Abstract
Mushrooms are eukaryotic organisms with absorptive heterotrophic nutrition, capable of feeding on organic matter rich in cellulose and lignocellulose. Since ancient times, they have been considered allies and, in certain cultures, they were seen as magical beings or food of the gods. Of the great variety of edible mushrooms identified worldwide, less than 2% are traded on the market. Although mushrooms have been valued for their multiple nutritional and healing benefits, some cultures perceive them as toxic and do not accept them in their culinary practices. Despite the existing skepticism, several researchers are promoting the potential of edible mushrooms. There are two main methods of mushroom cultivation: solid-state fermentation and submerged fermentation. The former is the most widely used and simplest, since the fungus grows in its natural environment; in the latter, the fungus grows suspended without developing a fruiting body. In addition, submerged fermentation is easily monitored and scalable. Both systems are important and have their limitations. This article discusses the main methods used to increase the performance of submerged fermentation with emphasis on the modes of operation used, types of bioreactors and application of morphological bioengineering of filamentous fungi, and especially the use of intelligent automatic control technologies and the use of non-invasive monitoring in fermentation systems thanks to the development of machine learning (ML), neural networks, and the use of big data, which will allow more accurate decisions to be made in the fermentation of filamentous fungi in submerged environments with improvements in production yields.
1. Introduction
The Basidiomycota constitutes the second largest higher taxonomic group of the Fungi after the Ascomycota and comprises over 30,000 species. Mycelial culture of Basidiomycota has already been studied since the 1950s for the production of antibiotics and other beneficial secondary metabolites [1]. It is estimated that more than 2000 species of mushrooms are considered edible [2], and around 25 of them are commercially available as food [3,4].
Commercial mushroom products are obtained from the fruiting bodies of field-cultivated mushrooms, which represent an intensive process over a long period of time [5]. It is reported that approximately 80–85% of edible mushrooms, whether in the form of powders, extracts, or protein hydrolysates, have been obtained from solid-state fermentation, while the remaining approximately 15% were obtained through submerged fermentation [6]. As a result of the increasing production of mushroom- and mycelium-derived products, the European Union incorporated these items into a new regulatory framework for novel foods in 2019, establishing a more structured approach to monitoring production systems and refining their categorization [7]. Currently, global mushroom production is led by Asia, Europe, and the United States, accounting for 76%, 17.2%, and 5.9%, respectively [8]. It is estimated that the global production of edible and medicinal mushrooms will likely exceed 50 million tons by 2025 [9].
Due to the reported nutritional benefits of edible mushrooms, the global mushroom industry has grown significantly. However, for mushrooms to become a resilient food option, they must be more economically viable. Mushrooms have a lower protein concentration compared to meat, and scaling up production would require substantial energy resources as well as the maintenance of temperature and humidity within specified ranges, raising concerns about their sustainability. New policies are needed to improve their technical and economic viability [10].
According to various studies, around 130 pharmacological properties of different fungi have been identified, including immunostimulant, hepatoprotective, antioxidant, hypoglycemic, and anticarcinogenic properties. Additionally, more than 600 clinical studies in different phases of development have been published worldwide [11].
Edible medicinal mushrooms constitute a resource for obtaining biomolecules with immunomodulatory and antitumor activity, with growing applications in treating cancer, immunodeficiencies, and infectious diseases. The genus Pleurotus sp. stands out as one of the most researched worldwide, being capable of producing substances that stimulate the host’s immune system [12,13,14]. Among the wide variety of substances, high molecular weight compounds such as β-(1,3)-(1,6)-D-glucans, proteins, proteoglycans, and polysaccharide–protein complexes are notable, as well as low molecular weight secondary metabolites, including terpenes [15]. Edible basidiomycetes have an enzymatic repertoire with biotechnological applications, particularly in synthesizing natural flavors and food-grade pigments, serving as healthier alternatives to artificial colorants [16].
Among the species of edible mushrooms that dominate the global market, the shiitake mushroom (Lentinula edodes) ranks first with 22%, followed by the oyster mushroom (Pleurotus spp.) with 19%, and Auricularia (known as wood ear) in third place by a narrow margin, with 18%. Subsequently, species such as Agaricus spp., Flammulina spp., Volvariella spp., and others follow with 15%, 11%, and 5%. Other species represent the remaining 10% [17,18].
The mycochemical industry is well-positioned globally and serves as a fundamental pillar for obtaining essential compounds with extensive applications in the chemical–pharmaceutical and food industries [19]. The production of bioactive compounds and fungal biomass from Basidiomycetes faces the primary challenge of achieving high yields in a shorter time while avoiding any signs of contamination [20].
Submerged fermentation (SmF) offers an efficient method for producing bioactive compounds from edible mushrooms, such as Pleurotus ostreatus. Compared to solid-state systems, SmF reduces fermentation time and contamination risks while optimizing workspace usage. However, challenges like high energy costs and shear stress on mycelial morphology must be addressed for industrial scaling [21,22].
2. Submerged Cultivation
Edible fungi serve as a sustainable source for the supply of unique compounds with demonstrated biological activity, emerging as valuable contributors to human well-being [23]. Currently, most mushroom-derived products come from fruiting bodies obtained from cultivation farms or collected in the field. For this reason, it is difficult to predict the quality of the final product due to the intrinsic heterogeneity of the substrates where the mushrooms grow and the climatic conditions in which they develop [21].
The fermentation of mushrooms in submerged conditions represents the future for industrial fermentation processes to obtain fungal biomass with high added value. It could be used as a source of food, nutraceuticals, and compounds with pharmacological activity, as it is a faster, easier method for maintaining optimal process conditions [24,25].
Submerged fermentation of edible mushrooms has garnered attention due to its industrial potential. However, successful commercial scale-up depends directly on addressing certain technical limitations, such as increasing product yield indicators, improving production process efficiency, and developing enhanced cultivation systems [26].
A wide variety of edible mushrooms can develop in agro-industrial by-products as submerged cultures, achieving high yields and reducing fermentation time [27]. Generally, these agricultural by-products—such as olive mill waste, second cheese whey, brewer’s spent grain, and wine distillery effluents—are rich in carbon and nitrogen. Carbon sources are important as they serve as structural blocks for organisms’ cellular maintenance and metabolic energy acquisition. Additionally, nitrogen sources are crucial in protein synthesis processes and proper metabolic development at the cellular level. It is emphasized that the use of these agricultural residues is important to promote sustainable and eco-friendly cultivation processes [28].
3. Morphology of Fungi in Submerged Fermentation
Filamentous fungi can grow in a dispersed form, or as small compact cotton-like specks called pellets, which are condensed hyphal structures that mostly form a defined core. It has been indicated that there is a relationship between aggregates’ morphology and the productivity of the fermentation process. Generally, in a culture, compact, small-volume pellets are required, as dispersed morphology increases the viscosity of the culture broth. In this sense, there is no optimal morphology related to productivity; it all depends on the strain and the operating conditions [29]. Productivity should be assessed from different product perspectives, as there is currently no harmonized rule regarding the correlation between fungal aggregate morphology, system rheology, and productivity [30].
In submerged cultivation, the morphology of mycelial aggregates can develop in various forms, and these morphological changes are directly related to fermentation process variables such as the strain, the composition of the culture medium, and the cultivation process conditions (Figure 1) [31]. On the other hand, genetic engineering targeting genes associated with fungal growth morphology has become a powerful tool to influence fungal morphology in submerged processes [19].
Pellet formation (compact spherical mycelial structures) in submerged fungal cultures critically depends on the strain’s coagulating capacity. Coagulating type strains exhibit hydrophobic conidia that aggregate upon inoculation, forming unified germination nuclei from which hyphae grow radially into pellets (see Figure 2). Conversely, noncoagulating strains display hydrophilic conidia that remain dispersed, germinating individually to generate disorganized filamentous networks. Reduced inoculum size is also reported to cause pellet formation [19,32]. Notably, pellet formation does not require spores in the culture medium, making it particularly suitable for asporogenous fungi or strains with limited sporulation in submerged cultures. Homogenizing mycelium with fragmentation devices provides an alternative route to generate pellets, offering a scalable and cost-effective inoculum production method for industrial applications [33,34,35].
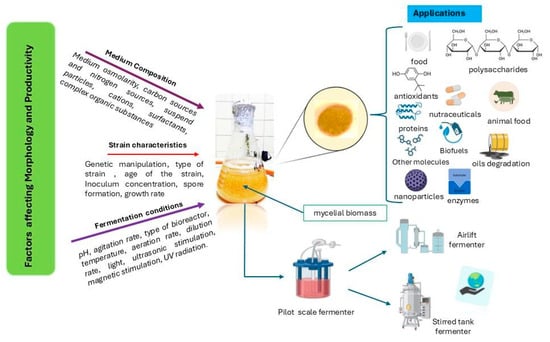
Figure 1.
Mushroom cultivated in submerged state fermentation. Figure constructed using information from Dzurendova, Losada [36] and El Enshasy [19]. This figure incorporates graphical elements partially created with BioRender Web, accessed via Mozilla Firefox browser on Windows 11 operating system, under the Free plan.
Figure 1.
Mushroom cultivated in submerged state fermentation. Figure constructed using information from Dzurendova, Losada [36] and El Enshasy [19]. This figure incorporates graphical elements partially created with BioRender Web, accessed via Mozilla Firefox browser on Windows 11 operating system, under the Free plan.
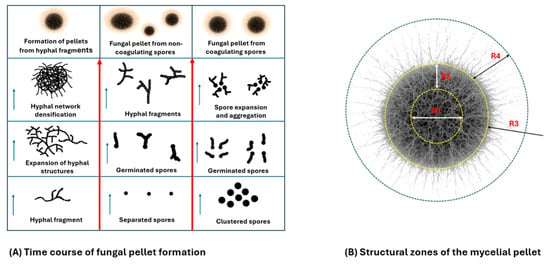
Figure 2.
(A) illustrates the main shapes from which a mycelial pellet is formed. The arrows indicate the direction of aggregate formation. (B) represents the different zones of a perfect fungal pellet. R1: Central core (necrotic/lysis zone)—a region with dead or autolyzing hyphae caused by nutrient limitation, buildup of metabolic by-products, and oxygen deprivation. R2: Middle region (transition zone)—features loosely arranged hyphae exhibiting slowed growth and early autolysis, resulting from oxygen and nutrient gradients. R3: Boundary Zone—a transitional, metabolically active region separating living and necrotic hyphae. R4: Peripheral Hairy Zone—characterized by outward-growing hyphae (“hairy” morphology), exhibiting maximal metabolic activity and viability due to direct access to oxygen and nutrients. Figure constructed using information from El Enshasy [19] and Veiter, Rajamanickam [29], With partial assistance from the online version of ChatGPT-4o by OpenAI.
Figure 2.
(A) illustrates the main shapes from which a mycelial pellet is formed. The arrows indicate the direction of aggregate formation. (B) represents the different zones of a perfect fungal pellet. R1: Central core (necrotic/lysis zone)—a region with dead or autolyzing hyphae caused by nutrient limitation, buildup of metabolic by-products, and oxygen deprivation. R2: Middle region (transition zone)—features loosely arranged hyphae exhibiting slowed growth and early autolysis, resulting from oxygen and nutrient gradients. R3: Boundary Zone—a transitional, metabolically active region separating living and necrotic hyphae. R4: Peripheral Hairy Zone—characterized by outward-growing hyphae (“hairy” morphology), exhibiting maximal metabolic activity and viability due to direct access to oxygen and nutrients. Figure constructed using information from El Enshasy [19] and Veiter, Rajamanickam [29], With partial assistance from the online version of ChatGPT-4o by OpenAI.
Fungal biomass in pellet form offers greater technological advantages, as it can be separated from the spent cultivation medium while exhibiting superior mixing and mass transfer properties, allowing for the optimization of energy and material resources in the process [37]. Additionally, this morphology is preferred at the industrial level, as it does not increase the rheology of the system compared to dispersed systems, allowing for the optimization of energy and material resources in the process [38]. The morphology of aggregates generally leads to a Newtonian behavior of the broth [39]. The formation of cellular aggregates (pellets) determines the existence of external and internal diffusion limitations [40,41]. The latter results in anoxia conditions, severe nutrient restriction, product intoxication, and cell autolysis. Inside the core of the pellet, nutrient transport is governed by diffusion, and therefore, it is related to the diameter of the pellet and is significantly slower than convective transfer within the liquid phase [42]. In cultures of Aspergillus terreus, cell death processes were evidenced inside aggregates, because oxygen only penetrated those with diameters smaller than 1400 μm [43].
In a study by [35] to optimize the submerged fermentation of the mushroom Inonotus hispidus and maximize polysaccharide and biomass production, two inoculum types were compared: dispersed mycelial fragments and aggregated clumps. Dispersed fragments demonstrated superior performance, exhibiting a shorter lag phase, higher biomass yield (10.43 vs. 5.29 g/L), and enhanced polysaccharide production, alongside more uniform pellet formation. Optimal fragmentation was achieved at 26,000 rpm for 3 min, improving nutrient transfer efficiency. Inoculum age (6 days) and recycling (up to 8 cycles) maintained productivity. When scaled to 100 L bioreactors in fed-batch mode, the process achieved 22.23 g/L biomass, 3.31 g/L EPS (Extracellular Polysaccharides), and 5.21 g/L IPS (Intracellular Polysaccharides), significantly surpassing smaller-scale results.
Pellet formation in fungal submerged fermentation is influenced by a complex interplay of factors. The geometry of the fermenter, culture mode, and rheological properties of the broth can play significant roles in determining pellet size, density, and uniformity. Initial inoculum size, medium pH, dissolved oxygen levels, agitation intensity, nutrient composition (such as carbon and nitrogen sources), trace metal concentrations, temperature, and the presence of additives or nucleating agents can affect the aggregation and growth patterns of fungal hyphae, leading to variations in pellet morphology.
3.1. Microparticles in Fungal Submerged Fermentation
The addition of microparticles in submerged fungal fermentation acts as nucleating agents, physically disrupting hyphal aggregation and promoting the formation of smaller, more uniform pellets, thereby further modulating fungal morphology during submerged fermentation. It has also been demonstrated that microparticle addition influences the expression of genes related to pigment synthesis. Huang, Guang [44] investigated the submerged fermentation of Monascus purpureus with microparticles (talc, Al2O3, SiO2, TiO2) to evaluate their effect on yellow pigment production. Furthermore, key genes (MrpigG, MrpigD, MrpigE, and MrpigH) involved in pigment biosynthesis were upregulated, indicating that microparticles modulate both fungal morphology and gene regulation to enhance metabolic productivity [44].
In Cordyceps sinensis fermentations, synergistic effects of talc microparticles (10 g/L, 2000-mesh) and high glucose (150 g/L) boost exopolysaccharide (EPS) yields by 1.8-fold to 4.21 g/L through targeted morphological engineering. Strategic microparticle timing induces critical structural shifts: smaller pellets and hypertrophied hyphae elevate oxygen/nutrient uptake. Concurrently, intracellular ROS-ATP surges activate polysaccharide synthases, with proteomic analysis confirming ADH activity as a key contributor in crude EPS. These findings position microparticle-mediated morphotype engineering as a scalable platform for fungal bioprocessing, albeit requiring strain-specific adaptation [45].
On the other hand, other researchers investigated how natural precursors and the addition of microparticles (silica) and macroparticles (glass beads) influence the morphology of Streptomyces toxytricini KD18 and its lipstatin productivity. Results demonstrated that particles reduced pellet size and increased lipstatin production by up to 4.5-fold. Combining all precursors in a bioreactor elevated production to 37.97 mg/mL (18.4-fold higher than the reference flask), with small, dispersed pellets (100 µm). Smaller, dispersed pellet morphology correlated with higher productivity, underscoring the importance of morphology engineering for industrial processes [46].
The addition of microparticles exerts mechanical stress on the mycelium of Paraisaria dubia, modifying both the cell wall structure and the extracellular matrix. These changes were confirmed by scanning electron microscopy (SEM): in submerged cultures supplemented with talc (15 g/L), the mycelium exhibited a rough surface and irregular morphology, along with reduced formation of dispersed aggregates. This phenomenon is attributed to the shear stress generated by the particles, which favored the dispersion of the mycelium into smaller and less dense pellets. The results demonstrate that microparticles not only physically modify morphology, but also transcriptionally reprogram the fungus, triggering adaptive responses mediated by the activation of key genes in the MAPK (Mitogen-Activated Protein Kinase) pathway, stress sensors (Wsc/Mid), cell integrity regulators (Rho1, Pkc1), and effectors such as the Slt2 kinase. This signaling triggered critical morphogenetic changes [47].
Similar results were obtained with the addition of talc (20 g/L) in submerged cultures of the nematophagous fungus Duddingtonia flagrans, aiming to optimize chlamydospore production. The addition of talc increased spore concentration by 42% without compromising their functionality. SEM analysis confirmed that the addition of talc microparticles induced variations in the structure of the chlamydospore cell walls compared to the control group. The morphology shifted from heterogeneous forms, including immature chlamydospores (type C: thin wall, less rigid), to mature and uniform chlamydospores (types A/B: rigid walls, with or without morulae), thereby improving the yield of mature chlamydospores—a key factor in their effectiveness as a nematophagous agent. It is noteworthy that talc promotes more dispersed and homogeneous mycelial forms [48].
Particles with sharp edges (talc and Al2O3) induced beneficial morphological changes in the mycelium of Monascus purpureus. These microparticles prevented spore and mycelium agglomeration through mechanical friction, generating smaller pellets and increasing the specific surface area (557.6 m2/kg in the group treated with talc at 4 g/L vs. 320.1 m2/kg in the control). This change improved nutrient transfer and, consequently, the productivity of yellow pigments. SEM analysis revealed significant differences between the groups: while the control showed smooth hyphae and dense aggregates, the microparticle treatments resulted in thinner, rougher hyphae with a notable reduction in mycelial aggregation [44]. Although the use of microparticles appears to be a cost-effective and straightforward technique for improving yields, several uncertainties remain regarding downstream processing, particularly the efficiency and economic feasibility of separating these particles from biomass or fermentation broth [49].
A broad range of microparticles is employed to modify fungal morphology in submerged culture. These include kaolin, nanoclay, florisil, aluminum silicate, titanium silicate, aluminum titanate, zirconium silicate, sodium octenyl succinate starch, talc, Al2O3, SiO2, ZnO, and titanium silicone oxide, as well as magnetite and forsterite (Mg2SiO4). Each exhibits distinct physical and chemical properties. However, there is a notable scarcity of literature addressing the mechanisms involved and the criteria for selecting specific microparticles for a given purpose [49,50,51]. Furthermore, from a food safety and toxicity point of view, these materials may pose potential risks, as their interactions with biological systems are not well understood. Depending on the specific particle, dose, and particle size, effects could range from severe gastrointestinal irritation and obstruction to systemic toxicity due to metals or impurities. Therefore, further research is needed to better understand these mechanisms and to explore the use of biodegradable biomaterials as safer alternatives.
3.2. Safety and Toxicity Issues of Microparticles in Fungal Submerged Fermentation
The use of microparticles, mainly metal oxides, for morphological control of filamentous microorganisms has proven to be an effective method for optimizing the productivity of these bioprocesses. However, the interaction of these oxides with a reducing biological medium, such as culture broth, could generate potentially toxic compounds (nanoparticles derived from degradation) due to their greater reactivity and cell penetration capacity. This raises concerns from a food safety perspective, especially when the products of these fermentations are intended for human and animal consumption [52]. Particles such as TiO2 can generate ROS under light conditions, leading to the oxidative degradation of valuable nutrients or metabolites in the culture medium. This process, known as the ‘ageing cycle’, could produce unwanted by-products with potential toxicity. It is suggested that the ions released from these oxides can act as catalysts in redox reactions, generating reactive oxygen species that damage microorganisms and induce oxidative stress in humans [53].
Titanium dioxide (TiO2) has been classified by the International Agency for Research on Cancer (IARC) as possibly carcinogenic to humans (Group 2B), after lung tumors were discovered in rats exposed to high concentrations of fine TiO2 particles for two years. However, this decision has been controversial [54]. It has been reported that frequent interaction with TiO2 nanoparticles produces harmful effects on the small intestine, liver, cardiac system, hippocampal neurons, and reproductive system, and also presents genotoxic effects that can lead to apoptosis and chromosomal instability [55].
Other compounds that have been used are titanates, although titanates are chemically inert under fermentation conditions, their long-term biocompatibility has not yet been explored. A study suggests that calcium titanate nanoparticles (CaTiO3NPs) have a low toxic effect on non-cancerous cells (HSF, human skin fibroblasts) even at high concentrations (up to 1670.65 µg/mL), but they do induce genotoxic damage and oxidative stress in cancer cells (A-549) [56].
To mitigate these risks in terms of food safety, the use of biodegradable microparticles such as chitosan, alginate, and microcrystalline cellulose is proposed. Chitosan, a polymer derived from chitin present in the cell walls of fungi and insects, has been used to immobilize microorganisms, improving fermentation yields thanks to its porous structure. Recent studies have shown that ZnO and TiO2 nanoparticles (NPs) have more toxic effects than microparticles, as their smaller size, greater surface area, and enhanced ability to cross the blood–brain barrier result in greater toxicological effects in cellular systems [57]. The use of composite microspheres based on sodium alginate (SA) and Tremella fuciformis polysaccharide has been shown to be biodegradable and biocompatible in food applications [58].
4. Bioreactors for Submerged Fungal Fermentation
A bioreactor is a biologically active vessel designed to maintain an optimal environment in which a biological agent can degrade a substrate. This biological agent may be an enzyme or a microorganism. Depending on the characteristics of the process, the bioreactor or fermenter may vary in complexity. The most well-known type of bioreactor, due to its advantages and versatility, is the stirred-tank bioreactor, which incorporates mechanical agitation systems. Additionally, there are other types of bioreactors, such as airlift reactors and bubble columns, which utilize air injection systems through compression, facilitating an ascending and descending flow of the liquid column [59].
A bioreactor functions as an integrated feedback-regulated system, where synergy between thermal regulation, gas management, nutrient dosing, ionic regulation, and fluid dynamics determines bioprocess efficiency. Its design must align with the specific biological requirements of the biocatalyst. The stirred-tank reactor (STR) is the most widely used fermentation system in the biotechnology industry due to its superior capability to maximize the volumetric oxygen transfer coefficient (KLa), a critical parameter in aerobic processes. Through mechanical agitation (Rushton impellers) and forced aeration (spargers) [60]. Emerging bioreactor architectures are increasingly biocatalyst-centric, with physiological adaptation, particularly in oxygen transfer and fluid dynamics, resolving long-standing metabolic constraints in diverse biological systems. In contrast, reactors for anaerobic culture are often very simple [61].
4.1. Stirred Tank Bioreactor (STR)
A stirred tank bioreactor consists of a vessel equipped with one or more impellers to maintain homogeneity within the system. The biomass and gases are contained within the liquid, forming a single mixture. Oxygen can be introduced into the vessel in several ways; the most common is through a perforated device placed at the bottom (“sparger”) that supplies a stream of air bubbles, which not only provides oxygen to the culture, but also helps maintain mixing between the liquid layers. However, this method of oxygenation is reported to be the source of the greatest mechanical stress for suspension cultures, even more so than that introduced by agitation [61,62].
Among the most commonly used impellers to ensure proper homogenization are the so-called “Rushton turbine” impellers, which are efficient at providing the high oxygen transfer rates required for high cell density fermentations. Stirred tank reactors have the advantage of good mixing properties and volumetric productivities, in contrast to the high energy cost of agitation and oxygen supply. Airlift bioreactors (ALRs) offer the advantage of lower energy input coupled with efficient heat transfer [63]. The main advantage offered by stirred tank bioreactors is their scalability. The largest industrial scale currently reported is 20,000 L for batch or fed-batch operations, and 2000 L for perfusion operations employing a “spinfilter” as a cell retention device [64].
4.2. Air Lift Bioreactor
Airlift bioreactors are cultivation systems that introduce oxygen-rich air into the system. These reactors feature a tall, tubular, or cylindrical design and can be scaled for large-scale operations. Mixing and homogenization occur when compressed air is injected through a sparger at the reactor’s base. The air rises as bubbles through the culture broth, reducing its density and generating convective upward and downward currents within the vessel to maintain circulation. A key advantage of these systems is their gentle handling of materials due to significantly low shear forces, making them ideal for sensitive applications. However, the downside is the high operational cost associated with gas compression, which can impact the overall economic feasibility of the process [65].
There are two main classifications of airlift bioreactors: the internal concentric tube type (think “tube-in-a-tube” setup) and the external loop type. The external loop bioreactors are less commonly used in large-scale industrial applications. Nevertheless, these bioreactors have demonstrated excellent performance in cultivating a wide range of organisms, including bacteria, yeast, filamentous fungi, animal cells, plant cells, and microalgae. They have also proven effective for enzyme biocatalysis [66].
This system is well-suited for the cultivation of filamentous fungi, as it facilitates oxygen distribution of up to 0.4 vol of air/vol of liquid/min. This is achieved through the presence of aerators that generate bubbles of varying sizes, ensuring effective homogenization of the medium. Nevertheless, a significant operational challenge that must be addressed is the occurrence of mixing dead spots, which restrict biomass productivity and the biosynthesis of target compounds [67].
An innovative study, based on optimization through computational fluid dynamics (CFD) and the strategic installation of an internal baffle in an airlift bioreactor for the fermentation of Fusarium venenatum, increased gas retention by 23.55% and mass transfer (Kla) by 30.07%, eliminating inactive flow zones. This improvement allowed for greater efficiency (reducing fermentation time from 72 to 48 h) and productivity (enhancing biomass conversion by 66.7% and glucose consumption by 54.32%) when validated with a real reactor [68].
The morphology of mycelial aggregates is affected by the shear stresses generated by mechanical agitation and mixing systems, which trigger changes in the shape and physiology of the process, thereby influencing yields. A favorable option to mitigate this phenomenon is the use of airlift bioreactors, where the volumetric oxygen transfer coefficient (KLa) is optimal. These systems have been implemented on a large scale with few microorganisms, such as Aspergillus and Fusarium venenatum. Their industrial-scale application has only been reported for Basidiomycetes in biomass production. Despite their advantages, they are not commonly used in high biomass density systems [69].
The growth of mycelium in a spherical form, termed pellets, presents limitations and challenges in gas transfer from the exterior to the interior of the pellet. This creates a zone in its core called the “dead zone,” resulting from the low metabolic rate within these mycelial aggregates. These limitations lead to reduced growth rates and negatively influence the production of secondary metabolites. Generally, fungi in submerged cultures require aerobic conditions of 0.05–0.4 volumes of air per volume of liquid per minute for optimal growth [67].
Some studies that have reported the effectiveness of using airlift reactors focus on wastewater purification processes and enzyme production. For example, the mycoremediation of vinasse using the fungus Pleurotus sajor-caju evaluated two air-lift bioreactor configurations (7.0 L) for vinasse treatment: reactor 1 (with an enlarged degassing zone) and reactor 2 (without an enlarged degassing zone). Results demonstrated that reactor 1 achieved reductions of 53% COD, 71% BOD5, and 68.5% decolorization of the residue, while reactor 2 showed 58% COD, 58% BOD5, and 62.0% decolorization. Notably, the bioreactors exhibited enzymatic activities (laccase and manganese peroxidase) 3–10 times higher than those in shake flasks. However, decolorization efficiency in shake flasks was superior (94% vs. ~65% in air-lift bioreactors). The authors propose that combining mycoremediation with air-lift bioreactor systems offers a sustainable solution for the sugarcane-alcohol industry, balancing pollutant removal, enzyme production, and scalability [70].
Other studies, based on the production of polysaccharides with biological activity, evaluated five basidiomycete strains (Trametes versicolor M9911, Lentinula edodes M3102, Hericium erinaceus M9514, Agaricus blazei M7700, and Agaricus bisporus ABS14). Their growth was assessed in batch and semi-continuous fermentations using an airlift bioreactor. Trametes versicolor exhibited the highest performance: in batch mode, it achieved a maximum biomass concentration of 15.7 g/L, while in semi-continuous operation, it reached 12.3 g/L of cell dry weight with a volumetric productivity of 0.48 g/L/h. The maximum volumetric productivity (0.87 g/L/h). The airlift bioreactor enabled prolonged cultivation under low shear stress conditions, demonstrating its suitability for filamentous fungal cultures [71].
A study on obtaining fungal biomass of Pleurotus flabellatus through submerged fermentation over 10 days demonstrated that the use of an airlift bioreactor enabled controlled and efficient mycelium production, outperforming traditional solid-state cultivation methods in terms of scalability and yield with a notable production of endopolysaccharides at a yield of 0.45 g/L, highlighting its potential for industrial applications in obtaining high-value biological compounds [72].
At an industrial level, the production of mycoprotein from Fusarium venenatum holds a prominent position in the scaling of airlift fermenters. The systems used by Quorn™ feature fermenters with volumes of up to 155 m3, allowing for productivities of up to 14,000 tonnes of fungal protein annually [73]. Fungal protein production methods utilizing fermentation are ecologically sustainable processes. These methods require sequential stages of fractionation, extraction, and, in some phases, antinutrient inactivation. They occupy a critical niche in the food industry, serving as meat analogs, enzymes, and additives [74].
5. Operational Modes Employed to Produce Mycelium and Metabolites Derived from Mushrooms
In the production of fungal mycelium derived from edible and medicinal mushrooms, various operational modes have been employed, each with its own advantages and limitations. The choice of a particular method depends on the specific characteristics of the process as well as its technical and economic feasibility.
5.1. Batch Mode
Batch fermentation, also known as discontinuous fermentation, can be considered a “closed system.” At the beginning of the operation, the sterile medium containing the nutrients is added, followed by the inoculum with the initial cell content (X0). During the fermentation, oxygen, an antifoaming agent, and a pH controller are added. The duration of a batch fermentation depends on the time required to achieve the desired product concentration or the substrate conversion level [75].
However, in practice, this operation involves relatively long unproductive periods such as cleaning and sterilization for a new setup, and, in cell cultures, the adaptation phase, during which the cells do not grow and no product is formed [76]. The conventional batch mode suffers from fundamental constraints stemming from accumulating metabolic waste products. These inhibitory compounds progressively impair microbial proliferation and ultimately limit the synthesis of high-value biomolecules through growth suppression and culture destabilization [77]. Nevertheless, this mode of operation has several advantages: it is a simple process, presents a low risk of contamination compared with other modes, and is used as a benchmark for initial studies [78].
Implementing an automated repeated-batch fermentation system, combining fungal growth in pellet form with sporulation supports and CGQ sensors for online monitoring, represents a significant technical advancement in the sustainable production of secondary metabolites. This approach addresses historical challenges such as biofouling and morphological instability, enhancing process efficiency and scalability [79].
However, the primary difficulty lies in residual mycelial growth in the upper regions of the reactor, particularly near the backscatter sensor, which distorts measurements. To mitigate this, validating the compatibility of nonionic surfactants during intermediate cleaning is proposed. These agents would reduce biomass adhesion on critical surfaces without interfering with spore viability or fungal metabolism. Their application would complement established strategies, such as optimizing the cleaning lance, using anti-adhesive materials, and redesigning sporulation supports. The integration of nonionic surfactants could specifically prevent mycelial accumulation in sensitive areas, improving sensor accuracy and ensuring consistent pellet growth. Together, these innovations not only minimize measurement errors but also reinforce system robustness, paving the way for more reliable and productive industrial applications in filamentous fungal biotechnology.
5.2. Fed-Batch Mode
Another operating system that leads the biotechnology industry due to its advantages is the fed-batch system. It is characterized by the fact that a certain volume of culture medium with the correct proportion of inoculum is added to the reactor, and then fresh medium is gradually fed in until the maximum operating volume is reached. This gradual addition of medium allows for dilution, which helps reduce the inhibitory effects caused by substrate consumption and metabolic by-products [80]. This type of system is used on a large scale due to its mentioned benefits, as it improves the yields of secondary metabolites [81].
For example, in a comparative study on submerged fermentation of the fungus Inonotus hispidus in a 100 L bioreactor, fed-batch cultivation achieved a biomass yield of 22.23 g/L, extracellular polysaccharides (EPS) of 3.31 g/L, and intracellular polysaccharides (IPS) of 5.21 g/L. In contrast, batch cultivation yielded 17.76 g/L biomass, 2.87 g/L EPS, and 4.81 g/L IPS. Notably, fed-batch cultivation reduced biomass growth time by one-third compared to the 5 L batch process (90 h vs. 144 h). These results demonstrate that fed-batch mode outperformed batch cultivation in both productivity and time efficiency, with 25% higher biomass, 15% higher EPS, and 8% higher IPS production. The reduced cultivation time and enhanced yields highlight the industrial potential of fed-batch strategies for scalable fungal fermentation processes [35].
In a similar study, 5 L stirred-tank bioreactors operated in fed-batch and batch modes were used to produce EPS and ergosterol using Agaricus brasiliensis. The fed-batch mode proved superior, significantly enhancing the production of both metabolites and biomass concentration (EPS = 1.67 ± 0.08 g/L [+26.9% vs. batch]; ergosterol = 2.50 ± 0.04 mg/100 mg DW [+17.2% vs. batch] at 144 h), attributed to controlled glucose feeding to prevent substrate inhibition and sustain productivity [82].
In a study on the valorization of animal fat by-products (category 3) through submerged fermentation using oleaginous fungi (Mucor circinelloides and Mortierella alpina), single-cell oils (SCOs) rich in polyunsaturated fatty acids (PUFAs), such as arachidonic acid (ARA) and gamma-linolenic acid (GLA), were produced. The process was optimized by comparing batch and fed-batch modes in a 15 L bioreactor. Results demonstrated that fed-batch fermentation was superior for Mortierella alpina, achieving higher biomass production (8.3 g/L), lipid content (55.8%), and ARA yield (23.8% of total lipids). However, for Mucor circinelloides, batch cultivation proved more effective due to technical challenges such as foam formation in the fed-batch mode, which compromised efficiency [83]. A research team developed a directed submerged fermentation process for the stable and efficient production of high molecular weight intracellular polysaccharides (IPS) from Ganoderma lucidum, focusing on their defined structure and bioactivity. Stirred-tank bioreactors (3 L, 50 L, and 500 L) were used for process scaling, while evaluating both batch and fed-batch operation modes. The fed-batch mode, implemented in the 500 L bioreactor with an initial glucose concentration of 20 g/L, proved superior, optimizing both biomass and high MW IPS production. This approach resulted in a 33.24% increase in high MW IPS content and a 17.16% increase in biomass compared to the 50 L bioreactor [84].
5.3. Continuous Fermentation Mode
A continuous bioreactor is a system where microorganisms (bacteria, yeast, fungi, etc.) are cultivated under controlled conditions, with a constant inflow of fresh medium and outflow of product, maintaining a constant volume in the reactor. Unlike batch or fed-batch systems, this mode operates in a steady state, where the microbial growth rate balances with the dilution rate (outflow rate). In prolonged cultures, strains may mutate or lose productivity. Furthermore, it is not suitable for products that require a stationary phase (secondary metabolites) [85,86].
The continuous submerged fermentation of edible and medicinal mushrooms has not been extensively explored, likely due to inherent technical and biological challenges. Key obstacles include the formation of viscous mycelial networks, which impede mixing efficiency and oxygen transfer. To date, batch and fed-batch processes remain dominant in industrial applications owing to their operational simplicity. To date, only one study has reported the use of continuous fermentation for Lentinus edodes biomass production, demonstrating a 14-fold increase in productivity compared to batch operation [87].
6. Solid-State Fermentation Systems for Edible Mushrooms
A solid-state fermentation (SSF) process is characterized by the presence of three fundamental phases: the solid phase, represented by the substrate, which serves as a support for mycelial growth; the liquid phase, associated with the free water present within the substrate; and the gaseous phase, which encompasses all gases involved in metabolism, including gaseous waste products and the supplied air necessary to maintain optimal fermentation conditions [88].
An ideal substrate in solid-state fermentation (SSF) provides all necessary nutrients for microbial growth. However, in most cases, substrates must be enriched with mineral salts or compounds that enhance fungal growth [89]. This includes carbohydrates, lipids, and proteins, and their catabolic processes at the cellular level provide energy for the cell. The solid substrate is a key element in solid-state fermentation (SSF). In addition to supplying nutrients such as carbon and nitrogen, the solid substrate also serves as a physical structure that supports the anchoring of filamentous fungi and promotes their growth [90].
These substrates can be classified into starch-rich substrates (Iding rice, barley, oats, cassava, wheat bran, cassava flour, corn flour, sweet potato residues, and banana peels); protein-rich substrates (by-products from food and agro-industrial industries, such as oil cakes canola, coconut, cottonseed, sunflower, and soybean); lignocellulosic and cellulosic substrates (rice straw, sugarcane bagasse, forest residues, and corn cobs); substrates with soluble sugar (fruit peels and coffee industry waste); and inert carriers (volcanic material, vermiculite, and polyurethane foam) [91].
Mushroom cultivation in solid-state systems is characterized by fungal expansion through the substrate via progressive mycelium colonization within the support material (or bed). The mushroom obtains the nutrients required for energy metabolism and cellular maintenance in this system. The latter aspect is directly linked to a parameter known as the substrate’s water activity (aw), a decisive factor in filamentous fungi cultivation, as it influences microbial development. Water activity refers to the free, unbound, or active water available to sustain fungal growth and should not be confused with the substrate’s total water content [92].
SSF has predominantly been studied using fungi and yeasts, based on the theoretical concept of water activity (aw). These microorganisms exhibit lower aw requirements (typically 0.5–0.6), in contrast to bacteria, which require higher aw values. Consequently, fungi and yeasts remain the most widely used microbiological models in industries employing SSF-based bioprocesses [93].
Mushrooms in solid-state systems require a certain amount of free water within the system, with its availability depending on both the substrate and the strain used. For instance, in the cultivation of Pleurotus ostreatus, the optimal free water content should be maintained between 65% and 75%. Values exceeding or falling below this range can negatively impact productivity, leading to lower cultivation yields or the development of fruiting bodies with limited commercial appeal [94].
From an ethnomycological perspective, SSF can be seen as a technique that has evolved from the empirical knowledge of Eastern societies [95]. Over the past three decades, solid-state fermentation (SSF) has gained much attention in biotechnology, allowing efficient production of feed, fuels, industrial enzymes, etc., accompanied by less wastewater and a lower risk of contamination than submerged fermentation (SmF). Meanwhile, mycoproteins obtained using plant biomass to culture fungi have good nutritional values and interesting functional properties. As the environmental burden of producing high-quality protein grows, there is an ongoing discussion about alternatives to conventional animal proteins; mycoprotein production via SSF may offer a potential solution [87].
Beyond their pharmacological potential, mushrooms represent a valuable source of protein that can meet the dietary needs of the population. The dry-weight protein content of edible and medicinal mushrooms ranges from approximately 19% to 37%, values comparable to or even higher than those of pork and chicken meat [27].
A comparative study analyzing the nutritional composition and amino acid profiles of 12 species of edible mushrooms, including Pleurotus ostreatus, Agaricus bisporus, and Agaricus brasiliensis, highlighted significant interspecific variability in essential amino acid content. The essential amino acids exhibited concentration ranges (expressed in g/100 g dry weight) as follows: histidine (His) 0.18–0.91, isoleucine (Ile) 0.39–1.26, leucine (Leu) 0.22–2.37, lysine (Lys) 0.29–1.92, methionine (Met) 0.08–0.62, phenylalanine (Phe) 0.27–1.21, threonine (Thr) 0.37–1.50, tryptophan (Trp) 0.19–0.55, and valine (Val) 0.12–1.58. Notably, Agaricus brasiliensis and Pleurotus ostreatus consistently displayed the highest concentrations across multiple amino acids. These findings emphasize the role of mushrooms as nutritionally complete and sustainable protein sources, with profiles rivaling or exceeding those of traditional animal-based proteins [96].
Edible mushroom cultivation can be divided into two parts: first, the cultivation of fruiting bodies, and second, the cultivation of mycelium in bioreactors. The former focuses on obtaining fruiting bodies through various techniques such as log cultivation, bag cultivation, tray cultivation, and bottle cultivation. The latter involves producing mycelium, which can be used as animal feed, to extract compounds with pharmacological properties (e.g., beta-glucans, polysaccharides, terpenoids, sterols, and enzymes), or spawn [97]. These bioreactors have been used for enzyme production, and they are also employed to produce spores, antibiotics, pigments, and chemicals. They have been classified into the following four categories, based on their mode of operation: tray bioreactor, packed bed bioreactor, air pressure pulsation bioreactor, and intermittent or continuously mixed SSF bioreactors [98].
7. Control Systems for Submerged Fermentation
In engineering, a control system refers to an interconnected set of components designed to monitor, regulate, and adjust the behavior of a dynamic process or machine to achieve desired outcomes. Control systems use sensors to measure variables such as temperature, pressure, or speed, while a controller processes this information to determine corrective actions, which are then executed by actuators. The goal is to ensure the system responds accurately and stably to changes or disturbances, often by employing feedback mechanisms that automatically correct deviations from target values.
Recent advances in control systems for submerged fermentation focus on integrating smart technologies, advanced sensors, and data-driven optimization to improve process efficiency, scalability, and product quality.
7.1. Smart Sensing and Online Monitoring
Modern submerged fermentation systems now employ optical, spectroscopic, electrochemical, and molecular (‘omics’) sensors for real-time, continuous measurement of critical parameters such as biomass, metabolites, and environmental conditions, including temperature, pH, and dissolved oxygen. These sensors enable online monitoring, allowing immediate detection of process deviations and more precise process management compared to traditional offline sampling. Internet of Things (IoT) systems focus on real-time data acquisition through connected sensors, remote monitoring of environmental variables (temperature, humidity, CO2, etc.), and automatic feedback-based control [99].
A group of researchers developed an efficient IoT-based system to automate the production of cordycepin, a compound with potent antiviral and tumor-suppressing properties. This system was applied to the submerged fermentation of Cordyceps militaris, a mushroom widely studied for its ability to produce this secondary metabolite. Traditionally, obtaining cordycepin using conventional methods is cumbersome, cost-ineffective, and lacks process homogeneity. Thanks to this automated system, the researchers achieved cordycepin concentrations of 1.44 g/L in a 5 L working volume, representing a significant advancement in process efficiency. Additionally, the use of big data enabled early detection of contamination during fermentation, improving process reliability and control. The authors emphasize that this technology applies to cordycepin production and can be extrapolated to other similar fermentation processes [100].
In this research, a closed-loop feedback control system was implemented, based on a Proportional-Integral-Derivative (PID) algorithm integrated with Pulse Width Modulation (PWM) to dynamically regulate airflow and control valve opening times. The objective was to create hypoxic stress conditions to stimulate the production of the secondary metabolite cordycepin. The system was equipped with CO2 sensors that transmitted real-time signals to a microcontroller, which generated adaptive control responses to maintain optimal fermentation conditions [100].
Continuous monitoring can be performed by image acquisition and processing capabilities. Wu, Xiao [101] used cameras in order to capture images through an observation window, and a Jetson Nano system (4GB RAM, Maxwell GPU) enabled in situ data analysis using computer vision models. The computer vision pipeline consisted of three stages: object detection, semantic segmentation, and morphological image processing using convolutional components. This approach allowed real-time calculation of biomass concentration through linear coefficient adjustments based on manually conducted experiments, achieving a correlation coefficient close to unity. The biomass concentration was visualized via a graphical user interface, enabling improved adjustments of nutrient supply and agitation power.
Moreover, the system utilized stored images and biomass values in the cloud as standard reference patterns, comparing them with real-time data to monitor the process. A decrease in biomass concentration, atypical morphology, and an increase in the carbon dioxide-to-temperature ratio were indicative of bacterial contamination. The authors emphasize the system’s applicability in industrial environments with low energy consumption [101].
Since the system employs in situ data analysis through computer vision models to calculate biomass concentration in real time and subsequently adjust operational parameters—such as nutrient supply and agitation power—it inherently integrates elements of intelligent and predictive control. This combination of real-time sensing with adaptive process management exemplifies the progression from smart sensing and online monitoring towards more advanced, data-driven control strategies.
7.2. Intelligent and Predictive Control
Model predictive control (MPC) uses mathematical models of the fermentation process to predict future system behavior and dynamically optimize control actions, improving consistency and yield.
Artificial Intelligence (AI) algorithms, including neural networks and support vector machines, analyze fermentation data in real time to identify patterns, optimize feeding strategies, and adaptively adjust process parameters for optimal outcomes. Integrating machine learning (ML) and Constraint-Based Modeling (CBM) offers a promising approach for analyzing and optimizing fermentation parameters, with the potential to predict and influence mycelial morphology in submerged fermentation. The accuracy of ML models heavily depends on the quality and quantity of data. High-quality, comprehensive datasets are essential for training reliable models. However, integrating ML and CBM can lead to complex models that require significant computational resources and expertise to develop and maintain. Experimental validation is crucial to ensure that the predictions made by the integrated models are accurate and applicable to real-world fermentation processes [102].
To enhance both mycelial growth and the production of bioactive metabolites, it is essential to optimize various nutritional and environmental factors. This process requires advanced data analysis tools, such as artificial neural networks (ANNs) and response surface methodology (RSM), which enable the evaluation and adjustment of optimal conditions to maximize system efficiency [103].
Digital twin technology combines real-time sensor data with multi-scale models (e.g., metabolic kinetics, hydrodynamics) to simulate and optimize the entire fermentation lifecycle, from laboratory to industrial scale. This enables rational scale-up and proactive process optimization.
The use of unique and non-invasive platforms integrated with computer vision, machine learning, real-time monitoring techniques, and flexible hardware enables the simultaneous detection and quantification of multiple events within an automated laboratory reactor. This technology can initiate operations in response to visual signals, optimizing control and efficiency in experimental processes [104].
In this regard, to enable non-invasive and real-time monitoring of Lyophyllum decastes biomass during submerged fermentation, an artificial vision system was developed based on edge computing (Edge CV) by implementing machine learning algorithms. The system successfully achieved continuous monitoring of 1000 L fermenters, accurately detecting growth phases with high precision. Additionally, the system demonstrated early warning capabilities for microbial contamination within a 12 to 24 h timeframe. This approach proved to be practically applicable, precise, energy-efficient, and scalable, reducing operational costs while enhancing the quality of fungal biomass production [101].
7.3. Integrated Process Management
Modern bioreactors feature integrated systems for precise control of mixing, aeration, pH, nutrient dosing, and temperature, all managed via advanced software platforms to ensure reproducibility and scalability. Supervisory Control and Data Acquisition (SCADA) systems integrate sensors, controllers, and data acquisition devices for centralized, real-time visualization, data logging, and alarm management, streamlining process supervision and troubleshooting
Machine learning (ML) and artificial neural networks (ANNs) can be integrated into bioprocess control in multiple ways [105]. According to recent studies, these models are used not only for offline optimization—such as model building, parameter estimation, and experimental planning—but also increasingly for real-time control actions within closed-loop systems. ML components can be embedded in control loops either by updating models offline and then deploying them during operation or by adapting models online during controller runtime, enabling improved performance, robustness, and safety. For example, ML-driven soft sensors and neural network models have been successfully incorporated into model predictive control (MPC) frameworks to replace or augment mechanistic models, allowing more accurate predictions and optimization in real time. However, the actual implementation of ML and ANNs in real-time control is still emerging and often combined with traditional control strategies like PID or MPC to form hybrid systems that leverage the strengths of both approaches. Thus, ML is used both for offline optimization and increasingly for real-time control, depending on process complexity and available computational resources.
7.4. Process Optimization and Scalability
Advances in manipulating nutrient composition and environmental factors (carbon/nitrogen ratio, aeration, rheology, osmotic pressure) allow targeted optimization of microbial growth and product formation, supporting both high yields and economic efficiency. New control strategies are designed to be robust across different scales, addressing challenges of process variability and ensuring consistent performance from laboratory to industrial production.
Control systems in submerged fermentation typically include inline probes for continuous monitoring, automated acid/base addition for pH regulation, spargers and agitators for oxygen transfer and mixing, and temperature control via jackets or internal coils. The liquid environment allows for homogeneous conditions, making it easier to maintain a steady state and optimize microbial growth and product formation. Bioreactors are usually closed stainless steel tanks equipped with advanced instrumentation for real-time process control, enabling reproducibility and scalability. However, submerged fermentation often demands higher energy and water inputs due to the need for agitation and aeration [106].
PID (Proportional-Integral-Derivative) controllers are widely used due to their simplicity and effectiveness in maintaining critical variables such as temperature, pH, and dissolved oxygen within desired ranges through continuous feedback actions. However, for more complex processes involving multiple variables, model predictive control (MPC) emerges as a superior strategy, as it employs a dynamic model of the process to anticipate future behavior and optimize control actions, even under operational constraints and external disturbances [107]. In practice, MPC can function as a supervisory controller that adjusts the setpoints of PID controllers, combining the robustness of PID with the predictive capability of MPC, thereby enhancing long-term process stability and efficiency. Therefore, the authors recommend implementing hybrid strategies that integrate PID controllers for basic regulation and MPC for optimization and supervision, tailored to the specific characteristics of fungal bioprocesses.
Solid-state fermentation (SSF), by contrast, involves microbial growth on moist solid substrates with limited free water. Control systems prioritize humidity and temperature regulation to maintain optimal moisture levels and prevent substrate drying or overheating. Gas exchange is managed mainly through aeration, as the solid matrix restricts mixing and mass transfer compared to liquid systems. Sensors for moisture and temperature are crucial, but pH control and nutrient dosing are less direct and more challenging. SSF bioreactors often have simpler designs, such as tray or packed-bed reactors, with less automation. The environment is more heterogeneous, making process control less precise but often more energy-efficient and suitable for fungi and the production of certain secondary metabolites [98].
7.5. Challenges in Bioreactor Control for Fungal Fermentation Systems
Fermentation of shear-sensitive fungi, such as filamentous or pellet-form fungi, presents important control challenges. These fungi tend to form extensive mycelial networks that are easily damaged by mechanical agitation and aeration. Shear stress can reduce biomass yield and product formation. Additionally, fungal overgrowth on bioreactor internals and sensors causes biofouling, compromising reliable online monitoring and control of the fermentation process.
In stirred-tank reactors, the selection of an appropriate impeller type can improve mixing efficiency as described by Wang, Xue [108]. These authors studied various impeller configurations in a 5 L bioreactor for the fermentation of nemadectin by Streptomyces cyaneogriseus ssp. noncyanogenus. Radial impellers generated high shear rates (50–200 s−1), leading to excessive hyphal breakage and consequently reducing nemadectin production. Conversely, axial impellers produced lower shear rates (30–110 s−1) but limited oxygen transfer and mycelial differentiation, thereby inhibiting production. Radial-axial impeller combinations provided an optimal average velocity field and a balanced turbulent energy dissipation rate, enhancing medium homogenization and oxygen transfer, which improved overall fermentation performance.
Maintaining fungal growth in pellet form improves rheological properties and reduces biofouling but requires careful process strategies, such as repeated-batch fermentation with intermediate cleaning and controlled aeration rates, to avoid spore dispersion and excessive biomass deposition. Non-invasive sensors, such as backscattered light sensors, can partially overcome measurement challenges but still face interference from biomass accumulation. Overall, the control difficulties revolve around balancing sufficient oxygen transfer and mixing while minimizing shear damage and biofouling, necessitating tailored bioreactor design and operational protocols to sustain productivity and process stability.
Mixing inefficiencies, shear stress, and oxygen transfer issues in stirred-tank and airlift bioreactors by applying advanced control strategies that optimize agitation and aeration parameters tailored to each system’s dynamics. Cheng, Wu [109] report that cell dispersion and sedimentation performance were improved by shear force control during the fermentation of Trichosporon fermentans. Their study highlights the use of computational fluid dynamics (CFD) simulations to identify flow patterns and optimize the placement of baffles and gas injectors within a stirred bioreactor, thereby enhancing reactor design and operational efficiency.
In airlift bioreactors, where fluid circulation is driven by gas flow rather than mechanical agitation, control focuses on regulating gas flow rates to optimize oxygen transfer while avoiding excessive shear stress. Zhu, Chen [68] emphasized the role of shear stress in airlift bioreactors during Fusarium venenatum fermentation. CFD optimization of the airlift fermenter aided in balancing flow patterns to reduce shear stress. The modified reactor has brought significant improvement to the fermentation.
The authors emphasize that robust, adaptive control systems are essential to reconcile efficient mixing, low shear stress, and sufficient oxygen transfer with the enhancement of process stability and productivity. One possibility is to integrate model predictive control or hybrid control schemes that respond to process disturbances in real time to enhance process reliability and safety. This could involve the incorporation of multiple sensors, providing redundancy in case one fails. Additionally, it may be beneficial to implement robust contamination control mechanisms; for example, if contamination is detected in a process line, the affected reactor could be isolated to prevent cross-contamination with other reactors, especially when several operate in parallel. Considering such measures could help safeguard the integrity of the entire system, minimize potential downtime, and maintain high standards of operational safety and product quality.
7.6. Sensor Failure Management in Fungi Cultivation Systems
Robust monitoring and control systems currently exist in bioprocesses. These employ virtual sensors (soft sensors), which use data from physical sensors such as pH, dissolved oxygen, carbon dioxide, temperature, among other variables, to estimate complex variables in a fermentation process using complex non-linear mathematical models. These systems use optimization methodologies such as genetic algorithms, bee colony optimization, and artificial bee colonies. However, they are not immune to errors from sensors and actuators [110]. To mitigate these inconveniences or failures in automated systems, fault-tolerant systems are created, because it is important in any process to counteract any deviation that generates economic losses, a decrease in product quality, or biological and environmental safety problems.
A study proposes a fault-tolerant mechanism for virtual sensors by integrating Long Short-Term Memory (LSTM) networks, a computational model that corrects disturbances in sensor systems using real-time historical reference data. This new approach was implemented in a simulated system for the fermentative production of penicillin, demonstrating its robustness in detecting the end time of fermentation and diagnosing the quality of the process [111]. Another study used historical data (penicillin production) from stable operation for the detection and isolation of faults caused by sensor errors, avoiding complex non-linear mathematical models. This approach was based on the parity-based residue generation technique with subspace aids, demonstrating its adaptability and accuracy in isolation by adjusting the model in real time according to the behavior of the process and classifying whether the fault comes from a sensor or an actuator, using a residue bank. This system presented a higher detection rate compared to previous methodologies (98% vs. 80–94.89%) and fewer false alarms (4.5% vs. 9–12.09%) [112].
8. Future Perspectives
In recent years, there has been significant growth in the adoption of solid-state fermentation (SSF) technology, owing to its numerous advantages over Submerged Bioprocessing (SmB). A bibliometric review highlights that solid-state fermentation for the utilization of agro-industrial by-products has gained the interest of the scientific community over the past 50 years [113].
The impact of change has created the need to develop new approaches for obtaining safe, accessible, and widely accepted food, while simultaneously respecting the food culture of each population. This principle aligns with the Sustainable Development Goals, promoting a more inclusive and sustainable food system [114]. Solid-state cultivation stands out as the most suitable alternative for producing pharmacologically active animal feed supplements, as the entire fermented solid substrate can be utilized as the final product [97]. New insights into fermentative processes have lowered the costs of products derived from fermentation. In this context, the design of bioreactors for solid-state systems is critical. However, current solid-state systems still face limitations, particularly in controlling operational variables and challenges in process scaling. These issues remain major hurdles to overcome in advancing this technology [115].
According to studies by the American Mushroom Institute, the production of edible mushrooms is one of the agricultural processes that generates the least greenhouse gases, emitting just 0.5 kg of CO2 per pound consumed. In comparison, other protein sources have a significantly higher carbon footprint: chicken emits 6.2 times more (3.1 kg CO2/pound), pork 11 times more (5.5 kg), salmon 10.8 times more (5.4 kg), cheese 12.2 times more (6.1 kg), eggs 4.4 times more (2.2 kg), and even broccoli, a vegetable, generates 1.8 times more (0.9 kg) [116].
However, a study conducted in France quantified the actual impact of producing 1 kg of Pleurotus ostreatus using Life Cycle Assessment (LCA), revealing that the system emitted three times more carbon dioxide than previously reported. This discrepancy can be explained by the fact that the analysis covered all stages, from cultivation to logistics and transport [117].
A systematic study on the environmental impact of mycoprotein production showed that obtaining fungal proteins in fermenters is superior due to their lower greenhouse gas emissions compared to traditional plant and animal protein sources. Mycoprotein (0.33 kgCO2eq/lb) has a lower carbon footprint than soy protein (0.55 kg CO2eq/kg) or pea protein concentrate (0.87 kg CO2eq/lb). Mycoprotein generates up to 90% fewer emissions than beef (which can exceed 27 kg CO2eq/lb). In addition to its low carbon dioxide emissions, the production of fungal protein concentrates requires fewer water resources and land, as well as zero emissions from fertilizers and pesticides [118]. Low-frequency ultrasounds and magnetic fields have been implemented as strategies to optimize productivity in submerged cultures of filamentous fungi [119]. A notable example is the cultivation of Lentinula edodes, where these techniques are used to enhance enzymes and biomass production. Studies have demonstrated that the application of ultrasound boosts the synthesis of laccases and polysaccharides [119], while magnetic stimulation increases biomass production [120]. These findings open new perspectives for industrial applications, proposing the use of non-invasive and sustainable methodologies to improve yields in fermentation processes, without compromising quality or causing pollution [121]. Some authors have explored the use of green and red light to enhance the polysaccharide production in Pleurotus ostreatus through submerged fermentation, representing a groundbreaking innovation in the field of fungal fermentations [122]. However, further optimization of lighting system design is required, as fungal growth may limit light distribution and absorption, potentially affecting process efficiency.
9. Conclusions
Submerged fermentation (SmF) and solid-state fermentation (SSF) of edible and medicinal fungi play complementary roles in biotechnology, where both techniques are equally relevant depending on the process objectives. The choice between SmF and SSF hinges on factors such as scalability, operational costs, substrate availability, and the desired characteristics of the final product. While SmF enables precise control of physicochemical parameters for producing soluble metabolites or specific biomolecules, SSF utilizes agro-industrial by-products as a support matrix, reducing expenses and optimizing the synthesis of enzymes or bioactive compounds bound to fibrous structures. Both strategies contribute to developing functional foods, pharmaceutical ingredients, and sustainable solutions, demonstrating that their relevance is not mutually exclusive but context-dependent. Innovative strategies have been developed to enhance yields in these processes, covering various areas of optimization. These include morphological engineering control in submerged fermentation, process automation using neural networks and machine learning, the implementation of early warning sensors, and new bioreactor design proposals tailored to the specific characteristics of each process. To achieve significant advancements, these findings could be integrated, exploring potential synergies between different operational conditions. However, it would be necessary to reduce material costs and optimize resources to improve the feasibility of these solutions.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidiomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Brugnari, T.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Biotechnological, nutritional and therapeutic uses of Pleurotus spp. (Oyster mushroom) related with its chemical composition: A review on the past decade findings. Trends Food Sci. Technol. 2016, 50, 103–117. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharma, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Hamza, A.; Mylarapu, A.; Krishna, K.V.; Kumar, D.S. An insight into the nutritional and medicinal value of edible mushrooms: A natural treasury for human health. J. Biotechnol. 2024, 381, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Horincar, V.-B.; Popa, A.; Parfene, G.; Balaes, T. Study of preliminary biotechnological conditions for Pleurotus ostreatus cultivation on submerged system. Innov. Rom. Food Biotechnol. 2014, 15, 58–62. Available online: https://www.gup.ugal.ro/ugaljournals/index.php/IFRB/article/view/3496 (accessed on 6 June 2025).
- Lindequist, U.; Niedermeyer, T.H.; Jülich, W.-D. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef]
- Molitorisová, A.; Monaco, A.; Purnhagen, K.P. An Analysis of the Regulatory Framework Applicable to Products Obtained from Mushroom and Mycelium; Adalbert Raps Stiftung: Kulmbach, Germany, 2021; SSRN 3955899. [Google Scholar] [CrossRef]
- Sande, D.; de Oliveira, G.P.; e Moura, M.A.F.; de Almeida Martins, B.; Lima, M.T.N.S.; Takahashi, J.A. Edible mushrooms as a ubiquitous source of essential fatty acids. Food Res. Int. 2019, 125, 108524. [Google Scholar] [CrossRef]
- Singh, M.; Kamal, S.; Sharma, V.P. Status and trends in world mushroom production-III-World Production of different mushroom species in 21st century. Mushroom Res. 2020, 29, 75. [Google Scholar] [CrossRef]
- Sangeeta, S.; Sharma, D.; Ramniwas, S.; Mugabi, R.; Uddin, J.; Nayik, G.A. Revolutionizing Mushroom processing: Innovative techniques and technologies. Food Chem. X 2024, 23, 101774. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms in human clinical studies. Part I. Anticancer, oncoimmunological, and immunomodulatory activities: A review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, A.; Tripathi, A. Biological activities of Pleurotus spp. polysaccharides: A review. J. Food Biochem. 2021, 45, e13748. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Vlassopoulou, M.; Paschalidis, N.; Savvides, A.L.; Saxami, G.; Mitsou, E.K.; Kerezoudi, E.N.; Koutrotsios, G.; Zervakis, G.I.; Georgiadis, P.; Kyriacou, A. Immunomodulating activity of Pleurotus eryngii mushrooms following their in vitro fermentation by human fecal microbiota. J. Fungi 2022, 8, 329. [Google Scholar] [CrossRef]
- Morris-Quevedo, H.J.; Llauradó-Maury, G.; Bermúdez-Savón, R.C.; Cos, P.; Lebeque-Pérez, Y.; Beltrán-Delgado, Y.; Tamayo-Ortega, V.; Fong-Lores, O.; Marcos-Albear, J.; Gaime-Perraud, I. Evaluation of the immunomodulatory activity of bioproducts obtained from the edible-medicinal mushroom Pleurotus ostreatus. Biotecnol. Apl. 2018, 35, 3511–3514. Available online: https://www.medigraphic.com/cgi-bin/new/resumenI.cgi?IDARTICULO=95629 (accessed on 6 June 2025).
- Berger, R.G.; Ersoy, F. Improved Foods Using Enzymes from Basidiomycetes. Processes 2022, 10, 726. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Wiley: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Pashaei, K.H.A.; Irankhah, K.; Namkhah, Z.; Sobhani, S.R. Edible mushrooms as an alternative to animal proteins for having a more sustainable diet: A review. J. Health Popul. Nutr. 2024, 43, 205. [Google Scholar] [CrossRef]
- El Enshasy, H.A. Fungal morphology: A challenge in bioprocess engineering industries for product development. Curr. Opin. Chem. Eng. 2022, 35, 100729. [Google Scholar] [CrossRef]
- Dudekula, U.T.; Doriya, K.; Devarai, S.K. A critical review on submerged production of mushroom and their bioactive metabolites. 3 Biotech 2020, 10, 337. [Google Scholar] [CrossRef]
- Tang, Y.-J.; Zhu, L.-W.; Li, H.-M.; Li, D.-S. Submerged culture of mushrooms in bioreactors–challenges, current state-of-the-art, and future prospects. Food Technol. Biotechnol. 2007, 45, 221–229. [Google Scholar]
- Vunduk, J.; Tura, D.; Biketova, A.Y. Medicinal mushroom nutraceutical commercialization: Two sides of a coin. In Wild Mushrooms; CRC Press: Boca Raton, FL, USA, 2022; pp. 89–131. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Guan, Y.-Y.; Hu, P.-F.; Chen, L.; Xu, G.-R.; Liu, L.; Cheung, P.C. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef]
- Horincar, V.-B.; Popa, A.-M.; Parfene, G.; Bahrim, G. Evaluation of some biotechnological parameters influencing the Pleurotus ostreatus biomass production by submerged cultivation. Ann. Univ. Dunarea Jos Galati. Fascicle VI Food Technol. 2015, 39, 55–63. Available online: https://www.proquest.com/scholarly-journals/evaluation-some-biotechnological-parameters/docview/1776148613/se-2 (accessed on 6 June 2025).
- Papaspyridi, L.-M.; Aligiannis, N.; Topakas, E.; Christakopoulos, P.; Skaltsounis, A.-L.; Fokialakis, N. Submerged fermentation of the edible mushroom Pleurotus ostreatus in a batch stirred tank bioreactor as a promising alternative for the effective production of bioactive metabolites. Molecules 2012, 17, 2714–2724. [Google Scholar] [CrossRef]
- Elisashvili, V.I. Submerged cultivation of medicinal mushrooms: Bioprocesses and products. Int. J. Med. Mushrooms 2012, 14, 211–239. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.; Shankar, M.P.; Chowdary, U.S.; Ghanekar, S.; Sahoo, S.; Krishnaiah, C.V.; Kumar, D.S. Submerged production of mycelium biomass and bioactive compounds from P. ostreatus in a controlled fermentation medium. Food Humanit. 2024, 2, 100302. [Google Scholar] [CrossRef]
- Veiter, L.; Rajamanickam, V.; Herwig, C. The filamentous fungal pellet—Relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [Google Scholar] [CrossRef]
- Meyer, V.; Cairns, T.; Barthel, L.; King, R.; Kunz, P.; Schmideder, S.; Müller, H.; Briesen, H.; Dinius, A.; Krull, R. Understanding and controlling filamentous growth of fungal cell factories: Novel tools and opportunities for targeted morphology engineering. Fungal Biol. Biotechnol. 2021, 8, 8. [Google Scholar] [CrossRef]
- Veiter, L.; Herwig, C. The filamentous fungus Penicillium chrysogenum analysed via flow cytometry—A fast and statistically sound insight into morphology and viability. Appl. Microbiol. Biotechnol. 2019, 103, 6725–6735. [Google Scholar] [CrossRef] [PubMed]
- Sohoni, S.V.; Bapat, P.M.; Lantz, A.E. Robust, small-scale cultivation platform for Streptomyces coelicolor. Microb. Cell Factories 2012, 11, 9. [Google Scholar] [CrossRef]
- Ferrer-Romero, J.C.; Mas-Diego, S.M.; Beltrán-Delgado, Y.; Rodríguez-Quiala, Y.; Morris Quevedo, H.J. Optimization of medium composition for the production of Pleurotus ostreatus biomass and phenols in submerged fermentation with response surface methodology. Tecnol. Química 2019, 39, 1–16. Available online: http://scielo.sld.cu/scielo.php?pid=S2224-61852019000100001&script=sci_arttext&tlng=pt (accessed on 6 June 2025).
- Ferrer-Romero, J.C.; Mas-Diego, S.M.; Beltrán-Delgado, Y.; Morris-Quevedo, H.J.; Díaz-Fernández, U. Kinetic study of the production of biomass and phenolic compounds for Pleurotus ostreatus in submerged phase. Rev. Cuba. Química 2019, 31, 16–30. Available online: http://scielo.sld.cu/scielo.php?pid=S2224-54212019000100016&script=sci_arttext (accessed on 6 June 2025).
- Shen, K.; Liu, Y.; Liu, L.; Khan, A.W.; Normakhamatov, N.; Wang, Z. Characterization, Optimization, and Scaling-up of Submerged Inonotus hispidus Mycelial Fermentation for Enhanced Biomass and Polysaccharide Production. Appl. Biochem. Biotechnol. 2024, 197, 1534–1555. [Google Scholar] [CrossRef] [PubMed]
- Dzurendova, S.; Losada, C.B.; Dupuy-Galet, B.X.; Fjær, K.; Shapaval, V. Mucoromycota fungi as powerful cell factories for modern biorefinery. Appl. Microbiol. Biotechnol. 2022, 106, 101–115. [Google Scholar] [CrossRef]
- Negi, B.B.; Das, C. Mycoremediation of wastewater, challenges, and current status: A review. Bioresour. Technol. Rep. 2023, 22, 101409. [Google Scholar] [CrossRef]
- Patel, A.K.; Agrawal, R.; Dong, C.-D.; Chen, C.-W.; Singhania, R.R.; Pandey, A. Filamentous Fungal Morphology in Industrial Aspects. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–217. [Google Scholar]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Rheological studies during submerged citric acid fermentation by Aspergillus niger in stirred fermentor using apple pomace ultrafiltration sludge. Food Bioprocess Technol. 2013, 6, 1240–1250. [Google Scholar] [CrossRef]
- Schmideder, S.; Barthel, L.; Müller, H.; Meyer, V.; Briesen, H. From three-dimensional morphology to effective diffusivity in filamentous fungal pellets. Biotechnol. Bioeng. 2019, 116, 3360–3371. [Google Scholar] [CrossRef] [PubMed]
- Schmideder, S.; Müller, H.; Barthel, L.; Friedrich, T.; Niessen, L.; Meyer, V.; Briesen, H. Universal law for diffusive mass transport through mycelial networks. Biotechnol. Bioeng. 2021, 118, 930–943. [Google Scholar] [CrossRef]
- Doran, P.M. Chapter 10—Mass Transfer. In Bioprocess Engineering Principles, 2nd ed.; Academic Press: London, UK, 2013; pp. 379–444. [Google Scholar] [CrossRef]
- Bizukojc, M.; Gonciarz, J. Influence of oxygen on lovastatin biosynthesis by Aspergillus terreus ATCC 20542 quantitatively studied on the level of individual pellets. Bioprocess Biosyst. Eng. 2015, 38, 1251–1266. [Google Scholar] [CrossRef]
- Huang, J.; Guan, H.W.; Huang, Y.Y.; Lai, K.S.; Chen, H.Y.; Xue, H.; Zhang, B.B. Evaluating the effects of microparticle addition on mycelial morphology, natural yellow pigments productivity, and key genes regulation in submerged fermentation of Monascus purpureus. Biotechnol. Bioeng. 2021, 118, 2503–2513. [Google Scholar] [CrossRef]
- Hao, F.; Zhong, B.; Shen, F.; Mao, Y.; Wu, Z. Macroparticle-enhanced morphology engineering of Cordyceps sinensis for high glucose fermentation to optimize the production of bioactive exopolysaccharides. Biochem. Eng. J. 2024, 211, 109470. [Google Scholar] [CrossRef]
- Khushboo; Dhaka, N.; Dubey, K.K. Effect of natural precursors and micro/macroparticles addition on the morphology modulation of Streptomyces toxytricini KD18 stimulates lipstatin productivity. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tong, L.-L.; Wang, Y.; Du, Y.-H.; Yuan, L.; Liu, M.-Z.; Mu, X.-Y.; Chen, Z.-L.; Zhang, Y.-D.; He, S.-J.; Li, X.-J.; et al. Transcriptomic analysis of morphology regulatory mechanisms of microparticles to Paraisaria dubia in submerged fermentation. Appl. Biochem. Biotechnol. 2022, 194, 4333–4347. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mira, A.; Castillo-Saldarriaga, C.; Uribe-Gutiérrez, L.; Céspedes-Gutíerrez, E.; Cortés-Rojas, D.; Gómez-Álvarez, M.; Cruz-Barrera, M. Culture media design and scaling-up of submerged fermentation for the nematophagous fungus Duddingtonia flagrans. Exp. Parasitol. 2025, 269, 108901. [Google Scholar] [CrossRef] [PubMed]
- Iram, A.; Özcan, A.; Yatmaz, E.; Turhan, İ.; Demirci, A. Effect of microparticles on fungal fermentation for fermentation-based product productions. Processes 2022, 10, 2681. [Google Scholar] [CrossRef]
- Etschmann, M.; Huth, I.; Walisko, R.; Schuster, J.; Krull, R.; Holtmann, D.; Wittmann, C.; Schrader, J. Improving 2-phenylethanol and 6-pentyl-α-pyrone production with fungi by microparticle-enhanced cultivation (MPEC). Yeast 2015, 32, 145–157. [Google Scholar] [CrossRef]
- Karahalil, E.; Coban, H.B.; Turhan, I. A current approach to the control of filamentous fungal growth in media: Microparticle enhanced cultivation technique. Crit. Rev. Biotechnol. 2019, 39, 192–201. [Google Scholar] [CrossRef]
- Laible, A.R.; Dinius, A.; Schrader, M.; Krull, R.; Kwade, A.; Briesen, H.; Schmideder, S. Effects and interactions of metal oxides in microparticle-enhanced cultivation of filamentous microorganisms. Eng. Life Sci. 2022, 22, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.S.; Lee, J.I.; Kang, D.H. TiO2-based photocatalyst Generated Reactive Oxygen Species cause cell membrane disruption of Staphylococcus aureus and Escherichia coli O157:H7. Food Microbiol. 2023, 109, 104119. [Google Scholar] [CrossRef]
- Driscoll, K.E. Review of lung particle overload, rat lung cancer, and the conclusions of the Edinburgh expert panel-It’s time to revisit cancer hazard classifications for titanium dioxide and carbon black. Front. Public Health 2022, 10, 907318. [Google Scholar] [CrossRef]
- Manzoor, Q.; Sajid, A.; Ali, Z.; Nazir, A.; Sajid, A.; Imtiaz, F.; Iqbal, S.; Younas, U.; Arif, H.; Iqbal, M. Toxicity spectrum and detrimental effects of titanium dioxide nanoparticles as an emerging pollutant: A review. Desalination Water Treat. 2024, 317, 100025. [Google Scholar] [CrossRef]
- Mohamed, H.R.H.; Shaheen, S.E.E.; Ibrahim, E.H.; Hussein, N.O.E.; Safwat, G. Calcium titanate nanoparticles-induced cytotoxicity, genotoxicity and oxidative stress in human non-small lung cancer cells. Sci. Rep. 2025, 15, 6373. [Google Scholar] [CrossRef]
- Grzybek, P.; Jakubski, Ł.; Dudek, G. Neat Chitosan Porous Materials: A Review of Preparation, Structure Characterization and Application. Int. J. Mol. Sci. 2022, 23, 9932. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, P.; Huang, R.; Huang, J.; Zhan, J.; Su, J.; You, R.; Lu, Y. Biomacromolecular composite microspheres based on sodium alginate and Tremella fuciformis polysaccharide for enhanced protection and delivery of Camellia oil: A comprehensive study in simulated digestion. Int. J. Biol. Macromol. 2025, 310, 143481. [Google Scholar] [CrossRef]
- Porto de Souza Vandenberghe, L.; Murawski de Mello, A.F.; Matte Borges Machado, C.; Biagini, G.; Gruening de Mattos, P.B.; Negreiros Piazenski, I.; Candelario, J.P.M.; Soccol, C.R. Alternative proteins production: Current scenario, bioreactor types, and scale-up strategies. Syst. Microbiol. Biomanuf. 2024, 5, 15–34. [Google Scholar] [CrossRef]
- Liu, S. Chapter 18—Bioreactor Design and Operation. In Bioprocess Engineering, 3rd ed.; Liu, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 819–856. [Google Scholar] [CrossRef]
- Doran, P.M. Chapter 14—Reactor Engineering. In Bioprocess Engineering Principles, 2nd ed.; Academic Press: London, UK, 2013; pp. 761–852. [Google Scholar] [CrossRef]
- Reuss, M. Stirred Tank Bioreactors: Of Bioreactor for Industrial. In Bioreactor System Design; CRC Press: Boca Raton, FL, USA, 1994; pp. 227–276. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781482277470-10/stirred-tank-bioreactors-matthias-reuss (accessed on 6 June 2025).
- Namdeo, N.; Kumar, B.; Jha, H. Bioreactor Design for the Production of Microbial Polysaccharides. In Microbial Exopolysaccharides; CRC Press: Boca Raton, FL, USA, 2024; pp. 94–112. Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003342687-5/bioreactor-design-production-microbial-polysaccharides-neha-namdeo-bikash-kumar-harit-jha (accessed on 6 June 2025).
- Merten, O.-W. Introduction to animal cell culture technology—Past, present and future. Cytotechnology 2006, 50, 1–7. [Google Scholar] [CrossRef]
- Barragán, L.P.; Figueroa, J.; Durán, L.R.; González, C.A.; Hennigs, C. Fermentative Production Methods. In Biotransformation of Agricultural Waste and By-Products; Elsevier: Amsterdam, The Netherlands, 2016; pp. 189–217. [Google Scholar] [CrossRef]
- Chisti, Y.; Moo-Young, M. Bioreactors. In Encyclopedia of Physical Science and Technology, 3rd ed.; Meyers, R.A., Ed.; Academic Press: New York, NY, USA, 2003; pp. 247–271. [Google Scholar] [CrossRef]
- Rawat, J.M.; Bhandari, A.; Raturi, M.; Rawat, B. Agrobacterium Rhizogenes Mediated Hairy Root Cultures: A Promising Approach for Production of Useful Metabolites. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 103–118. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, W.; Chen, Y.; Amin, F.R.; Li, Y.; Lu, M.; Li, D. CFD optimization of an air lift fermenter for Fusarium venenatum fermentation. Bioresour. Technol. Rep. 2025, 29, 102024. [Google Scholar] [CrossRef]
- Cerrone, F.; O’Connor, K.E. Cultivation of filamentous fungi in airlift bioreactors: Advantages and disadvantages. Appl. Microbiol. Biotechnol. 2025, 109, 41. [Google Scholar] [CrossRef] [PubMed]
- Aragão, M.S.; Menezes, D.B.; Ramos, L.C.; Oliveira, H.S.; Bharagava, R.N.; Romanholo Ferreira, L.F.; Teixeira, J.A.; Ruzene, D.S.; Silva, D.P. Mycoremediation of vinasse by surface response methodology and preliminary studies in air-lift bioreactors. Chemosphere 2020, 244, 125432. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, F.; Lochlainn, C.Ó.; Callaghan, T.; McDonald, P.; O’Connor, K.E. Airlift bioreactor–based strategies for prolonged semi-continuous cultivation of edible Agaricomycetes. Appl. Microbiol. Biotechnol. 2024, 108, 377. [Google Scholar] [CrossRef]
- Klaus, A.; Wan-Mohtar, W.A.A.Q.I.; Nikolić, B.; Cvetković, S.; Vunduk, J. Pink oyster mushroom Pleurotus flabellatus mycelium produced by an airlift bioreactor—The evidence of potent in vitro biological activities. World J. Microbiol. Biotechnol. 2021, 37, 17. [Google Scholar] [CrossRef]
- Whittaker, J.A.; Johnson, R.I.; Finnigan, T.J.A.; Avery, S.V.; Dyer, P.S. The Biotechnology of Quorn Mycoprotein: Past, Present and Future Challenges. In Grand Challenges in Fungal Biotechnology; Nevalainen, H., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 59–79. [Google Scholar] [CrossRef]
- Wang, B.; Shi, Y.; Lu, H.; Chen, Q. A critical review of fungal proteins: Emerging preparation technology, active efficacy and food application. Trends Food Sci. Technol. 2023, 141, 104178. [Google Scholar] [CrossRef]
- Galindo, E.; Peña, C.; Leobardo, S.-C. Domesticar microorganismos en un biorreactor: Los retos del bioingeniero. Claridades Agropecu. 2017, 5, 131–144. Available online: https://ibt.unam.mx/documentos/general/ibt-libro-25-aniv-capitulo12-1300.pdf (accessed on 6 June 2025).
- Liu, S. Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Croughan, M.S.; Konstantinov, K.B.; Cooney, C. The future of industrial bioprocessing: Batch or continuous? Biotechnol. Bioeng. 2015, 112, 648–651. [Google Scholar] [CrossRef]
- Yang, Y.; Sha, M. A beginner’s guide to bioprocess modes–batch, fed-batch, and continuous fermentation. Enfield CT Eppendorf Inc 2019, 408, 1–16. Available online: www.eppendorf.com (accessed on 6 June 2025).
- Soerjawinata, W.; Kockler, I.; Wommer, L.; Frank, R.; Schüffler, A.; Schirmeister, T.; Ulber, R.; Kampeis, P. Novel bioreactor internals for the cultivation of spore-forming fungi in pellet form. Eng. Life Sci. 2022, 22, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.C.; Todaro, C.M. Fermentation and Biochemical Engineering Handbook Principles, Process Design, and Equipment, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Zou, X. Fed-batch fermentation for hyperproduction of polysaccharide and ergosterol by medicinal mushroom Agaricus brasiliensis. Process Biochem. 2006, 41, 970–974. [Google Scholar] [CrossRef]
- Gaykawad, S.S.; Ramanand, S.S.; Blomqvist, J.; Zimmermann, B.; Shapaval, V.; Kohler, A.; Oostindjer, M.; Boccadoro, C. Submerged Fermentation of Animal Fat By-Products by Oleaginous Filamentous Fungi for the Production of Unsaturated Single Cell Oil. Fermentation 2021, 7, 300. [Google Scholar] [CrossRef]
- Guo, J.; Tang, C.; Liu, Y.; Shi, J.; Vunduk, J.; Tang, C.; Feng, J.; Zhang, J. Innovative submerged directed fermentation: Producing high molecular weight polysaccharides from Ganoderma lucidum. Food Chem. 2025, 471, 142759. [Google Scholar] [CrossRef]
- Xie, D. Continuous biomanufacturing with microbes—Upstream progresses and challenges. Curr. Opin. Biotechnol. 2022, 78, 102793. [Google Scholar] [CrossRef] [PubMed]
- Asadollahzadeh, M.; Mohammadi, M.; Lennartsson, P.R. 2—Fungal Biotechnology. In Current Developments in Biotechnology and Bioengineering; Taherzadeh, M.J., Ferreira, J.A., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 31–66. [Google Scholar] [CrossRef]
- Fukushima, Y.; Okada, K.; Kawai, G.; Motai, H. Efficient production of mycelium of Lentinus edodes by a continuous culture and the effect of lignin on growth. J. Ferment. Bioeng. 1993, 76, 45–48. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Krishna, C. Solid-state fermentation systems—An overview. Crit. Rev. Biotechnol. 2005, 25, 1–30. [Google Scholar] [CrossRef]
- Rodríguez Couto, S. Exploitation of biological wastes for the production of value-added products under solid-state fermentation conditions. Biotechnol. J. Healthc. Nutr. Technol. 2008, 3, 859–870. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Manan, M.A.; Webb, C. Solid-State Fermentation of Food Industry Wastes. In Food Industry Wastes; Elsevier: Amsterdam, The Netherlands, 2020; pp. 135–161. [Google Scholar] [CrossRef]
- Hu, C.; Meguro, S.; Kawachi, S. Effects of physical properties of wood on the water activity of wood meal media for the cultivation of edible mushrooms. J. Wood Sci. 2004, 50, 365–370. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Wiesnerová, L.; Hřebečková, T.; Jablonský, I.; Koudela, M. Effect of different water contents in the substrate on cultivation of Pleurotus ostreatus Jacq. P. Kumm. Folia Hortic. 2023, 35, 25–31. [Google Scholar] [CrossRef]
- Manan, M.; Webb, C. Design aspects of solid state fermentation as applied to microbial bioprocessing. J. Appl. Biotechnol. Bioeng. 2017, 4, 511–532. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Jiang, Q.; Roubík, H.; Xu, Q.; Gharsallaoui, A.; Cai, M.; Yang, K.; Sun, P. Fungal solid-state fermentation of crops and their by-products to obtain protein resources: The next frontier of food industry. Trends Food Sci. Technol. 2023, 138, 628–644. [Google Scholar] [CrossRef]
- Berovic, M. Cultivation of Medicinal Mushroom Biomass by Solid-State Bioprocessing in Bioreactors. In Solid State Fermentation, Research and Industrial Applications; Springer: Cham, Switzerland, 2019; pp. 3–25. [Google Scholar] [CrossRef]
- Arora, S.; Rani, R.; Ghosh, S. Bioreactors in solid state fermentation technology: Design, applications and engineering aspects. J. Biotechnol. 2018, 269, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Adeleke, I.; Nwulu, N.; Adebo, O.A. Internet of Things (IoT) in the food fermentation process: A bibliometric review. J. Food Process Eng. 2023, 46, e14321. [Google Scholar] [CrossRef]
- Chien, T.-Y.; Lo, H.-C.; Liu, M.-L.; Hsu, T.-H.; Lee, S.-C.; Hsu, W.-K.; Yang, J.-S.; Yang, S.-F.; Chao, S.-C. Internet of Things (loT)−Driven Fermentation System for Enhanced Cordycepin Production in Cordyceps militaris (Ascomycetes) under Hypoxic Conditions. Int. J. Med. Mushrooms 2025, 27, 57–69. [Google Scholar] [CrossRef]
- Wu, L.; Xiao, G.; Huang, D.; Zhang, X.; Ye, D.; Weng, H. Edge Computing-Based Machine Vision for Non-Invasive and Rapid Soft Sensing of Mushroom Liquid Strain Biomass. Agronomy 2025, 15, 242. [Google Scholar] [CrossRef]
- Khaleghi, M.K.; Savizi, I.S.P.; Lewis, N.E.; Shojaosadati, S.A. Synergisms of machine learning and constraint-based modeling of metabolism for analysis and optimization of fermentation parameters. Biotechnol. J. 2021, 16, 2100212. [Google Scholar] [CrossRef]
- Lu, Z.-M.; Lei, J.-Y.; Xu, H.-Y.; Shi, J.-S.; Xu, Z.-H. Optimization of fermentation medium for triterpenoid production from Antrodia camphorata ATCC 200183 using artificial intelligence-based techniques. Appl. Microbiol. Biotechnol. 2011, 92, 371–379. [Google Scholar] [CrossRef] [PubMed]
- El-Khawaldeh, R.; Guy, M.; Bork, F.; Taherimakhsousi, N.; Jones, K.N.; Hawkins, J.M.; Han, L.; Pritchard, R.P.; Cole, B.A.; Monfette, S. Keeping an “eye” on the experiment: Computer vision for real-time monitoring and control. Chem. Sci. 2024, 15, 1271–1282. [Google Scholar] [CrossRef]
- Hamza, A.; Khalad, A.; Kumar, D.S. Enhanced production of mycelium biomass and exopolysaccharides of Pleurotus ostreatus by integrating response surface methodology and artificial neural network. Bioresour. Technol. 2024, 399, 130577. [Google Scholar] [CrossRef]
- Chai, W.Y.; Teo, K.T.K.; Tan, M.K.; Tham, H.J. Fermentation process control and optimization. Chem. Eng. Technol. 2022, 45, 1731–1747. [Google Scholar] [CrossRef]
- Nikita, S.; Banerjee, S.; Jesubalan, N.G.; Kulkarni, A.; Gupta, K.; Rathore, A.S. Holistic process control framework for smart biomanufacturing: A deep learning driven approach. Comput. Chem. Eng. 2024, 187, 108742. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, J.; Sun, H.; Zhao, M.; Wang, Y.; Chu, J.; Zhuang, Y. Evaluation of mixing effect and shear stress of different impeller combinations on nemadectin fermentation. Process Biochem. 2020, 92, 120–129. [Google Scholar] [CrossRef]
- Cheng, J.; Wu, S.; Wang, X.; Shi, Y.; Guo, J.; Qiao, N.; Zhang, X.; Yu, D. The dispersion of Trichosporon fermentans was controlled by optimizing the shear environment in the reactor to improve the cell sedimentation and separation performance. J. Clean. Prod. 2023, 414, 137649. [Google Scholar] [CrossRef]
- Zhu, X.; Rehman, K.U.; Wang, B.; Shahzad, M. Modern Soft-Sensing Modeling Methods for Fermentation Processes. Sensors 2020, 20, 1771. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, D.; Wang, Z. A Fault-Tolerant Soft Sensor Algorithm Based on Long Short-Term Memory Network for Uneven Batch Process. Processes 2024, 12, 495. [Google Scholar] [CrossRef]
- Abbasi, M.A.; Khan, A.Q.; Mustafa, G.; Abid, M.; Khan, A.S.; Ullah, N. Data-driven fault diagnostics for industrial processes: An application to Penicillin fermentation process. IEEE Access 2021, 9, 65977–65987. [Google Scholar] [CrossRef]
- Yafetto, L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric analysis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef] [PubMed]
- Xena, Y.C. Geography: Origin of the Complexity of the Food System. In Sustainable Development Goals in Europe: A Geographical Approach; Springer: Cham, Switzerland, 2023; pp. 23–41. [Google Scholar] [CrossRef]
- Krishania, M.; Sindhu, R.; Binod, P.; Ahluwalia, V.; Kumar, V.; Sangwan, R.S.; Pandey, A. Chapter 5—Design of Bioreactors in Solid-State Fermentation. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 83–96. [Google Scholar] [CrossRef]
- American Mushroom Institute. Sustainability. Available online: https://www.americanmushroom.org/main/sustainability/ (accessed on 26 June 2025).
- Dorr, E.; Koegler, M.; Gabrielle, B.; Aubry, C. Life cycle assessment of a circular, urban mushroom farm. J. Clean. Prod. 2021, 288, 125668. [Google Scholar] [CrossRef]
- Shahid, M.; Shah, P.; Mach, K.; Rodgers-Hunt, B.; Finnigan, T.; Frost, G.; Neal, B.; Hadjikakou, M. The environmental impact of mycoprotein-based meat alternatives compared to plant-based meat alternatives: A systematic review. Future Foods 2024, 10, 100410. [Google Scholar] [CrossRef]
- Li, W.; Ma, H.; He, R.; Ren, X.; Zhou, C. Prospects and application of ultrasound and magnetic fields in the fermentation of rare edible fungi. Ultrason. Sonochem. 2021, 76, 105613. [Google Scholar] [CrossRef]
- Diego, S.M.M. Efectos biológicos del campo electromagnético en el crecimiento de microorganismos. Mecanismos de acción. Rev. Cuba. Química 2005, 17, 161. [Google Scholar]
- Veiga, M.C.; Seibel, J.; Díaz, H.J.N.; Santos, L.O.; Mazutti, M.A. Magnetic Field and Ultrasound to Intensify the Production of Biomass, Polysaccharides and Enzymes by Lentinula edodes Cultivated in Submerged Fermentation. Ind. Biotechnol. 2023, 19, 347–354. [Google Scholar] [CrossRef]
- Bakratsas, G.; Tsoumanis, C.; Stamatis, H.; Katapodis, P. Exopolysaccharide Production in Submerged Fermentation of Pleurotus ostreatus under Red and Green Light. Fermentation 2024, 10, 313. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
