3.1. Fermentation Results
The fermentations were performed using a full 22 factorial design, which analyzed the influence of yeast type and temperature on ethanol production for each substrate: S1, S2, and S3. For substrates S1 and S2, the rejected fruits’ peels, seeds, and pulp were used, while for S3, coffee mucilage was employed. The pH was set to 4.5, and the operational time was fixed at seven days for all trials.
Guava was selected as substrate S1 due to its high antioxidant content, including vitamin C, vitamin E, phenols, and carotenoids [
38]. In addition, it contains reducing sugars ranging from 2.1% to 6%, and total sugars from 2.25% to 4.05% [
39]. The seeds also enhance the fermentation process due to their fatty acid content, which provides antifoaming properties during fermentation. This helps prevent microbial contamination and reduces substrate loss [
20].
Papaya was used as substrate S2 because it contains about 14.21% protein, which plays an important role in fermentation [
40]. It also has a sugar composition of sucrose (48.13%), glucose (29.8%), and fructose (21.9%) [
21]. Furthermore, papaya provides vitamins (A, B1, B2, C, D) and minerals such as calcium, potassium, sodium, and magnesium [
41], which act as essential cofactors in yeast metabolism [
42].
Coffee was employed as substrate S3 because it contains 22.65% reducing sugars by dry weight, including D-fructose (in its α and β anomers), D-glucose (in both anomers), sucrose (14.1%), D-galactose (10.1%), and inositol (1.2%). These fermentable sugars enhance ethanol production while minimizing the formation of by-products, like vinasse [
43,
44]. Additionally, coffee contains proteins, tannins, and other compounds that promote ethanol production [
45].
The yeast used for ethanol production was
Saccharomyces cerevisiae, a common strain in the industry due to its robustness, high ethanol tolerance, and efficient yield [
46].
S. cerevisiae also exhibits resistance to a low pH, bacterial contamination, and is readily available commercially [
47].
The fermentation temperatures were set to 21 °C and 30 °C. Studies have shown that ethanol production increases gradually at 30 °C, but decreases at higher fermentation temperatures [
48,
49]. In general,
S. cerevisiae thrives at optimal growth temperatures in the ranges of 25–30 °C or 30–33 °C, with a pH range from 3.0 to 7.0 [
50,
51].
Fermentation using substrates S1, S2, and S3 was performed at a pH of 4.5, which falls within the recommended range for ethanol production (4.5–5.0) [
49,
50]. Both commercial and native yeasts exhibit optimal growth at a pH between 5.0 and 5.5 [
52]. Despite this, pH does not influence the outcome of the fermentation process within the range from 4 to 5; when the pH drops below 4, the formation of acetic acid is favored [
53,
54]. Moreover, lower pH values help minimize bacterial contamination [
55]. Therefore, the initial pH of the substrate was not adjusted, in order to avoid the use of chemical reagents and to facilitate process scale-up.
The fermentation time was fixed at seven days to ensure sufficient yeast activity and complete sugar conversion [
56]. This duration optimizes ethanol concentration while preventing yeast stress, which could reduce yields [
57]. Moreover, it guarantees the complete conversion of reducing sugars into ethanol through the yeast’s metabolic pathway [
49].
Table 4 presents the design matrix and the ethanol concentration results (%
v/
v) obtained using substrates S1, S2, and S3.
The results were analyzed using Statgraphics Centurion XIX® (Statgraphics Technologies, Version 19.0, The Plains, VA, USA), with ethanol concentration (% v/v) as the response variable, yeast type (YC = +1, YN = −1), and temperature (21 °C, 30 °C) as the factors.
To determine whether the calculated effects were statistically significant, Student’s t-test was applied. At a 95% confidence level (α = 0.05) and with 4 degrees of freedom, the two-tailed critical t-value was 3.1824 for all experimental designs.
Figure 3,
Figure 4 and
Figure 5 show the Pareto charts, where the factors and their interactions are ordered based on the absolute values of their standardized effects. The vertical line in each chart represents the critical t-value. Any effect surpassing the vertical line is considered statistically significant. In this case, yeast type, temperature, and their interaction were found to be statistically significant for ethanol production across substrates S1, S2, and S3.
To evaluate the fit of the experimental data to the proposed mathematical model, we performed a linear regression analysis using yeast type and temperature as independent variables. The ethanol concentration (%
v/
v) was modeled for each substrate (S1: guava, S2: papaya, S3: coffee) based on Equation (18):
where
is the ethanol concentration (%
v/
v),
is the intercept,
represents the effect of
Yeast type (YC = +1, YN = −1), and
represents the effect of temperature (21 °C or 30 °C).
To assess the accuracy of the models, the coefficient of determination (R
2), root mean square error (RMSE), standard error, and
p-value were calculated for each substrate. The results are summarized in
Table 5.
These results indicate a strong correlation between the experimental data and the model predictions, with R2 values above 0.99 for all substrates. The low RMSE, standard error values, and statistically significant p-values (p < 0.05) further confirm the robustness of the models.
According to the full 2
2 factorial design, the factor levels for maximizing ethanol production are presented in
Table 6.
The results show that the commercial yeast
S. cerevisiae outperformed the native yeast isolated from the substrates. This was reflected in the higher ethanol concentrations obtained at the end of fermentation for all substrates (S1, S2, and S3). The highest ethanol concentrations were achieved when commercial yeast and a temperature of 30 °C were used, both at their upper levels. The ethanol concentrations obtained under these conditions align with the reported values in the literature, which range between 3% and 8% [
58].
The maximum ethanol concentrations were 4.78% (
v/
v) for S1, 5.69% (
v/
v) for S2, and 6.79% (
v/
v) for S3. These results may have been influenced in part by the C/N ratios: 6.33 for S1, 16.08 for S2, and 21.09 for S3 [
59]. Maintaining a C/N ratio between 20 and 35 provides energy and supports the formation of new cellular structures [
60]. Microorganisms utilize carbon as an energy source and nitrogen for protein and nucleic acid synthesis [
61]. To maintain that ratio in their cells, they have been found to perform best with a C/N ratio of 24 [
62].
A study by Manikandan and Viruthagiri highlighted the effect of the C/N ratio on ethanol concentration and biomass production, finding that a C/N ratio of 35.2 in the fermentation medium resulted in a maximum ethanol concentration of 8.85 g/L [
63].
The ethanol concentration obtained using coffee mucilage (S3) was 29.5% higher than that obtained with guava (S1) and 17.1% higher than with papaya (S2).
Studies show that the ethanol yield from coffee mucilage (0.47 g ethanol/g sugar) is higher than that of other agro-industrial residues, such as barley hay (0.3 g/g), barley straw (0.31 g/g), millet hay (0.27 g/g), sweet sorghum hay (0.31 g/g), triticale hay (0.34 g/g), and wheat straw (0.31 g/g). Additionally, raw coffee mucilage does not require any pretreatment or supplemental nutrients prior to fermentation, making it attractive for various fermentation applications [
50].
Given the typical composition of coffee mucilage, and the compounds that are produced by the metabolic pathway in alcoholic fermentation with
S. cerevisiae, the literature reviewed indicates that during such fermentation, various metabolites are generated. These include sugars, organic acids, alcohols, and amino acids [
64]. Ethanol is a primary product, with yields up to 0.47 g ethanol/g sugars reported [
50]. Other metabolites include lactic acid and ethyl acetate [
65]. The fermentation process also impacts volatile compounds, enhancing aroma complexity with increases in benzeneacetaldehyde, 2-heptanol, and benzylalcohol [
65].
3.2. Selection of Ethanol to Be Dehydrated
Based on the multi-criteria evaluation, coffee residues emerged as the most favorable option for ethanol production according to the selected criteria. The complete priority matrix for selecting the fermentation process to be used in the dehydration system is presented in
Table 7.
Among the highest-weighted criteria is the number of hectares cultivated in Colombia, reflecting crop availability and the ability to maintain a steady supply of residual biomass. Coffee holds a significant advantage with 844,744 hectares under cultivation [
45], ensuring a year-round availability of residues, which is crucial for sustainable industrial processes. In comparison, guava has 18,700 hectares [
66], representing moderate availability, while papaya, with only 7903 hectares, poses a significant limitation in terms of biomass supply [
72].
Despite its higher commercial cost, coffee offers decisive advantages. Its high ethanol yield (6.8%) in fermentation, combined with the 80% residual biomass content and the extensive cultivated area in Colombia [
20,
45,
77], ensures a steady and substantial flow of raw materials for large-scale bioprocesses.
Utilizing coffee residues would also enhance sustainability in Colombia’s production chain by optimizing the use of agricultural by-products that would otherwise be wasted. This makes coffee residues a promising alternative for producing ethanol with applications in energy, food, pharmaceuticals, or cosmetics [
79].
Although papaya offers a low commercial cost and year-round availability, its limited biomass content reduces its attractiveness for producing significant ethanol volumes [
72,
74]. Similarly, guava is less viable due to its limited biomass and production cycles [
66,
68,
69].
In addition to ethanol production, coffee residues offer great potential for various industrial applications. These residues can be used to manufacture bio-based composites [
39], biodegradable polymers [
80], biofuels, and oil extraction [
81], contributing to a circular economy and generating high-value products. This approach not only enhances the sustainability of the process but also opens new opportunities for the bioproducts industry, maximizing the value of coffee by-products and fostering a greater integration of green technologies in agriculture and chemical industries.
3.3. Ethanol Dehydration by Bioadsorption
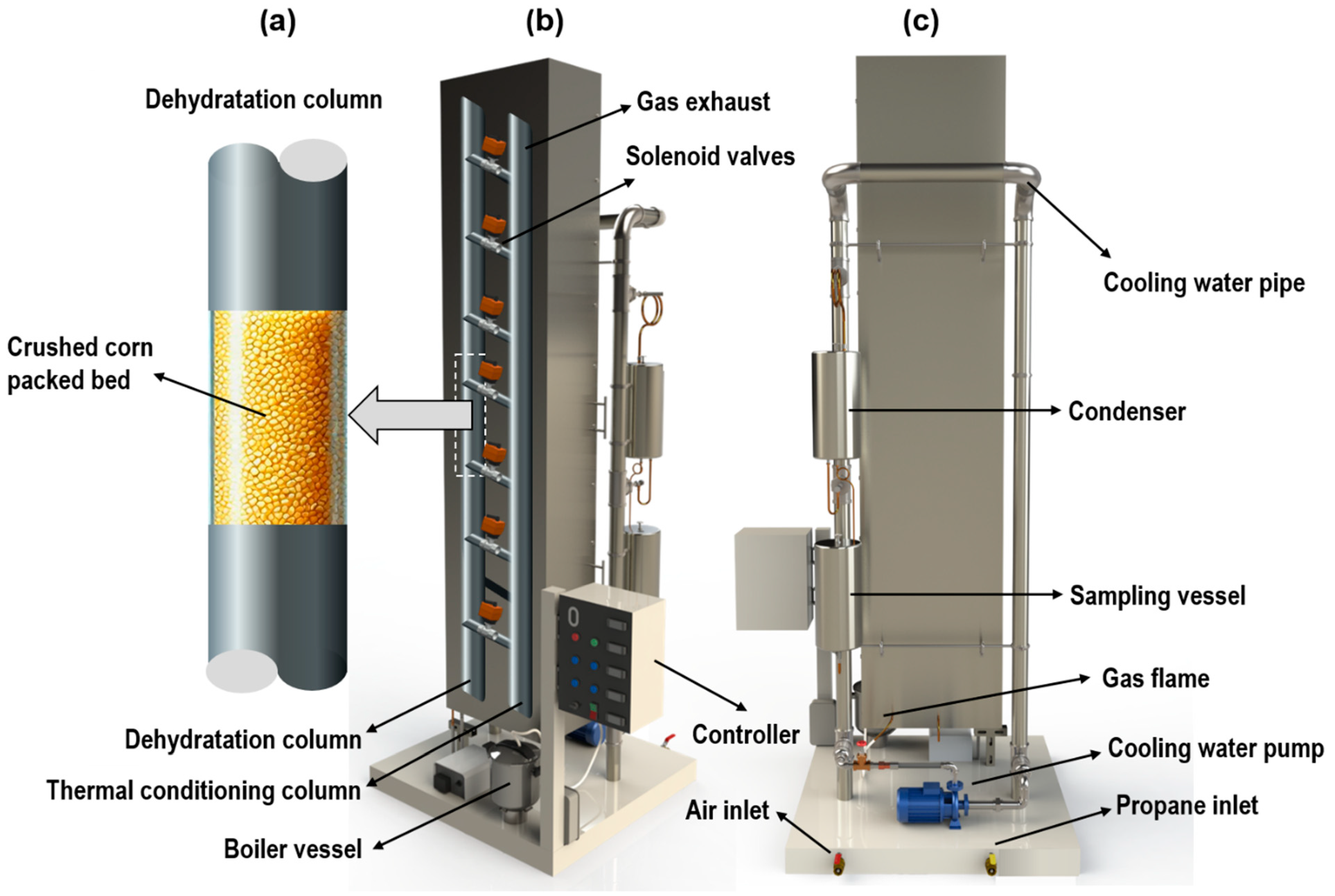
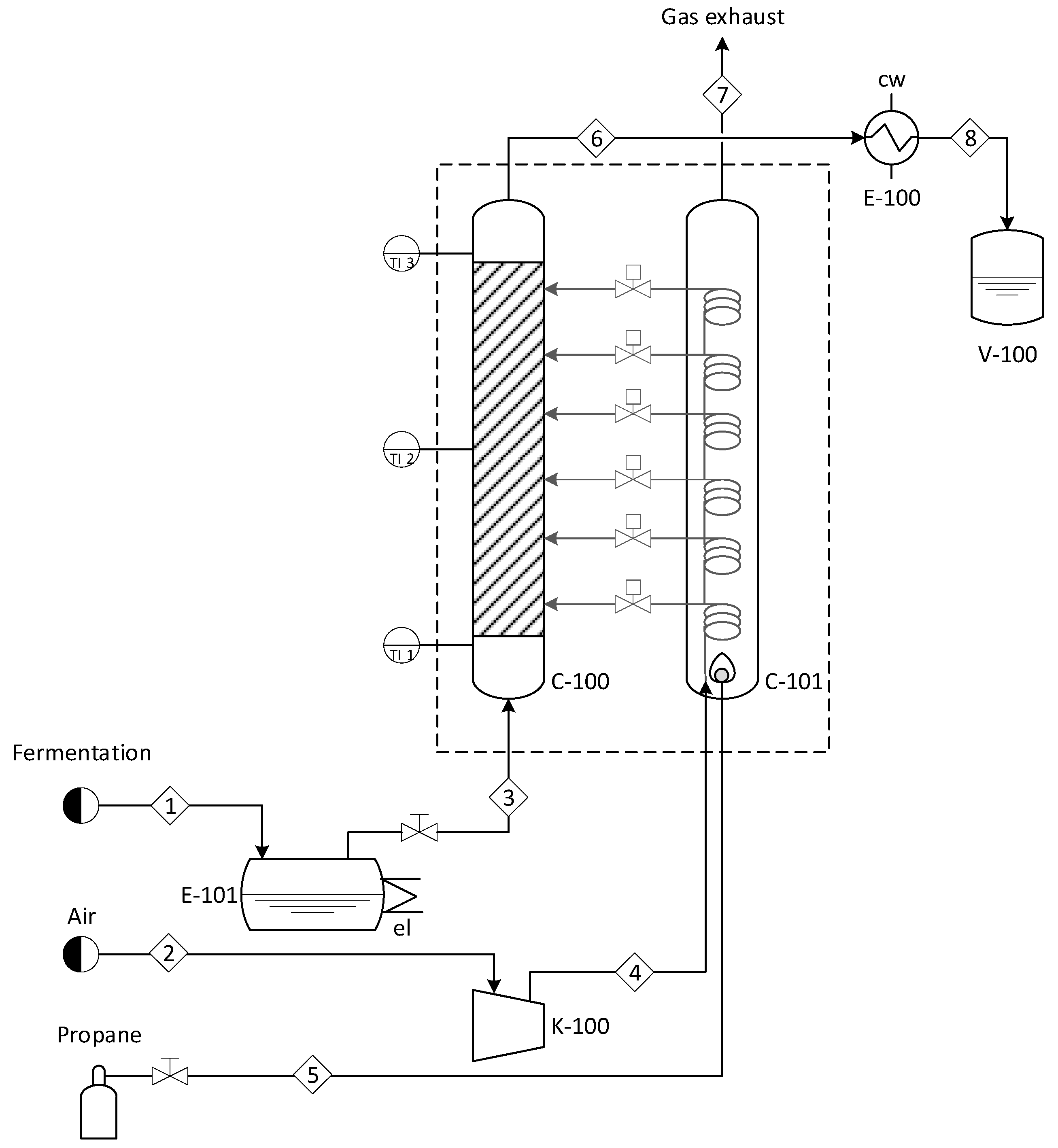
To address the limitations of conventional distillation, this study implemented a green bioadsorption technology for the dehydration of ethanol produced through fermentation. The system consists of two main components, a thermal conditioning column and a packed bed of crushed corn, which acts as the main adsorbent for water removal from ethanol vapor. The packed bed allows for adsorbent regeneration through a hot air injection, reducing operating costs and enhancing sustainability.
Corn was selected as the adsorbent material for vapor-phase ethanol dehydration due to its strong hydrophilic properties, renewable nature, and cost-effectiveness. Starch-based materials, such as corn, have been shown to exhibit high water affinity, making them effective in breaking ethanol–water azeotropes through adsorption processes without requiring chemical entrainers [
82]. Additionally, studies indicate that cornmeal-based adsorption is significantly more energy-efficient than conventional distillation, reducing energy consumption while maintaining high ethanol purity [
83]. The abundance and low cost of corn further enhance its feasibility as a sustainable and scalable alternative for bioethanol dehydration, aligning with green technology principles.
The performance of the packed column was analyzed by estimating the pressure drop, superficial velocity, and saturation time of the maize-based adsorbent. The pressure drop across the packed bed was 18.42 Pa, as calculated using the Ergun equation for gas-phase flow. This low pressure drop suggests minimal resistance to vapor flow, ensuring efficient mass transfer and stable operational conditions within the column.
The superficial velocity of the ethanol–water vapor mixture was determined to be 0.112 m/s, reflecting a stable and uniform flow through the packed bed. This velocity ensures prolonged contact between the vapor and the adsorbent material without excessive pressure losses or channeling effects. The system operated efficiently under steady-state conditions, confirming its suitability for ethanol dehydration.
The saturation time of the maize adsorbent was estimated at 2.84 h, based on its water adsorption capacity and the mass flow rate of water in the vapor phase. This represents the operational time before the adsorbent reaches its maximum water retention capacity, after which its efficiency decreases. Since the system allows adsorbent regeneration, the need for frequent material replacement is significantly reduced, lowering operational costs.
A comprehensive mass balance was performed to validate the efficiency of the bioadsorption system. The results confirm that the packed column successfully increased ethanol concentration from 6.7% to 98.9%
v/
v through two sequential adsorption stages. The total mass flow of ethanol and water through the packed bed is detailed in
Table 8, demonstrating the system’s ability to effectively remove water and improve ethanol purity with low energy consumption.
The key advantage of this technology is that it does not involve azeotropic phenomena, unlike conventional separation processes. This enables the system to dehydrate ethanol without the high energy requirements associated with extractive or azeotropic distillation. Additionally, the system integrates spiral heat exchangers for energy recovery, reducing energy consumption by up to five times compared to traditional dehydration technologies. The use of natural and reusable adsorbents, such as crushed maize, further enhances the environmental sustainability of the process.
3.4. Mass and Energy Balance of the Dehydration Process
A global mass and energy balance were performed for the ethanol dehydration system using the bioadsorption process with crushed maize as the adsorbent. Two sequential dehydration runs were conducted: the first increased the ethanol concentration from 6.7% to 49.5%
v/
v, and the second achieved a final concentration of 98.9%
v/
v. The mass balance results are summarized in
Table 8.
The mass balance shows that 101 min of processing were required to obtain high-concentration ethanol after pre-heating the dehydration system and running two adsorption stages. The results confirm that the bioadsorption system effectively removed water, progressively increasing ethanol purity.
The energy efficiency of the dehydration process was also analyzed. Conventional ethanol separation by distillation consumes more than 50% of the total energy used in distilleries, with thermodynamic efficiency values as low as in the range from 5% to 10%, primarily due to azeotropic limitations [
27]. In contrast, the adsorption-based system used in this study bypasses azeotropic constraints, enabling direct ethanol dehydration without the need for chemical entrainers or additional separation stages.
The estimated specific energy consumption for ethanol dehydration in this system was 3.68 MJ/kg of ethanol recovered, which is significantly lower than conventional distillation, which can consume up to 6.0 MJ/kg for diluted ethanol solutions [
84]. Compared to vapor-assisted membrane distillation (VAMS), which achieves 4.0 MJ/kg, the bioadsorption system demonstrates competitive energy efficiency with a simpler operational design [
85,
86].
To sustain the adsorption-driven dehydration process, propane combustion was used as the heat source. The propane flow rate was 2.2 L/min, equivalent to 3.72 × 10⁻
5 m
3/s under standard conditions, supplying the necessary heat to maintain ethanol volatilization at 78.3 °C [
79]. The composition of the combustion gases (stream 7 in the process flow diagram) is summarized in
Table 9, confirming efficient combustion.
The final adiabatic flame temperature was estimated at 2449.6 K, with a total combustion heat generation () of 2195.1 W, ensuring the effective thermal conditioning of the dehydration system. Heat loss analysis confirmed that radiative losses, , accounted for 7.2% of the total thermal energy, while convective losses, , were more significant at 951.3 W, aligning with the expectations for high-surface-area heat exchange systems.
The specific energy consumption for ethanol recovery in the dehydrator, based on the previous mass and energy balances, was estimated at 3.68 MJ/kg. This value is intermediate compared to other separation technologies, such as conventional distillation and hybrid membrane separation systems.