Free Radical Formation in a Pharmaceutical Product Containing Bisoprolol Fumarate Stored Under Different Physical Conditions
Abstract
1. Introduction
2. Materials and Methods
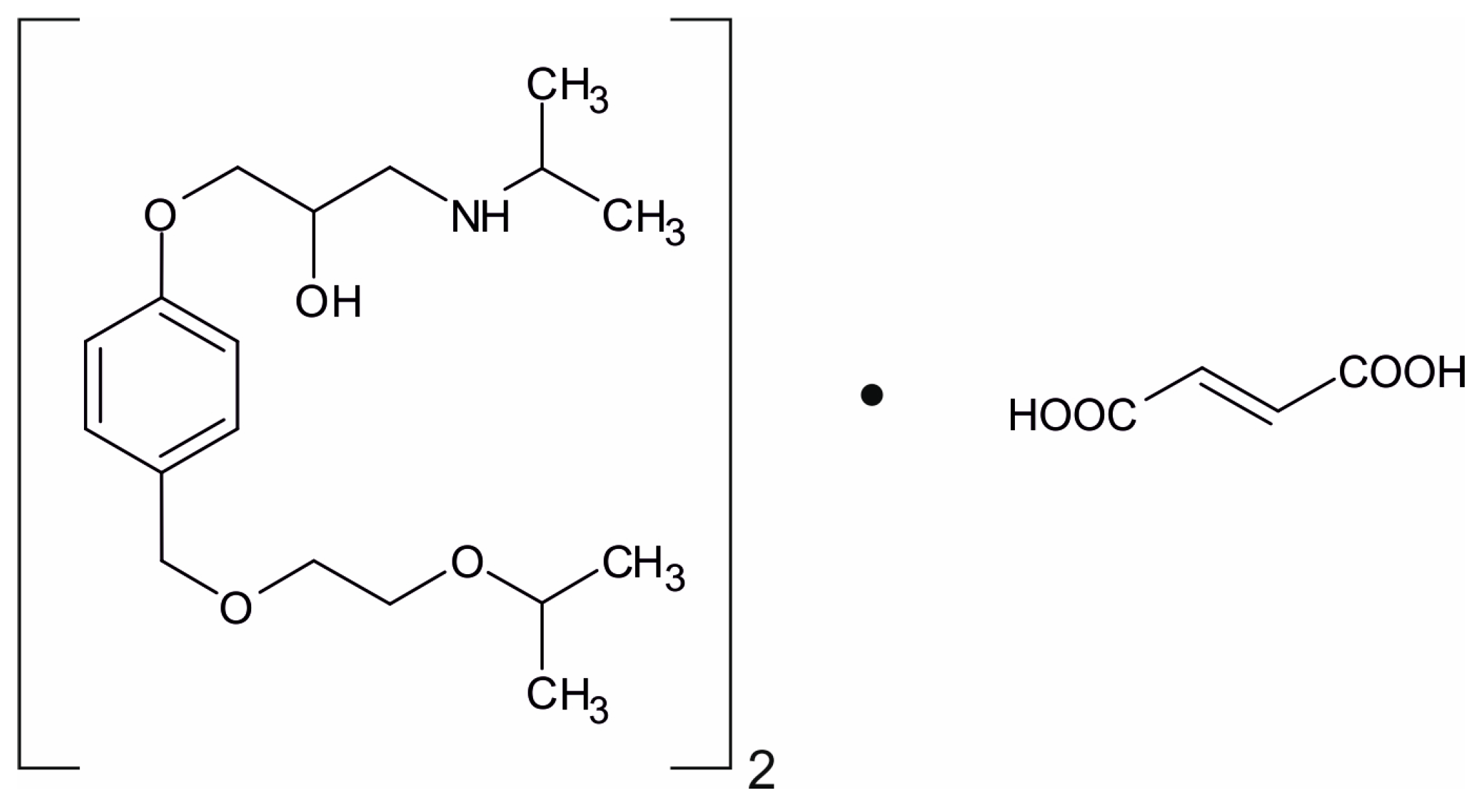
2.1. Samples
- − Temperature of 50 °C (30 min);
- − UVA radiation (30 min);
- − UVA radiation (30 min) and then a temperature of 50 °C (30 min).
2.2. EPR Measurements
3. Results and Discussion
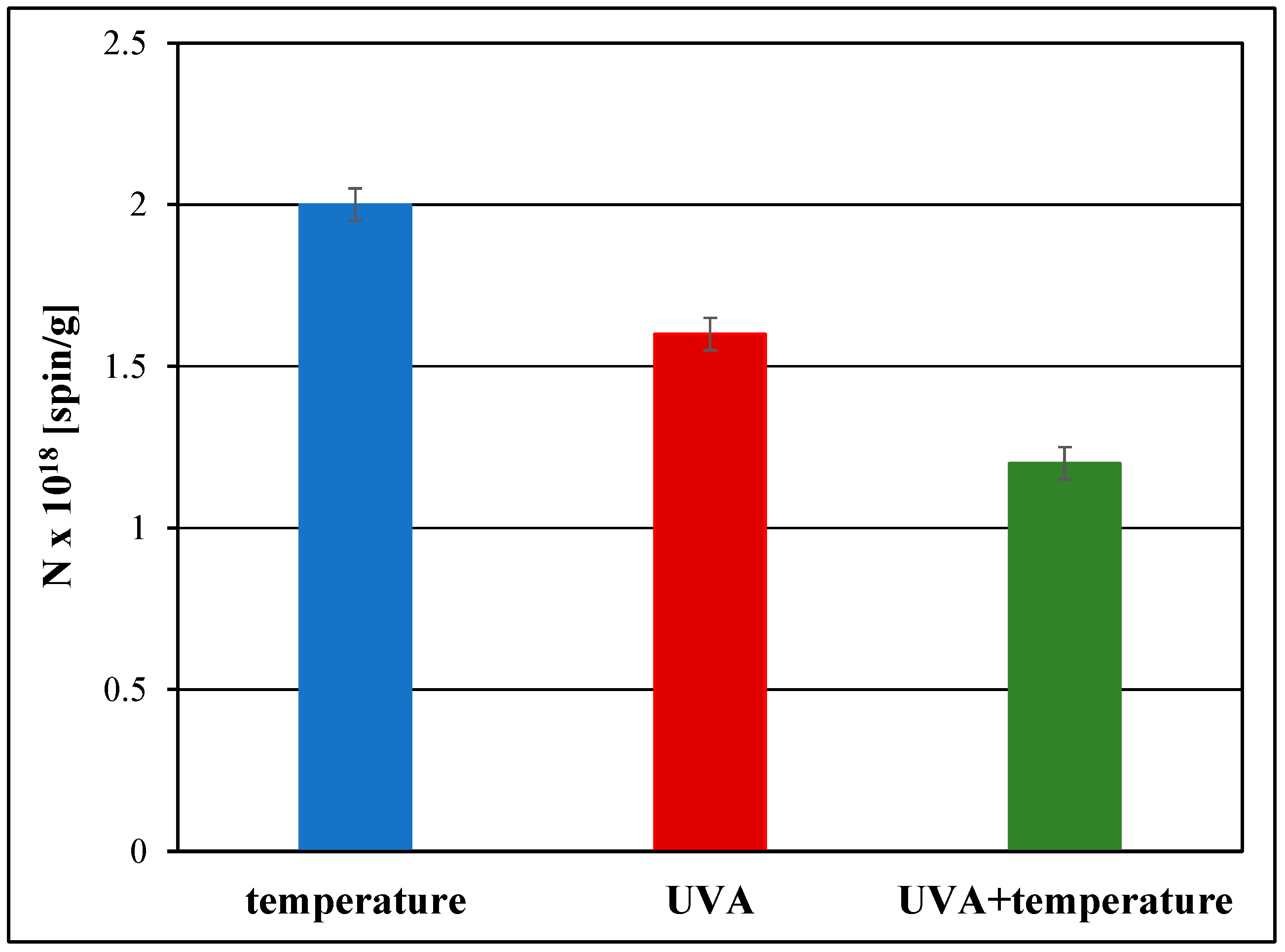
3.1. Free Radical Formation in the Examined Pharmaceutical Product Stored Under Different Physical Conditions
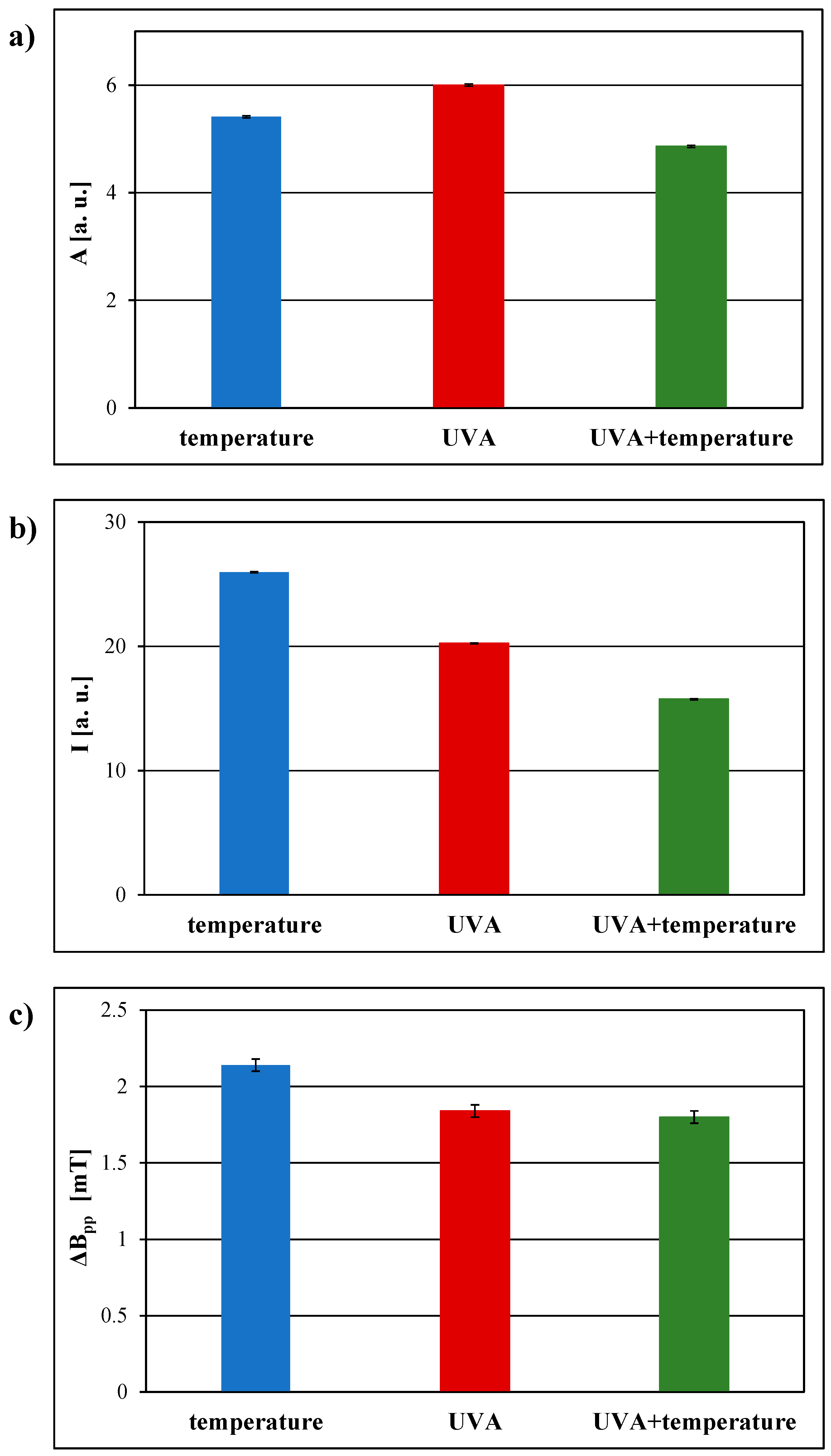
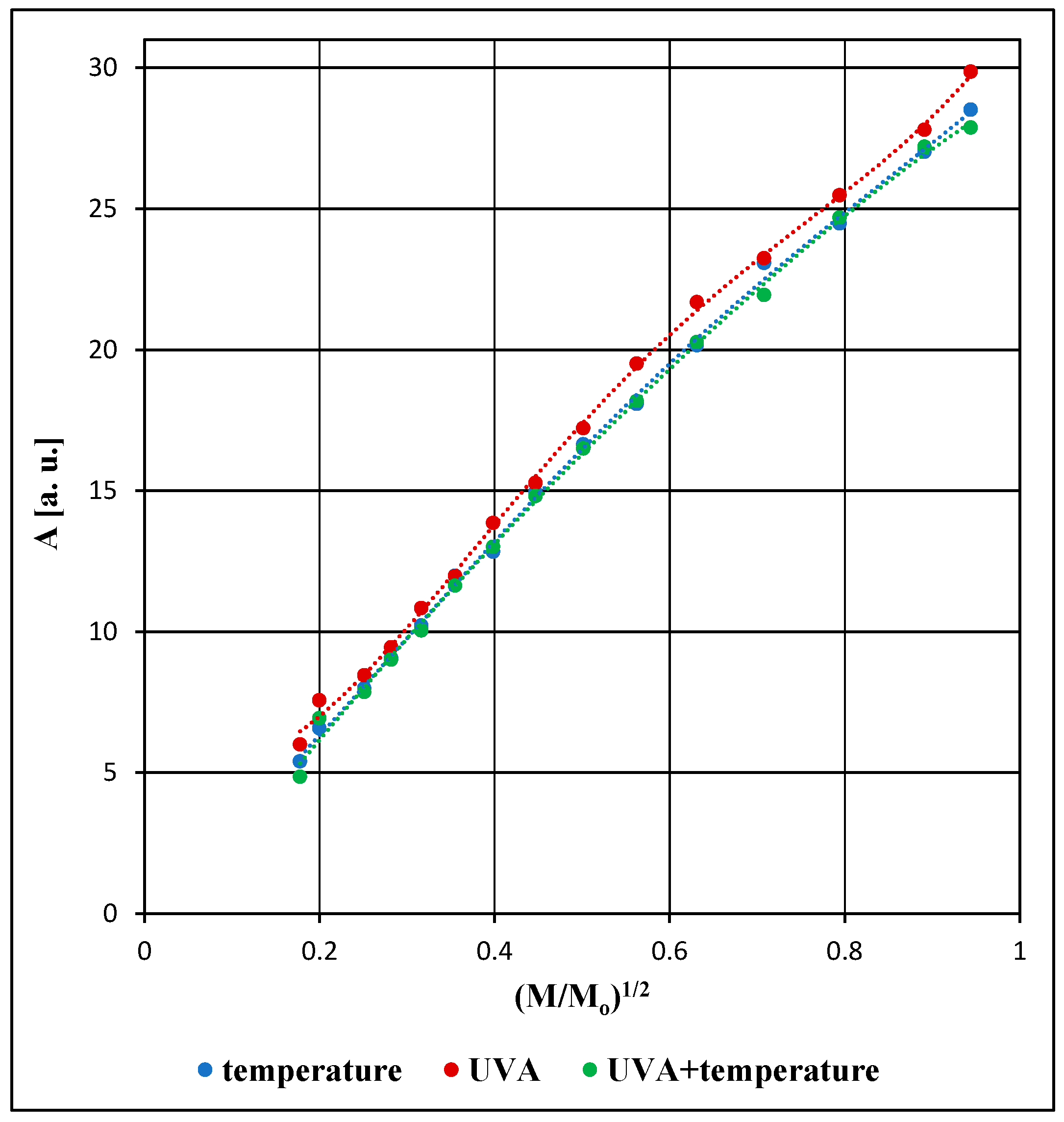
3.2. Comparison of the EPR Spectral Parameters for the Examined Pharmaceutical Product Stored Under Different Physical Conditions
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pindi, S.R.; Gollapalli, V.R.; Sanasi, P.D.; Chavakula, R.; Nandi, S. Synthesis and characterization of related compounds of Bisoprolol fumarate: A β-blocker agent. Chem. Pap. 2024, 78, 1335–1345. [Google Scholar] [CrossRef]
- Prichard, B.N.C. Bisoprolol: A new beta-adrenoceptor blocking drug. Eur. Heart J. 1987, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Sezai, A.; Sekino, H.; Taoka, M.; Osaka, S.; Tanaka, M. A Comparative Study to Investigate the Effects of Bisoprolol in Patients with Chronic Heart Failure and Hypertension When Switched from Tablets to Transdermal Patches. J. Pers. Med. 2023, 13, 785. [Google Scholar] [CrossRef]
- Charoo, N.A.; Shamsher, A.A.; Lian, L.Y.; Abrahamsson, B.; Cristofoletti, R.; Groot, D.W.; Kopp, S.; Langguth, P.; Polii, J.; Shah, V.P.; et al. Biowaiver Monograph for Immediate-Release Solid Oral Dosage Forms: Bisoprolol Fumarate. J. Pharm. Sci. 2014, 103, 378–391. [Google Scholar] [CrossRef]
- Verma, M.; Pandey, P.; Gidwani, B.; Pandey, R.K.; Shukla, S.S. Bisoprolol Fumarate: An Exploration on its Properties and Analytical Methods. PMPJ 2024, 9, 483–496. [Google Scholar] [CrossRef]
- Bakheit, A.H.; Ali, R.; Alshahrani, A.D.; El Azab, A.S. Bisoprolol: A comprehensive profile. Profiles Drug Subst. Excip. Relat. Methodol. 2021, 46, 51–89. [Google Scholar] [CrossRef] [PubMed]
- Lithell, H.; Weiner, L.; Vessby, B.; Nordström, M.L. Effects of small doses of bisoprolol on blood pressure and lipoprotein concentrations in hypertensive patients. Eur. J. Clin. Pharmacol. 1993, 44, 19–22. [Google Scholar] [CrossRef]
- Schuchert, A. Bisex Investigators. Effects of bisoprolol treatment for chronic heart failure initiated and followed up by primary care physicians. Eur. J. Heart Fail. 2005, 7, 604–611. [Google Scholar] [CrossRef]
- Hori, M.; Nagai, R.; Izumi, T.; Matsuzaki, M. Efficacy and safety of bisoprolol fumarate compared with carvedilol in Japanese patients with chronic heart failure: Results of the randomized, controlled, double-blind, Multistep Administration of bisoprolol IN Chronic Heart Failure II (MAIN-CHF II) study. Heart Vessels 2014, 29, 238–247. [Google Scholar] [CrossRef]
- Lazarevska, E.; Todevska, M.; Piponski, M.; Stefova, M. Forced degradation studies and structural characterization of related substances of bisoprolol fumarate in finished drug product using LC–UV–MS/MS. J. Serb. Chem. Soc. 2022, 87, 1185–1202. [Google Scholar] [CrossRef]
- Mackiewicz, U.; Klemenska, E.; Beręsewicz, A. Receptory beta-adrenergiczne w zdrowym i niewydolnym sercu. Kardiol. Pol. 2007, 65, 294–302. [Google Scholar] [PubMed]
- Zejc, A.; Gorczyca, M. Chemia leków. In Podręcznik dla Studentów Farmacji i Farmaceutów; PZWL: Warszawa, Poland, 2013. [Google Scholar]
- Pawłowski, M. Chemia Leków; PZWL: Warszawa, Poland, 2020. [Google Scholar]
- Graham, P. Chemia Medyczna; PWN: Warszawa, Poland, 2019. [Google Scholar]
- Steinhilber, D.; Schubert-Zsilavecz, M.; Hermann, J.R. Chemia Medyczna; MedPharm Polska: Wrocław, Poland, 2012. [Google Scholar]
- Kostowski, W.; Herman, Z. Farmakologia. In Podstawy Farmakoterapii; PZWL: Warszawa, Poland, 2010. [Google Scholar]
- Mutschler, E.; Geisslinger, G.; Kroemer, H.K.; Menzel, S.; Ruth, P. Farmakologia i Toksykologia; MedPharm Polska: Wrocław, Poland, 2016. [Google Scholar]
- Olszanecki, R.; Wołkow, P.; Jawień, J. Farmakologia; PZWL: Warszawa, Poland, 2017. [Google Scholar]
- Janiec, W. (Ed.) Kompendium Farmakologii; PZWL: Warszawa, Poland, 2021. [Google Scholar]
- Janiec, W. Farmakodynamika. In Podręcznik dla Studentów Farmacji; PZWL: Warszawa, Poland, 2013. [Google Scholar]
- Leopold, G. Balanced pharmacokinetics and metabolism of bisoprolol. J. Cardiovasc. Pharmacol. 1986, 8 (Suppl. S11), S16–S20. [Google Scholar] [CrossRef] [PubMed]
- Doroszko, A.; Dobrowolski, P.; Prejbisz, A.; Filipiak, K.J.; Januszewicz, A.; Narkiewicz, K.; Olszanecka, A.; Tykarski, A.; Wolf, J. Nebiwolol i bisoprolol w terapii nadciśnienia tętniczego i chorób towarzyszących—Konsensus ekspertów. Via Medica 2022, 8, 1–16. [Google Scholar]
- Tykarski, A. Miejsce leku złożonego bisoprolol-kwas acetylosalicylowy w leczeniu nadciśnienia tętniczego i jego powikłań. Via Medica 2014, 18, 43–46. [Google Scholar]
- Rogowska, E.; Matuszkiewicz, E.; Biel, M.; Plucińska, K.; Przepiórkiewicz, H.; Skonieczna, P.; Stępniak, P.; Szafranek, S.; Zgoła, D. Farmy Kompendium; Corpusmind: Warszawa, Poland, 2020. [Google Scholar]
- Turner, R.M.; Fontana, V.; Bayliss, M.; Whalley, S.; Castelazo, A.S.; Pirmohamed, M. Development, validation and application of a novel HPLC-MS/MS method for the quantification of atorvastatin, bisoprolol and clopidogrel in a large cardiovascular patient cohort. J. Pharm. Biomed. 2018, 159, 272–281. [Google Scholar] [CrossRef]
- Mahmoud, O.A.; Omran, A.A.; Binsaleh, A.Y.; Almalki, M.A.; Mohamed, M.A. Chromatographic Techniques for Assessment of Bisoprolol Fumarate and Perindopril Arginine in Solid Formulations under Various Stress Conditions and Application to Six Sigma, Content Uniformity, and Comparative Dissolution Approaches. J. AOAC Int. 2023, 106, 1165–1179. [Google Scholar] [CrossRef]
- Sodeifian, G.; Garlapati, C.; Razmimanesh, F.; Sodeifian, F. Solubility of Amlodipine Besylate (Calcium Channel Blocker Drug) in Supercritical Carbon Dioxide: Measurement and Correlations. J. Chem. Eng. Data 2021, 66, 1119–1131. [Google Scholar] [CrossRef]
- Shafaat, K.; Hussain, A.; Kumar, B.; Hasan, R.; Prabhat, P.; Yadav, V.K. An overview: Storage of pharmaceutical products. WJPPS 2014, 2, 2499–2515. [Google Scholar]
- Tejada, E.T.; Pérez, B.G.; Muner, D.S. Review of Drug Storage Conditions, A Case Report. Hosp. Pharm. 2023, 58, 252–254. [Google Scholar] [CrossRef]
- World Health Organization. Good storage and distribution practices for medical products. WHO Drug Inf. 2019, 33, 195–225. [Google Scholar]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar] [CrossRef]
- Shultz, J.; Harvie, M.; McDonald, D.; Manley, J.; Cole, M. Standardizing the storage and labelling of medications: Part 2. Can. J. Hosp. Pharm. 2007, 60, 101–104. [Google Scholar]
- Morrish, A.H. Fizyczne Podstawy Magnetyzmu; PWN: Warszawa, Poland, 1970. [Google Scholar]
- Eaton, G.R.; Eaton, S.S.; Salikhov, K.M. (Eds.) Foundations of Modern EPR; World Scientific: Singapore; Hackensack, NJ, USA; London, UK; Hong Kong, China, 1998. [Google Scholar]
- Stankowski, J.; Hilczer, W. Wstęp do Spektroskopii Rezonansów Magnetycznych; PWN: Warszawa, Poland, 2005. [Google Scholar]
- Dyrek, K. Elektronowy rezonans paramagnetyczny. In Fizyczne Metody Badań w Biologii, Medycynie i Ochronie Środowiska; Hrynkiewicz, A.Z., Rokita, E., Eds.; PWN: Warszawa, Poland, 2013; pp. 136–159. [Google Scholar]
- Wertz, J.E.; Bolton, J.R. Electron Spin Resonance. Theory and Practical Applications; Springer: New York, NY, USA; London, UK, 1986. [Google Scholar]
- Weil, J.A.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2007. [Google Scholar]
- Kęcki, Z. Podstawy Spektroskopii Molekularnej; PWN: Warszawa, Poland, 1992. [Google Scholar]
- Pilawa, B.; Ramos, P. Spektroskopia EPR. Ćwiczenia dla Studentów Analityki Medycznej i Medycyny; Śląski Uniwersytet Medyczny w Katowicach: Katowice, Poland, 2017. [Google Scholar]
- Bartosz, G. Druga Twarz Tlenu. Wolne Rodniki w Przyrodzie; PWN: Warszawa, Poland, 2021. [Google Scholar]
- Alkadi, H. A Review on free radicals and antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef]
- Pambuk, C.I.A.; Muhammad, F.M. Free radicals: The types generated in biological system. Mol. Cell Sci. Rep. 2018, 5, 72–73. [Google Scholar] [CrossRef]
- Gutowski, M.; Kowalczyk, S. A study of free radical chemistry: Their role and pathophysiological significance. Acta Biochim. Pol. 2013, 60, 1–16. [Google Scholar] [CrossRef]
- McCord, J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Bergendi, L.; Benes, L.; Durackova, Z.; Ferencik, M. Chemistry, physiology and pathology of free radicals. Life Sci. 1999, 65, 1865–1874. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Ismail, R.; Tannous, J.H. Free radical quantification in chemical systems: Challenges and future perspectives. Chem. Eng. Res. Des. 2024, 210, 97–111. [Google Scholar] [CrossRef]
- Więckowski, A.B.; Wojtowicz, W.; Śliwa-Nieściór, J. Temperature dependence of the EPR linewidth of ultramarine blue. Magn. Reson. Chem. (Magn. Reson. Porous Mater.) 1999, 37, S150–S153. [Google Scholar] [CrossRef]
- Kowalak, S.; Jankowska, A.; Zeidler, S.; Więckowski, A.B. Sulfur radicals embedded in various cages of ultramarine analogs prepared from zeolites. J. Sol. State Chem. 2007, 180, 1119–1124. [Google Scholar] [CrossRef]
- Ramos, P.; Pilawa, B. Application of EPR spectroscopy to examine free radicals evolution during storage of the thermally sterilized Ungentum ophthalmicum. Pharm. Dev. Technol. 2018, 23, 834–837. [Google Scholar] [CrossRef] [PubMed]
- Bogacz, A.; Stec, M.; Ramos, P.; Pilawa, B. UV-irradiation influence on free radical formation and radical scavenging ability of caffeic acid—EPR, UV-Vis, and colorimetric examination. J. Food Process Eng. 2021, 33, e13700. [Google Scholar] [CrossRef]
- Pierzchała, E.; Ramos, P.; Pilawa, B. Application of EPR spectroscopy in comparative analysis of free radicals thermally formed during storage in the antibacterial ointments containing fusidic acid and neomycin. Acta Pol. Pharm. Drug Res. 2017, 74, 1017–1078. [Google Scholar]
- Pierzchała, E.; Ramos, P.; Pilawa, B. EPR study of free radicals formed in fusidic acid and neomycin under UV irradiation. Acta Pol. Pharm. Drug Res. 2019, 76, 215–223. [Google Scholar] [CrossRef]
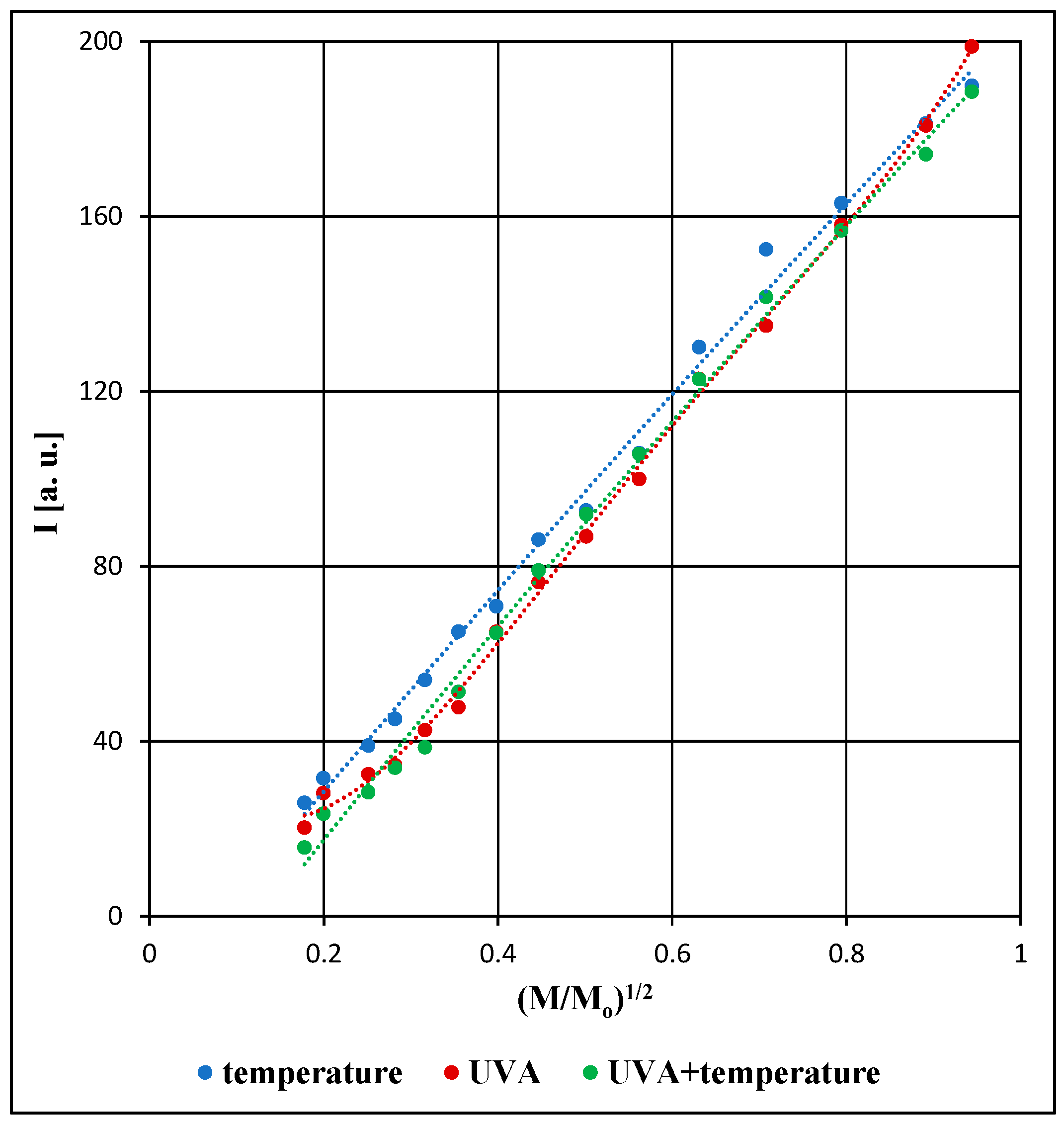
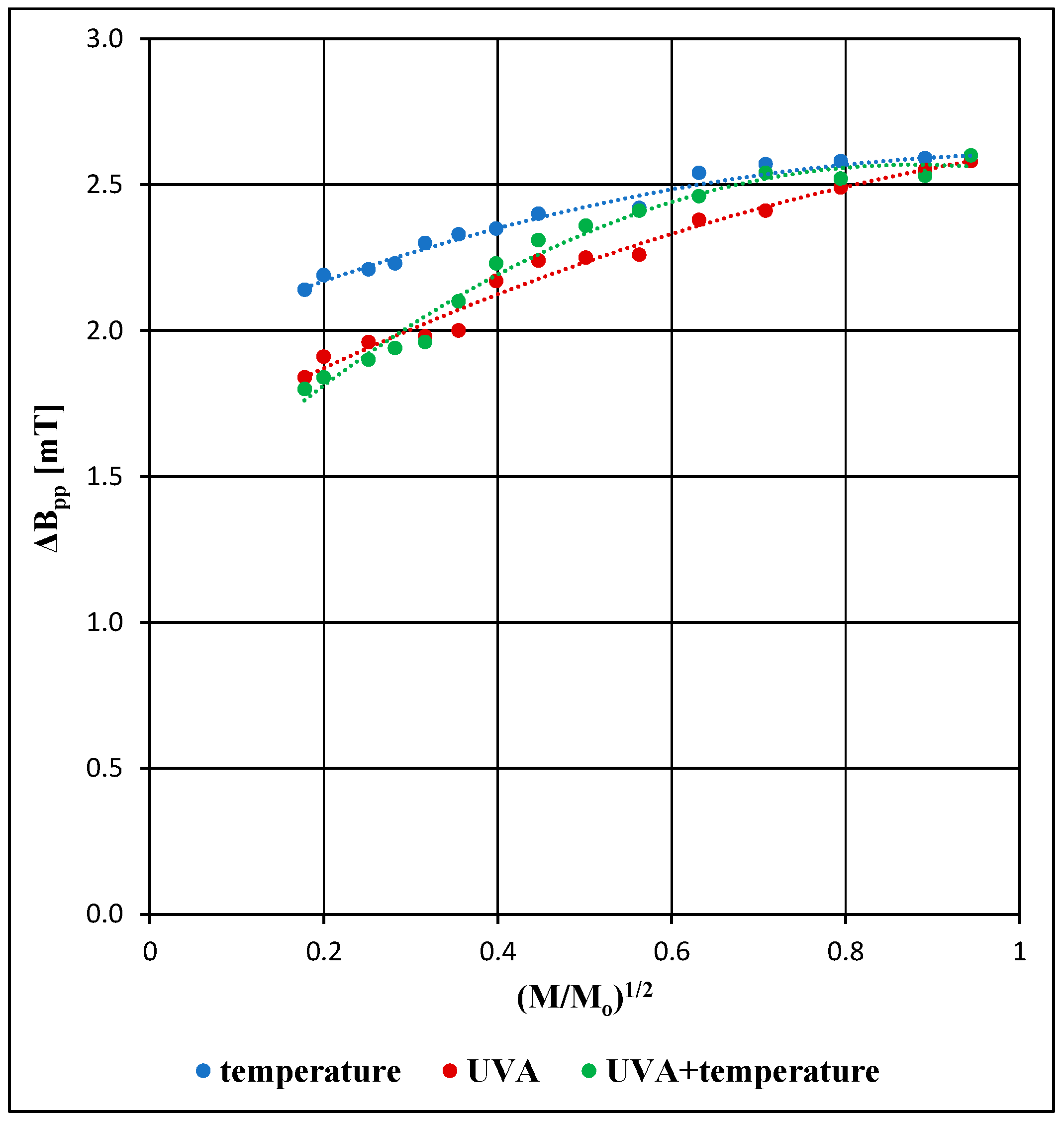
| Parameters of the EPR Spectra | M [mW] | ||
|---|---|---|---|
| 2.2 | 7 | 55 | |
| A [a. u.] [±0.02] | 5.41 | 10.22 | 27.02 |
| I [a. u.] [±0.04] | 25.96 | 54.06 | 181.22 |
| ΔBpp [mT] [±0.04] | 2.14 | 2.30 | 2.59 |
| Parameters of the EPR Spectra | M [mW] | ||
|---|---|---|---|
| 2.2 | 7 | 55 | |
| A [a. u.] [±0.02] | 6.00 | 10.84 | 27.81 |
| I [a. u.] [±0.04] | 20.23 | 42.51 | 180.81 |
| ΔBpp [mT] [±0.04] | 1.84 | 1.98 | 2.55 |
| Parameters of the EPR Spectra | M [mW] | ||
|---|---|---|---|
| 2.2 | 7 | 55 | |
| A [a. u.] [±0.02] | 4.86 | 10.05 | 27.21 |
| I [a. u.] [±0.04] | 15.74 | 38.60 | 174.20 |
| ΔBpp [mT] [±0.04] | 1.80 | 1.96 | 2.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, K.; Pilawa, B.; Zdybel, M.; Chodurek, E. Free Radical Formation in a Pharmaceutical Product Containing Bisoprolol Fumarate Stored Under Different Physical Conditions. Processes 2025, 13, 1742. https://doi.org/10.3390/pr13061742
Sobczak K, Pilawa B, Zdybel M, Chodurek E. Free Radical Formation in a Pharmaceutical Product Containing Bisoprolol Fumarate Stored Under Different Physical Conditions. Processes. 2025; 13(6):1742. https://doi.org/10.3390/pr13061742
Chicago/Turabian StyleSobczak, Kacper, Barbara Pilawa, Magdalena Zdybel, and Ewa Chodurek. 2025. "Free Radical Formation in a Pharmaceutical Product Containing Bisoprolol Fumarate Stored Under Different Physical Conditions" Processes 13, no. 6: 1742. https://doi.org/10.3390/pr13061742
APA StyleSobczak, K., Pilawa, B., Zdybel, M., & Chodurek, E. (2025). Free Radical Formation in a Pharmaceutical Product Containing Bisoprolol Fumarate Stored Under Different Physical Conditions. Processes, 13(6), 1742. https://doi.org/10.3390/pr13061742






