Samarium-Doped PbO2 Electrocatalysts for Environmental and Energy Applications: Theoretical Insight into the Mechanisms of Action Underlying Their Carbendazim Degradation and OER Properties
Abstract
1. Introduction
2. Materials and Methods
3. Results
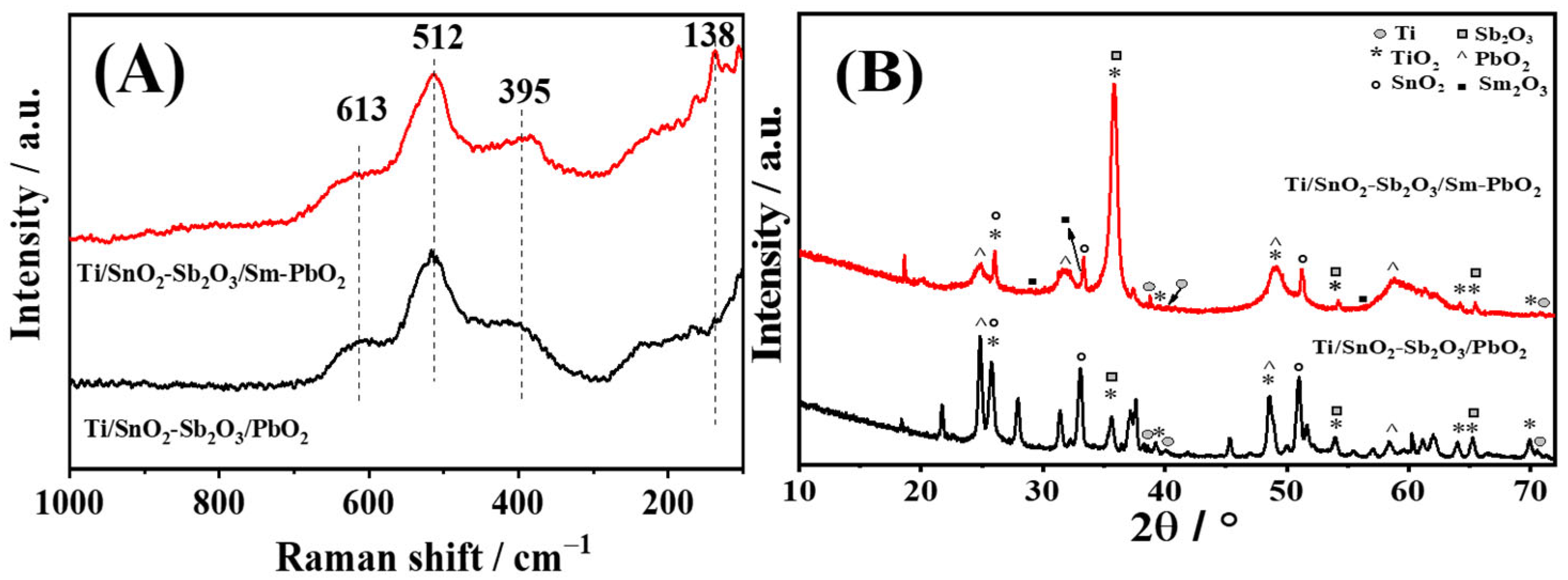
3.1. Characterization of Ti/Sb-SnO2/Sm-PbO2 Electrocatalyst
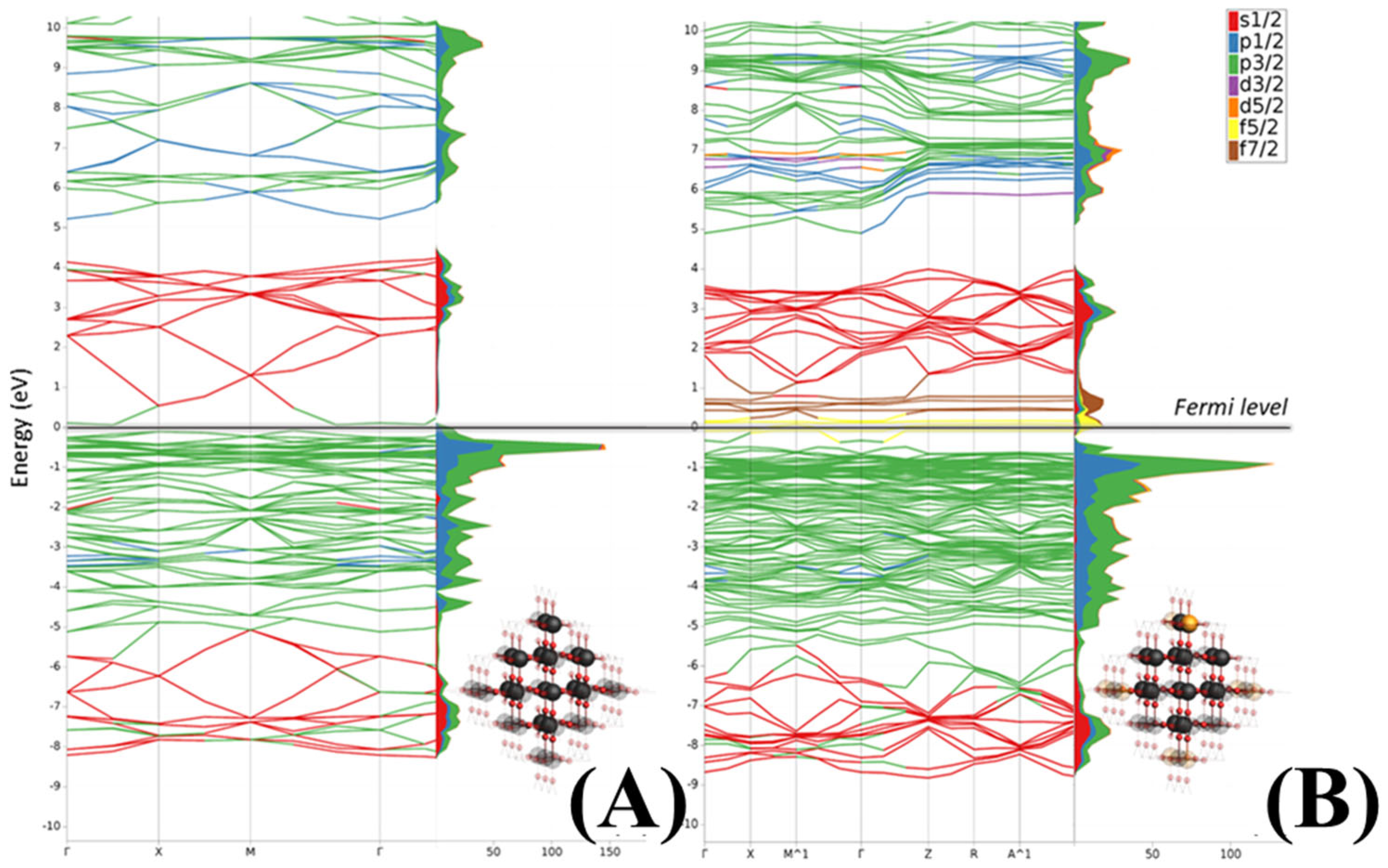
3.2. Obtained Theoretical Results
3.3. Oxygen Evolution Reaction Investigation
| OER Electrocatalysts | Electrolyte | Eonset/V | ηonset/mV | b/mV dec−1 | j at 2 V/mA cm−2 | Ref. |
|---|---|---|---|---|---|---|
| Ti/Sb-SnO2/PbO2 | 1 M KOH (pH = 14) | 1.83 | 630 | 389 | 67.1 | This work |
| Ti/Sb-SnO2/Sm-PbO2 | 1 M KOH (pH = 14) | 1.80 | 600 | 489 | 168.4 | This work |
| EPD-1.0/PbO2 | 0.25 M Na2SO4 (pH = 6) | ~2.25 | 1050 | / | ~35 | [70] |
| EPD-2.0/PbO2 | 0.25 M Na2SO4 (pH = 6) | ~2.25 | 1050 | / | ~30 | [70] |
| EPD-5.0/PbO2 | 0.25 M Na2SO4 (pH = 6) | ~2.38 | 1180 | / | ~45 | [70] |
| Ti-PbO2-reference | 0.1 M Na2SO4 (pH = 6.2) | ~3.12 | 1920 | / | / | [71] |
| Ti-PbO2-2 | 0.1 M Na2SO4 (pH = 6.2) | ~2.44 | 1240 | / | / | [71] |
| Ti-PbO2-4 | 0.1 M Na2SO4 (pH = 6.2) | ~2.44 | 1240 | / | / | [71] |
| PbO2-MnO2 | 0.5 M H2SO4 (pH = 0.3) | ~1.83 | ~630 | 123 | / | [68] |
| PbO2 | 0.5 M H2SO4 (pH = 0.3) | ~1.93 | ~730 | 152 | / | [68] |
| GFC20600 | 1 M KOH (pH = 14) | / | / | 139 | / | [77] |
| GFC20700 | 1 M KOH (pH = 14) | / | / | 154 | / | [77] |
| GFC20800 | 1 M KOH (pH = 14) | / | / | 180 | / | [77] |
| CeO2 | 0.1 M KOH (pH = 13) | 1.36 | 110 | 148 | / | [72] |
| CeO2/rGO, | 0.1 M KOH (pH = 13) | 1.31 | 160 | 138 | / | [72] |
| La2O3/rGO | 0.1 M KOH (pH = 13) | / | / | 210 | / | [72] |
| Ce0.95Ni0.05O2−δ | 0.1 M KOH (pH = 13) | 1.65 | 450 | 281 | 0.9 | [73] |
| Ce0.90Ni0.10O2−δ | 0.1 M KOH (pH = 13) | 1.58 | 380 | 232 | 1.8 | [73] |
3.4. AOP Application of Ti/Sb-SnO2/Sm-PbO2 Electrode for CBZ Removal
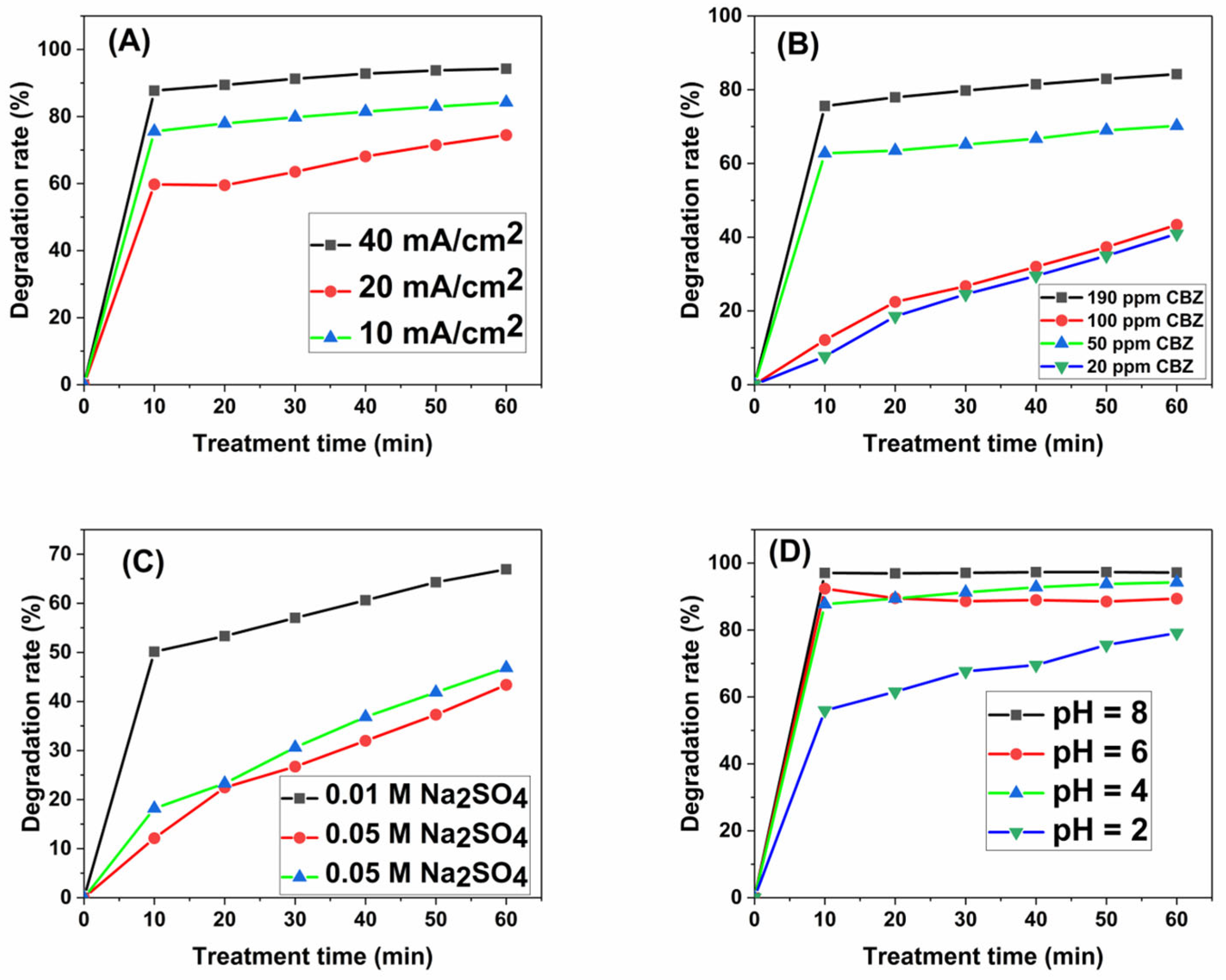
3.4.1. Effect of Applied Current Density
3.4.2. Effect of Initial Concentration of CBZ
3.4.3. Effect of Supporting Electrolyte Concentration
3.4.4. Effect of Initial pH Value
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Merel, S.; Benzing, S.; Gleiser, C.; Di Napoli-Davis, G.; Zwiener, C. Occurrence and Overlooked Sources of the Biocide Carbendazim in Wastewater and Surface Water. Environ. Pollut. 2018, 239, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, N.; Kumar, V.; Datta, S.; Wani, A.B.; Singh, D.; Singh, K.; Singh, J. Toxicity, Monitoring and Biodegradation of the Fungicide Carbendazim. Environ. Chem. Lett. 2016, 14, 317–329. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Shi, X.-C.; Liu, F.-Q.; Laborda, P. Chromatographic Methods for Detection and Quantification of Carbendazim in Food. J. Agric. Food Chem. 2020, 68, 11880–11894. [Google Scholar] [CrossRef]
- Wang, D.; Yang, G.; Yun, X.; Luo, T.; Guo, H.; Pan, L.; Du, W.; Wang, Y.; Wang, Q.; Wang, P.; et al. Carbendazim Residue in Plant-Based Foods in China: Consecutive Surveys from 2011 to 2020. Environ. Sci. Ecotechnology 2024, 17, 100301. [Google Scholar] [CrossRef] [PubMed]
- Regulation-396/2005-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2005/396/oj (accessed on 25 July 2024).
- Zhou, T.; Guo, T.; Wang, Y.; Wang, A.; Zhang, M. Carbendazim: Ecological Risks, Toxicities, Degradation Pathways and Potential Risks to Human Health. Chemosphere 2023, 314, 137723. [Google Scholar] [CrossRef]
- Marković, M.; Jović, M.; Stanković, D.; Kovačević, V.; Roglić, G.; Gojgić-Cvijović, G.; Manojlović, D. Application of Non-Thermal Plasma Reactor and Fenton Reaction for Degradation of Ibuprofen. Sci. Total Environ. 2015, 505, 1148–1155. [Google Scholar] [CrossRef]
- Marković, A.; Savić, S.; Kukuruzar, A.; Konya, Z.; Manojlović, D.; Ognjanović, M.; Stanković, D.M. Differently Prepared PbO2/Graphitic Carbon Nitride Composites for Efficient Electrochemical Removal of Reactive Black 5 Dye. Catalysts 2023, 13, 328. [Google Scholar] [CrossRef]
- Jović, M.; Manojlović, D.; Stanković, D.; Dojčinović, B.; Obradović, B.; Gašić, U.; Roglić, G. Degradation of Triketone Herbicides, Mesotrione and Sulcotrione, Using Advanced Oxidation Processes. J. Hazard. Mater. 2013, 260, 1092–1099. [Google Scholar] [CrossRef]
- Savić, B.G.; Stanković, D.M.; Živković, S.M.; Ognjanović, M.R.; Tasić, G.S.; Mihajlović, I.J.; Brdarić, T.P. Electrochemical Oxidation of a Complex Mixture of Phenolic Compounds in the Base Media Using PbO2-GNRs Anodes. Appl. Surf. Sci. 2020, 529, 147120. [Google Scholar] [CrossRef]
- Temgoua, R.C.T.; Bussy, U.; Alvarez-Dorta, D.; Galland, N.; Hémez, J.; Thobie-Gautier, C.; Tonlé, I.K.; Boujtita, M. Using Electrochemistry Coupled to High Resolution Mass Spectrometry for the Simulation of the Environmental Degradation of the Recalcitrant Fungicide Carbendazim. Talanta 2021, 221, 121448. [Google Scholar] [CrossRef]
- Vlahović, F.; Ognjanović, M.; Djurdjić, S.; Kukuruzar, A.; Antić, B.; Dojčinović, B.; Stanković, D. Design of an Ethidium Bromide Control Circuit Supported by Deep Theoretical Insight. Appl. Catal. B Environ. 2023, 334, 122819. [Google Scholar] [CrossRef]
- Bian, X.; Xia, Y.; Zhan, T.; Wang, L.; Zhou, W.; Dai, Q.; Chen, J. Electrochemical Removal of Amoxicillin Using a Cu Doped PbO2 Electrode: Electrode Characterization, Operational Parameters Optimization and Degradation Mechanism. Chemosphere 2019, 233, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, Y.; Dai, Q. Electrochemical Degradation of Chloramphenicol with a Novel Al Doped PbO2 Electrode: Performance, Kinetics and Degradation Mechanism. Electrochimica Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- Dai, Q.; Xia, Y.; Sun, C.; Weng, M.; Chen, J.; Wang, J.; Chen, J. Electrochemical Degradation of Levodopa with Modified PbO2 Electrode: Parameter Optimization and Degradation Mechanism. Chem. Eng. J. 2014, 245, 359–366. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- da Costa, E.P.; Bottrel, S.E.C.; Starling, M.C.V.M.; Leão, M.M.D.; Amorim, C.C. Degradation of Carbendazim in Water via Photo-Fenton in Raceway Pond Reactor: Assessment of Acute Toxicity and Transformation Products. Environ. Sci. Pollut. Res. 2018, 26, 4324–4336. [Google Scholar] [CrossRef]
- Jović, M.; Stanković, D.; Manojlović, D.; Anđelković, I.; Milić, A.; Dojčinović, B.; Roglić, G. Study of the Electrochemical Oxidation of Reactive Textile Dyes Using Platinum Electrode. Int. J. Electrochem. Sci. 2013, 8, 16. [Google Scholar] [CrossRef]
- Bouya, H.; Errami, M.; Chakir, A.; Hammouti, B. Comparison of BDD and SnO2 Electrodes for Carbendazim Electro-Oxidation. Chem. Sci. Rev. Lett. 2014, 3, 151–158. [Google Scholar]
- Popov, N.; Ristić, M.; Bošković, M.; Perović, M.; Musić, S.; Stanković, D.; Krehula, S. Influence of Sn Doping on the Structural, Magnetic, Optical and Photocatalytic Properties of Hematite (α-Fe2O3) Nanoparticles. J. Phys. Chem. Solids 2022, 161, 110372. [Google Scholar] [CrossRef]
- Popov, N.; Krehula, S.; Ristić, M.; Kuzmann, E.; Homonnay, Z.; Bošković, M.; Stanković, D.; Kubuki, S.; Musić, S. Influence of Cr Doping on the Structural, Magnetic, Optical and Photocatalytic Properties of α-Fe2O3 Nanorods. J. Phys. Chem. Solids 2021, 148, 109699. [Google Scholar] [CrossRef]
- Andjelkovic, I.; Stankovic, D.; Nesic, J.; Krstic, J.; Vulic, P.; Manojlovic, D.; Roglic, G. Fe Doped TiO2 Prepared by Microwave-Assisted Hydrothermal Process for Removal of As(III) and As(V) from Water. Ind. Eng. Chem. Res. 2014, 53, 10841–10848. [Google Scholar] [CrossRef]
- Manojlović, D.; Lelek, K.; Roglić, G.; Zherebtsov, D.; Avdin, V.; Buskina, K.; Sakthidharan, C.; Sapozhnikov, S.; Samodurova, M.; Zakirov, R.; et al. Efficiency of Homely Synthesized Magnetite: Carbon Composite Anode toward Decolorization of Reactive Textile Dyes. Int. J. Environ. Sci. Technol. 2020, 17, 2455–2462. [Google Scholar] [CrossRef]
- Stanković, D.M.; Kukuruzar, A.; Savić, S.; Ognjanović, M.; Janković-Častvan, I.M.; Roglić, G.; Antić, B.; Manojlović, D.; Dojčinović, B. Sponge-like Europium Oxide from Hollow Carbon Sphere as a Template for an Anode Material for Reactive Blue 52 Electrochemical Degradation. Mater. Chem. Phys. 2021, 273, 125154. [Google Scholar] [CrossRef]
- Wang, K.; Xing, X.; Liu, W.; Jiang, Y.; Li, H.; Lu, Y.; Chen, H.; Ren, H. Fabrication of a Novel PbO2 Electrode with Rare Earth Elements Doping for p-Nitrophenol Degradation. J. Environ. Chem. Eng. 2023, 11, 109513. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Zeng, W.; Gong, Y. Luminescence and Storage Properties of Sm-Doped Alkaline-Earth Atannates—IOPscience. J. Electrochem. Soc. 2011, 158, J305. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Xia, X.; Fan, R.; Su, T.; Shi, Y.; Yu, J.; Li, L.; Jiang, Y. Band Edge Movement in Dye Sensitized Sm-Doped TiO2 Solar Cells: A Study by Variable Temperature Spectroelectrochemistry. RSC Adv. 2015, 5, 70512–70521. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Li, C.; Li, J.; He, H.; Li, Y.; Li, W. Hydrothermal Sm-Doped Tungsten Oxide Vertically Plate-like Array Photoelectrode and Its Enhanced Photoelectrocatalytic Efficiency for Degradation of Organic Dyes. J. Mater. Sci. Mater. Electron. 2016, 28, 4004–4013. [Google Scholar] [CrossRef]
- Wan, Y.; Xie, X.; Zhou, S.; Li, W.; Ma, J.; Zhou, Y.; Song, Y.; Zhou, J.; Pan, A. Sm Doping-Enhanced Li3VO4/C Electrode Kinetics for High-Performance Lithium-Ion Batteries. ACS Appl. Energy Mater. 2025, 8, 3581–3591. [Google Scholar] [CrossRef]
- Bibi, I.; Alrowaily, A.W.; Alotaibi, B.M.; Alyousef, H.A.; Alotiby, M.F.; Dahshan, A.; Ahmad, K.; Saleem, M. Sol–Gel Fabrication of Sm-Doped MnTiO3 Perovskite Electrode for Enhanced Oxygen Evaluation Reaction. Appl. Organomet. Chem. 2025, 39, e7850. [Google Scholar] [CrossRef]
- Narsimulu, D.; Rao, B.N.; Nagaraju, G.; Yu, J.S.; Satyanarayana, N. Enhanced Energy Storage Performance of Nanocrystalline Sm-Doped CoFe2O4 as an Effective Anode Material for Li-Ion Battery Applications. J. Solid State Electrochem. 2020, 24, 225–236. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Rakočević, L.; Lazarević, S.; Šljukić, B. Porous Cerium-Zeolite Bifunctional ORR/OER Electrocatalysts in Alkaline Media. J. Electroanal. Chem. 2023, 944, 117668. [Google Scholar] [CrossRef]
- Milikić, J.; Knežević, S.; Ognjanović, M.; Stanković, D.; Rakočević, L.; Šljukić, B. Template-Based Synthesis of Co3O4 and Co3O4/SnO2 Bifunctional Catalysts with Enhanced Electrocatalytic Properties for Reversible Oxygen Evolution and Reduction Reaction. Int. J. Hydrog Energy 2023, 48, 27568–27581. [Google Scholar] [CrossRef]
- Andrić, S.; Milikić, J.; Sevim, M.; Santos, D.M.F.; Šljukić, B. Frontiers | Effect of Carbon Support on the Activity of Monodisperse Co45Pt55 Nanoparticles for Oxygen Evolution in Alkaline Media. Front. Chem. 2023, 11, 1244148. [Google Scholar] [CrossRef] [PubMed]
- Milikić, J.; Balčiūnaitė, A.; Sukackienė, Z.; Mladenović, D.; Santos, D.M.F.; Tamašauskaitė-Tamašiūnaitė, L.; Šljukić, B. Bimetallic Co-Based (CoM, M = Mo, Fe, Mn) Coatings for High-Efficiency Water Splitting. Materials 2020, 14, 92. [Google Scholar] [CrossRef]
- Milikić, J.; Stojanović, S.; Damjanović-Vasilić, L.; Vasilić, R.; Šljukić, B. Efficient Bifunctional Cerium-Zeolite Electrocatalysts for Oxygen Evolution and Oxygen Reduction Reactions in Alkaline Media. Synth. Met. 2023, 292, 117231. [Google Scholar] [CrossRef]
- Milikić, J.; Vasić, M.; Amaral, L.; Cvjetićanin, N.; Jugović, D.; Hercigonja, R.; Šljukić, B. NiA and NiX Zeolites as Bifunctional Electrocatalysts for Water Splitting in Alkaline Media. Int. J. Hydrog Energy 2018, 43, 18977–18991. [Google Scholar] [CrossRef]
- Gusmão, F.M.B.; Đurić, T.; Milikić, J.; Radinović, K.; Santos, D.M.F.; Stanković, D.; Šljukić, B. Transition Metal Polyoxometalates with Reduced Graphene Oxide for High-Performance Air-Electrode of Metal-Air Batteries. Int. J. Hydrog Energy 2024, 71, 763–774. [Google Scholar] [CrossRef]
- Duan, X.; Ma, F.; Yuan, Z.; Chang, L.; Jin, X. Comparative Studies on the Electro-Catalytic Oxidation Performance of Surfactant–Carbon Nanotube-Modified PbO2 Electrodes. J. Electroanal. Chem. 2012, 677–680, 90–100. [Google Scholar] [CrossRef]
- Parr, R.G.; Weitao, Y. Density-Functional Theory of Atoms and Molecules; Oxford University Press: Oxford, UK, 1994. [Google Scholar]
- Koch, W.; Max, C. Holthausen A Chemist’s Guide to Density Functional Theory, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- te Velde, G.; Bickelhaupt, F.M.; Baerends, E.J.; Fonseca Guerra, C.; van Gisbergen, S.J.A.; Snijders, J.G.; Ziegler, T. Chemistry with ADF. J. Comput. Chem. 2001, 22, 931–967. [Google Scholar] [CrossRef]
- te Velde, G.; Baerends, E.J. Precise Density-Functional Method for Periodic Structures. Phys. Rev. B 1991, 44, 7888–7903. [Google Scholar] [CrossRef]
- Philipsen, H.T.; Velde, G.T.; Baerends, E.J.; Berger, J.A.; de Boeij, P.L.; Franchini, M.; Groeneveld, J.A.; Kadantsev, E.S.; Klooster, R.; Kootstra, F.; et al. BAND 2024.1, SCM, Theoretical Chemistry; Vrije Universiteit: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.; Burke, K. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef] [PubMed]
- Kadantsev, E.S.; Klooster, R.; De Boeij, P.L.; Ziegler, T. The Formulation and Implementation of Analytic Energy Gradients for Periodic Density Functional Calculations with STO/NAO Bloch Basis Set. Mol. Phys. 2007, 105, 2583–2596. [Google Scholar] [CrossRef]
- Cuyacot, B.J.R.; Novotný, J.; Berger, R.J.F.; Komorovsky, S.; Marek, R. Relativistic Spin–Orbit Electronegativity and the Chemical Bond Between a Heavy Atom and a Light Atom. Chem.—Eur. J. 2022, 28, e202200277. [Google Scholar] [CrossRef]
- Raupach, M.; Tonner, R. A Periodic Energy Decomposition Analysis Method for the Investigation of Chemical Bonding in Extended Systems. J. Chem. Phys. 2015, 142, 194105. [Google Scholar] [CrossRef]
- Mitoraj, M.P.; Michalak, A.; Ziegler, T. A Combined Charge and Energy Decomposition Scheme for Bond Analysis. J. Chem. Theory Comput. 2009, 5, 962–975. [Google Scholar] [CrossRef]
- Mitoraj, M.; Michalak, A. Applications of Natural Orbitals for Chemical Valence in a Description of Bonding in Conjugated Molecules. J. Mol. Model. 2008, 14, 681–687. [Google Scholar] [CrossRef]
- Costantini, I.; Lottici, P.P.; Bersani, D.; Pontiroli, D.; Casoli, A.; Castro, K.; Madariaga, J.M. Darkening of Lead- and Iron-Based Pigments on Late Gothic Italian Wall Paintings: Energy Dispersive X-Ray Fluorescence, μ-Raman, and Powder X-Ray Diffraction Analyses for Diagnosis: Presence of β-PbO2 (Plattnerite) and α-PbO2 (Scrutinyite). J. Raman Spectrosc. 2020, 51, 680–692. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman Spectra of Titanium Dioxide. J. Solid State Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Dan, Y.; Lu, H.; Liu, X.; Lin, H.; Zhao, J. Ti/PbO2 + Nano-Co3O4 Composite Electrode Material for Electrocatalysis of O2 Evolution in Alkaline Solution. Int. J. Hydrog Energy 2011, 36, 1949–1954. [Google Scholar] [CrossRef]
- Comisso, N.; Armelao, L.; Cattarin, S.; Fasolin, S.; Mattarozzi, L.; Musiani, M.; Rancan, M.; Vázquez-Gómez, L.; Verlato, E. Deposition of FeOOH Layers onto Porous PbO2 by Galvanic Displacement and Their Use as Electrocatalysts for Oxygen Evolution Reaction. J. Electroanal. Chem. 2021, 880, 114844. [Google Scholar] [CrossRef]
- Shamsi, F.; Rezaei, M. Anodic Electrodeposition of PbO2 on ATO/Ti with Simultaneous Doping of F, Co, and Fe as Super-Hydrophilic, Highly Active, and Durable Electrocatalyst for Oxygen Evolution Reaction in Acidic Solution. Colloids Surf. Physicochem. Eng. Asp. 2023, 670, 131608. [Google Scholar] [CrossRef]
- Kong, J.; Shi, S.; Kong, L.; Zhu, X.; Ni, J. Preparation and Characterization of PbO2 Electrodes Doped with Different Rare Earth Oxides. Electrochimica Acta 2007, 53, 2048–2054. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Jia, L.; Li, C.; Liu, H.; Wang, S.; Zhou, S.; Dong, F. Ti/PbO2-Sm2O3 Composite Based Electrode for Highly Efficient Electrocatalytic Degradation of Alizarin Yellow R. J. Colloid Interface Sci. 2019, 533, 750–761. [Google Scholar] [CrossRef]
- Gržeta, B.; Tkalčec, E.; Goebbert, C.; Takeda, M.; Takahashi, M.; Nomura, K.; Jakší, M. Structural Studies of Nanocrystalline SnO2 Doped with Antimony: XRD and Mössbauer Spectroscopy. J. Phys. Chem. Solids 2002, 63, 765–772. [Google Scholar] [CrossRef]
- Akgul, F.A.; Gumus, C.; Er, A.O.; Farha, A.H.; Akgul, G.; Ufuktepe, Y.; Liu, Z. Structural and Electronic Properties of SnO2. J. Alloys Compd. 2013, 579, 50–56. [Google Scholar] [CrossRef]
- Tigau, N.; Ciupina, V.; Prodan, G.; Rusu, G.I.; Vasile, E. Structural Characterization of Polycrystalline Sb2O3 Thin Films Prepared by Thermal Vacuum Evaporation Technique. J. Cryst. Growth 2004, 269, 392–400. [Google Scholar] [CrossRef]
- Ghosh, P.; Kundu, S.; Kar, A.; Ramanujachary, K.V.; Lofland, S.; Patra, A. Synthesis and Characterization of Different Shaped Sm2O3 Nanocrystals. J. Phys. Appl. Phys. 2010, 43, 405401. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Yanagi, G.; Furukawa, M.; Tateishi, I.; Katsumata, H.; Kaneco, S. Optimization of Operating Conditions for Electrochemical Decolorization of Methylene Blue with Ti/α-PbO2/β-PbO2 Composite Electrode. J. Compos. Sci. 2021, 5, 117. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Chen, K.; Yu, X.; Sun, F.; Wang, M.; Cheng, Z.; Zhang, J.; Niu, Q.J. Effects of PbO2/Pb3O4 Ratio Alteration for Enhanced Electrochemical Advanced Oxidation Performance. J. Solid State Chem. 2021, 301, 122277. [Google Scholar] [CrossRef]
- Nenadović, S.S.S.; Kljajević, L.M.; Ivanović, M.M.; Mirković, M.M.; Radmilović, N.; Rakočević, L.Z.; Nenadović, M.T. Structural and Chemical Properties of Geopolymer Gels Incorporated with Neodymium and Samarium. Gels 2021, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, D.O.; Kehoe, A.B.; Watson, G.W.; Jones, M.O.; David, W.I.F.; Payne, D.J.; Egdell, R.G.; Edwards, P.P.; Walsh, A. Nature of the Band Gap and Origin of the Conductivity of PbO2 Revealed by Theory and Experiment. Phys. Rev. Lett. 2011, 107, 246402. [Google Scholar] [CrossRef]
- Payne, D.J.; Paolicelli, G.; Offi, F.; Panaccione, G.; Lacovig, P.; Beamson, G.; Fondacaro, A.; Monaco, G.; Vanko, G.; Egdell, R.G. A Study of Core and Valence Levels in β-PbO2 by Hard X-Ray Photoemission. J. Electron Spectrosc. Relat. Phenom. 2009, 169, 26–34. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Jiang, W.; Chen, C.; Wei, J.; Yu, B.; Chen, B.; Xu, R.; Yang, L. MnCo2O4 Decorating Porous PbO2 Composite with Enhanced Activity and Durability for Acidic Water Oxidation. Fuel 2023, 338, 127344. [Google Scholar] [CrossRef]
- Yang, H.; Liang, J.; Zhang, L.; Liang, Z. Electrochemical Oxidation Degradation of Methyl Orange Wastewater by Nb/PbO2 Electrode. Int. J. Electrochem. Sci. 2016, 11, 1121–1134. [Google Scholar] [CrossRef]
- Man, S.; Luo, D.; Sun, Q.; Yang, H.; Bao, H.; Xu, K.; Zeng, X.; He, M.; Yin, Z.; Wang, L.; et al. When MXene (Ti3C2Tx) Meet Ti/PbO2: An Improved Electrocatalytic Activity and Stability. J. Hazard. Mater. 2022, 430, 1–14. [Google Scholar] [CrossRef]
- Lyu, J.; Han, H.; Wu, Q.; Ma, H.; Ma, C.; Dong, X.; Fu, Y. Enhancement of the Electrocatalytic Oxidation of Dyeing Wastewater (Reactive Brilliant Blue KN-R) over the Ce-Modified Ti-PbO2 Electrode with Surface Hydrophobicity. J. Solid State Electrochem. 2019, 23, 847–859. [Google Scholar] [CrossRef]
- Sun, L.; Zhou, L.; Yang, C.; Yuan, Y. CeO2 Nanoparticle-Decorated Reduced Graphene Oxide as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution Reactions. Int. J. Hydrog Energy 2017, 42, 15140–15148. [Google Scholar] [CrossRef]
- Milikić, J.; Fuentes, R.O.; Tasca, J.E.; Santos, D.M.F.; Šljukić, B.; Figueiredo, F.M.L. Nickel-Doped Ceria Bifunctional Electrocatalysts for Oxygen Reduction and Evolution in Alkaline Media. Batteries 2022, 8, 100. [Google Scholar] [CrossRef]
- Kapałka, A.; Fóti, G.; Comninellis, C. Determination of the Tafel Slope for Oxygen Evolution on Boron-Doped Diamond Electrodes. Electrochem. Commun. 2008, 10, 607–610. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Jiang, L.; Xu, W.; Lv, X.; Li, J.; Liu, Y. Electrochemical Behaviors of Co-Deposited Pb/Pb-MnO2 Composite Anode in Sulfuric Acid Solution—Tafel and EIS Investigations. J. Electroanal. Chem. 2012, 671, 16–23. [Google Scholar] [CrossRef]
- Mladenović, D.; Daş, E.; Santos, D.M.F.; Bayrakçeken Yurtcan, A.; Šljukić, B. Highly Efficient Oxygen Electrode Obtained by Sequential Deposition of Transition Metal-Platinum Alloys on Graphene Nanoplatelets. Materials 2023, 16, 3388. [Google Scholar] [CrossRef] [PubMed]
- Omari, E.; Omari, M. Cu-Doped GdFeO3 Perovskites as Electrocatalysts for the Oxygen Evolution Reaction in Alkaline Media. Int. J. Hydrog Energy 2019, 44, 28769–28779. [Google Scholar] [CrossRef]
- Duan, X.; Ma, F.; Yuan, Z.; Chang, L.; Jin, X. Electrochemical Degradation of Phenol in Aqueous Solution Using PbO2 Anode. J. Taiwan Inst. Chem. Eng. 2013, 44, 95–102. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, K.; Han, W.; Wang, L.; Sun, X.; Li, J. Electrochemical Degradation of Tricyclazole in Aqueous Solution Using Ti/SnO2–Sb/PbO2 Anode. J. Electroanal. Chem. 2013, 705, 68–74. [Google Scholar] [CrossRef]
- Yao, Y.; Li, M.; Yang, Y.; Cui, L.; Guo, L. Electrochemical Degradation of Insecticide Hexazinone with Bi-Doped PbO2 Electrode: Influencing Factors, Intermediates and Degradation Mechanism. Chemosphere 2019, 216, 812–822. [Google Scholar] [CrossRef]
- Yang, Y.; Xia, Y.; Wei, F.; Zhang, L.; Yao, Y. Electrochemical Oxidation of the Pesticide Nitenpyram Using a Gd-PbO2 Anode: Operation Parameter Optimization and Degradation Mechanism. J. Chem. Technol. Biotechnol. 2020, 95, 2120–2128. [Google Scholar] [CrossRef]
- Yao, Y.; Teng, G.; Yang, Y.; Huang, C.; Liu, B.; Guo, L. Electrochemical Oxidation of Acetamiprid Using Yb-Doped PbO2 Electrodes: Electrode Characterization, Influencing Factors and Degradation Pathways. Sep. Purif. Technol. 2019, 211, 456–466. [Google Scholar] [CrossRef]

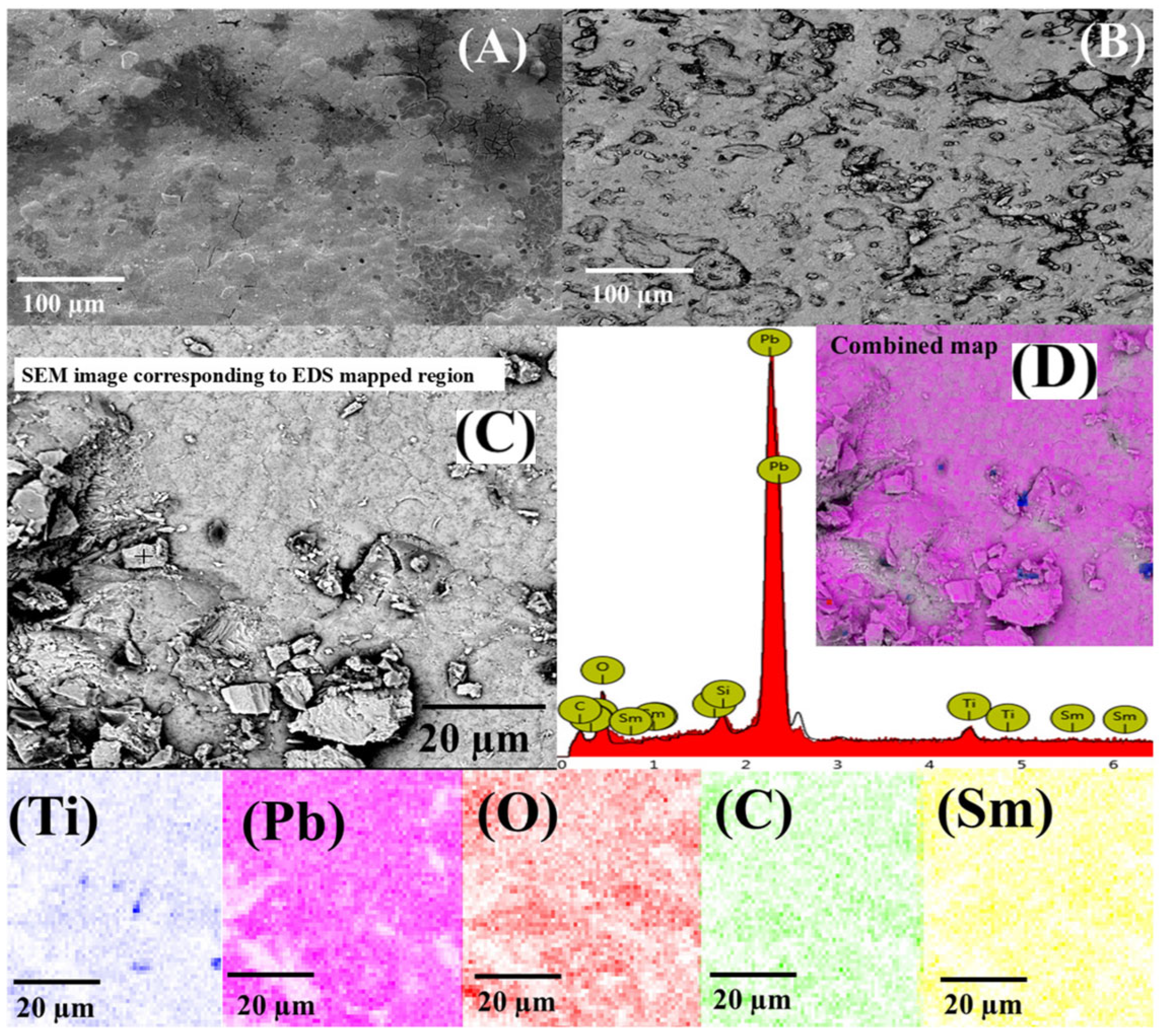



| Element | Atomic Concentration (%) | Weight Concentration (wt %) |
|---|---|---|
| Pb | 37.96 ± 2.99 | 87.88 ± 3.33 |
| O | 40.98 ± 3.52 | 7.33 ± 1.06 |
| C | 17.75 ± 2.01 | 2.38 ± 0.24 |
| Sm | 0.68 ± 0.31 | 1.14 ± 0.51 |
| Ti | 2.03 ± 1.23 | 1.08 ± 0.66 |
| Si | 0.60 ± 0.18 | 0.19 ± 0.05 |
| Electrocatalyst | Rs (Ω) | Re (Ω) | Rct (Ω) | Qe (mF) | Qdl (mF) |
|---|---|---|---|---|---|
| Ti/Sb-SnO2/PbO2 | 7.2 | 0.18 | 16.5 | 2.4 × 10−3 | 3.8 × 10−3 |
| Ti/Sb-SnO2/Sm-PbO2 | 8.4 | 1.05 | 20.5 | 3.3 × 10−3 | 9.2 × 10−3 |
| Electrode | Electrolyte | Analyte | Applied Current Ma 1 | Degradation Rate % | Treatment Time Min | Reference |
|---|---|---|---|---|---|---|
| Ti/Sb-SnO2/Sm-PbO2 | Na2SO4 | Carbendazim | 40 | 94.2 | 60 | This work |
| BDD | NaCl | Carbendazim | 80 | 62.5 | 180 | [19] |
| Ti/SnO2–Sb2O3/Bi-PbO2 | Na2SO4 | Hexazinone | 120 | 97.9 | 120 | [78] |
| Ti/SnO2–Sb/Yb-PbO2 | Na2SO4 | Acetamiprid | 120 | 97.9 | 120 | [79] |
| Ti/Sb-SnO2/PbO2 | Na2SO4 | Hexazinone | 120 | 78.1 | 120 | [80] |
| Gd-PbO2 | Na2SO4 | Nitenpyram | 70 | 95.4 | 120 | [81] |
| Ti/Sb-SnO2/PbO2 | Na2SO4 | Phenol | 50 | 72 | 60 | [82] |
| Ti/Sn–SbOx/Al–PbO2 | Na2SO4 | Chloramphenicol | 30 | 87.3 | 150 | [14] |
| Ti/Sn–SbOx/La–PbO2 | Na2SO4 | Levodopa | 50 | 87.3 | 120 | [15] |
| Ti/Sn–SbOx/La-Gd–PbO2 | Na2SO4 | Levodopa | 50 | 100 | 120 | [15] |
| Ti/Sn–SbOx/Gd–PbO2 | Na2SO4 | Levodopa | 50 | 93.2 | 120 | [15] |
| Cu–PbO2 | Na2SO4 | Amoxicillin | 30 | 99.4 | 150 | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaluđerović, M.; Savić, S.; Bajuk-Bogdanović, D.; Jovanović, A.Z.; Rakočević, L.; Vlahović, F.; Milikić, J.; Stanković, D. Samarium-Doped PbO2 Electrocatalysts for Environmental and Energy Applications: Theoretical Insight into the Mechanisms of Action Underlying Their Carbendazim Degradation and OER Properties. Processes 2025, 13, 1459. https://doi.org/10.3390/pr13051459
Kaluđerović M, Savić S, Bajuk-Bogdanović D, Jovanović AZ, Rakočević L, Vlahović F, Milikić J, Stanković D. Samarium-Doped PbO2 Electrocatalysts for Environmental and Energy Applications: Theoretical Insight into the Mechanisms of Action Underlying Their Carbendazim Degradation and OER Properties. Processes. 2025; 13(5):1459. https://doi.org/10.3390/pr13051459
Chicago/Turabian StyleKaluđerović, Milica, Slađana Savić, Danica Bajuk-Bogdanović, Aleksandar Z. Jovanović, Lazar Rakočević, Filip Vlahović, Jadranka Milikić, and Dalibor Stanković. 2025. "Samarium-Doped PbO2 Electrocatalysts for Environmental and Energy Applications: Theoretical Insight into the Mechanisms of Action Underlying Their Carbendazim Degradation and OER Properties" Processes 13, no. 5: 1459. https://doi.org/10.3390/pr13051459
APA StyleKaluđerović, M., Savić, S., Bajuk-Bogdanović, D., Jovanović, A. Z., Rakočević, L., Vlahović, F., Milikić, J., & Stanković, D. (2025). Samarium-Doped PbO2 Electrocatalysts for Environmental and Energy Applications: Theoretical Insight into the Mechanisms of Action Underlying Their Carbendazim Degradation and OER Properties. Processes, 13(5), 1459. https://doi.org/10.3390/pr13051459









