Nano Water Ion Technology for VOCs Degradation: Insights into the Synergistic Mechanism of Hydrogen-Containing and Non-Hydrogen-Containing Reactive Oxygen Species
Abstract
1. Introduction
2. Materials and Methods
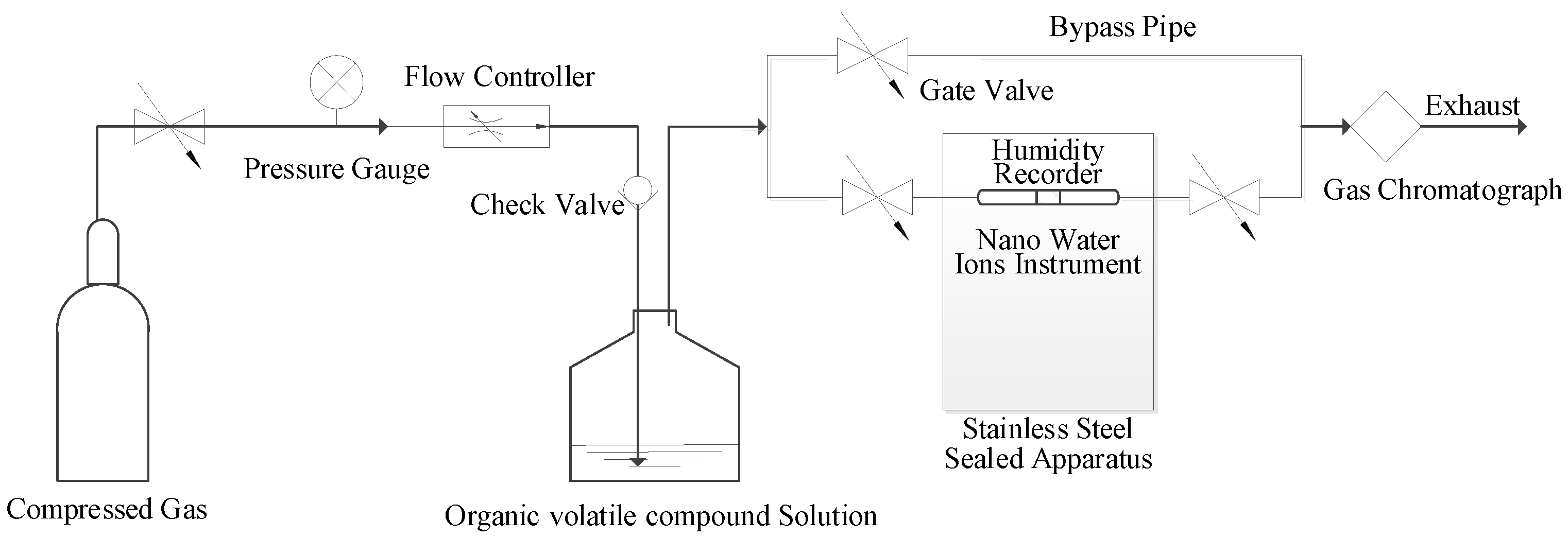
2.1. Experimental Materials and Instruments
2.2. Experimental Methods and Procedures
2.2.1. Purification Efficiency Experiment
2.2.2. Purification Mechanism Experiment
2.3. Data Analysis and Evaluation
3. Results and Discussion
3.1. Degradation Kinetics and Response to Environmental Parameters
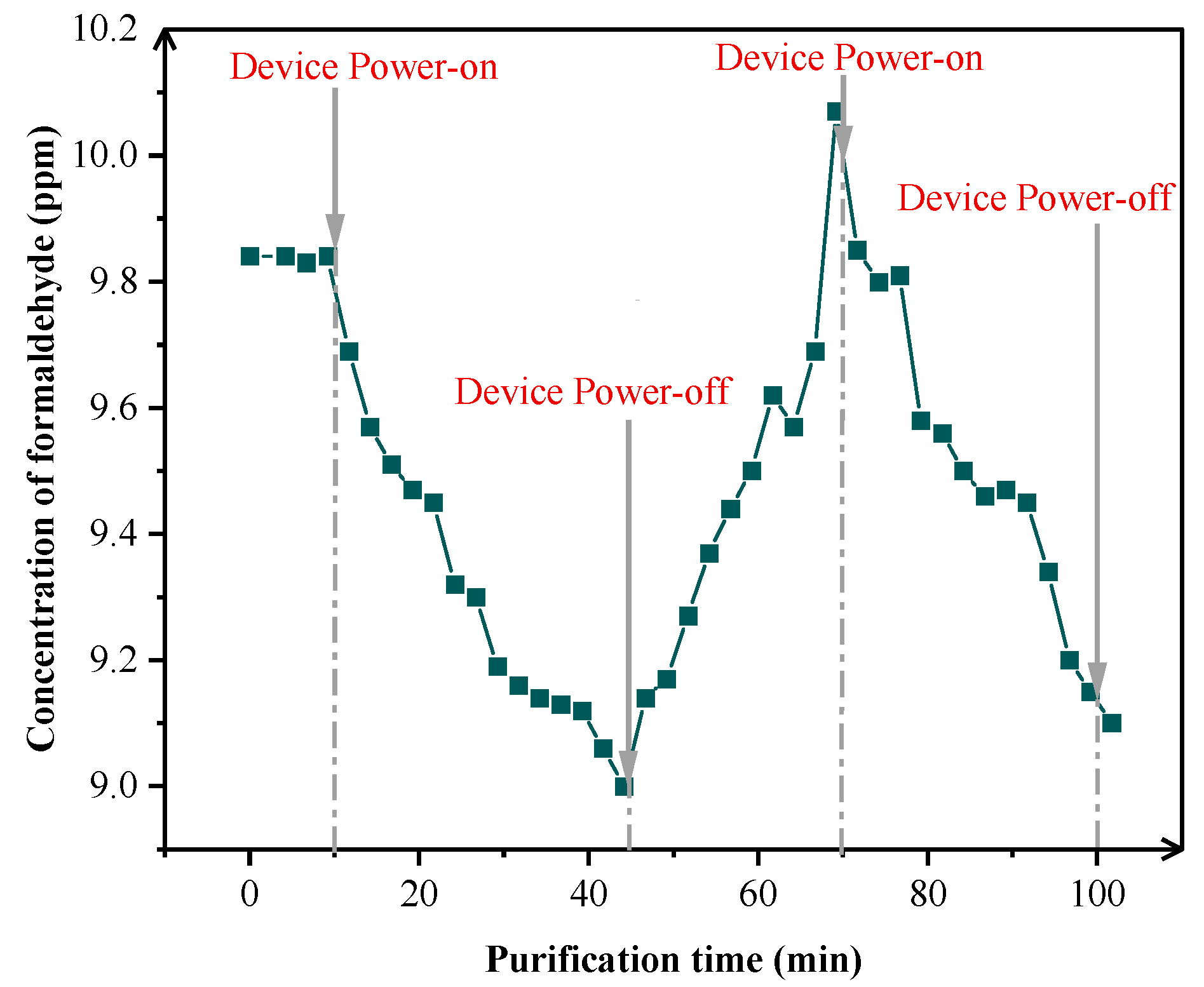
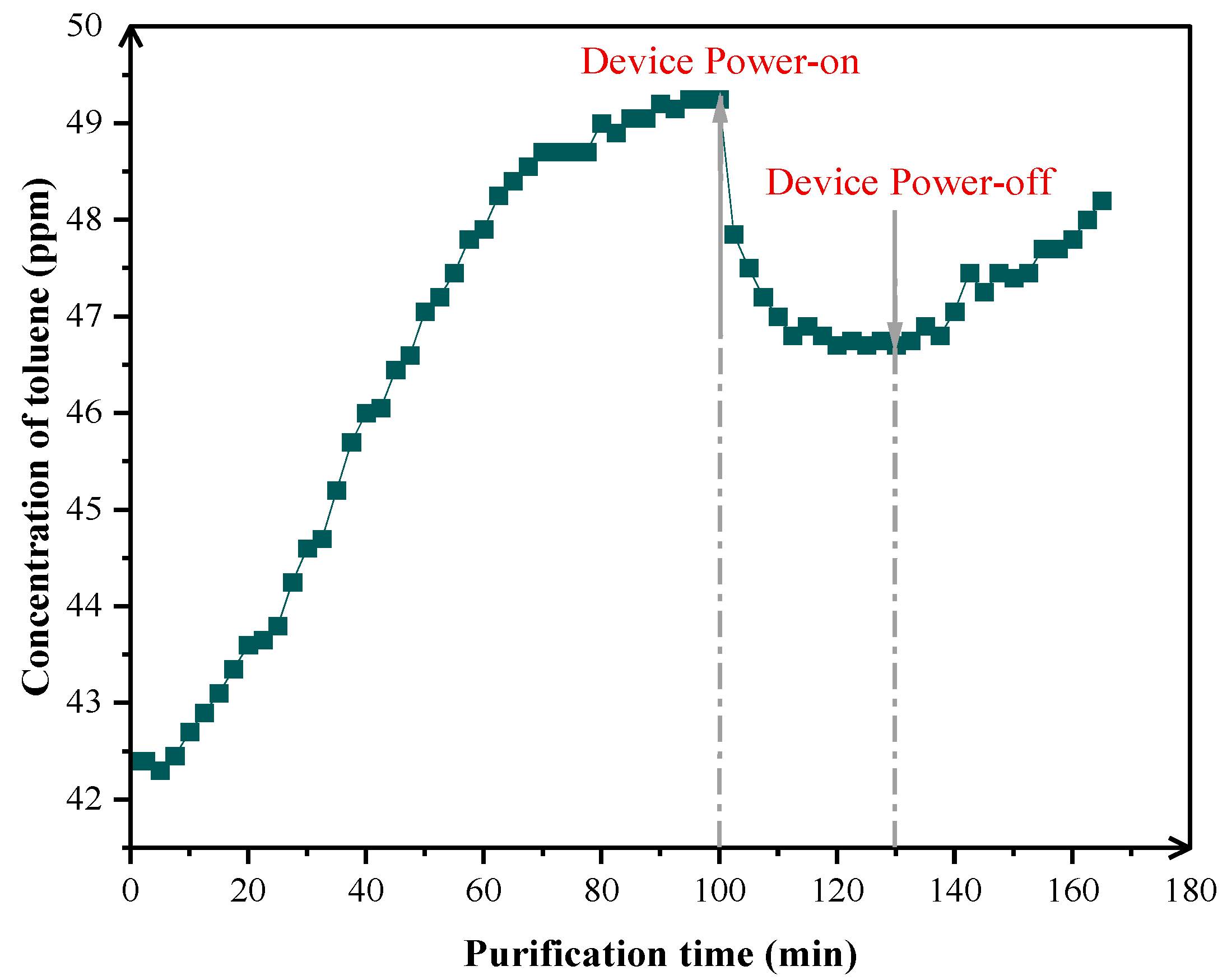
3.1.1. Verification of Temporal Response Between NWIT and VOCs Degradation
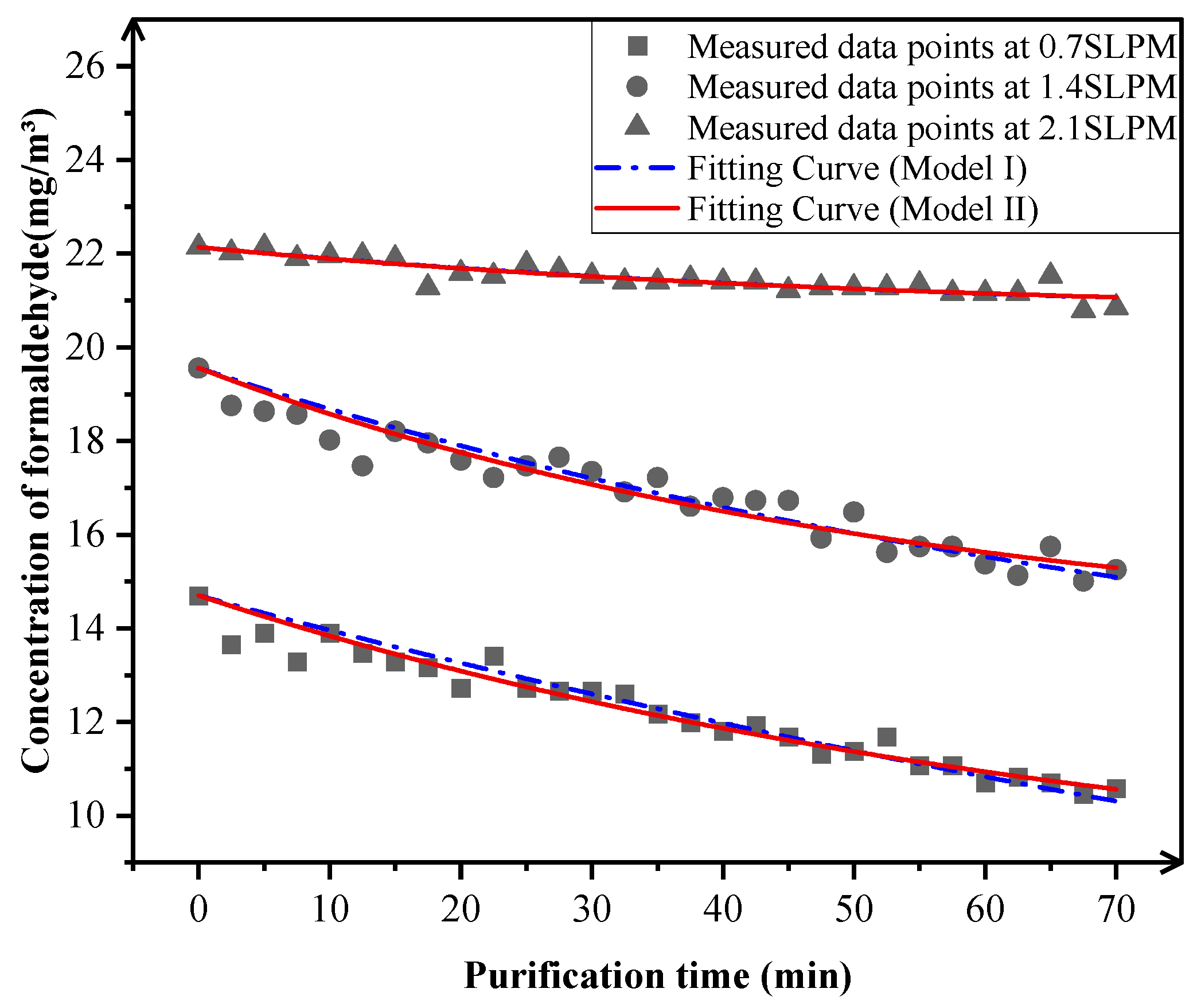
3.1.2. Flow Decay-Induced Mass Transfer Limitations
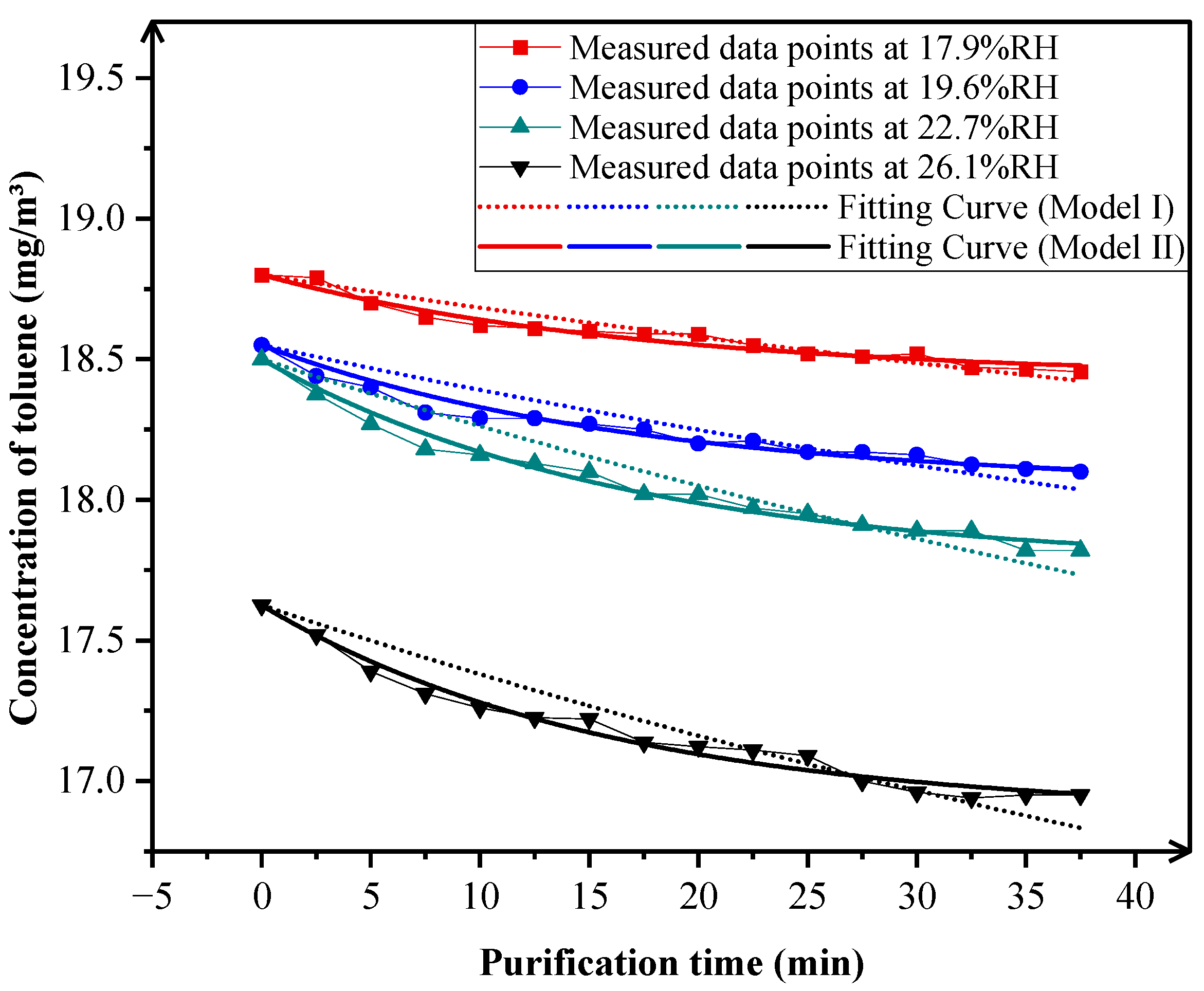
3.1.3. Impact of Relative Humidity on Degradation Efficiency
3.2. Analysis of Synergistic Interaction Mechanisms of ROS
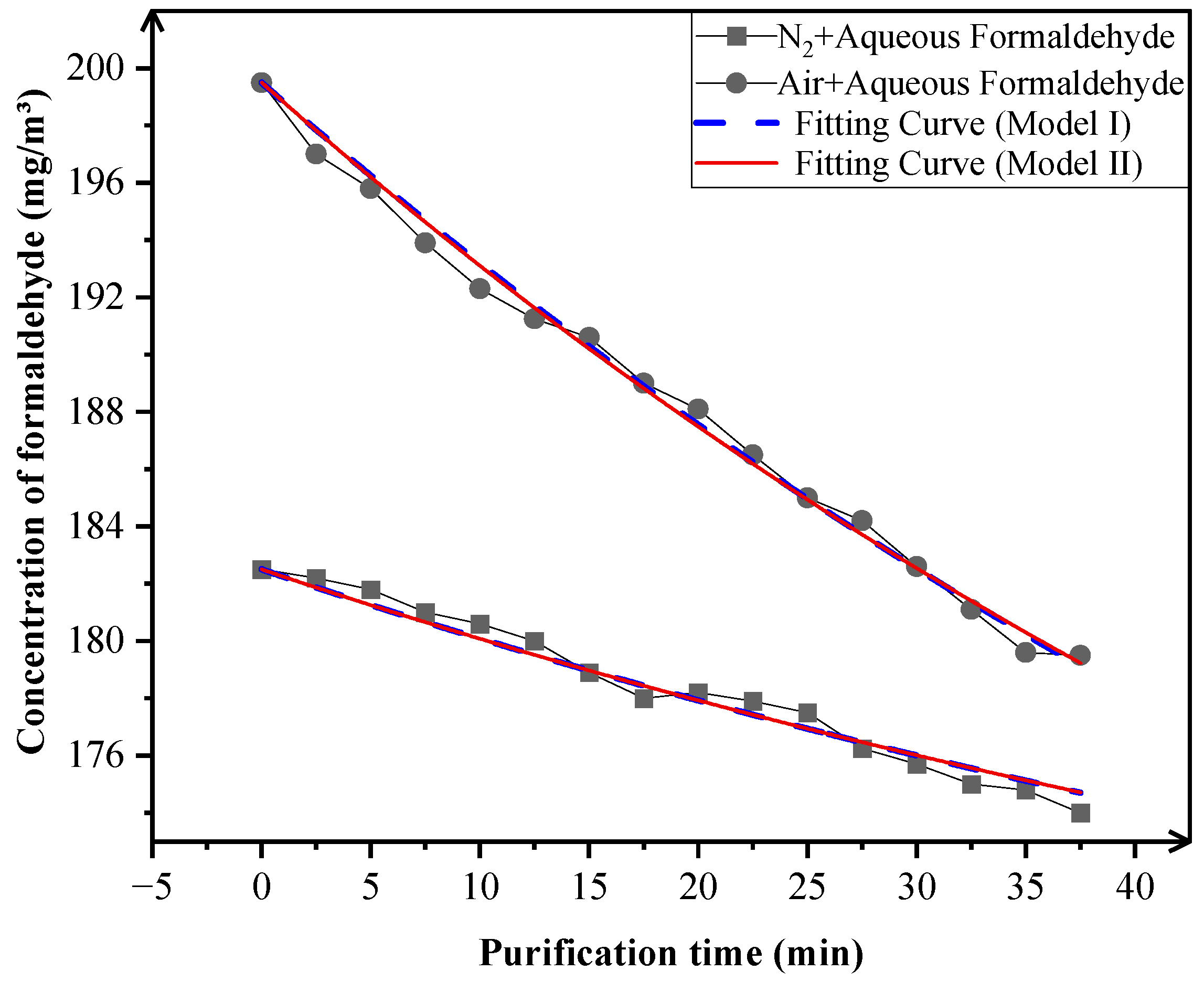
3.2.1. Mechanistic Study on Formaldehyde Purification
3.2.2. Mechanistic Study on Toluene Purification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mai, J.-L.; Yang, W.-W.; Zeng, Y.; Guan, Y.-F.; Chen, S.-J. Volatile organic compounds (VOCs) in residential indoor air during interior finish period: Sources, variations, and health risks. Hyg. Environ. Health Adv. 2024, 91, 100087. [Google Scholar] [CrossRef]
- Jindamanee, K.; Keawboonchu, J.; Pinthong, N.; Meeyai, A.; Inchai, P.; Thepanondh, S. Environmental impacts and emission profiles of volatile organic compounds from petroleum refineries. Sci. Rep. 2025, 15, 15509. [Google Scholar] [CrossRef] [PubMed]
- Montero-Montoya, R.; López-Vargas, R.; Arellano-Aguilar, O. Volatile Organic Compounds in Air: Sources, Distribution, Exposure and Associated Illnesses in Children. Ann. Glob. Health 2018, 84, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Tunga, S.; Sibel, M.; Rainer, M. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar]
- Jiménez-Garza, O.; Baccarelli, A.A.; Byun, H.; Márquez-Gamiño, S.; Barrón-Vivanco, B.S.; Albores, A. CYP2E1 epigenetic regulation in chronic, low-level toluene exposure: Relationship with oxidative stress and smoking habit. Toxicol. Appl. Pharmacol. 2015, 286, 207–215. [Google Scholar] [CrossRef]
- Li, X.; Li, Z. Safe or unsafe: Role of exposure time and interactions between volatile organic compounds in mixtures. Build. Environ. 2025, 27, 112619. [Google Scholar] [CrossRef]
- Wen, H.; Yuan, L.; Wei, C.; Zhao, Y.; Qian, Y.; Ma, P.; Ding, S.; Yang, X.; Wang, X. Effects of combined exposure to formaldehyde and benzene on immune cells in the blood and spleen in Balb/c mice. Environ. Toxicol. Pharmacol. 2016, 45, 265–273. [Google Scholar] [CrossRef]
- Wang, Y.; Bi, R.; Ge, J.; Xu, P. VOCs absorption using ionic liquids. Sep. Purif. Technol. 2025, 363, 132265. [Google Scholar] [CrossRef]
- Takeuchi, M.; Hidaka, M.; Anpo, M. Efficient removal of toluene and benzene in gas phase by the TiO2/Y-zeolite hybrid photocatalyst. J. Hazard. Mater. 2012, 237, 133–139. [Google Scholar] [CrossRef]
- Chang, T.; Wang, Y.; Wang, Y.; Zhao, Z.; Shen, Z.; Huang, Y.; Veerapandian, S.K.P.; De Geyter, N.; Wang, C.; Chen, Q.; et al. A critical review on plasma-catalytic removal of VOCs: Catalyst development, process parameters and synergetic reaction mechanism. Sci. Total environment 2022, 828, 154290. [Google Scholar] [CrossRef]
- Linley, S.; Thomson, N.R. Environmental Applications of Nanotechnology: Nano-enabled Remediation Processes in Water, Soil and Air Treatment. Water Air Soil Pollut. 2021, 232, 59. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Bhardwaj, N.; Kim, K.H.; Kumar, S. Recent advances in photocatalytic removal of airborne pathogens in air. Sci. Total Environ. 2021, 794, 148477. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Chew, L.S.N.; Low, M.; Tan, M.K. Plasma-Activated Water: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, H.; Xu, Z.; Zhang, Z.; Cheng, C.; Ni, G.; Lan, Y.; Meng, Y.; Xia, W.; Chu, P.K. Preferential Production of Reactive Species and Bactericidal Efficacy of Gas-liquid Plasma Discharge. Chem. Eng. J. 2019, 362, 402–412. [Google Scholar] [CrossRef]
- Nidheesh, V.P.; Boczkaj, G.; Ganiyu, O.S.; Oladipo, A.A.; Fedorov, K.; Xiao, R. Generation, properties, and applications of singlet oxygen for wastewater treatment: A review. Environ. Chem. Lett. 2024, 23, 195–240. [Google Scholar] [CrossRef]
- Xie, Z.-H.; He, C.-S.; Pei, D.-N.; Dong, Y.; Yang, S.-R.; Xiong, Z.; Zhou, P.; Pan, Z.-C.; Yao, G.; Lai, B. Review of characteristics, generation pathways and detection methods of singlet oxygen generated in advanced oxidation processes (AOPs). Chem. Eng. J. 2023, 468, 143778. [Google Scholar] [CrossRef]
- Kamal, S.M.; Razzak, A.S.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Wang, S.; Wu, X.; Chen, X. Detailed mechanism study of volatile organic compound decomposition and oxidation removal based on a ReaxFF MD method. RSC Adv. 2024, 14, 5863–5874. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Zhao, D.; Liu, R.; Liu, S.; Fu, J.; Zhang, Y.; Ding, H. Review on Catalytic Oxidation of VOCs at Ambient Temperature. Int. J. Mol. Sci. 2022, 23, 13739. [Google Scholar] [CrossRef]
- Dai, Z.; Li, D.; Ao, Z.; Wang, S.; An, T. Theoretical exploration of VOCs removal mechanism by carbon nanotubes through persulfate-based advanced oxidation processes: Adsorption and catalytic oxidation. J. Hazard. Mater. 2021, 405, 124684. [Google Scholar] [CrossRef]
- Hou, X.; Qi, L.; Li, W.; Zhao, J.; Liu, S. Theoretical study on water adsorption and dissociation on the nickel surfaces. J. Mol. Model. 2021, 27, 36. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Qi, L.; Li, W.; Zhao, J.; Liu, S. Theoretical study on water behavior on the copper surfaces. J. Mol. Model. 2021, 27, 149. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, W.; Peng, M.; Zhai, G.; Hu, L.; Mao, L. The role of S and Mo doping on the dissociation of water molecule on FeOCl surface: Experimental and theoretical analysis. Chem. Eng. J. 2021, 426, 131353. [Google Scholar] [CrossRef]
- Reese, J.A.; Gelin, S.; Maalouf, M.; Wadehra, N.; Schlom, D.; Dabo, I.; Suntivich, J. Tracking Water Dissociation on Well-Defined Ruthenium Oxide Surfaces. Electrochem. Soc. Meet. Abstr. 2024, MA2024-02, 3895. [Google Scholar] [CrossRef]
- Reese, J.A.; Gelin, S.; Maalouf, M.; Wadehra, N.; Zhang, L.; Hautier, G.; Schlom, D.G.; Dabo, I.; Suntivich, J. Tracking Water Dissociation on RuO2(110) Using Atomic Force Microscopy and First-Principles Simulations. J. Am. Chem. Soc. 2024, 146, 32080–32087. [Google Scholar] [CrossRef]
- Shirato, M.; Ikai, H.; Nakamura, K.; Hayashi, E.; Kanno, T.; Sasaki, K.; Kohno, M.; Niwano, Y. Synergistic effect of thermal energy on bactericidal action of photolysis of H2O2 in relation to acceleration of hydroxyl radical generation. Antimicrob. Agents chemotherapy 2012, 56, 295–301. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Meng, J.; Song, M.; Jiao, M.; Jiang, H.; Chen, Y. Electrocatalytic Lignin Valorization via Enhanced H2O2 Generation Using a MWNCT-Modified Gas Diffusion Electrode. ChemPlusChem 2025, 90, e202400769. [Google Scholar] [CrossRef]
- Rawal, S.H.; McKeea, W.C.; Xu, Y. Estimation of electric field effects on the adsorption of molecular superoxide species on Au based on density functional theory. Phys. Chem. Chem. Phys. 2017, 19, 32626–32635. [Google Scholar] [CrossRef]
- Chen, H.J.; Li, T.W.; Cai, Y.K.; Tu, H.-J.; Long, Z.-T.; Akhtar, S.; Liu, L.-D. Proton-coupled electron transfer controls peroxide activation initiated by a solid-water interface. Nat. Commun. 2025, 16, 3789. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry—Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Kiselev, M.V.; Kislyakov, M.I.; Burchinov, N.A. Generation of singlet oxygen on the surface of metal oxides. Opt. Spectrosc. 2016, 120, 520–528. [Google Scholar] [CrossRef]
- Safin, R.R.; Ershov, K.; Valiulin, S.; Pyryaeva, A. Singlet Oxygen Generation by Photoexcitation of Gold Nanoparticles. High Energy Chem. 2024, 57 (Suppl. S3), S464–S469. [Google Scholar] [CrossRef]
- Ershov, K.; Valiulin, S.; Pyryaeva, A. Singlet Oxygen Generaion via Silver Nanoparticles UV-Photoexcitation. Russ. J. Phys. Chem. B 2024, 18, 289–295. [Google Scholar] [CrossRef]
- Sha, N.; Xu, S.; Wan, B.; Zhao, K.-H. Light-oxygen-voltage (LOV) domain-derived photosensitizers with the highest quantum yield for superoxide anion or singlet oxygen. J. Photochem. Photobiol. A Chem. 2024, 452, 115591. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, N.; Pan, Y.; Fu, L.; Zhang, Y. The degradation pathways of contaminants by reactive oxygen species generated in the Fenton/Fenton-like systems. Chin. Chem. Lett. 2024, 35, 109579. [Google Scholar] [CrossRef]
- Lew, M.M.; Rickly, S.P.; Bottorff, P.B.; Reidy, E.; Sklaveniti, S.; Léonardis, T.; Locoge, N.; Dusanter, S.; Kundu, S.; Wood, E.; et al. OH and HO2 radical chemistry in a midlatitude forest: Measurements and model comparisons. Atmos. Chem. Phys. 2020, 20, 9209–9230. [Google Scholar] [CrossRef]
- Wang, Z.; Berbille, A.; Feng, Y.; Li, S.; Zhu, L.; Tang, W.; Wang, Z.L. Contact-electro-catalysis for the degradation of organic pollutants using pristine dielectric powders. Nat. Commun. 2022, 13, 130. [Google Scholar] [CrossRef]
- Dan, W.; Le, Y. Effects of airflow rate and plant species on formaldehyde removal by active green walls. Environ. Sci. Pollut. Res. Int. 2022, 29, 88812–88822. [Google Scholar]
- Zhang, T.; Li, X.; Rao, Y.; Liu, Y.; Zhao, Q. Removal of formaldehyde in urban office building by the integration of ventilation and photocatalyst-coated window. Sustain. Cities Soc. 2020, 55, 102050. [Google Scholar] [CrossRef]
- Nikita, M.; Gordon, M.S. Stabilities and Energetics of Inorganic Benzene Isomers: Prismanes. J. Am. Chem. Soc. 1994, 116, 11407–11419. [Google Scholar]
- Swain, K.C.; Scheiner, S. Comparison of Various Theoretical Measures of Aromaticity within Monosubstituted Benzene. Molecules 2024, 29, 2260. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]




| Experimental Variables | Parameter Gradient | Types of VOCs | Detection Indicators |
|---|---|---|---|
| Gas flow rate | 0.7/1.4/2.1 SLPM (Standard Liters Per Minute) | Formaldehyde | Concentration decay rate; Model fitting goodness |
| Gas flow rate | 0.7/1.4/2.1 SLPM | Toluene | Concentration decay rate; Model fitting goodness |
| Relative humidity | 40.1~58.0% RH | Formaldehyde | Humidity Efficiency Response Curve |
| Relative humidity | 17.9~26.1% RH | Toluene | Humidity Efficiency Response Curve |
| Experiment Group | O2 Condition | H2O Condition | Types of VOCs | Objective Mechanistic Analysis |
|---|---|---|---|---|
| 1 | Absent | Present (Aqueous Solution) | Formaldehyde | To verify the necessity of H2O for the generation of ROS |
| 2 | Present | Present (Aqueous Solution) | Formaldehyde | To evaluate the synergistic effect of O2 and H2O on ROS, and to analyze the enhancement effect of dual factors on the total reactive oxygen concentration |
| 3 | Absent | Absent (Pure Toluene) | Toluene | To verify the inhibitory effect of the absence of O2 and H2O on the generation of ROS |
| 4 | Absent | Present (Aqueous Solution) | Toluene | To quantify the degradation efficiency of ROS under the sole action of H2O |
| 5 | Present | Absent (Pure Toluene) | Toluene | To evaluate the degradation efficiency of ROS under the sole action of O2 |
| 6 | Present | Present (Aqueous Solution) | Toluene | To analyze the mechanism of the combined action of O2 and H2O on the degradation of ROS |
| Condition Parameters | Fitting Results | Model I | Model II |
|---|---|---|---|
| 0.7 SLPM | m (μg/min) | 12.6251 | —— |
| r (m3/min) | —— | 9.93108 × 10−4 | |
| RSS | 2.83466 | 2.34754 | |
| COD | 0.92619 | 0.93661 | |
| 1.4 SLPM | m (μg/min) | 13.9769 | —— |
| r (m3/min) | —— | 8.05983 × 10−4 | |
| RSS | 3.74935 | 3.46405 | |
| COD | 0.90876 | 0.91258 | |
| 2.1 SLPM | m (μg/min) | 2.89781 | —— |
| r (m3/min) | —— | 1.34534 × 10−4 | |
| RSS | 0.684448 | 0.683936 | |
| COD | 0.80691 | 0.79990 |
| Condition Parameters | Fitting Results | Model I | Model II |
|---|---|---|---|
| 0.7 SLPM | m (μg/min) | 72.2535 | —— |
| r (m3/min) | —— | 6.73161 × 10−3 | |
| RSS | 0.31584 | 0.14735 | |
| COD | 0.78113 | 0.89004 | |
| 1.4 SLPM | m (μg/min) | 28.1592 | —— |
| r (m3/min) | —— | 2.39838 × 10−3 | |
| RSS | 0.03934 | 0.02015 | |
| COD | 0.96777 | 0.98222 | |
| 2.1 SLPM | m (μg/min) | 17.8185 | —— |
| r (m3/min) | —— | 1.29153 × 10−3 | |
| RSS | 0.01165 | 0.00728 | |
| COD | 0.98763 | 0.99168 |
| Relative Humidity | r (m3/min) | Purification Efficiency S (Mean ± Variance) |
|---|---|---|
| 40.1% RH | 3.305877 × 10−3 | (3.444081 × 10−3 ± 8.25172 × 10−9) × C(t) |
| 48.7% RH | 3.435519 × 10−3 | |
| 53.8% RH | 3.478326 × 10−3 | |
| 58.0% RH | 3.556600 × 10−3 |
| Relative Humidity | r (m3/min) | Purification Efficiency S (Mean ± Variance) |
|---|---|---|
| 17.9% RH | 5.630876 × 10−3 | (5.895664 × 10−3 ± 5.71536 × 10−8) × C(t) |
| 19.6% RH | 5.780087 × 10−3 | |
| 22.7% RH | 5.895053 × 10−3 | |
| 26.1% RH | 6.276641 × 10−3 |
| Condition Parameters | r (m3/min) | Purification Efficiency S (Mean ± Variance) |
|---|---|---|
| N2 + Aqueous Formaldehyde | 3.69981 × 10−5 | (1.05947 × 10−4 ± 4.75389 × 10−9) × C(t) |
| Air + Aqueous Formaldehyde | 1.74895 × 10−4 |
| Condition Parameters | r (m3/min) | Purification Efficiency S (Mean ± Variance) |
|---|---|---|
| N2 + Pure Toluene | 1.570383 × 10−3 | (5.291789 × 10−3 ± 5.69704 × 10−6) × C(t) |
| N2 + Aqueous Toluene | 5.490227 × 10−3 | |
| Air + Pure Toluene | 5.892607 × 10−3 | |
| Air + Aqueous Toluene | 8.213937 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wei, Y.; Sun, F.; Maihemuti, M. Nano Water Ion Technology for VOCs Degradation: Insights into the Synergistic Mechanism of Hydrogen-Containing and Non-Hydrogen-Containing Reactive Oxygen Species. Processes 2025, 13, 1959. https://doi.org/10.3390/pr13071959
Zhang G, Wei Y, Sun F, Maihemuti M. Nano Water Ion Technology for VOCs Degradation: Insights into the Synergistic Mechanism of Hydrogen-Containing and Non-Hydrogen-Containing Reactive Oxygen Species. Processes. 2025; 13(7):1959. https://doi.org/10.3390/pr13071959
Chicago/Turabian StyleZhang, Ge, Yujia Wei, Fulu Sun, and Maierfeina Maihemuti. 2025. "Nano Water Ion Technology for VOCs Degradation: Insights into the Synergistic Mechanism of Hydrogen-Containing and Non-Hydrogen-Containing Reactive Oxygen Species" Processes 13, no. 7: 1959. https://doi.org/10.3390/pr13071959
APA StyleZhang, G., Wei, Y., Sun, F., & Maihemuti, M. (2025). Nano Water Ion Technology for VOCs Degradation: Insights into the Synergistic Mechanism of Hydrogen-Containing and Non-Hydrogen-Containing Reactive Oxygen Species. Processes, 13(7), 1959. https://doi.org/10.3390/pr13071959









