Abstract
This study evaluated the efficacy of active packaging containing albumin (ALB) and pectin (PEC) cryogels loaded with pink pepper essential oil in preserving strawberries during 7 days of storage at 4 °C. The cryogels were prepared in different ratios (ALB:PEC 50:50 and 30:70) and applied in sachets within the strawberry packaging, varying the amounts from 0.4 g to 1.0 g. Analyses included the evaluation of mass loss, soluble solids, pH, titratable acidity, color, firmness, anthocyanin content, volatile compound composition and release from the essential oil (GC-FID and GC-MS), and microbiological analyses. Results showed that the cryogels maintained the stability of soluble solids and pH, and did not significantly affect the color or anthocyanin content. Strawberry firmness was influenced by the amount of cryogel, with 0.4 g of the ALB:PEC 30:70 cryogel’s best-preserving texture. A GC-MS analysis identified monoterpenes (α-pinene, 3-carene, and D-limonene) and sesquiterpenes (caryophyllene and germacrene D) as the major volatile compounds of the essential oil, with a controlled release over time. Cryogels, especially ALB:PEC 30:70, reduced the count of mesophilic aerobic bacteria (1 g) and yeasts and molds (0.4 g). This formulation extends shelf life and preserves the quality of strawberries through controlled antimicrobial release and firmness preservation.
1. Introduction
The United Nations General Assembly declared the International Year of Fruits and Vegetables as a strategy to raise awareness about their nutritional and health benefits [1]. Fruits are a natural source of bioactive compounds and macro- and micronutrients [2]. Consumers especially appreciate the strawberry (Fragaria x ananassa) due to its sensory properties, such as flavor, color, and texture. Furthermore, it is rich in carbohydrates (7.68 g/100 g), proteins (0.67 g/100 g), fats (0.30 g/100 g), and dietary fiber, the latter being beneficial for the digestive system [3]. It also contains anthocyanins, which are known for their antitumor, neuroprotective, and anti-inflammatory activity [3,4]. However, the strawberry is a non-climacteric fruit with a post-harvest time of two to three days, it possesses a high respiration rate, undergoes significant mass loss, and is susceptible to fungal attack, which results in a significant loss of its quality [5,6].
Active packaging has emerged as a promising alternative for fruit preservation. These systems can incorporate antimicrobial agents, such as silver ions, silver oxides, copper or zinc, and TiO2 nanoparticles. However, due to the potentially harmful effects on human health, there is a growing demand for antimicrobial substances of natural origins [7]. For this reason, essential oils have gained interest as a potential option to act as natural antifungal agents. Their chemical composition, characterized by the presence of alcohols, phenols, terpenes, and other bioactive compounds [7], along with their volatile nature, allow them to interact with the product’s surface, reduce microbial growth, and extend the shelf life of fruits and vegetables [8,9,10]. One approach to using active packaging with essential oils has been the design of the coating. For example, Iqbal et al. [11] developed edible coatings from hydroxypropyl cellulose (HMPC), beeswax, and essential oils of thyme, cinnamon, and mint for cherry preservation. These coatings demonstrated antibacterial and antifungal activity, maintaining the physicochemical attributes of the fruits.
On the other hand, Wigati et al. [12] used jicama starch and agarwood essential oil coatings on strawberries. They reported that the coatings maintained color parameters and the bioactive compound content for 16 days of refrigerated storage. Frazão et al. [13] showed that applying cassava starch, chitosan, and Myrcia ovata Cambessedes essential oil coatings helped control microbial growth in mangabas during storage. Researchers have also studied the effect of coatings in the post-harvest treatment of tomatoes stored at room temperature, using a coating prepared with arrowroot starch, beeswax, and clove essential oil, which resulted in an extended shelf life of 48 days, compared to 20 days for uncoated samples [14]. However, using coatings can cause changes in the sensory characteristics of the fruit’s surface [15].
For this reason, researchers have explored encapsulating essential oils and applying them as indirect additives, which overcomes the difficulty presented by coatings [16]. Researchers implemented mint and lime essential oil encapsulation in mangosteen preservation, leveraging their antifungal activity and ability to delay ripening [17]. Pinto et al. [18] evaluated the effect of red thyme oil on fungal development and the quality and shelf-life parameters of oranges stored at 7 °C for 12 days, showing that the essential oil significantly reduced spore production and did not affect quality parameters such as color, mass loss, pH, and ascorbic acid content. They also used carvacrol, oregano, and cinnamon essential oils in a 70:10:20 ratio, encapsulated in β-cyclodextrin inclusion complexes, and applied in cardboard boxes to preserve mandarins, which allowed for a one-week increase in shelf life [16]. Locali-Pereira et al. [15] encapsulated pink pepper oil emulsions. They applied them to polyethylene terephthalate (PET) boxes as a thin coating or within sachets for the post-harvest storage of cherry tomatoes. In our study, we used pink pepper oil (Schinus terebinthifolius Raddi) due to its antioxidant, antimicrobial, and insecticidal properties [19], which Dannenberg et al. [20] associated with the presence of monoterpenes such as α-pinene, myrcene, and limonene. In a previous study, we encapsulated pink pepper essential oil in albumin and low-methoxyl amidated pectin cryogels. We observed the effectiveness of this wall material in encapsulating the essential oil [21]. This characteristic is attractive for application in active packaging, allowing us to leverage the oil’s potential antimicrobial and antioxidant activity. Chaux-Gutiérrez et al. [21] focused on determining the encapsulation capacity of the wall material and verifying the antimicrobial activity of pink peppercorn oil against Staphylococcus aureus, Escherichia coli, and Bacillus cereus using the agar diffusion method, total phenolic content, and antioxidant activity by UV-vis spectrophotometry. However, it is still necessary to explore its effectiveness against spoilage microorganisms such as mesophilic aerobic bacteria, molds, and yeasts and to examine its effect on the physical and chemical characteristics during the storage of strawberries. For this reason, this study aimed to apply pink pepper essential oil-loaded cryogels in active packaging to evaluate their effect on strawberries’ physicochemical and microbiological characteristics during storage.
2. Materials and Methods
Strawberries (Fragaria x ananassa) cv. “San Andreas” were purchased from the local market of São Jose do Rio Preto (São Paulo, Brazil). Pink pepper (Schinus terebinthifolius Raddi) essential oil extracted from fruits was obtained from Ferquima (Vargem Grande Paulista, São Paulo, Brazil). Powdered egg albumin (ALB) was purchased from Neovita Foods Eirelli (São Paulo, São Paulo, Brazil; 83.3% protein, 5.0% carbohydrates, and 0.0% fat). Amidated low-methoxyl pectin (PEC) was purchased from Danisco (GRINDSTED LA 210; 34% esterification degree and 17% amidation, Barueri, São Paulo, Brazil). Sodium hydroxide, calcium chloride, potassium chloride, sodium acetate, and hydrochloric acid were obtained from Êxodo Científica (Sumaré, Sao Paulo, Brazil). Phenolthalein, methanol, and acetone were purchased from Synth (Diadema, São Paulo, Brazil). Mueller–Hinton agar, plate count agar (PCA) for the enumeration of aerobic mesophilic bacteria and potato dextrose agar (PDA), and Peptone water phosphate buffer (HiMedia, Sumaré, São Paulo, Brazil) were purchased. The n-alkanes (C7-C30) standard mixture and hexane (≥97%) were provided by Sigma-Aldrich® (St. Louis, MO, USA). All reagents were analytical grade.
2.1. Encapsulation of Pink Pepper Essential Oil in Cryogels
The encapsulation of pink pepper essential oil was performed following the methodology proposed by Chaux-Gutiérrez et al. [22] and Volić et al. [23], with some modifications. Dispersions of ALB (5% w/w, based on the total mass of the dispersion) and PEC (5% w/w, based on the total mass of the dispersion) were prepared separately at room temperature with constant stirring for 3 h to ensure complete dispersion, using a magnetic stirrer. To prepare the ALB:PEC hydrogels, the ALB dispersion was adjusted to a pH of 8 using 0.1 M HCl and 0.1 M NaOH solutions. The mixture was then heated to 85 °C and stirred continuously for 15 min before cooling to 40 °C. The dispersion was mixed at different ALB:PEC ratios of 50:50 and 30:70 while maintaining constant stirring for 5 min. Then, 3% (w/w, based on the total mass of the dispersion) of pink pepper essential oil was added, and the mixture was homogenized at 14,000 rpm for 5 min using an Ultra-Turrax® IKA T25 (IKA-Werke GmbH, Staufen, Baden-Württemberg, Germany). A calcium chloride solution (2% w/v) was prepared and added to the mixture, which was maintained under constant stirring for 10 min. The resulting mixture was stored in a Petri dish at 4 °C until gel formation. Finally, the gels were frozen at −18 °C for 24 h and then freeze-dried (model L-101, Liotop, São Carlos, São Paulo, Brazil) at 40 µmHg for 48 h to obtain cryogels. The lyophilized samples were stored in metallized bags within desiccators at 25 °C.
2.2. Application of Cryogels Loaded with Pink Pepper Oil in Strawberry Packaging
This study evaluated the effects of active packaging containing pink pepper essential oil encapsulated in cryogels on soluble solids, titratable acidity, pH, color, firmness, and microbiological analyses of strawberries stored for 7 days at 4 °C. Samples were collected for physicochemical characterization at 0, 3, 5, and 7 days storage. Previously, the strawberries were manually selected under aseptic laboratory conditions using nitrile gloves to minimize cross-contamination. Fruits were chosen based on uniformity in size, an average weight of 15 g, ripeness (intense red coloration), and the absence of physical damage or microbial decay. We did not apply chemical washing or disinfection, as distributors often transport unwashed fruits to avoid deterioration caused by moisture. Approximately 100 g of strawberries were stored in thermoformed poly(ethylene terephthalate) boxes (12 cm (length) × 10.3 cm (width) × 4.5 cm (height)). The cryogel-to-strawberry mass ratios were maintained at 1:250 (0.4 g cryogel:100 g fruit) or 1:100 (1.0 g cryogel:100 g fruit). Lyophilized cryogel particles containing pink pepper oil were placed in small fabric sachets at the bottom of the packages, using amounts of 0.4 g and 1.0 g. The boxes (two per sampling period) were stored in a BOD chamber (411D, New Ethics, EtickTechnology, Vargem Grande Paulista, SP, Brazil) at 4 °C. Control samples were also stored without cryogel throughout the storage period.
2.2.1. Weight Loss
Mass loss was calculated using Equation (1) [8]. The analyses were carried out in triplicate.
where .
2.2.2. Soluble Solids, pH, and Titratable Acidity
Total soluble solids were determined using a refractometer (Atago, Minato, Tokyo, Japan). The pH measurements were performed with a calibrated pH meter using standard buffer solutions (pH 4.0 and 7.00) (Akso, São Leopoldo, Rio Grande do Sul, Brazil) at 25 °C. To determine titratable acidity, 2 g of homogenized strawberries was diluted in 20 mL of distilled water. This mixture was then titrated with a 0.1 M NaOH solution, using 0.1% phenolphthalein as an indicator [24]. The analyses were carried out in triplicate.
2.2.3. Color
The color of the strawberry was determined using a ColorFlex model 45/0 spectrophotometer (Hunterlab, Reston, VA, USA) with the D65 illuminant and observer at 10º. The 4.10 version universal software was used to determine the absolute values of L*, a*, and b*. The chroma, which expresses the degree of intensity or saturation of the color (Equation (2)); and Hue angle, which represents the tonality of the color (Equation (3)); and ∆E, the total color difference from the standard sample (Equation (4)), were also calculated. The results were expressed as the average of twelve replicates for each treatment.
2.2.4. Anthocyanin Content
Anthocyanin extraction was conducted using an aqueous solution of methanol and acetone in a 2:2:1 (v/v) ratio, which was acidified with 0.1% formic acid. The monomeric anthocyanin content was determined using the pH-differential spectrophotometric method. The pH was adjusted to 1.0 and 4.5 with potassium chloride buffer (0.025 M) and sodium acetate buffer (0.04 M), respectively. Absorbance was measured at 520 nm and 700 nm using a UV/Vis spectrophotometer (UV-3000, Shanghai Mapada Instruments Co., Ltd., Shanghai, China) [25]. The analyses were carried out in triplicate.
2.2.5. Firmness
Fruit firmness was measured using a texture analyzer (TA.XT/Plus/50) equipped with a cylindrical plunger probe. The results were expressed in Newtons (N) [26]. The results were expressed as the average of twelve replicates for each treatment.
2.3. GC-FID and GC-MS Analysis
The quantification of compounds present in pink pepper essential oil and their respective content within the cryogels was conducted using a gas chromatograph equipped with a flame ionization detector (GC-FID) (GC-2014, Shimadzu, Japan). An Rtx-5 silica capillary column (30 m × 0.25 mm × 0.25 µm) was used, with hydrogen as the carrier gas at a 1 mL/min flow rate. Gas chromatography coupled with mass spectrometry (GC-MS QP2010 SE, Shimadzu, Japan) was used to identify volatile compounds. An Rtx-5MS-fused silica capillary column (30 m × 0.25 mm × 0.25 μm) was employed, using helium as the carrier gas at a constant flow rate of 1 mL/min. The same chromatographic parameters applied in GC-FID were also used for GC-MS. The interface and ionization source temperatures were set at 240 °C and 230 °C, respectively, with electron impact ionization at 70 eV and a mass range of 35 to 350 m/z. Peak identification was performed by comparing experimental mass spectral with NIST MS Search version 2.0. A standard mixture of alkanes (C7–C30) (Sigma–Aldrich®) was diluted 1:2 in hexane and injected into both GC-FID and GC-MS under identical conditions to calculate the linear retention index. The experimental retention index of each volatile compound was compared with the retention index described in the literature on a column of the same polarity [27,28,29]. The analyses were carried out in triplicate.
Release of Volatile Compounds
The percentage of release (Release (%)) of pink pepper essential oil during the storage of strawberries (for 7 days) was determined according to Equation (5) [15]. The analyses were carried out in triplicate.
where and .
2.4. Microbiological Analyses
The total counts of aerobic mesophilic bacteria and yeasts and molds (log CFU/g) in the stored strawberries were determined following the APHA 08:2015 guidelines [30] and methodology of Siquiera [31]. Twenty-five grams of fruit, packaged with cryogels and without cryogels (control), was diluted in 225 mL of peptone water and homogenized under aseptic conditions. Serial dilutions of the prepared suspension were then performed. One hundred microliters of each sample was transferred to plates containing plate count agar (PCA) for the enumeration of aerobic mesophilic bacteria and potato dextrose agar (PDA) for the enumeration of yeasts and molds. Finally, the PCA plates were incubated at 35 °C for 24 h, while the PDA plates were incubated at 25 °C for 5 days. The analyses were carried out in triplicate.
2.5. Statistical Analyses
The analyses were carried out in triplicate. All measurements were performed in triplicate, and the results were expressed as mean ± standard deviation. Statistical analyses were conducted using a one-way analysis of variance (ANOVA) in the Minitab 21® program, with a significance level set at 5%. Tukey’s test was used to compare differences among the mean values of the samples.
3. Results and Discussion
3.1. Weight Loss and Physicochemical Properties
Table 1 presents the weight loss of strawberries packaged with cryogels at ALB:PEC ratios of 50:50 and 30:70, along with the control, stored for 7 days at 4 °C and 65–70% RH. There were no significant differences in weight loss among the different cryogel types (50:50 and 30:70) or the different cryogel quantities (1 g and 0.4 g). A significant difference in weight loss was observed between the control and the treatments over time (p < 0.05). The control exhibited the highest weight loss in 7 days. This difference indicates that the release of pink pepper oil generates a hydrophobic barrier that limits moisture loss and reduces transpiration. Oliveira Filho et al. [32] conducted a similar study with strawberries using Cymbopogon martini and Mentha spicata essential oils. According to Fu et al. [33], the use of active packaging with chitosan/pectin-based multi-active films containing epigallocatechin gallate reduced the weight loss of strawberries by 30% after 12 days of storage at 5 °C and 90% relative humidity. This behavior was like that of Locali-Pereira et al. [26], who also evidenced a lower mass loss in tomatoes stored in active packaging with pink pepper essential oil.

Table 1.
Quality parameters of strawberries stored at 4 °C for 7 days.
In a similar study with tangerines, López-Gómez et al. [16] found that the use of active packaging with encapsulated essential oils resulted in a weight loss of only 2% after 42 days of refrigerated storage, while the control and gelatin-based coating showed losses of 8% and 6%, respectively. However, it is important to highlight that Owolabi et al. [17] did not find significant changes in the mass loss of Mangosteen (Garcinia mangostana) when using peppermint oil and lime oil in active packaging, which could indicate that effectiveness depends on the specific interaction between the essential oil, the type of packaging, and the type of fruit. Guo et al. [34] demonstrated that the incorporation of punicalagin into films based on soy protein isolate and apple pectin at a concentration of 0.5% resulted in a weight loss of only 5.3% in strawberries stored at 25 °C for 7 days, maintaining an edibility rate of 93.3%. Takahashi et al. [35] developed an edible coating based on mung bean starch (MBS) and citric acid (CA) containing terpinene-4-ol (TP4O), a component of tea tree oil with antimicrobial properties. They observed that the 0.5% concentration of TP4O in the MBS/CA coating resulted in a significant increase in the weight loss of strawberries, while the 0.25% concentration had no significant effect. These results emphasize the importance of the ideal concentration of the compounds released by the cryogel to be used in active packaging, as a very high concentration can adversely affect weight loss.
Regarding soluble solids, a progressive decrease in soluble solids content was observed in the control over time, which is typical of the ripening process and the possible degradation of sugars in the fruits. The cryogels, in general, showed a positive effect in conserving soluble solids, as the content remained relatively stable over time compared to the control. These results suggest that cryogels could be more effective in preserving the sweetness and quality of the fruits. At 7 days, there was a significant difference in soluble solids content between the control group, the cryogels with ALB:PEC 30:70, and the treatment with ALB:PEC 50:50. This highlights the capacity that the cryogel ALB:PEC 50:50 (0.4 g) showed, especially to maintain the quality and preserve the sweetness of the fruits over a more extended storage period. In a similar study with tangerines, López-Gómez et al. [16] found that the use of active packaging with encapsulated essential oils resulted in the maintenance of higher levels of total soluble solids (12 °Brix) after 42 days of refrigerated storage, compared to the control groups (10 °Brix) and gelatin-based coating (11 °Brix). These results indicate that encapsulated essential oils can contribute to the preservation of sugars in fruits, an important factor in maintaining the sensory and nutritional qualities.
The pH data reveal that, in general, the control exhibited a slight decrease in pH over time, which is the typical behavior of fruits during ripening due to the accumulation of organic acids from fungal growth that acidify the fruit as it decomposes [32]. In contrast, the fruits in contact with the cryogels ALB:PEC 50:50 or 30:70 showed a more stable pH, similar to the initial pH. Titratable acidity (TA) did not present differences among treatments and storage time. The lack of significant changes in titratable acidity is consistent with the observation that pH remained relatively stable across all treatments. This result suggests that the cryogels do not interfere with ripening processes involving organic acid production. The stability of titratable acidity and the stability of pH and soluble solids suggest that cryogels help preserve the overall quality of the fruits during storage. These results contrast with the meta-analysis results of Taban et al. [36], who reported that the addition of essential oils to edible coatings increased the chromaticity (27.7%) and titratable acidity (21.8%), while the addition of extracts increased the retention of firmness (90.3%) and weight (39.4%).
3.2. Color Parameters of Stored Strawberries
Figure 1 shows the strawberries used in stability analyses during the 4 °C storage period. Lightness (L*) varied over time in all treatments, including the control. There was no significant change in luminosity in the control group and the treatments. The strawberries’ redness intensity (a*) among treatments and the control did not show significant differences (Table 2). On the last day (day 7), only the ALB:PEC (0.4 g) 50:50 treatment showed a significantly lower redness intensity (a*) compared to the control group. The other treatments did not differ significantly from the control on this day (Table 2).
Figure 1.
Appearance of strawberries during storage time with and without cryogel incorporation.

Table 2.
Color parameters of strawberries stored in active packaging using cryogels loaded with pink pepper oil.
Regarding yellow intensity (b*), it was observed that the treatments had a significant effect on this parameter. The significant differences in yellow intensity (b*) between the control and the treatments occurred on days 5 and 7, mainly with the ALB:PEC (0.4 g) 50:50 treatment. However, a variable trend in the b* value was observed over time in the different treatments, regardless of the application of cryogels (Table 2). Cryogels may have had a punctual effect on the yellow intensity at some points, but there was no consistent and significant effect throughout the entire storage period. Chroma represents the intensity or saturation of color. The higher the chroma, the more vivid and intense the color. Lower values indicate opaque colors. The ALB:PEC (0.4 g) 50:50 treatment stands out for presenting the lowest chroma values on days 3, 5, and 7, suggesting that this specific treatment may have affected the color intensity of the strawberries. Significant variations in the hue angle occurred over time in all treatments, indicating that the hue (strawberry hue) naturally changes during storage. There were significant punctual differences between treatments on some days; for example, the hue differed significantly between the control and the 30:70 ALB:PEC treatment on day 5, but without a consistent pattern that related the presence of the cryogel to the hue parameter.
Conversely, when other conservation methodologies were used, such as that reported by Hernández-Carillo et al. [37] for strawberries coated with edible coatings made from pectin and lemon essential oil, the chroma parameter decreased with increasing storage time, which was related to the degradation of anthocyanins and fungal growth. The control sample exhibited the highest ΔE values on days 3 and 5 (Table 2). The data for the different combinations of wall material ALB: PEC 50:50 and 30:70 show that the coloration of the strawberries naturally changed during storage (7 days), as expected in perishable products. However, cryogels significantly reduced color degradation compared to the control, suggesting that cryogels can retard physical degradation processes in strawberries, and delay color changes during ripening [32]. The data suggest that the cryogel did not cause drastic and consistent changes in the color parameters of the strawberries over time. The variations observed in the treatments also occurred in the control group, indicating that they were more related to the natural ripening process. Color is important in post-harvest strawberries, as it directly influences their quality and consumer perception. This result is promising, as it suggests the feasibility of applying cryogels as active packaging for fruit preservation.
3.3. Anthocyanin Content
Regarding the anthocyanin content in the strawberries (Table 3), it was observed that all samples did not show significant changes in the levels of these bioactive compounds during storage. It is important to highlight that the presence of cryogels loaded with pink pepper essential oil did not interfere with the anthocyanin concentration in the fruits, indicating that the active packaging technology does not harm the bioactive compounds present in the strawberry. A similar result was reported by Oliveira et al. [32], who coated strawberries with an edible film from arrowroot starch, nanocrystal cellulose, carnauba wax nanoemulsion, and essential oils of Cymbopogon martini, and Mentha spicata. They found no changes in the anthocyanin content of the strawberries after 12 days of storage at refrigeration temperature.

Table 3.
Anthocyanin content in strawberries stored in active packaging using cryogels loaded with pink pepper oil.
3.4. Firmness Analysis
The evaluation of the cryogel mass used is a crucial factor to ensure the effectiveness of active packaging. Low quantities may not provide the desired barrier. On the other hand, high quantities can lead to adverse effects, such as changes in the sensory properties or texture of the packaged product. In the case of strawberries, firmness plays an important role in consumer acceptance. Figure 2 presents the evolution of firmness in strawberries subjected to different treatments over 7 days of storage at 4 °C.
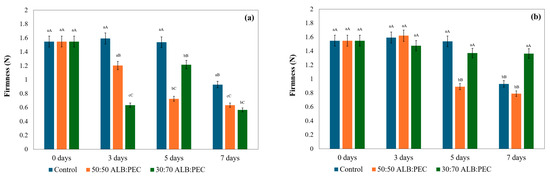
Figure 2.
Firmness of strawberries stored in active packaging using cryogels loaded with pink pepper oil using 1 g of cryogel (a) and 0.4 g of cryogel (b). Different superscript lowercase letters (within each row) show differences between treatment groups within the same analysis day (p < 0.05). Different superscript uppercase letters (within each column) show differences between the storage time within the same analysis group (p < 0.05).
The presence of the cryogel in active packaging influenced this characteristic (Figure 2). Although the firmness of the strawberries decreased with increasing storage time, the use of 1 g of cryogel with encapsulated pink pepper oil caused an even more marked reduction in this parameter compared to the control sample. The results suggest that cryogels, especially at a concentration of 1 g, may affect firmness in the initial days of storage (Figure 2).
In the first 3 days, the 1 g cryogel treatments (50:50 and 30:70) exhibited significantly lower firmness than the control group. Their effect was more significant on firmness than the control on day 7. In the case of 30:70 cryogels with 0.4 g, the firmness remained relatively stable over the 7 days of storage, with values ranging from 1.365 ± 0.559 to 1.549 ± 0.410 N. Meanwhile, the 50:50 cryogel treatment with 0.4 g did not show significant differences with the control group on day 7. The preservation of firmness with the use of 30:70 cryogel and 0.4 g is an important indicator of the potential of pink pepper in strawberry conservation, and the quantity used plays an important role in maintaining the structural integrity of the tissue. Fu et al. [33] reported similar results: they observed that applying active packaging with multi-active films containing epigallocatechin gallate maintained the firmness and color of strawberries during storage, positively affecting these quality parameters. López-Gómez et al. [16] reported that the firmness of tangerines was significantly higher in the groups with encapsulated essential oils (25 N) after 42 days of storage, compared to the control group (18 N) and the gelatin-based coating group (20 N).
3.5. Identification and Release of Volatile Compounds from Pink Pepper Oil
The chromatographic analysis showed that the major volatile compounds of pink pepper essential oil were monoterpenes such as α-pinene, α-phellandrene, 3-carene, o-cymene, and D-limonene (Table 4). In addition to monoterpenes, sesquiterpenes were identified, with caryophyllene and germacrene D being the most prominent (Table 4), as also reported by Dannenberg et al. [20]. The proportions of the identified compounds can vary depending on factors such as the plant’s origin, the method of essential oil extraction, and the analysis conditions.

Table 4.
Volatile compounds of pink pepper oil encapsulated in ALB:PEC 50:50 and 30:70 cryogels.
The composition of the pink pepper essential oil encapsulated in albumin and pectin (ALB:PEC) cryogels at 50:50 and 30:70 ratios showed some variations compared to pure essential oil. In the 50:50, cryogel had the following distributions of major monoterpenes: α-phellandrene (37.53%), D-limonene (19.40%), 3-carene (16.85%), o-cymene (8.99%), and α-pinene (8.81%). These five compounds together represented 91% of the total monoterpenes, sesquiterpenes, Caryophyllene, and germacrene D, showing 3.57% and 3.15%, respectively. These sesquiterpenes suggest that the albumin–pectin wall material also allowed the encapsulation of less volatile and higher molecular weight compounds. Furthermore, there were some differences between the composition of the oil encapsulated in the two cryogels. Caryophyllene was found in a higher proportion in the 30:70 cryogel (6.11%) than in the 50:50 cryogel (3.57%). The proportion of minor monoterpenes such as β-phellan drene, β-pinene, β-myrcene, and 2-carene remained relatively constant between the 30:70 and 50:50 cryogels (Table 4). The variations in the distributions of the components were associated with factors such as the interaction with the matrix components (albumin and pectin), the encapsulation process conditions, and the possible loss of some compounds during lyophilization. The composition of the essential oil remained similar in the cryogels, which suggests that the encapsulation process did not drastically alter the composition of the volatile compounds.
3.6. Release of Volatile Compounds
Table 5 shows the release profile of the main compounds of pink pepper essential oil during storage. Regarding the ALB:PEC 50:50 cryogel, α-pinene showed a gradual and sustained release over time, reaching 18.7% in 7 days. Furthermore, 3-carene and o-cymene showed a gradual and sustained release, reaching 3.8% and 3.2% in 7 days, respectively. While α-phellandrene was the most abundant component initially (37.53%), its release was remarkably low (0.8% at 7 days). Similarly, D-limonene showed a slow and gradual release (0.1% at 7 days). Several compounds showed a more gradual release over time, including β-pinene, reaching a 4.3% release, and 2-carene at 5.4%. However, 4-carene, despite being one of the least abundant components initially, had a remarkably high and rapid release, reaching 59.9% in 7 days. Regarding sesquiterpenes, Caryophyllene showed a rapid and continuous release, reaching 52.2%, and germacrene D reached a 54.4% release in 7 days. The ALB:PEC 30:70 cryogel results showed that α-pinene reached a release of 19.9% in 7 days. Alpha-phellandrene, being the most abundant component initially with an area of 37.17%, showed a low release of 0.5% in 7 days. Furthermore, 3-carene had a release of 9.4%. Meanwhile, D-limonene showed a very slow and gradual release, reaching 4.8% in 7 days. Regarding sesquiterpenes, Caryophyllene and germacrene D showed a rapid and continuous release, reaching 76.8% and 62.5% in 7 days, respectively. In general, the release of monoterpenes and sesquiterpenes was higher in the ALB:PEC 30:70 cryogel than in the 50:50. The gradual and sustained release observed for α-pinene, 3-carene, and D-limonene reasonably indicates their greater availability as antimicrobial agents, and they could be mainly responsible for the inhibitory effects, consequently, for a more significant antimicrobial potential and increased shelf life.

Table 5.
Percentage of volatile compound release from pepper essential oil (Schinus terebinthifolius Raddi) encapsulated in cryogel ALB:PEC 50:50 and 30:70 cryogel during storage for 7 days at 4 °C.
3.7. Microbiological Analysis
3.7.1. Mesophilic Aerobic Bacteria
At the beginning of storage (time zero), all samples, including the control and the ALB:PEC 50:50 and ALB:PEC 30:70 treatments, both 1 g and 0.4 g, showed a uniform initial count of 2.27 log CFU/g of mesophilic aerobic bacteria. After 7 days of storage (4 °C), the control showed a count of 3.20 log CFU/g. The cryogels showed ALB:PEC 50:50 (1 g) 2.48 log CFU/g and ALB:PEC 50:50 (0.4 g) 2.98 log CFU/g. The cryogel with the ALB:PEC 30:70 ratio showed (1 g) 2.00 log CFU/g and ALB:PEC 30:70 (0.4 g) 2.70 log CFU/g. The results at 7 days showed the significant effect of the treatments. The cryogels, especially with a mass of 1 g, significantly reduced the count of mesophilic aerobic bacteria compared to the control. The ALB:PEC 30:70 cryogel with 1 g had the lowest count of mesophilic aerobes. The difference between the 1 g and 0.4 g concentrations highlights the importance of a cryogel dosage in microbial inhibition efficacy (Figure 3). The transient increase in mesophilic aerobic bacteria for ALB:PEC 50:50 and 30:70 (0.4 g) at day 5 (Figure 3b) may reflect sublethal stress responses induced by the release of lower molecular weight compounds, which temporarily disrupt microbial membranes without immediate lethality, triggering stress adaptation pathways [38,39,40]. This phase may coincide with the nutrient release from lysed cells, providing residual substrates for surviving populations. However, the delayed release kinetics of less volatile and higher molecular weight compounds from the cryogel matrix ensured progressive antimicrobial accumulation, leading to eventual inhibition (days 5–7).
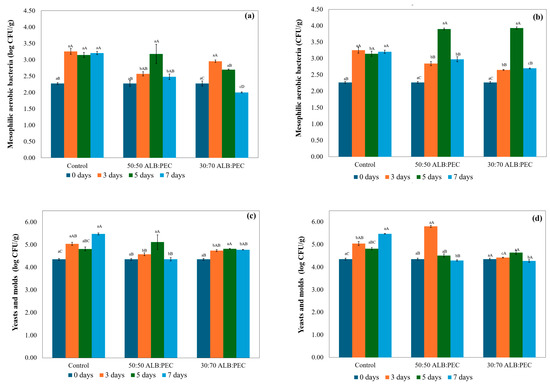
Figure 3.
Microbiological analysis of mesophilic aerobic bacteria in strawberries using 1 g of cryogel (a) and 0.4 g of cryogel (b), and of yeasts and molds using 1 g of cryogel (c) and 0.4 g of cryogel (d), during storage time. Different superscript lowercase letters (within each row) show differences between treatment groups within the same analysis day (p < 0.05). Different superscript uppercase letters (within each column) show differences between the storage time within the same analysis group (p < 0.05).
3.7.2. Yeasts and Molds
The control showed a gradual increase in mold and yeast growth, from 4.36 log CFU/g at the beginning to 5.48 log CFU/g on the seventh day. The ALB:PEC 30:70 cryogel with a mass of 0.4 g stood out for its greater inhibitory efficacy, reaching 4.28 log CFU/g on the seventh day, showing a count below the initial value, which indicated that in addition to the inhibitory capacity, it had a fungicidal effect. On the other hand, the ALB:PEC 50:50 cryogel, at a mass of 0.4 g, showed an increase in growth on the third day, reaching 5.81 log CFU/g before decreasing to 4.30 log CFU/g on the seventh day (Figure 3). The direct comparison between the masses showed that the 0.4 g dosage using the ALB:PEC 30:70 cryogel was superior in inhibiting yeasts and molds during the 7 days, compared to mesophilic aerobic bacteria, where the 1 g mass offered high inhibition. The more significant inhibitory effect in the ALB:PEC 30:70 cryogel for mesophilic aerobes with a count of 2.00 log CFU/g (1 g), and for yeasts and molds, with 4.28 log CFU/g (0.4 g) in 7 days, was associated with the higher percentage of mono- and sesquiterpenes released, mainly those observed for α-pinene, 3-carene, and D-limonene, along with the sesquiterpenes caryophyllene and germacrene D. The synergy between its different compounds in pink pepper essential oil led to improved antimicrobial activity.
Therefore, a specific combination of mono- and sesquiterpenes may have a more pronounced inhibitory effect than the release of a single compound. The low release of α-phellandrene observed in both treatments suggests a significant interaction with the ALB:PEC matrix, consequently limiting its direct influence on microbial inhibition; its functional efficacy was significantly contingent upon synergistic interactions with other compounds, notably D-limonene. This interplay suggests that the observed antimicrobial potency, as detailed in the microbiological analysis (Figure 3 and Table 5), was not solely attributable to α-phellandrene’s action but rather a result of the combinatorial effect. For instance, the sustained release of α-pinene and 3-carene, as shown in Table 5, correlated with the reduction in mesophilic aerobic bacteria and yeasts and molds observed in Figure 3. These synergistic effects among the released volatile compounds contributed significantly to the overall antimicrobial activity of active packaging. These results agree with those observed also by Celaya et al. [41] and Radice et al. [42]. The microbiological results and the analysis of the compound release profile indicate that cryogels can be effective in the microbiological control of strawberries. Several factors affected the cryogels’ effectiveness, including the ratio of cryogel wall components (ALB:PEC), the type of microorganism, and storage time. In terms of the ratio of wall components, for example, in the case of coatings, Guerreiro et al. [43] showed that specific combinations of coating components can have a more pronounced effect on a specific characteristic; thus, pectin and eugenol-based edible coatings were more effective in preserving the firmness of strawberries stored at 0.5 °C for 14 days, while alginate coatings showed more excellent antioxidant activity and were more effective in reducing microbial growth. However, the results observed with ALB:PEC cryogels indicate that the combination of biopolymers in the cryogel wall material brought advantages, and the optimization of the composition and structure of the cryogels can modulate the release of compounds, and, consequently, antimicrobial activity. The gradual and sustained release of bioactive compounds ensures efficacy over time.
The results of the microbiological study show the efficacy of cryogels in reducing the count of mesophilic aerobic bacteria and molds/yeasts in strawberries, complementing the evidence of antimicrobial activity against S. aureus previously reported [21]. The ALB:PEC 30:70 cryogel also exhibited a remarkable ability to inhibit bacterial growth, requiring only 5 mg to produce significant inhibition zones. The higher proportion of pectin in the cryogel matrix (30:70) appeared to optimize the release of these compounds, enhancing the antimicrobial effect [21].
The results also reinforce the hypothesis that low methoxyl amidated pectin is crucial in modulating essential oil release and configuring an effective delivery system for antimicrobial applications in active packaging. Therefore, the ALB:PEC 30:70 cryogel was the most promising formulation, demonstrating a broad spectrum of antimicrobial activity against Gram-positive bacteria and food spoilage microorganisms. Furthermore, the 30:70 cryogel resulted in less firmness loss, especially at 0.4 g, configuring an effective delivery system for applications in food safety and perishable product conservation.
Pink pepper essential oil, therefore, has antimicrobial properties and exhibits an equivalent or similar effect in inhibiting spoilage microorganisms in strawberries, in agreement with works such as that reported by Oliveira Filho et al. [32], who observed that the incorporation of spearmint and fennel essential oils in edible bio-nanocomposite coatings resulted in the inhibition of microbial growth against mesophilic aerobic bacteria and fungi (including yeasts) in strawberries during refrigerated storage. The effectiveness of pink pepper oil and its application in active packaging also aligns with the results of Wu et al. [44], who used an edible nanofiber film containing cinnamaldehyde and thymol to inhibit microbial growth in strawberries during refrigerated storage. Guo et al. [34] demonstrated that incorporating punicalagin, a compound present in pomegranate peel, into films based on soy protein isolate and apple pectin at a concentration of 0.5% demonstrated antimicrobial activity against S. aureus and E. coli. Gharibzahedi and Altintas [45] developed fennel essential oil (EEO) nanoemulsions with high antioxidant activity, which were effective in preserving the physicochemical quality of strawberries stored at 4 °C for 14 days; the study also demonstrated the inhibition of total aerobic bacteria and mold and yeast growth in strawberries coated with nanoemulsions. Cai et al. [46] developed chitosan and gelatin-based films incorporating curcumin encapsulated in γ-CD-MOFs, which demonstrated effective antimicrobial activity against E. coli and S. aureus, in addition to preserving the sensory quality of strawberries stored at 4 °C for 12 days.
4. Conclusions
The results demonstrated the feasibility of using cryogels composed of albumin and pectin as matrices for encapsulating and releasing pink pepper essential oil for strawberry preservation. The composition of the cryogels, the ALB:PEC 30:70 ratio, significantly influenced the release of volatile compounds and preserved fruit firmness. The observed reduction in strawberry mass loss, along with the stabilization of the evaluated physicochemical parameters, suggests the effectiveness of cryogels in maintaining fruit quality during storage. The ability of cryogels to inhibit the growth of mesophilic aerobic bacteria and mold and yeast reinforces their potential as an active packaging strategy. The influence of cryogel composition on the release rate of these compounds highlights the importance of formulation optimization for specific applications. The ALB:PEC 30:70 cryogel, particularly at a dosage of 0.4 g, in maintaining strawberry firmness and microbial control, indicates the pectin ratio’s importance in the matrix.
Author Contributions
Conceptualization, A.M.C.-G., E.J.P.-M. and V.R.N.; methodology, A.M.C.-G., N.S.J. and M.G.C.; formal analysis, A.M.C.-G. and E.J.P.-M.; investigation, A.M.C.-G., E.J.P.-M., N.S.J. and M.G.C.; resources, V.R.N. and M.R.d.M.; data curation, A.M.C.-G. and E.J.P.-M.; writing—original draft preparation, A.M.C.-G. and E.J.P.-M.; writing—review and editing, A.M.C.-G., E.J.P.-M., V.R.N., M.G.C., F.A.A. and M.R.d.M.; visualization, A.M.C.-G.; supervision, M.R.d.M.; project administration, M.R.d.M.; funding acquisition, A.M.C.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Coordination for the Improvement of Higher Education Personnel (CAPES) and Capes-Print program for a scholarship (code 88887.890935/2023-00).
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed at the corresponding author.
Acknowledgments
The authors are grateful to Jessica Thaís do Prado Silva for the use of the gas chromatograph equipped with a flame ionization detector (GC-FID).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. Fruit and Vegetables—Your Dietary Essentials. The International Year of Fruits and Vegetables, 2021; FAO: Rome, Italy, 2020. [Google Scholar]
- Chen, Y.; Martynenko, A. Combination of Hydrothermodynamic (HTD) Processing and Different Drying Methods for Natural Blueberry Leather. LWT 2018, 87, 470–477. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Jayakumar, A.; de Souza, C.K.; Rhim, J.W.; Kim, J.T. Advances in Strawberry Postharvest Preservation and Packaging: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13417. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Jiang, X.; He, F.; Bai, W. Qualitative and Quantitative Methods to Evaluate Anthocyanins. eFood 2020, 1, 339–346. [Google Scholar] [CrossRef]
- Wang, D.; Shao, S.; Wang, B.; Guo, D.; Tan, L.; Chen, Q. Fabrication of Chitosan/Guar Gum/Polyvinyl Alcohol Films Incorporated with Polymethoxyflavone-Rich Citrus Extracts: Postharvest Shelf-Life Extension of Strawberry Fruits. Prog. Org. Coat. 2024, 194, 108611. [Google Scholar] [CrossRef]
- Priyadarshi, R.; El-Araby, A.; Rhim, J.W. Chitosan-Based Sustainable Packaging and Coating Technologies for Strawberry Preservation: A Review. Int. J. Biol. Macromol. 2024, 278, 134859. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.G.; Ferreira, R.R.; Paula, L.C.; Mitra, S.K.; Rosa, D.S. Starch-Based Films Enriched with Nanocellulose-Stabilized Pickering Emulsions Containing Different Essential Oils for Possible Applications in Food Packaging. Food Packag. Shelf Life 2021, 27, 100615. [Google Scholar] [CrossRef]
- Wigati, L.P.; Wardana, A.A.; Tanaka, F.; Tanaka, F. Strawberry Preservation Using Combination of Yam Bean Starch, Agarwood Aetoxylon Bouya Essential Oil, and Calcium Propionate Edible Coating during Cold Storage Evaluated by TOPSIS-Shannon Entropy. Prog. Org. Coat. 2023, 175, 107347. [Google Scholar] [CrossRef]
- Bodbodak, S.; Rafiee, Z. Recent Trends in Active Packaging in Fruits and Vegetables. In Eco-Friendly Technology for Postharvest Produce Quality; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 77–125. ISBN 9780128043844. [Google Scholar]
- Aguado, R.J.; Saguer, E.; Tarrés, Q.; Fiol, N.; Delgado-Aguilar, M. Antioxidant and Antimicrobial Emulsions with Amphiphilic Olive Extract, Nanocellulose-Stabilized Thyme Oil and Common Salts for Active Paper-Based Packaging. Int. J. Biol. Macromol. 2024, 279, 135110. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Hussain, M.; Ali, H.; Haider, A.; Ali, S.; Hussain, A.; Javed, M.A.; Jawaid, M. Preparation and Application of Hydroxypropyl Methylcellulose Blended with Beeswax and Essential Oil Edible Coating to Enhance the Shelf Life of Sweet Cherries. Int. J. Biol. Macromol. 2024, 272, 132532. [Google Scholar] [CrossRef]
- Wigati, L.P.; Wardana, A.A.; Jothi, J.S.; Leonard, S.; Van, T.T.; Yan, X.; Tanaka, F.; Tanaka, F. Biochemical and Color Stability Preservation of Strawberry Using Edible Coatings Based on Jicama Starch/Calcium Propionate/Agarwood Bouya Essential Oil during Cold Storage. J. Stored Prod. Res. 2024, 107, 102324. [Google Scholar] [CrossRef]
- Frazão, G.G.S.; Blank, A.F.; de Aquino Santana, L.C.L. Optimisation of Edible Chitosan Coatings Formulations Incorporating Myrcia Ovata Cambessedes Essential Oil with Antimicrobial Potential against Foodborne Bacteria and Natural Microflora of Mangaba Fruits. LWT 2017, 79, 1–10. [Google Scholar] [CrossRef]
- Lakshan, N.D.; Senanayake, C.M.; Liyanage, T.; Lankanayaka, A. Clove Essential Oil Emulsions-Loaded Arrowroot Starch-Beeswax-Based Edible Coating Extends the Shelf Life and Preserves the Postharvest Quality of Fresh Tomatoes (Solanum lycopersicum L.) Stored at Room Temperature. Sustain. Food Technol. 2024, 2, 1052–1068. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Lopes, N.A.; Menis-Henrique, M.E.C.; Janzantti, N.S.; Nicoletti, V.R. Modulation of Volatile Release and Antimicrobial Properties of Pink Pepper Essential Oil by Microencapsulation in Single- and Double-Layer Structured Matrices. Int. J. Food Microbiol. 2020, 335, 108890. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, A.; Ros-Chumillas, M.; Buendía-Moreno, L.; Navarro-Segura, L.; Martínez-Hernández, G.B. Active Cardboard Box with Smart Internal Lining Based on Encapsulated Essential Oils for Enhancing the Shelf Life of Fresh Mandarins. Foods 2020, 9, 590. [Google Scholar] [CrossRef]
- Owolabi, I.O.; Songsamoe, S.; Matan, N. Combined Impact of Peppermint Oil and Lime Oil on Mangosteen (Garcinia mangostana) Fruit Ripening and Mold Growth Using Closed System. Postharvest Biol. Technol. 2021, 175, 111488. [Google Scholar] [CrossRef]
- Pinto, L.; Cefola, M.; Bonifacio, M.A.; Cometa, S.; Bocchino, C.; Pace, B.; De Giglio, E.; Palumbo, M.; Sada, A.; Logrieco, A.F.; et al. Effect of Red Thyme Oil (Thymus vulgaris L.) Vapours on Fungal Decay, Quality Parameters and Shelf-Life of Oranges during Cold Storage. Food Chem. 2021, 336, 127590. [Google Scholar] [CrossRef]
- Oliveira, K.C.; Franciscato, L.M.S.S.; Mendes, S.S.; Barizon, F.M.A.; Gonçalves, D.D.; Barbosa, L.N.; Faria, M.G.I.; Valle, J.S.; Casalvara, R.F.A.; Gonçalves, J.E.; et al. Essential Oil from the Leaves, Fruits and Twigs of Schinus Terebinthifolius: Chemical Composition, Antioxidant and Antibacterial Potential. Molecules 2024, 29, 469. [Google Scholar] [CrossRef]
- Dannenberg, S.; Funck, G.D.; Padilha, W.; Fiorentini, Â.M. Essential Oil from Pink Pepper (Schinus terebinthifolius Raddi): Chemical Composition, Antibacterial Activity and Mechanism of Action. Food Control 2019, 95, 115–120. [Google Scholar] [CrossRef]
- Chaux-Gutiérrez, A.M.; Pérez-Monterroza, E.J.; Cattelan, M.G.; Nicoletti, V.R.; Moura, M.R. de Encapsulation of Pink Pepper Essential Oil (Schinus terebinthifolius Raddi) in Albumin and Low-Methoxyl Amidated Pectin Cryogels. Processes 2024, 12, 1681. [Google Scholar] [CrossRef]
- Chaux-Gutiérrez, A.M.; Pérez-Monterroza, E.J.; Granda-Restrepo, D.M.; Mauro, M.A. Cryogels from Albumin and Low Methoxyl Amidated Pectin as a Matrix for Betalain Encapsulation. J Food Process Preserv 2020, 44, 1–10. [Google Scholar] [CrossRef]
- Volić, M.; Pajić-Lijaković, I.; Djordjević, V.; Knežević-Jugović, Z.; Pećinar, I.; Stevanović-Dajić, Z.; Veljović, Đ.; Hadnadjev, M.; Bugarski, B. Alginate/Soy Protein System for Essential Oil Encapsulation with Intestinal Delivery. Carbohydr. Polym. 2018, 200, 15–24. [Google Scholar] [CrossRef] [PubMed]
- AOAC Official Methods of Analysis, 16th ed.; Chemists, Association of Official Agricultural: Washington, DC, USA, 1995.
- Martynenko, A.; Chen, Y. Degradation Kinetics of Total Anthocyanins and Formation of Polymeric Color in Blueberry Hydrothermodynamic (HTD) Processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Locali-Pereira, A.R.; Scarpin Guazi, J.; Conti-Silva, A.C.; Nicoletti, V.R. Active Packaging for Postharvest Storage of Cherry Tomatoes: Different Strategies for Application of Microencapsulated Essential Oil. Food Packag. Shelf Life 2021, 29, 100723. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, D. A Generalization Of The Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Acree, T.E.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 7 March 2025).
- PubChem PubChem Identifier. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 7 March 2025).
- Ryser, E.T.; Schuman, J.D. Mesophilic Aerobic Plate Count. In Compendium of Methods for the Microbiological Examination of Foods; Salfinger, Y., Tortorello, M.L., Eds.; American Public Health Association (APHA): Washington, DC, USA, 2015; pp. 95–101. [Google Scholar]
- de Siqueira, R.S. Manual de Microbiologia de Alimentos; EMBRAPA: Rio de Janeiro, Brazil, 1995. [Google Scholar]
- de Oliveira Filho, J.G.; Albiero, B.R.; Calisto, Í.H.; Bertolo, M.R.V.; Oldoni, F.C.A.; Egea, M.B.; Bogusz Junior, S.; de Azeredo, H.M.C.; Ferreira, M.D. Bio-Nanocomposite Edible Coatings Based on Arrowroot Starch/Cellulose Nanocrystals/Carnauba Wax Nanoemulsion Containing Essential Oils to Preserve Quality and Improve Shelf Life of Strawberry. Int. J. Biol. Macromol. 2022, 219, 812–823. [Google Scholar] [CrossRef]
- Fu, X.; Chang, X.; Xu, S.; Xu, H.; Ge, S.; Xie, Y.; Wang, R.; Xu, Y.; Luo, Z.; Shan, Y.; et al. Development of a Chitosan/Pectin-Based Multi-Active Food Packaging with Both UV and Microbial Defense Functions for Effectively Preserving of Strawberry. Int. J. Biol. Macromol. 2024, 254, 127968. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, A.; Huang, G.; Jin, X.; Xiao, Y.; Gan, R.Y.; Gao, H. Development of Apple Pectin/Soy Protein Isolate-Based Edible Films Containing Punicalagin for Strawberry Preservation. Int. J. Biol. Macromol. 2024, 273, 133111. [Google Scholar] [CrossRef]
- Takahashi, M.; Nkede, F.N.; Tanaka, F.; Tanaka, F. Development and Characterization of Mung Bean Starch and Citric Acid Active Packaging Combined with Terpinen-4-Ol and Its Application to Strawberry Preservation. J. Food Meas. Charact. 2024, 18, 2280–2292. [Google Scholar] [CrossRef]
- Taban, A.; Haghighi, T.M.; Mousavi, S.S.; Sadeghi, H. Are Edible Coatings (with or without Essential Oil/Extract) Game Changers for Maintaining the Postharvest Quality of Strawberries? A Meta-Analysis. Postharvest Biol. Technol. 2024, 216, 113082. [Google Scholar] [CrossRef]
- Hernández-Carrillo, J.G.; Orta-Zavalza, E.; González-Rodríguez, S.E.; Montoya-Torres, C.; Sepúlveda-Ahumada, D.R.; Ortiz-Rivera, Y. Evaluation of the Effectivity of Reuterin in Pectin Edible Coatings to Extend the Shelf-Life of Strawberries during Cold Storage. Food Packag. Shelf Life 2021, 30, 100760. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Celaya, L.S.; Alabrudzińska, M.H.; Molina, A.C.; Viturro, C.I.; Moreno, S. The Inhibition of Methicillin-Resistant Staphylococcus Aureus by Essential Oils Isolated from Leaves and Fruits of Schinus Areira Depending on Their Chemical Compositions. Acta Biochim. Pol. 2014, 1, 41–46. [Google Scholar] [CrossRef]
- Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life 2022, 12, 1602. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The Use of Polysaccharide-Based Edible Coatings Enriched with Essential Oils to Improve Shelf-Life of Strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; He, S.; Liu, J.; Shao, W. Development of an Edible Food Packaging Gelatin/Zein Based Nanofiber Film for the Shelf-Life Extension of Strawberries. Food Chem. 2023, 426, 136652. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Altintas, Z. Eryngo Essential Oil Nanoemulsion Stabilized by Sonicated-Insect Protein Isolate: An Innovative Edible Coating for Strawberry Quality and Shelf-Life Extension. Food Chem. 2025, 463, 141150. [Google Scholar] [CrossRef]
- Cai, X.; Chen, L.; Yang, X.; Wang, Y.; Xu, J.; Zhang, R.; Ling, S.; Liu, Y. Active Curcumin-Loaded γ-Cyclodextrin-Metal Organic Frameworks as Nano Respiratory Channels for Reinforcing Chitosan/Gelatin Films in Strawberry Preservation. Food Hydrocoll. 2025, 159, 110656. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).