Advancing Municipal Solid Waste Management Through Gasification Technology
Abstract
1. Introduction
| Region | 2016 (Million Tonnes/Year) | 2030 (Million Tonnes/Year) | 2050 (Million Tonnes/Year) |
|---|---|---|---|
| Middle East and North Africa | 129 | 177 | 255 |
| Sub-Saharan Africa | 174 | 269 | 516 |
| Latin America and the Caribbean | 231 | 290 | 396 |
| North America | 289 | 342 | 396 |
| South Asia | 334 | 466 | 661 |
| Europe and Central Asia | 392 | 440 | 490 |
| East Asia and the Pacific | 468 | 602 | 714 |
2. Methodology
- Publication period
- Publication type
- Scope
- Language
- Number of references
- Total references
3. Municipal Solid Waste Management
4. Gasification Process for the Conversion of MSW to Energy
- Drying Zone
- Pyrolysis
- Oxidation/combustion
- Reduction
4.1. Effect of Operating Parameters
4.1.1. Effect of Temperature
4.1.2. Effect of Pressure
4.1.3. Effect of Feedstock
| Operating Parameter | Effect | Observation/Result | Benefit | References |
|---|---|---|---|---|
| Temperature | Influences the syngas composition and reaction kinetics Promotes CO yield through the Boudouard reaction Enhances tar cracking and production of H2 | Optimal range: 800–900 °C | Improved carbon conversion efficiency Clean syngas with high energy content | [134,147,148] |
| Pressure | Influences chemical equilibrium and gas production | Reduces H2 yield and increases CH4 production Promotes feedstock conversion | Beneficial when CH4-rich syngas is required | [149,150] |
| Feedstock | Helps to determine syngas composition, yield, and reactor design Co-gasification of plastics with biomass results in higher CO and H2 yield | High volatile, low moisture, and ash lead to an improved gasification process | Improved synergies through co-gasification | [151,152] |
4.2. Effect of Gasification Agents
4.2.1. Air
4.2.2. Oxygen
4.2.3. Steam
4.2.4. CO2
4.3. Effect of the Catalyst
4.3.1. Akali and Alkaline Earth-Based Metal (AAEM) Catalysts
4.3.2. Effect of Transition-Metal-Based Catalysts
Nickel-Based and Other Metal Oxide Catalysts
Olivine-Based Catalysts
4.4. Effect of Residence Time
4.5. Effect of Equivalent Ratio
5. Syngas Cleaning Strategies
5.1. Particulate Removal
5.2. Tar Removal
5.3. Metal and Trace Contaminants Removal
5.4. Acid Gas Removal (CO2, H2S)
6. Recent Innovations in Gasification Technology
6.1. Chemical Looping Gasification (CLG)
6.2. Plasma Gasification
6.3. Integration with a Carbon Capture System
6.4. Solar-Assisted Gasification
6.5. AI-Based (IoT) Assisted Gasification Technologies
7. Prospective and Future Research Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaman, A.U.; Lehmann, S. Challenges and Opportunities in Transforming a City into a “Zero Waste City”. Challenges 2011, 2, 73–93. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Liang, X.; O’callaghan, E.; Goh, H.; Othman, M.H.D.; Avtar, R.; Kusworo, T.D. Transformation of Solid Waste Management in China: Moving towards Sustainability through Digitalization-Based Circular Economy. Sustainability 2022, 14, 2374. [Google Scholar] [CrossRef]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liu, H.M. Evaluation of Greenhouse Gas Emissions and the Feed-in Tariff System of Waste-to-Energy Facilities Using a System Dynamics Model. Sci. Total Environ. 2021, 792, 148445. [Google Scholar] [CrossRef] [PubMed]
- Alves Bruna Global Waste Generation—Statistics & Facts. Available online: https://www.statista.com/topics/4983/waste-generation-worldwide/ (accessed on 6 June 2025).
- United Nations Environmental Program Global Waste Management Outlook 2024. Available online: https://www.unep.org/ietc/resources/report/global-waste-management-outlook-2024 (accessed on 6 June 2025).
- Al Rayaan, M.B. Recent Advancements of Thermochemical Conversion of Plastic Waste to Biofuel—A Review. Clean Eng. Technol. 2021, 2, 100062. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0. A Global Snapshot of Solid Waste Management to 2050; World Bank Publications: Washington, DC, USA, 2018. [Google Scholar]
- U.S Environmental Protection Agency Wastes-Non-Hazardous Waste-Municipal Solid Waste. Available online: https://archive.epa.gov/epawaste/nonhaz/municipal/web/html/ (accessed on 20 May 2025).
- EPA Sustainable Materials Management: Non-Hazardous Materials and Waste Management Hierarchy. Available online: https://www.epa.gov/smm/sustainable-materials-management-non-hazardous-materials-and-waste-management-hierarchy (accessed on 20 May 2025).
- Zhang, L.H.; Gong, Q.C.; Duan, F.; Chyang, C.S.; Huang, C.Y. Emissions of Gaseous Pollutants, Polychlorinated Dibenzo-p-Dioxins, and Polychlorinated Dibenzo-Furans from Medical Waste Combustion in a Batch Fluidized-Bed Incinerator. J. Energy Inst. 2020, 93, 1428–1438. [Google Scholar] [CrossRef]
- Sharma, H.B.; Vanapalli, K.R.; Samal, B.; Cheela, V.R.S.; Dubey, B.K.; Bhattacharya, J. Circular Economy Approach in Solid Waste Management System to Achieve UN-SDGs: Solutions for Post-COVID Recovery. Sci. Total Environ. 2021, 800, 149605. [Google Scholar] [CrossRef]
- Achi, C.G.; Snyman, J.; Ndambuki, J.M.; Kupolati, W.K. Advanced Waste-to-Energy Technologies: A Review on Pathway to Sustainable Energy Recovery in a Circular Economy. Nat. Environ. Pollut. Technol. 2024, 23, 1239–1259. [Google Scholar] [CrossRef]
- Wang, F.; Peng, W.; Zeng, X.; Sun, D.; Cui, G.; Han, Z.; Wang, C.; Xu, G. Insight into Staged Gasification of Biomass Waste: Essential Fundamentals and Applications. Sci. Total. Environ. 2024, 953, 175954. [Google Scholar] [CrossRef]
- Chandra, C.S.J.; Sasi, S.; Sharmila, T.K.B.; Varghese, J.R. Modern Biomass Conversion Technologies. In Handbook of Biomass; Sabu, T., Mahesh, H., Daniel, P., Cintil, J.C., Eds.; Springer: Singapore, 2024; pp. 1037–1067. [Google Scholar]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An Overview of Advances in Biomass Gasification. Energy Env. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Hameed, Z.; Aslam, M.; Khan, Z.; Maqsood, K.; Atabani, A.E.; Ghauri, M.; Khurram, M.S.; Rehan, M.; Nizami, A.S. Gasification of Municipal Solid Waste Blends with Biomass for Energy Production and Resources Recovery: Current Status, Hybrid Technologies and Innovative Prospects. Renew. Sustain. Energy Rev. 2021, 136, 110375. [Google Scholar] [CrossRef]
- Hoang, A.T.; Varbanov, P.S.; Nižetić, S.; Sirohi, R.; Pandey, A.; Luque, R.; Ng, K.H.; Pham, V.V. Perspective Review on Municipal Solid Waste-to-Energy Route: Characteristics, Management Strategy, and Role in Circular Economy. J. Clean. Prod. 2022, 359, 131897. [Google Scholar] [CrossRef]
- Raheem, A.; He, Q.; Mangi, F.H.; Areeprasert, C.; Ding, L.; Yu, G. Roles of Heavy Metals during Pyrolysis and Gasification of Metal-Contaminated Waste Biomass: A Review. Energy Fuels 2022, 36, 2351–2368. [Google Scholar] [CrossRef]
- Glavič, P.; Pintarič, Z.N.; Levičnik, H.; Dragojlović, V.; Bogataj, M. Transitioning towards Net-Zero Emissions in Chemical and Process Industries: A Holistic Perspective. Processes 2023, 11, 2647. [Google Scholar] [CrossRef]
- Voss, R.; Lee, R.P.; Seidl, L.; Keller, F.; Fröhling, M. Global Warming Potential and Economic Performance of Gasification-Based Chemical Recycling and Incineration Pathways for Residual Municipal Solid Waste Treatment in Germany. Waste Manag. 2021, 134, 206–219. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.K. Review on Biomass Gasification: Gasifiers, Gasifying Mediums, and Operational Parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification. A Review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent Advances in the Development of Biomass Gasification Technology: A Comprehensive Review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Khan, S.; Anjum, R.; Raza, S.T.; Ahmed Bazai, N.; Ihtisham, M. Technologies for Municipal Solid Waste Management: Current Status, Challenges, and Future Perspectives. Chemosphere 2022, 288, 132403. [Google Scholar] [CrossRef]
- Kaur, A.; Bharti, R.; Sharma, R. Municipal Solid Waste as a Source of Energy. Mater. Today Proc. 2021, 81, 904–915. [Google Scholar] [CrossRef]
- García, A.J.; Esteban, M.B.; Márquez, M.C.; Ramos, P. Biodegradable Municipal Solid Waste: Characterization and Potential Use as Animal Feedstuffs. Waste Manag. 2005, 25, 780–787. [Google Scholar] [CrossRef]
- Bagyaraj, D.J. Microorganisms in Sustainable Agriculture. Proc. Indian. Natl. Sci. Acad. USA 2014, 80, 357. [Google Scholar] [CrossRef]
- Zheng, W.; Phoungthong, K.; Lü, F.; Shao, L.M.; He, P.J. Evaluation of a Classification Method for Biodegradable Solid Wastes Using Anaerobic Degradation Parameters. Waste Manag. 2013, 33, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Oladele, I.O.; Okoro, C.J.; Adelani, S.O.; Agbeboh, N.I.; Betiku, O.T. Current Application of Recycled Waste Plastics as a Sustainable Materials: A Review on Availability, Processing and Application. J. Thermoplast. Compos. Mater. 2024, 38, 277–301. [Google Scholar] [CrossRef]
- Safiuddin, M.; Jumaat, Z.; Salam, M.A.; Hashim, R. Utilization of Solid Wastes in Construction Materials. Int. J. Phys. Sci. 2010, 5, 1952–1963. [Google Scholar]
- Sarmah, P.; Katsumi, T.; Takai, A.; Gathuka, L.W.; Yamawaki, A. Leaching Behavior of Inert Waste Landfills. Waste Manag. 2024, 182, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Mallick, J.; Ahmed, M.; Saleem, M. Hazardous Wastes and Its Impact on Human Health. In Proceedings of the IOP Conference Series: Materials Science and Engineering; Institute of Physics Publishing: Bristol, UK, 2020; Volume 804. [Google Scholar]
- El-Khateeb, M.A. Household Hazardous Waste: Handling, Precaution and Hazard Reduction. Egypt. J. Chem. 2022, 65, 625–642. [Google Scholar] [CrossRef]
- Mundada, M.N.; Kumar, S.; Shekdar, A.V. E-Waste: A New Challenge for Waste Management in India. Int. J. Environ. Stud. 2004, 61, 265–279. [Google Scholar] [CrossRef]
- Himantha Kelaniyagama, S.; Gannoruwa, A.; Renuka Nilmini, A.H.L. Synthesize and Applications of Biodegradable Plastics as a Solution for Environmental Pollution Due to Non-Biodegradable Plastics, a Review. Sustain. Polym. Energy 2024, 2, 10011. [Google Scholar] [CrossRef]
- Ncube, L.K.; Ude, A.U.; Ogunmuyiwa, E.N.; Zulkifli, R.; Beas, I.N. An Overview of Plasticwaste Generation and Management in Food Packaging Industries. Recycling 2021, 6, 12. [Google Scholar] [CrossRef]
- Janik-Karpinska, E.; Brancaleoni, R.; Niemcewicz, M.; Wojtas, W.; Foco, M.; Podogrocki, M.; Bijak, M. Healthcare Waste—A Serious Problem for Global Health. Healthcare 2023, 11, 242. [Google Scholar] [CrossRef]
- Likhitha, A.S.; Ahad, H.A.; Haranath, C.; Harsha, S.S.; Thanmayadivya, K.; Sumanth, G.; Nagarajeswari, A. Effective Disposal of Biological and Pharmaceutical Waste: A Note to the Health Care Professional. Int. J. Life Sci. Pharma Res. 2022, 11, 52–58. [Google Scholar] [CrossRef]
- Curran, A.; Williams, I.D. The Role of Furniture and Appliance Re-Use Organisations in England and Wales. Resour. Conserv. Recycl. 2010, 54, 692–703. [Google Scholar] [CrossRef]
- Czekała, W.; Drozdowski, J.; Łabiak, P. Modern Technologies for Waste Management: A Review. Appl. Sci. 2023, 13, 8847. [Google Scholar] [CrossRef]
- Marchetti, R.; Vasmara, C.; Bertin, L.; Fiume, F. Conversion of Waste Cooking Oil into Biogas: Perspectives and Limits. Appl. Microbiol. Biotechnol. 2020, 104, 2833–2856. [Google Scholar] [CrossRef] [PubMed]
- Posti, L.; Bhamoriya, V.; Kumar, R.; Khare, R. How Productive Is Liquid Waste Management Practices in Indian Informal Micro, Small and Medium Enterprises? Manag. Environ. Qual. Int. J. 2024, 35, 314–340. [Google Scholar] [CrossRef]
- Bhange, V.; Vaidya, A.; Bhange, V.P.; William, S.P.; Vaidya, A.N.; Chokhandre, A.R. Green Waste As a Resource for Value Added Product Generation: A Review. Int. J. Recent. Trends Sci. Technol. 2012, 4, 22–33. [Google Scholar]
- Neugebauer, M.; Sołowiej, P. The Use of Green Waste to Overcome the Difficulty in Small-Scale Composting of Organic Household Waste. J. Clean. Prod. 2017, 156, 865–875. [Google Scholar] [CrossRef]
- Abubakar, I.R.; Maniruzzaman, K.M.; Dano, U.L.; AlShihri, F.S.; AlShammari, M.S.; Ahmed, S.M.S.; Al-Gehlani, W.A.G.; Alrawaf, T.I. Environmental Sustainability Impacts of Solid Waste Management Practices in the Global South. Int. J. Environ. Res. Public. Health 2022, 19, 12717. [Google Scholar] [CrossRef]
- Jbara, A.A. Environmental and Health Impacts Resulting From Burning Solid Waste Near Residential Areas in Diyala Governorate, Iraq. Acad. Open 2024, 9, 10–21070. [Google Scholar] [CrossRef]
- Adeyemi Aderinoye-Abdulwahab, S.; Adesokan, J.B.; Benapugha Owutuamor, Z. Assessment of Waste Management Practices of Rural Dwellers in Kwara State, Nigeria. J. Community Dev. Res. 2022, 15, 71–84. [Google Scholar]
- Potter, J.W.; Jones, K.B.; Barrott, J.J. Sarcoma—The Standard-Bearer in Cancer Discovery. Crit. Rev. Oncol. Hematol. 2018, 126, 1–5. [Google Scholar] [CrossRef]
- Khan, A.H.; López-Maldonado, E.A.; Alam, S.S.; Khan, N.A.; López, J.R.L.; Herrera, P.F.M.; Abutaleb, A.; Ahmed, S.; Singh, L. Municipal Solid Waste Generation and the Current State of Waste-to-Energy Potential: State of Art Review. Energy Convers. Manag. 2022, 267, 115905. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.H.; Kumar, P.; Kim, K.H.; Lee, S.S.; Bhattacharya, S.S. Solid Waste Management: Scope and the Challenge of Sustainability. J. Clean. Prod. 2019, 228, 658–678. [Google Scholar] [CrossRef]
- Demirbas, A. Waste Management, Waste Resource Facilities and Waste Conversion Processes. Energy Convers. Manag. 2011, 52, 1280–1287. [Google Scholar] [CrossRef]
- Chen, Y.C.; Lo, S.L. Evaluation of Greenhouse Gas Emissions for Several Municipal Solid Waste Management Strategies. J. Clean. Prod. 2016, 113, 606–612. [Google Scholar] [CrossRef]
- Jagun, Z.T.; Daud, D.; Ajayi, O.M.; Samsudin, S.; Jubril, A.J.; Rahman, M.S.A. Waste Management Practices in Developing Countries: A Socio-Economic Perspective. Environ. Sci. Pollut. Res. 2023, 30, 116644–116655. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Municipal Solid Waste Management and Landfilling Technologies: A Review. Env. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- Vaverková, M.D. Landfill Impacts on the Environment—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- De Gioannis, G.; Muntoni, A.; Cappai, G.; Milia, S. Landfill Gas Generation after Mechanical Biological Treatment of Municipal Solid Waste. Estimation of Gas Generation Rate Constants. Waste Manag. 2009, 29, 1026–1034. [Google Scholar] [CrossRef]
- Takeda, C.M.; Maciel-Silva, F.W.; Forster-Carneiro, T.; Miguel, M.G. Methane Generation Potential of the Easily Degradable Group of Landfilled Municipal Solid Waste. Methane 2024, 3, 569–583. [Google Scholar] [CrossRef]
- Moraes, C.A.; de Lima Casseres dos Santos, L.; de Oliveira, A.C.L.; Botelho, D.F.; Moltó Berenguer, J.; dos Santos Renato, N. Biogas-Based Electricity Production from Landfills in Places of Irregular Disposal: Overview for the Southeast Region of Brazil. Energy 2024, 290, 130161. [Google Scholar] [CrossRef]
- Gu, W.; Liu, D.; Wang, C. Energy Recovery Potential from Incineration Using Municipal Solid Waste Based on Multi-Scenario Analysis in Beijing. Environ. Sci. Pollut. Res. 2021, 28, 27119–27131. [Google Scholar] [CrossRef] [PubMed]
- Di Maria, F.; Mastrantonio, M.; Uccelli, R. The Life Cycle Approach for Assessing the Impact of Municipal Solid Waste Incineration on the Environment and on Human Health. Sci. Total Environ. 2021, 776, 145785. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Mustafa, A.B.; Liu, M.; Huang, G.; Shang, N.; Liu, X.; Wei, K.; Wang, P.; Dong, H. Environmental, Energy, and Techno-Economic Assessment of Waste-to-Energy Incineration. Sustainability 2024, 16, 4140. [Google Scholar] [CrossRef]
- Liu, A.; Ren, F.; Lin, W.Y.; Wang, J.Y. A Review of Municipal Solid Waste Environmental Standards with a Focus on Incinerator Residues. Int. J. Sustain. Built Environ. 2015, 4, 165–188. [Google Scholar] [CrossRef]
- Vamvuka, D. Bio-Oil, Solid and Gaseous Biofuels from Biomass Pyrolysis Processes-An Overview. Int. J. Energy Res. 2011, 35, 835–862. [Google Scholar] [CrossRef]
- Rekos, K.C.; Charisteidis, I.D.; Tzamos, E.; Palantzas, G.; Zouboulis, A.I.; Triantafyllidis, K.S. Valorization of Hazardous Organic Solid Wastes towards Fuels and Chemicals via Fast (Catalytic) Pyrolysis. Sustain. Chem. 2022, 3, 91–111. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the Lignocellulosic Biomass Pyrolysis for Biofuel Production toward Environmental Sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Maya, D.M.Y.; Sarmiento, A.L.E.; Oliveira, C.A.V.B.; Lora, E.S.; Andrade, R. Gasification of Municipal Solid Waste for Power Generation in Brazil, a Review of Available Technologies and Their Environmental Benefits. J. Chem. Chem. Eng. 2016, 10. [Google Scholar] [CrossRef]
- Yan, B. IEA Bioenergy Task 33. Actual Deployment of Gasification (China Updates) 2023 Semiannual Meeting. 2023. Available online: https://task33.ieabioenergy.com/wp-content/uploads/sites/33/2023/11/China.pdf (accessed on 6 June 2025).
- OPET Finland. Review of Finnish Biomass Gasification Technologies. 2002. Available online: https://cris.vtt.fi/en/publications/review-of-finnish-biomass-gasification-technologies (accessed on 6 June 2025).
- United Nations Environment Programm Report of the Intergovernmental Negotiating Committee to Develop an International Legally Binding Instrument on Plastic, Including in the Marine Environment, on the Work of Its Second Session. Paris. 2023. Available online: https://www.unep.org/inc-plastic-pollution (accessed on 6 June 2025).
- Hrbek, J. Gasification Developments in Europe and the USA. Vienna. 2021. Available online: https://www.ieabioenergy.com/wp-content/uploads/2021/03/Hrbek-Gasification-developments-in-Europe-USA.pdf (accessed on 6 June 2025).
- Kolb, T.; Eberhard, M. Country Report Germany, Task 33 Thermal Gasification of Biomass; Karlsruhe. 2019. Available online: https://task33.ieabioenergy.com/wp-content/uploads/sites/33/2022/06/Germany-7.pdf (accessed on 6 June 2025).
- Segakweng Tshiamo. Gasification in South Africa Task 33AIE Bioenergy Meeting. South Africa. 2023. Available online: https://task33.ieabioenergy.com/wp-content/uploads/sites/33/2023/11/S_Africa.pdf (accessed on 6 June 2025).
- Ren, J.; Liu, Y.L.; Zhao, X.Y.; Cao, J.P. Biomass Thermochemical Conversion: A Review on Tar Elimination from Biomass Catalytic Gasification. J. Energy Inst. 2020, 93, 1083–1098. [Google Scholar] [CrossRef]
- González, R.; Peña, D.C.; Gómez, X. Anaerobic Co-Digestion of Wastes: Reviewing Current Status and Approaches for Enhancing Biogas Production. Appl. Sci. 2022, 12, 8884. [Google Scholar] [CrossRef]
- Lee, D.J. Gasification of Municipal Solid Waste (MSW) as a Cleaner Final Disposal Route: A Mini-Review. Bioresour. Technol. 2022, 344, 126217. [Google Scholar] [CrossRef]
- Lombardi, L.; Carnevale, E.; Corti, A. A Review of Technologies and Performances of Thermal Treatment Systems for Energy Recovery from Waste. Waste Manag. 2015, 37, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Torres, W.; Pansare, S.S.; Goodwin, J.G. Hot Gas Removal of Tars, Ammonia, and Hydrogen Sulfide from Biomass Gasification Gas. Catal. Rev. Sci. Eng. 2007, 49, 407–456. [Google Scholar] [CrossRef]
- Mahinpey, N.; Gomez, A. Review of Gasification Fundamentals and New Findings: Reactors, Feedstock, and Kinetic Studies. Chem. Eng. Sci. 2016, 148, 14–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Wan, L.; Guan, J.; Xiong, Q.; Zhang, S.; Jin, X. A Review on Biomass Gasification: Effect of Main Parameters on Char Generation and Reaction. Energy Fuels 2020, 34, 13438–13455. [Google Scholar] [CrossRef]
- Dejtrakulwong, C.; Patumsawad, S. Four Zones Modeling of the Downdraft Biomass Gasification Process: Effects of Moisture Content and Air to Fuel Ratio. Energy Procedia 2014, 52, 142–149. [Google Scholar] [CrossRef]
- Deng, N.; Li, D.; Zhang, Q.; Zhang, A.; Cai, R.; Zhang, B. Simulation Analysis of Municipal Solid Waste Pyrolysis and Gasification Based on Aspen Plus. Front. Energy 2019, 13, 64–70. [Google Scholar] [CrossRef]
- Chanthakett, A.; Arif, M.T.; Khan, M.M.K.; Oo, A.M.T. Performance Assessment of Gasification Reactors for Sustainable Management of Municipal Solid Waste. J. Environ. Manag. 2021, 291, 112661. [Google Scholar] [CrossRef]
- Keifer, D. Enthalpy and the Second Law of Thermodynamics. J. Chem. Educ. 2019, 96, 1407–1411. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K. Why Combustions Are Always Exothermic, Yielding about 418 KJ per Mole of O2. J. Chem. Educ. 2015, 92, 2094–2099. [Google Scholar] [CrossRef]
- Cooper, A.; Johnson, C.M.; Lakey, J.H.; Nollmann, M. Heat Does Not Come in Different Colours: Entropyenthalpy Compensation, Free Energy Windows, Quantum Confinement, Pressure Perturbation Calorimetry, Solvation and the Multiple Causes of Heat Capacity Effects in Biomolecular Interactions. Biophys. Chem. 2001, 93, 215–230. [Google Scholar] [CrossRef]
- Ramírez Rubio, S.; Sierra, F.E.; Guerrero, C.A. Ingeniería e Investigación. Ing. E Investig. 1981, 31, 17–25. [Google Scholar] [CrossRef]
- Janajreh, I.; Adeyemi, I.; Raza, S.S.; Ghenai, C. A Review of Recent Developments and Future Prospects in Gasification Systems and Their Modeling. Renew. Sustain. Energy Rev. 2021, 138, 110505. [Google Scholar] [CrossRef]
- Masmoudi, M.A.; Halouani, K.; Sahraoui, M. Comprehensive Experimental Investigation and Numerical Modeling of the Combined Partial Oxidation-Gasification Zone in a Pilot Downdraft Air-Blown Gasifier. Energy Convers. Manag. 2017, 144, 34–52. [Google Scholar] [CrossRef]
- Baratieri, M.; Baggio, P.; Fiori, L.; Grigiante, M. Biomass as an Energy Source: Thermodynamic Constraints on the Performance of the Conversion Process. Bioresour. Technol. 2008, 99, 7063–7073. [Google Scholar] [CrossRef]
- Zainal, Z.A.; Ali, R.; Lean, C.H.; Seetharamu, K.N. Prediction of Performance of a Downdraft Gasifier Using Equilibrium Modeling for Different Biomass Materials. Energy Convers. Manag. 2001, 42, 1499–1515. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P. Overview of Recent Advances in Thermo-Chemical Conversion of Biomass. Energy Convers. Manag. 2010, 51, 969–982. [Google Scholar] [CrossRef]
- Bryden, K.M.; Ragland, K.W.; Rutland, C.J. Modeling Thermally Thick Pyrolysis of Wood. Biomass Bioenergy 2002, 22, 41–53. [Google Scholar] [CrossRef]
- Bryden, K.M.; Hagge, M.J. Modeling the Combined Impact of Moisture and Char Shrinkage on the Pyrolysis of a Biomass Particle. Fuel 2003, 82, 1633–1644. [Google Scholar] [CrossRef]
- Tinaut, F.V.; Melgar, A.; Pérez, J.F.; Horrillo, A. Effect of Biomass Particle Size and Air Superficial Velocity on the Gasification Process in a Downdraft Fixed Bed Gasifier. An Experimental and Modelling Study. Fuel Process. Technol. 2008, 89, 1076–1089. [Google Scholar] [CrossRef]
- Chan, W.-C.R.; Kelbon, M.; Krieger, B.B. Modelling and Experimental Verification of Physical and Chemical Processes during Pyrolysis of a Large Biomass Particle. Fuel 1985, 64, 1505–1513. [Google Scholar] [CrossRef]
- Elbaba, I.F.; Williams, P.T. Two Stage Pyrolysis-Catalytic Gasification of Waste Tyres: Influence of Process Parameters. Appl. Catal. B 2012, 125, 136–143. [Google Scholar] [CrossRef]
- Demirbas, A. Effect of Temperature on Pyrolysis Products from Biomass. Energy Sources Part. A Recovery Util. Environ. Eff. 2007, 29, 329–336. [Google Scholar] [CrossRef]
- Faraji, M.; Saidi, M. Hydrogen-Rich Syngas Production via Integrated Configuration of Pyrolysis and Air Gasification Processes of Various Algal Biomass: Process Simulation and Evaluation Using Aspen Plus Software. Int. J. Hydrogen Energy 2021, 46, 18844–18856. [Google Scholar] [CrossRef]
- Sharma, G.; Dewangan, A.K.; Yadav, A.K.; Ahmad, A. Feasibility of Waste-to-Hydrogen Generation System Based on Gasification/Pyrolysis: A Comprehensive Review of Experimental Studies. J. Therm. Anal. Calorim. 2024, 1–23. [Google Scholar] [CrossRef]
- Baruah, D.; Baruah, D.C. Modeling of Biomass Gasification: A Review. Renew. Sustain. Energy Rev. 2014, 39, 806–815. [Google Scholar] [CrossRef]
- Izquierdo-Horna, L.; Kahhat, R.; Vázquez-Rowe, I. Reviewing the Influence of Sociocultural, Environmental and Economic Variables to Forecast Municipal Solid Waste (MSW) Generation. Sustain. Prod. Consum. 2022, 33, 809–819. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass Gasification for Sustainable Energy Production: A Review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Giltrap, D.L.; McKibbin, R.; Barnes, G.R.G. A Steady State Model of Gas-Char Reactions in a Downdraft Biomass Gasifier. Sol. Energy 2003, 74, 85–91. [Google Scholar] [CrossRef]
- Hernández, J.J.; Aranda-Almansa, G.; Bula, A. Gasification of Biomass Wastes in an Entrained Flow Gasifier: Effect of the Particle Size and the Residence Time. Fuel Process. Technol. 2010, 91, 681–692. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Effects of Biomass Particle Size on Slow Pyrolysis Kinetics and Fast Pyrolysis Product Distribution. Waste Biomass Valorization 2018, 9, 465–477. [Google Scholar] [CrossRef]
- Altafini, C.R.; Wander, P.R.; Barreto, R.M. Prediction of the Working Parameters of a Wood Waste Gasifier through an Equilibrium Model. Energy Convers. Manag. 2003, 44, 2763–2777. [Google Scholar] [CrossRef]
- Couto, N.; Rouboa, A.; Silva, V.; Monteiro, E.; Bouziane, K. Influence of the Biomass Gasification Processes on the Final Composition of Syngas. Energy Procedia 2013, 36, 596–606. [Google Scholar] [CrossRef]
- Rennard, D.; French, R.; Czernik, S.; Josephson, T.; Schmidt, L. Production of Synthesis Gas by Partial Oxidation and Steam Reforming of Biomass Pyrolysis Oils. Int. J. Hydrogen Energy 2010, 35, 4048–4059. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-Gasification and Recent Developments on Waste-to-Energy Conversion: A Review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Ramzan, N.; Ashraf, A.; Naveed, S.; Malik, A. Simulation of Hybrid Biomass Gasification Using Aspen plus: A Comparative Performance Analysis for Food, Municipal Solid and Poultry Waste. Biomass Bioenergy 2011, 35, 3962–3969. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Dogru, M.; Howarth, C.R.; Akay, G.; Keskinler, B.; Malik, A.A. Gasification of Hazelnut Shells in a Downdraft Gasifier. Energy 2002, 27, 415–427. [Google Scholar] [CrossRef]
- Di Blasi, C. Combustion and Gasification Rates of Lignocellulosic Chars. Prog. Energy Combust. Sci. 2009, 35, 121–140. [Google Scholar] [CrossRef]
- Hernández, J.J.; Ballesteros, R.; Aranda, G. Characterisation of Tars from Biomass Gasification: Effect of the Operating Conditions. Energy 2013, 50, 333–342. [Google Scholar] [CrossRef]
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic Steam Reforming of Biomass Tar: Prospects and Challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, J., II; Jung, D.; Miyawaki, J.; Yoon, S.H.; Mochida, I. Low-Temperature Catalytic Conversion of Lignite: 1. Steam Gasification Using Potassium Carbonate Supported on Perovskite Oxide. J. Ind. Eng. Chem. 2014, 20, 216–221. [Google Scholar] [CrossRef]
- Prabhansu; Karmakar, M.K.; Chandra, P.; Chatterjee, P.K. A Review on the Fuel Gas Cleaning Technologies in Gasification Process. J. Env. Chem. Eng. 2015, 3, 689–702. [Google Scholar] [CrossRef]
- Hoffmann, B.S.; Szklo, A. Integrated Gasification Combined Cycle and Carbon Capture: A Risky Option to Mitigate CO2 Emissions of Coal-Fired Power Plants. Appl. Energy 2011, 88, 3917–3929. [Google Scholar] [CrossRef]
- Kanhar, A.H.; Chen, S.; Wang, F. Incineration Fly Ash and Its Treatment to Possible Utilization: A Review. Energies 2020, 13, 6681. [Google Scholar] [CrossRef]
- Imam, T.; Capareda, S. Characterization of Bio-Oil, Syn-Gas and Bio-Char from Switchgrass Pyrolysis at Various Temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, J.; Meng, Z.; Zhong, W. Gasification Characteristics of Refuse Derived Fuels in a Fluidized Bed: Effect of Process Parameters and Catalytic Reforming. J. Energy Inst. 2023, 111, 101435. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Gasification of Sewage Sludge. In Industrial and Municipal Sludge: Emerging Concerns and Scope for Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 575–593. ISBN 9780128159071. [Google Scholar]
- Zhu, H.L.; Zhang, Y.S.; Materazzi, M.; Aranda, G.; Brett, D.J.L.; Shearing, P.R.; Manos, G. Co-Gasification of Beech-Wood and Polyethylene in a Fluidized-Bed Reactor. Fuel Process. Technol. 2019, 190, 29–37. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; André, R.N.; Tavares, C.; Dias, M.; Gulyurtlu, I.; Cabrita, I. Effect of Experimental Conditions on Co-Gasification of Coal, Biomass and Plastics Wastes with Air/Steam Mixtures in a Fluidized Bed System. Fuel 2003, 82, 1967–1976. [Google Scholar] [CrossRef]
- Peng, M.Q.; Chen, T.H.; Jin, T.; Su, Y.C.; Luo, S.T.; Xu, H. A Novel First-Order Kinetic Model for Simultaneous Anaerobic–Aerobic Degradation of Municipal Solid Waste in Landfills. Processes 2024, 12, 2225. [Google Scholar] [CrossRef]
- Maitlo, G.; Ali, I.; Mangi, K.H.; Ali, S.; Maitlo, H.A.; Unar, I.N.; Pirzada, A.M. Thermochemical Conversion of Biomass for Syngas Production: Current Status and Future Trends. Sustainability 2022, 14, 2596. [Google Scholar] [CrossRef]
- Alvarado-Flores, J.J.; Alcaraz-Vera, J.V.; Ávalos-Rodríguez, M.L.; Guzmán-Mejía, E.; Rutiaga-Quiñones, J.G.; Pintor-Ibarra, L.F.; Guevara-Martínez, S.J. Thermochemical Production of Hydrogen from Biomass: Pyrolysis and Gasification. Energies 2024, 17, 537. [Google Scholar] [CrossRef]
- Alouani, Y.; Saifaoui, D.; Alouani, A.; Alouani, M.A. Municipal Solid Waste Gasification to Produce Hydrogen: Integrated Simulation Model and Performance Analysis. Int. J. Energy Res. 2022, 46, 20068–20078. [Google Scholar] [CrossRef]
- Briesemeister, L.; Kremling, M.; Fendt, S.; Spliethoff, H. Air-Blown Entrained-Flow Gasification of Biomass: Influence of Operating Conditions on Tar Generation. Energy Fuels 2017, 31, 10924–10932. [Google Scholar] [CrossRef]
- Ascher, S. Environmental and Techno-Economic Analysis of Biomass and Waste Gasification Facilitated by Machine Learning. 2024. Available online: https://theses.gla.ac.uk/84564/ (accessed on 6 June 2025).
- Fu, X.; Chan, W.P.; Chin, V.; Boon, Y.Z.; Chen, W.; Zhao, Y.; Heberlein, S.; Gu, Y.; Oh, J.; Lisak, G. Converting Sludge to Slag through a High Temperature Slagging Co-Gasification Process: An Evaluation Based on a Demonstration Trial and Life Cycle Assessment. Chem. Eng. J. 2023, 468, 143475. [Google Scholar] [CrossRef]
- Sajid, M.; Raheem, A.; Ullah, N.; Asim, M.; Ur Rehman, M.S.; Ali, N. Gasification of Municipal Solid Waste: Progress, Challenges, and Prospects. Renew. Sustain. Energy Rev. 2022, 168, 112815. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S.; et al. Syngas Production from Biomass Gasification: Influences of Feedstock Properties, Reactor Type, and Reaction Parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Szul, M.; Głód, K.; Iluk, T. Influence of Pressure and CO2 in Fluidized Bed Gasification of Waste Biomasses. Biomass Convers. Biorefinery 2021, 11, 69–81. [Google Scholar] [CrossRef]
- Krūmiņš, J.; Kļaviņš, M. Integrated Circulating Fluidized Bed Gasification System for Sustainable Municipal Solid Waste Management: Energy Production and Heat Recovery. Energies 2023, 16, 5203. [Google Scholar] [CrossRef]
- Kertthong, T.; Schmid, M.; Scheffknecht, G. Non-Catalytic Partial Oxidation of Methane in Biomass-Derived Syngas with High Steam and Hydrogen Content Optimal for Subsequent Synthesis Process. J. Energy Inst. 2022, 105, 251–261. [Google Scholar] [CrossRef]
- Lestander, T.A.; Weiland, F.; Grimm, A.; Rudolfsson, M.; Wiinikka, H. Gasification of Pure and Mixed Feedstock Components: Effect on Syngas Composition and Gasification Efficiency. J. Clean. Prod. 2022, 369, 133330. [Google Scholar] [CrossRef]
- Williams, C.L.; Westover, T.L.; Emerson, R.M.; Tumuluru, J.S.; Li, C. Sources of Biomass Feedstock Variability and the Potential Impact on Biofuels Production. Bioenergy Res. 2016, 9, 1–14. [Google Scholar] [CrossRef]
- De, S.; Kumar, A.; Moholkar, V.S.; Thallada, B. (Eds.) Coal and Biomass Gasification Energy, Environment, and Sustainability Recent Advances and Future Challenges; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Raheem, A.; Dupont, V.; Channa, A.Q.; Zhao, X.; Vuppaladadiyam, A.K.; Taufiq-Yap, Y.H.; Zhao, M.; Harun, R. Parametric Characterization of Air Gasification of Chlorella Vulgaris Biomass. Energy Fuels 2017, 31, 2959–2969. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Abdul Salam, P. Estimation of Higher Heating Value of Biomass from Proximate Analysis: A New Approach. Fuel 2012, 99, 55–63. [Google Scholar] [CrossRef]
- Basha, M.H.; Sulaiman, S.A.; Uemura, Y. Co-Gasification of Palm Kernel Shell and Polystyrene Plastic: Effect of Different Operating Conditions. J. Energy Inst. 2020, 93, 1045–1052. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; Andre, R.N.; Miranda, M.; Gulyurtlu, I.; Cabrita, I. Co-Gasification Study of Biomass Mixed with Plastic Wastes. Fuel 2002, 81, 291–297. [Google Scholar] [CrossRef]
- Zhuang, X.; Zhu, N.; Li, F.; Lin, H.; Liang, C.; Dang, Z.; Zou, Y. Hydrogen-Rich Syngas Production from Waste Textile Gasification Coupling with Catalytic Reforming under Steam Atmosphere. Processes 2024, 12, 1790. [Google Scholar] [CrossRef]
- Ishak, M.Z.; Samiran, N.A.; Yu, Y.X.; Ishak, I.A.; Hamid, M.S.S. Effect of Feedstock Blends and Equivalence Ratio on the Thermal Arc Plasma Assisted Co-Gasification of Biomass and Plastic Using Air-Blown Downdraft Reactor. J. Adv. Res. Fluid. Mech. Therm. Sci. 2024, 121, 1–16. [Google Scholar] [CrossRef]
- Zubek, K.; Czerski, G.; Porada, S. Determination of Optimal Temperature and Amount of Catalysts Based on Alkali and Alkaline Earth Metals for Steam Gasification Process of Bituminous Coal. Thermochim. Acta 2018, 665, 60–69. [Google Scholar] [CrossRef]
- Sadhwani, N.; Adhikari, S.; Eden, M.R. Biomass Gasification Using Carbon Dioxide: Effect of Temperature, CO2/C Ratio, and the Study of Reactions Influencing the Process. Ind. Eng. Chem. Res. 2016, 55, 2883–2891. [Google Scholar] [CrossRef]
- Mishra, A.; Gautam, S.; Sharma, T. Effect of Operating Parameters on Coal Gasification. Int. J. Coal Sci. Technol. 2018, 5, 113–125. [Google Scholar] [CrossRef]
- Tuomi, S.; Kaisalo, N.; Simell, P.; Kurkela, E. Effect of Pressure on Tar Decomposition Activity of Different Bed Materials in Biomass Gasification Conditions. Fuel 2015, 158, 293–305. [Google Scholar] [CrossRef]
- Ajorloo, M.; Ghodrat, M.; Scott, J.; Strezov, V. Experimental Analysis of the Effects of Feedstock Composition on the Plastic and Biomass Co-Gasification Process. Renew. Energy 2024, 231. [Google Scholar] [CrossRef]
- Wang, B.; Gupta, R.; Bei, L.; Wan, Q.; Sun, L. A Review on Gasification of Municipal Solid Waste (MSW): Syngas Production, Tar Formation, Mineral Transformation and Industrial Challenges. Int. J. Hydrogen Energy 2023, 48, 26676–26706. [Google Scholar] [CrossRef]
- Islam, M.W. Effect of Different Gasifying Agents (Steam, H2O2, Oxygen, CO2, and Air) on Gasification Parameters. Int. J. Hydrogen Energy 2020, 45, 31760–31774. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass Gasification Technology: The State of the Art Overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Kihedu, J.H.; Yoshiie, R.; Naruse, I. Performance Indicators for Air and Air-Steam Auto-Thermal Updraft Gasification of Biomass in Packed Bed Reactor. Fuel Process. Technol. 2016, 141, 93–98. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Q.; Du, J.; Chen, J. Oxygen-Enriched Air Gasification of Biomass Materials for High-Quality Syngas Production. Energy Convers. Manag. 2019, 199, 111628. [Google Scholar] [CrossRef]
- Singh Siwal, S.; Zhang, Q.; Sun, C.; Thakur, S.; Kumar Gupta, V.; Kumar Thakur, V. Energy Production from Steam Gasification Processes and Parameters That Contemplate in Biomass Gasifier—A Review. Bioresour. Technol. 2020, 297, 122481. [Google Scholar] [CrossRef] [PubMed]
- Alipour Moghadam Esfahani, R.; Osmieri, L.; Specchia, S.; Yusup, S.; Tavasoli, A.; Zamaniyan, A. H2-Rich Syngas Production through Mixed Residual Biomass and HDPE Waste via Integrated Catalytic Gasification and Tar Cracking plus Bio-Char Upgrading. Chem. Eng. J. 2017, 308, 578–587. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Y.; Bie, X.; Li, Q.; Zhang, Y.; Zhou, H. Mechanisms in CO2 Gasification and Co-Gasification of Combustible Solid Waste: A Critical Review. Gas. Sci. Eng. 2024, 128, 205368. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, T.; Cheng, M.; Xie, M.; Shi, N.; Liu, T.; Huang, Z.; Zhao, Y.; Huang, Q.; Liu, Z.; et al. Recent Advances in Organic Waste Pyrolysis and Gasification in a CO2 Environment to Value-Added Products. J. Environ. Manag. 2024, 356, 120666. [Google Scholar] [CrossRef]
- Jamro, I.A.; Chen, G.; Baloch, H.A.; Wenga, T.; Ma, W. Optimization of Municipal Solid Waste Air Gasification for Higher H2 Production along with the Validation via Kinetics and Statistical Approaches. Fuel 2022, 322, 124137. [Google Scholar] [CrossRef]
- Wei, P.; Chen, G.; Zhi, F.; Zhang, A.; Deng, H.; Wen, X.; Wang, F.; Yu, C. Numerical Simulation and Experimental Study of Hydrogen Production from Chili Straw Waste Gasification Using Aspen Plus. Int. J. Hydrogen Energy 2024, 95, 377–388. [Google Scholar] [CrossRef]
- Liu, W.; Tian, Y.; Yan, H.; Zhou, X.; Tan, Y.; Yang, Y.; Li, Z.; Yuan, L. Gasification of Biomass Using Oxygen-Enriched Air as Gasification Agent: A Simulation Study. Biomass Convers. Biorefin 2023, 13, 15993–16000. [Google Scholar] [CrossRef]
- Restrepo, S.Y.G.; Rocha, M.H.; Lora, E.E.S.; Venturini, O.J.; Cobas, V.R.M.; Maya, D.M.Y. Design and Operation of a Gas Cleaning System for Biomass Gasification in a Two-Stage Air-Blown Downdraft Gasifier to Meet Quality Requirements of Solid Oxide Fuel Cells. Biomass Convers. Biorefin 2023, 13, 8239–8265. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Li, Z.; Chen, G.; Lei, T.; Gupta, A.K. Biomass Gasification Characteristic in an Auto-Thermal Fixed-Bed Gasifier Using Air and O2-Enriched Air. J. Energy Inst. 2025, 119. [Google Scholar] [CrossRef]
- Adnan, M.A.; Susanto, H.; Binous, H.; Muraza, O.; Hossain, M.M. Feed Compositions and Gasification Potential of Several Biomasses Including a Microalgae: A Thermodynamic Modeling Approach. Int. J. Hydrogen Energy 2017, 42, 17009–17019. [Google Scholar] [CrossRef]
- Gomes, H.G.M.F.; Matos, M.A.A.; Tarelho, L.A.C. Influence of Oxygen/Steam Addition on the Quality of Producer Gas during Direct (Air) Gasification of Residual Forest Biomass. Energies 2023, 16, 2427. [Google Scholar] [CrossRef]
- Raibhole, V.N.; Sapali, S.N. Simulation of Biomass Gasification with Oxygen/Air as Gasifying Agent by ASPEN Plus. Adv. Mater. Res. 2013, 622, 633–638. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, S.Y.; Jeong, Y.O.; Han, G.H.; Seo, Y.C. Effects of Oxygen Enrichment in Air Oxidants on Biomass Gasification Efficiency and the Reduction of Tar Emissions. Energies 2018, 11, 2664. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.; Cao, J.; Liu, C.; Dong, L.; Xu, L.; Zha, J. Experimental Study of Biomass Gasification with Oxygen-Enriched Air in Fluidized Bed Gasifier. Sci. Total Environ. 2018, 626, 423–433. [Google Scholar] [CrossRef]
- de Diego, L.F.; Ortiz, M.; García-Labiano, F.; Adánez, J.; Abad, A.; Gayán, P. Hydrogen Production by Chemical-Looping Reforming in a Circulating Fluidized Bed Reactor Using Ni-Based Oxygen Carriers. J. Power Sources 2009, 192, 27–34. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, H.; Wang, S.; Cai, J.; Qin, Y.; Zhou, L. Syngas Production by Chemical Looping Co-Gasification of Rice Husk and Coal Using an Iron-Based Oxygen Carrier. Fuel 2022, 309, 122100. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A Comprehensive Review of Primary Strategies for Tar Removal in Biomass Gasification. Energy Convers. Manag. 2022, 276, 116496. [Google Scholar] [CrossRef]
- Khan, M.J.; Al-attab, K.A. Steam Gasification of Biomass for Hydrogen Production—A Review and Outlook. J. Adv. Res. Fluid. Mech. Therm. Sci. 2022, 98, 175–204. [Google Scholar] [CrossRef]
- Shafiq, H.; Azam, S.U.; Hussain, A. Steam Gasification of Municipal Solid Waste for Hydrogen Production Using Aspen Plus® Simulation. Discov. Chem. Eng. 2021, 1, 4. [Google Scholar] [CrossRef]
- Fu, L.; Cao, Y.; Du, J. H2-Rich Syngas Produced from Steam Gasification of Municipal Solid Waste: A Modeling Approach. Clean. Technol. Environ. Policy 2022, 24, 1001–1007. [Google Scholar] [CrossRef]
- Singh, D.; Yadav, S.; Bharadwaj, N.; Verma, R. Low Temperature Steam Gasification to Produce Hydrogen Rich Gas from Kitchen Food Waste: Influence of Steam Flow Rate and Temperature. Int. J. Hydrogen Energy 2020, 45, 20843–20850. [Google Scholar] [CrossRef]
- Elbl, P.; Baláš, M.; Lisý, M.; Lisá, H. Sewage Sludge and Digestate Gasification in an Atmospheric Fluidized Bed Gasifier. Biomass Convers. Biorefin 2024, 14, 21821–21829. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current Technology Development for CO2 Utilization into Solar Fuels and Chemicals: A Review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Li, X.; Ge, Y.; Gao, F.; Chen, H.; Hao, Q.; Ma, X. Syngas Production by Integrating CO2 Partial Gasification of Pine Sawdust and Methane Pyrolysis over the Gasification Residue. Int. J. Hydrogen Energy 2019, 44, 19742–19754. [Google Scholar] [CrossRef]
- Cho, D.W.; Kwon, G.; Ok, Y.S.; Kwon, E.E.; Song, H. Reduction of Bromate by Cobalt-Impregnated Biochar Fabricated via Pyrolysis of Lignin Using CO2 as a Reaction Medium. ACS Appl. Mater. Interfaces 2017, 9, 13142–13150. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohammadi, M.; Mohamed, A.R. Conversion of the Greenhouse Gas CO2 to the Fuel Gas CO via the Boudouard Reaction: A Review. Renew. Sustain. Energy Rev. 2015, 41, 615–632. [Google Scholar] [CrossRef]
- Butterman, H.C.; Castaldi, M.J. CO2 as a Carbon Neutral Fuel Source via Enhanced Biomass Gasification. Env. Sci. Technol. 2009, 43, 9030–9037. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.; Miranda, M.; Neves, D.; Varela, F.; Santos, J. Effect of Gasification Agent on Co-Gasification of Rice Production Wastes Mixtures. Fuel 2016, 180, 407–416. [Google Scholar] [CrossRef]
- Sadhwani, N.; Adhikari, S.; Eden, M.R.; Li, P. Aspen plus Simulation to Predict Steady State Performance of Biomass-CO2 Gasification in a Fluidized Bed Gasifier. Biofuels Bioprod. Biorefining 2018, 12, 379–389. [Google Scholar] [CrossRef]
- Mauerhofer, A.M.; Müller, S.; Bartik, A.; Benedikt, F.; Fuchs, J.; Hammerschmid, M.; Hofbauer, H. Conversion of CO2 during the DFB Biomass Gasification Process. Biomass Convers. Biorefinery 2021, 11, 15–27. [Google Scholar] [CrossRef]
- Temaja, I.W.; Winaya, I.N.S.; Wirawan, I.K.G.; Sucipta, M.; Swamardika, I.B.A.; Darma, I.W.A.; Leite, I.d.J. Optimizing Syngas Production from Municipal Solid Waste Gasification: A Dual Reactor Fluidized Bed Study with Steam and CO2 as Gasification Agents. J. Adv. Res. Fluid. Mech. Therm. Sci. 2024, 123, 222–232. [Google Scholar] [CrossRef]
- Rakesh, N.; Dasappa, S. A Critical Assessment of Tar Generated during Biomass Gasification—Formation, Evaluation, Issues and Mitigation Strategies. Renew. Sustain. Energy Rev. 2018, 91, 1045–1064. [Google Scholar] [CrossRef]
- De Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The Influence of Catalysts in Biomass Steam Gasification and Catalytic Potential of Coal Bottom Ash in Biomass Steam Gasification: A Review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Alptekin, F.M.; Celiktas, M.S. Review on Catalytic Biomass Gasification for Hydrogen Production as a Sustainable Energy Form and Social, Technological, Economic, Environmental, and Political Analysis of Catalysts. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef]
- Arzamendi, G.; Arguiñarena, E.; Campo, I.; Zabala, S.; Gandía, L.M. Alkaline and Alkaline-Earth Metals Compounds as Catalysts for the Methanolysis of Sunflower Oil. Catal. Today 2008, 133–135, 305–313. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A Review of the Effects of Alkali and Alkaline Earth Metal Species on Biomass Gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.; Ramasamy, K.K.; Li, Z.; Klinger, J.L.; Schaidle, J.A.; Wang, H. Catalyst Deactivation and Its Mitigation during Catalytic Conversions of Biomass. ACS Catal. 2022, 12, 13555–13599. [Google Scholar] [CrossRef]
- Zubek, K.; Czerski, G.; Porada, S. Comparison of Catalysts Based on Individual Alkali and Alkaline Earth Metals with Their Composites Used for Steam Gasification of Coal. Energy Fuels 2018, 32, 5684–5692. [Google Scholar] [CrossRef]
- Ren, J.; Li, Y.; Jin, X.; Huang, X.; Li, Y.; Deng, L.; Che, D. Effects of Inherent AAEMs on Catalytic Gasification of Biomass with Large Particle Size under Oxygen-Steam Atmosphere. J. Anal. Appl. Pyrolysis 2024, 177, 106313. [Google Scholar] [CrossRef]
- Lv, P.; Bai, Y.; Wang, J.; Song, X.; Su, W.; Yu, G.; Ma, Y. Investigation into the Interaction of Biomass Waste with Industrial Solid Waste during Co-Pyrolysis and the Synergetic Effect of Its Char Gasification. Biomass Bioenergy 2022, 159. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, S.; Chen, Y.; Yang, Y.; Yang, H. Alkali and Alkaline Earth Metals Catalytic Steam Gasification of Ashless Lignin: Influence of the Catalyst Type and Loading Amount. Fuel 2024, 356, 129549. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, X.; Wu, C.; Wang, C.; Xie, J.; Zhou, Z.; Ma, L.; Li, H. Effects of Metal Catalysts on CO2 Gasification Reactivity of Biomass Char. Biotechnol. Adv. 2009, 27, 568–572. [Google Scholar] [CrossRef]
- Wang, G.; Ren, S.; Zhang, J.; Ning, X.; Liang, W.; Zhang, N.; Wang, C. Influence Mechanism of Alkali Metals on CO2 Gasification Properties of Metallurgical Coke. Chem. Eng. J. 2020, 387, 124093. [Google Scholar] [CrossRef]
- Li, S.; Gong, J. Strategies for Improving the Performance and Stability of Ni-Based Catalysts for Reforming Reactions. Chem. Soc. Rev. 2014, 43, 7245–7256. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Shen, B.; Wu, C. Preparation, Modification and Development of Ni-Based Catalysts for Catalytic Reforming of Tar Produced from Biomass Gasification. Renew. Sustain. Energy Rev. 2018, 94, 1086–1109. [Google Scholar] [CrossRef]
- Ngo, T.N.L.T.; Chiang, K.Y.; Liu, C.F.; Chang, Y.H.; Wan, H.P. Hydrogen Production Enhancement Using Hot Gas Cleaning System Combined with Prepared Ni-Based Catalyst in Biomass Gasification. Int. J. Hydrogen Energy 2021, 46, 11269–11283. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, Y.; Cao, W.; Jin, H.; Guo, L.; Huo, Z. Transition Metal Oxides as Catalysts for Hydrogen Production from Supercritical Water Gasification of Glucose. Catal. Lett. 2017, 147, 828–836. [Google Scholar] [CrossRef]
- Gonçalves, G.; Lenzi, M.K.; Santos, O.A.A.; Jorge, L.M.M. Preparation and Characterization of Nickel Based Catalysts on Silica, Alumina and Titania Obtained by Sol-Gel Method. J. Non Cryst. Solids 2006, 352, 3697–3704. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Co-Precipitation, Impregnation and so-Gel Preparation of Ni Catalysts for Pyrolysis-Catalytic Steam Reforming of Waste Plastics. Appl. Catal. B 2018, 239, 565–577. [Google Scholar] [CrossRef]
- Zhang, B.; Biswal, B.K.; Zhang, J.; Balasubramanian, R. Hydrothermal Treatment of Biomass Feedstocks for Sustainable Production of Chemicals, Fuels, and Materials: Progress and Perspectives. Chem. Rev. 2023, 123, 7193–7294. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, C.V.; Srikant, D.; Gurav, H.R. Catalyst Deactivation and Regeneration. In Industrial Catalytic Processes for Fine and Specialty Chemicals; Elsevier: Amsterdam, The Netherlands, 2016; pp. 187–219. ISBN 9780128014578. [Google Scholar]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Naseem, M.T.; Ali, S.; Zaman, W. Metal-Based Catalysts in Biomass Transformation: From Plant Feedstocks to Renewable Fuels and Chemicals. Catalysts 2025, 15, 40. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Q.; Wang, W.; Wang, Y. Processes of Metal Oxides Catalyst on Conversion of Spent Coffee Grounds into Rich-Synthesis Gas by Gasification. Processes 2024, 12, 2232. [Google Scholar] [CrossRef]
- Farooq, A.; Song, H.; Park, Y.K.; Rhee, G.H. Effects of Different Al2O3 Support on HDPE Gasification for Enhanced Hydrogen Generation Using Ni-Based Catalysts. Int. J. Hydrogen Energy 2021, 46, 18085–18092. [Google Scholar] [CrossRef]
- Liu, D.; Yu, R.; Yuan, P. Estuaries Are Promising Sites for Olivine Dissolution Engineering: Insights from Olivine Mineralogy. Ocean-Land-Atmos. Res. 2024, 3, 0039. [Google Scholar] [CrossRef]
- Soomro, A.; Chen, S.; Ma, S.; Xiang, W. Catalytic Activities of Nickel, Dolomite, and Olivine for Tar Removal and H2-Enriched Gas Production in Biomass Gasification Process. Energy Environ. 2018, 29, 839–867. [Google Scholar] [CrossRef]
- Savuto, E.; May, J.; Di Carlo, A.; Gallucci, K.; Di Giuliano, A.; Rapagnà, S. Steam Gasification of Lignite in a Bench-Scale Fluidized-Bed Gasifier Using Olivine as Bed Material. Appl. Sci. 2020, 10, 2931. [Google Scholar] [CrossRef]
- Tian, Y.; He, D.; Zeng, Y.; Hu, L.; Du, J.; Luo, Z.; Ma, W.; Zhang, Z. Experimental Research on Hydrogen-Rich Syngas Yield by Catalytic Biomass Air-Gasification over Ni/Olivine as in-Situ Tar Destruction Catalyst. J. Energy Inst. 2023, 108. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, S.; Liu, Y.; Qiao, C. Catalytic Steam Co-Gasification of Biomass and Coal in a Dual Loop Gasification System with Olivine Catalysts. J. Energy Inst. 2020, 93, 1074–1082. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, Y.; Du, J. Study on Gasification Characteristics of Pine Sawdust Using Olivine as In-Bed Material for Combustible Gas Production. J. Energy Inst. 2021, 96, 168–172. [Google Scholar] [CrossRef]
- Su, E.C.; Fu, R.C.; Lin, C.L. Application of Modified Olivine to a Two-Stage Gasification Process to Evaluate the Effects on Hydrogen Generation and Retention of Heavy Metals. Appl. Therm. Eng. 2024, 236, 121665. [Google Scholar] [CrossRef]
- Gao, N.; Salisu, J.; Quan, C.; Williams, P. Modified Nickel-Based Catalysts for Improved Steam Reforming of Biomass Tar: A Critical Review. Renew. Sustain. Energy Rev. 2021, 145, 111023. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Amutio, M.; Bilbao, J.; Olazar, M. Fe/Olivine as Primary Catalyst in the Biomass Steam Gasification in a Fountain Confined Spouted Bed Reactor. J. Ind. Eng. Chem. 2021, 99, 364–379. [Google Scholar] [CrossRef]
- Vinita, R. The Potential and Environmental Implications of Enhanced Olivine Weathering as Negative CO2 Emission Technology in Europe Ii. 2020. Available online: https://studenttheses.uu.nl/handle/20.500.12932/37063 (accessed on 6 June 2025).
- Atong, D.; Pechyen, C.; Aht-Ong, D.; Sricharoenchaikul, V. Synthetic Olivine Supported Nickel Catalysts for Gasification of Glycerol. Appl. Clay Sci. 2011, 53, 244–253. [Google Scholar] [CrossRef]
- Rapagnà, S.; Virginie, M.; Gallucci, K.; Courson, C.; Di Marcello, M.; Kiennemann, A.; Foscolo, P.U. Fe/Olivine Catalyst for Biomass Steam Gasification: Preparation, Characterization and Testing at Real Process Conditions. Catal. Today 2011, 176, 163–168. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, T.; Hou, B.; Yang, H.; Hu, N.; Zhang, M. A Comprehensive Review of Biomass Gasification Characteristics in Fluidized Bed Reactors: Progress, Challenges, and Future Directions. Fluids 2025, 10, 147. [Google Scholar] [CrossRef]
- Andrew, R.; Gokak, D.T.; Sharma, P.; Sharma, J.; Somkuwar, N.; Gupta, S. Practical Achievements on Biomass Steam Gasification in a Rotary Tubular Coiled-Downdraft Reactor. Procedia Environ. Sci. 2016, 35, 818–825. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, L.; Wei, W.; Jin, H.; Guo, L. Hydrogen Production by Sewage Sludge Gasification in Supercritical Water with High Heating Rate Batch Reactor. Energy 2022, 238, 121740. [Google Scholar] [CrossRef]
- Martínez, I.; Grasa, G.; Callén, M.S.; López, J.M.; Murillo, R. Optimised Production of Tailored Syngas from Municipal Solid Waste (MSW) by Sorption-Enhanced Gasification. Chem. Eng. J. 2020, 401, 126067. [Google Scholar] [CrossRef]
- Cerone, N.; Zimbardi, F.; Contuzzi, L.; Baleta, J.; Cerinski, D.; Skvorčinskienė, R. Experimental Investigation of Syngas Composition Variation along Updraft Fixed Bed Gasifier. Energy Convers. Manag. 2020, 221, 113116. [Google Scholar] [CrossRef]
- Upadhyay, D.S.; Sakhiya, A.K.; Panchal, K.; Patel, A.H.; Patel, R.N. Effect of Equivalence Ratio on the Performance of the Downdraft Gasifier—An Experimental and Modelling Approach. Energy 2019, 168, 833–846. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, Y.; Du, J. Air-Steam Gasification of Biomass Based on a Multi-Composition Multi-Step Kinetic Model: A Clean Strategy for Hydrogen-Enriched Syngas Production. Sci. Total Environ. 2021, 753, 141690. [Google Scholar] [CrossRef] [PubMed]
- Sidek, F.N.; Abdul Samad, N.A.F.; Saleh, S. Review on Effects of Gasifying Agents, Temperature and Equivalence Ratio in Biomass Gasification Process. IOP Conf. Ser. Mater. Sci. Eng. 2020, 863, 012028. [Google Scholar] [CrossRef]
- Han, S.W.; Lee, J.J.; Tokmurzin, D.; Lee, S.H.; Nam, J.Y.; Park, S.J.; Ra, H.W.; Mun, T.Y.; Yoon, S.J.; Yoon, S.M.; et al. Gasification Characteristics of Waste Plastics (SRF) in a Bubbling Fluidized Bed: Effects of Temperature and Equivalence Ratio. Energy 2022, 238, 121944. [Google Scholar] [CrossRef]
- Zin, M.A.M.; Samiran, N.A.; Chiong, M.C.; Ishak, M.Z.; Hamid, M.S.S. Effect of Equivalence Ratio on EFB Pellet Gasification Characteristic Using Thermal Arc Plasma Suction Downdraft Gasifier. J. Adv. Res. Appl. Sci. Eng. Technol. 2023, 30, 92–102. [Google Scholar] [CrossRef]
- Putro, F.A.; Pranolo, S.H.; Waluyo, J.; Basworo, A.T.; Norman, H.; Kristiani, A.; Hidayati, L.N. Tar Removal of Palm Kernel Shell Syngas Using Wet Scrubber. J. Rekayasa Kim. Lingkung. 2024, 19, 18–25. [Google Scholar] [CrossRef]
- Pereira, E.G.; Da Silva, J.N.; De Oliveira, J.L.; MacHado, C.S. Sustainable Energy: A Review of Gasification Technologies. Renew. Sustain. Energy Rev. 2012, 16, 4753–4762. [Google Scholar] [CrossRef]
- Courson, C.; Gallucci, K. Gas Cleaning for Waste Applications (Syngas Cleaning for Catalytic Synthetic Natural Gas Synthesis). In Substitute Natural Gas from Waste: Technical Assessment and Industrial Applications of Biochemical and Thermochemical Processes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–220. ISBN 9780128155547. [Google Scholar]
- Mondal, P.; Dang, G.S.; Garg, M.O. Syngas Production through Gasification and Cleanup for Downstream Applications—Recent Developments. Fuel Process. Technol. 2011, 92, 1395–1410. [Google Scholar] [CrossRef]
- Ghosh, A.; Debnath, B.; Ghosh, S.K.; Das, B.; Sarkar, J.P. Sustainability Analysis of Organic Fraction of Municipal Solid Waste Conversion Techniques for Efficient Resource Recovery in India through Case Studies. J. Mater. Cycles Waste Manag. 2018, 20, 1969–1985. [Google Scholar] [CrossRef]
- Matei, E.; Râpă, M.; Mateș, I.M.; Popescu, A.F.; Bădiceanu, A.; Balint, A.I.; Covaliu-Mierlă, C.I. Heavy Metals in Particulate Matter—Trends and Impacts on Environment. Molecules 2025, 30, 1455. [Google Scholar] [CrossRef] [PubMed]
- Haydary, J.; Rapčanová, E.; Škulec, M. Purification of Syngas from Refuse-Derived Fuel (RDF) Gasification: Techno-Economic Analysis. Therm. Sci. Eng. Prog. 2023, 44, 102024. [Google Scholar] [CrossRef]
- Morselli, N.; Parenti, M.; Puglia, M.; Tartarini, P. Use of Fabric Filters for Syngas Dry Filtration in Small-Scale Gasification Power Systems. AIP Conf. Proc. 2019, 2191, 020117. [Google Scholar]
- Parihar, A.K.S.; Joshi, C.; Sridhar, G. The Performance of Cyclones in Producer Gas Cleaning: Experimental and Modeling Studies. Proc. Inst. Mech. Eng. Part A J. Power Energy 2012, 226, 776–793. [Google Scholar] [CrossRef]
- Bianchini, A.; Cento, F.; Golfera, L.; Pellegrini, M.; Saccani, C. Performance Analysis of Different Scrubber Systems for Removal of Particulate Emissions from a Small Size Biomass Boiler. Biomass Bioenergy 2016, 92, 31–39. [Google Scholar] [CrossRef]
- Ji, X.; Huang, J.; Teng, L.; Li, S.; Li, X.; Cai, W.; Chen, Z.; Lai, Y. Advances in Particulate Matter Filtration: Materials, Performance, and Application. Green. Energy Environ. 2023, 8, 673–697. [Google Scholar] [CrossRef]
- Parihar, A.K.S.; Hammer, T.; Sridhar, G. Development and Testing of Tube Type Wet ESP for the Removal of Particulate Matter and Tar from Producer Gas. Renew. Energy 2015, 74, 875–883. [Google Scholar] [CrossRef]
- Chan, W.P.; Veksha, A.; Lei, J.; Da Oh, W.; Dou, X.; Giannis, A.; Lisak, G.; Lim, T.T. A Hot Syngas Purification System Integrated with Downdraft Gasification of Municipal Solid Waste. Appl. Energy 2019, 237, 227–240. [Google Scholar] [CrossRef]
- Lei, Z.; Hao, S.; Yusu, W.; Yang, J. Study on Dry Desulfurization Performance of MnOx Hydrothermally Loaded Halloysite Desulfurizer. Env. Technol. Innov. 2022, 26, 102308. [Google Scholar] [CrossRef]
- Wang, T.C.; Wei, L.W.; Huang, H.L.; Lin, K.S.; Wang, H.P. High-Temperature Syngas Desulfurization and Particulate Filtration by ZnO/Ceramic Filters. ACS Omega 2023, 8, 13813–13818. [Google Scholar] [CrossRef]
- Peck, D.; Zappi, M.; Gang, D.; Guillory, J.; Hernandez, R.; Buchireddy, P. Review of Porous Ceramics for Hot Gas Cleanup of Biomass Syngas Using Catalytic Ceramic Filters to Produce Green Hydrogen/Fuels/Chemicals. Energies 2023, 16, 2334. [Google Scholar] [CrossRef]
- Chan, W.P.; Yusoff, S.A.M.B.; Veksha, A.; Giannis, A.; Lim, T.T.; Lisak, G. Analytical Assessment of Tar Generated during Gasification of Municipal Solid Waste: Distribution of GC–MS Detectable Tar Compounds, Undetectable Tar Residues and Inorganic Impurities. Fuel 2020, 268, 117348. [Google Scholar] [CrossRef]
- Tri Setioputro, N.; Muchammad, M.; Yohana, E.; Fahd Fachrizal, M.; Ariyanto, H.D.; Kosim, M. Evaluation of Gasification Performance for Soaked Bamboo and Dried Bamboo as Feedstock in an Open Downdraft Gasifier. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=5223479 (accessed on 6 June 2025).
- Ud Din, Z.; Zainal, Z.A. Tar Reduction Mechanism via Compression of Producer Gas. J. Clean. Prod. 2018, 184, 1–11. [Google Scholar] [CrossRef]
- Tepamatr, P.; Rungsri, P.; Daorattanachai, P.; Laosiripojana, N. Maximizing H2 Production from a Combination of Catalytic Partial Oxidation of CH4 and Water Gas Shift Reaction. Molecules 2025, 30, 271. [Google Scholar] [CrossRef] [PubMed]
- Pranolo, S.H.; Waluyo, J.; Prasetiyo, J.; Hanif, M.I. Application of Recycle System on a Cocoa Pod Husks Gasification in a Fixed-Bed Downdraft Gasifier to Produce Low Tar Fuel Gas. J. Rekayasa Kim. Lingkung. 2019, 14, 120–129. [Google Scholar] [CrossRef][Green Version]
- Khajeh, A.; Masoumi, S.; Wang, L.; Shahbazi, A. Effects of Various Carbon-Supported Iron Catalysts on Tar Removal Efficiency and Syngas Yield during Catalytic Biomass Gasification. J. Env. Chem. Eng. 2023, 11, 110884. [Google Scholar] [CrossRef]
- Maulana, S.; Karnowo; Yunawan, N.R.; Ardani, A.A.; Putra, B.W.D.P.; Al-Janan, D.H. The Effect of Absorbent Type on Scrubber to Reduce Tar in Syngas. J. Adv. Res. Fluid. Mech. Therm. Sci. 2023, 108, 126–135. [Google Scholar] [CrossRef]
- Wiyono, A.; Aziz, M.; Sholehudin, A.; Sukrawan, Y.; Purnawan; Anggrainy, R.; Kadja, G.T.M.; Pambudi, N.A. Syngas Optimization and Tar Reduction via Multistage Catalytic Gasification: Effects of Catalyst Pore, Catalyzer Stage, and Temperature Profile. S. Afr. J. Chem. Eng. 2024, 48, 246–253. [Google Scholar] [CrossRef]
- Giannopoulos, D.; Katsifis, I.; Katsourinis, D.; Rentizelas, A.; Founti, M. An Assessment of Liquid Biofuel Value Chains from Heavy-Metal Contaminated Feedstock. Fuels 2022, 3, 509–532. [Google Scholar] [CrossRef]
- Pudasainee, D.; Paur, H.R.; Fleck, S.; Seifert, H. Trace Metals Emission in Syngas from Biomass Gasification. Fuel Process. Technol. 2014, 120, 54–60. [Google Scholar] [CrossRef]
- Mutyavaviri, L.C.; Chihobo, C.H.; Makepa, D.C. The Ecological Effects and Valorization of Coal Fines—A Review. Environ. Sci. Pollut. Res. 2024, 31, 51045–51063. [Google Scholar] [CrossRef]
- Fang, H.; Huang, L.; Wang, J.; He, G.; Reible, D. Environmental Assessment of Heavy Metal Transport and Transformation in the Hangzhou Bay, China. J. Hazard. Mater. 2016, 302, 447–457. [Google Scholar] [CrossRef]
- Dayton, D.C.; Turk, B.; Gupta, R. 5 Syngas Cleanup, Conditioning, and Utilization. 2019. Available online: https://onlinelibrary.wiley.com/doi/10.1002/9781119417637.ch5 (accessed on 6 June 2025).
- Ngoc Lan Thao, N.T.; Chiang, K.Y. The Migration, Transformation and Control of Trace Metals during the Gasification of Rice Straw. Chemosphere 2020, 260, 127540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, J.; Jin, L.; Zhou, Q.; Zhou, L.; Lu, Y.; Li, B. Mercury Removal from Syngas by Metal Oxides Based Adsorbent: A Review. Fuel 2022, 327, 125057. [Google Scholar] [CrossRef]
- Szul, M.; Iluk, T.; Sobolewski, A. High-Temperature, Dry Scrubbing of Syngas with Use of Mineral Sorbents and Ceramic Rigid Filters. Energies 2020, 13, 1528. [Google Scholar] [CrossRef]
- Sonia Sepahi, M.R.R. Advances in Synthesis Gas: Methods, Technologies and Applications. In Syngas Products and Usages; Mohammad Reza Rahimpour, M., Makarem, A., Meshksar, M., Eds.; Elsivier: Amsterdam, The Netherlands, 2023; Volume 3, pp. 111–146. [Google Scholar]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to Solution: Capture and Sequestration of Carbon Dioxide (CO2) and Its Utilization as a Renewable Energy Source for a Sustainable Future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Rey, J.R.C.; Longo, A.; Rijo, B.; Pedrero, C.M.; Tarelho, L.A.C.; Brito, P.S.D.; Nobre, C. A Review of Cleaning Technologies for Biomass-Derived Syngas. Fuel 2024, 377, 132776. [Google Scholar] [CrossRef]
- Gatti, M.; Martelli, E.; Marechal, F.; Consonni, S. Review, Modeling, Heat Integration, and Improved Schemes of Rectisol®-Based Processes for CO2 Capture. Appl. Therm. Eng. 2014, 70, 1123–1140. [Google Scholar] [CrossRef]
- Maneeintr, K.; Luemunkong, T.; Charinpanitkul, T. Removal of Acid Gases from Biomass-to-Liquid Process Syngas Used as Raw Materials for Fischer-Tropsch Technology. Available online: https://www.jstage.jst.go.jp/article/jie/93/11/93_1227/_pdf (accessed on 6 June 2025).
- Castrillon, M.C.; Moura, K.O.; Alves, C.A.; Bastos-Neto, M.; Azevedo, D.C.S.; Hofmann, J.; Möllmer, J.; Einicke, W.D.; Gläser, R. CO2 and H2S Removal from CH4-Rich Streams by Adsorption on Activated Carbons Modified with K2CO3, NaOH, or Fe2O3. Energy Fuels 2016, 30, 9596–9604. [Google Scholar] [CrossRef]
- Taheri, M.; Zhu, R.; Yu, G.; Lei, Z. Ionic Liquid Screening for CO2 Capture and H2S Removal from Gases: The Syngas Purification Case. Chem. Eng. Sci. 2021, 230, 116199. [Google Scholar] [CrossRef]
- Qayyum, A.; Ali, U.; Ramzan, N. Acid Gas Removal Techniques for Syngas, Natural Gas, and Biogas Clean up—A Review. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 46, 13136–13159. [Google Scholar] [CrossRef]
- Abotaleb, A.; Gladich, I.; Alkhateeb, A.; Mardini, N.; Bicer, Y.; Sinopoli, A. Chemical and Physical Systems for Sour Gas Removal: An Overview from Reaction Mechanisms to Industrial Implications. J. Nat. Gas Sci. Eng. 2022, 106, 104755. [Google Scholar] [CrossRef]
- Chan, Y.H.; Loy, A.C.M.; Cheah, K.W.; Chai, S.Y.W.; Ngu, L.H.; How, B.S.; Li, C.; Lock, S.S.M.; Wong, M.K.; Yiin, C.L.; et al. Hydrogen Sulfide (H2S) Conversion to Hydrogen (H2) and Value-Added Chemicals: Progress, Challenges and Outlook. Chem. Eng. J. 2023, 458, 141398. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Djellabi, R.; Vaccari, M.; Prasad, S.; M Aminabhavi, T.; Rtimi, S. Emerging Technologies and Sustainable Strategies for Municipal Solid Waste Valorization: Challenges of Circular Economy Implementation. Clean. Prod. 2023, 423, 138708. [Google Scholar] [CrossRef]
- Acharya, B.; Dutta, A.; Basu, P. Chemical-Looping Gasification of Biomass for Hydrogen-Enriched Gas. Energy Fuels 2009, 23, 5077–5083. [Google Scholar] [CrossRef]
- Sayyed, F.A.; Sayyed, N.; Solanki, F. Plasma Gasification: Transforming Waste into Energy. Available online: https://www.ijfmr.com/research-paper.php?id=19267 (accessed on 6 June 2025).
- Emun, F.; Gadalla, M.; Majozi, T.; Boer, D. Integrated Gasification Combined Cycle (IGCC) Process Simulation and Optimization. Comput. Chem. Eng. 2010, 34, 331–338. [Google Scholar] [CrossRef]
- Deshmukh, S.; Santhosh, R. Solar-Assisted Gasification of Agriculture Residues for Green Hydrogen Production. Bioresour. Technol. Rep. 2023, 22, 101506. [Google Scholar] [CrossRef]
- Zhang, S.; Asadullah, M.; Dong, L.; Tay, H.L.; Li, C.Z. An Advanced Biomass Gasification Technology with Integrated Catalytic Hot Gas Cleaning. Part II: Tar Reforming Using Char as a Catalyst or as a Catalyst Support. Fuel 2013, 112, 646–653. [Google Scholar] [CrossRef]
- Alfarra, F.; Ozcan, H.K.; Cihan, P.; Ongen, A.; Guvenc, S.Y.; Ciner, M.N. Artificial Intelligence Methods for Modeling Gasification of Waste Biomass: A Review. Environ. Monit. Assess. 2024, 196, 309. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; Dieringer, P.; Epple, B. Biomass-Based Chemical Looping Gasification: Overview and Recent Developments. Appl. Sci. 2021, 11, 7069. [Google Scholar] [CrossRef]
- Li, F.; Kim, H.R.; Sridhar, D.; Wang, F.; Zeng, L.; Chen, J.; Fan, L.S. Syngas Chemical Looping Gasification Process: Oxygen Carrier Particle Selection and Performance. Energy Fuels 2009, 23, 4182–4189. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Wang, Y.; Huo, R.; Huang, Z.; Liu, M.; Wei, G.; Zhao, Z.; Li, H.; Fang, Y. Review of Biomass Chemical Looping Gasification in China. Energy Fuels 2020, 34, 7847–7862. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J.; Wang, M.; Ma, X.; Jiang, E.; Hu, Z. Syngas Production by Chemical Looping Gasification of Rice Husk Using Fe-Based Oxygen Carrier. J. Energy Inst. 2020, 93, 1261–1270. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Feng, Y.; Zhao, K.; Zheng, A.; Chang, S.; Wei, G.; Zhao, Z.; Li, H. Biomass Char Direct Chemical Looping Gasification Using NiO-Modified Iron Ore as an Oxygen Carrier. Proc. Energy Fuels 2014, 28, 183–191. [Google Scholar] [CrossRef]
- Miccio, F.; Polchri, L.; Murri, A.N.; Landi, E.; Medri, V. Chemical Looping Gasification of Biomass Char in Fluidized Bed and CO2-Enriched Atmosphere. Biomass Convers. Biorefinery 2024, 15, 11561–11571. [Google Scholar] [CrossRef]
- Yang, G.; Hu, Q.; Hu, J.; Yang, H.; Yan, S.; Chen, Y.; Wang, X.; Chen, H. Hydrogen-Rich Syngas Production from Biomass Gasification Using Biochar-Based Nanocatalysts. Bioresour. Technol. 2023, 379, 129005. [Google Scholar] [CrossRef]
- Ge, H.; Shen, L.; Gu, H.; Jiang, S. Effect of Co-Precipitation and Impregnation on K-Decorated Fe2O3/Al2O3 Oxygen Carrier in Chemical Looping Combustion of Bituminous Coal. Chem. Eng. J. 2015, 262, 1065–1076. [Google Scholar] [CrossRef]
- Hai-bo, Z.; Li-ming, L.; Di, X.; Chu-guang, Z.; Guo-jun, L.; Lin-lin, J. NiO/NiAl2O4 Oxygen Carriers Prepared by Sol-Gel for Chemical-Looping Combustion Fueled by Gas. J. Fuel Chem. Technol. 2008, 36, 261–266. [Google Scholar]
- Adánez, J.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J.M. Selection of Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Jacobs, M.; van der Kolk, T.; Albertsen, K.; Mattisson, T.; Lyngfelt, A.; Snijkers, F. Synthesis and Upscaling of Perovskite Mn-Based Oxygen Carrier by Industrial Spray Drying Route. Int. J. Greenh. Gas. Control 2018, 70, 68–75. [Google Scholar] [CrossRef]
- Chang, Y.J.; Chang, J.S.; Lee, D.J. Gasification of Biomass for Syngas Production: Research Update and Stoichiometry Diagram Presentation. Bioresour. Technol. 2023, 387, 129535. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Shen, L.; Xiao, J.; Chen, D.; Gu, H.; Zhang, S. Nitrogen Transfer of Fuel-N in Chemical Looping Combustion. Combust. Flame 2012, 159, 1286–1295. [Google Scholar] [CrossRef]
- Mihai, O.; Chen, D.; Holmen, A. Catalytic Consequence of Oxygen of Lanthanum Ferrite Perovskite in Chemical Looping Reforming of Methane. Ind. Eng. Chem. Res. 2011, 50, 2613–2621. [Google Scholar] [CrossRef]
- Wang, S.; Song, T.; Yin, S.; Hartge, E.U.; Dymala, T.; Shen, L.; Heinrich, S.; Werther, J. Syngas, Tar and Char Behavior in Chemical Looping Gasification of Sawdust Pellet in Fluidized Bed. Fuel 2020, 270, 117464. [Google Scholar] [CrossRef]
- Wang, S.; Wu, F.; Wang, X. Experimental and Kinetics Analysis on Biomass Chemical Looping Gasification Using Lean Iron Ore as Oxygen Carrier. Chem. Eng. J. 2023, 474, 145855. [Google Scholar] [CrossRef]
- Gao, N.; Milandile, M.H.; Quan, C.; Rundong, L. Critical Assessment of Plasma Tar Reforming during Biomass Gasification: A Review on Advancement in Plasma Technology. J. Hazard. Mater. 2022, 421, 126764. [Google Scholar] [CrossRef]
- Moustakas, K.; Fatta, D.; Malamis, S.; Haralambous, K.; Loizidou, M. Demonstration Plasma Gasification/Vitrification System for Effective Hazardous Waste Treatment. J. Hazard. Mater. 2005, 123, 120–126. [Google Scholar] [CrossRef]
- Chang, J.S.; Gu, B.W.; Looy, P.C.; Chu, F.Y.; Simpson, C.J. Thermal Plasma Pyrolysis of Used Old Tires for Production of Syngas. J. Env. Sci. Health A Tox Hazard. Subst. Env. Eng. 1996, 31, 1781–1799. [Google Scholar] [CrossRef]
- Munir, M.T.; Mardon, I.; Al-Zuhair, S.; Shawabkeh, A.; Saqib, N.U. Plasma Gasification of Municipal Solid Waste for Waste-to-Value Processing. Renew. Sustain. Energy Rev. 2019, 116, 109461. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.; Zhang, Y.; Li, T.; Wei, X. Performance Investigation of the Gasification for the Kitchen Waste Powder in a Direct Current Plasma Reactor. J. Energy Inst. 2022, 100, 170–176. [Google Scholar] [CrossRef]
- Mazzoni, L.; Janajreh, I. Plasma Gasification of Municipal Solid Waste with Variable Content of Plastic Solid Waste for Enhanced Energy Recovery. Int. J. Hydrogen Energy 2017, 42, 19446–19457. [Google Scholar] [CrossRef]
- Messerle, V.E.; Mosse, A.L.; Ustimenko, A.B. Processing of Biomedical Waste in Plasma Gasifier. Waste Manag. 2018, 79, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Gorokhovski, M.; Karpenko, E.I.; Lockwood, F.C.; Messerle, V.E.; Trusov, B.G.; Ustimenko, A.B. Plasma Technologies for Solid Fuels: Experiment and Theory. J. Energy Inst. 2005, 78, 157–171. [Google Scholar] [CrossRef]
- Dong, K.; Chen, S.; Guo, Z.; Chu, C.; Chen, G.; De Filippis, P.; de Caprariis, B.; Ma, W. Plasma Gasification of Municipla Solid Waste to Produce High H2 Syngas 2022. Available online: https://europepmc.org/article/ppr/ppr556504 (accessed on 6 June 2025).
- Mallick, R.; Vairakannu, P. CO2 Plasma Gasification of Bakelite-Based Electrical Switch Waste Feedstock. J. Clean. Prod. 2023, 423, 138813. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Hosseini, S.S. A Novel Integration of Plasma Gasification Melting Process with Direct Carbon Fuel Cell. Int. J. Hydrogen Energy 2024, 50, 388–401. [Google Scholar] [CrossRef]
- Qi, Y.; Muhammad, U.; Zhang, W.; Song, Y.; Zhang, M.; Wang, M.; Xu, C.; Xu, Y.; Cai, S.; Han, C.; et al. A Comprehensive Evaluation of Recent Advancement in Municipal Solid Waste Gasification: Research Status, Technical Challenges and Perspectives. Sep. Purif. Technol. 2024, 358, 130443. [Google Scholar] [CrossRef]
- Chari, S.; Sebastiani, A.; Paulillo, A.; Materazzi, M. The Environmental Performance of Mixed Plastic Waste Gasification with Carbon Capture and Storage to Produce Hydrogen in the U.K. ACS Sustain. Chem. Eng. 2023, 11, 3248–3259. [Google Scholar] [CrossRef]
- Chai, Y.; Packham, N.; Wang, M. Process Improvement Analysis of Pyrolysis/Gasification of Biomass and Waste Plastics with Carbon Capture and Utilisation through Process Simulation. Fuel 2022, 324, 124571. [Google Scholar] [CrossRef]
- Haaf, M.; Peters, J.; Hilz, J.; Unger, A.; Ströhle, J.; Epple, B. Combustion of Solid Recovered Fuels within the Calcium Looping Process—Experimental Demonstration at 1 MWth Scale. Exp. Therm. Fluid. Sci. 2020, 113, 110023. [Google Scholar] [CrossRef]
- Dashtestani, F.; Nusheh, M.; Siriwongrungson, V.; Hongrapipat, J.; Materic, V.; Pang, S. CO2 Capture from Biomass Gasification Producer Gas Using a Novel Calcium and Iron-Based Sorbent through Carbonation-Calcination Looping. Ind. Eng. Chem. Res. 2020, 59, 18447–18459. [Google Scholar] [CrossRef]
- Detchusananard, T.; Im-orb, K.; Ponpesh, P.; Arpornwichanop, A. Biomass Gasification Integrated with CO2 Capture Processes for High-Purity Hydrogen Production: Process Performance and Energy Analysis. Energy Convers. Manag. 2018, 171, 1560–1572. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Wang, Y.; Yang, Z.; Du, Q.; Jiao, K. Assessment of CO2 Enrichment Mechanism in Integrated Coal Gasification Fuel Cell Combined Cycle System with Carbon Capture. Front. Energy Res. 2023, 10, 1017829. [Google Scholar] [CrossRef]
- Cormos, C.C. Integrated Assessment of IGCC Power Generation Technology with Carbon Capture and Storage (CCS). Energy 2012, 42, 434–445. [Google Scholar] [CrossRef]
- Shi, B.; Xu, W.; Wu, E.; Wu, W.; Kuo, P.C. Novel Design of Integrated Gasification Combined Cycle (IGCC) Power Plants with CO2 Capture. J. Clean. Prod. 2018, 195, 176–186. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Bellouard, Q.; Abanades, S.; Rodat, S.; Dupassieux, N. A High Temperature Drop-Tube and Packed-Bed Solar Reactor for Continuous Biomass Gasification. AIP Conf. Proc. 2017, 1850, 100001. [Google Scholar]
- Kodama, T.; Enomoto, S.I.; Hatamachi, T.; Gokon, N. Application of an Internally Circulating Fluidized Bed for Windowed Solar Chemical Reactor with Direct Irradiation of Reacting Particles. J. Sol. Energy Eng. 2008, 130, 0145041–0145044. [Google Scholar] [CrossRef]
- Wieckert, C.; Obrist, A.; Zedtwitz, P.; Von Maag, G.; Steinfeld, A. Syngas Production by Thermochemical Gasification of Carbonaceous Waste Materials in a 150 KWth Packed-Bed Solar Reactor. Energy Fuels 2013, 27, 4770–4776. [Google Scholar] [CrossRef]
- Xu, D.; Gu, X.; Dai, Y. Concentrating Solar Assisted Biomass-to-Fuel Conversion through Gasification: A Review. Front. Energy Res. 2023, 10, 1029477. [Google Scholar] [CrossRef]
- Gómez-Barea, A.; Suárez-Almeida, M.; Ghoniem, A. Analysis of Fluidized Bed Gasification of Biomass Assisted by Solar-Heated Particles. Biomass Convers. Biorefinery 2021, 11, 143–158. [Google Scholar] [CrossRef]
- Müller, F.; Patel, H.; Blumenthal, D.; Poživil, P.; Das, P.; Wieckert, C.; Maiti, P.; Maiti, S.; Steinfeld, A. Co-Production of Syngas and Potassium-Based Fertilizer by Solar-Driven Thermochemical Conversion of Crop Residues. Fuel Process. Technol. 2018, 171, 89–99. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Performance Assessment of Hydrogen Production from a Solar-Assisted Biomass Gasification System. Int. J. Hydrogen Energy 2013, 38, 6120–6129. [Google Scholar] [CrossRef]
- Sun, Z.; Aziz, M. Solar-Assisted Biomass Chemical Looping Gasification in an Indirect Coupling: Principle and Application. Appl. Energy 2022, 323, 119635. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I. Revolutionizing Waste Management: Unleashing the Power of Artificial Intelligence and Machine Learning. In Advances in Energy from Waste; Elsevier: Amsterdam, The Netherlands, 2024; pp. 225–279. ISBN 9780443138478. [Google Scholar]
- Shafik, W. IoT-Enabled Model and Waste Management Technologies for Sustainable Agriculture. In Sustainable Environmental Management; Parray, A.J., Haghi, A.K., Meraj, G., Eds.; Springer: Cham, Switzerland, 2024; Volume 227, pp. 137–163. [Google Scholar]
- ECTI DAMT & NCON 2019. In Proceedings of the 4th International Conference on Digital Arts, Media and Technology and 2nd ECTI Northern Section Conference on Electrical, Electronics, Computer and Telecommunications Engineering, Nan, Thailand, 30 January–2 February 2019; ISBN 9781538680728.
- Salou, H.; Fidele, N.J.; Ousmane, Z.; Frederic, O.; Gerard, S.B. Monitoring the Air-Fuel Ratio (AFR) of a Biomass Gasification Process with an Arduino Uno 3 and a Narrow Band Lambda Oxygen Sensor. Curr. J. Appl. Sci. Technol. 2024, 43, 46–58. [Google Scholar] [CrossRef]
- Mighani, M.; Covella, K.; Örs, E.; Rauch, J.F.; Mönch, H.P.; Gräbner, M. CO2 Reduction by Advanced Process Control in Gasification Processes. Comput. Aided Chem. Eng. 2020, 48, 1279–1284. [Google Scholar] [CrossRef]
- Aboughaly, M.; Fattah, I.M.R. Environmental Analysis, Monitoring, and Process Control Strategy for Reduction of Greenhouse Gaseous Emissions in Thermochemical Reactions. Atmosphere 2023, 14, 655. [Google Scholar] [CrossRef]
- Dev, P.R.; Badcha, Y.M.; Priya, M.; Muthuveerappan, S.; Karthikeyan, S.; Britto, P.J. Iot Enabled Sensors and Wireless Networks for Efficient Environmental Tracking Systems. In Proceedings of the 2023 3rd International Conference on Pervasive Computing and Social Networking, ICPCSN, Salem, India, 19–20 June 2023; pp. 1387–1393. [Google Scholar]
- In Proceedings of the 2018 Fourth International Conference on Computing Communication Control and Automation (ICCUBEA). Pune, India, 16–18 August 2018; IEEE; Piscataway, NJ, USA; ISBN 9781538652572.
- Waters, M.; Waszczuk, P.; Ayre, R.; Dreze, A.; McGlinchey, D.; Alkali, B.; Morison, G. Open Source IIoT Solution for Gas Waste Monitoring in Smart Factory. Sensors 2022, 22, 2972. [Google Scholar] [CrossRef]
- Forootan, M.M.; Larki, I.; Zahedi, R.; Ahmadi, A. Machine Learning and Deep Learning in Energy Systems: A Review. Sustainability 2022, 14, 4832. [Google Scholar] [CrossRef]
- Mutlu, A.Y.; Yucel, O. An Artificial Intelligence Based Approach to Predicting Syngas Composition for Downdraft Biomass Gasification. Energy 2018, 165, 895–901. [Google Scholar] [CrossRef]
- Guo, X.; Bouteraa, Y.; Khishe, M.; Li, C.; Martín, D. Intelligent Optimization of Steam Gasification Catalysts for Palm Oil Waste Using Support Vector Machine and Adaptive Transition Marine Predator Algorithm. Complex. Intell. Syst. 2024, 10, 6283–6303. [Google Scholar] [CrossRef]
- Pan, I.; Pandey, D.S. Incorporating Uncertainty in Data Driven Regression Models of Fluidized Bed Gasification: A Bayesian Approach. Fuel Process. Technol. 2016, 142, 305–314. [Google Scholar] [CrossRef]
- Kačur, J.; Durdán, M.; Laciak, M.; Flegner, P. A Comparative Study of Data-Driven Modeling Methods for Soft-Sensing in Underground Coal Gasification. Acta Polytech. 2019, 59, 322–351. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Mustapa, S.I.; Kanthasamy, R.; Mohammad, N.; AlTurki, A.; Babu, T.S. Performance Analysis of Support Vector Machine, Gaussian Process Regression, Sequential Quadratic Programming Algorithms in Modeling Hydrogen-Rich Syngas Production from Catalyzed Co-Gasification of Biomass Wastes from Oil Palm. Int. J. Hydrogen Energy 2022, 47, 41432–41443. [Google Scholar] [CrossRef]
- Ranjan, R. AI-Enhanced Predictive Maintenance Systems for Industrial Equipment: Developing Machine Learning Models to Forecast Failures, Optimize Maintenance Schedules, and Minimize Downtime. Available online: https://www.researchgate.net/publication/388122620_AI-Enhanced_Predictive_Maintenance_Systems_for_Industrial_Equipment_Developing_Machine_Learning_Models_to_Forecast_Failures_Optimize_Maintenance_Schedules_and_Minimize_Downtime (accessed on 6 June 2025).
- Wu, H.; Zhao, J. Deep Convolutional Neural Network Model Based Chemical Process Fault Diagnosis. Comput. Chem. Eng. 2018, 115, 185–197. [Google Scholar] [CrossRef]
- Wang, K.; Shang, C.; Liu, L.; Jiang, Y.; Huang, D.; Yang, F. Dynamic Soft Sensor Development Based on Convolutional Neural Networks. Ind. Eng. Chem. Res. 2019, 58, 11521–11531. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, J.; Shang, C.; Huang, D. Operation Optimization of Shell Coal Gasification Process Based on Convolutional Neural Network Models. Appl. Energy 2021, 292, 116847. [Google Scholar] [CrossRef]
- Aentung, T.; Patcharavorachot, Y.; Wu, W. Co-Gasification of Plastic Waste Blended with Biomass: Process Modeling and Multi-Objective Optimization. Processes 2024, 12, 1906. [Google Scholar] [CrossRef]
- Raj, K.; Srivastava, A.K. Real Time Estimation of GHG Emissions Using IoT Integrated Sensor Fusion. In Proceedings of the 2023 International Conference on Data Science and Informatics, ICDSI, Bhubaneswar, India, 12–13 August 2023; pp. 286–290. [Google Scholar]
- Barnwal, R.P.; Bharti, S.; Misra, S.; Obaidat, M.S. UCGNet: Wireless Sensor Network-Based Active Aquifer Contamination Monitoring and Control System for Underground Coal Gasification. Int. J. Commun. Syst. 2017, 30, e2852. [Google Scholar] [CrossRef]
- Kabugo, J.C.; Jämsä-Jounela, S.L.; Schiemann, R.; Binder, C. Industry 4.0 Based Process Data Analytics Platform: A Waste-to-Energy Plant Case Study. Int. J. Electr. Power Energy Syst. 2020, 115, 105508. [Google Scholar] [CrossRef]
- Buratto, W.G.; Muniz, R.N.; Nied, A.; Barros, C.F.d.O.; Cardoso, R.; Gonzalez, G.V. A Review of Automation and Sensors: Parameters Control of Thermal Treatments for Electricity Generation. Sensors 2024, 24, 967. [Google Scholar] [CrossRef]
- Ji, Z.; Yang, S.H.; Cao, Y.; Wang, Y.; Zhou, C.; Yue, L.; Zhang, Y. Harmonizing Safety and Security Risk Analysis and Prevention in Cyber-Physical Systems. Process Saf. Environ. Prot. 2021, 148, 1279–1291. [Google Scholar] [CrossRef]
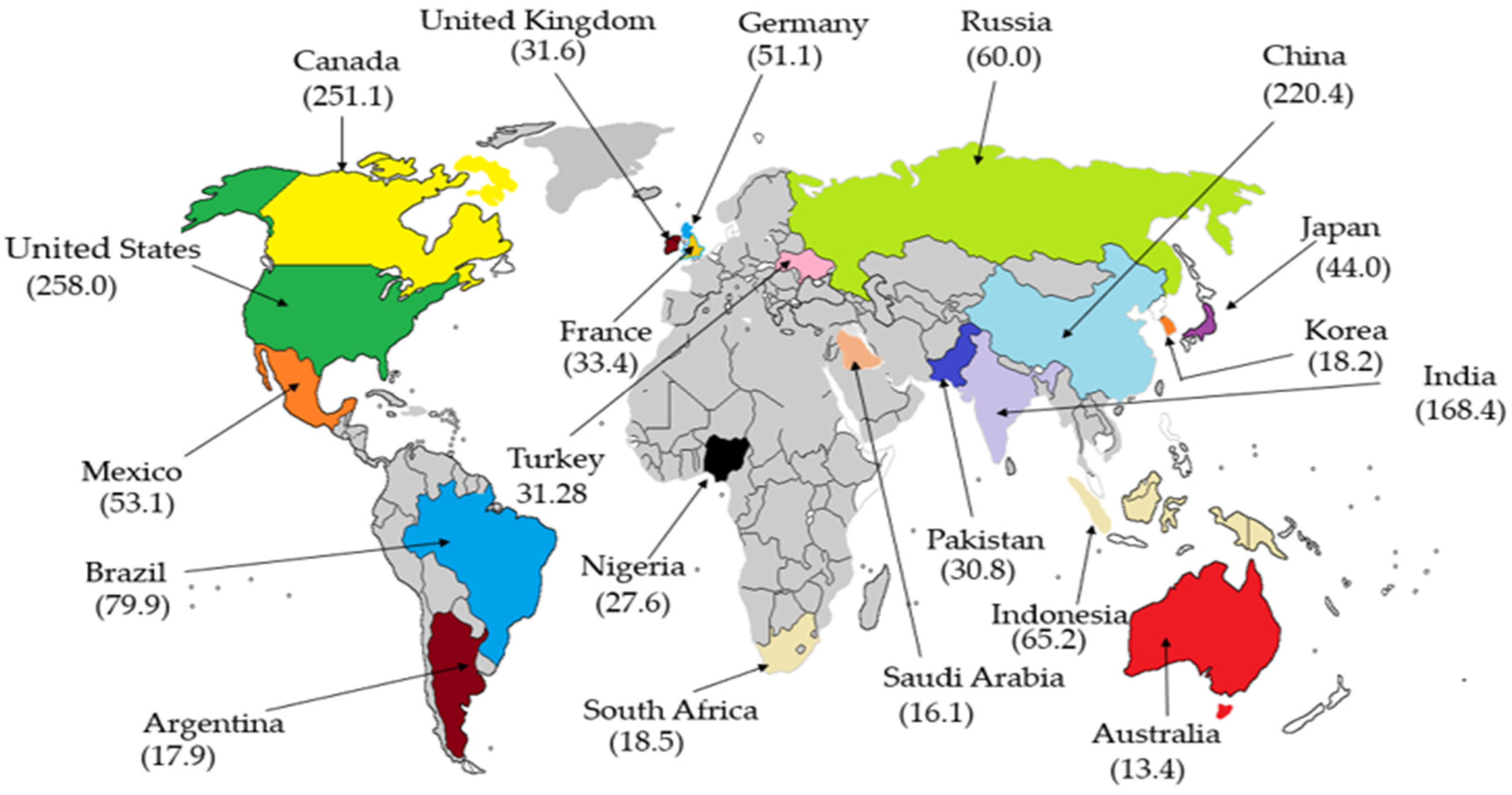

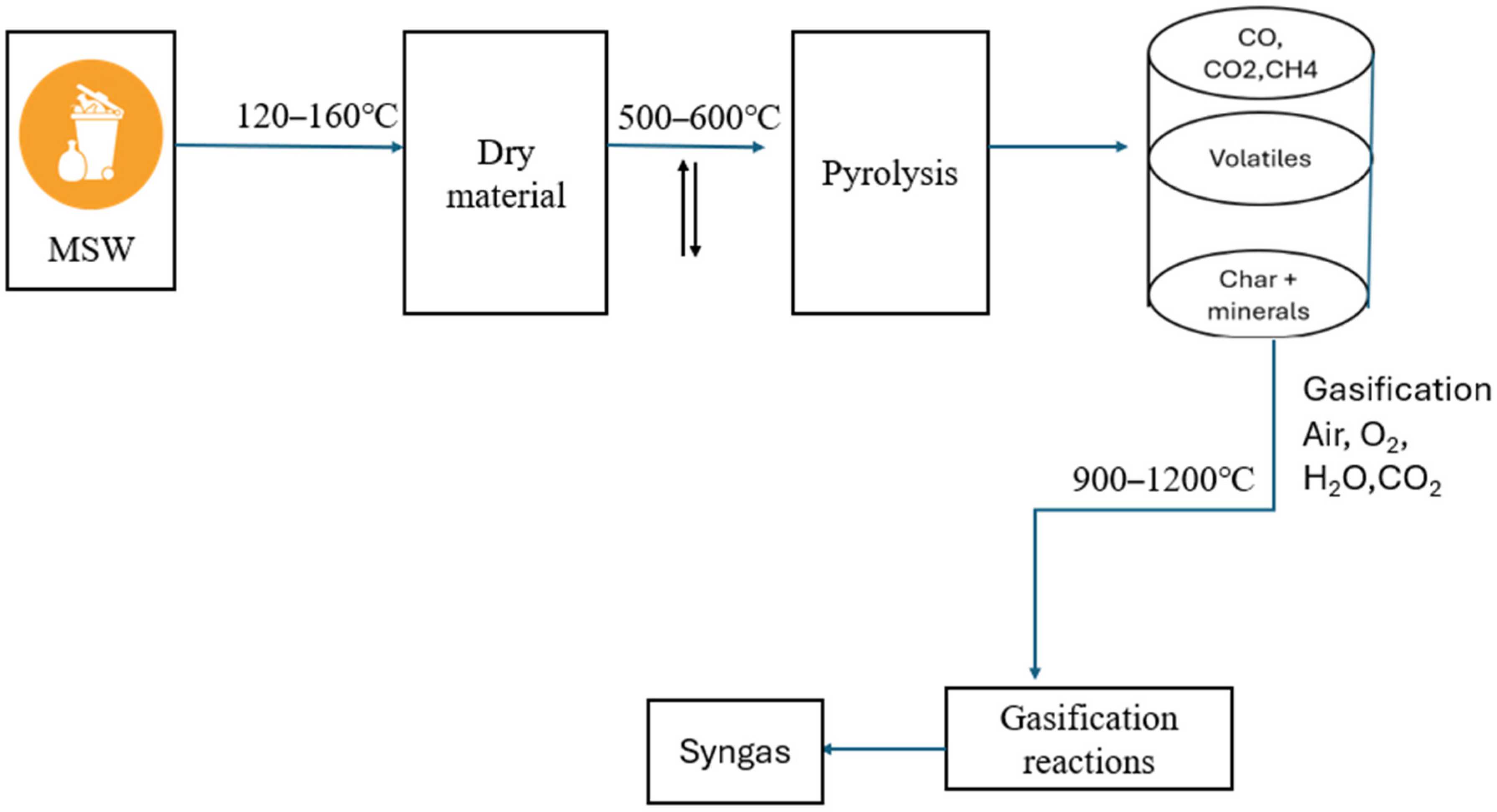







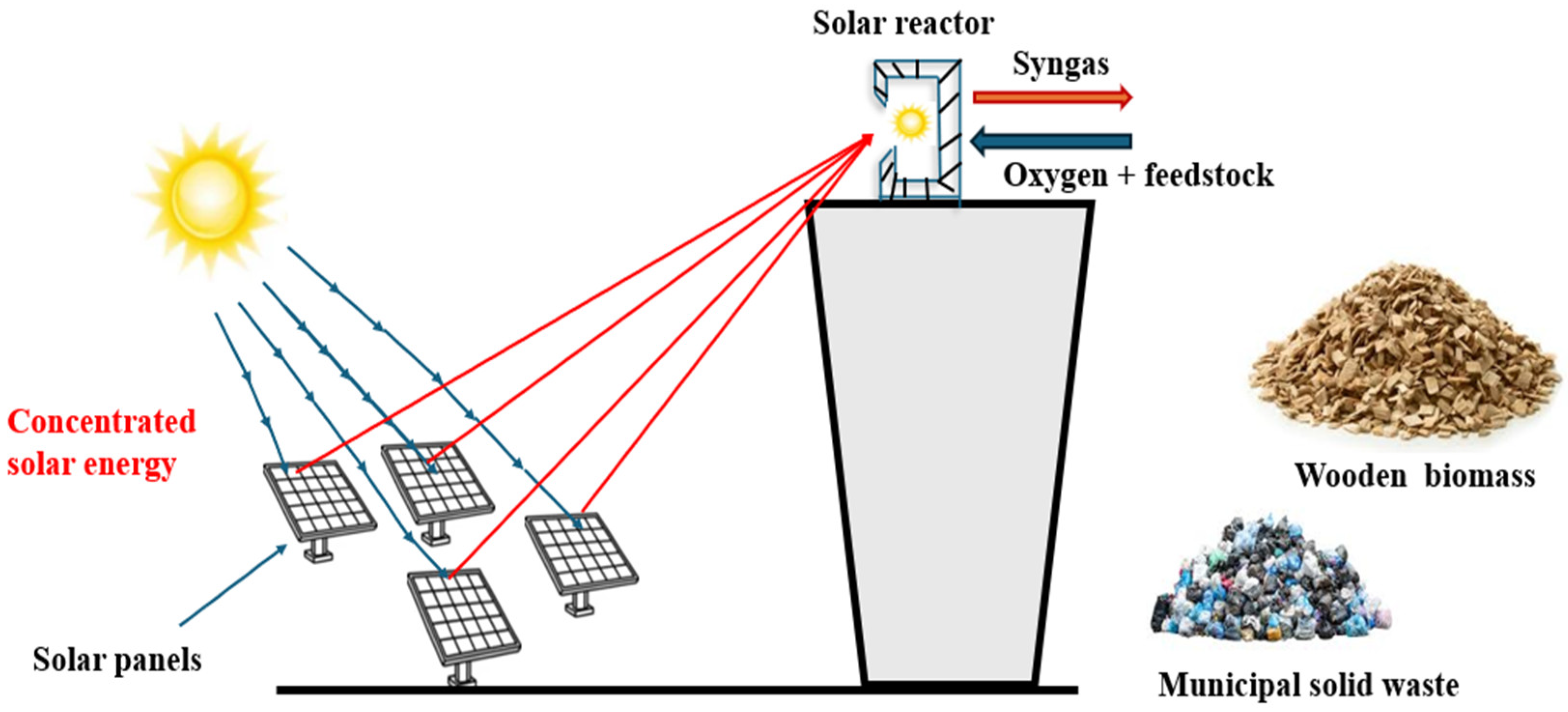
| MSW Type | Composition | Examples | References |
|---|---|---|---|
| Biodegradable Waste | Waste that decomposes naturally by microorganisms. | Food, yard waste, and paper. | [28,29] |
| Recyclable Waste | Waste materials that can be recovered, processed, and reused. | Plastic, glass, metals, and cardboard. | [30,31] |
| Inert Waste | Non-reactive, non-biodegradable, and has negligible or no negative environmental impacts. | Concrete, stones, ceramics, and construction debris. | [32] |
| Hazardous Waste | Materials causing a risk to human health or the environment. | Batteries, paints, chemicals, and e-waste. | [33,34] |
| E-Waste | Discarded electronic devices. | Computers, smartphones, and televisions. | [35] |
| Plastic Waste | Non-biodegradable synthetic polymers. | Plastic bottles, bags, and food packaging. | [36,37] |
| Biomedical Waste | Waste from healthcare facilities and activities. | Syringes, medical gloves, and pharmaceuticals. | [38,39] |
| Bulky Waste | Large items not suited for regular waste systems. | Furniture, appliances, and mattresses. | [40,41] |
| Liquid Waste | Waste in liquid form, often from households or businesses. | Used cooking oil, wastewater, and sludges. | [42,43] |
| Green Waste | Organic waste generated from gardening or agricultural activities. | Grass clippings, pruning waste, and weeds. | [44,45] |
| Aspect | Gasification [22,44,118,119] | Incineration [62,120] | Pyrolysis [120,121] |
|---|---|---|---|
| Primary Output | Syngas (H2, CO, CH4) | Heat and electricity | Bio-oil, biochar, syngas |
| Emission Levels | Low (with cleaning systems) | Higher NOx, SOx, dioxins, and furans | Minimal air emissions |
| Feedstock Flexibility | High | Moderate | Moderate |
| Energy Efficiency | High | Moderate | Moderate |
| By-product Usability | Recyclable slag or ash | Toxic fly ash requires treatment | Biochar (limited reuse potential) |
| Scalability | High | Large-scale only | Suitable for small- to medium-scale |
| Carbon Capture Feasibility | Feasible due to syngas use | Not feasible | Limited feasibility |
| Environmental Impact | Minimal with advanced technologies | Higher environmental burden | Lower than incineration |
| Gasification Agent | Effect on Gasification | Advantages | Limitations | References |
|---|---|---|---|---|
| Air | Low calorific value of 3–5 MJ/Nm3, low syngas yield due to nitrogen dilution | Cost-effective The need for an external oxidant is eliminated | Due to N2 contamination, the syngas heating value is low | [154,155] |
| Oxygen | High CO and H2 yield | No nitrogen contamination High calorific value | Higher heating value than air gasification Higher syngas quality | [153,156] |
| Steam | Enhances H2 through the water–gas shift and steam reforming reactions | Promotes syngas yield and tar cracking | May require heat input Excess steam may affect the CO and CH4 yield | [157,158] |
| CO2 | Promotes CO yield via the Boudouard reaction | Supports systems with a low carbon footprint and promotes carbon recycling | Reduces H2 yield Promotes CO yield | [158,159,160] |
| Fuel | Gasifying Agent | Syngas Composition | ||||
|---|---|---|---|---|---|---|
| H2 | CO | CO2 | CH4 | CV (MJ/kg) | ||
| Indian coal | Air | 8.8 | 41.8 | 0.623 | 17.3 | 12.59 |
| Oxygen | 15.3 | 60.1 | 0.003 | 0.23 | 19.55 | |
| Rice husk | Air | 22.9 | 18.4 | 13.0 | 0.8 | 5.49 |
| Oxygen | 36.5 | 21.8 | 20.2 | 0.6 | 9.14 | |
| Wood pellets | Air | 32.1 | 29.8 | 7.9 | 0.9 | 9.22 |
| oxygen | 4.07 | 37.8 | 11.3 | 1.7 | 13.19 | |
| Benefits | Description | References |
|---|---|---|
| Process efficiency | Efficiency is enhanced through real-time process parameter monitoring and control. | [331,332] |
| Minimization of emissions | Precise control of parameters reduces GHG and pollutant emissions. | [333,334] |
| Improved safety | Safety is enhanced by monitoring harmful gases and activating safety measures. | [335,336] |
| Cost-effectiveness | Low-cost IoT-based solutions lower operating and implementation expenses. | [336,337] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kun, U.H.; Ksepko, E. Advancing Municipal Solid Waste Management Through Gasification Technology. Processes 2025, 13, 2000. https://doi.org/10.3390/pr13072000
Kun UH, Ksepko E. Advancing Municipal Solid Waste Management Through Gasification Technology. Processes. 2025; 13(7):2000. https://doi.org/10.3390/pr13072000
Chicago/Turabian StyleKun, Uzeru Haruna, and Ewelina Ksepko. 2025. "Advancing Municipal Solid Waste Management Through Gasification Technology" Processes 13, no. 7: 2000. https://doi.org/10.3390/pr13072000
APA StyleKun, U. H., & Ksepko, E. (2025). Advancing Municipal Solid Waste Management Through Gasification Technology. Processes, 13(7), 2000. https://doi.org/10.3390/pr13072000







