Furthering the Application of a Low-Moisture Anhydrous Ammonia Pretreatment of Corn Stover
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass
2.2. Biomass Composition Analysis
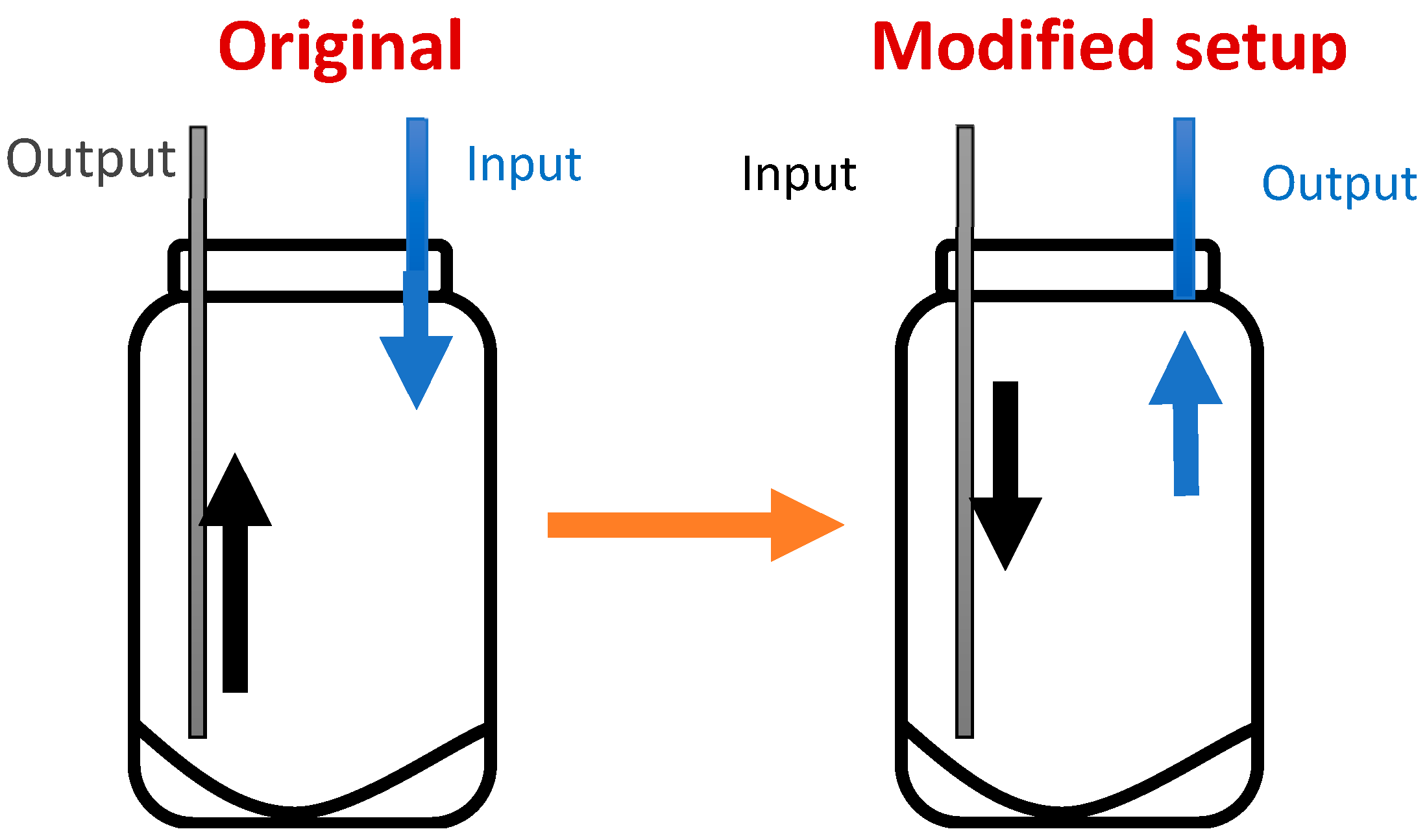
2.3. Ammoniation Reactor for LMAA Pretreatment
2.4. LMAA Pretreatment
2.5. Enzymatic Hydrolysis
2.6. Kinetics of Glucan Digestibility Through Enzymatic Hydrolysis
3. Results and Discussion
3.1. Effect of LMAA Treatment with Higher Ammonia Loading on Biomass Composition
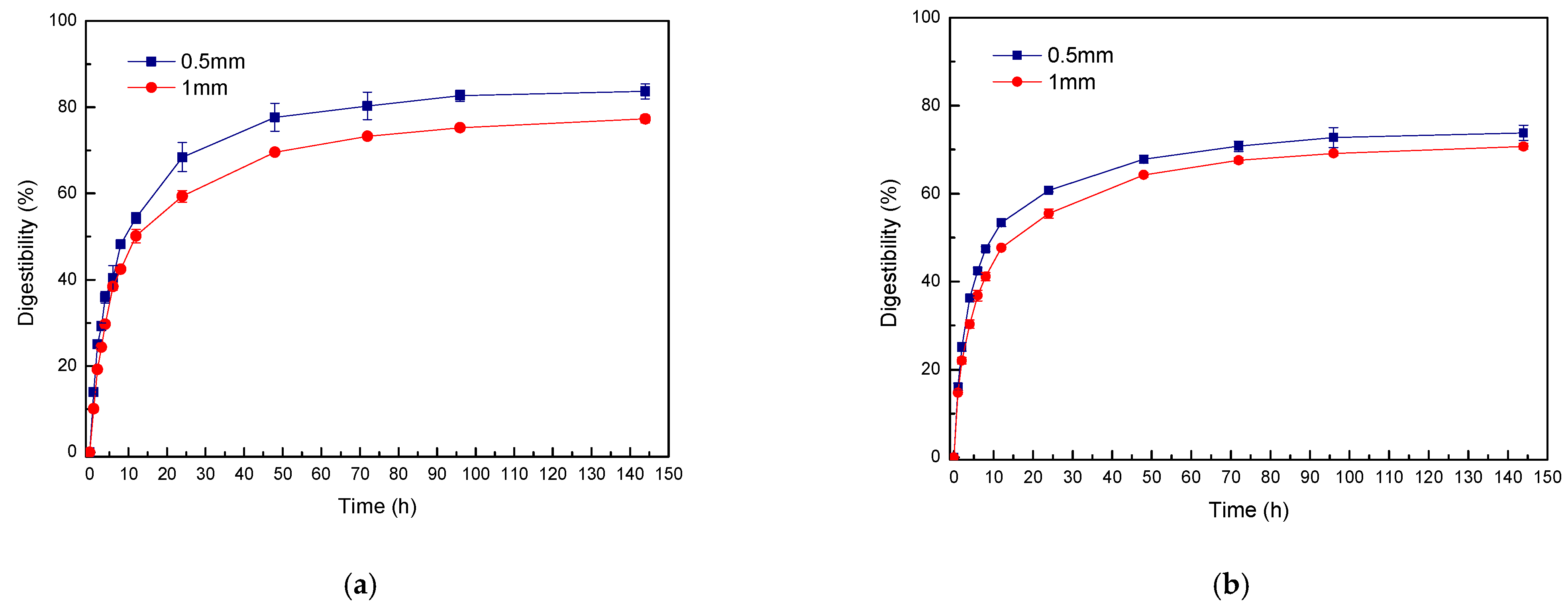
3.2. Effect of LMAA Treatment on Glucan Digestibility via Enzymatic Hydrolysis
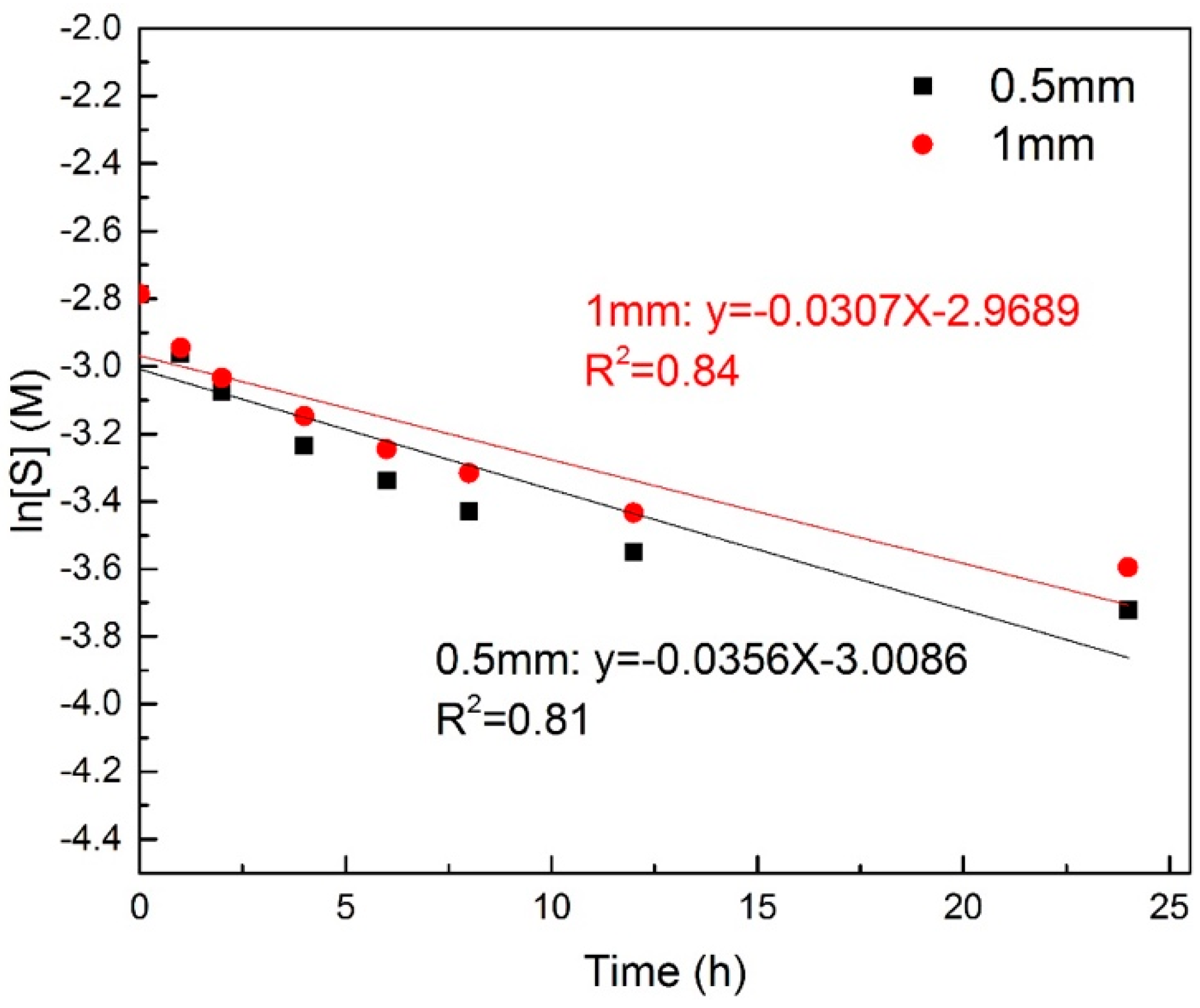
3.3. Kinetics of Enzymatic Hydrolysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Segers, B.; Nimmegeers, P.; Spiller, M.; Tofani, G.; Jasiukaitytė-Grojzdek, E.; Dace, E.; Kikas, T.; Marchetti, J.M.; Rajić, M.; Yildiz, G.; et al. Lignocellulosic biomass valorisation: A review of feedstocks, processes and potential value chains and their implications for the decision-making process. RSC Sustain. 2024, 2, 3730–3749. [Google Scholar] [CrossRef]
- Tumbalam, P.; Thelen, K.D.; Adkins, A.; Dale, B.; Balan, V.; Gunawan, C.; Gao, J. Corn stover ethanol yield as affected by grain yield, Bt trait, and environment. Biomass Bioenergy 2016, 85, 119–125. [Google Scholar] [CrossRef]
- Xu, G.-C.; Ding, J.-C.; Han, R.-Z.; Dong, J.-J.; Ni, Y. Enhancing cellulose accessibility of corn stover by deep eutectic solvent pretreatment for butanol fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Saha, B.C.; Qureshi, N.; Kennedy, G.J.; Cotta, M.A. Biological pretreatment of corn stover with white-rot fungus for improved enzymatic hydrolysis. Int. Biodeterior. Biodegrad. 2016, 109, 29–35. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Kadhum, H.J.; Murthy, G.S.; Dien, B.S.; Singh, V. High solids loading biorefinery for the production of cellulosic sugars from bioenergy sorghum. Bioresour. Technol. 2020, 318, 124051. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Chen, X.; Kuhn, E.; Jennings, E.W.; Nelson, R.; Tao, L.; Zhang, M.; Tucker, M.P. DMR (deacetylation and mechanical refining) processing of corn stover achieves high monomeric sugar concentrations (230 g L−1) during enzymatic hydrolysis and high ethanol concentrations (>10% v/v) during fermentation without hydrolysate purification or concentration. Energy Environ. Sci. 2016, 9, 1237–1245. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Chang, V.S.; Holtzapple, M.T. Fundamental Factors Affecting Biomass Enzymatic Reactivity. Appl. Biochem. Biotechnol. 2000, 84, 5–37. [Google Scholar] [CrossRef]
- García-Negrón, V.; Stoklosa, R.J.; Toht, M.J. Effects of NaOH and Na2CO3 pretreatment on the saccharification of sweet sorghum bagasse. Front. Chem. Eng. 2024, 6, 1449114. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Kim, S.B.; Lee, Y.Y. Fractionation of herbaceous biomass by ammonia-hydrogen peroxide percolation treatment. Appl. Biochem. Biotechnol. 1996, 57, 147–156. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, J.S.; Sunwoo, C.; Lee, Y.Y. Pretreatment of corn stover by aqueous ammonia. Bioresour. Technol. 2003, 90, 39–47. [Google Scholar] [CrossRef]
- Yang, J.; Gao, C.; Yang, X.; Su, Y.; Shi, S.; Han, L. Effect of combined wet alkaline mechanical pretreatment on enzymatic hydrolysis of corn stover and its mechanism. Biotechnol. Biofuels Bioprod. 2022, 15, 31. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Preethi, M.G.; Banu, J.R. Indexing energy and cost of the pretreatment for economically efficient bioenergy generation. Front. Energy Res. 2023, 10, 1060599. [Google Scholar] [CrossRef]
- Kim, T.H.; Lee, Y.Y. Pretreatment of Corn Stover by Soaking in Aqueous Ammonia. Appl. Biochem. Biotechnol. 2005, 124, 1119–1132. [Google Scholar] [CrossRef]
- Akus-Szylberg, F.; Antczak, A.; Zawadzki, J. Effects of soaking aqueous ammonia pretreatment on selected properties and enzymatic hydrolysis of poplar (Populus trichocarpa) wood. Bioresources 2021, 16, 5618–5627. [Google Scholar] [CrossRef]
- Li, X.; Kim, T.H. Low-liquid pretreatment of corn stover with aqueous ammonia. Bioresour. Technol. 2011, 102, 4779–4786. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, N.; Rosentrater, K.A. Low Moisture Anhydrous Ammonia Pretreatment of Four Lignocellulosic Materials—Distillers Dried Grains with Solubles, Corn Gluten Feed, Corn Fiber, and Oil Palm Frond. Front. Energy Res. 2021, 9, 682522. [Google Scholar] [CrossRef]
- Mohammadi, M.; Alian, M.; Dale, B.; Ubanwa, B.; Balan, V. Multifaced application of, A.F.;EX-pretreated biomass in producing second-generation biofuels, ruminant animal feed, and value-added bioproducts. Biotechnol. Adv. 2024, 72, 108341. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.G.; Nghiem, N.P.; Hicks, K.B.; Kim, T.H. Pretreatment of corn stover using low-moisture anhydrous ammonia (LMAA) process. Bioresour. Technol. 2011, 102, 10028–10034. [Google Scholar] [CrossRef]
- Yang, M.; Rosentrater, K.A. Small-scale low-moisture anhydrous ammonia (LMAA) pretreatment of corn stover. Biomass Bioenergy 2017, 97, 38–42. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Huang, H.; Dien, B.S.; Singh, V. The costs of sugar production from different feedstocks and processing technologies. Biofuels Bioprod. Biorefining 2019, 13, 723–739. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. In Technical Report; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Resch, M.G.; Baker, J.O.; Decker, S.R. Low Solids Enzymatic Saccharification of Lignocellulosic Biomass. In Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2015; NREL/TP-5100-63351. [Google Scholar]
- Chen, M.; Xia, L.; Xue, P. Enzymatic hydrolysis of corncob and ethanol production from cellulosic hydrolysate. Int. Biodeterior. Biodegrad. 2007, 59, 85–89. [Google Scholar] [CrossRef]
- Gao, K.; Rehmann, L. ABE fermentation from enzymatic hydrolysate of NaOH-pretreated corncobs. Biomass Bioenergy 2014, 66, 110–115. [Google Scholar] [CrossRef]
- Balan, V.; Mohammadi, M.; Dale, B.E. Advancements in ammonia-based pretreatment: Key benefits and industry applications. RSC Sustain. 2025; in press. [Google Scholar] [CrossRef]
- Wu, J.; Li, L.; Wang, W. Greater Importance of Structural Changes Over Lignin Removal in Impacting the Enzymatic Hydrolysis of Crop Straws. ACS Omega 2023, 8, 26556–26560. [Google Scholar] [CrossRef]
- Xie, T.; Pei, X.; Ni, B.; Fan, M. Evaluation on the efficiency of “lignin-first” dissolution and biomass fractionation in alcohols solvents systems. Int. J. Biol. Macromol. 2025, 319, 145420. [Google Scholar] [CrossRef] [PubMed]
- Kululo, W.W.; Habtu, N.G.; Abera, M.K.; Sendekie, Z.B.; Fanta, S.W.; Yemata, T.A. Advances in various pretreatment strategies of lignocellulosic substrates for the production of bioethanol: A comprehensive review. Discov. Appl. Sci. 2025, 7, 476. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, L.; Li, M.; Peng, H.; Tan, Y.; Arvelli, S.; Huang, Y.; Neves, A.C.; Oh, E.J.; Zhao, J. One-Pot Biomass Pretreatment for Ethanol Production by Engineered Saccharomyces cerevisiae. ACS Sustain. Chem. Eng. 2025, 13, 5201–5209. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef] [PubMed]

| Factor | Levels | Glucan (%) | Xylan (%) | AIL (%) | ASL (%) |
|---|---|---|---|---|---|
| Time (h) | 72 | 36.55 (1.70) b | 27.03 (1.04) b | 13.31 (1.57) c | 2.87 (0.15) b |
| 144 | 39.74 (1.46) a | 24.82 (0.80) c | 15.03 (0.87) b | 2.71 (0.16) b | |
| Particle Size (mm) | 0.5 | 37.05 (2.33) b | 25.95 (1.85) b | 15.93 (0.95) b | 2.93 (0.22) a |
| 1 | 39.25 (1.67) a | 25.89 (1.09) b | 14.88 (1.61) c | 2.91 (0.12) a | |
| Raw Materials | 34.73 (1.20) c | 38.8 (1.08) a | 17.87 (1.09) a | 3.19 (0.14) a | |
| Factor | Cellulose | Hemicellulose | AIL | ASL |
|---|---|---|---|---|
| Time | <0.0001 | <0.0001 | <0.0001 | 0.001 |
| Particle Size | 0.02 | 0.93 | 0.03 | 0.75 |
| Time × Particle Size | 0.14 | 0.62 | 0.04 | 0.40 |
| Factor | Levels | Digestibility (%) | p-Value |
|---|---|---|---|
| Time (h) | 72 | 72.29 (2.05) b | <0.0001 |
| 144 | 80.50 (3.71) a | ||
| Particle Size (mm) | 0.5 | 78.76 (5.61) a | 0.0003 |
| 1 | 74.03 (3.67) b | ||
| Time × Particle Size | 144 × 0.5 | 83.69 (1.72) a | 0.0662 |
| 144 × 1 | 77.31 (0.94) b | ||
| 72 × 0.5 | 73.83 (1.72) b,c | ||
| 72 × 1 | 70.74 (0.65) c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-H.; Rosentrater, K.A. Furthering the Application of a Low-Moisture Anhydrous Ammonia Pretreatment of Corn Stover. Processes 2025, 13, 2643. https://doi.org/10.3390/pr13082643
Cheng M-H, Rosentrater KA. Furthering the Application of a Low-Moisture Anhydrous Ammonia Pretreatment of Corn Stover. Processes. 2025; 13(8):2643. https://doi.org/10.3390/pr13082643
Chicago/Turabian StyleCheng, Ming-Hsun, and Kurt A. Rosentrater. 2025. "Furthering the Application of a Low-Moisture Anhydrous Ammonia Pretreatment of Corn Stover" Processes 13, no. 8: 2643. https://doi.org/10.3390/pr13082643
APA StyleCheng, M.-H., & Rosentrater, K. A. (2025). Furthering the Application of a Low-Moisture Anhydrous Ammonia Pretreatment of Corn Stover. Processes, 13(8), 2643. https://doi.org/10.3390/pr13082643








