Extraction-Based Pretreatment of End-of-Life Plastics from Waste Electrical and Electronic Equipment for Brominated Flame Retardant Removal and Subsequent Valorization via Pyrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Analytical Methods
2.2.2. Chemical Recycling Method—Pyrolysis
2.2.3. Soxhlet Extraction Method
3. Results
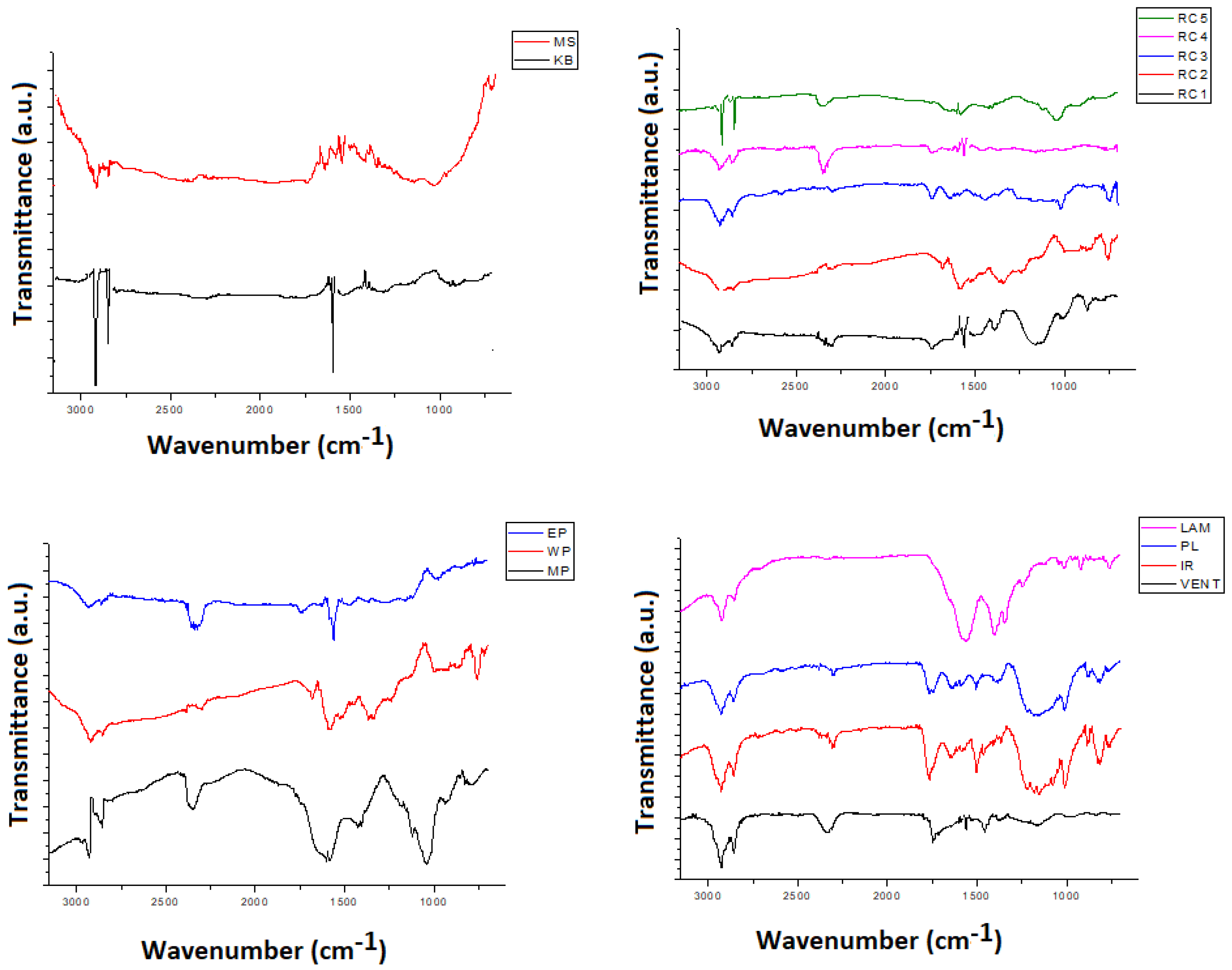
3.1. Chemical Characteristics of the Samples—FTIR Results
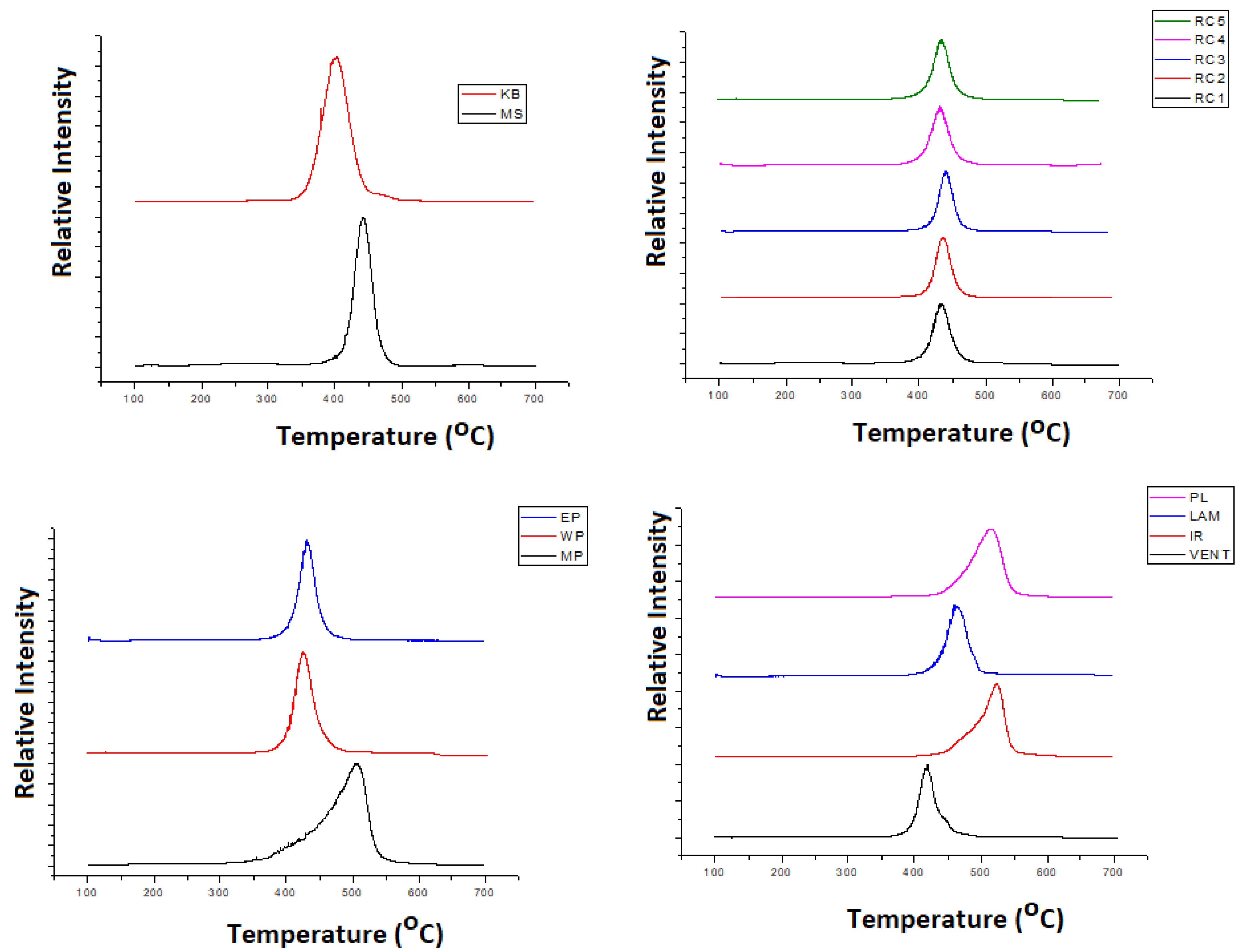
3.2. Thermal Characteristicsof the Samples—EGA Results
3.3. XRF Results and Debromination Efficiency
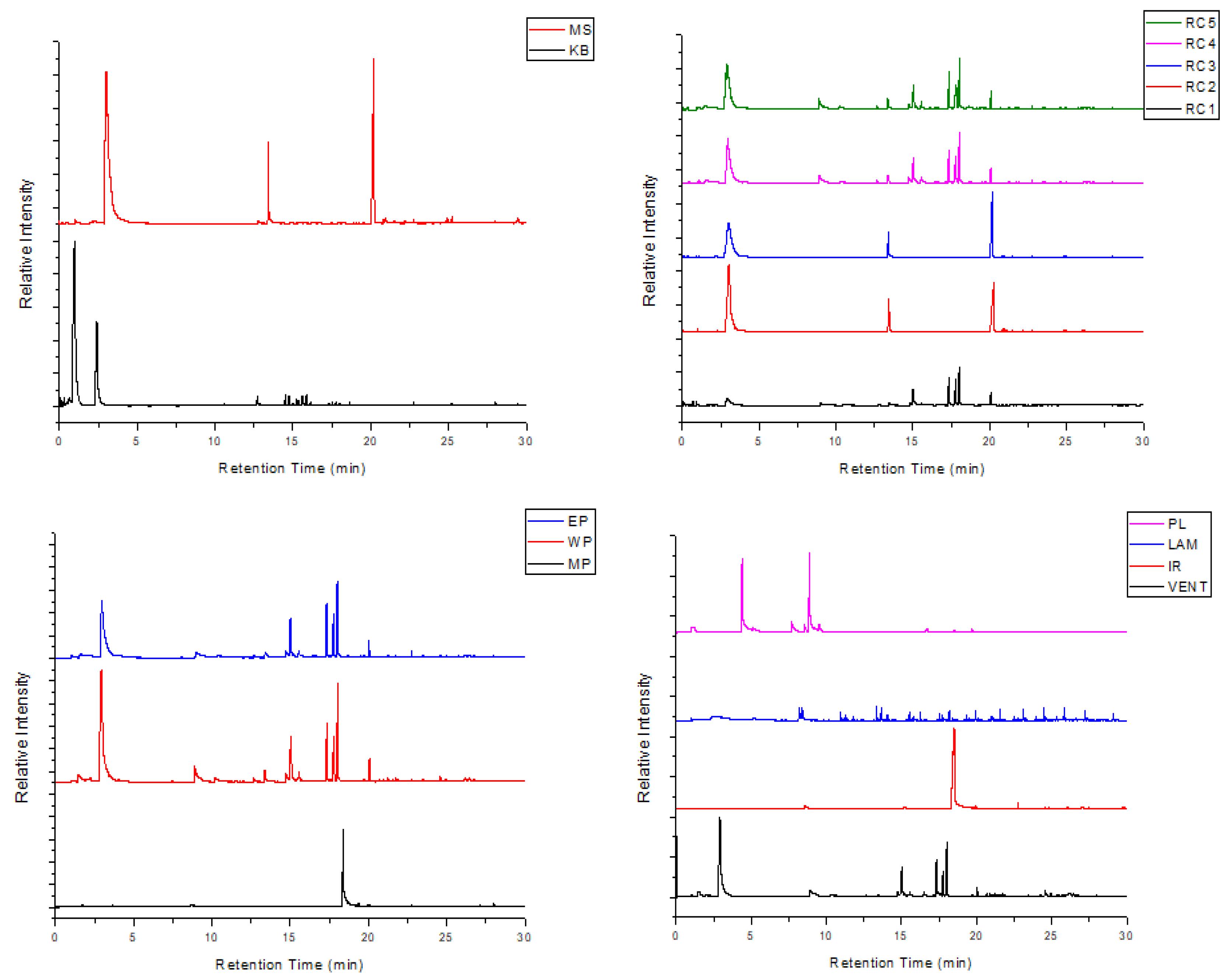
3.4. Thermal Pyrolysis Results
3.5. DSC Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Achilias, D.S.; Charitopoulou, M.-A.; Ciprioti, S.V. Thermal and Catalytic Recycling of Plastics from Waste Electrical and Electronic Equipment—Challenges and Perspectives. Polymers 2024, 16, 2538. [Google Scholar] [CrossRef] [PubMed]
- Baldé, C.P.; Kuehr, R.; Yamamoto, T.; McDonald, R.; D’Angelo, E.; Althaf, S.; Bel, G.; Deubzer, O.; Fernandez-Cubillo, E.; Forti, V.; et al. The Global E-Waste Monitor 2024; International Telecommunication Union (ITU): Geneva, Switzerland; United Nations Institute for Training and Research (UNITAR): Bonn, Germany, 2024; ISBN 978-92-61-38791-4. [Google Scholar]
- Cardamone, F.G.; Ardolino, F.; Arena, U. About the environmental sustainability of the European management of WEEE plastics. Waste Manag. 2021, 126, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Dong, L.; Lu, H.; Zhan, L.; Xu, Z. Debromination process of Br-containing PS of E-wastes and reuse with virgin PS. J. Hazard. Mater. 2022, 431, 128526. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Sun, L.; Xiang, J.; Hu, S.; Su, S. Pyrolysis and dehalogenation of plastics from waste electrical and electronic equipment (WEEE): A review. Waste Manag. 2013, 33, 462–473. [Google Scholar] [CrossRef]
- Buekens, A.; Yang, J. Recycling of WEEE plastics: A review. J. Mater. Cycles Waste Manag. 2014, 16, 415–434. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Leeke, G.A.; El-Eskandarany, M.S.; Van Haute, M.; Constantinou, A.; Dewil, R.; Baeyens, J. On the implementation of the circular economy route for E-waste management: A critical review and an analysis for the case of the state of Kuwait. J. Environ. Manag. 2022, 323, 116181. [Google Scholar] [CrossRef]
- Mtibe, A.; Mokhena, T.C.; John, M.J. Sustainable valorization and conversion of e-waste plastics into value-added products. Curr. Opin. Green Sustain. Chem. 2023, 40, 100762. [Google Scholar] [CrossRef]
- Kike, O.; Sindiku, O.; Osibanjo, O.; Weber, R. Polybrominated diphenyl ethers (PBDEs) concentrations in soil, sediment and water samples around electronic wastes dumpsites in Lagos, Nigeria. Emerg. Contam. 2022, 8, 206–215. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Chemical recycling of brominated flame retarded plastics from e-waste for clean fuels production: A review. Renew. Sustain. Energy Rev. 2016, 61, 433–450. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Wang, B.; Song, Z.; Xiang, J.; Hu, S.; Su, S.; Sun, L. Catalytic pyrolysis of flame retarded high impact polystyrene over various solid acid catalysts. Fuel Process. Technol. 2017, 155, 32–41. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Lappas, A.A.; Achilias, D.S. Thermo-chemical recycling of plastics retrieved from waste electric and electronic equipment (WEEE) by pyrolysis: Identification of the polymer type, removal of bromine compounds from plastics based on an environmentally-friendly process and characterization of the pyrolysates. Sustain. Chem. Pharm. 2023, 35, 101210. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Kirim, Y. Material recycling. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1018–1042. [Google Scholar]
- Beccagutti, B.; Cafiero, L.; Pietrantonio, M.; Pucciarmati, S.; Tuffi, R.; Vecchio Ciprioti, S. Characterization of Some Real Mixed Plastics from WEEE: A Focus on Chlorine and Bromine Determination by Different Analytical Methods. Sustainability 2016, 8, 1107. [Google Scholar] [CrossRef]
- Ma, C.; Yu, J.; Chen, T.; Yan, Q.; Song, Z.; Wang, B.; Sun, L. Influence of Fe based ZSM-5 catalysts on the vapor intermediates from the pyrolysis of brominated acrylonitrile-butadiene-styrene copolymer (Br-ABS). Fuel 2018, 230, 390–396. [Google Scholar] [CrossRef]
- Charitopoulou, M.A.; Stefanidis, S.D.; Lappas, A.A.; Achilias, D.S. Catalytic pyrolysis of polymers with brominated flame-retardants originating in waste electric and electronic equipment (WEEE) using various catalysts. Sustain. Chem. Pharm. 2022, 26, 100612. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A review on co-pyrolysis of biomass: An optional technique to obtain a high-grade pyrolysis oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Ma, C.; Yan, Q.; Yu, J.; Chen, T.; Wang, D.; Liu, S.; Bikane, K.; Sun, L. The behavior of heteroatom compounds during the pyrolysis of waste computer casing plastic under various heating conditions. J. Clean. Prod. 2019, 219, 461–470. [Google Scholar] [CrossRef]
- Bifulco, A.; Chen, J.; Sekar, A.; Wu Klingler, W.; Gooneie, A.; Gaan, S. Recycling of flame retardant polymers: Current technologies and future perspectives. J. Mater. Sci. Technol. 2024, 199, 156–183. [Google Scholar] [CrossRef]
- Evangelopoulos, P.; Arato, S.; Persson, H.; Kantarelis, E.; Yang, W. Reduction of brominated flame retardants (BFRs) in plastics from waste electrical and electronic equipment (WEEE) by solvent extraction and the influence on their thermal decomposition. Waste Manag. 2019, 94, 165–171. [Google Scholar] [CrossRef]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Microwave-assisted extraction for qualitative and quantitative determination of brominated flame retardants in styrenic plastic fractions from waste electrical and electronic equipment (WEEE). Talanta 2009, 78, 33–39. [Google Scholar] [CrossRef]
- Kousaiti, A.; Hahladakis, J.N.; Savvilotidou, V.; Pivnenko, K.; Tyrovola, K.; Xekoukoulotakis, N.; Astrup, T.F.; Gidarakos, E. Assessment of tetrabromobisphenol-A (TBBPA) content in plastic waste recovered from WEEE. J. Hazard. Mater. 2020, 390, 121641. [Google Scholar] [CrossRef] [PubMed]
- Charitopoulou, M.A.; Papadopoulou, L.; Achilias, D.S. Microwave-assisted extraction as an effective method for the debromination of brominated flame retarded polymeric blends with a composition that simulates the plastic part of waste electric and electronic equipment (WEEE). Sustain. Chem. Pharm. 2022, 29, 100790. [Google Scholar] [CrossRef]
- Zhang, C.-C.; Zhang, F.-S. Removal of brominated flame retardant from electrical and electronic waste plastic by solvothermal technique. J. Hazard. Mater. 2012, 221–222, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Charitopoulou, M.A.; Papadopoulou, L.; Achilias, D.S. Effect of brominated flame retardant on the pyrolysis products of polymers originating in WEEE. Environ. Sci. Pollut. Control Ser. 2022, 29, 29570–29582. [Google Scholar] [CrossRef]
- Rosi, L.; Bartoli, M.; Frediani, M. Microwave assisted pyrolysis of halogenated plastics recovered from waste computers. Waste Manag. 2018, 73, 511–522. [Google Scholar] [CrossRef]
- Parshin, A.M.; Gunyakov, V.A.; Zyryanov, V.Y.; Shabanov, V.F. Domain Structures in Nematic Liquid Crystals on a Polycarbonate Surface. Int. J. Mol. Sci. 2013, 14, 16303–16320. [Google Scholar] [CrossRef]
- Achilias, D.S. Chemical recycling of poly(methyl methacrylate) by pyrolysis. Potential use of the liquid fraction as a raw material for the reproduction of the polymer. Eur. Polym. J. 2007, 43, 2564–2575. [Google Scholar] [CrossRef]
| Sample Category | Samples | Sample Name | |
|---|---|---|---|
| Computer peripheral equipment | Mouse | MS |  |
| Keyboard | KB | ||
| Remote controls | Television remote | RC1 | |
| Television remote | RC2 |  | |
| Television remote | RC3 | ||
| Television remote | RC4 |  | |
| PlayStation remote | RC5 |  | |
| Telephones and accessories | Mobile phone | MP |  |
| Wireless phone | WP |  | |
| Earphones | EP | ||
| Miscellaneous household equipment | Bathroom ventilator | VENT |  |
| Iron | IR |  | |
| Plug | PL |  | |
| Lamp | LAM |
| Sample Name | Tmax (°C) | Bromine Content (ppm) |
|---|---|---|
| MS | 443 | 2630 ± 154 |
| KB | 394 | 0 |
| RC1 | 433 | 0 |
| RC2 | 436 | 0 |
| RC3 | 439 | 1100 ± 69 |
| RC4 | 432 | 0 |
| RC5 | 434 | 2300 ± 131 |
| MP | 489 | 0 |
| WP | 426 | 0 |
| EP | 432 | n.a. * |
| VENT | 420 | 0 |
| IR | 523 | 0 |
| PL | 517 | 0 |
| LAM | 459 | 0 |
| Sample Name | Solvent: Isopropanol | Solvent: Butanol | ||
|---|---|---|---|---|
| ppm Br | % Br Reduction | ppm Br | % Br Reduction | |
| MS | 729 ± 42 | 72 | 408 ± 22 | 85 |
| RC3 | 428 ± 25 | 61 | BDL * | 100 |
| RC5 | 669 ± 40 | 71 | 654 ± 37 | 72 |
| Peak | Retention Time (min) | Compound |
|---|---|---|
| KB (computer peripheral equipment) | ||
| 1 | 0.59 | Acrylonitrile |
| 2 | 0.96 | Methyl methacrylate |
| 3 | 2.40 | Styrene |
| 4 | 12.70 | 2-Heptenoic acid, 7-(methylenecyclopropyl)-, methyl ester |
| 5–6 | 14.50; 14.73 | 2-Methyl-4-phenyl-butyric acid, methyl ester |
| 7 | 15.36 | Pentadecanoic acid, 14-methyl-, methyl ester |
| 8 | 15.59 | Cyclohexane, 1-ethenyl-3-methylene-5-(1-propenylidene)- |
| 9 | 15.86 | 1,6-Heptadiene, 2-methyl-6-phenyl- |
| MS (computer peripheral equipment) and RC2 and RC3 (remote controls) | ||
| 1 | 3.00 | Styrene |
| 2 | 13.41 | 3-Butynylbenzene |
| 3 | 20.22 | Cyclohexane, 1,3,5-triphenyl- |
| RC1, RC4 and RC5 (remote controls); WP and EP (telephones and accessories); and VENT (miscellaneous household equipment) | ||
| 1 | 2.92 | Styrene |
| 2 | 8.97 | Propanedinitrile, (1-methylethenyl)(phenylmethyl)- |
| 3 | 13.41 | 3-Butynylbenzene |
| 4 | 15.01 | 4-Isopropylphenylacetonitrile |
| 5 | 17.33 | 7-Ethyl-1,3,5-cycloheptatriene |
| 6 | 17.78 | 4-Isopropylphenylacetonitrile |
| 7 | 18.02 | [1-(3-Phenyl-3-butenyl)cyclopropyl]benzene |
| 8 | 20.07 | Cyclohexane, 1,3,5-triphenyl- |
| MP (telephones and accessories) and IR (miscellaneous household equipment) | ||
| Peak | Retention Time (min) | Compound |
| 1 | 8.61 | Phenol, p-tert-butyl- |
| 2 | 15.25 | Phenol, 4-(1-methyl-1-phenylethyl)- |
| 3 | 18.40 | Bisphenol A |
| 4 | 20.03 | Cyclohexane, 1,3,5-triphenyl- |
| PL (miscellaneous household equipment) | ||
| 1 | 4.38 | Phenol |
| 2 | 7.67 | p-Isopropylphenol |
| 3 | 8.53 | Phenol, p-tert-butyl- |
| 4 | 8.89 | 2-(2-Propenyl)-phenol |
| 5 | 18.49 | Bisphenol A |
| LAM (miscellaneous household equipment) | ||
| 1–3 | 8.16; 8.38; 10.90 | 1-Undecene, 7-methyl- |
| 4 | 11.74 | Cyclohexane, 3-ethyl-5-methyl-1-propyl- |
| 5–7 | 13.34; 13.65; 13.98 | 1-Undecene, 7-methyl- |
| 8 | 14.09 | Cyclohexane, 1,1′-(1,2-dimethyl-1,2-ethanediyl)bis- |
| 9–10 | 15.50; 15.76 | Cyclooctacosane |
| 11 | 16.22 | Cyclopentane, 1,2-dibutyl- |
| 12 | 18.16 | Cyclohexane, 1,3,5-trimethyl-2-octadecyl- |
| 13–14 | 19.92; 21.56 | Cyclooctane, 1-methyl-3-propyl- |
| 15 | 23.08 | Cyclohexane, 1,3,5-trimethyl-2-octadecyl- |
| 16 | 24.49 | Cyclooctane, 1-methyl-3-propyl- |
| 17–19 | 25.81; 27.18; 29.07 | 1-Cyclopentyleicosane |
| Sample Category | Samples | Sample Name | Polymer Type |
|---|---|---|---|
| Computer peripheral equipment | Mouse | MS | HIPS |
| Keyboard | KB | ABS/PMMA | |
| Remote controls | Television remote | RC1 | ABS |
| Television remote | RC2 | HIPS | |
| Television remote | RC3 | HIPS | |
| Television remote | RC4 | ABS | |
| PlayStation remote | RC5 | ABS | |
| Telephones and accessories | Mobile phone | MP | PC |
| Wireless phone | WP | ABS | |
| Earphones | EP | ABS | |
| Miscellaneous household equipment | Bathroom ventilator | VENT | ABS |
| Iron | IR | PC | |
| Plug | PL | PC | |
| Lamp | LAM | PP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charitopoulou, M.-A.; Papadimitriou, M.; Papadopoulou, L.; Achilias, D.S. Extraction-Based Pretreatment of End-of-Life Plastics from Waste Electrical and Electronic Equipment for Brominated Flame Retardant Removal and Subsequent Valorization via Pyrolysis. Processes 2025, 13, 1458. https://doi.org/10.3390/pr13051458
Charitopoulou M-A, Papadimitriou M, Papadopoulou L, Achilias DS. Extraction-Based Pretreatment of End-of-Life Plastics from Waste Electrical and Electronic Equipment for Brominated Flame Retardant Removal and Subsequent Valorization via Pyrolysis. Processes. 2025; 13(5):1458. https://doi.org/10.3390/pr13051458
Chicago/Turabian StyleCharitopoulou, Maria-Anna, Maria Papadimitriou, Lambrini Papadopoulou, and Dimitriοs S. Achilias. 2025. "Extraction-Based Pretreatment of End-of-Life Plastics from Waste Electrical and Electronic Equipment for Brominated Flame Retardant Removal and Subsequent Valorization via Pyrolysis" Processes 13, no. 5: 1458. https://doi.org/10.3390/pr13051458
APA StyleCharitopoulou, M.-A., Papadimitriou, M., Papadopoulou, L., & Achilias, D. S. (2025). Extraction-Based Pretreatment of End-of-Life Plastics from Waste Electrical and Electronic Equipment for Brominated Flame Retardant Removal and Subsequent Valorization via Pyrolysis. Processes, 13(5), 1458. https://doi.org/10.3390/pr13051458









