Abstract
Coal resources still occupy a dominant position in the energy consumption structure, and the prevention and control of coal dust explosion has become an important measure to ensure the safe production of coal. To this end, a new type of environmentally friendly, economical, and efficient composite powder explosion suppressant has been developed. CMS@C12H22O14Fe was prepared by an anti-solvent crystallization method using Chinese Maifan stone (CMS) as the carrier and ferrous gluconate (C12H22O14Fe) as the active component. The physicochemical properties of the explosion suppressant were analyzed using characterization techniques such as SEM and FT-IR. At the same time, the Hartmann tube experimental device was utilized to study the inhibition effect of the detonation suppressor on the coal powder flame, and to determine the optimal loading amount of the active component and the addition amount of the detonation suppressor. The results show that the composite powder synthesized by the anti-solvent crystallization method has a uniform particle size and good structure. The flame was almost completely suppressed when the active component loading was 50 wt.% and the additive amount of the detonation suppressant was 30 wt.%. Finally, a physicochemical synergistic inhibition mechanism of CMS@C12H22O14Fe for coal dust explosion is proposed.
1. Introduction
In recent years, coal dust explosions have occurred frequently. For instance, in 2020, a coal dust explosion took place at the Liang Baosi coal mine, resulting in seven deaths and nine injuries; in 2024, a coal dust explosion occurred at the Zao Quan coal mine in Ningxia, with one death and six injuries; and in 2021, a domestic coal mine in Colombia experienced a gas and coal dust explosion, causing eleven fatalities. The aforesaid accidents indicate that coal dust explosions remain a serious safety concern that cannot be overlooked globally. The destructive consequence of a coal dust explosion is remarkable, with the explosion temperature capable of reaching 2300 to 2500 °C, the flame propagation rate exceeding 1120 m/s, and the shock wave speed reaching 2340 m/s [1]. Such an explosion will not only result in severe casualties and property losses but also bring about destructive harm to coal mining and transportation facilities. This remains an important factor endangering the safe production of China’s coal mines and coal chemical enterprises, and it significantly restricts the safe and stable development of the coal industry [2,3,4].
As an effective approach for reducing the intensity of the explosion and confining the explosion of coal dust, powder suppressants have garnered extensive attention from scholars both at home and abroad [5,6,7]. Generally, powder suppressants can be classified into two types: a single powder suppressant and composite powder suppressant. Generally speaking, the suppression performance of the composite powder suppressant is superior to that of the single powder suppressant. Yu et al. [8] developed a modified kaolin composite suppressant and studied its explosion suppression performance and mechanism in the coal dust/methane system. Compared to the two monomer inhibitors, the composite inhibitor has the better inhibition effect. Wang et al. [9] explored the surface modification and compounding technology of the detonation inhibitor and developed a PSE coal dust inhibitor, which is composed of potassium chloride, urea, and other components; these demonstrated an outstanding detonation inhibition effect when compared with that of the single detonation inhibitor. Shang et al. [10] investigated the inhibition of aluminum dust explosion by alumina of different particle sizes and compared the explosion suppression performance levels between nanoscale and micron-sized alumina. The results indicated that the explosion suppression effect of nanoscale alumina as a constituent of the composite powder suppressant was superior to that of micron-sized alumina alone. As the composite powder suppressant is easy to store and transport and has a physicochemical dual synergistic effect, its application in coal dust explosion prevention and control is escalating [11,12,13].
Currently, in the research on composite powder detonation inhibitors, the structures and performance levels of new composite powder detonation inhibitors have emerged as research focuses. Dai et al. [14] investigated the urea/zeolite composite detonation inhibitor, and experiments have demonstrated that the inhibition effect is greatest when the ratio is 1:1. Ni et al. [15] fabricated a composite NaHCO3/zeolite nanoparticle fire extinguishing material with a core–shell structure by utilizing porous zeolite as a carrier in conjunction with nanosynthesis technology. Jiang et al. [16] synthesized a composite detonation suppressant with a porous structure of PA@NH2MCM-41, and it was discovered that the composite detonation suppressant containing phosphorus could prominently inhibit the flame spread in dust explosions and the explosion pressure. Yuan et al. [17] investigated a porous mineral (MTS)–ammonium polyphosphate (APP) composite powder’s inhibition effect in a methane–air mixed explosion, and the results indicate that the composite powder’s explosion suppression mechanism encompasses both physical and chemical effects. Chen et al. [18], conversely, fabricated a new type of core–shell structural inhibitor and discovered that the addition of potassium oxalate and ferric citrate could markedly enhance its inhibition performance at a loading level of 30 wt.%. Li et al. [19] synthesized a metal–organic framework (PAPP@Ni-MOF) inhibition material. The propagation of PP dust explosion flame could be entirely suppressed when the addition amount of the suppression material was 50 wt.%. Although various composite powder suppressants have been developed, there are still some problems such as explosion suppression decomposition products polluting the environment, the need to improve performance, and a high cost in practical applications. Hence, the development of new green, high-performance, and low-cost composite powder explosion suppressants has become a key research direction for coal dust explosion suppression powder.
Chinese Maifan stone, classified as a subvolcanic ore, is enriched with over 20 trace elements such as iron, magnesium, potassium, and sodium [20,21], among which some have the potential to inhibit coal dust combustion. The material has a good porous structure and makes a high-quality adsorbent material as its surface is rich in hydroxyl and other groups, which likely have good adsorption properties for combustion-explosive radicals. Hence, it is of great significance to study Chinese Maifan stone and explore its application in the field of coal dust explosion. Meanwhile, as a green bioactive component, ferrous gluconate has been widely used in various fields. It has reached industrial production, and its production cost is low and gradually decreasing.
In this study, the composite powder inhibitor CMS@C12H22O14Fe with different loadings was fabricated by loading ferrous gluconate onto CMS through the anti-solvent crystallization method, with Chinese Maifan stone (CMS) as the carrier and ferrous gluconate (C12H22O14Fe) as the active component. The samples of coal dust before and after adding the inhibitor were characterized by SEM, TG, and so on. The Hartmann device was used to study the flame propagation characteristics. By analyzing the detonation inhibition effect of coal dust under different loadings and additive amounts of the inhibitor, the optimal loadings and additive amounts were studied. Furthermore, based on the characterization results and the coal dust flame propagation process and flame deflagration behavior after adding detonation suppressants, the inhibition mechanism of the new composite powder detonation suppressant in inhibiting the flame propagation of coal dust was revealed.
2. Experiment
2.1. Preparation of Suppressors
2.1.1. Experimental Raw Materials
Ferrous gluconate (C12H22O14Fe, AR, 98%), anhydrous ethanol (AR), and deionized water were procured from Qingdao Jingke Instrument Reagent Co., Ltd., Qingdao, China. Chinese Maifan stone (CMS) was obtained from Pingdingshan, Naimanqi in Tongliao City, Inner Mongolia, China, and lignite was supplied by the Xinjiang coal mine in China. The dried 200-mesh lignite samples underwent industrial analysis and particle size distribution analysis. The results of the industrial analysis are presented in Table 1, and the particle size distribution is depicted in Figure 1. Table 1 indicates that, when using the 5E-MAG6600B industrial analyzer, the moisture (Mad) of lignite is 1.89, the ash (Aad) is 9.07, the fixed carbon content (FCad) is 52.48, and the volatile content (Vad) is 36.56. These contents are important for the assessment of the explosion risk of coal dust; in relatively dry conditions, volatile matter and the fixed carbon content are higher, while conditions of lower ash show significant explosive potential and make it more difficult to suppress the explosion. From the above results, it can be inferred that the Vad and FCad of lignite are extremely high, signifying that lignite is prone to combustion and has a high risk of explosion [22,23]. There is thus significant value in using lignite to test the efficacy of the explosion suppression agent. From Figure 1, it can be seen that the median particle size (D50) of coal dust is 22.713 µm, while the particle size of lignite is less than 100 µm, with 90% of the particles having a size less than 75 µm.

Table 1.
Analysis and particle size distribution data of the lignite industry.
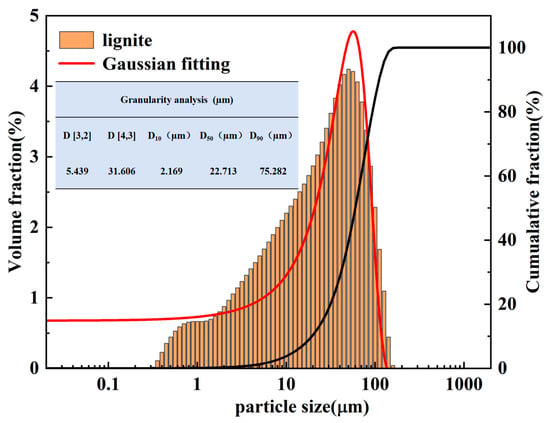
Figure 1.
Particle size distribution of lignite.
2.1.2. Preparation of Suppressor
The CMS was first ground using a 300-mesh sieve and then roasted at 500 °C for 4 h to remove moisture and impurities. The solubility levels of C12H22O14Fe in deionized water and CMS in anhydrous ethanol were analyzed at 20 °C, with the results presented in Table 2. Based on these solubility data, a saturated C12H22O14Fe solution was prepared. The treated CMS was uniformly dispersed in anhydrous ethanol and stirred to form a suspension. The treated CMS served as the carrier, while C12H22O14Fe acted as the active component of the inhibitor. An anti-solvent crystallization method was employed to prepare powder explosive inhibitors (CMS@C12H22O14Fe) with different loadings (10%, 20%, 30%, 40%, 50%, and 60%) [24]. The specific steps were as follows: A saturated C12H22O14Fe solution was added gradually and at a constant rate to the CMS suspension, with uniform stirring throughout to ensure an even dispersion of CMS particles in the solution. The mixture was then allowed to settle, filtered, and dried at 50 °C for 12 h. Finally, the dried solid was ground and sieved to obtain a 200-mesh detonation inhibitor powder. The preparation process is illustrated in Figure 2.

Table 2.
Solubility of CMS and C12H22O14Fe (25 °C).

Figure 2.
Preparation flow chart of CMS@C12H22O14Fe.
The loadings of C12H22O14Fe () are calculated using Equation (1):
where is the mass of C12H22O14Fe (g) and is the mass of CMS (g).
The preparation of CMS@C12H22O14Fe with varying loadings was carried out following the steps outlined above.
The 10% CMS@C12H22O14Fe is labeled as CMS@C12H22O14Fe-10, while the other samples are labeled as CMS@C12H22O14Fe-20, CMS@C12H22O14Fe-30, CMS@C12H22O14Fe-40, CMS@C12H22O14Fe-50, and CMS@C12H22O14Fe-60.
2.2. Characterization and Analysis of Experimental Materials
2.2.1. Particle Size Distribution
The particle size distributions of CMS and CMS@C12H22O14Fe were determined using a laser particle size analyzer. The results are shown in Figure 3, and the particle size statistics are presented in Table 3. As shown in Figure 3, the small CMS particles become larger composite particles as the loading of the active component C12H22O14Fe increases. According to Table 3, the median particle size (D50) of CMS was 19.751 µm, with most particles smaller than 100 µm, and 90% of CMS particles had a size less than 69 µm. The median particle size (D50) of CMS@C12H22O14Fe was 28.258 µm, with most particles smaller than 100 µm, and 90% of CMS@C12H22O14Fe particles had a size less than 77 µm. After loading C12H22O14Fe, the active components are partially coated on the CMS surface and tend to form a certain degree of agglomeration, thus increasing the median diameter (D50) to about 28 µm, which also leads to an increase in D90 to 76.947 µm. The particles increase in size, and the particle size distribution becomes more concentrated, indicating the successful preparation of CMS@C12H22O14Fe.
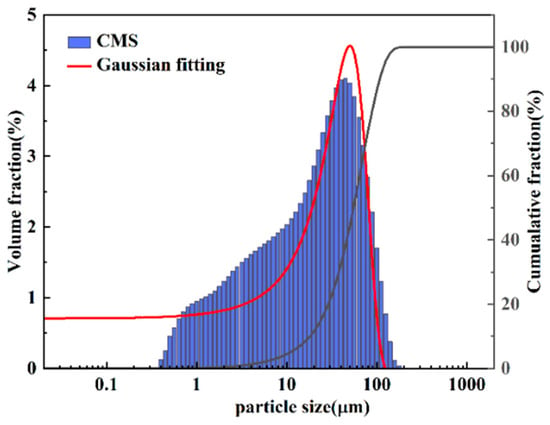
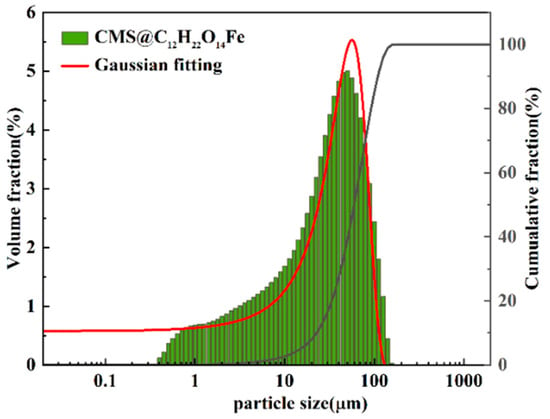
Figure 3.
Particle size distribution of CMS and CMS@C12H22O14Fe.

Table 3.
Granularity distribution data of CMS and CMS@C12H22O14Fe.
2.2.2. Morphological Analysis
Figure 4 presents SEM images of CMS, C12H22O14Fe, and CMS@C12H22O14Fe. CMS exhibits an irregular, blocky shape with a smooth, shiny surface, well-defined angles, and a fine concave–convex structure [25,26]. In contrast, the surface of C12H22O14Fe is relatively rough and exhibits an irregular morphology [27]. CMS@C12H22O14Fe combines the smooth areas of CMS with the rough, irregular surface of C12H22O14Fe, indicating that the active ingredient was successfully loaded on the carrier.
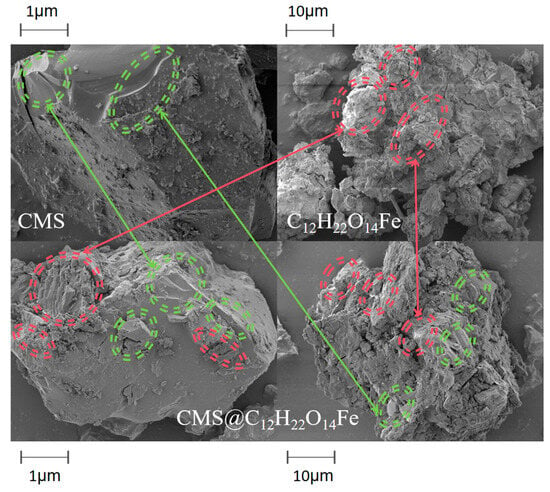
Figure 4.
SEM images of CMS, C12H22O14Fe, and CMS@C12H22O14Fe.
Figure 5 presents SEM images and mapping diagrams of CMS, C12H22O14Fe, and CMS@C12H22O14Fe. CMS exhibits an irregular morphology with a relatively clear hierarchical structure and a smooth surface. In contrast, C12H22O14Fe has a rough and irregular surface. Cracks are observed in the structure of CMS@C12H22O14Fe, with the smooth regions resembling that of CMS and its rough, uneven areas resembling that of C12H22O14Fe. Mapping analysis of the Fe element reveals that CMS contains only trace amounts of Fe, while C12H22O14Fe has a relatively high Fe content. Notably, after loading the active component C12H22O14Fe, the Fe content in CMS@C12H22O14Fe significantly increased and was uniformly distributed. This result further confirms the successful preparation of CMS@C12H22O14Fe.
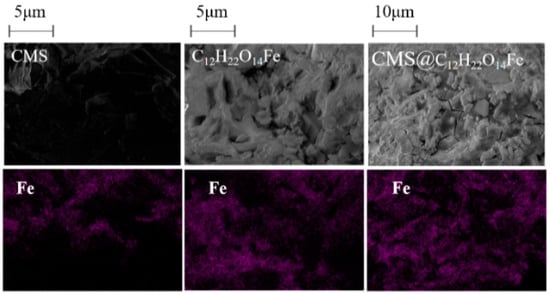
Figure 5.
SEM and mapping images of CMS, C12H22O14Fe, and CMS@C12H22O14Fe.
2.2.3. X-Ray Diffraction Analysis
Figure 6 presents the outcomes of the X-ray diffraction patterns for CMS, C12H22O14Fe, and CMS@C12H22O14Fe. Based on the positions of the diffraction peaks in the plots, CMS exhibited distinct diffraction peaks at 2θ values of 12.54°, 22.14°, 25.18°, 26.74°, 28.08°, 50.24°, 60.06°, and 68.24° [28,29], while C12H22O14Fe presented characteristic diffraction peaks at 2θ values of 16.92°, 19.20°, 20.74°, 22.58°, 24.80°, and 30.74° [30]. After the CMS is loaded with C12H22O14Fe, the characteristic diffraction peaks of C12H22O14Fe barely appear, suggesting that for the suppressant prepared by the anti-solvent crystallization method, no aggregation of C12H22O14Fe particles occurs. Due to the good structural properties of CMS, and with the appropriate preparation method applied, C12H22O14Fe particles could be dispersed uniformly on the surface of CMS, which is favorable to enhance the deflagration inhibition performance of CMS@C12H22O14Fe [31,32,33]. It is also because of this that part of the structure of CMS may be transformed from an ordered state to a partially amorphous state after loading, leading to a decrease in the intensity of some peaks or even a slight shift.
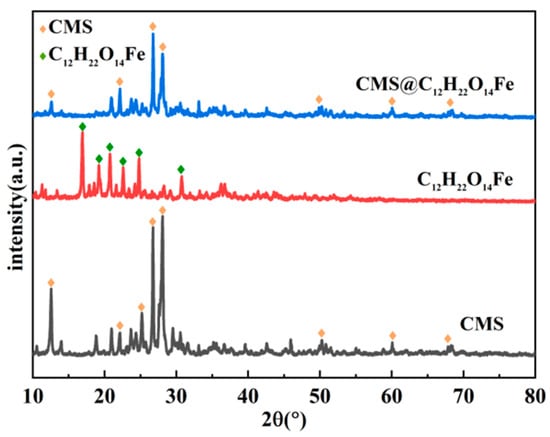
Figure 6.
X-ray diffraction patterns of CMS, C12H22O14Fe, and CMS@C12H22O14Fe.
2.2.4. Infrared Spectrum Analysis
Figure 7 presents the IR spectra of CMS, C12H22O14Fe, and CMS@C12H22O14Fe. From the figure, it can be seen that CMS exhibits Si-O4 and Al-O4 vibrational bands in the range of 1320–1000 cm⁻1, C=O vibrational bands at 1750–1600 cm⁻1, and Si-OH, Al-OH, and -OH vibrational bands in the 3600–3200 cm⁻1 region. For C12H22O14Fe, the -CH2 vibrational bands appear at 1450–1400 cm⁻1, alongside C=O vibrational bands also observed in the 1750–1600 cm⁻1 range. The spectrum of CMS@C12H22O14Fe displays both common vibrational bands and those specific to CMS and C12H22O14Fe, confirming the successful combination of the two materials.

Figure 7.
Infrared spectra of CMS, C12H22O14Fe, and CMS@C12H22O14Fe.
2.2.5. Thermogravimetric Analysis
Figure 8 depicts the TG-DTG plot of CMS@C12H22O14Fe, which represents the TG-DTG curve of pyrolysis and oxidation commencing at an initial temperature of 30 °C in nitrogen with a heating rate of 10 °C per minute.

Figure 8.
TG-DTG diagram of CMS@C12H22O14Fe.
It can be seen from the result that the weight loss before 100 °C is mainly due to the removal of free water in the inhibitor, while the weight loss from 100 °C to 240 °C is due to the decomposition of C12H22O14Fe, triggered by the removal of water and carbon dioxide. From 240 °C to 660 °C, C12H22O14Fe not only removes water and carbon dioxide but also decomposes to produce a very small amount of CO, which can react with O2 in the detonation process and generate CO2 [30]. When the temperature reaches about 800 °C, the sample loses very little weight. In the explosion reaction, the released water helps to dilute the O2 concentration and remove heat.
2.3. Experimental Equipment
The setup primarily comprises an ignition system, a Hartmann tube, a high-pressure powder coating system, a high-speed photography device, and a control system [34], as shown in Figure 9. During the experiment, 1 g of coal powder was mixed with inhibitors at varying mass ratios and evenly distributed around a mushroom-shaped nozzle. The ignition electrodes, positioned 2/3 of the way up from the bottom of the tube, were separated by a distance of 3 mm. The powder coating pressure was maintained at 0.4 MPa. A high-pressure dedusting system sprayed the coal powder into the Hartmann tube, forming a dust cloud. The subsequent flame propagation was recorded using high-speed photography [35].
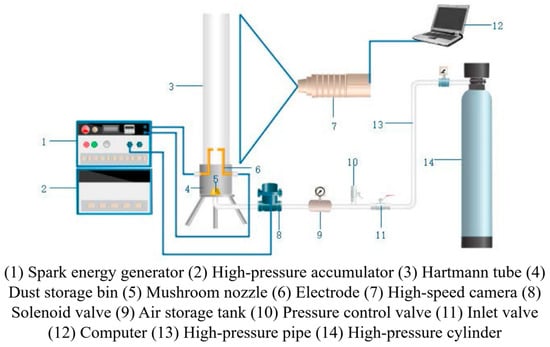
Figure 9.
Hartmann tube experimental setup.
The mass fraction of CMS@C12H22O14Fe added to the mixed powder, expressed in wt.%, was calculated using Equation (2):
In the equation, represents the mass of CMS@C12H22O14Fe (g), and represents the mass of lignite coal powder (g).
3. Results and Analysis
3.1. Analysis of Flame Propagation Inhibition of Lignite Coal Dust by CMS@C12H22O14Fe
Figure 10 presents the flame propagation process of coal dust, captured using a high-speed camera. As shown in the figure, lignite flame propagation is continuous, reflecting a relatively uniform dust distribution. The flame front remains neat and stable throughout propagation, with the luminous flame transitioning from an initial bright yellow to predominantly bright white flames. Additionally, the flame appears full and highly luminous.

Figure 10.
Flame propagation process of lignite.
In this work, we set up a two-step screening process: firstly, we fixed the proportion (10 wt.%) of the blast inhibitor when mixed with the coal dust, in order to quickly compare the blast inhibition effects of different loadings (10~60%), and select the loading amount of the ‘highly effective blast inhibition without wasting the active ingredient’; after obtaining the preliminary optimal loading amount (50 wt.%), then we systematically explored the blast inhibitor in the coal dust (10 wt.%, 20 wt.%, 30 wt.%, 20 wt.%). After obtaining the initial optimal loading (50 wt.%), we then systematically explored the additive proportion of the inhibitor in the coal dust (10 wt.%, 20 wt.%, 30 wt.%, etc.), and ultimately sought to determine the ‘active ingredient loading + the amount of inhibitor added to the’ optimal combination. Figure 11a–f illustrates the flame propagation inhibition of lignite with varying loadings (10%, 20%, 30%, 40%, 50%, 60%) of the suppressant CMS@C12H22O14Fe, under conditions where the suppressant was added at 10 wt.%. Figure 11a–e show that as the CMS@C12H22O14Fe loading increases, flame brightness gradually decreases, the time taken for the flame to reach its highest point extends, and the flame length shortens. This demonstrates a positive correlation between loading and the suppression effect for loading between 10% and 50% . However, as shown in Figure 11e,f, the suppression effect deteriorates at a loading of 60% , indicating that the optimal loading is 50% .
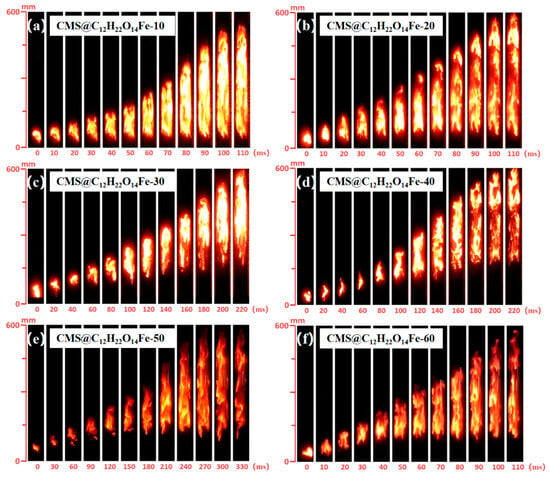
Figure 11.
Different 10 wt.% CMS@C12H22O14Fe inhibit the flame propagation process of lignite.
In the flame propagation experiments, we compared the flame height, brightness, and morphology by taking images at key time points with high-speed photography. Figure 12 illustrates the flame propagation inhibition of lignite coal dust at 60 ms with varying suppressant loadings. Among the tested loadings, when the loading of CMS@C12H22O14Fe was 50% , it demonstrated the most effective inhibition.
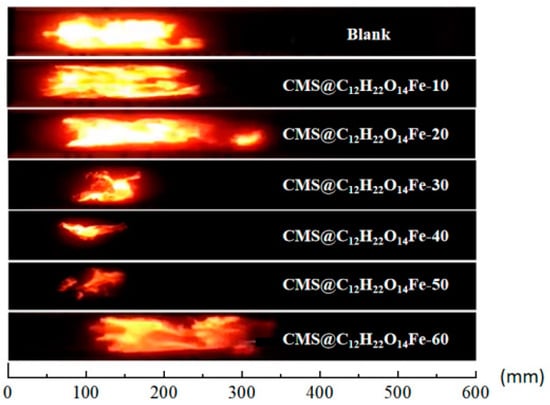
Figure 12.
Different 10 wt.% CMS@C12H22O14Fe flame propagation patterns at 60 ms.
Figure 13 presents the flame front heights for blank lignite and lignite with varying additions of CMS@C12H22O14Fe. The figure shows that the loading amount of CMS@C12H22O14Fe does not exhibit a positive correlation with flame front height. However, as the loading increases, the time required for the flame to reach its highest point also increases, particularly at 60% loading. A comprehensive comparison indicates that the optimal flame suppression effect occurs at CMS@C12H22O14Fe-50.

Figure 13.
Blank lignite pulverized coal and pulverized coal added to different 10 wt.% CMS@C12H22O14Fe flame front heights.
Figure 14 illustrates the flame propagation velocities for blank lignite and lignite with different 10 wt.% CMS@C12H22O14Fe. The curves show an initial decrease followed by a gradual increase, with the increase becoming notably gentle beyond a loading of 30%. As the loading of CMS@C12H22O14Fe increases, the propagation speed of the coal dust flame slows, and the rate of change diminishes. A comprehensive analysis indicates that the optimal effect on coal dust explosion inhibition is achieved with CMS@C12H22O14Fe-50.
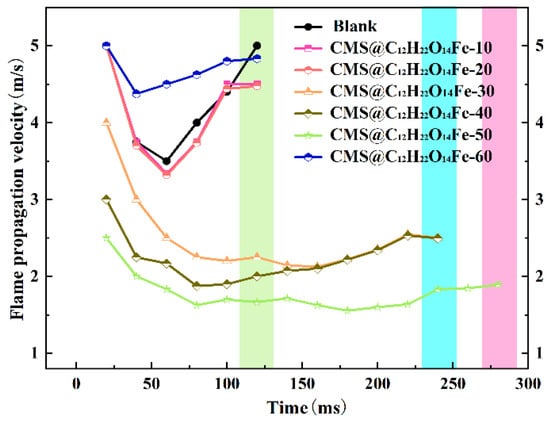
Figure 14.
Comparison of flame propagation velocities of different 10 wt.% CMS@C12H22O14Fe coal dusts.
The effects of varying additions (wt.% = 10, 20, 30) of CMS@C12H22O14Fe-50 on lignite flame propagation were investigated. As shown in Figure 15a–c, the flame propagation process was increasingly inhibited as the CMS@C12H22O14Fe-50 addition increased. The inhibition effect strengthened progressively with higher additive amounts, achieving near-complete inhibition at 30 wt.% CMS@C12H22O14Fe-50.
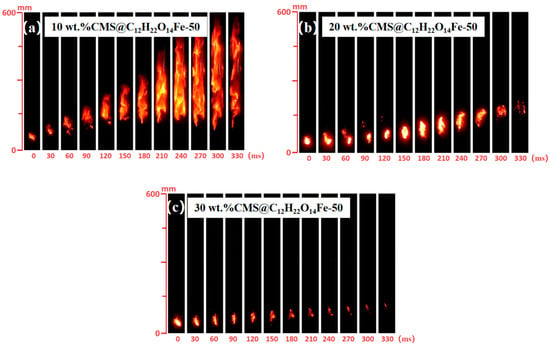
Figure 15.
Flame propagation inhibited by different additions of CMS@C12H22O14Fe-50.
In order to better observe and compare the addition of different additives to the same point in time flame height and morphology, we chose an intermediate time point of 180 ms. Figure 16 depicts the flame propagation of lignite coal dust with varying wt.% of CMS@C12H22O14Fe-50 at 180 ms. As can be seen from the figure alone, at 30 wt.% CMS@C12H22O14Fe-50, the coal dust explosion flame exhibited the lowest brightness and shortest length, achieving near-complete suppression. To improve this conclusion’s accuracy, we measured the flame height and propagation speed during flame propagation.

Figure 16.
Flame propagation diagram inhibited by different additions of CMS@C12H22O14Fe-50 at 60 ms.
Figure 17 and Figure 18 show the change in height and propagation velocity of the flame with time after different additions of detonation suppressants, respectively. As can be seen from the figure, at the same point in time, the flame height of the 30 wt.% CMS@C12H22O14Fe-50 is the lowest, and the propagation speed is the slowest; furthermore, in the same time period, the flame height difference is also the lowest. It can be concluded that the 30 wt.% CMS@C12H22O14Fe-50 has the best explosion suppression effect.
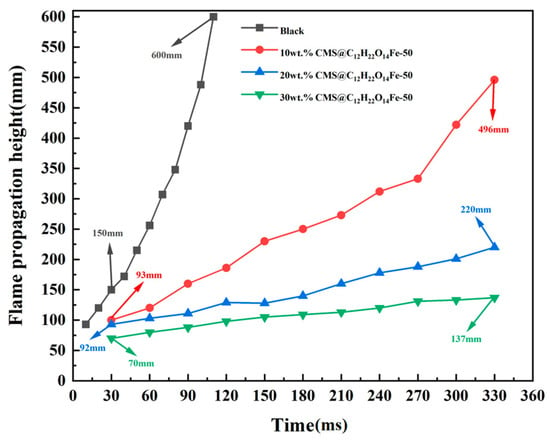
Figure 17.
Blank lignite pulverized coal and pulverized coal added to different wt.% CMS@C12H22O14Fe-50 flame front heights.
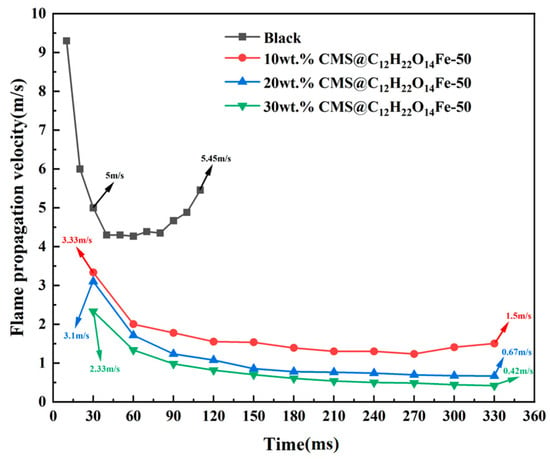
Figure 18.
Comparison of flame propagation velocities of different wt.% CMS@C12H22O14Fe-50 for blank lignite pulverized coal and pulverized coal.
3.2. Infrared Analysis of Lignite Pulverized Coal Flame Suppression by CMS@C12H22O14Fe
Figure 19 depicts the comparison of infrared spectra of lignite coal before and after the explosion. Examination of this figure reveals that the vibrational bands of the chemical structure of the lignite coal samples are primarily concentrated in the wave number ranges of 3700–3600 cm⁻1 (hydroxyl), 3000–2800 cm⁻1 (aliphatic hydrocarbons), approximately 1600 cm⁻1 (carbonyl), and 1300–1000 cm⁻1 (aromatic hydrocarbons) [36,37,38,39,40]. In contrast to the infrared spectral analysis results of lignite prior to the explosion, the vibrational peaks of hydroxyl and the aliphatic hydrocarbon vibrational bonds vanished, while the vibrational peaks of carbonyl and the aromatic hydrocarbon vibrational bonds were attenuated, indicating that chemical structures such as hydroxyl and the aliphatic and aromatic hydrocarbon vibrational bonds in lignite were implicated in the explosion reaction process.
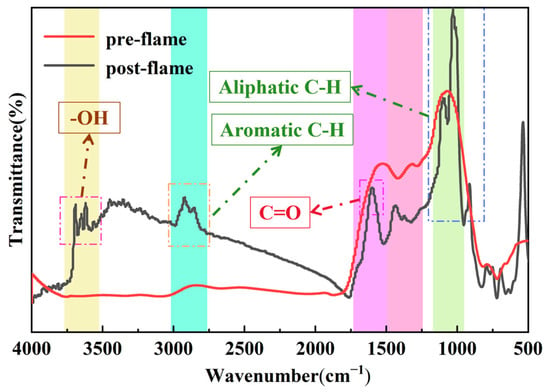
Figure 19.
Comparison of infrared spectra before and after lignite flame.
Figure 20 presents the comparative infrared spectra of lignite flames before and after the addition of 30 wt.% CMS@C12H22O14Fe-50. Infrared analysis shows that after the addition of the best powder inhibitor, coal dust explosion products in a variety of chemical structures in the vibration peak area remain largely unchanged, some of the peaks almost overlap, and the peak intensity has been weakened. Compared with Figure 19, the inhibition effect of the suppressant on the coal dust can be clearly seen. These results confirm the excellent lignite explosion suppression performance of the suppressant.
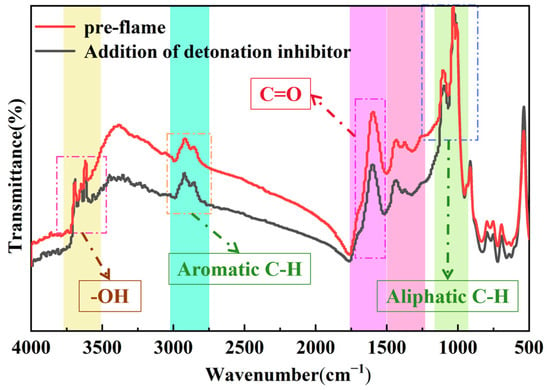
Figure 20.
Comparison of infrared spectra before lignite flame and after adding 30 wt.% CMS@C12H22O14Fe-50 flame of lignite pulverized coal.
3.3. Mechanism of Coal Dust Detonation Suppression by CMS@C12H22O14Fe
Figure 21 illustrates the detailed process of coal dust explosions and the inhibition mechanism of CMS@C12H22O14Fe. When coal dust forms a cloud of sufficient concentration and is exposed to adequate energy and O2, a coal dust explosion is triggered, causing the rapid breakage of chemical bonds in the coal and the release of hydrocarbon gases and CO2 [41]. CMS@C12H22O14Fe demonstrates an excellent explosion suppression performance through highly efficient physical and chemical synergistic effects.
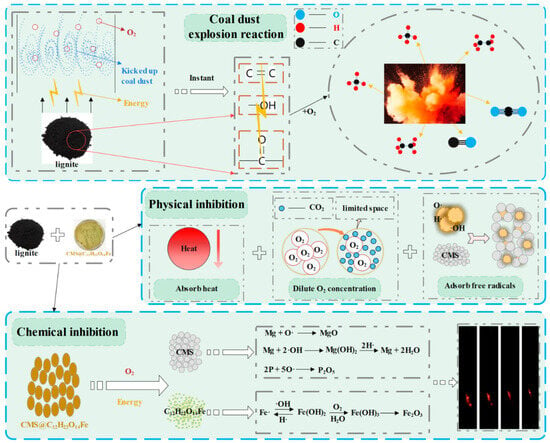
Figure 21.
Inhibition mechanism of the CMS@C12H22O14Fe suppressant for coal dust detonation.
- (1)
- Physical inhibition
When heated, CMS@C12H22O14Fe undergoes a dehydration and heat-absorption process. The adsorbed H2O evaporates and is released, producing a cooling effect. C12H22O14Fe decomposes via heat absorption, generating CO2 and a very small amount of CO, which significantly reduces O2 concentration within space and effectively dilutes the O2 in the explosion area. The decomposition products adhere to the surface of coal dust particles, forming a protective layer that isolates unburned particles. This inhibits the breakage of chemical bonds in functional groups, preventing the coal dust from participating in subsequent explosive reactions. CMS, with its good pore structure, exhibits an excellent adsorption capacity. It efficiently absorbs free radicals involved in the explosion’s chain reaction, thereby disrupting the reaction and suppressing the detonation [42].
- (2)
- Chemical inhibition
During the heating process, C12H22O14Fe mainly decomposes into iron oxides and CO2. At the same time, it can react with O2. The Fe element produced during decomposition consumes OH• and O•, and thus represents a key component of the explosion reaction, thereby interrupting the chain reaction [18]. Additionally, CMS contains a certain amount of elements such as Mg and P, which further suppress the reaction by chemically adsorbing OH• and O•, effectively blocking the explosive chain reaction.
4. Conclusions
In this study, an environmentally friendly composite powder detonation suppression agent was successfully prepared using anti-solvent crystallization technology, with ferrous gluconate as the active ingredient and Chinese Maifan stone as the carrier. The structure of the detonation suppression agent was analyzed through various characterization techniques. Additionally, flame propagation experiments were conducted to examine the morphological structure, propagation speed, and other combustion characteristics of the flame. These experiments were used to determine the optimal loading of the active component and the ideal addition of the powder detonation inhibitor.
- (1)
- The optimal loading of the active component was ascertained by regulating the loading of CMS@C12H22O14Fe when the addition was 10 wt.%. After a comprehensive analysis of the flame morphology, color, and propagation speed, it was discovered that with the increase in loading capacity, the flame propagation inhibited by the explosion suppressant showed a trend of first increasing and then decreasing.
- (2)
- When the loading of C12H22O14Fe was 50 wt.% in the suppressant, the flame suppression effect was the best.
- (3)
- The addition amount of CMS@C12H22O14Fe was further altered, and through re-analyzing the morphology, color, and propagation speed of the flame, it was ascertained that with the increase in the addition amount, the effect of explosion suppression was gradually enhanced. The flame could be suppressed when the addition amount of CMS@C12H22O14Fe-50 was 30 wt.%.
- (4)
- The FT-IR results of coal dust combustion before and after adding the 30 wt.% CMS@C12H22O14Fe-50 were compared. This comparative analysis further corroborates that the suppressant exhibits an excellent inhibitory effect on coal dust. In combination with the characterization analysis, this shows that the CMS@C12H22O14Fe suppressant undergoes the processes of dehydration, heat absorption, and chemical decomposition, which reveals the physical and chemical inhibitory mechanism of the CMS@C12H22O14Fe suppressant on coal dust.
- (5)
- The preparation of CMS@C12H22O14Fe composite powders by anti-solvent crystallization is an efficient and environmentally friendly technological route, but its process stability is limited by key parameters: the solubility of the active component (C12H22O14Fe) in deionized water, and the dispersion characteristics of the carrier material (CMS) in anhydrous ethanol. These parameters have a direct impact on the loading uniformity and coating efficiency of the active component. Especially in large-scale production scenarios, the control of particle size distribution and consistency of inhibitor loading may face engineering challenges. In the future, automated process control systems need to be developed to achieve industrial-grade batch stability by monitoring solvent ratios in real-time.
Author Contributions
Y.Z. (Yansong Zhang): Funding Acquisition, Validation, Supervision; Y.Y.: Investigation, Formal Analysis, Writing—Original Draft, Methodology, Conceptualization, Validation, Writing—Review and Editing; J.H.: Validation, Formal Analysis; S.D.: Data Curation, Methodology; F.W.: Data Curation; W.L.: Investigation, Validation; L.W.: Investigation, Methodology; Y.Z. (Yang Zhang): Investigation, Methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 51974179).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Author Yang Zhang was employed by Shandong Luqing Safety Assessment Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open-Access Journals |
| TLA | Three-letter acronym |
| LD | Linear dichroism |
References
- Cui, K. Dictionary of Safety Engineering; Chemical Industry Press: Beijing, China, 1995; p. 295. [Google Scholar]
- Wu, D.; Zhao, P.; Spitzer, S.H.; Krietsch, A.; Amyotte, P.; Krause, U. A review on hybrid mixture explosions: Safety parameters, explosion regimes and criteria, flame characteristics. J. Loss Prev. Process Ind. 2023, 82, 104969. [Google Scholar] [CrossRef]
- Guo, R.; Xu, C.; Li, N.; Huang, Y.; Zhang, Y.; Zhang, X. Suppression effect of nanocomposite inhibitor on Methane-Coal dust explosion flame propagation. Adv. Powder Technol. 2024, 35, 104314. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, Y.; Yan, K.; Chen, J.; Shi, W.; Li, R.; Yang, J. A novel ATH/SBA-15 suppressant prepared by in-situ synthesis and its inhibition mechanism on PE dust deflagration flame. Adv. Powder Technol. 2022, 33, 103871. [Google Scholar] [CrossRef]
- Cao, W.; Zhang, X.; Jia, Y.; Zhou, Z.; Li, W.; Xiao, Q.; Xu, S.; Jiao, F.; Zhao, F.; Xu, S.; et al. Energy output characteristics and safety design of Al-AlH3 composite dust for energetic material additive. Combust. Flame 2023, 254, 112842. [Google Scholar] [CrossRef]
- Jin, S.; Gao, W.; Huang, Z.; Bi, M.; Jiang, H.; Si, R.; Wen, G. Suppression characteristics of methane/coal dust explosions by active explosion suppression system in the large mining tunnel. Fire Saf. J. 2024, 150, 104251. [Google Scholar] [CrossRef]
- Qin, X.; Wei, X.; Shi, J.; Yan, Y.; Zhang, Y. Research on the Inhibition Effect of NaCl on the Explosion of Mg-Al Alloy Powder. ACS Omega 2024, 9, 8048–8054. [Google Scholar] [CrossRef]
- Yu, M.; Wang, F.; He, T.; Li, H.; Han, S.; Lou, R.; Zheng, K.; Yu, Y. Experimental exploration and mechanism analysis of the deflagration pressure of methane/pulverized coal blenders inhibited by modified Karoline-containing compound inhibitors. Fuel 2023, 333, 126353. [Google Scholar] [CrossRef]
- Wang, X. Study on the Inhibition Characteristics and Mechanism of Composite Powder Explosion Suppressant on Coal Dust Explosion. Ph.D. Thesis, Shandong University of Science and Technology, Qingdao, China, 2019. [Google Scholar] [CrossRef]
- Shang, S.; Bi, M.; Zhang, T.; Jiang, H.; Zhang, S.; Gao, W. Synthesis of green nanomaterial and discussion on its suppression performance and mechanism to aluminum dust explosion. Process Saf. Environ. Prot. 2021, 151, 355–364. [Google Scholar] [CrossRef]
- Kuang, K.; Chow, W.; Ni, X.; Ni, X.; Yang, D.; Zeng, W.; Liao, G. Fire suppressing performance of superfine potassium bicarbonate powder. Fire Mater. 2011, 35, 353–366. [Google Scholar] [CrossRef]
- Jiang, H.; Bi, M.; Gao, W.; Gan, B.; Zhang, D.; Zhang, Q. Inhibition of aluminum dust explosion by NaHCO3 with different particle size distributions. Hazard. Mater. 2018, 344, 902–912. [Google Scholar] [CrossRef]
- Wen, H.; Cao, W.; Wang, K.; Cheng, F.M. Experimental study on ABC dry powder to repress gas explosion. J. Saf. Sci. Technol. 2011, 7, 9–12. [Google Scholar]
- Dai, H.; Liang, G.; Yin, H. Experimental investigation on the inhibition of coal dust explosion by the composite inhibitor of carbamide and zeolite. Fuel 2021, 308, 121981. [Google Scholar] [CrossRef]
- Ni, X.; Kuang, K.; Yang, D.; Jin, X.; Liao, G. A new type of fire suppressant powder of NaHCO3/zeolite nanocomposites with core-shell structure. Fire Saf. J. 2009, 44, 968–975. [Google Scholar] [CrossRef]
- Jiang, H.; Bi, M.; Zhang, T.; Shang, S.; Gao, W. A novel reactive P-containing composite with an ordered porous structure for suppressing nano-Al dust explosions. Chem. Eng. J. 2021, 416, 129156. [Google Scholar] [CrossRef]
- Yuan, B.; Tao, H.; Sun, Y.; Wang, L.; Chen, X.; Tan, H. Study on synergistic suppression of methane explosion by porous mineral materials-ammonium polyphosphate composite powder. China Saf. Sci. J. 2021, 31, 41–46. [Google Scholar]
- Chen, J.; Chen, K.; Shi, W.; Pan, Z.; Yang, J.; Zhang, G.; Meng, X.; Zhang, Y. The preparation of novel core-shell suppressor and its suppression mechanism on coal dust explosion flame. Fuel 2022, 313, 122997. [Google Scholar] [CrossRef]
- Li, Y.; Meng, X.; Song, S.; Chen, J.; Ding, J.; Yu, X.; Zhu, Y.; Qin, Z. Piperazine pyrophosphate-functionalized Ni-MOF metal framework: Fabrication and synergistic explosion suppression mechanisms. Chem. Eng. J. 2024, 499, 155870. [Google Scholar] [CrossRef]
- Trace element content of Chinese Maifanshi (mg/kg). J. Feed Rev. 2002, 62. Available online: https://webvpn.sdust.edu.cn/https/77726476706e69737468656265737421fbf952d2243e635930068cb8/kcms2/article/abstract?v=tOz5m-jLbAXLLo4jissbpuxgPLYYYWwaD929DJw-IvcgNTsi-XscykNU5ZNksVFWmuLjr8kS4H6tDYvn8DO-OLwku7G9oRmYTdE_QomFNNHgVtbaYGqPoXEVJorwJM0TnYSyJemw4J1gr5b5gdz6PeD-nX00SK2sFSMK_D86ZVLONR7FA0nJFOnRM5X89ojr&uniplatform=NZKPT&language=CHS (accessed on 5 January 2025).
- Chen, L.; Li, F.; Fu, D.; Wang, H.; Zhao, Y. Determination of surface properties and adsorption mechanism of Chinese Maifan stone. J. Inn. Mong. Petrochem. Ind. 1998, 4, 19–21. [Google Scholar]
- Onifade, M.; Genc, B. A review of research on spontaneous combustion of coal. Int. J. Min. Sci. Technol. 2020, 30, 303–311. [Google Scholar] [CrossRef]
- Youssefi, R.; Segers, T.; Norman, F.; Maier, J.; Scheffknecht, G. Experimental Investigations of the Ignitability of Several Coal Dust Qualities. Energy 2021, 14, 6323. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, Y.; Zhang, P.; Li, L.; Chen, J.; Pan, Z.; Li, R.; He, M. Study on the preparation of green suppressors and their characteristics in coal dust flame propagation inhibition. Adv. Powder Technol. 2022, 33, 103749. [Google Scholar] [CrossRef]
- Guo, X.; Hu, Z.; Fu, S.; Dong, Y.; Jiang, G.; Li, Y. Experimental study of the remediation of acid mine drainage by Maifan stones combined with SRB. PLoS ONE 2022, 17, e0261823. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, Z.; Dong, Y.; Fu, S.; Li, Y. Study of the preparation of Maifan stone and SRB immobilized particles and their effect on treatment of acid mine drainage. RSC Adv. 2022, 12, 4595–4604. [Google Scholar] [CrossRef]
- Popoola, A.; Sanni, O.; Loto, C.A.; Popoola, O.M. Corrosion inhibition: Synergistic influence of gluconates on mild steel in different corrosive environments. synergetic interactions of corrosion inhibition tendency of two different gluconates on mild steel in different corrosive environments. Port. Electrochim. Acta 2015, 33, 353–370. [Google Scholar] [CrossRef]
- Ren, J.; Liu, J.; Feng, L. Research on the process of extracting minerals from Chinese Maifan stone. Compr. Util. Miner. 2020, 75–81+70. [Google Scholar]
- Ren, J.; Liu, J.; Feng, L. Adsorption of heavy metal ions by Chinese Maifan stone. Water Purif. Technol. 2018, 37, 88–94. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, J.; Chen, S.; Zhang, P. Stability of ferrous gluconate solid by thermal analysis. J. China Pharm. Univ. 1987, 16–20. [Google Scholar]
- Liu, B. Preparation and desulfurization performance of LaMeOx/SBA-15 for hot coal gas. Appl. Catal. B Environ. 2011, 102, 27–36. [Google Scholar] [CrossRef]
- Chen, J. Surface chemistry and catalytic performance of amorphous NiB/Hβ catalyst for n -hexane isomerization. Appl. Surf. Sci. 2016, 390, 157–166. [Google Scholar] [CrossRef]
- Moosavi, A. Synthesis of mesoporous ZnO/SBA-15 composite via sonochemical route. Micro Nano Lett. 2012, 7, 130–133. [Google Scholar] [CrossRef]
- Liu, J.; Meng, X.; Yan, K.; Wang, Z.; Dai, W.; Wang, Z.; Li, F.; Yang, P.; Liu, Y. Study on the Effect and Mechanism of Ca(H2PO4)2 and CaCO3 Powders on inhibiting the explosion of Titanium Powder. Powder Technol. 2022, 395, 158–167. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, P.; Chen, C.; Zhang, X.; Zhou, Z.; Geng, S.; Zhang, Y.; Lan, Y.; Shi, X.; Cao, W. Flame propagation characteristics and surface functional groups changes of corn starch dust during the combustion process. Powder Technol. 2023, 430, 118995. [Google Scholar] [CrossRef]
- Zhang, D. Study on Dynamic Adsorption Spreading of Different Non-Ionic Surfactants and Wetting Mechanism of Coal Dust. Ph.D. Thesis, Taiyuan University of Technology, Taiyuan, China, 2016. [Google Scholar]
- Wang, P.; Tan, X.; Zhang, L.; Li, Y.; Liu, R. Influence of particle diameter on the wettability of coal dust and the dust suppression efficiency via spraying. Process Saf. Environ. Prot. 2019, 132, 189–199. [Google Scholar] [CrossRef]
- Feng, L.; Zhao, G.; Zhao, Y.; Zhao, M.; Tang, J. Construction of the molecular structure model of the Shingle Lignite using TG-GC/MS and FTIR spectrometry data. Fuel 2017, 203, 924–931. [Google Scholar] [CrossRef]
- Jiang, H. Suppression mechanism of ultrafine water mist containing phosphorus compounds in methane/coal dust explosions. Energy 2022, 239, 121987. [Google Scholar] [CrossRef]
- Jiang, H. Effect of turbulence intensity on flame propagation and extinction limits of methane/coal dust explosions. Energy 2022, 239, 122246. [Google Scholar] [CrossRef]
- Gong, J.; Nie, B.; Peng, C.; Ge, Z. Research on the Explosion Reaction Mechanism of Volatile Gases from Coal Dust Pyrolysis. Combust. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Chen, J.; Yang, J.; Wang, F.; Pan, Z.; Shi, W.; Dongye, S.; Zhao, W. Study on the inhibition mechanism of green suppressants zinc borate and zinc silicate for oil shale based on flame propagation experiment and thermodynamic analysis. Energy 2023, 283, 129014. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).