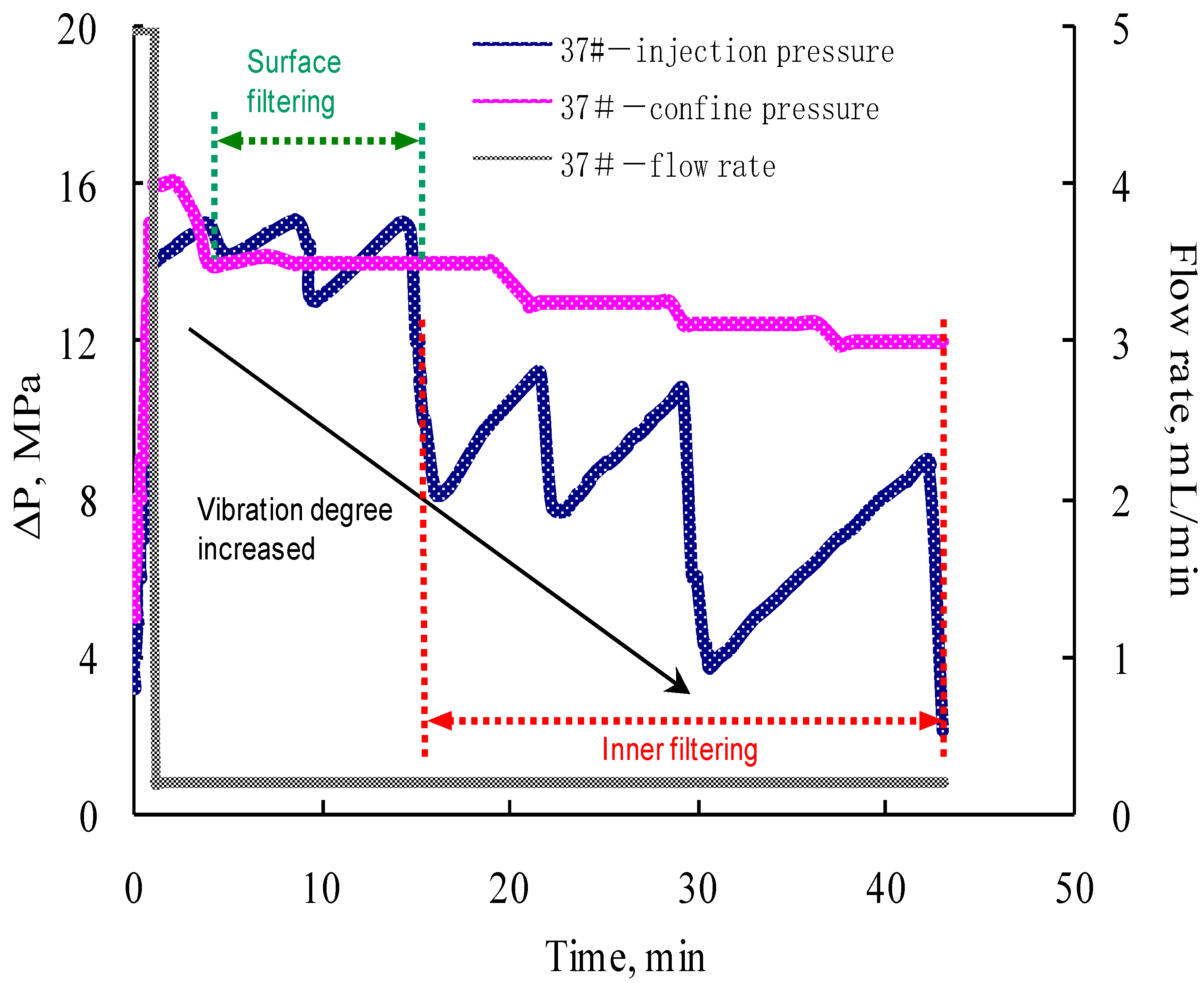
3.3. Single Core Flooding Experiments
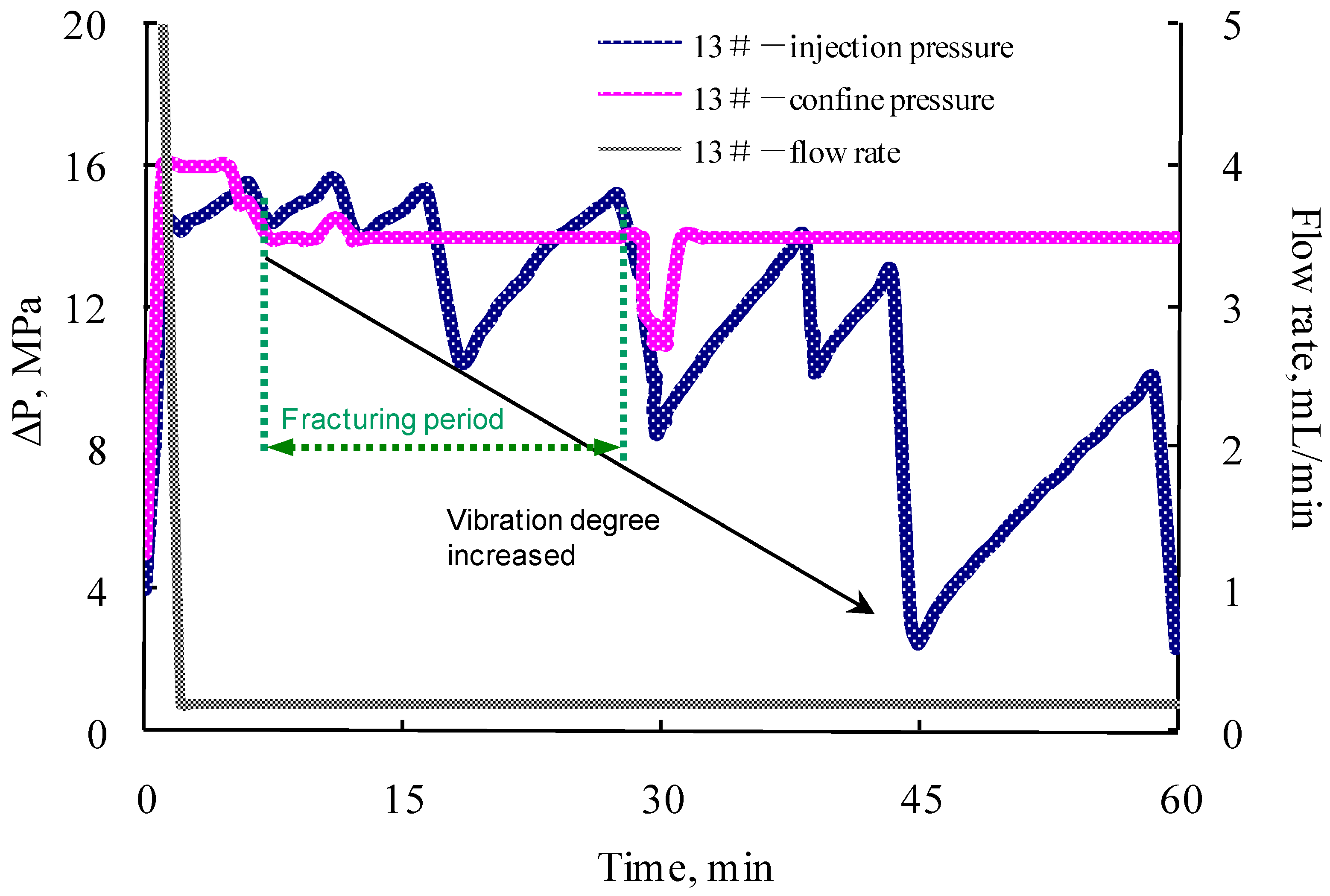
3.4. Parallel Core Flooding Experiment
3.5. Mechanism of VES on Carbonate Rocks with Uninvaded Matrix
4. Conclusions and Future Work
- (1)
- As the concentration of VES and CaCl2 increases, the viscosity of the system increases. The generated Ca2+ during the reaction contributes to the association of VES which results in increasing the viscosity of the solution and reducing the mass transfer rate of H+.
- (2)
- Foam in-situ produced during the etching process is the major contribution to the fluid viscosity enhancement. The permeability of the fracture, formed on the surface of the core, is more sensitive to the confining pressure.
- (3)
- For carbonate rocks with the uninvaded matrix, an appropriate increase in the rate of filtration loss will help improve the effect of acid fracturing. While for cores with high permeability, the extremely high fluid loss will lead to acid fracturing failure.
- (4)
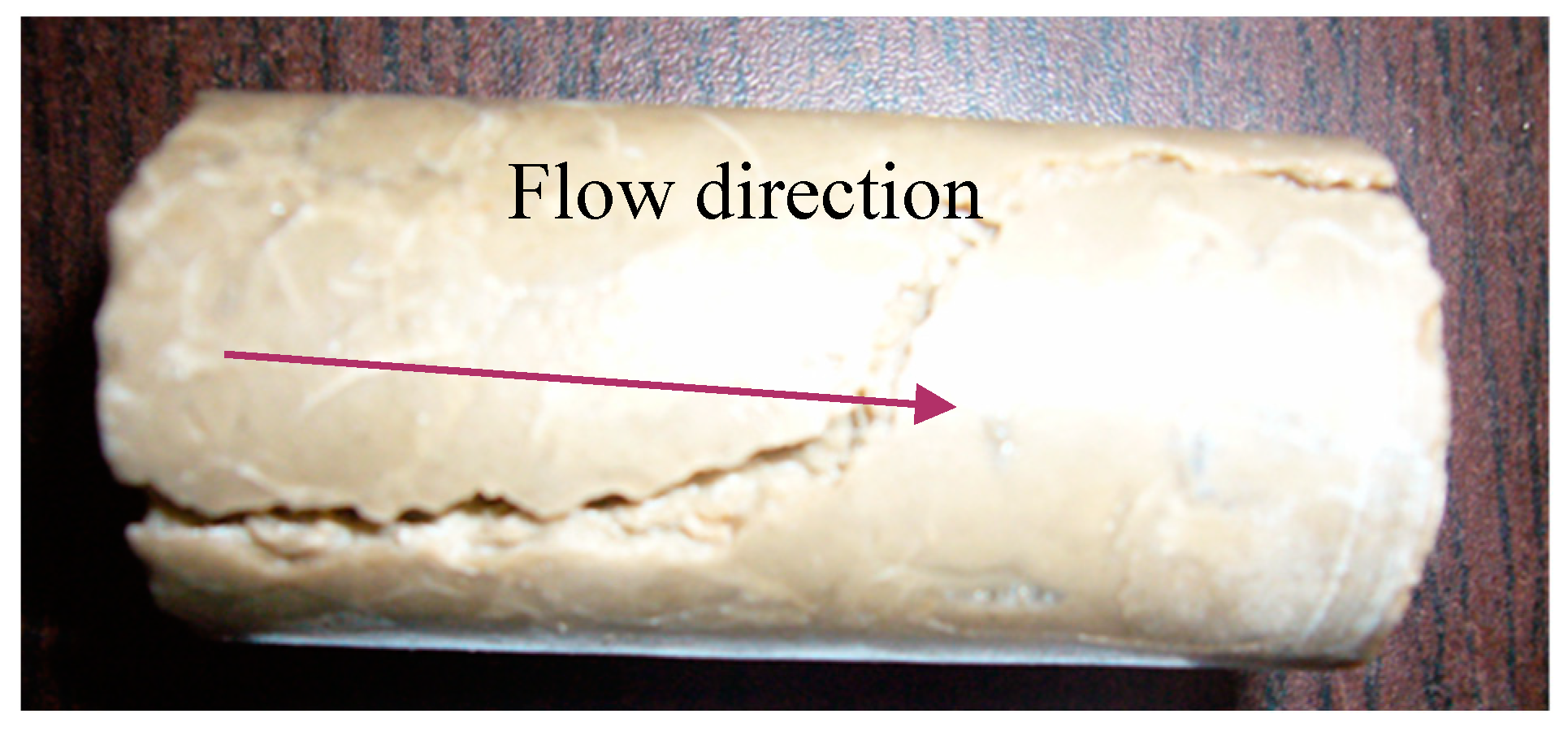
- The formation and propagation of the VES-foam system change the injection pressure and varies periodically when the confining pressure is set lower than the injection pressure. Fractures are created on the surface and formed after the acid breakthrough.
- (5)
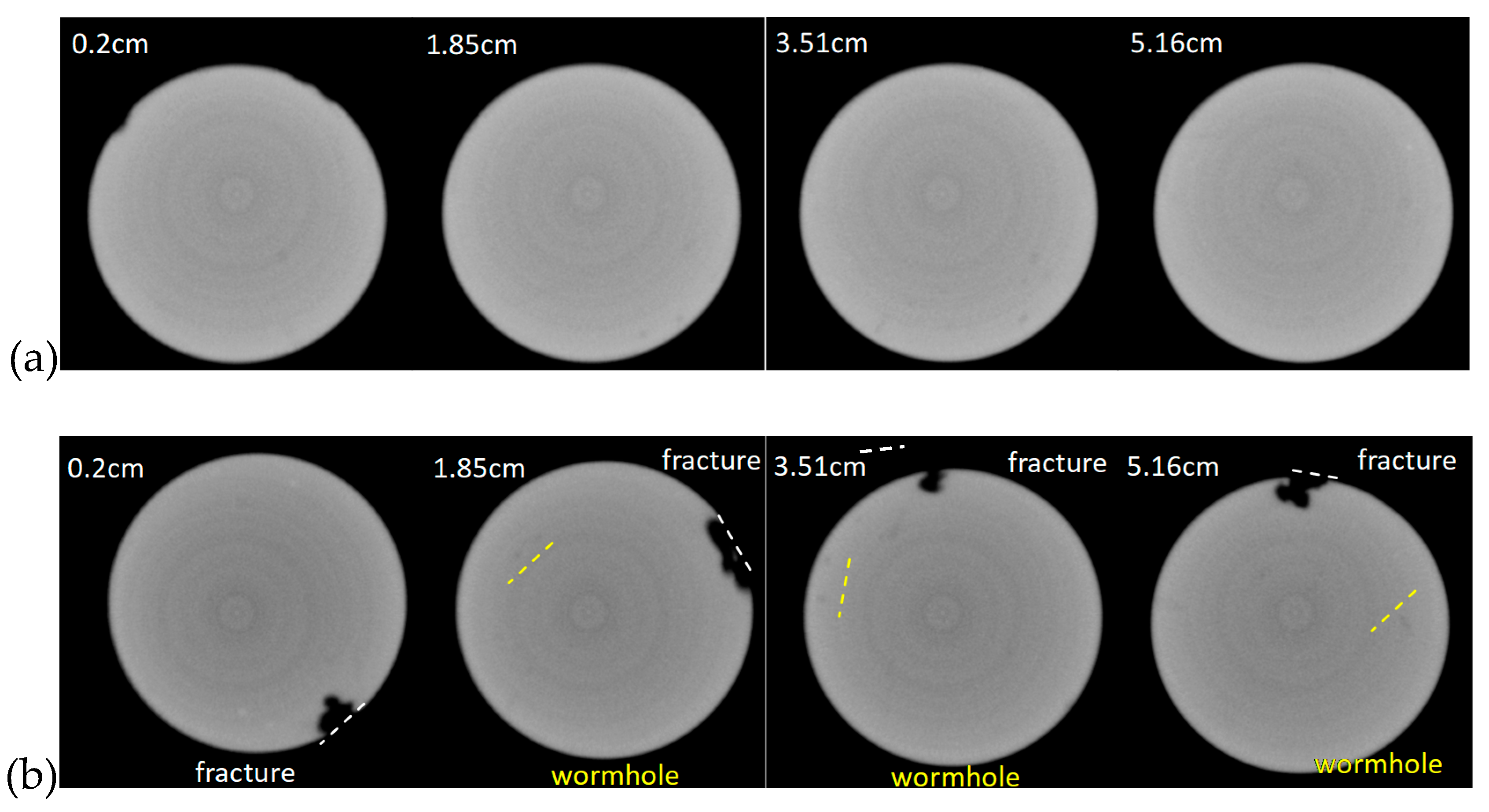
- Wormholes are preferably produced around the pre-developed micro-cracks, which depends on the original characteristics of the fracture distribution of the reservoir, and have a major control on the treatment effect, causing the uncertainty of the treatment effect to be noticeably increased.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hull, K.L.; Sayed, M.; Al-Muntasheri, G.A. Recent Advances in Viscoelastic Surfactants for Improved Production from Hydrocarbon Reservoirs. SPE J. 2016, 21, 1–340. [Google Scholar] [CrossRef]
- Chang, F.F.; Love, T.; Affeld, C.J.; Blevins, J.B., III; Thomas, R.L.; Fu, D.K. January. Case study of a novel acid-diversion technique in carbonate reservoirs. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: Houston, TX, USA, 1999. [Google Scholar]
- Lungwitz, B.R.; Fredd, C.N.; Brady, M.E.; Miller, M.J.; Ali, S.A.; Hughes, K.N. Diversion and Cleanup Studies of Viscoelastic Surfactant-Based Self-Diverting Acid; Society of Petroleum Engineers: Houston, TX, USA, 2007. [Google Scholar] [CrossRef]
- Jardim Neto, A.T.; Silva, C.A.M.; Torres, R.S.; Farias, R.L.; Prata, F.G.M.; Souza, L.A.M.; Sandes, E.F. Self-diverting acid for effective carbonate stimulation offshore Brazil: A successful history. In SPE European Formation Damage Conference & Exhibition; Society of Petroleum Engineers: Houston, TX, USA, 2013. [Google Scholar]
- Alleman, D.; Qi, Q.; Keck, R. The development and successful field use of viscoelastic surfactant-based diverting agents for acid stimulation. In International Symposium on Oilfield Chemistry; Society of Petroleum Engineers: Houston, TX, USA, 2003. [Google Scholar]
- Gomaa, A.M.; Wang, G.; Nasr-El-Din, H.A. An Experimental Study of a New VES Acid System: Considering the Impact of CO2 Solubility; Society of Petroleum Engineers: Houston, TX, USA, 2011. [Google Scholar] [CrossRef]
- Wang, G.; Gomaa, A.M.; Nasr-El-Din, H.A. Effect of Initial HCl Concentration on the Performance of New VES Acid System; Society of Petroleum Engineers: Houston, TX, USA, 2011. [Google Scholar] [CrossRef]
- Mou, J.; Liu, M.; Zheng, K.; Zhang, S. Diversion Conditions for Viscoelastic-Surfactant-Based Self-Diversion Acid in Carbonate Acidizing; Society of Petroleum Engineers: Houston, TX, USA, 2015. [Google Scholar] [CrossRef]
- Tan, X.; Weng, X.; Cohen, C.-E. An Improved Wormhole Propagation Model with a Field Example; Society of Petroleum Engineers: Houston, TX, USA, 2016. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Bazargan, M.; Rostami, B.; Ayatollahi, S. Modeling of Wormhole Propagation in Carbonate Rocks by Use of In-Situ-Gelled Acids; Society of Petroleum Engineers: Houston, TX, USA, 2017. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Kalia, N.; Balakotaiah, V. Modeling, analysis and simulation of wormhole formation in carbonate rocks with in situ cross-linked acids. Chem. Eng. Sci. 2013, 90, 179–199. [Google Scholar] [CrossRef]
- Nasr-El-Din, H.A.; Al-Ghamdi, A.H.; Al-Qahtani, A.A.; Samuel, M.M. Impact of acid additives on the rheological properties of a viscoelastic surfactant and their influence on field application. SPE J. 2008, 13, 35–47. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Influence of transport and reaction on wormhole formation in porous media. AIChE J. 1998, 44, 1933–1949. [Google Scholar] [CrossRef]
- Valko, P.; Economides, M.J. Volume equalized constitutive equations for foamed polymer solutions. J. Rheol. 1992, 36, 1033–1055. [Google Scholar] [CrossRef]
- Bernadiner, M.G.; Thompson, K.E.; Fogler, H.S. Effect of Foams Used During Carbonate Acidizing. SPE Prod. Eng. 1992, 7, 350. [Google Scholar] [CrossRef]







| Core-Flow Experiments | Core Sample | Length (cm) | Permeability (×10−3 μm2) | VES Concentration (%) | HCl Concentration (%) |
|---|---|---|---|---|---|
| Single | 37 | 5.334 | 0.002 | 1.5 | 27 |
| 13 | 5.325 | 0.020 | 1.5 | 27 | |
| Parallel | 14 | 5.365 | 0.025 | 1.5 | 18.2 |
| 17 | 5.498 | 0.063 | 1.5 | 18.2 |
| VES Concentration, %wt | Fitting Results (Constant Shear Rate, 100 s−1) |
|---|---|
| 1 | y = 0.891e−0.0376x, R2 = 0.6201 |
| 2 | y = 3.5434e−0.0763x, R2 = 0.8244 |
| 4 | y = 9.1183e−0.0987x, R2 = 0.9034 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Luo, W.; Jia, X.; Fang, H.; Wang, H.; Yu, S. Application of VES Acid System on Carbonate Rocks with Uninvaded Matrix for Acid Etching and Fracture Propagation. Processes 2019, 7, 159. https://doi.org/10.3390/pr7030159
Wu Y, Luo W, Jia X, Fang H, Wang H, Yu S. Application of VES Acid System on Carbonate Rocks with Uninvaded Matrix for Acid Etching and Fracture Propagation. Processes. 2019; 7(3):159. https://doi.org/10.3390/pr7030159
Chicago/Turabian StyleWu, Yahong, Weiwei Luo, Xunan Jia, Haoqing Fang, Honggang Wang, and Shuai Yu. 2019. "Application of VES Acid System on Carbonate Rocks with Uninvaded Matrix for Acid Etching and Fracture Propagation" Processes 7, no. 3: 159. https://doi.org/10.3390/pr7030159
APA StyleWu, Y., Luo, W., Jia, X., Fang, H., Wang, H., & Yu, S. (2019). Application of VES Acid System on Carbonate Rocks with Uninvaded Matrix for Acid Etching and Fracture Propagation. Processes, 7(3), 159. https://doi.org/10.3390/pr7030159




