1. Introduction
According to the pillars of the circular bioeconomy strategy, AD was reported as one of the most environmentally friendly management approaches for OFMSW recycling [
1]. AD of OFMSW produces an energy vector (biogas) and a digested fraction (digestate) that can be used in agriculture as a nutrient-rich product. The digestate from AD of OFMSW cannot be directly landspread but must be appropriately treated with processes such as composting to guarantee its sanitary safety based on Regulation (EU) 2019/1009. In 2023, in Italy, 3,357,018 tons of OFMSW were treated through the AD process in 61 integrated AD and composting plants (Rif. ISPRA, catalogo nazionale dei rifiuti). Considering that for each ton of biomass treated in an anaerobic digester, approximately 50–85% of the weight is transformed into digestate, depending on the water content of the influent and the post-digestion treatment, it can be estimated that in 2023, in Italy, between 1,700,000 and 2,900,000 tons of digestate were subjected to the composting process. However, the industrial composting of the digestate from AD of OFMSW is a process that requires a large amount of energy, large spaces, and a long time to be completed [
2]. An alternative pathway is further methane recovery through thermochemical processes such as pyrolysis. Preliminary evaluations conducted on a bench-scale pilot plant equipped with a downstream catalytic methanation section suggest that approximately 0.15–0.2 Nm
3 of methane per kilogram of dry digestate could be obtained through membrane separation. Considering the total amount of digestate produced annually in Italy, this corresponds to an additional potential methane production of approximately 38–65 million Nm
3/year via thermochemical conversion. However, these results are based on bench-scale data and should be considered preliminary. Further investigations are required to fully assess the potential and economic viability of scaling up the technology. In particular, detailed energy analyses and process optimization—including heat recovery strategies—are essential to reduce the energy demand of pyrolysis. For instance, heat recovered from the combustion of secondary streams (e.g., char and pyrolysis oils) or from downstream units (e.g., methanation and CO
2 separation) could be reused to support digestate drying or sustain the pyrolysis process itself.
Pyrolysis is a thermochemical process occurring usually at temperatures above 400 °C, in an inert atmosphere that degrades the organic matter into char, oil, and gas. The relative distribution of char, pyrolysis oil, and gas is severely related to the pyrolysis temperature, feedstock, residence time of the feedstock in the reactor, and heating rate.
Char is a solid carbonaceous material that contains the metals of the feedstock; it is a precursor for activated carbons or composite material for hazardous pollutants removal [
3,
4]. It has recently attracted scientists for its application as a soil amendment [
5]. The pyrolysis oil is a metastable dark brown complex mixture composed of water and more than 300 organic molecules (acids, alcohols, aldehydes, esters, ketones, sugars, phenols, furans, guaiacols, syringols, and alkenes, as well as aromatic and nitrogen compounds) [
6]. Its composition and chemical–physical properties can largely vary as a function of the adopted pyrolysis process. From a theoretical point of view, it could be upgraded to recovery chemicals or fuels. Anyway, several challenges should be faced as bio-oil shows many undesirable characteristics such as high acidity, viscosity, high reactivity, and high aqueous content [
7]. The main components of the pyrolysis gas are methane, light hydrocarbons, carbon monoxide and dioxide, and hydrogen. The relative amounts of the components are more closely related to the feedstock used for pyrolysis and process conditions. The gas obtained from pyrolysis may be used as a gaseous biofuel after appropriate purification. Anyway, this application was limitedly investigated [
8]. In this regard, this paper is devoted to assessing pyrolysis as a technology to dispose of digestate with the production of a tailored methane-rich syngas, which could be mixed with the biogas from the AD process. The integration of biological disposal technologies, such as anaerobic digestion for MSW, and thermochemical disposal technologies such as pyrolysis for digestate, is a virtuous synergic pathway to sustainably close the MSW cycle.
The scientific community has recently been focusing on the thermal treatment of digestate. In the following, digestate pyrolysis works are debated and concisely summarized in
Table 1. To date, experimental works about digestate pyrolysis have been carried out by using a laboratory thermogravimetric balance in order to establish the basic mechanisms of feedstock pyrolysis and to determine kinetic parameters and deviations of thermodynamic state functions. Wang et al. [
3] identified five stages in the pyrolysis of digestate residue; each stage corresponded to one of the five most pronounced weight loss peaks in the DTG diagram.
The stages correspond to water evaporation (1), light volatile decomposition (2), decomposition of hemicellulose, cellulose, and extracts including protein, starch, carbohydrate, and aliphatic (3), macromolecular substances and lignin decomposition (4), and decomposition of calcium carbonate resulting from the decomposition of organic calcium compounds (5). Basina et al. [
9] analogously determined the steps of the digestate pyrolysis by thermogravimetric analysis, and they carried out the digestate pyrolysis in a batch fixed-bed reactor in the temperature range between 300 and 700 °C. From the chemical and physical analyses of the produced biochars, they screened the biochar produced at 500 °C as the most favorable for the improvement of soil fertility in the long term. On the other hand, the biochar produced at 700 °C exhibited the highest surface area and was the optimal choice among biochars for the absorption of pollutants, the prevention of nutrient leaching, and the improvement of the water retention capacity of a soil. Moreover, its large surface area also permits an optimal CO
2 and H
2S retention activity and mitigation of free NH
3, further promoting AD. Petrovic et al. [
10] carried out pyrolysis tests of digestate and its mixture with biomass by thermogravimetric balance. They determined kinetic and thermodynamic parameters that revealed a complex degradation mechanism of digestate. The digestate-derived biochar exhibited excellent performance in germination tests, especially at concentrations between 6 and 10 wt.%. Other scientists have carried out digestate pyrolysis tests at the bench scale using batch reactors; they acquired useful information about the technical feasibility of the process at a reduced operational scale. Wiśniewski et al. pyrolyzed digestate at 500 °C in a batch reactor at bench scale. During this pyrolysis, the gas composition was checked, and the gas calorific value was assessed, as well [
11]. Zhao et al. [
12] executed pyrolysis experiments of digestate in a fixed-bed reactor in batch mode (10 gr/batch), in a temperature range between 500 and 800 °C. Char, oil, and gas were quantified and analyzed. Char and oil decreased with increasing temperature; conversely, gas increased. Regarding the gas composition, they observed clear tendencies in the gas macroconstituents with increasing pyrolysis temperature, e.g., a net CO
2 decrease, a net H
2 increase, and a light CO increase were detected. Methane and hydrocarbons concentrations were about 25–30 vol %. Alghashm et al. [
13] pyrolyzed digestate in a laboratory muffle at 300 °C, 500 °C, and 700 °C. They investigated the chemical and physical characteristics of the biochars, and they tested the effects of the biochars on AD performance. Biochar prepared at 700 °C improved the biogas production yields by 10.0%. Tayibi et al. [
14] carried out pyrolysis of 300 g of dried digestate in a batch fixed-bed reactor at 500 °C for 1 h. The pyrolysis product distribution (char, oil, and gas) was assessed, as well as the syngas composition as a function of the process temperature. The overall gas was composed of CO
2 30 vol %, CO 23 vol %, and CH
4 14 vol %, followed by H
2 and light hydrocarbons.
The novelty of this work is to perform the pyrolysis of dried digestate in a rotary kiln having a digestate mass rate of about 0.5 kg/h, equipped with facilities to quantify product distribution (char, oil, and gas) as well as their chemical and physical characterization. To date, to the best of our knowledge, digestate pyrolysis has not been investigated in a continuous rotary kiln reactor at the proposed industrially relevant scale. Furthermore, another very interesting aspect of the experimentation is the integration of the digestate pyrolysis with the CO
2 chemisorption by CaO inside the rotary kiln. By this trick, a methane-rich pyrolysis gas is obtained. About this topic, from the works discussed in
Table 1, it results that the CO
2 molar fraction in the pyrolysis gas reaches a value of 30–35 mol %, especially at the lower pyrolysis temperature (500 °C). The removal of CO
2 from the pyrolysis gas has the great advantage of reducing the amount of gas to handle and of increasing its CH
4 concentration. CO
2 chemisorption by CaO-based sorbents, such as limestone or dolomite, was investigated by several scientists, mainly by IFK Stuttgart in Germany and TU Wien in Austria, using different laboratory- and pilot-scale plants based on the dual fluidized bed principle for lignocellulosic biomass gasification [
15,
16,
17]. As concerns pyrolysis, to date, to the best of our knowledge, this work is the first in the world that couples digestate pyrolysis in a bench-scale rotary kiln with CO
2 sorption by CaO.
2. Materials and Methods
This section provides a concise description of the experimental facility and the methodologies employed to evaluate the chemical and physical properties of the solid feedstock and the output streams from the bench-scale pyrolysis plant.
2.1. Feedstock Characterization: Chemical and Physical Properties
The feedstock used in the pyrolysis process, namely the digestate, was characterized through proximate analysis, ultimate analysis, metals, and calorific value determination. These analyses were conducted at the ENEA laboratories in Trisaia Research Centre.
Digestate samples were provided by Calabra Maceri e Servizi S.p.A. in the form available during the anaerobic digestion (AD) process. Digestate was obtained by low-thermophilic (43–45 °C), dry (TS = 27–30%), plug-flow (HRT = 21 days) industrial AD of OFMSW separately collected in the municipalities of the province of Cosenza (Italy). The industrial AD plant characteristics were detailed elsewhere [
18].
To mitigate potential biological risks, the samples were dried prior to characterization. The drying process was carried out by placing the wet digestate—characterized by a moisture content of 84.5% (as reported in
Table 1)—in an industrial oven at 105 °C for approximately 12 h, maintaining a constant temperature throughout the procedure.
The dried material, exhibiting a residual moisture content of 2.3%, was subsequently used for bench-scale thermochemical testing. Representative samples for characterization were carefully extracted from the homogenized batch, which had been thoroughly mixed and milled to a particle size of 20 µm. The prepared samples were then stored in an oven at 105 °C until further use.
Proximate analysis was performed using a PerkinElmer TGA 7 thermogravimetric analyzer (PerkinElmer, Waltham, MA, USA) following the ASTM D1762-84 standard method. Initially, the solid samples were heated to 900 °C under an inert nitrogen atmosphere to determine moisture and volatile matter content. Subsequently, the residual material was combusted at 550 °C in an oxidative environment to quantify ash content and fixed carbon.
Ultimate analysis was carried out using a Vario Macro Cube CHNS analyzer (Elementar, Langenselbold, Germany). During the procedure, the samples were combusted in a high-purity helium stream within a reducing environment, ensuring complete conversion of the elements into CO2, N2, H2O, and SO2. The resulting gaseous products were then directed to their respective detectors for quantification. Nitrogen was directly measured using a thermal conductivity detector (TCD), while the remaining components were quantified through dedicated detection systems.
The lower heating value (LHV) was determined in accordance with the ASTM D5865 standard method, using an IKA Werke C5003 bomb calorimeter (IKA, Staufen, Germany) operating at constant volume and with a reference temperature of 25 °C. Benzoic acid was employed as the calibration standard.
Metal quantification was performed following a pretreatment step aimed at transferring the metal content from the solid matrix into the liquid phase, enabling subsequent analysis via inductively coupled plasma optical emission spectrometry (ICP-OES). Mineralization was conducted using approximately 40 mg of sample, which was digested with a mixture of hydrogen peroxide (H
2O
2), nitric acid (HNO
3), and hydrofluoric acid (HF). The resulting liquid solution was filtered through a 0.45 µm membrane prior to instrumental quantification. The main results are summarized in
Table 2 and
Table 3.
Char, condensable products, and non-condensable gases obtained from the pyrolysis process were appropriately collected and characterized, as shown in
Figure 1. Proximate and ultimate analyses, along with lower heating value (LHV) determination—as previously described—were performed on char samples collected at the end of each test conducted at different temperatures. Condensable products (including pyrolytic oils, water, and other condensable compounds) were subjected to analyses for organic compound identification, LHV, viscosity, and water content. Organic compounds were identified using a GC-MS system (Agilent T5975, Agilent Technologies, Santa Clara, CA, USA), consisting of an HP 6890 gas chromatograph coupled with a quadrupole mass spectrometer equipped with electron impact (EI) ionization. The system employed a 30 m HP-5MS capillary column, a split/splitless injector (SS), and electronic control of helium as the carrier gas. Quantitative analysis of major organic compounds was performed using a multilevel calibration with external standards.
The water content in the syngas was determined by measuring the water fraction in the condensable phase using the Karl Fischer titration method, in accordance with ASTM E203. Prior to analysis, the oily mixture was thoroughly homogenized, and a defined aliquot (0.05 g) was extracted and dissolved in 1 mL of methanol. The resulting solution was titrated using a Mettler DL18 titrator (Mettler-Toledo GmbH, Greifensee, Schweiz) with Hydranal Composite 5 as the titrant.
Kinematic viscosity of the pyrolytic oil was determined using a Cannon-Fenske viscometer (CANNON Instrument Company, State College, PA, USA), in accordance with ASTM D445, ASTM D446, and ISO 3105 standards.
The composition of the syngas—specifically H2, CO, CO2, CH4, CnHm, N2, and O2—was determined through continuous sampling at the plant outlet, upstream of the flare line. Real-time analysis was conducted using an Agilent 3000A mobile gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with two parallel columns (Molsieve 5A and Paraplot Q) and a thermal conductivity detector (TCD) for volumetric gas composition measurement. Argon was used as the carrier gas. Instrument control and data acquisition were managed automatically via dedicated software. Prior to analysis, the system was calibrated using external gas mixtures with varying compositions, employing at least three calibration points for each analyte.
2.2. Experimental Setup
The experimental activity was carried out using a laboratory-scale rotary kiln reactor, schematically illustrated in
Figure 2. The setup allowed for the adjustment of key operating parameters, including reactor inclination, rotation speed, feed rate, and process temperature. All tests were performed with a fixed reactor inclination (β
reactor) of 3°, a rotation speed (w
reactor) of 10 rpm, and a solid feed rate (Q
feed) of 0.5 kg/h, while varying the reaction temperature between 600 °C and 800 °C. To investigate the effect of in situ CO
2 abatement, additional pyrolysis tests were conducted under the same operating conditions by co-feeding a sorbent material with the digestate at a weight ratio of 5:1. All operating parameters were selected to achieve an estimated mean residence time of the solid fraction inside the reactor between 7 and 10 min, as calculated using Sullivan’s correlation [
19].
With reference to the layout of the bench-scale pyrolysis system shown in
Figure 2, the feedstock was loaded in the hopper (1). An electrical furnace (2) surrounds the rotary kiln reactor (3) to provide the thermal energy required for the pyrolysis process. A screw conveyor fed the feedstock to the reactor. To prevent vapor formation from the feedstock, the walls of the screw conveyor were cooled by circulating water through a surrounding jacket. When a sorbent was used, it was premixed with the digestate before loading into the hopper (1). During the pyrolysis process, in addition to the solid feed, a controlled stream of nitrogen was introduced. This allowed for the quantification of the produced syngas by determining the nitrogen mole fraction in the gas stream. The pyrolysis products were char, condensable liquids, and gas. Condensable products and gas crossed the plenum chamber (4), whose temperature was kept above the condensation point of the vapor fraction. Char was collected in a bottom reservoir (5). Condensable products, mainly pyrolysis oils and water, were liquified in the condensation section (6), while the gas crossed it.
A small amount of gas crosses two impingers (7) and (8) before being analyzed by the online gas chromatograph (9), which measures the concentrations of the main components (H
2, CO, CH
4, CO
2, C
nH
m, N
2, and O
2) with an acquisition frequency of one measurement every three minutes. Most of the gas is burned by a torch (10). The main specifications of the rotary kiln test facility are summarized in
Table 4.
Some details of the bench-scale rotary kiln pyro-gasifier test facility are shown in
Figure 3. The images clearly show (a) the rotary kiln reactor equipped with electric heaters and its temperature control system, and (b) the condenser where the liquid fraction is collected at the end of each test.
Prior to each pyrolysis test, all sections of the plant and associated pipelines were purged with nitrogen to ensure an inert atmosphere. Once a nitrogen mole fraction of approximately 95–98% was detected, the solid feedstock was introduced, and the nitrogen flow rate was stabilized at the desired residual value. Similarly, at the end of each test, nitrogen was again circulated to prevent oxidation of the residual solid material—mainly char and unreacted feedstock—which needed to be quantified for mass balance closure. Nitrogen purging continued until the reactor temperature dropped to approximately 150 °C. Once the shutdown procedures were fully completed, the different product fractions (liquid and solid) were collected and quantified, while the reactor and pipelines were thoroughly cleaned and restored in preparation for the next test.
3. Results and Discussion
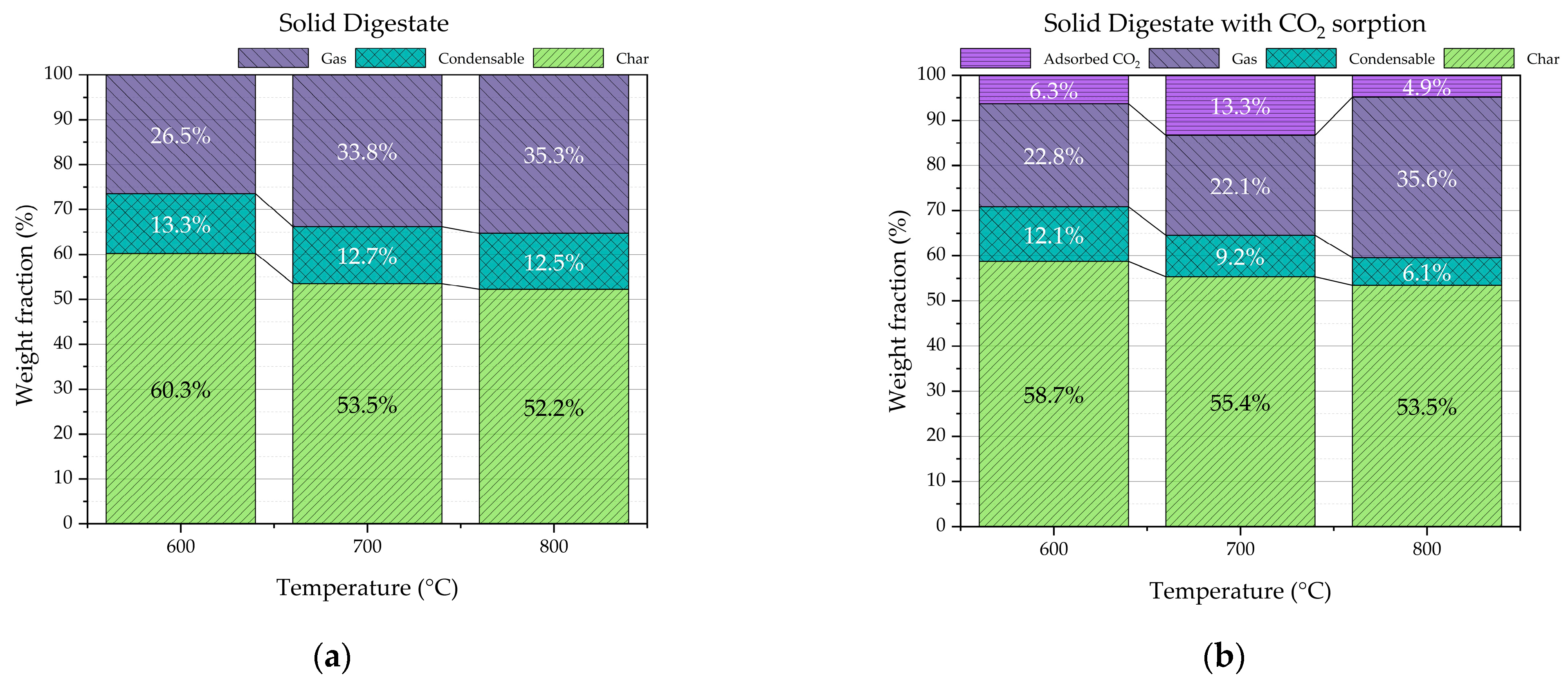
Pyrolysis products were char, condensable products, and permanent pyrolysis gas. Char, shown in
Figure 1b, was a black solid that approximately preserved the shape of the starting digestate feedstock. Condensable products were a bad-smelling metastable mixture of water, organic compounds having good affinity with water, and small amounts of organic water-insoluble sticky waxes. The permanent pyrolysis gas was composed of hydrogen, carbon monoxide, carbon dioxide, methane, and light hydrocarbons. Both for the digestate pyrolysis and the digestate pyrolysis with CO
2 sorption by CaO, similar trends were observed as concerns char, condensable products, and gas, as a function of pyrolysis temperature. Char and condensable products decrease with the temperature; on the contrary, gas increases.
It is very instructive to observe that at all the investigated process temperatures, the condensable products yield when CaO is added to the process is systematically lower compared to that detected for the sole digestate pyrolysis. Very probably, CaO works as a catalyst in conversion reactions of condensable organic molecules that produce permanent gases such as CO, H
2, CH
4, and C
2 and C
3 hydrocarbons [
20,
21]. According to Corella and Islam, the main removal reactions of condensable organic molecules are steam reforming, steam dealkylation, thermal cracking, and dry reforming [
22,
23]. The effect of the catalytic activity of CaO is more marked at the higher temperature. E.g., at 800 °C, comparing
Figure 4a,b, a reduction in condensable products of about 6 wt.% (12.5 vs. 6.1 wt.%) and a total gas (considering both permanent gas fraction and CO
2 adsorbed) increase of about 5 wt.% (35.3 vs. 40.4 wt.%) were detected.
Figure 5 shows the syngas composition of the pyrolysis tests. The CO volumetric concentration, for both the processes, with and without CaO, shows a similar increasing trend. The effect of chemisorption of CO
2 by CaO is evident from
Figure 5b, which shows, at the investigated pyrolysis temperature, a lower CO
2 concentration compared to the case of pyrolysis of sole digestate (
Figure 5a). The decrease in CO
2 is particularly evident at the lower temperatures of 600 and 700 °C, where the thermodynamic equilibrium of the exothermic reaction (1) is shifted towards the products. Moreover, the driving force of the reaction (1) is the partial pressure of CO
2, which is higher at the lower temperature (see
Figure 5a).
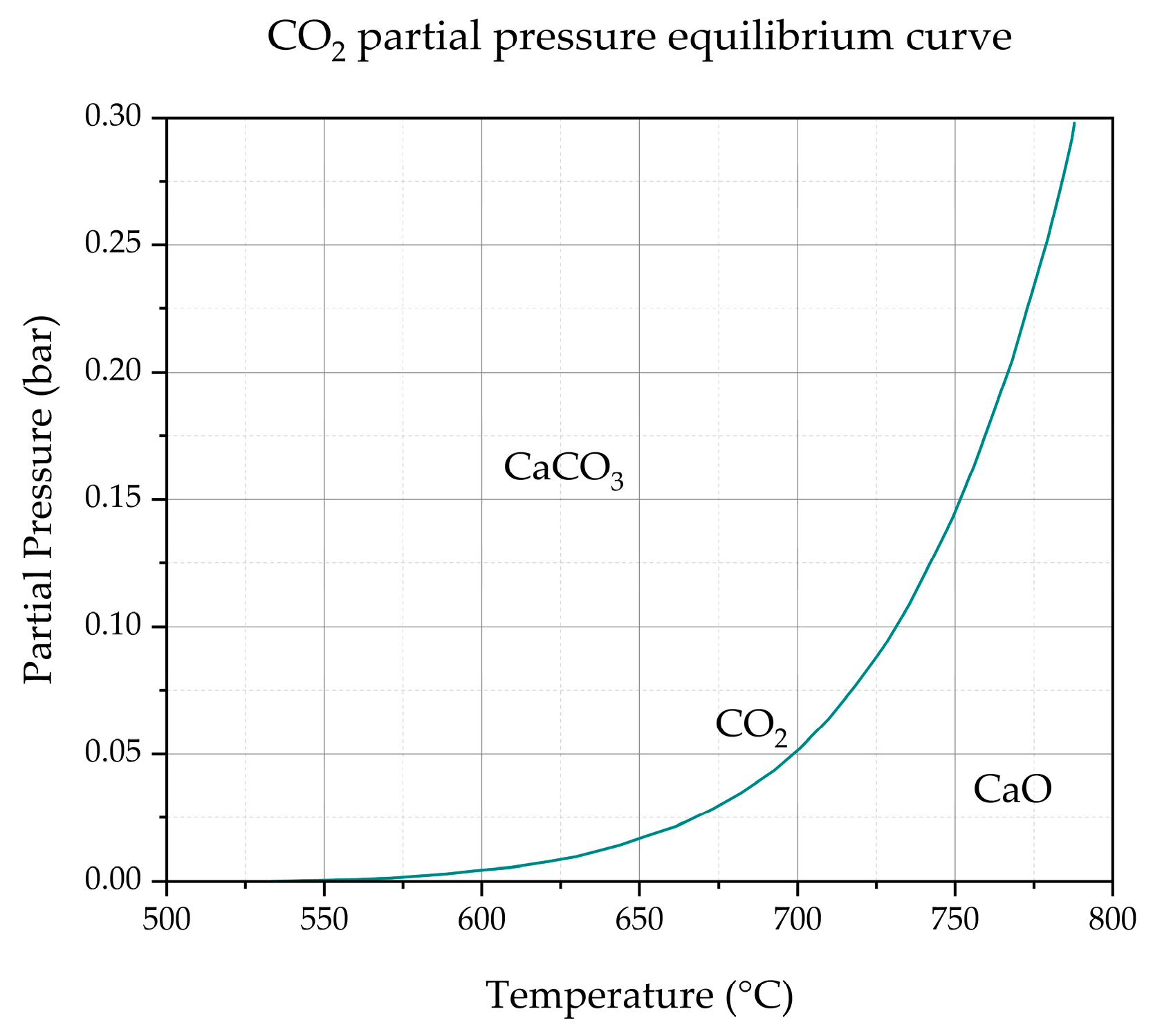
By calculating the CO
2 partial pressure from
Figure 5a, and considering CO
2 partial pressure in equilibrium with CaO and CaCO
3 plotted in
Figure 6 versus the temperature, it is clear that the CaCO
3 is enhanced at pyrolysis temperatures of 600 and 700 °C. At a pyrolysis temperature of 800 °C, the sorption probably occurs in the reactor volume not covered by the electrical resistances, just upstream of the char reservoir, where a temperature drop up to 250 °C is obtained.
The sorption of CO
2 by CaO has the effect of increasing the hydrogen content (
Figure 5a,b), at all the investigated temperatures by two mechanisms: the first is the concentration effect caused by the CO
2 sorption, the second is the application of the Le Chatelier’s principle to the water gas shift reaction that shifts the chemical equilibrium towards the hydrogen formation.
Analogously, the sorption of CO
2 by CaO has the effect of increasing the methane and light hydrocarbons content, at all the investigated temperature by two mechanisms: the first is the concentration effect caused by the CO
2 sorption, the second is the application of the Le Chatelier’s principle to the dry reforming of methane and light hydrocarbons (Reactions 3 and 4) that shifts the chemical equilibriums towards the methane and light hydrocarbons formation.
The maximum heating value of syngas (16–17 MJ/kg) was coherently detected with the CO
2 sorption tests and at 600–700 °C, where the methane and light hydrocarbons concentrations were the highest (30–25 vol%) (See
Figure 5a,b).
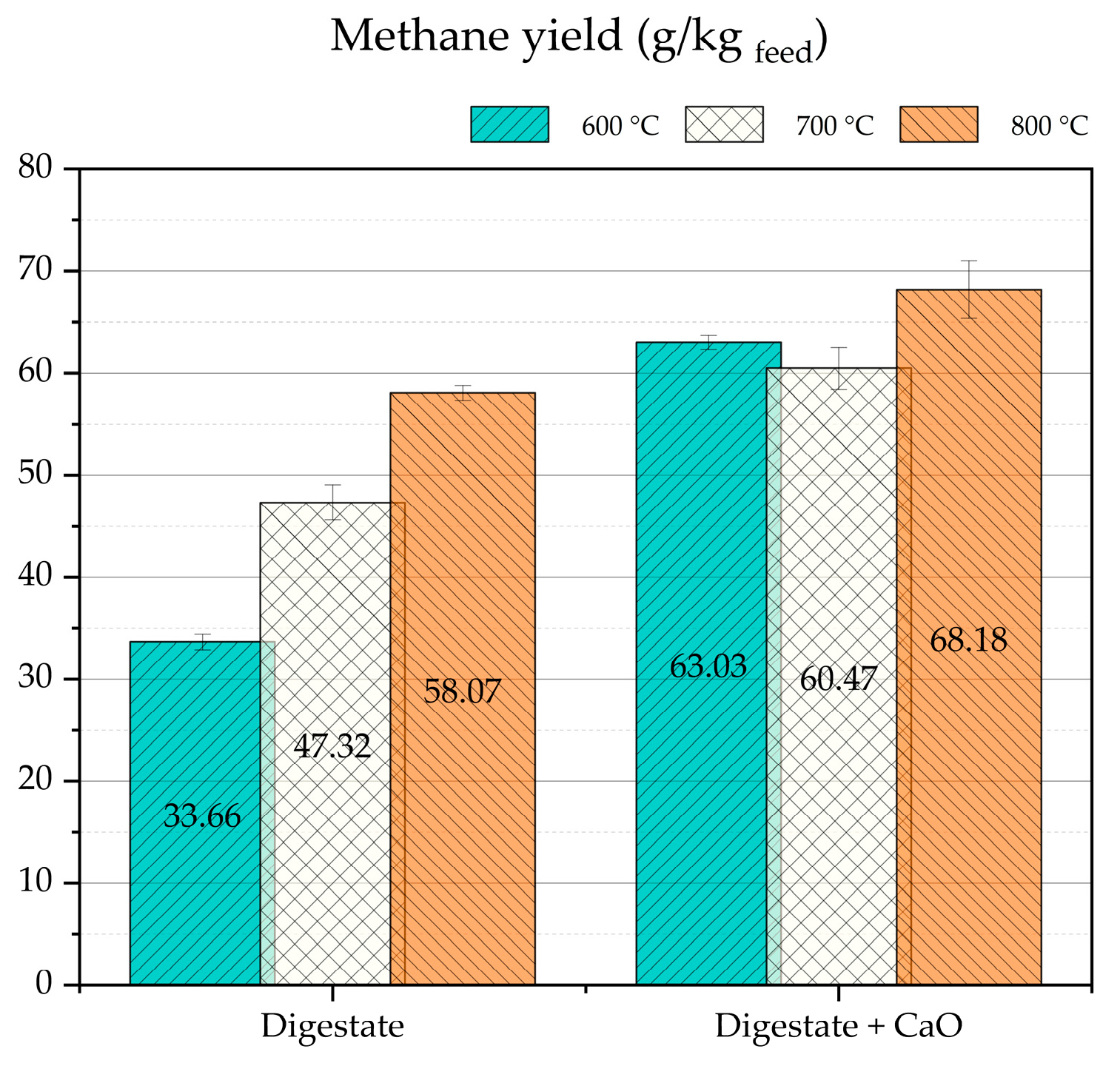
Considering the goal of this work, to obtain a methane-rich syngas by thermochemical conversion of digestate, it is very useful to evaluate quantitatively the methane yield for a digestate mass unit. About this parameter,
Figure 7 shows that, with the CaO addition, the higher methane yields were obtained. Particularly, the highest value of about 68 g/kg
digestate was obtained at 800 °C. Moreover, the data in
Figure 7 demonstrate that the presence of CaO significantly mitigates the effect of pyrolysis temperature on methane yield. Very probably, at 700 and 800 °C, CaO does not chemisorb CO
2 (Reaction (1)) as efficiently as at 600 °C (see
Figure 6); consequently, at the higher temperatures, the CO
2 that was not sorbed converts the methane by dry reforming (Reaction (3)). This mitigation of methane is evident in the apparent minimum methane yield observed at 700 °C, although this value could still fall within the error bars. Preliminary thermodynamic simulations have shown that, under the investigated experimental conditions, the theoretical methane yield is below 10 g/kg
digestate. This indicates that the thermochemical process is under kinetic control.
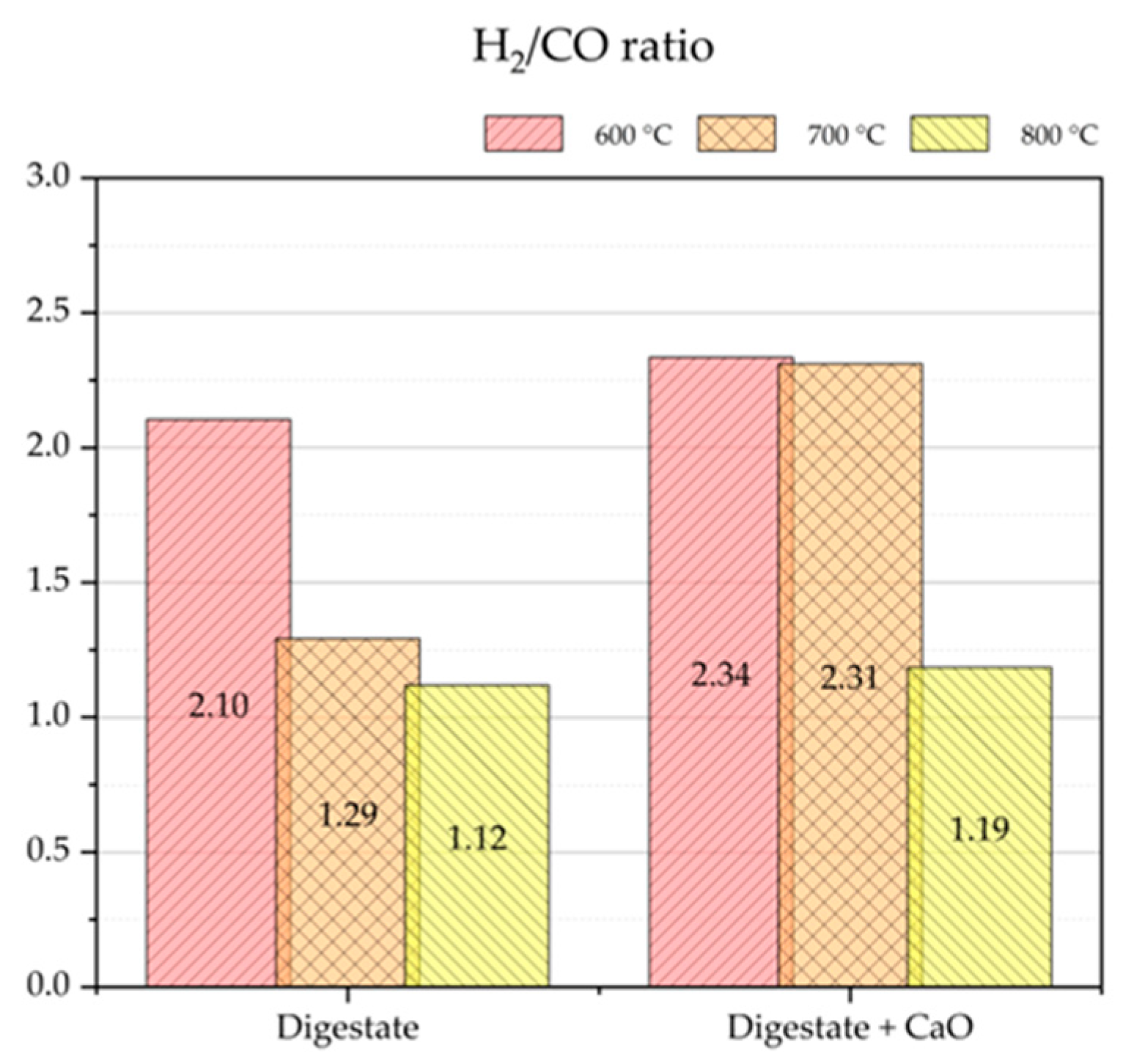
From a theoretical point of view, the H
2/CO molar ratio of syngas is of paramount importance for a hypothetical successive syngas catalytic methanation. An H
2/CO molar ratio equal to three satisfies the stoichiometric requirements of the methanation reaction (5).
The syngas with the highest molar ratio H
2/CO equal to 2.34 was detected when digestate was pyrolyzed at 600 °C with CaO (
Figure 8).
The pyrolysis carried out with CaO at 700 °C seems to give an optimum compromise between syngas with low CO2 and relatively high CH4 content, good H2/CO molar ratio, and good methane yield. The liquid output stream from the pyrolysis process proposed here was called “pyrolysis condensable products” because, as written above, it is a metastable mixture of water, water-soluble organic compounds, fine particulates, and waxes. Their heterogeneity was very challenging for an accurate chemical and physical characterization. Despite this, by opportune shaking, quite representative samples for the chemical and physical analysis were obtained from which it was assessed that chemical and physical properties are quite far from the promising pyrolysis oil obtained by fast pyrolysis.
This macroscopic difference is reasonably coherent with the different conditions of the pyrolysis experiment conducted here, characterized by a residence time of gas and vapours in the reactor in the range of some tens of seconds and a heating rate of the feedstock in the range of a few hundred °C/min.
Table 5 shows that the water content of condensable products ranges from 66.7% wt. for the pyrolysis of digestate to 47.0% wt. in the case of pyrolysis of digestate at 800 °C with CaO. As a general trend, the CaO sorbent addition to the pyrolysis decreases the water content of condensable products. These experimental data are in accordance with the catalytic activity of CaO in the steam reforming of organic molecules (Reaction (6)), where water is consumed.
The relatively high value of water content probably determines the relatively low value of kinematic viscosity, which ranged from 2 to 3 cSt for the six samples. Regarding the heating value of condensable products, it can be argued that they are quite low to consider the condensable products as bad fuels.
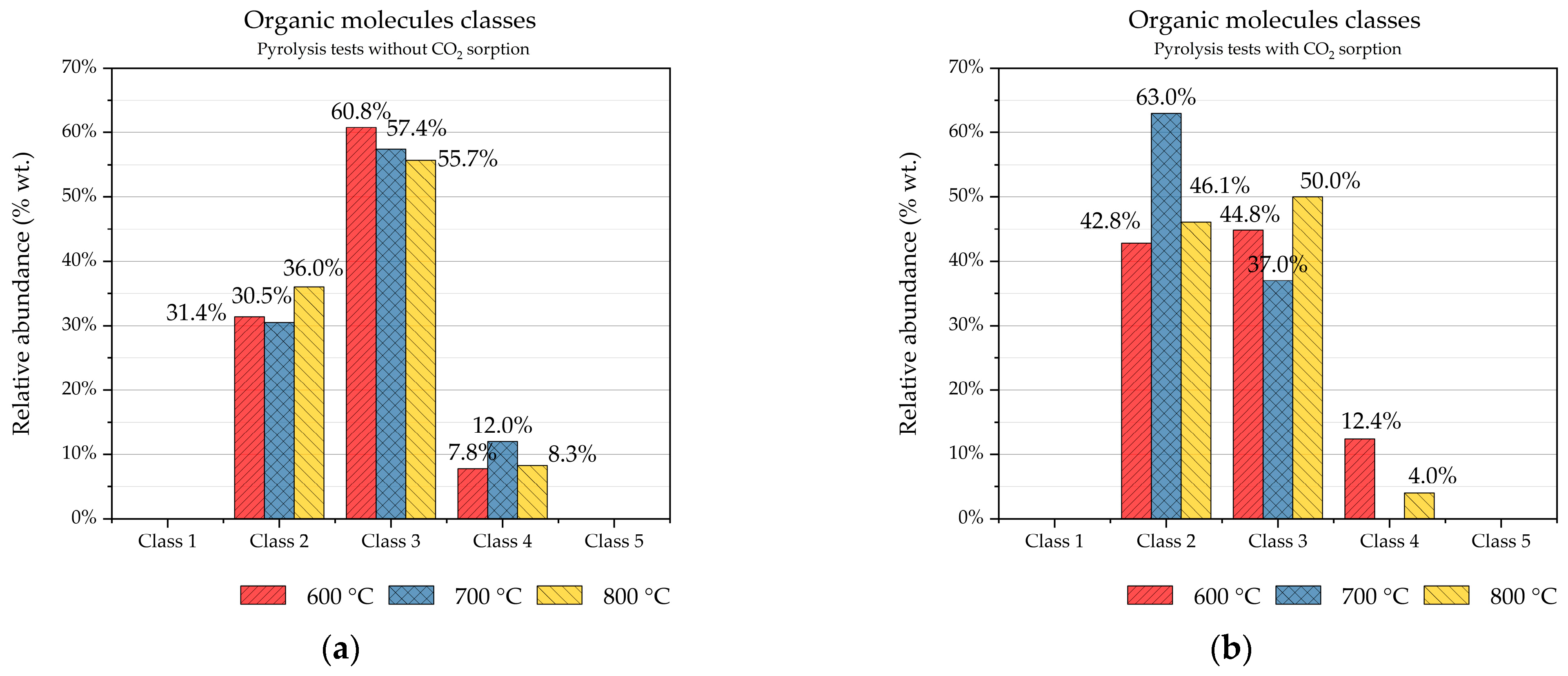
The organic fraction of condensable products was analysed by GC-MS. The detected organic molecules were classified according to the ECN tar classification to have a general framework and immediacy in the comprehension of the process. The relative abundances of the organic molecule classes of the condensable products of the six pyrolysis tests are shown in
Figure 9. In the case of pyrolysis of digestate, class III is the most abundant one (
Figure 9a). Chemical compounds with one aromatic ring, such as xylene, styrene, and toluene, belong to this class. When CaO is added to the pyrolysis process, an evident reduction in chemical compounds of class III was observed, as well as a reduction in chemical compounds belonging to class IV, which are organic molecules with two or three aromatic rings, such as naphthalene, phenanthrene, anthracene, and fluorene. Very probably, this reduction is caused by the catalytic effect of CaO in steam, dry reforming, and cracking reactions of the molecules of classes III and IV. In recent years, there has been growing interest in innovative materials capable of promoting the catalytic cracking of aromatic molecules in syngas, as extensively documented in the scientific literature [
26,
27]. The organic molecules of class II are heterocyclic components (like phenol, pyridine, cresol), generally having high water solubility, due to their polarity. From the comparison of
Figure 9a,b, CaO does not have an evident catalytic action on these molecules.
4. Conclusions
To date, the treatment of the digestate from AD of OFMSW industrially occurs by composting, which is a process that requires a large amount of energy, large spaces, and a long time to be completed. In this paper, the pyrolysis of digestate is proposed as an alternative, efficient, and scalable technology to dispose of digestate with the production of relatively methane-rich syngas. The experimental campaign was conducted using a bench-scale rotary kiln reactor, which demonstrated high operational reliability, with all tests completed without technical issues. In addition to baseline pyrolysis tests, in situ CO2 capture was investigated by co-feeding calcium oxide (CaO) as a sorbent. The syngas produced from digestate pyrolysis exhibited CO2 concentrations ranging from 9.2 to 13.9 vol.%.
The addition of CaO not only contributed to CO2 removal but also significantly enhanced methane production. The most notable increase in methane yield was observed at 600 °C, where the yield rose from 34 to 63 g CH4/kgdigestate with CaO addition. Correspondingly, the methane concentration in the syngas increased from 16.1 to 29.4 vol.%. At this temperature, the highest H2/CO molar ratio of 2.34 was also recorded. However, pyrolysis at 700 °C appeared to offer the best compromise, yielding a syngas with low CO2 content (9.2 vol.%), high CH4 concentration (24.9 vol.%), a favorable H2/CO ratio (2.31), and a methane yield of 60.47 g/kgdigestate. These conditions are particularly promising for subsequent catalytic methanation, where a high H2/CO ratio is advantageous.
The presence of CaO likely also played a catalytic role in the removal of organic vapours, promoting steam reforming, dry reforming, and cracking reactions. This was evidenced by a marked reduction in class III compounds and polycyclic aromatic hydrocarbons (PAHs) such as naphthalene, phenanthrene, anthracene, and fluorene (class IV compounds).
In conclusion, while pyrolysis presents a promising route for digestate conversion and syngas-based biomethane production, further research is required. Detailed energy assessments are necessary to evaluate the economic feasibility of the overall process. Additionally, plant optimization and heat recovery strategies should be explored to reduce the energy demand of pyrolysis. For instance, heat recovered from the combustion of secondary streams (e.g., char and pyrolysis oils) or from downstream units (e.g., methanation and CO2 separation) could be reused for digestate drying or to sustain the pyrolysis process itself. These aspects will be the focus of future investigations.