Investigation of the Possibilities for the Recycling of Mixed Heterogeneous Lead Refinery Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Procedure
2.3. Instrumental Testing
3. Results
3.1. Chemical Analysis
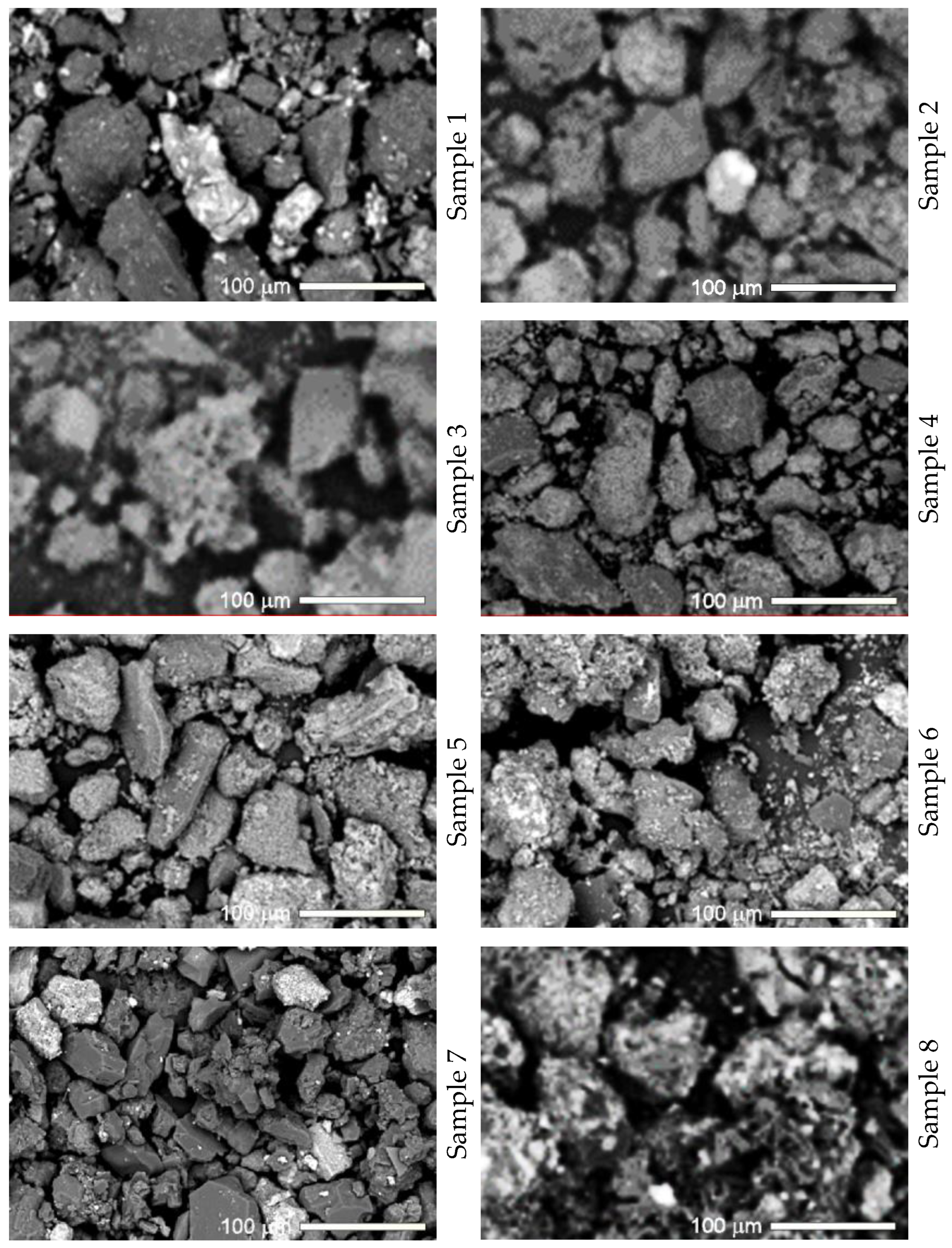
3.2. SEM-EDS Analysis
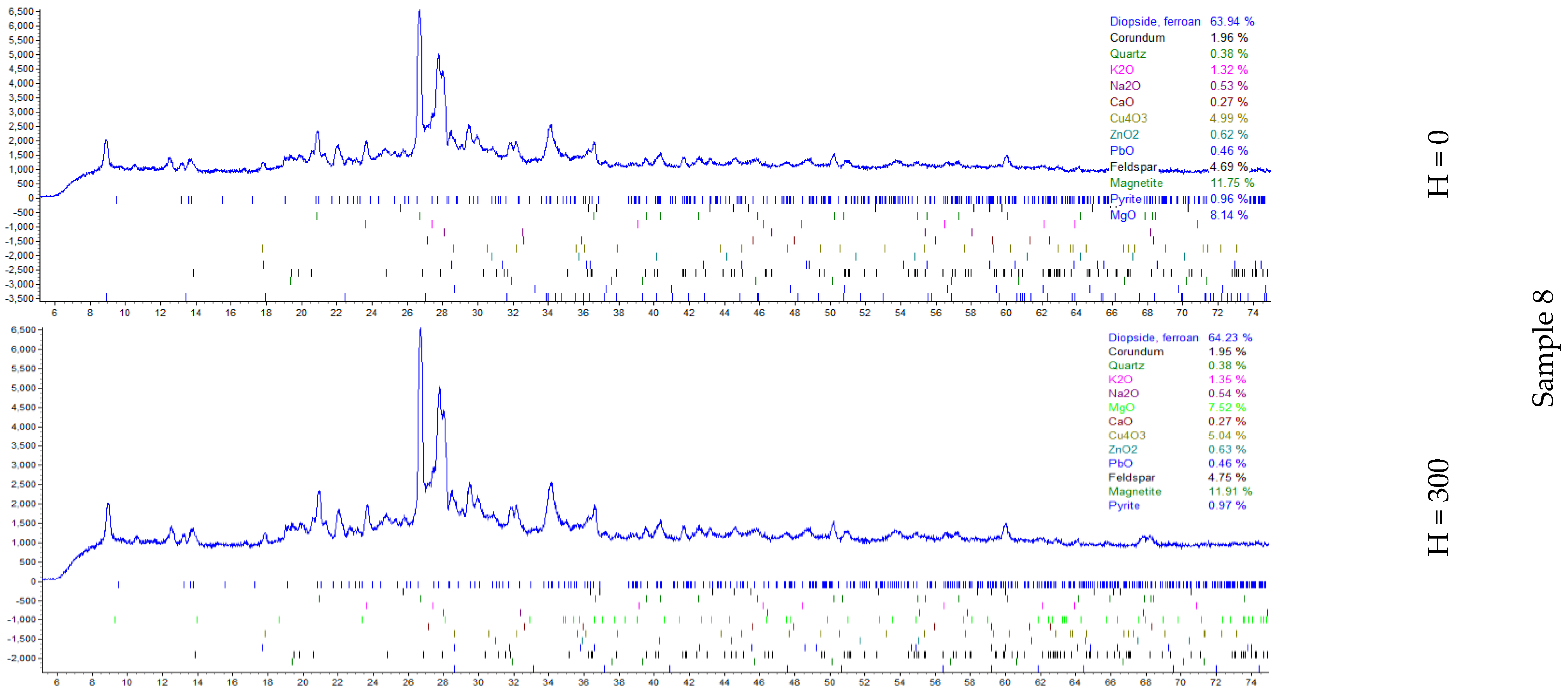
3.3. Mineralogical Analysis
4. Discussion of the Results
5. Conclusions and Recommendations for the Future
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lassin, A.; Piantone, P.; Burnol, A.; Bodénan, F.; Chateau, L.; Lerouge, C.; Crouzet, C.; Guyonnet, D.; Bailly, L. Reactivity of waste generated during lead recycling: An integrated study. J. Hazard. Mater. 2007, 139, 430–437. [Google Scholar] [PubMed]
- Ellis, T.W.; Mirza, A.H. The refining of secondary lead for use in advanced lead-acid batteries. J. Power Sources 2010, 195, 4525–4529. [Google Scholar]
- Sahu, K.K.; Agrawal, A.; Pandey, B.D. Recent trends and current practices for secondary processing of zinc and lead. Part II: Zinc recovery from secondary sources. Waste Manag. Res. J. Int. Solid Wastes Public Cleansing Assoc. Iswa. 2004, 22, 248–254. [Google Scholar]
- Milentijević, G.; Nedeljković, B.; Đokić, J. Implementation the environmental protection system in the territory of the Kosovska Mitrovica and Zvečan municipalities. Min. Metall. Eng. Bor. 2014, 4, 193–216. [Google Scholar]
- Kelmendi, M.; Sadiku, M.; Kadriu, S.; Dobroshi, F.; Igrishta, L.; Baruti, B. Research of heavy metals on the agricultural land in bajgora region, kosovo. Acta Chem. Iasi. 2018, 26, 105–122. [Google Scholar]
- Kelmendi, M.; Baruti, B.; Hyseni, S.; Durmishaj, B.; Nikshiqi Kadriu, S. The influence of industrial deposition in earth pollution with heavy metals in Mitrovica. In Proceedings of the 11th International Multidisciplinary Scientific Geo Conference SGEM, Albena, Bulgaria, 18–24 June 2011; Volume 3, pp. 701–708. [Google Scholar]
- Prathumratana, L.; Kim, R.; Kim, K.W. Lead contamination of the mining and smelting district in Mitrovica, Kosovo. Environ. Geochem. Health. 2020, 42, 1033–1044. [Google Scholar]
- Nannoni, F.; Protano, G.; Riccobono, F. Fractionation and geochemical mobility of heavy elements in soils of a mining area in northern Kosovo. Geoderma 2011, 161, 63–73. [Google Scholar]
- Nikolić, B.; Jakšić, L. Historical review and actual situation of metallurgy at Kosovo and Metohija. J. Min. Metall. 2005, 41B, 135–140. [Google Scholar]
- Castillo, S.; Jesús, D.; De La Campa, A.M.S.; González-Castanedo, Y.; Fernández-Caliani, J.C.; Gonzalez, I.; Romero, A. Contribution of mine wastes to atmospheric metal deposition in the surrounding area of an abandoned heavily polluted mining district (Rio Tinto mines, Spain). Sci. Total Environ. 2013, 449, 363–372. [Google Scholar]
- Gholizadeh, A.; Borůvka, L.; Seberioon, M.M.; Kozák, J.; Vašát, R.; Němeček, K. Comparing different data pre-processing methods for monitoring soil heavy metals based on soil spectral features. Soil Water Res. 2015, 10, 218–227. [Google Scholar]
- Amponsah-Dacosta, F. A field-scale performance evaluation of erosion control measures for slopes of mine tailings dams. In Proceedings of the 10th International Conference on Acid Rock Drainage and IMWA Annual Conference, Santiago, Chile, 21–24 April 2015. [Google Scholar]
- Souissi, R.; Souissi, F.; Chakroun, H.K.; Bouchardon, J.L. Mineralogical and Geochemical Characterization of Mine Tailings and Pb, Zn, and Cd Mobility in a Carbonate Setting (Northern Tunisia). Mine Water Environ. 2012, 32, 16–27. [Google Scholar]
- Gray, N.F. Acid mine drainage composition and the implications for its impact on lotic systems. Water Res. 1998, 32, 2122–2134. [Google Scholar]
- Dedic, J.; Djokic, J.; Galjak, J.; Milentijevic, G.; Lazarevic, D.; Šarkočević, Ž.; Lekic, M. An Experimental Investigation of the Environmental Risk of a Metallurgical Waste Deposit. Minerals 2022, 12, 661. [Google Scholar] [CrossRef]
- Galjak, J.; Đokić, J.; Dervišević, I.; Milentijević, G.; Mojsić, M.; Živković, B. Assessment of Pollution and Distribution of Heavy Metals in the Soil Near the Flotation Tailings Gornje Polje. Pol. J. Environ. Stud. 2022, 31, 4097–4106. [Google Scholar] [CrossRef]
- Milentijević, G.; Nedeljković, B.; Lekić, M.; Nikić, Z.; Ristović, I.; Djokić, J. Application of a Method for Intelligent Multi-Criteria Analysis of the Environmental Impact of Tailing Ponds in Northern Kosovo and Metohija. Energies 2016, 9, 935. [Google Scholar] [CrossRef]
- Đokić, J.; Arsić, N.; Milentijević, G. Natural Disasters in Industrial Areas. In Natural Risk Management and Engineering; Springer Tracts in Civil Engineering; Gocić, M., Aronica, G., Stavroulakis, G., Trajković, S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- European Commission. Report on Critical Raw Materials in the Circular Economy. 2018. Available online: http://publications.europa.eu/en/publication-detail/-/publication/2d43b7e2-66ac-11e7-b2f2-01aa75ed71a1/language-en/format-PDF/source-32064602 (accessed on 7 January 2025).
- European Commission. Critical Raw Materials for the EU: 2023 Final Report. 2023. Available online: https://ec.europa.eu (accessed on 8 January 2025).
- Brown, G.E., Jr.; Foster, A.L.; Ostergren, J.D. Mineral surfaces and bioavailability of heavy metals: A molecular-scale perspective. Proc. Natl. Acad. Sci. USA 1999, 96, 3388–3395. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; Alvarez-Valero, A.M.; Almodôvar, G.R. Mineralogy of the hardpan formation processes in the interface between sulfide-rich sludge and fly ash: Applications for acid mine drainage mitigation. Am. Mineral. 2007, 92, 1966–1977. [Google Scholar]
- Lottermoser, B.; Ashley, P. Mobility and retention of trace elements in hardpan-cemented cassiterite tailings, north Queensland, Australia. Environ. Geol. 2006, 50, 835–846. [Google Scholar]
- Valente, T.; Lealgomes, C. Occurrence, properties and pollution potential of environmental minerals in acid mine drainage. Sci. Total Environ. 2009, 407, 3. [Google Scholar]
- Brill, H.; Floc’h, J.P. Le devenir des metaux provenant des anciennes mines; l’Exemple du Massif Central français. Geologues 2001, 130, 233–241. [Google Scholar]
- Heikkinen, P.M.; Räisänen, M.L.; Johnson, R.H. Geochemical characterization of seepage and drainage water quality from two sulphide mine tailings impoundments: Acid mine drainage versus neutral mine drainage. Mine Water Environ. 2009, 28, 38–49. [Google Scholar]
- Hammarstrom, J.M.; Seal, R.R., II; Meier, A.L.; Jackson, J.C. Weathering of sulfidic shale and copper mine waste: Secondary minerals and metal cycling in Great Smoky Mountains National Park, Tennessee, and North Carolina, USA. Environ. Geol. 2003, 45, 35–57. [Google Scholar]
- Harris, D.L.; Lottermoser, B.G.; Duchesne, J. Ephemeral acid mine drainage at the Montalbion silver mine, North Queensland. Aust. J. Earth Sci. 2003, 50, 797–809. [Google Scholar]
- Šajn, R.; Alijagić, J. Secondary deposits as a potential REEs source in South-Eastern Europe. Minerals 2024, 14, 120. [Google Scholar] [CrossRef]
- Šajn, R.; Ristović, I. Mining and metallurgical waste as potential secondary sources of metals—A case study for the West Balkan region. Minerals 2022, 12, 547. [Google Scholar] [CrossRef]
- Yıldız, T.D.; Tombal-Kara, T.D.; Kurşun-Ünver, İ. Challenges and recovery opportunities in waste management during the mining and enrichment processes of rare earth elements containing ores. Chapter 11. In Trash or Treasure: Entrepreneurial Opportunities in Waste Management; Singh, P., Borthakur, A., Eds.; Springer: Cham, Switzerland, 2024; pp. 277–306. ISBN 978-3-031-55130-7. [Google Scholar] [CrossRef]
- Souza, A.G.O.; Aliprandini, P.; Espinosa, D.C.R.; Tenório, J.A.S. Scandium Extraction from Nickel Processing Waste Using Cyanex 923 in Sulfuric Medium. JOM 2019, 71, 2003–2009. [Google Scholar]
- Hu, J.; Zou, D.; Chen, J.; Li, D. A novel synergistic extraction system for the recovery of scandium (III) by Cyanex272 and Cyanex923 in sulfuric acid medium. Sep. Purif. Technol. 2020, 233, 115977. [Google Scholar]
- Cao, X.; Zhang, T.-A.; Zhang, W.; Lv, G. Solvent Extraction of Sc(III) by D2EHPA/TBP from the Leaching Solution of Vanadium Slag. Metals 2020, 10, 790. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, H.; Liu, S.-F.; Ning, S.-Y.; Wei, Y.-Z. Scandium recovery from ion-adsorption rare earth concentrate with HEHEHP as extractant. J. Cent. South Univ. 2021, 28, 679–689. [Google Scholar]
- Batchu, N.K.; Vander Hoogerstraete, T.; Banerjee, D.; Binnemans, K. Separation of rare-earth ions from ethylene glycol (+LiCl) solutions by non-aqueous solvent extraction with Cyanex 923. RSC Adv. 2017, 7, 45351–45362. [Google Scholar]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar]
- Chen, Y.; Ma, S.; Ning, S.; Zhong, Y.; Wang, X.; Fujita, T.; Wei, Y. Highly efficient recovery and purification of scandium from the waste sulfuric acid solution from titanium dioxide production by solvent extraction. J. Environ. Chem. Eng. 2021, 9, 106226. [Google Scholar]
- Hedwig, S.; Yagmurlu, B.; Huang, D.; von Arx, O.; Dittrich, C.; Constable, E.C.; Friedrich, B.; Lenz, M. Nanofiltration-Enhanced Solvent Extraction of Scandium from TiO2 Acid Waste. ACS Sustain. Chem. Eng. 2022, 10, 6063–6071. [Google Scholar]
- Agrawal, S.; Dhawan, N. Process flowsheet for extraction of Fe, Al, Ti, Sc, and Ga values from red mud. Miner. Eng. 2022, 184, 107601. [Google Scholar]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar]
- Habibi, H.; Mokmeli, M.; Shakibania, S.; Pirouzan, D.; Pourkarimi, Z. Separation and recovery of titanium and scandium from the red mud. Sep. Purif. Technol. 2023, 317, 123882. [Google Scholar]
- Liu, H.; Liu, H.; Nie, C.; Zhang, J.; Steenari, B.-M.; Ekberg, C. Comprehensive treatments of tungsten slags in China: A critical review. J. Environ. Manag. 2020, 270, 110927. [Google Scholar]
- Anawati, J.; Azimi, G. Integrated carbothermic smelting—Acid baking—Water leaching process for extraction of scandium, aluminum, and iron from bauxite residue. J. Clean. Prod. 2022, 330, 129905. [Google Scholar]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Comparative Analysis of Processes for Recovery of Rare Earths from Bauxite Residue. JOM 2016, 68, 2958–2962. [Google Scholar]
- Meng, F.; Li, X.; Shi, L.; Li, Y.; Gao, F.; Wei, Y. Selective extraction of scandium from bauxite residue using ammonium sulfate roasting and leaching process. Miner. Eng. 2020, 157, 106561. [Google Scholar]
- Ray, A.R.; Mishra, S. Hydro metallurgical technique as better option for the recovery of rare earths from mine tailings and industrial wastes. Sustain. Chem. Pharm. 2023, 36, 101311. [Google Scholar]
- Mostajeran, M.; Bondy, J.-M.; Reynier, N.; Cameron, R. Mining value from waste: Scandium and rare earth elements selective recovery from coal fly ash leach solutions. Miner. Eng. 2021, 173, 107091. [Google Scholar]
- Li, X.; Azimzadeh, B.; Martinez, C.E.; McBride, M.B. Pb Mineral Precipitation in Solutions of Sulfate, Carbonate and Phosphate: Measured and Modeled Pb Solubility and Pb2+ Activity. Minerals 2021, 11, 620. [Google Scholar] [CrossRef]
- Peelman, S.; Sun, Z.H.I.; Sietsma, J.; Yang, Y. Hydrometallurgical Extraction of Rare Earth Elements from Low Grade Mine Tailings. In Rare Metal Technology 2016; Alam, S., Kim, H., Neelameggham, N.R., Ouchi, T., Oosterhof, H., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Wilson, D.J. Hazardous Waste Site Soil Remediation: Theory and Application of Innovative Technologies, 1st ed.; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Nancharaiah, Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances. J. Environ. Manag. 2018, 211, 138–153. [Google Scholar]
- Galjak, J.; Đokić, J.; Gurešić, D.; Jovic, S.; Milentijević, G. Evaluation of acid mine drainage kinetics in the lead-zinc mine. Arab. J. Geosci. 2020, 13, 354. [Google Scholar] [CrossRef]
- Gunarathne, V.; Rajapaksha, A.U.; Vithanage, M.; Alessi, D.S.; Selvasembian, R.; Naushad, M.; You, S.; Oleszczuk, P.; Ok, Y.S. Hydrometallurgical processes for heavy metals recovery from industrial sludges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 1022–1062. [Google Scholar]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.-V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Demol, J.; Ho, E.; Soldenhoff, K.; Senanayake, G. The sulfuric acid bake and leach route for processing of rare earth ores and concentrates: A review. Hydrometallurgy 2019, 188, 123–139. [Google Scholar] [CrossRef]




| Samples | H, mm | Elements mg/kg | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ag | As | Ba | Ca | Cd | Co | Cr | Cu | Fe | Li | Mg | Na | Ni | Pb | S | Sb | Sc | Si | Zn | ||
| Sample 1 | 0 | 114.2 | 2428.1 | 59.2 | 7366.5 | 13.8 | 41.7 | 39.1 | 13,921 | 22,348.9 | 4.2 | 4682.5 | 1044.8 | 149.1 | 82,811.6 | 259.4 | 2.4 | 170,325.4 | 7953.4 | |
| 300 | 14.2 | 1428.1 | - | 14,376.5 | - | 24.6 | 19.8 | 923.2 | 42,168.5 | - | 668.3 | 1763.2 | 54.8 | 64,527.8 | 232.4 | 37.7 | 21,242.4 | 4963.6 | ||
| Sample 2 | 0 | 134.6 | 53.6 | 3362.5 | 11.6 | 31.4 | 59.1 | 23,624.2 | 52,646.9 | 21.2 | 76,824.5 | 12,144.8 | 172.1 | 21,861.6 | 162.4 | 13 | 14,1365.4 | 9983.4 | ||
| 300 | 94.2 | 52.2 | 6386.5 | - | 51.2 | 42.6 | 13,641.2 | 41,368.9 | - | 56,682.5 | 11,494.8 | 143.5 | 12,381.6 | 263.7 | 23 | 16,1326.4 | 69,853.4 | |||
| Sample 3 | 0 | 214.6 | 24,328.1 | - | 36,356.5 | 23.8 | 61.9 | - | 6921.7 | 202,358.9 | - | 64,682.5 | - | 184.1 | 2811.4 | 92,742.8 | 62.4 | 61 | 71,325.4 | 3963.4 |
| 300 | - | 6478.1 | - | 31,876.5 | - | 43.2 | - | 942.6 | 195,635.9 | - | 16,482.5 | - | 52.4 | 862.6 | 68,578.4 | - | 30.6 | 62,325.4 | 253.4 | |
| Sample 4 | 0 | 134.2 | 14,368.1 | 42.2 | 376.5 | - | 61.7 | 142.1 | 10,021.6 | 232,578.9 | - | - | 7494.8 | 132.1 | 72,821.6 | - | 169.4 | 56.1 | 71,325.6 | 7853.4 |
| 300 | 91.2 | 4438.1 | 23.2 | 176.5 | - | 62.7 | 163.1 | 13,964.2 | 164,358.7 | - | - | 1104.6 | 119.4 | 22,411.6 | - | 82.4 | 34.1 | 81,325.4 | 5943.7 | |
| Sample 5 | 0 | 87.9 | 4745.4 | 84.6 | 63,963.9 | 13.7 | 14.2 | 136.1 | 3170.9 | 101,677.3 | 4.7 | 7463.3 | 5433.7 | 135.8 | 32,944.6 | - | 6485.9 | 6.3 | - | 9339 |
| 300 | 54.9 | 1755.4 | 74.6 | 396,23.9 | 11.4 | 24.6 | 132.7 | 6370.2 | 112,657.3 | - | 2463.1 | 2483.7 | - | 12,644.6 | - | - | 6.3 | - | 4379 | |
| Sample 6 | 0 | 66.3 | 7062.9 | 68 | 25,982.3 | 11.5 | 26.2 | 178.9 | 9278 | 39,837.9 | 5.3 | 20,225.9 | 32,529.5 | 305.2 | 154,027.5 | 21,326.4 | 514.7 | 5.1 | - | 1.259 |
| 300 | - | 8462.9 | 48 | 24,852.3 | - | - | 124.9 | 6988.2 | 86,847.8 | - | 68,235.9 | 224,39.5 | - | 36,837.5 | 10,234.5 | 51.7 | 3.7 | - | 18,592.6 | |
| Sample 7 | 0 | 76.3 | - | 94.2 | 28,592.3 | 21.5 | 22.6 | 128.6 | 12898 | 52,847.4 | 15.3 | 14,235.9 | 31,359.5 | 35.2 | 54,537.5 | - | 18,514.7 | 59.5 | - | 25,934.6 |
| 300 | 14.3 | 62.9 | 41.2 | 5852.3 | - | - | - | 282.7 | 112,847.9 | - | 20,265.7 | - | - | 4037.5 | - | - | 75.3 | - | 12,574.8 | |
| Sample 8 | 0 | 24.3 | 62.9 | 36 | 15,962.3 | 31.8 | 54.2 | 198.6 | 19,688.2 | 38,947.9 | 14.3 | 61,235.7 | 31,539.4 | 314.7 | 74,537.5 | - | 414.7 | 60.9 | - | 42,695.4 |
| 300 | - | 162.9 | - | 12,982.3 | - | - | 198.6 | 28,298 | 62,847.9 | - | 29,235.9 | 32,529.5 | 20,315.2 | 14,837.5 | - | - | 62.7 | - | 4592.6 | |
| Samples | H, mm | Detected Elements on the Sample Surface (mass. %) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | Na | Mg | Al | Si | S | K | As | Cl | Fe | Cu | Zn | Pb | Ca | Ti | Mn | Sb | ||
| Sample 1 | 0 | 52.76 | 1.19 | 0.85 | 6.3 | 17.55 | - | 1.47 | - | - | 5.77 | 2.42 | 1.84 | 8.55 | 0.86 | - | - | - |
| 300 | 57.15 | 1.79 | 0.57 | 7.27 | 18.65 | - | 1.61 | - | 0.53 | 4.66 | 0.53 | - | 6.39 | 1.1 | 0.27 | - | - | |
| Sample 2 | 0 | 73.51 | 0.83 | 0.79 | 4.83 | 12.17 | 1.56 | 0.86 | - | - | 4.93 | - | - | - | - | 0.2 | - | - |
| 300 | 74.24 | 0.63 | 0.89 | 4.61 | 13.15 | - | 0.81 | - | - | 4.86 | 0.21 | - | - | 0.35 | 0.25 | - | - | |
| Sample 3 | 0 | 49.24 | - | 8.46 | 1.64 | 5.64 | 8.21 | 0.36 | 1.91 | - | 21.21 | - | 0.14 | - | 2.63 | - | 0.65 | - |
| 300 | 52.26 | - | - | 0.98 | 4.28 | 12.08 | 0.29 | 0.65 | - | 25.44 | - | - | - | 3.18 | - | 0.36 | - | |
| Sample 4 | 0 | 48.61 | 0.47 | 1.23 | 5.38 | 7.76 | 0.24 | 1.08 | 0.15 | 26.17 | 0.64 | 0.77 | 7.5 | - | - | - | - | |
| 300 | 49.55 | - | - | 1.7 | 3.59 | 11.15 | 0.27 | 0.86 | - | 17.05 | 0.84 | - | 7.39 | 6.18 | - | - | - | |
| Sample 5 | 0 | 50.78 | 1.19 | 0.91 | 2.06 | 6.99 | 8.15 | 1.16 | 2.45 | 16.3 | 0.81 | 1.55 | 4.09 | 3.42 | - | - | 2.14 | |
| 300 | 74.98 | - | - | 1.44 | 3.33 | 7.45 | - | 0.31 | - | 7.98 | 0.26 | 0.55 | 1.05 | 2.65 | - | - | - | |
| Sample 6 | 0 | 49.28 | 5.09 | 1.97 | 3.41 | 10.27 | 3.32 | 1.01 | 1.5 | 0.32 | 6.16 | 1.31 | 2.98 | 11.72 | 1.69 | - | - | - |
| 300 | 48.28 | 2.19 | 8.86 | 5.75 | 13.73 | 1.85 | 0.86 | 0.96 | 0.13 | 9.54 | 0.89 | 1.55 | 2.45 | 2.96 | - | - | - | |
| Sample 7 | 0 | 50.46 | 2.74 | 0.65 | 6.42 | 19.27 | - | 1.99 | - | - | 6.59 | 1.34 | 2.68 | 4.24 | 1.42 | - | - | 2.2 |
| 300 | 59.35 | 2.51 | 2.69 | 5.02 | 15.98 | - | 1.61 | - | - | 10.36 | 0.98 | 0.86 | 0.63 | 0.98 | - | - | - | |
| Sample 8 | 0 | 48.37 | 3.7 | 8.95 | 3.59 | 9.81 | 6.8 | 1.17 | - | 0.3 | 2.88 | 0.94 | 3.79 | 8.23 | 0.94 | - | - | - |
| 300 | 59.76 | 2.91 | 3.56 | 5.02 | 15.98 | - | 1.61 | - | - | 7.36 | - | 0.86 | 2.43 | 0.9 | - | - | - | |
| Sample | pH Value | Conductivity (μS/cm) |
|---|---|---|
| 1 | 5.23 | 318 |
| 2 | 7.15 | 236 |
| 3 | 6.67 | 1075 |
| 4 | 4.27 | 1273 |
| 5 | 2.86 | 2630 |
| 6 | 2.80 | 2110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dedić, J.; Đokić, J.; Milentijević, G.; Dervišević, I.; Petrović, M. Investigation of the Possibilities for the Recycling of Mixed Heterogeneous Lead Refinery Waste. Processes 2025, 13, 1380. https://doi.org/10.3390/pr13051380
Dedić J, Đokić J, Milentijević G, Dervišević I, Petrović M. Investigation of the Possibilities for the Recycling of Mixed Heterogeneous Lead Refinery Waste. Processes. 2025; 13(5):1380. https://doi.org/10.3390/pr13051380
Chicago/Turabian StyleDedić, Jasmina, Jelena Đokić, Gordana Milentijević, Irma Dervišević, and Maja Petrović. 2025. "Investigation of the Possibilities for the Recycling of Mixed Heterogeneous Lead Refinery Waste" Processes 13, no. 5: 1380. https://doi.org/10.3390/pr13051380
APA StyleDedić, J., Đokić, J., Milentijević, G., Dervišević, I., & Petrović, M. (2025). Investigation of the Possibilities for the Recycling of Mixed Heterogeneous Lead Refinery Waste. Processes, 13(5), 1380. https://doi.org/10.3390/pr13051380







