Impact of Sourdoughs, Enzymes, and Their Combinations on Gluten-Based Bread Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Enzymes
2.3. Determination of Pasting Properties of Flours
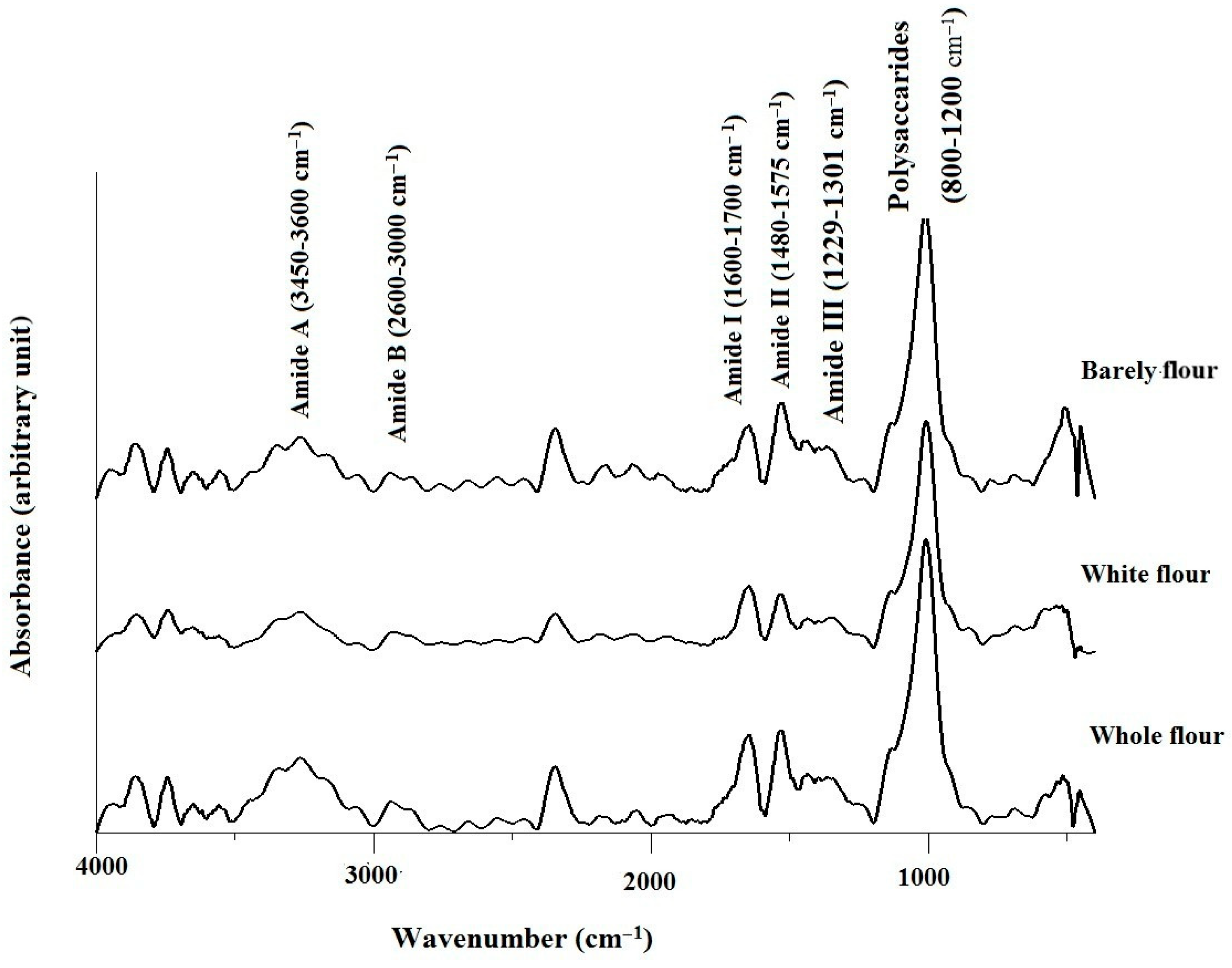
2.4. Fourier Transformed Infrared (FT-IR) Analysis of Flours
2.5. Sourdough Preparation, Incorporation Levels, and Shelf Life Evaluation
2.5.1. Sourdough Preparation Method
2.5.2. Incorporation Levels
2.5.3. Shelf Life Monitoring
- CB: Control Bread (made with yeast),
- GBBS1: gluten-based bread with S1 (white wheat flour sourdough),
- GBBS2: gluten-based bread with S2 (wholemeal wheat flour sourdough),
- GBBS3: gluten-based bread with S3 (barley flour sourdough).
- Textural properties, specific volume, and moisture content at intervals of 1 h, 24 h, 48 h, and 72 h post-baking were determined. The samples were stored in airtight plastic bags at room temperature (20 °C ± 2 °C).
2.6. Bread Making Procedure
2.7. Enzyme Incorporation into Optimized Sourdough Bread
- Individual Enzyme Incorporation:
- -
- Laccase (lacc): 10 ppm and 20 ppm,
- -
- Lipase (lipa): 30 ppm and 50 ppm,
- -
- Hemicellulase (hemi): 5 ppm and 10 ppm;
- Combination Enzyme Incorporation:
- -
- Enzyme combination (EnzComb): Laccase 10 ppm + Lipase 30 ppm + Hemicellulase 5 ppm.
2.8. Bread Quality Evaluation
2.8.1. Specific Volume
2.8.2. Moisture Content
2.8.3. Texture Profile Analysis (TPA)
2.9. Statistical Analysis
3. Results and Discussion
3.1. Pasting Properties of Flours
3.2. Structural Properties of Flours and Protein Secondary Structure Analysis
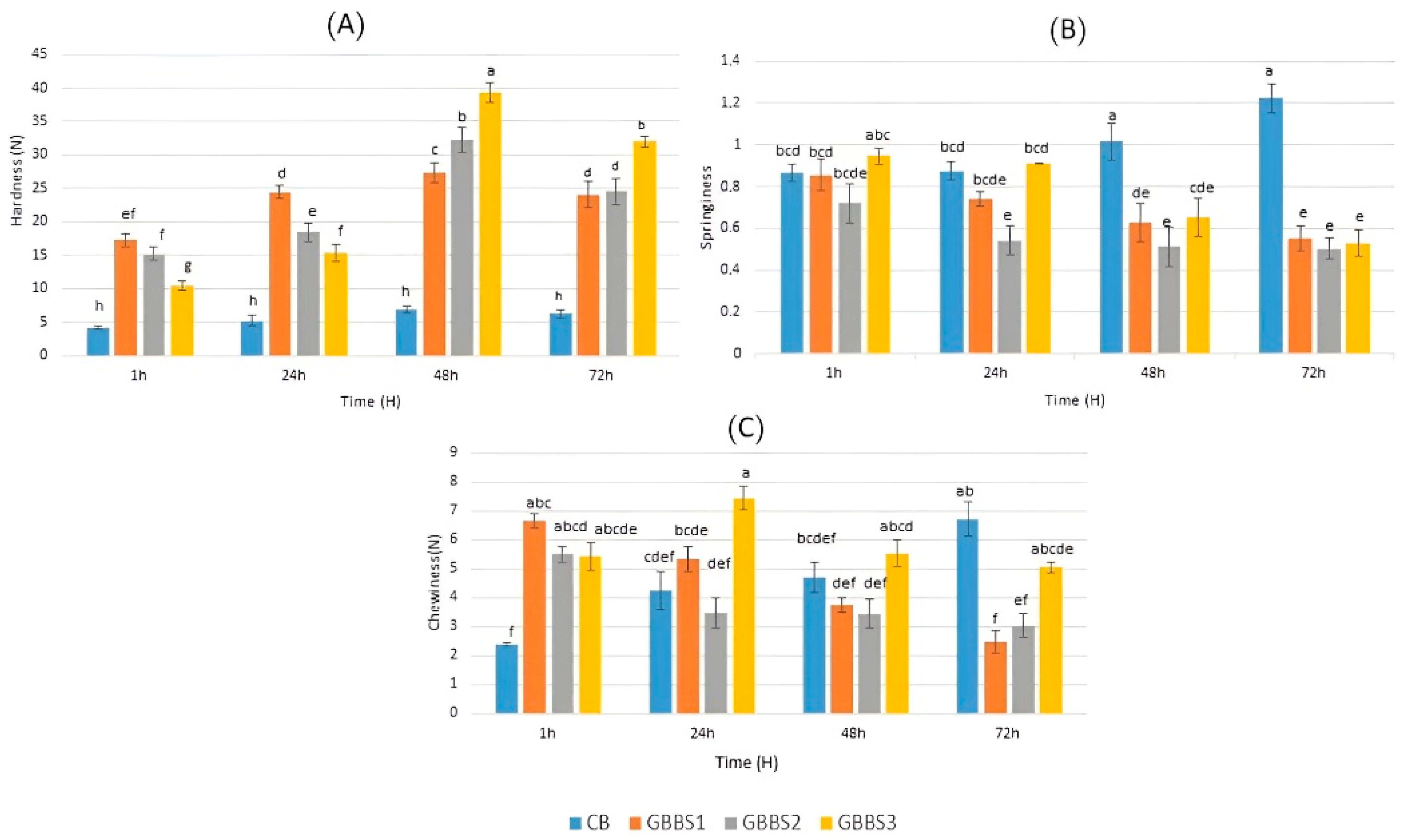
3.3. Effect of Sourdough Incorporation Level on Physical Properties of Breads
3.4. Shelf Life and Technological Quality Evaluation of Selected Sourdough Breads
3.5. Enzymes’ Effect on the Textural Properties of Sourdough Breads
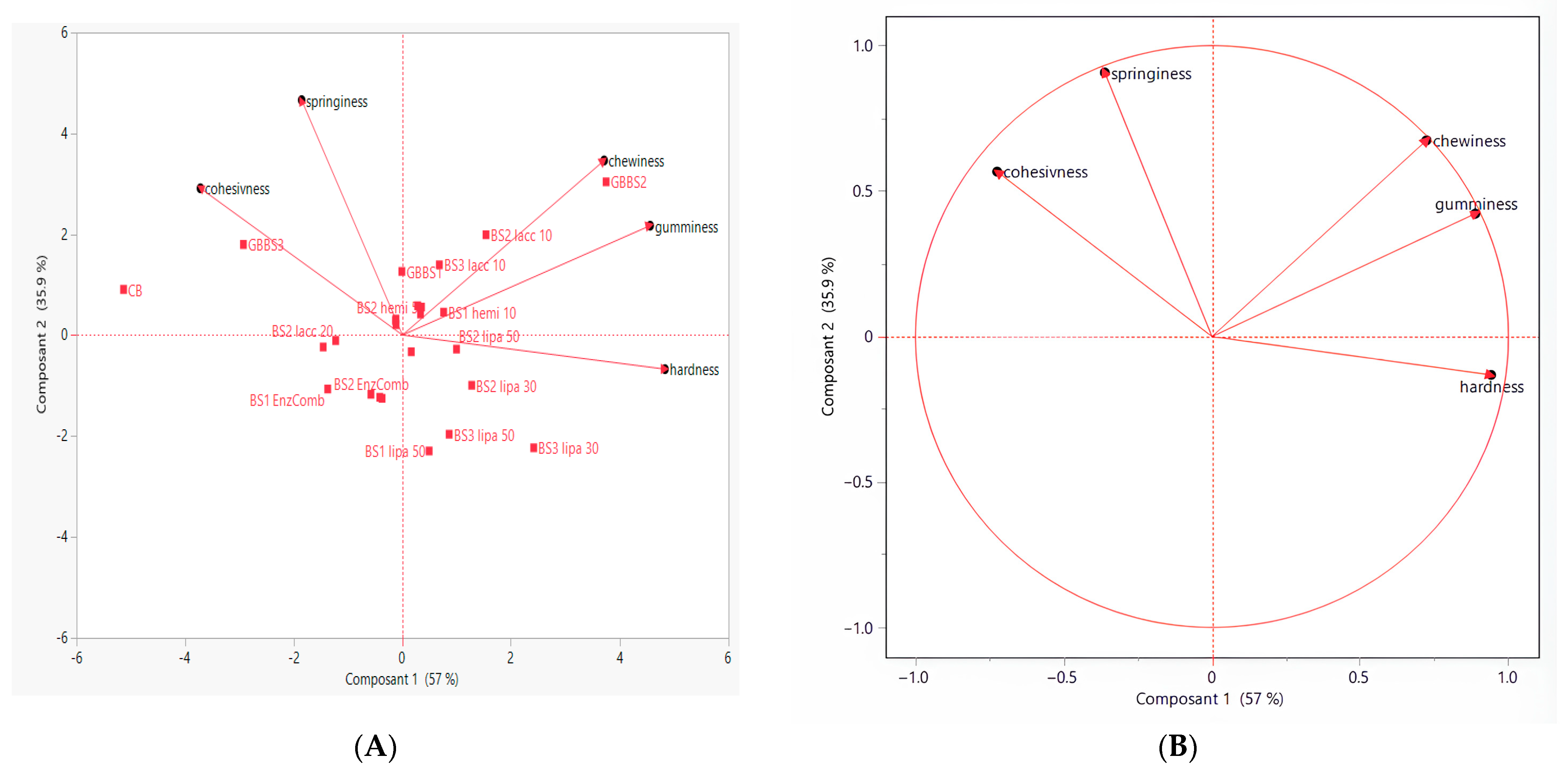
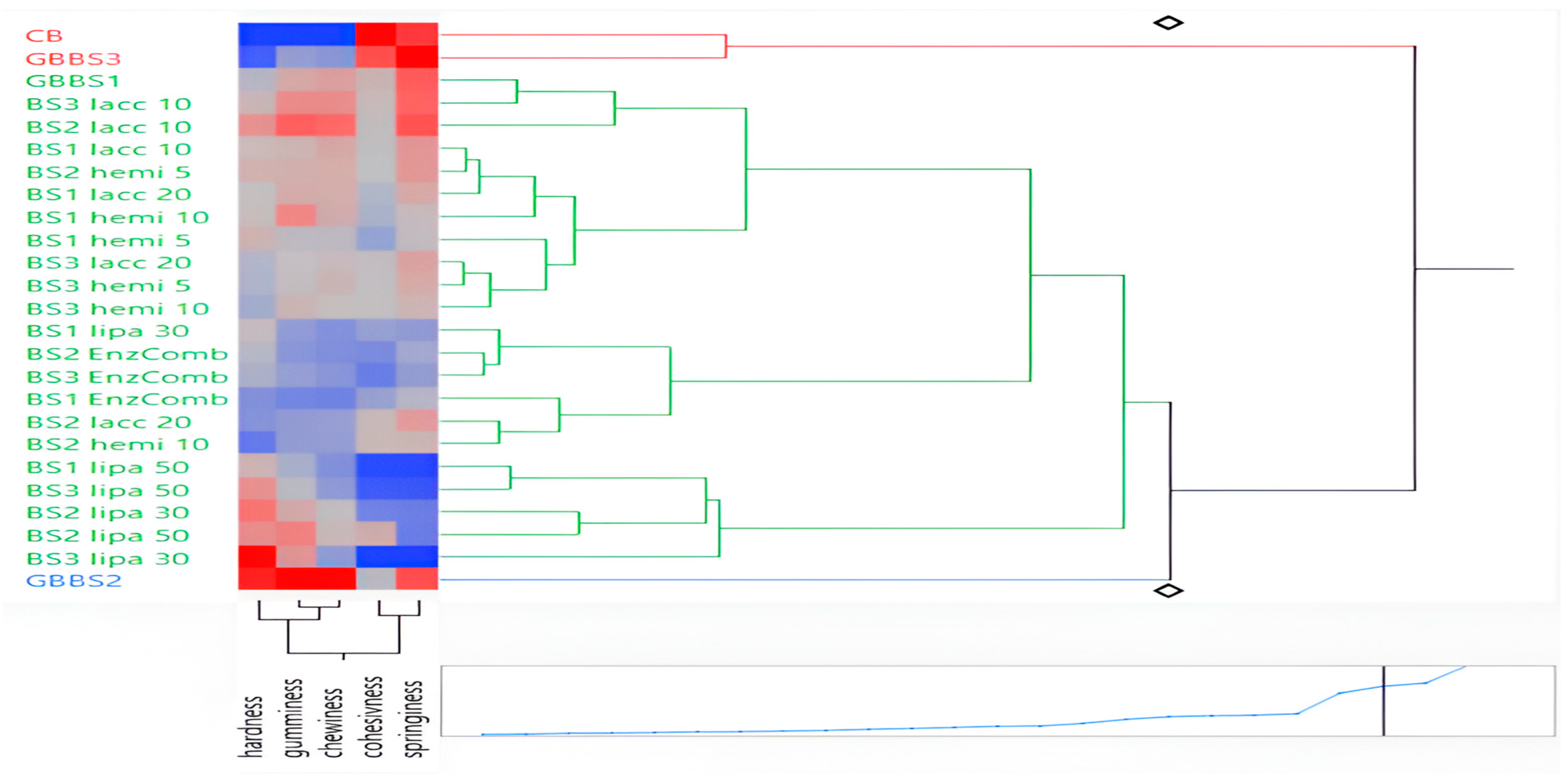
3.6. Principal Component, Cluster, and Hierarchical Classification Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taglieri, I.; Macaluso, M.; Bianchi, A.; Sanmartin, C.; Quartacci, M.F.; Zinnai, A.; Venturi, F. Overcoming bread quality decay concerns: Main issues for bread shelf life as a function of biological leavening agents and different extra ingredients used in formulation. J. Sci. Food Agric. 2020, 100, 4709–4721. [Google Scholar] [CrossRef]
- Giannone, V.; Lauro, M.R.; Spina, A.; Pasqualone, A.; Auditore, L.; Puglisi, I.; Puglisi, G. A novel α-amylase–lipase formulation as anti-staling agent in durum wheat bread. LWT-Food Sci. Technol. 2016, 65, 381–389. [Google Scholar] [CrossRef]
- Karaoǧlu, M.M. Effect of initial baking and storage time on pasting properties and aging of par-baked and rebaked rye bread. Int. J. Food Prop. 2006, 9, 583–596. [Google Scholar] [CrossRef]
- Rayas-Duarte, P.; Murtini, E.S. Bread staling. In Breadmaking, 3rd ed.; Cauvain, S.P., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 561–585. [Google Scholar] [CrossRef]
- Chen, Y.; Eder, S.; Schubert, S.; Gorgerat, S.; Boschet, E.; Baltensperger, L.; Städeli, C.; Kuster, S.; Fischer, P.; Windhab, E.J. Influence of amylase addition on bread quality and bread staling. ACS Food Sci. Technol. 2021, 1, 1143–1150. [Google Scholar] [CrossRef]
- Fadda, C.; Sanguinetti, A.M.; Del Caro, A.; Collar, C.; Piga, A. Bread staling: Updating the view. Compr. Rev. Food Sci. Food Saf. 2014, 13, 473–492. [Google Scholar] [CrossRef]
- Haros, M.; Rosell, C.M.; Benedito, C. Effect of different carbohydrases on fresh bread texture and bread staling. Eur. Food Res. Technol. 2002, 215, 425–430. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Leroy, F. Microbial ecology and process technology of sourdough fermentation. In Advances in Applied Microbiology; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: London, UK, 2017; Volume 100, pp. 49–160. [Google Scholar] [CrossRef]
- De Vuyst, L.; Comasio, A.; Van Kerrebroeck, S. Sourdough production: Fermentation strategies, microbial ecology, and use of non-flour ingredients. Crit. Rev. Food Sci. Nutr. 2021, 63, 2447–2479. [Google Scholar] [CrossRef] [PubMed]
- Decock, P.; Cappelle, S. Bread technology and sourdough technology. Trends Food Sci. Technol. 2005, 16, 113–120. [Google Scholar] [CrossRef]
- Hernández Figueroa, R.H.; Mani López, E.; Palou, E.; López Malo, A. Sourdoughs as natural enhancers of bread quality and shelf life: A review. Fermentation 2024, 10, 7. [Google Scholar] [CrossRef]
- Martín Garcia, A.; Riu-Aumatell, M.; López Tamames, E. Influence of process parameters on sourdough microbiota, physical properties and sensory profile. Food Rev. Int. 2021, 39, 334–348. [Google Scholar] [CrossRef]
- Clarke, C.I.; Schober, T.J.; Arendt, E.K. Effect of single-strain and traditional mixed-strain starter cultures on rheological properties of wheat dough and on bread quality. Cereal Chem. 2002, 79, 640–647. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; De Marco, B.; Balestrieri, F.; Paoletti, F.; Russi, L.; Rossi, J. Combined effect of sourdough lactic acid bacteria and additives on bread firmness and staling. J. Agric. Food Chem. 2000, 48, 3044–3051. [Google Scholar] [CrossRef] [PubMed]
- Crowley, P.; Schober, T.J.; Clarke, C.I.; Arendt, E.K. The effect of storage time on textural and crumb grain characteristics of sourdough wheat bread. Eur. Food Res. Technol. 2002, 214, 489–496. [Google Scholar] [CrossRef]
- Galle, S.; Arendt, E.K. Exopolysaccharides from sourdough lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2014, 54, 891–901. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Guo, X.; Wang, F.; Huang, J.; Sun, B.; Wang, X. Sourdough improves the quality of whole wheat flour products: Mechanisms and challenges—A review. Food Chem. 2021, 360, 130038. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.; Alonso-Hernando, A.; Martínez-Castro, M.; Morán-Pérez, J.A.; Cabrero-Lobato, P.; Pascual-Maté, A.; Téllez-Jiménez, E.; Mujico, J.R. Sourdough Biotechnology Applied to Gluten-Free Baked Goods: Rescuing the Tradition. Foods 2021, 10, 1498. [Google Scholar] [CrossRef]
- Dura, A.; Rosell, C.M. Enzymes in baking. In Microbial Enzyme Technology in Food Applications, 1st ed.; Ray, R.C., Rosell, C.M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; p. 20. [Google Scholar] [CrossRef]
- Pourmohammadi, K.; Abedi, E. Enzymatic modifications of gluten protein: Oxidative enzymes. Food Chem. 2021, 356, 129679. [Google Scholar] [CrossRef]
- Melis, S.; Meza Morales, W.R.; Delcour, J.A. Lipases in wheat flour bread making: Importance of an appropriate balance between wheat endogenous lipids and their enzymatically released hydrolysis products. Food Chem. 2019, 298, 125002. [Google Scholar] [CrossRef]
- Tian, B.; Han, X.; Bai, Y.; Zhang, T.; Shen, X.; Li, J. Monitoring the effects of hemicellulase on the different proofing stages of wheat aleurone-rich bread dough and bread quality. Foods 2021, 10, 2427. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Zhang, H.; Lv, R.; Han, Y.; Zheng, X.; Mu, Y. Effect of a bacterial laccase on the quality and micro-structure of whole wheat bread. J. Microbiol. Biotechnol. 2023, 33, 1671–1680. [Google Scholar] [CrossRef] [PubMed]
- Kupryaniuk, K.; Oniszczuk, T.; Combrzyński, M.; Wójtowicz, A.; Mitrus, M. Effect of extrusion-cooking conditions on the physical properties of Jerusalem artichoke straw. Int. Agrophys. 2020, 34, 509–517. [Google Scholar] [CrossRef]
- Susi, H.; Byler, D. Protein structure by Fourier transform infrared spectroscopy: Second derivative spectra. Biochem. Biophys. Res. Commun. 1983, 115, 391–397. [Google Scholar] [CrossRef]
- Seabourn, B.W.; Chung, O.K.; Seib, P.A.; Mathewson, P.R. Determination of secondary structural changes in gluten proteins during mixing using Fourier transform horizontal attenuated total reflectance spectroscopy. J. Agric. Food Chem. 2008, 56, 4236–4243. [Google Scholar] [CrossRef]
- Fetouhi, A.; Sujak, A.; Bentallah, L.; Nawrocka, A.; Szymańska-Chargot, M.; Tomczyńska-Mleko, M.; Wójtowicz, A.; Zidoune, M.N. Development of new gluten-free maize-field bean bread dough: Relationships between rheological properties and structure of non-gluten proteins. Pol. J. Food Nutr. Sci. 2021, 71, 161–175. [Google Scholar] [CrossRef]
- Bock, J.E.; Damodaran, S. Bran-induced changes in water structure and gluten conformation in model gluten dough studied by Fourier transform infrared spectroscopy. Food Hydrocoll. 2013, 31, 146–155. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Application of FT-IR and Raman spectroscopy for the study of biopolymers in breads fortified with fibre and polyphenols. Food Res. Int. 2013, 50, 574–585. [Google Scholar] [CrossRef]
- Van Velzen, E.J.J.; van Duynhoven, J.P.M.; Pudney, P.; Weegels, P.L.; van der Maas, J.H. Factors associated with dough stickiness as sensed by attenuated total reflectance infrared spectroscopy. Cereal Chem. 2003, 80, 378–382. [Google Scholar] [CrossRef]
- Yang, H.; Yang, S.; Kong, J.; Dong, A.; Yu, S. Obtaining information about protein secondary structures in aqueous solution using Fourier transform IR spectroscopy. Nat. Protoc. 2015, 10, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Naji-Tabasi, S.; Shahidi-Noghabi, M.; Hosseininezhad, M. Improving the quality of traditional Iranian bread by using sourdough and optimizing the fermentation conditions. SN Appl. Sci. 2022, 4, 148. [Google Scholar] [CrossRef]
- Bourekoua, H.; Różyło, R.; Gawlik-Dziki, U.; Dziki, D.; Zidoune, M.N.; Benatallah, L. Enhancing gluten-free bread-making: Process optimization of starch blend in premix with rice and corn flour enriched with various natural flours. Cereal Res. Commun. 2025, 1–20. [Google Scholar] [CrossRef]
- AACC. American Association of Cereal Chemists Approved Methods; AACC: St. Paul, MN, USA, 2000. [Google Scholar]
- ICC. Standard Methods of the International Association for Cereal Science and Technology; ICC: Vienna, Austria, 1996. [Google Scholar]
- Różyło, R.; Dziki, D.; Gawlik-Dziki, U.; Cacak-Pietrzak, G.; Miś, A.; Rudy, S. Physical properties of gluten-free bread caused by water addition. Int. Agrophys. 2015, 29, 353–364. [Google Scholar] [CrossRef]
- Bourekoua, H.; Benatallah, L.; Róźylo, R. Panification Traditionnelle Sans Gluten Type «Khobz Eddar». Ph.D. Thesis, University of Constantine, Constantine, Algeria, 2018. [Google Scholar]
- Collar, C.; Santos, E.; Rosell, C.M. Significance of dietary fiber on the viscometric pattern of pasted and gelled flour-fiber blends. Cereal Chem. 2006, 83, 370–376. [Google Scholar] [CrossRef]
- Brennan, C.S.; Suter, M.; Luethi, T.; Matia-Merino, L.; Qvortrup, J. The relationship between wheat flour and starch pasting properties and starch hydrolysis: Effect of non-starch polysaccharides in a starch gel system. Starch/Stärke 2008, 60, 23–33. [Google Scholar] [CrossRef]
- Ma, S.; Liu, D.; Guo, X.; Wang, P.; Yu, L.; Wang, M.; Wang, L.; Liu, J. Supplementation of wheat flour products with wheat bran dietary fiber: Purpose, mechanisms, and challenges. Trends Food Sci. Technol. 2022, 123, 281–289. [Google Scholar] [CrossRef]
- Symons, L.J.; Brennan, C.S. The effect of barley β-glucan fiber fractions on starch gelatinization and pasting characteristics. J. Food Sci. 2004, 69, FCT257–FCT261. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim Biophys Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Fetouhi, A.; Sujak, A.; Bentallah, L.; Nawrocka, A.; Szymańska-Chargot, M.; Tomczyńska-Mleko, M.; Wójtowicz, A.; Zidoune, M.N. Investigation of viscoelastic behaviour of rice-field bean gluten-free dough using the bio-physical characterization of proteins and starch: A FT-IR study. J. Food Sci. Technol. 2019, 56, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dhital, S.; Zhao, C.; Ye, F.; Chen, J.; Zhao, G. Dietary fiber-gluten protein interaction in wheat flour dough: Analysis, consequences and proposed mechanisms. Food Hydrocol. 2021, 111, 106203. [Google Scholar] [CrossRef]
- Nawrocka, A.; Miś, A.; Niewiadomski, Z. Dehydration of gluten matrix as a result of dietary fibre addition—A study on model flour with application of FT-IR spectroscopy. J. Cereal Sci. 2017, 74, 86–94. [Google Scholar] [CrossRef]
- Pézolet, M.; Bonenfant, S.; Dousseau, F.; Popineau, Y. Conformation of wheat gluten proteins: Comparison between functional and solution states as determined by infrared spectroscopy. FEBS Lett. 1992, 299, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Verdonck, C.; De Bondt, Y.; Pradal, I.; Bautil, A.; Langenaeken, N.A.; Brijs, K.; Goos, P.; De Vuyst, L.; Courtin, C.M. Impact of process parameters on the specific volume of wholemeal wheat bread made using sourdough- and baker’s yeast-based leavening strategies. Int. J. Food Microbiol. 2023, 396, 110193. [Google Scholar] [CrossRef]
- Picozzi, C.; Rizzello, C.G.; Gobbetti, M.; Collins, F.W.; Coda, R. Development of a Type I gluten-free sourdough. Lett. Appl. Microbiol. 2016, 62, 119–125. [Google Scholar] [CrossRef]
- Aplevicz, K.S.; Ogliari, P.J.; Sant’Anna, E.S. Influence of fermentation time on characteristics of sour-dough bread. Braz. J. Pharm. Sci. 2013, 49, 233–239. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Dal Bello, F. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Corsetti, A.; Settanni, L. Lactobacilli in sourdough fermentation. Food Res. Int. 2007, 40, 539–558. [Google Scholar] [CrossRef]
- Djurle, S.; Andersson, A.A.M.; Andersson, R. Effects of baking on dietary fibre, with emphasis on β-glucan and resistant starch, in barley breads. J. Cereal Sci. 2018, 79, 449–455. [Google Scholar] [CrossRef]
- Shalaby, M.T.; Abo-Rya, M.A.; Motawei, A.-Z.M. Effect of baking process on β-glucan content in whole barley balady bread. J. Food Dairy Sci. 2014, 5, 481–490. [Google Scholar] [CrossRef]
- Novotni, D.; Vitale, F.; Schoenlechner, R.; Brunner, M.; Berghofer, E.; Becker, T. Influence of barley sourdough and vacuum cooling on shelf life quality of partially baked bread. Food Technol. Biotechnol. 2017, 55, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yao, Y.; Li, J.; Ju, X.; Wang, L. Impact of exopolysaccharide-producing lactic acid bacteria on the chemical, rheological properties of buckwheat sourdough and the quality of buckwheat bread. Food Chem. 2023, 425, 136369. [Google Scholar] [CrossRef] [PubMed]
- Schmiele, M.; Jaekel, L.Z.; Patricio, S.M.C.; Steel, C.J.; Chang, Y.K. Rheological properties of wheat flour and quality characteristics of pan bread as modified by partial additions of wheat bran or whole grain wheat flour. Int. J. Food Sci. Technol. 2012, 47, 2141–2150. [Google Scholar] [CrossRef]
- Boukid, F. Comprehensive review of barley dietary fibers with emphasis on arabinoxylans. Bioact. Carbohydr. Diet. Fibre 2024, 31, 100410. [Google Scholar] [CrossRef]
- Galle, S.; Schwab, C.; Dal Bello, F.; Coffey, A.; Gänzle, M.G.; Arendt, E.K. Influence of in-situ synthesized exopolysaccharides on the quality of gluten-free sorghum sourdough bread. Int. J. Food Microbiol. 2012, 155, 105–112. [Google Scholar] [CrossRef]
- Terrazas-Avila, P.; Palma-Rodríguez, H.M.; Navarro-Cortez, R.O.; Hernández-Uribe, J.P.; Piloni-Martini, J.; Vargas-Torres, A. The effects of fermentation time on sourdough bread: An analysis of texture profile, starch digestion rate, and protein hydrolysis rate. J. Texture Stud. 2024, 55, e12831. [Google Scholar] [CrossRef]
- Casado, A.; Álvarez, A.; González, L.; Fernández, D.; Marcos, J.L.; Tornadijo, M.E. Effect of fermentation on microbiological, physicochemical and physical characteristics of sourdough and impact of its use on bread quality. Czech J. Food Sci. 2017, 35, 496–506. [Google Scholar] [CrossRef]
- Islam, M.A.; Islam, S. Sourdough Bread Quality: Facts and Factors. Foods 2024, 13, 2132. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.; Wu, F.; Jin, Y.; Xu, X.; Gänzle, M.G. Comparison of the functionality of exopolysaccharides produced by sourdough lactic acid bacteria in bread and steamed bread. J. Agric. Food Chem. 2020, 68, 8907–8914. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, Z.; Qiu, Y.; Tang, Y.; Jin, Z.; Xu, X.; Gänzle, M.G. Effects of sourdough on bread staling rate: From the perspective of starch retrogradation and gluten depolymerization. Food Biosci. 2024, 59, 103877. [Google Scholar] [CrossRef]
- Perri, G.; Coda, R.; Rizzello, C.G.; Celano, G.; Ampollini, M.; Gobbetti, G.; De Angelis, M.; Calasso, M. Sourdough fermentation of whole and sprouted lentil flours: In situ formation of dextran and effects on the nutritional, texture and sensory characteristics of white bread. Food Chem. 2021, 355, 129638. [Google Scholar] [CrossRef]
- Chochkov, R.; Savov, M.; Gotcheva, V.; Papageorgiou, M.; Rocha, J.M.; Baev, V.; Angelov, A. Effects of sourdough on rheological properties of dough, quality characteristics and staling time of wholemeal wheat croissants. Ital. J. Food Sci. 2023, 35, 115–129. [Google Scholar] [CrossRef]
- Corsetti, A.; Gobbetti, M.; Balestrieri, F.; Paoletti, F.; Russi, L.; Rossi, J. Sourdough lactic acid bacteria effects on bread firmness and staling. J. Food Sci. 1998, 63, 347–351. [Google Scholar] [CrossRef]
- Molina, M.A.; Cazzaniga, A.; Milde, L.B.; Sgroppo, S.C.; Zapata, P.D.; Fonseca, M.I. Purification and characterization of a fungal laccase expressed in Kluyveromyces lactis suitable for baking. J. Food Sci. 2023, 88, 1365–1377. [Google Scholar] [CrossRef] [PubMed]
- Cuq, B.; Abecassis, J.; Guilbert, S. State diagrams to help describe wheat bread processing. Int. J. Food Sci. Technol. 2003, 38, 759–766. [Google Scholar] [CrossRef]
- Tembhurkar, V.R.; Kulkarni, M.B.; Peshwe, S.A. Optimization of lipase production by Pseudomonas spp. in submerged batch process in shake flask culture. Sci. Res. Rep. 2012, 2, 46–50. Available online: https://api.semanticscholar.org/CorpusID:54841219 (accessed on 11 July 2025).
- Park, Y.-H.; Jung, L.-H.; Jeon, E.-R. Quality characteristics of bread using sourdough. Prev. Nutr. Food Sci. 2006, 11, 323–327. [Google Scholar] [CrossRef]
- Gandra, K.M.; Del Bianchi, M.; Godoy, V.P.; Queiroz, F.P.C.; Steel, C.J. Aplicação de lipase e monoglicerídeo em pão de forma enriquecido com fibras. Food Sci. Technol. 2008, 28, 182–192. [Google Scholar] [CrossRef][Green Version]



| Flour Type | OGT (°C) | PVT (°C) | OGV (mPas) | PV (mPas) | FV (mPas) |
|---|---|---|---|---|---|
| WF | 63.85 ± 0.07 a | 89.60 ± 0.14 b | 18.50 ± 0.71 a | 353.50 ± 2.12 a | 526.50 ± 3.54 a |
| WMF | 66.15 ± 1.63 a | 92.05 ± 0.78 a | 19.00 ± 0.00 a | 234.00 ± 7.07 b | 415.00 ± 0.00 b |
| BF | 66.20 ± 0.14 a | 81.70 ± 0.00 c | 19.00 ± 0.00 a | 120.50 ± 0.71 c | 91.00 ± 1.41 c |
| β-Sheet (%) | α-Helix (%) | β-Turn (%) | |
|---|---|---|---|
| WF | 47 | 29 | 24 |
| WMF | 51 | 32 | 17 |
| BF | 47 | 33 | 20 |
| SV (cm3/g) | Moisture (%) | Hardness (N) | Chewiness (N) | Springiness (–) | |
|---|---|---|---|---|---|
| CB | 5.54 ± 0.09 a | 31.15 ± 0.37 bcd | 3.53 ± 0.26 f | 3.4 ± 0.03 g | 1.01 ± 0.01 d |
| GBBS1 20% | 3.15 ± 0.15 c | 29.65 ± 0.33 cde | 20.87 ± 0.90 d | 13.22 ± 0.71 d | 1.00 ± 0.00 de |
| GBBS1 30% | 3.38 ± 0.18 fg | 26.26 ± 1.74 e | 14.00 ± 2.01 e | 7.94 ± 0.70 e | 0.92 ± 0.01 b |
| GBBS1 40% | 3.59 ± 0.06 b | 26.55 ± 2.39 e | 17.22 ± 0.07 de | 11.45 ± 0.75 d | 0.99 ± 0.01 e |
| GBBS2 20% | 2.13 ± 0.05 e | 27.99 ± 3.01 de | 49.27 ± 2.4 a | 28.29 ± 1.23 a | 1.00 ± 0.00 de |
| GBBS2 30% | 2.23 ± 0.04 de | 29.24 ± 0.52 cde | 40.96 ± 1.35 b | 23.81 ± 0.47 b | 1.00 ± 0.001 de |
| GBBS2 40% | 2.32 ± 0.04 d | 32.02 ± 2.57 bc | 33.21 ± 6.45 c | 20.11 ± 3.76 c | 1.00 ± 0.001 de |
| GBBS3 20% | 3.31 ± 0.06 g | 33.67 ± 0.48 ab | 8.41 ± 0.56 f | 5.07 ± 0.21 efg | 0.95 ± 0.006 c |
| GBBS3 30% | 3.39 ± 0.11 fg | 35.93 ± 0.55 a | 7.06 ± 0.05 f | 4.79 ± 0.24 fg | 0.94 ± 0.007 c |
| GBBS3 40% | 3.44 ± 0.11 f | 34.23 ± 0.09 ab | 6.99 ± 0.22 f | 7.33 ± 0.15 ef | 1.06 ± 0.007 a |
| Hardness (N) | Gumminess (–) | Cohesiveness (–) | Springiness (–) | Chewiness (N) | |
|---|---|---|---|---|---|
| GBBS1 | 17.22 ± 0.07 c | 11.53 ± 0.62 ab | 0.67 ± 0.03 a | 0.99 ± 0.01 a | 11.45 ± 0.74 a |
| Lacc 10 ppm | 19.27 ± 1.36 ab | 11.04 ± 1.16 bcd | 0.61 ± 0.02 b | 0.94 ± 0.04 b | 11.07 ± 0.75 a |
| Lacc 20 ppm | 18.59 ± 1.39 bc | 11.52 ± 0.80 abc | 0.59 ± 0.02 bc | 0.92 ± 0.01 bc | 10.64 ± 0.83 a |
| Lipa 30 ppm | 19.27 ± 1.20 ab | 8.27 ± 1.39 ef | 0.55 ± 0.06 cd | 0.85 ± 0.05 c | 6.94 ± 0.81 c |
| Lipa 50 ppm | 20.08 ± 0.94 ab | 9.43 ± 0.49 de | 0.40 ± 0.03 d | 0.74 ± 0.02 d | 6.98 ± 0.56 c |
| Hemi 5 ppm | 20.40 ± 0.88 a | 10.09 ± 1.24 cde | 0.55 ± 0.04 c | 0.90 ± 0.03 c | 9.02 ± 0.95 b |
| Hemi 10 ppm | 19.25 ± 1.28 ab | 13.04 ± 2.24 a | 0.58 ± 0.04 bc | 0.91 ± 0.03 bc | 10.84 ± 1.06 a |
| EnzComb ppm | 12.68 ± 1.28 d | 7.01 ± 0.29 f | 0.56 ± 0.04 c | 0.89 ± 0.01 c | 6.21 ± 0.18 c |
| Hardness (N) | Gumminess (–) | Cohesiveness (–) | Springiness (–) | Chewiness (N) | |
|---|---|---|---|---|---|
| GBBBS2 | 33.21 ± 6.40 a | 18.08 ± 0.92 a | 0.60 ± 0.004 ab | 1.00 ± 0.001 a | 20.11 ± 3.70 a |
| Lacc 10 ppm | 23.24 ± 1.44 c | 14.66 ± 0.63 ab | 0.63 ± 0.01 ab | 1.00 ± 0.01 a | 14.63 ± 0.60 b |
| Lacc 20 ppm | 12.97 ± 1.75 f | 8.30 ± 1.10 c | 0.64 ± 0.02 ab | 0.94 ± 0.01 ab | 7.76 ± 1.02 c |
| Lipa 30 ppm | 26.11 ± 1.42 b | 11.59 ± 1.57 bc | 0.50 ± 0.02 b | 0.81 ± 0.03 c | 9.34 ± 0.94 c |
| Lipa 50 ppm | 23.59 ± 0.37 c | 16.65 ± 7.23 a | 0.69 ± 0.33 a | 0.81 ± 0.01 c | 14.12 ± 1.75 b |
| Hemi 5 ppm | 20.39 ± 1.58 d | 11.32 ± 1.97 bc | 0.62 ± 0.02 ab | 0.94 ± 0.04 ab | 10.69 ± 2.36 bc |
| Hemi 10 ppm | 9.69 ± 0.36 g | 8.38 ± 2.05 c | 0.65 ± 0.07 ab | 0.91 ± 0.02 b | 7.61 ± 1.70 c |
| EnzComb ppm | 17.56 ± 1.12 e | 7.92 ± 1.53 c | 0.51 ± 0.02 b | 0.87 ± 0.02 bc | 6.87 ± 1.29 c |
| Hardness (N) | Gumminess (–) | Cohesiveness (–) | Springiness (–) | Chewiness (N) | |
|---|---|---|---|---|---|
| GBBS3 | 6.99 ± 0.21 e | 8.53 ± 0.29 d | 1.01 ± 0.03 a | 1.06 ± 0.007 a | 7.33 ± 0.15 c |
| Lacc 10 ppm | 19.99 ± 0.79 c | 12.78 ± 0.48 a | 0.64 ± 0.02 b | 0.98 ± 0.03 b | 12.55 ± 0.77 a |
| Lacc 20 ppm | 16.95 ± 1.09 d | 10.61 ± 1.56 bc | 0.61 ± 0.01 b | 0.93 ± 0.01 bc | 9.89 ± 1.62 b |
| Lipa 30 ppm | 35.72 ± 0.96 a | 12.26 ± 1.66 ab | 0.39 ± 0.03 d | 0.73 ± 0.10 d | 7.62 ± 0.80 c |
| Lipa 50 ppm | 23.48 ± 1.18 b | 10.18 ± 0.39 cd | 0.43 ± 0.03 d | 0.76 ± 0.06 d | 7.49 ± 0.67 c |
| Hemi 5 ppm | 16.9 ± 1.20 d | 10.26 ± 1.44 cd | 0.61 ± 0.05 b | 0.92 ± 0.05 b | 10.3 ± 1.51 b |
| Hemi 10 ppm | 15.71 ± 0.77 d | 11.17 ± 1.65 abc | 0.61 ± 0.03 b | 0.91 ± 0.02 bc | 9.57 ± 0.24 b |
| EnzComb ppm | 16.69 ± 1.60 d | 8.54 ± 0.63 d | 0.48 ± 0.03 c | 0.85 ± 0.05 c | 7.24 ± 0.45 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yahia, D.F.; Bourekoua, H.; Fetouhi, A.; Wójcik, M.; Wójtowicz, A.; Mitrus, M.; Siar, E.H.; Różyło, R. Impact of Sourdoughs, Enzymes, and Their Combinations on Gluten-Based Bread Quality. Processes 2025, 13, 2796. https://doi.org/10.3390/pr13092796
Yahia DF, Bourekoua H, Fetouhi A, Wójcik M, Wójtowicz A, Mitrus M, Siar EH, Różyło R. Impact of Sourdoughs, Enzymes, and Their Combinations on Gluten-Based Bread Quality. Processes. 2025; 13(9):2796. https://doi.org/10.3390/pr13092796
Chicago/Turabian StyleYahia, Djihane Faten, Hayat Bourekoua, Awatif Fetouhi, Monika Wójcik, Agnieszka Wójtowicz, Marcin Mitrus, El Hocine Siar, and Renata Różyło. 2025. "Impact of Sourdoughs, Enzymes, and Their Combinations on Gluten-Based Bread Quality" Processes 13, no. 9: 2796. https://doi.org/10.3390/pr13092796
APA StyleYahia, D. F., Bourekoua, H., Fetouhi, A., Wójcik, M., Wójtowicz, A., Mitrus, M., Siar, E. H., & Różyło, R. (2025). Impact of Sourdoughs, Enzymes, and Their Combinations on Gluten-Based Bread Quality. Processes, 13(9), 2796. https://doi.org/10.3390/pr13092796










