Abstract
Strict gaseous emission standards are applied globally to regulate the maximum amounts of pollutant emissions that can be produced from all vehicles. The exhaust aftertreatment systems used by automotive manufacturers rely on the utilization of precious metals (Pt, Pd, Rh). However, much effort has been devoted on the reduction or the replacement of the amount of Platinum Group Metals (PGMs) in three-way catalysts (TWC), both from a cost-effectiveness as well as an environmental point of view. PROMETHEUS catalyst, which was recently homologated for Euro 6 applications, is a low-cost, Cu-based TWC, which consists of a significantly lower quantity of PGMs compared to conventional state-of-the-art catalysts and achieves similar or even better catalytic efficiencies. In this review paper, a complex reaction scheme is proposed for the first time for a catalytic converter utilizing Cu and PGMs, following an extensive literature investigation of the available models. The scheme also accounts for the surface reaction mechanisms of the main processes and the side reactions potentially taking place during the TWC operation in the presence of Cu and at least one of the following PGMs: Pt, Pd or Rh. At a next step, the proposed reaction scheme will be validated based on experimental data, using mathematical modelling of a PROMETHEUS catalytic converter incorporating Cu and PGM nanoparticles.
1. Introduction
Increasingly stringent emission standards for vehicles have been implemented globally over the last decades, aiming at reducing air pollution and address climate change. These emission standards typically regulate the amount of pollutants, such as carbon monoxide (CO), hydrocarbons (HC), nitrogen oxides (NOx) and particulate matter (PM) emitted by vehicles equipped with internal combustion engines [1,2,3,4,5]. At the same time, several incentives and regulations worldwide promote the transition to ’green’ mobility. At European level, Regulation (EU) 2023/851 has been adopted by the European Parliament and the Council, which sets a zero tailpipe CO2 emission target for new cars and vans produced after 2035 [6]. At the same time, the Council of the European Union has recently adopted the Euro 7 regulation, which aims to further reduce air pollutant emissions from exhausts as well as brakes of vehicles. The new emission standard is a single legal act covering simultaneously all new cars, vans and heavy-duty vehicles, and it imposes stricter lifetime requirements compared to the previous standards [7].
While electrification is the ultimate long-term zero-emission alternative, the transition to battery electric vehicles is not an immediate or universal alternative. Thus, most road transport is still powered by internal combustion engines, which primarily run on liquid fuels derived from petroleum and their blends with biofuels. It should be noted that hybrid electric vehicles also utilize internal combustion engines, so they still need to comply with the current emission limits in terms of harmful gaseous pollutants emissions [8,9]. The main measures taken by the automotive manufacturers in their efforts to address the further reduction of gaseous emissions are a combination of measures in the engine and the powertrain, along with the development of suitable exhaust after-treatment systems [1,9,10,11,12]. Concerning the latter, three-way catalysts (TWC) have been used for several decades in spark-ignited vehicles (fueled with gasoline, natural gas, Liquified Petroleum Gas (LPG)) in order to reduce gaseous emissions. A TWC consists of a ceramic (usually cordierite) or metallic monolithic substrate with a honeycomb-like structure. The catalytically active powder is deposited (washcoated) on the surface of the monolith channels. Typical catalytic washcoat formulations developed commercially include platinum group metals (PGM; i.e., Pt, Pd and Rh) supported on γ-Al2O3, CeO2 and other oxides; mainly, ceria-zirconia solid solutions are used to increase the oxygen storage capacity (OSC) of the catalyst and improve its thermal stability [4,10,13,14,15]. The main reactions that take place during the operation of an efficient TWC are the oxidation of CO and unburnt HC toward CO2 and H2O, and the reduction of nitrogen monoxide (NO) for the production of nitrogen and CO2 (Equations (1)–(3)):
The increase of PGM content in automotive catalysts is one of the commonly used means by manufacturers to further increase the efficiency and meet the increasingly stringent emission limits. In addition, the design of highly efficient catalysts requires the complete understanding of the elemental reaction steps that can define the performance of the catalysts under the complex real-world operating conditions [16]. For this reason, the mechanistic steps of the operation of commercial catalysts incorporating PGMs have been studied by several authors over the last decades [16,17,18,19,20,21]. In these studies, the role of each metal on the formation of surface intermediate species is documented, while kinetic models have been developed based on the proposed reaction schemes to predict the performance of the catalysts based on their formulation [17,22,23,24,25,26].
Conversely, the critical importance and high cost of PGMs (average price since 1 January 2025 for Rh is USD 164.27/g, for Pd is USD 34.23/g and for Pt is USD 33.89/g [27]) have significantly accelerated research efforts aimed at reducing or even replacing PGMs in TWC applications [10,28,29,30]. Numerous studies have examined the performance of PGM-free catalysts, primarily focusing on perovskites and transition metal catalysts for potential use in TWC. These investigations are typically based on model reactions [30,31,32,33,34,35,36,37,38,39,40]. While perovskite-based catalysts have demonstrated potential as alternatives to traditional noble metal catalysts due to their favorable catalytic properties [30,31,37,38], further advancements are necessary to overcome existing limitations before they can be widely implemented in commercial automotive catalytic converters. Among the various transition metal catalysts explored as PGM substitutes in TWC formulations, copper (Cu) has emerged as a highly attractive, low-cost alternative (Cu metal price: <USD 10/kg [41]) for exhaust gas purification. Its appeal stems from its reported high activity in oxidation reactions and effective NO reduction [36,40,42,43,44,45,46,47]. In view of the above, the recycling and reuse of PGM and Cu-based TWCs could offer several advantages both from an economic and environmental perspective. Monolithos Catalysts & Recycling Ltd. has specialized in recycling PGMs from End-Of-Life catalysts (EOL) by applying an environmentally friendly hydrometallurgical method [28,48,49], with high PGM recovery rates (100% for Pt, 92% for Pd, ~60% for Rh). These PGMs, can be reused as precursors to produce new catalysts with the same activity as commercially available metal precursors [50]. This process, that could be modified also for Cu recovery, offers a sustainable and environmentally friendly approach to catalyst lifecycle management when compared to the environmental concerns of Cu and especially PGM mining due to their scarcity.
PROMETHEUS is a novel TWC catalyst developed by Monolithos Catalysts & Recycling Ltd., incorporating copper (Cu), palladium (Pd) and rhodium (Rh) nanoparticles as the active catalytic material supported on a CeO2-ZrO2 mixed oxide characterized by high oxygen storage capacity [51,52,53,54]. The novelty of PROMETHEUS catalyst lies in its use of Cu nanoparticles in the active catalytic phase, with a total metal loading of 2 wt% and a molar ratio of Cu/Pd/Rh = 21/7/1, which leads to a reduction of PGMs loading by up to 85% [52]. This results in a catalytic converter that offers comparable or even enhanced performance relative to state-of-the-art commercial automotive catalysts, both as fresh as well as after being subjected to hydrothermal aging [52]. At the same time, the reduction of PGMs content in the PROMETHEUS catalyst allows for a significant reduction of the cost of the catalytic converter production, which can reach up to 50% compared to the state-of-the-art PGMs-based catalytic converters, depending on the content of PGMs used. In addition, PROMETHEUS recently became the first copper-based catalyst to achieve homologation for a Euro 6 application (Approval No.: E24*103R00/04*0796*00). The homologation has been granted by the National Standards Authority of Ireland (NSAI—NSAI|National Standards Authority of Ireland) and is valid for Euro 6 applications up to 1.2lt engines in the European Union. The emission tests have been conducted by TÜV SÜD Auto Service GmbH (Munchen, Germany), showing that a reduction of 25–55% of all regulated emissions (CO, NOx, Total Hydrocarbons (THC) and Non-Methane Hydrocarbons (NMHC)) compared to the legislative Euro 6 limits is feasible using the full-scale PROMETHEUS catalyst. The superior performance of the PROMETHEUS catalyst compared to the conventional PGM-based commercial catalysts has also been previously reported, despite the fact that it incorporates 50–85% less PGMs through their partial substitution by copper [51,52]. A positive synergy between the different functionalities of metal elements (Cu, PGMs and CeO2-ZrO2 carrier) has been proposed to be the main driver of its high observed catalytic performance. However, the specific impact of copper, when combined with PGMs on the catalyst support, on the elementary steps of the overall reaction network remains unclear.
The existing literature on Cu-based catalysts as potential candidates for TWC development primarily relies on model reactions to gain insights into the underlying reaction mechanisms. Although there are some available studies regarding model Cu catalysts or Cu-containing polymetallic catalysts employing transition metals, to the best of our knowledge, there is no study dealing with the potential reaction network of a catalyst comprising Cu in combination with PGMs for TWC applications emphasizing on the role of each metal on the catalyst performance. In this work, we propose, for the first time, a comprehensive reaction scheme for a Cu-based catalyst containing PGMs, following an extensive review of the surface reaction mechanisms of elementary steps. This includes insights derived from both model reactions and realistic simulated streams, potentially occurring during TWC operation in the presence of Cu and at least one of the following PGMs: Pt, Pd or Rh. As mentioned above, Cu-based catalytic systems have been widely tested in the literature for the main TWC reactions and have been reported to exhibit exceptionally interesting catalytic properties, especially in oxidation reactions. Since the high activity of Cu-based catalysts in catalyzing oxidation reactions is well documented in the related scientific literature, the following sections initially report the widely accepted mechanistic steps involved in the oxidation of CO and HC, with specific attention being given to the available studies including catalyst formulations combining Cu with PGMs. Furthermore, the NOx reduction in the presence of Cu and PGMs, which is considered to be more complex, and thus, the main challenge in such type of TWC formulations, is examined in detail. Additionally, the potential occurrence of reactions leading to the formation of undesired by-products is also thoroughly discussed. Finally, the role of the carrier, mainly due to its oxygen storage capacity is also described, while the potential of a TWC catalyst containing Cu and PGMs for dioxin formation is addressed. In Figure 1, the outline of the review paper described above is graphically illustrated.
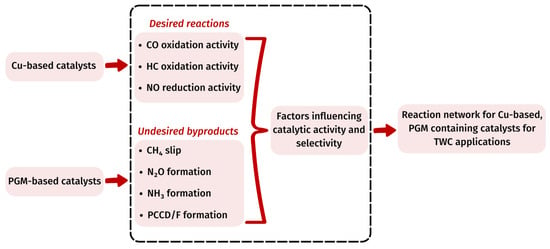
Figure 1.
Schematic illustration of the review of the reaction network for Cu-based, PGM-containing catalysts for TWC applications.
The results of the present review paper in terms of the proposed reaction scheme will be integrated in the currently ongoing mathematical modelling of the PROMETHEUS catalytic converter and will be further calibrated and validated with experimental results. The results of modelling activities will be included in a subsequent publication.
2. Potential Reaction Mechanism in TWC Containing Cu and PGMs as Active Phase
2.1. CO Oxidation Reaction
Various catalytic systems of supported Cu catalysts have been applied successfully in the oxidation of CO [44,55,56,57,58,59,60,61,62]. A Langmuir–Hinshelwood type mechanism between adsorbed oxygen and CO has been widely proposed for CO oxidation on CuO-based catalysts [32] and could safely be extended to several Cu-containing catalyst formulations. At temperatures below 250 °C, O2 is dissociatively adsorbed on the catalyst surface requiring two adjacent surface sites (Equation (4)), while CO is adsorbed non-dissociatively on a surface site of Cu (Equation (5)). The CO molecules and O atoms initially disperse on the surface of the catalyst and when a CO molecule and O atom interact, they recombine and produce CO2. The following reaction scheme is proposed based on the literature:
The reaction rate in this process is proportional to the surface exposure of Oad and COad.
Significant attention has been focused on copper-promoted ceria-oxide (CuO/CeO2) catalysts due to their distinctive, unique activity for effectively catalyzing low-temperature CO oxidation. The defect sites and oxygen vacancies are most desirable active species for CO oxidation [32,63,64]. These processes occur at high reaction rates if the CO molecule adsorbed on the metal particle is able to interact with oxygen adsorbed on the highly reducible metal oxide support, with the reactions taking place at the metal–support boundary [32,65]. CO migration toward the interfacial region in the case of supported catalysts is critical for facilitating reactions at the metal–support interface and can be influenced by several key parameters, such as reaction temperature, oxygen mobility and the concentration of oxygen vacancies at the support, that dictate how easily CO can move from one site to another. Thus, the outstanding catalytic activity of catalysts based on CuO/CeO2 has been attributed to interfacial regions between the CuO and CeO2 domains [61,66]. The synergy arises from ceria’s capacity to store and release oxygen atoms through a redox process, along with the presence of a special CuO−CO interaction. In fact, the oxygen storage/release ability of CeO2 is intensified in the presence of CuO. As the CO molecules are adsorbed on the CuO surface, they migrate to the interfacial region with the CeO2, where O2 is added to the interface passing through the oxygen vacancies on the CeO2 phase [66]. Higher oxygen mobility of the support is favored at slightly higher reaction temperatures. Furthermore, for a gas–solid reaction, the large surface area and high mesoporosity of catalysts favor easy access of reactants to active sites and diffusion of products, thereby increasing the reaction efficiency over the material surface, improving the catalytic performance. In this mechanism, the adsorbed CO (COad) undergoes oxidation by lattice oxygen (OL). The COad is oxidized by the adsorbed oxygen (Oad), which is the rate-determining process. Therefore, the mobility of active oxygen (Oad and/or OL) is generally considered as crucial for the CO oxidation reaction [32,67,68,69,70,71].
Remarkable selectivity toward CO oxidation has also been documented in the literature for catalysts composed of Cu-containing alloys on CeO2-based oxide supports [72,73,74,75,76]. Table 1 includes a comparison of the literature data of light-off temperatures (T50) for monometallic and bimetallic Cu- and PGM-based catalysts for the CO oxidation reaction studied under different experimental conditions. Based on the data of Table 1, in most cases, Cu presence in combination with PGMs has a promotional effect, reducing significantly the measured light-off temperatures compared to the monometallic PGM- or Cu \-based catalysts.

Table 1.
Comparison of light-off temperatures of monometallic Cu or PGM and bimetallic Cu-promoted PGM-containing catalysts for the CO oxidation reaction.
In addition, the related literature studies suggest that the promotional effect of Cu for the CO oxidation through its alloy with several noble metals can be explained by the formation of a Cu+ species when Cu and its alloy are supported on a CeO2-containing material, which is reported to play a role as highly selective chemisorption site for CO [73,75,83]. Concerning the influence of Cu presence on CO oxidation kinetics, Table 2 summarizes several literature values of apparent activation energies for the oxidation of CO over Cu, PGM and bimetallic Cu-containing catalysts. Judging from the apparent activation energies (Ea) summarized in Table 2, Cu and Pd ceria-based catalysts are the most active for the CO oxidation reaction. It seems from the above that the support plays a pivotal role in the catalytic activity, with CeO2 yielding the best catalytic results due to its defective structure and high oxygen storage capacity (OSC). The catalytic activity of Cu ceria-based catalysts is comparable to the Pd-based catalysts and better when compared to Rh and Pt monometallic catalysts. Nikolaev et al. [84] investigated the synergistic effect of Pd–Cu alloy catalysts for low-temperature CO oxidation and found enhanced light-off activities and lower activation energies compared to the monometallic catalysts. This suggests that CO oxidation in the presence of Cu/Pd occurs predominantly at new sites. The promotional effect of Cu, in terms of catalytic activity and stability, was also shown by Singhania et al. [81] on Pd-supported catalysts on activated carbon (AC). In general, the values of apparent activation energies lie in the same range both for Cu-containing and PGM-based catalysts, suggesting a common reaction mechanism in all cases.

Table 2.
Apparent activation energies (Ea) calculated for different PGM- and Cu-based catalysts for the CO oxidation reaction.
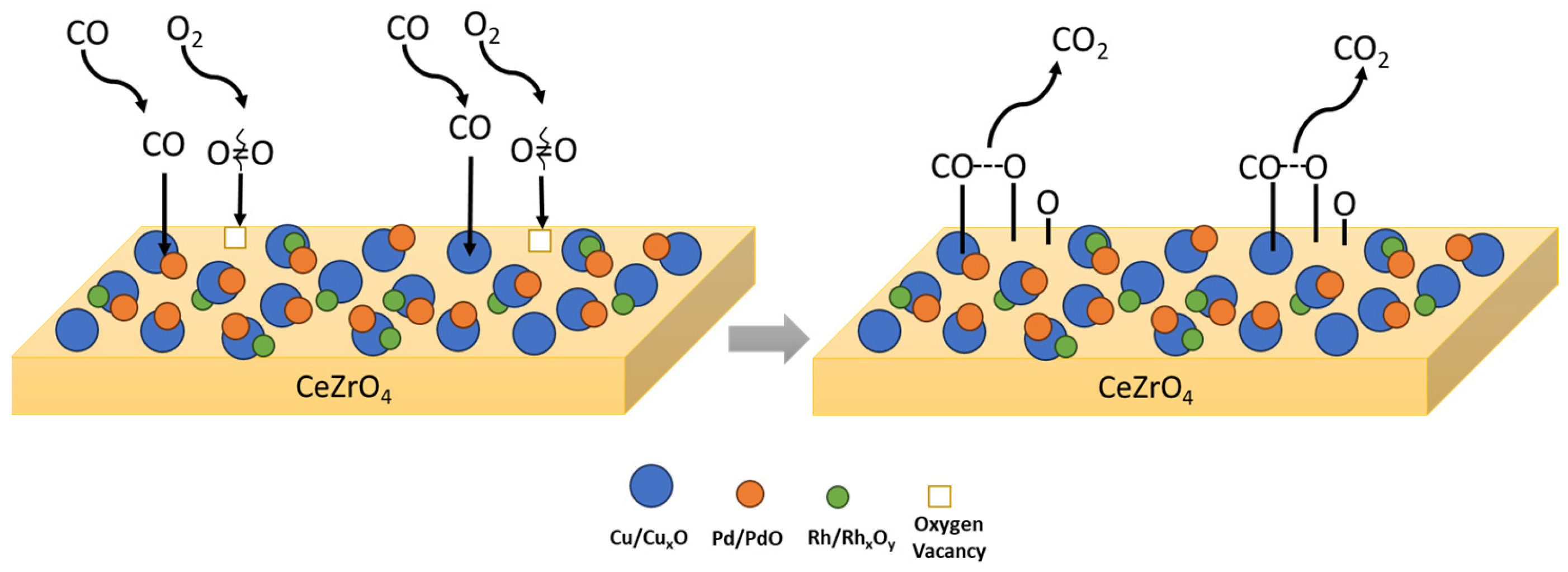
Concerning the interaction of Cu with Pd, as well as the impact of alloy formation on the catalytic CO oxidation, theoretical studies have shown that Cu can significantly modify the valence state of Pd by injecting charge into the sp sub-band [79,97]. Consequently, this electronic modification in Pd-Cu alloys is able to facilitate the dissociation of CO compared to Pd, altering the nature of CO interaction with Pd surface centers from almost purely covalent (in the case of pure metal) to a mixture with an ionic component. The latter ionic component increases with increasing Cu content in the alloy, leading to the progressive weakening of the C-O bond [79]. Taking the above into consideration a proposed mechanism followed for CO oxidation over a CuPdRh-ceria-based catalyst involves the molecular adsorption of CO mainly on the surface of Cu and CuPd metal particles, which then reacts in the metal–support interface with Oad being formed on the surface of the CeZrO4 oxide producing gaseous CO2. The proposed scheme is graphically illustrated in Figure 2.
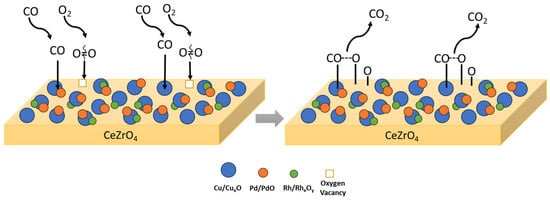
Figure 2.
Proposed mechanistic pathway for CO oxidation followed on CuPdRh-ceria-based catalysts. Cu–Pd alloys and Cu/CuxO act as the active sites for CO adsorption.
2.2. HC Oxidation Reaction
The oxidation of HCs can be considered similar to that of CO [14,98]. However, the oxidation of HCs, for example, C3H8 (propane) or CH4 (methane), needs higher temperatures because of the difficulty in breaking the C-H bond in saturated hydrocarbons. In most of the literature studies, the oxidation of C3H6 (propene) and C3H8 has been investigated as representative of fast and slow oxidizing hydrocarbons, respectively. In addition, the variety of adsorbed surface species that can be produced by the partial oxidation of hydrocarbons make the overall oxidation reaction quite complex, while the oxygen coverage could be reduced favoring different reaction paths [17].
Kareem et al. [99] performed a detailed assessment of the chemical and intermediate species formed during the catalytic oxidation of hydrocarbons in palladium-based systems and proposed the following general reaction steps for C3H8 oxidation:
- (1)
- Initial C-H bond cleavage on Pd sites by reacting with adsorbed O species followed by adsorption of C3H(8-n) (n = 1, 2, …)
- (2)
- Reaction of activated C3H(8-n) species with activated O species leading to cleavages of the first C-C bond and additional C-H bonds to form intermediate species.
- (3)
- Reaction of the first-C-C bond cleaved intermediate species with activated O species leading to cleavages of the second C-C bond and additional C-H bonds to form intermediate species.
- (4)
- Decomposition and release of the adsorbed intermediate species as CO2 and H2O.
The above-mentioned reaction steps can be adopted for the catalytic oxidation of any hydrocarbon that may be present in the feed gas stream, taking into account the different stoichiometric quantities for each component. Concerning the kinetics of HC oxidation, several developed reaction mechanism models available in the literature simulating three-way monolithic catalysts containing Pd/Rh [100] and Pt/Pd/Rh [16] have reported activation energies for the oxidation of C3H8, C3H6 and CH4, corresponding to 112–125, 105 and 121 kJ/mol, respectively. In general, based on the reported activation energy values, it is evident that CH4 oxidation is more challenging compared to C3H8 and C3H6.
Courtois et al. [101,102] investigated a series of bimetallic copper–rhodium catalysts for their performance as three-way catalysts. By investigating γ-Al2O3-supported Cu and CuRh catalysts, the authors observed an increase in catalytic activity for CO and C3H6 conversions at low temperatures in the bimetallic CuRh catalyst, compared to a monometallic Rh catalyst. This enhancement was attributed to the combined properties of the two metals, specifically the oxidation activity of Cu, which facilitated the initiation of CO and hydrocarbon oxidation reactions at lower temperatures [101]. In a subsequent study, they expanded their research to include Cu and Rh monometallic and bimetallic catalysts on supports of different oxygen storage capacity for their performance in TWC application [102]. The conversion of C3H6 was found to be slightly increased by the addition of Rh to Cu, as well as by the redox properties of the support, mainly in the case of CeO2-ZrO2. Moreover, as discussed above, the presence of high Cu content in the catalyst formulation was suggested by the authors to have a positive effect in catalytic activity by acting as an OSC component.
CH4 Oxidation Reaction
In addition to the oxidation of NMHCs and the corresponding mechanism presented above, the mechanistic aspects of CH4 oxidation in TWC are of major importance both for gasoline- as well as natural-gas-fueled vehicles. CH4 is classified as a strong greenhouse gas; thus, the abatement of methane emissions from vehicles is considered as an additional challenge for catalytic aftertreatment systems. Vehicles equipped with internal combustion engines running on natural gas attracted increased attention over the recent years, since natural gas has been considered as a more sustainable and clean fuel compared to the conventional fuels [103,104,105,106,107,108,109].
In the commercial catalytic converter formulations (Pt, Pd, Rh), the highest activity for CH4 oxidation has been documented for Pd-containing catalysts [104,110,111,112,113,114,115,116,117]. Thus, most of the available studies reviewing the CH4 oxidation mechanism are focused on the performance of commercial TWC formulations, including PGMs (mainly Pt and Pd, which are exceptional oxidation catalysts, and Rh, in order to simultaneously promote NO reduction reaction) [104,111,118,119,120]. Several literature studies have suggested that CH4 oxidation reaction over Pd-based catalysts is dominated by the Pd0 ↔ PdO phase transformations. These transformations depend on the catalyst structure and reaction conditions and are accompanied by distinct mechanisms [104,118,119]. More specifically, PdO species have been reported to be more active than reduced Pd, following the Mars-van Krevelen redox mechanism, in which the PdO phase facilitates the dissociative adsorption of methane, while the oxide support provides lattice oxygen, which is replenished by the feed gas O2 [104,118,121,122,123]. On the other hand, over reduced Pd0 species, the CH4 oxidation reaction has been suggested to be catalyzed following a Langmuir–Hinshelwood mechanism where both hydrocarbon and oxygen are chemisorbed on the catalyst surface (competitively) and react according to a bimolecular reaction [118,122,123]. In addition, the role of CeO2 in stabilizing PdO is also well documented, leading to an increase of PdO decomposition temperature [114,119].
Cu-based catalysts have also been investigated for their activity regarding CH4 oxidation reactions, showing high efficiencies over CuO, especially in combination with CeO2supports characterized by high OSC [111,124]. A similar Mars-van Krevelen mechanism has also been reported for CuO/CeO2 catalysts, as the redox cycle of the interface is promoted by the interfacial metal–support interaction. CH4 is bound at the surface of CuO, while O2 is adsorbed on the abundant oxygen vacancies of CeO2 [124,125].
It should be noted that only a few studies have been carried out regarding the activity of bimetallic or polymetallic catalysts containing Cu and at least one PGM for the oxidation of methane. Reyes et al. [117] investigated the effect of Cu addition and preparation methods on the catalytic performance of a Cu-Pd bimetallic catalyst supported on SiO2 for the oxidation of CH4. Their results showed that the addition of Cu did not lead to an increase of the activity, a fact that the authors attributed to both structural and mechanistic reasons. The negative effect caused by the Cu–Pd interaction was attributed to the decreased accessibility of the reactant to the Pd active sites caused by the Cu loading increase, resulting in the partial coverage of Pd particles as well as the changing of the active sites for the reaction.
On the other hand, an interesting research paper by Heo et al. [80] investigated the simultaneous CO and HC oxidation activity of a cerium zirconium mixed oxide containing copper (CZCu) under simulated diesel exhaust conditions. The authors found improved activity compared to a reference PGM-based catalyst containing Pt and Pd supported on γ-Al2O3, even though significant deactivation was observed. To address the latter, they integrated the CZCu catalyst into the conventional PGM-based catalyst, and their results showed significant improvement of activity for light hydrocarbon oxidation reaction, while the heavy hydrocarbon oxidation efficiency was also increased. They attributed the observed improvement to the fact that fast CO oxidation takes place at low temperatures due to the presence of CZCu. CO and C3H6 oxidation share the same active sites; thus, the increased CO activity at lower temperatures resulted in lower inhibition by CO over the active sites of the support.
The high activity of Pd-based catalysts for CH4 oxidation reaction is also confirmed by studying the apparent activation energy values reported in the literature, which are shown in Table 3.

Table 3.
Apparent activation energies (Ea) calculated for different PGM- and Cu-based catalysts for the CH4 oxidation reaction.
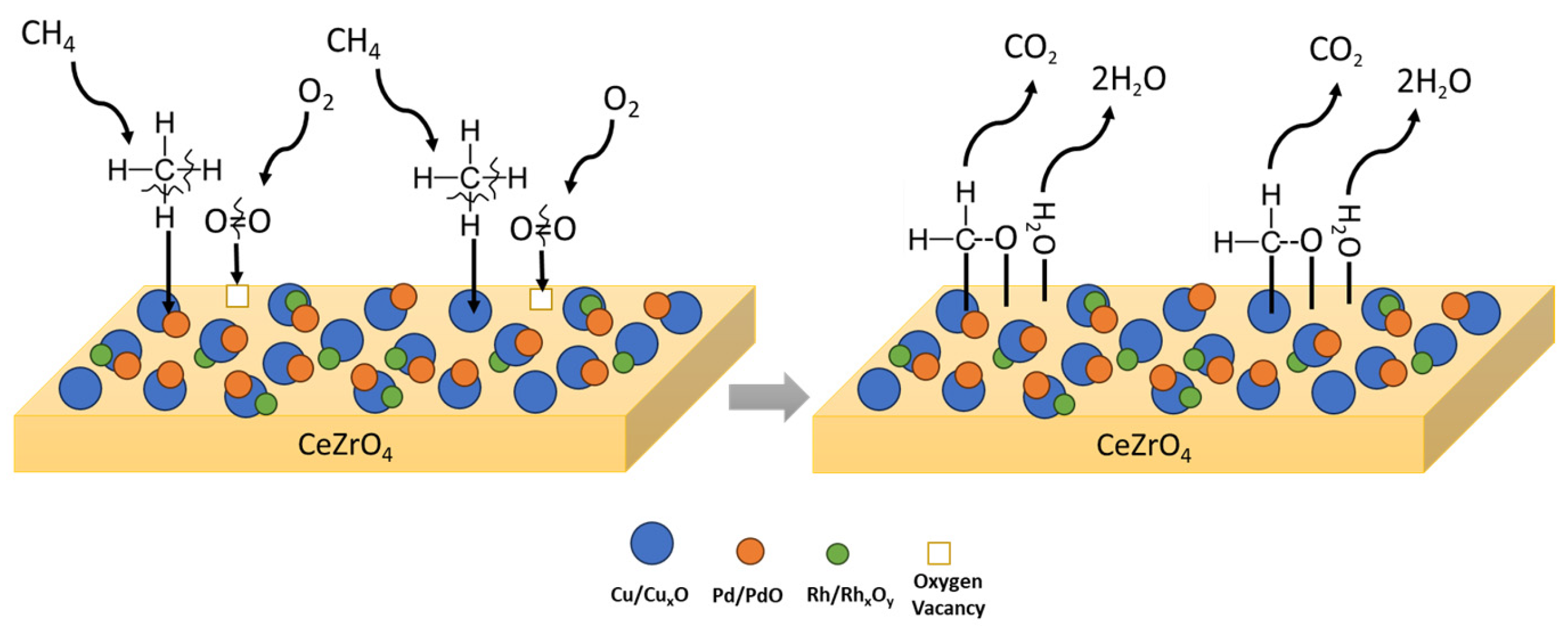
Considering the values of the apparent activation energies (Table 3), higher reaction rates are expected for Pd-based catalysts, which are the most active for the CH4 oxidation reaction compared to Pt- and Cu-based catalysts. Concerning Pt-based catalysts, it seems that the support plays a crucial role, as the ceria-based Pt catalysts exhibit lower activation energies compared to the alumina-based ones. Finally, Cu-based catalysts are found to exhibit similar or even lower activation energies when compared with Pt alumina-based catalysts. In addition, similarities in apparent activation energies observed over various catalysts for the oxidation of CH4 suggest a common reaction mechanism both in the presence of PGM as well as Cu as the active phase in catalyst formulation. A proposed mechanism for CH4 oxidation is graphically illustrated in Figure 3.
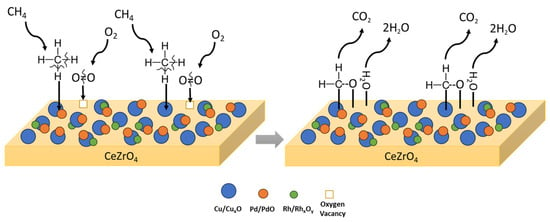
Figure 3.
Proposed mechanistic pathway for CH4 oxidation followed on CuPdRh-ceria-based catalysts.
2.3. NO Reduction Reaction
One of the most important functionalities of TWC is the reduction of nitrogen oxides (NOx) into nitrogen gas (N2) under near-stoichiometric conditions present in spark ignited (SI) engines. The latter implies that the concentrations of reductants (such as CO, HCs and H2) and NOx species are near the stoichiometric equivalents. It should be noted that the mechanism of NOx reduction has been reported to depend strongly on the reductant used [137,138]. However, several open issues still exist regarding the elementary steps of the reaction. The NO reduction mechanism over Cu- [31,43,46,139,140,141,142] as well as PGM-based catalysts [13,20,21,100,143,144,145] has been extensively studied in the literature, while in most of the cases, the interaction of NO−CO is the prevailing reaction investigated as the model TWC reaction. In general, the reaction in commercial TWCs is proposed to proceed with the decomposition of NO on the surface of a platinum group metal [20,21,137,146,147]. Thus, the elementary steps in the heterogeneous reduction of NO by CO include the molecular adsorption of CO (Equation (5)) and NO (Equation (11)), the dissociation of the NO (Equation (12)), the reassembling of the surface species and finally, the desorption of the products (Equations (13)–(18)):
Several authors have suggested an additional surface reaction that can potentially play a role in NO reduction to N2, which includes the formation of adsorbed isocyanate species NCOad (Equation (19)) and that can further react with NO or O2, producing additional N2 (Equations (20) and (21)) [20,21,145,148].
Commercial state-of-the-art TWCs incorporate Pt or Pd supported on Al2O3 as well as Rh promoted with Ce. Rh is generally perceived as the most active metal since it selectively decomposes NO to Nad and Oad to yield N2 [137]. The reduction of NO involves reduced metallic sites [144]. Carbon monoxide (CO) can react with Oad to produce CO2 (Equation (6)), creating reduced Rh0 sites. At the same time, N-O bond cleavage (Equation (12)) takes place on these sites (Rh0) due to the high extent of electron back donation into the molecular antibonding π* orbital of ΝO leaving atomic nitrogen and oxygen adsorbed on the surface of the catalyst [149]. Subsequently, nitrogen is produced by the combination of two adsorbed atomic nitrogen species (Equation (18)). Remaining Oad species are removed by CO as presented above, resulting in the restoration of required reduced metal sites. It has been reported that the same surface reaction sequence described above takes place in the presence of O2 in the feed, although with lower reaction rates [39,150,151]. The widely accepted Rh superiority in NO reduction by CO, especially in the presence of O2, is often ascribed to the fact that each Rh atom has the ability to exchange electrons, while in metals such as Pd and Pt, only two electrons are exchanged during the reversible redox process between the M0 and MOx states [152]. The high activity of Rh-based catalysts and the role of the support for NO reduction reaction with CO is also confirmed by studying the apparent activation energy values reported in the literature. In the case of a Pt/Al2O3-based catalyst [153], the activation energy for N2 formation has been reported at 120 kJ/mol, while in the case of Rh/Al2O3 [154], the activation energy for N2 formation ranged from 88 to 130 kJ/mol, depending on the temperature, and the mechanism followed. In addition, in the study performed by Oh [155], the author showed that the addition of Ce to a low loaded Rh/Al2O3 catalyst modified the kinetics of the CO-NO reaction, decreasing the apparent activation energy Ea from 159 to 75 kJ/mol, depending on Ce loading, while, at the same time, suppressed N2O formation.
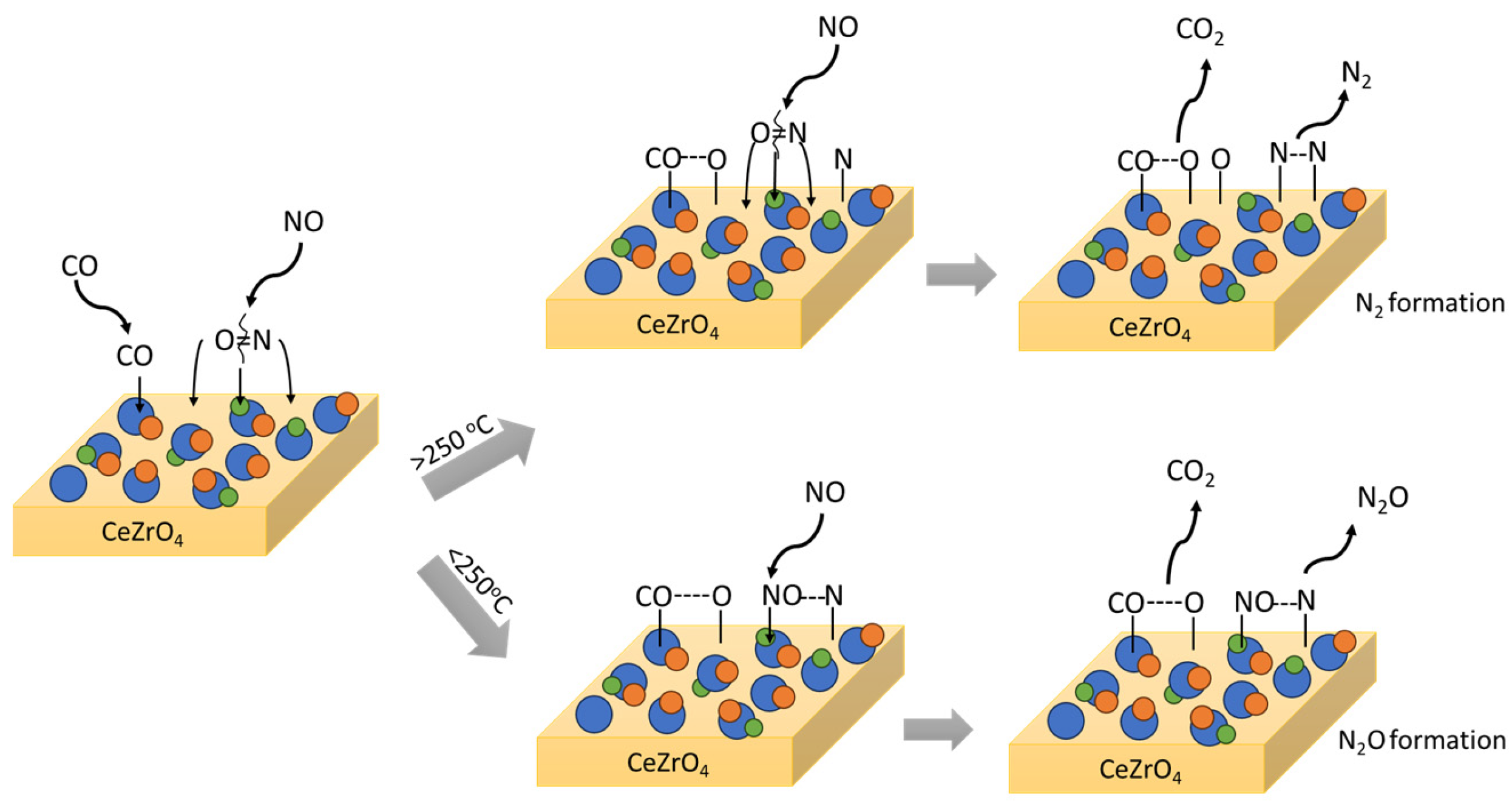
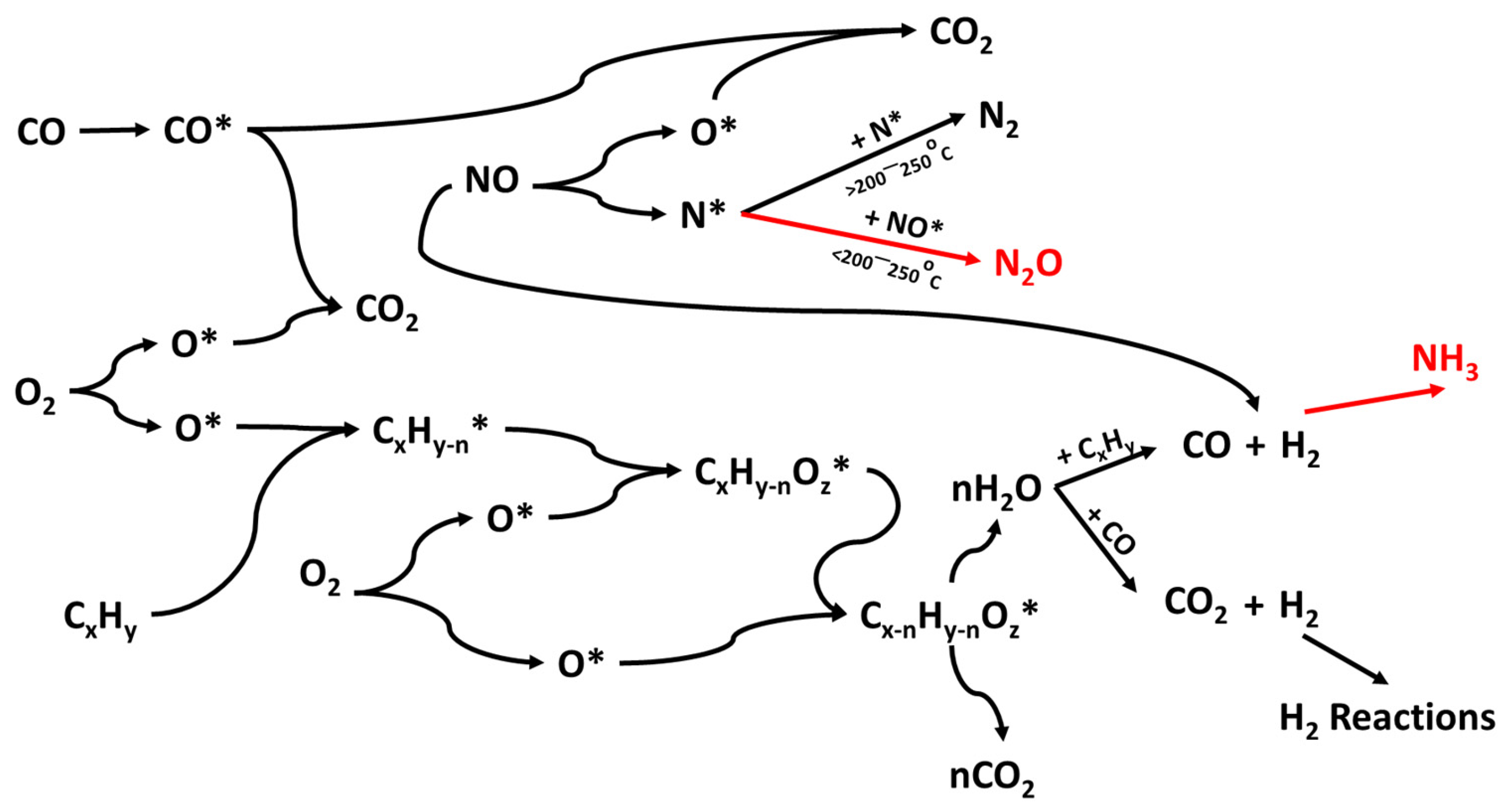
The available studies on NO reduction by CO on the surface of Cu-based catalysts widely suggest that the NO reduction mechanism depends on the reaction temperature, with a critical threshold around 200–250 °C [47,139,140,141]. N2O is formed as the intermediate or the main product of NO + CO reaction at lower temperatures, while N2 is the direct product at higher temperatures. The two mechanistic pathways that lead either to N2 or N2O, depending on the temperature, are graphically illustrated in Figure 4.
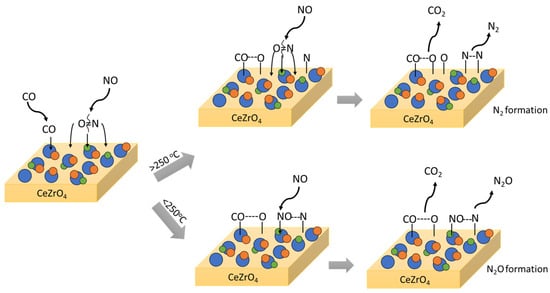
Figure 4.
N2 and N2O mechanistic pathway formation through the NO reaction with CO followed on CuPdRh-ceria-based catalysts. Rh-based alloys act as the active sites for NO adsorption and NO bond cleavage.
As presented above, the reduction of NO in the presence of CO could proceed in two steps, the first one being a partial reduction of NO to N2O, and the second one a subsequent reduction of N2O to N2 [46]. Both of these steps involve the oxidation of a reduced site. Most of the oxygen coming from NO is liberated with the simultaneous formation of a more positive site. Thus, the redox cycling of CuO is proved to be beneficial, since Cu2+ is reported to act as the NO adsorption site, while Cu+ acts as the CO adsorption site and the recipient of the dissociation oxygen [156]. In the fundamental work of London et al. [157] that combined spectral studies with kinetic experiments, it was reported that both Cu and Cu+ reduced sites were essential for the reaction, since NO was firstly dissociated on the surface of CuO. Highly active CuO contained both Cu and Cu2O under reaction conditions, indicating that the active sites for reduction might be located at the boundary between the two reduced phases. N2O was reported as an intermediate product which was further reduced to N2 at higher temperatures and longer residence times [47,158,159].
CuO-CeO2/γ-Al2O3 catalysts have been also investigated as potentially active catalysts for NO reduction by CO [43,46]. It has been reported that the beneficial role of ceria in the catalyst formulation depends on the metal–support interaction due to the synergistic activity of copper species and ceria, which can be influenced by the catalyst preparation methods [160]. Moreover, it should be noted that the reaction mechanism involving the formation of isocyanate species has been also reported by several authors, suggesting the dissociation of N-O bond and its combination with CO molecules [43,159,161].
Another available study of NO reduction by CO has been performed by Liu et al. [142], who investigated a series of CuO catalysts supported on CexZr1-xO2 catalysts with different Ce:Zr molar ratios in order to correlate their structural characteristics with observed performance. Their results suggested that the crystal structure of the support could largely influence the dispersion of CuO on the mixed oxide support. A ceria-rich pseudocubic (t″) phase was effective in stabilizing the active copper species compared to the zirconia-rich supports studied, resulting in increased activity due to the synergistic interaction of Cu and CeO2 support.
Most of the above-mentioned literature studies regarding Cu-based catalysts have been performed by studying the model CO-NO reaction in the absence of O2 in the feed. Chen et al. [47] recently published a review article summarizing the mechanistic aspects of NO reduction by CO on the surface of Cu-based catalysts with O2 being present in the feed. By combining the available literature studies’ results, the authors concluded that under O2-containing conditions, the catalytic activity can be greatly influenced by both the Cu content of the catalyst, as well as the O2 concentration in the feed. Depending on the catalyst formulation, O2 can either inhibit NO reduction reactions or promote the formation of N2. O2 present in the gas steam can be adsorbed on the active surface sites instead of NO and then preferentially react with COad for the formation of CO2, thus decreasing the catalytic activity for NO reduction. The formation of surface oxygen vacancies, however, can subsequently facilitate the adsorption of NO which can be decomposed to Oad and Nad. The latter can react to form N2, while Oad can react with NO to form NO2, which finally reacts with available CO to produce CO2 and additional N2. The formation of NO2 is promoted with the increase of O2 concentration in the feed; however, under high O2 excess conditions, CO is no longer available due to its oxidation for CO2 production [162].
The interaction between Cu and PGMs in TWC catalyst formulations has been previously reported by Courtois et al. [102], who documented the superior activity of Rh for NO reduction reaction even in the presence of Cu. Their study demonstrated that at low conversion, particularly when oxygen was present in the reaction mixture, the rhodium phase exhibited significantly higher activity than copper for NO reduction, and therefore the conversion was practically unaffected neither by the presence of copper nor by OSC of the support. They attributed the observed activity of bimetallic CuRh catalysts exclusively on the rhodium content. The fact that the NO conversion increased with Rh content was solely attributed to the intrinsic activity of rhodium [101]. At higher conversion, the support OSC may influence the conversion by mitigating composition fluctuations, leading to less or no limitation of the conversion depending on the rhodium content and the support’s OSC. Finally, the authors demonstrated that rhodium presented the same properties in Cu-Rh bimetallic as in Rh monometallic catalysts.
Based on the above observations, it can be concluded that no synergistic effect is expected in the case of catalyst formulations, including Rh in combination with Cu and other PGMs. The presence of Rh in the catalyst formulation provides the active sites for NO reduction, while metals characterized by high activity in oxidation reactions (e.g., Cu or Pd) are responsible for the oxidation of CO and hydrocarbons.
2.3.1. Formation of By-Products During NO Reduction Reaction
Although N2 is the desired product of NO reduction during the operation of the TWCs, the formation of undesired by-products such as N2O and NH3 can also take place during the operation of the catalyst under specific conditions related with the catalyst formulation, operation temperature, air-to-fuel ratio, fuel used and catalyst deactivation [18,137,163,164,165]. N2O is a strong greenhouse gas (300 times greater greenhouse potential over a 100-year period than CO2) that contributes to climate change and stratospheric ozone depletion [166]. NH3, on the other hand, is a corrosive gas known to significantly contribute to several environmental problems, including the acidification and eutrophication of ecosystems [165,167], has adverse effects on human health [168,169] and contributes to PM2.5 pollution via the formation of ammonium (sulfate and nitrate) salts [165,170,171].
It is generally accepted that N2O can be formed mainly at temperatures lower than 350 °C, where nitrogen oxide can react with reducing components present in the feed stream to produce N2O and N2 following the reactions presented above. When the exhaust gas temperature is in the range of 350–600 °C, in fuel-rich operation, partial oxidation of HCs and steam reforming reactions are possible, generating CO and H2. At the same time, NO can react (be reduced) with H2, HCs and other H-containing components to form NH3 and N2 [164].
Below, the formation of these two main by-products (N2O and NH3) is discussed, focusing on the mechanistic steps involved, as well as the main factors affecting their formation, with special focus on the potential role of each metal. Finally, some measures proposed in the literature to control their emissions are presented.
N2O Formation
Several researchers have studied the N2O formation mechanism during the operation of a TWC under near stoichiometric conditions, employing in situ DRIFTS analysis to identify the surface intermediates in the reactions network, coupled with activity measurements [26,172,173]. In addition to the already mentioned possible formation of N2O by the coupling reaction of an adsorbed nitrogen (Nad) with a molecularly adsorbed NOad according to Equation (13) (Figure 4), or through an adsorbed (NO) dimer (Equation (15)), an alternate proposed mechanism involves the formation of isocyanate intermediate (Equation (19)) through the surface reaction of molecularly adsorbed COad, or COad coming from the partial oxidation of hydrocarbons (Equation (22)), with Nad originating from the dissociative adsorption of NOad. The adsorbed surface NCOad species can further react with NO, leading to the formation of N2O, according to Equation (23).
The production of N2O during TWC operation depends on the operating conditions (mainly reaction temperature), and the metals present on the catalyst. The selectivity toward undesired N2O has been reported to vary based on different catalyst formulations. As mentioned above, N2O formation can potentially take place at temperatures lower than 350 °C. At this temperature range, the dissociation of NO takes place, but at a slow rate that favors N2O formation, following one of the mechanisms presented above. On the other hand, at higher temperatures (normal operating temperatures of a warmed up TWC), the water–gas shift reaction as well as the steam reforming reaction of hydrocarbons can contribute to the production of dissociated hydrogen species which facilitate the N2O decomposition reaction leading to N2O minimization in the gas stream, producing additional N2 [172].
In general, N2O formation has been reported for several catalyst formulations studied in the literature using both monometallic Pt, Pd an Rh, or combinations of PGMs as well as Cu-based catalysts [18,19,31,47,137,158,159,162,165]. N2O emissions of gasoline-fueled vehicles equipped with state-of-the-art PGM-based catalytic converters have been reported not to exceed 14mg/km over a wide range of driving conditions [174,175,176]. Comparing a commercial Pd-only high-precious-metal loading closed coupled catalyst and a Pd/Rh low-precious-metal loading commercial TWC catalyst, DiGiulio et al. [137] found that the bimetallic Pd/Rh catalyst produced less N2O under slightly lean conditions. The authors concluded that the reduced N2O formation of the bimetallic catalyst was likely due to the presence of Rh, which exhibits high activity for the selective reduction of NOx to N2, as well as the inclusion of CeO2 in the low PGM catalyst studied [18,137]. In addition, similar observations have been made by other authors, suggesting that higher amounts of N2O are produced over Pd-based, compared to Rh-based, samples [177,178,179].
The literature studies have suggested that, in the presence of Cu and CeO2, the number of Cu+ species (which can be increased by doping CeO2 with ZrO2), the dispersion of CuO, as well as the presence of surface oxygen vacancies in the catalyst, can result in catalysts that are highly active and selective toward the formation of N2. This is attributed to the rapid reduction of formed N2O both in the presence and absence of O2 in the feed [158,159,160].
NH3 Formation
Potentially high NH3 emissions over TWCs have been widely reported in the literature, using both model reactions as well as simulated laboratory conditions and real-world vehicle measurements [137,164,165,180,181]. It should be noted that NH3 is primarily formed as a secondary pollutant over the catalyst and is not generated during engine operation [182,183].
Concerning the reaction mechanism, NH3 is formed on the surface of commercial TWCs containing PGMs through the hydrogenation of N adatoms, with Had originating from dissociatively adsorbed hydrogen [18,137,164,184,185]. For the reaction to occur, a minimum stoichiometric H2 to NO ratio equal to 2.5 is required. As mentioned above, hydrogen can originate from the water–gas shift reaction and the steam reforming reactions of hydrocarbons. At lower H2 concentrations, an alternative NH3 formation mechanism has been proposed in the relevant literature. This mechanism involves the reaction of NO with CO and H2, producing NH3 and CO2 as products. The process proceeds through the formation of isocyanic acid as intermediate, which is subsequently hydrolyzed to yield NH3 and CO2 [137,186]. Several authors have suggested that the formation of NH3 in TWC operation is affected by the operation temperature, the Air Fuel Ratio (AFR), the presence of CO, as well as the metals in the catalyst formulation and its OSC [18,137,165,181,185,187,188,189,190]. In general, at a given temperature, richer AFR leads to increased NH3 formation due to the higher availability of CO. The presence of CO can promote the removal of O atoms (via CO oxidation), which would otherwise react with hydrogen and/or ammonia resulting in higher NH3 production. Higher temperatures favor the subsequent reaction of the formed NH3 with NO resulting in increased N2 formation. It has been reported that the NH3 emissions of gasoline cars equipped with state-of-the-art PGM-based catalysts over various driving conditions lie between 15 and 49 mg/km with a wide scatter of values [174,175,191].
Among the various PGM-based TWC catalyst formulations studied in terms of NH3 emissions, Rh has been reported to exhibit the lowest activity for ammonia formation both in monometallic form and in combination with Pt and/or Pd. This is attributed to Rh’s high activity for NO dissociation [137,188], which leads to the selective reduction of NOx toward N2 instead of NH3, [18,185,189,192]. At the same time, the oxygen storage component of the catalyst also contributes to the reduction of NH3 formation due to its ability to promote the re-oxidation of the undesired secondary emissions downstream [181,190]. It is stressed that there are no studies in the literature that address the potential formation of NH3 in the case of Cu-based catalysts for TWC applications under either laboratory-simulated conditions or real-world emission testing.
2.4. Role of OSC Properties of the Support
Gasoline-fueled engines are typically operated under near stoichiometric conditions to facilitate the functioning of TWC exhaust emission control systems. In this context, the air-to-fuel ratio values fluctuate around the stoichiometric value. Thus, ceria emerges as a crucial component of the washcoat in these dynamic oxidation-reduction conditions, due to its increased OSC (Oxygen Storage Capacity) properties [100]. Zirconia (ZrO2) is often combined with ceria to form a CeO2-ZrO2 (CZ) solid solution, characterized by improved OSC, enhanced mechanical and thermal stability that prevents sintering in high operating temperatures, optimized electronic and ionic conductivity and enhanced redox properties of Ce, favoring the formation of oxygen vacancies. As a result, CZ solid solution is widely employed across the industry in catalytic converter applications.
The superiority of CZ in TWC conversion originates from CeO2’s capacity to actively participate in redox reactions through the reversible transformation between Ce3+ and Ce4+.This redox capability enables the material to store and release oxygen, thereby enhancing its catalytic performance [13,14,143]. In the presence of oxygen in the gas phase, CO oxidation occurs rapidly; however, in the absence of gaseous-phase oxygen, CO oxidation depends initially on surface oxygen species of CeO2 and subsequently on the availability of bulk oxygen, once these surface species are depleted. Consequently, the high OSC activity plays an important role in facilitating CO oxidation at low temperatures.
Furthermore, CeO2 exhibits exceptional metal-support interaction [143,193,194], which is considered the primary factor contributing to the superior activity of the CeO2 supported catalyst family. While various hypotheses have been made, the mechanism of CeO2 interaction with noble metal nanoparticles is generally related to the migration of lattice oxygen and the formation of strong bonding between the metal and the support, M-O-Ce. The inclusion of CeO2 in TWC formulations is also important because of its reported ability to promote high noble metal dispersion, enhance thermal stability of the catalyst, promote water gas shift (WGS) and steam reforming activity, and facilitate CO oxidation [14]. CeO2 has also been found to enhance NO decomposition through the spillover of oxygen onto partially reduced CeO2, and to beneficially alter the kinetics for CO oxidation and NOx reduction [195]. Moreover, it is important to emphasize that the Oxygen Storage Capacity (OSC) properties of the support are particularly crucial in the case of nanocatalysts, as the strong metal-support interaction can ensure the high dispersion and prevention of sintering of metal nanoparticles over CeO2.
2.5. Potential Polychlorinated Dibenzo-Para-Dioxins (PCCD) and Polychlorinated Dibenzo Furans (PCDF) Formation
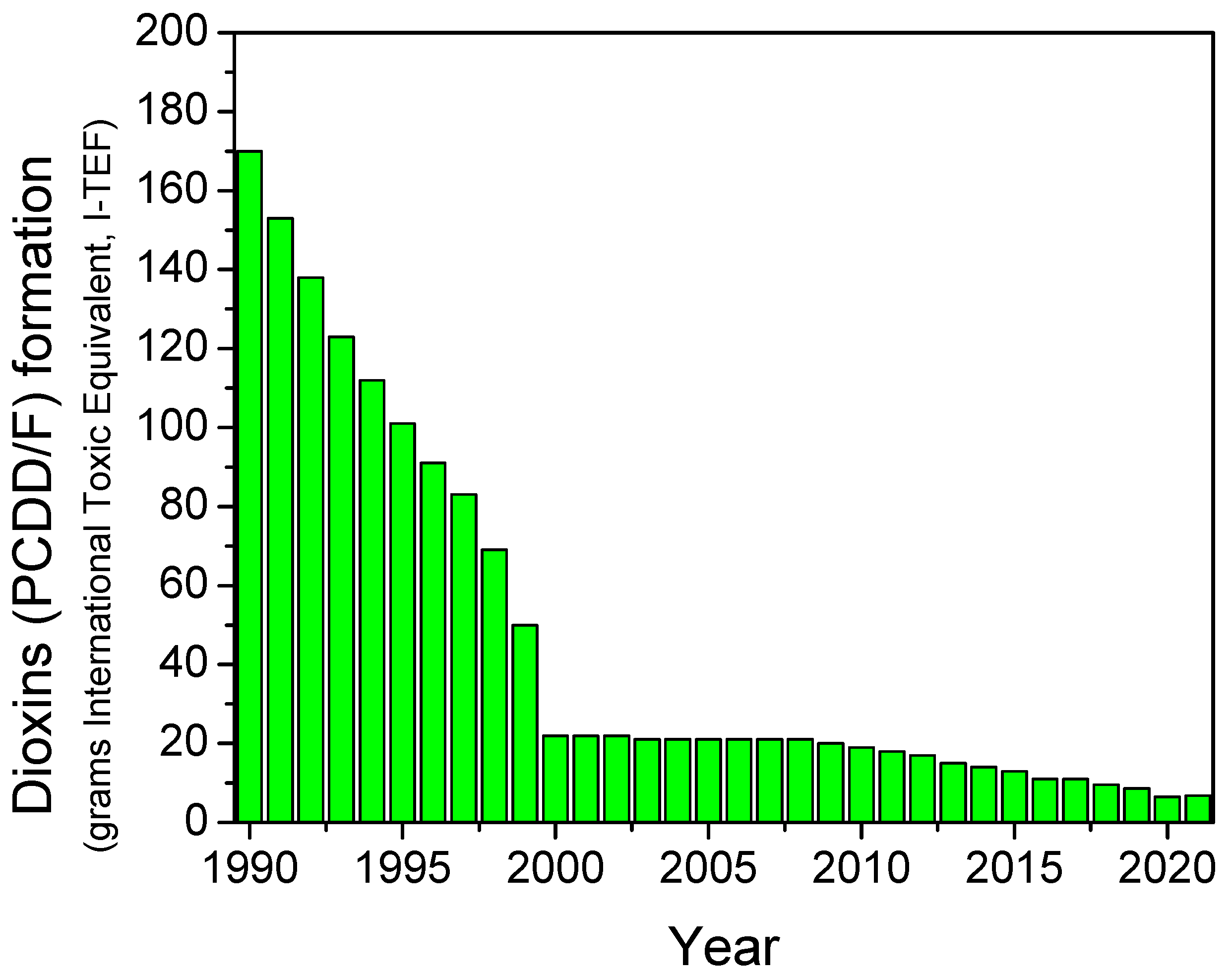
According to the World Health Organization (WHO), dioxin is a term commonly used to describe a group of chlorinated chemical compounds, polychlorinated dibenzo-para-dioxins (PCDDs) and polychlorinated dibenzo furans (PCDFs), characterized by similar chemical structure and properties [196]. The group of dioxins is made up of a total of 75 polychlorinated dibenzodioxins (PCDDs) and 135 polychlorinated dibenzofurans (PCDFs), called congeners or isomers [197]. Due to their chemical properties, dioxins are highly toxic and persistent in the environment [198]. To this end, there are significant efforts undertaken worldwide to control known sources of dioxin emissions [196,199]. Dioxins can be potentially formed in all combustion processes in which chlorine and a carbon source are present at a specific temperature (usually between 200 and 800 °C) [197,199,200,201,202,203]. Dioxin emissions from road transport are mainly related to compounds previously added to leaded petrol. On the other hand, diesel-fueled vehicles have been also reported to contribute to dioxin formation. As a consequence, in recent years, the total dioxins emissions coming from road transport have been significantly decreased, as it can be seen in Figure 5 (total emissions for 2021 were decreased by 95% and 69% since 1990 and 2001, respectively) [204]. In this context, vehicles with internal combustion engines do emit dioxins, and thus, they have been the subject of several studies in the literature, although the exact mechanism of formation is not well documented [197,205]. De novo synthesis of dioxins has been reported to be applicable as the mechanism of formation in vehicles (mainly in diesel-fueled vehicles), involving the solid-phase reactions with carbon, chlorine and oxygen on combustion-generated particles promoted by copper chloride as a catalyst [197,205,206,207,208], with the optimal temperatures for de novo dioxin formation being 200–400 °C. Alternatively, a mechanism for the gas-phase formation from precursors catalyzed by the presence of a transition metal such as copper is also reported in the literature [205,206,207].
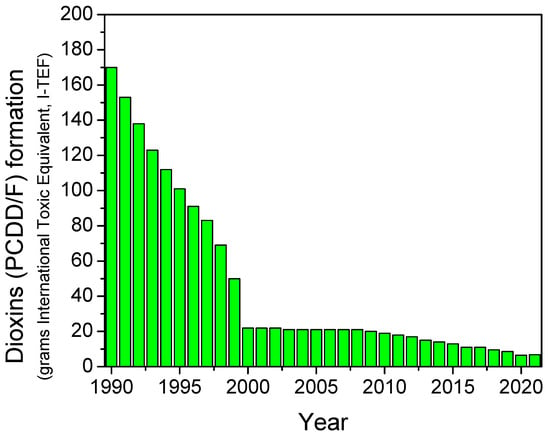
Figure 5.
Dioxins (PCDD/F) formation coming from transport sector (expressed in grams International Toxic Equivalent) between 1990 and 2021. Source: NAEI [204].
Diesel particle filters (DPF), which equip all modern diesel vehicles, trap soot, fly ash and related carbonaceous deposits on and within the DPF channel walls. These can form the required carbon phase needed for the de novo formation of dioxins. Thus, in the presence of chlorine (may be present in the fuel, additives or lubricating oils used), the formation of dioxins may be favored, while, at the same time, the presence of metals, such as Cu and Fe, could result in catalyzing the PCDD/F formation at temperatures between 250 and 400 °C. CuCl2 has been identified by several authors as a very reactive catalyst for dioxin formation [202,207,208,209].
A similar mechanism could be possible for dioxin formation in gasoline-fueled vehicles operating near stoichiometric conditions, where the formation of PM also takes place, and gasoline particulate filters (GPF) are often used in combination with TWC in closed coupled configurations. However, it should be noted that dioxin emissions from petrol fueled vehicles have been reported to have significantly decreased—by 99% between 1990 and 2021 [204].
The literature studies have suggested that the dioxin emission factors of gasoline-fueled vehicles equipped with exhaust aftertreatment systems are lower than the corresponding emission factors of diesel-fueled vehicles [197]. Moreover, chlorine concentration in petrol fuels, as well as in lubricating oils, has been decreased over the last decades [197,199], suggesting that the main factor influencing PCDD/F emissions (Cl concentration) is significantly reduced. Even in the presence of trace amounts of soot deposits, the formation of dioxins could be possible [210], especially considering a catalyst formulation including Cu as an active material. However, several recent studies have shown that in the case of modern vehicles equipped with state-of-the-art exhaust aftertreatment systems, the presence of Cu along with PGMs did not lead to increased PCDD/F emissions, at least compared to the engine out emissions [200,201,207]. Evidently, the overall status of engine tuning and the condition of the emission control system can further prohibit dioxin production.
3. Proposed Reaction Scheme for a Three-Way Catalytic Converter Containing Cu and PGMs
Based on the literature studies of elemental reaction pathways presented above for reactions occurring during the operation of a TWC incorporating Cu and PGMs under near stoichiometric conditions, a global reaction mechanism can be proposed to summarize the key findings. This is the first time that such a reaction mechanism is proposed for a catalyst incorporating copper in combination with PGMs under TWC conditions. The proposed overall reaction network is presented in Table 4 and is derived from Langmuir–Hinshelwood–Hougen–Watson principles. The reaction network takes into account all the main chemical species contained in the exhaust gas under all relevant operating conditions. It should be emphasized that CeO2 is used in the proposed reaction network included in Table 4 to represent the reactions taking place with the participation of the OSC material of the support, which is a CeZrO4 mixed oxide. The presence of ZrO2 enhances the carrier’s properties, without altering the mechanistic pathways followed.

Table 4.
Proposed overall reaction network for a Cu-based PGM-containing three-way catalyst.
A graphical summary of the suggested reaction network is also provided in Figure 6 that follows.
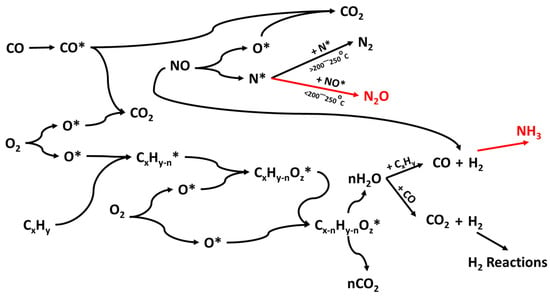
Figure 6.
Graphical summary of the proposed reaction network over a three-way catalytic converter containing Cu and PGMs (* indicates adsorbed compounds).
It should be noted that the reaction network does not differ significantly from that of a conventional PGM-based three-way catalyst [16,17,22,25]. This is expected given the similarities outlined in the previous sections regarding the activation energy values reported in the literature for the individual reactions on catalysts containing only PGMs, Cu or combinations of Cu and PGMs, which suggest a common reaction mechanism.
4. Conclusions/Summary
In this paper, a comprehensive literature review was conducted on existing studies evaluating the performance of commercial PGM-based catalysts and model Cu-containing formulations for TWC operation under near-stoichiometric conditions. Emphasis was placed on the mechanistic aspects of the individual steps involved in the reaction network. By synthesizing these findings, a potential reaction network for a TWC incorporating Cu nanoparticles alongside PGM nanoparticles, supported on a CeZrO4 mixed oxide carrier with high oxygen storage capacity, was proposed for the first time. This network was identified as crucial based on the exceptional activity results demonstrated by the recently homologated PROMETHEUS catalyst in our previous studies, when compared with commercial PGM-based automotive catalysts [51,52].
In summary, the overall network involves CO and CxHy oxidation reactions occurring on Cu and Pd (and their alloys), supported on a high oxygen storage capacity CeO2-ZrO2 support. The reduction of NO by CO is also included, with the activity of Rh playing a key role in determining the overall catalyst performance. For CO oxidation reaction specifically, the presence of Cu in combination with PGMs has been shown to enhance the reaction rate, as evidenced by the lower values of apparent activation energy reported in the literature, significantly reducing the light-off temperatures. However, the formation of by-products such as N2O or NH3 cannot be ruled out. Depending on the temperature and the fuel used (gasoline or CNG), the water gas shift reaction, steam reforming reactions, as well as reactions involving H2 (mainly from CH4 decomposition or steam reforming), may also contribute to the overall reaction network. The role of the high-OSC CeO2-ZrO2 mixed oxide is integral to the overall reaction scheme.
Based on the desired activity, by carefully adjusting the ratio between the metals, as well as the dispersion and nanoparticle size, CO and CxHy oxidation reactions could be favored due to the synergy between Cu and Pd nanoparticles, with a CeZrO4 carrier also participating in the reaction mechanism. As discussed, the oxygen storage/release ability of CeO2 is intensified in the presence of CuO. Addition of metallic Rh in the catalyst formulation, whose high activity in catalyzing the NO reduction by CO has been found to prevail even in multimetallic formulations, can prohibit the formation of undesired N2O. The formation of NH3 by-product cannot be ruled out, but the high CO oxidation activity of the catalyst is expected to prohibit its formation, since CO availability in the gaseous mixture has been directly related with the generation of NH3. At the same time, improving the OSC of TWCs and effectively controlling the lambda value near 1.0 (avoiding extended rich-burn operation) can further effectively prevent NH3 emissions. Moreover, although concern regarding the potential formation of dioxins could be valid since the presence of Cu could theoretically favor the formation of PCDD/F, low Cl content in fuels and lubricating oils, and the proper conditioning of exhaust aftertreatment systems and engine tuning should sufficiently control the formation of dioxins.
With a focus on future research aimed at deepening the understanding of the previously reported exceptional performance of PROMETHEUS TWC, which incorporates Cu and PGMs (partial substitution of PGMs in state-of-the-art catalytic converter formulations, resulting in significant cost reduction compared to the PGM-based catalysts), the proposed mechanism requires further validation. Thus, an extensive mechanistic study will be conducted in our future work to confirm the proposed mechanism and elucidate the performance of PROMETHEUS TWC by identifying the surface intermediate species formed during the operation of the catalyst. Thus, the reaction network proposed in Table 4 will be used in our subsequent study for the development of a suitable model for the simulation of chemical phenomena in such a three-way catalytic converter, enabling the calculation of kinetic parameters in conjunction with experimental data.
5. Patents
European Patent of Prometheus: copper and noble metal polymetallic catalysts for engine exhaust gas treatment, EP3569309. Applicant: Monolithos Catalysts and Recycling Ltd. Inventor: Iakovos Yakoumis.
Author Contributions
Conceptualization, I.Y. and Z.S.; methodology, A.M.M. and A.D.; validation, C.P., M.K. and A.D.; formal analysis, C.P. and M.K.; investigation, C.P. and M.K.; writing—original draft preparation, M.K., C.P., A.M.M. and A.D.; writing—review and editing, all authors; supervision, I.Y. and Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been funded by the VERA project, which has received funding from the European Union’s Horizon Europe research and innovation programme under grant agreement No. 101056893.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
Authors Christos Papadopoulos, Marios Kourtelesis, Anastasia Maria Moschovi, and Iakovos Yakoumis were employed by the Monolithos Catalysts & Recycling Limited. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Johnson, T.; Joshi, A. Review of Vehicle Engine Efficiency and Emissions. SAE Int. J. Engines 2018, 11, 1307–1330. [Google Scholar] [CrossRef]
- Johnson, T. Vehicular Emissions in Review. SAE Int. J. Engines 2016, 9, 1258–1275. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Melas, A.; Martini, G.; Dilara, P. Overview of Vehicle Exhaust Particle Number Regulations. Processes 2021, 9, 2216. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Deeba, M.; Alerasool, S. Gasoline automobile catalysis and its historical journey to cleaner air. Nat. Catal. 2019, 2, 603–613. [Google Scholar] [CrossRef]
- Dey, S.; Mehta, N.S. Automobile pollution control using catalysis. Resour. Environ. Sustain. 2020, 2, 100006. [Google Scholar] [CrossRef]
- Regulation (EU) 2023/851 of the European Parliament and of the Council of 19 April 2023 Amending Regulation (EU) 2019/631 as Regards Strengthening the CO2 Emission Performance Standards for New Passenger Cars and New Light Commercial Vehicles in Line with the Union’s Increased Climate Ambition. 2023. Available online: https://eur-lex.europa.eu/eli/reg/2023/851/oj/eng (accessed on 7 February 2025).
- Council of the EU. Euro 7: Council Adopts New Rules on Emission Limits for Cars, Vans and Trucks. 12 April 2024. Available online: https://www.consilium.europa.eu/en/press/press-releases/2024/04/12/euro-7-council-adopts-new-rules-on-emission-limits-for-cars-vans-and-trucks/ (accessed on 7 February 2025).
- Robles-Lorite, L.; Dorado-Vicente, R.; Torres-Jiménez, E.; Bombek, G.; Lešnik, L. Recent Advances in the Development of Automotive Catalytic Converters: A Systematic Review. Energies 2023, 16, 6425. [Google Scholar] [CrossRef]
- Boger, T.; Rose, D.; He, S.; Joshi, A. Developments for future EU7 regulations and the path to zero impact emissions—A catalyst substrate and filter supplier’s perspective. Transp. Eng. 2022, 10, 100129. [Google Scholar] [CrossRef]
- Datye, A.K.; Votsmeier, M. Opportunities and challenges in the development of advanced materials for emission control catalysts. Nat. Mater. 2021, 20, 1049–1059. [Google Scholar] [CrossRef]
- Hassdenteufel, A.; Schünemann, E.; Neubert, V.; Hirchenhein, A. Gasoline powertrain solutions with ultra low tailpipe emissions. Transp. Eng. 2022, 8, 100109. [Google Scholar] [CrossRef]
- Aminzadegan, S.; Shahriari, M.; Mehranfar, F.; Abramović, B. Factors affecting the emission of pollutants in different types of transportation: A literature review. Energy Rep. 2022, 8, 2508–2529. [Google Scholar] [CrossRef]
- Rood, S.; Eslava, S.; Manigrasso, A.; Bannister, C. Recent advances in gasoline three-way catalyst formulation: A review. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2019, 234, 936–949. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A review on oxygen storage capacity of CeO2-based materials: Influence factors, measurement techniques, and applications in reactions related to catalytic automotive emissions control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Hughes, A.E.; Haque, N.; Northey, S.A.; Giddey, S. Platinum Group Metals: A Review of Resources, Production and Usage with a Focus on Catalysts. Resources 2021, 10, 93. [Google Scholar] [CrossRef]
- Koltsakis, G.C.; Kandylas, I.P.; Stamatelos, A.M. Three-way catalytic converter modeling and applications. Chem. Eng. Commun. 1998, 164, 153–189. [Google Scholar] [CrossRef]
- Chatterjee, D.; Deutschmann, O.; Warnatz, J. Detailed surface reaction mechanism in a three-way catalyst. Faraday Discuss. 2002, 119, 371–384. [Google Scholar] [CrossRef]
- Oh, S.H.; Triplett, T. Reaction pathways and mechanism for ammonia formation and removal over palladium-based three-way catalysts: Multiple roles of CO. Catal. Today 2014, 231, 22–32. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Xu, Y.; Wu, Y.; Yang, W.; Huang, C.; Wang, X.; Zhong, L.; Wang, J.; Chen, Y. The formation mechanism of N2O and NH3 on PtRh three-way catalyst of natural gas vehicles. Mol. Catal. 2023, 547, 113392. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Grange, P.; Delmon, B. Catalytic removal of NO. Catal. Today 1998, 46, 233–316. [Google Scholar] [CrossRef]
- Burch, R. Knowledge and Know-How in Emission Control for Mobile Applications. Catal. Rev. 2004, 46, 271–334. [Google Scholar] [CrossRef]
- Kočí, P.; Kubíček, M.; Marek, M. Modeling of Three-Way-Catalyst Monolith Converters with Microkinetics and Diffusion in the Washcoat. Ind. Eng. Chem. Res. 2004, 43, 4503–4510. [Google Scholar] [CrossRef]
- Koltsakis, G.C.; Konstantinidis, P.A.; Stamatelos, A.M. Development and application range of mathematical models for 3-way catalytic converters. Appl. Catal. B Environ. 1997, 12, 161–191. [Google Scholar] [CrossRef]
- Montenegro, G.; Onorati, A. 1D Thermo-Fluid Dynamic Modeling of Reacting Flows inside Three-Way Catalytic Converters. SAE Int. J. Engines 2009, 2, 1444–1459. [Google Scholar] [CrossRef]
- Pontikakis, G.N.; Konstantas, G.S.; Stamatelos, A.M. Three-Way Catalytic Converter Modeling as a Modern Engineering Design Tool. J. Eng. Gas Turbines Power 2004, 126, 906–923. [Google Scholar] [CrossRef]
- Zeng, F.; Finke, J.; Olsen, D.; White, A.; Hohn, K.L. Modeling of three-way catalytic converter performance with exhaust mixtures from dithering natural gas-fueled engines. Chem. Eng. J. 2018, 352, 389–404. [Google Scholar] [CrossRef]
- PGM Prices and Trading. Available online: https://matthey.com/products-and-markets/pgms-and-circularity/pgm-management (accessed on 5 February 2025).
- Grilli, M.L.; Slobozeanu, A.E.; Larosa, C.; Paneva, D.; Yakoumis, I.; Cherkezova-Zheleva, Z. Platinum Group Metals: Green Recovery from Spent Auto-Catalysts and Reuse in New Catalysts—A Review. Crystals 2023, 13, 550. [Google Scholar] [CrossRef]
- Nakayama, H.; Kanno, Y.; Nagata, M.; Zheng, X. Development of TWC and PGM Free Catalyst Combination as Gasoline Exhaust Aftertreatment. SAE Int. J. Engines 2016, 9, 2194–2200. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Y.; Hu, L.; Yin, H.; Guo Wang, W. Synthesis, Characterization, and Catalytic Activity of Mn-doped Perovskite Oxides for Three-Way Catalysis. Chem. Eng. Technol. 2015, 38, 291–296. [Google Scholar] [CrossRef]
- Perin, G.; Fabro, J.; Guiotto, M.; Xin, Q.; Natile, M.M.; Cool, P.; Canu, P.; Glisenti, A. Cu@LaNiO3 based nanocomposites in TWC applications. Appl. Catal. B Environ. 2017, 209, 214–227. [Google Scholar] [CrossRef]
- Dey, S.; Chandra Dhal, G. Controlling carbon monoxide emissions from automobile vehicle exhaust using copper oxide catalysts in a catalytic converter. Mater. Today Chem. 2020, 17, 100282. [Google Scholar] [CrossRef]
- Yoshida, H.; Yamashita, N.; Ijichi, S.; Okabe, Y.; Misumi, S.; Hinokuma, S.; Machida, M. A Thermally Stable Cr–Cu Nanostructure Embedded in the CeO2 Surface as a Substitute for Platinum-Group Metal Catalysts. ACS Catal. 2015, 5, 6738–6747. [Google Scholar] [CrossRef]
- Yoshida, H.; Okabe, Y.; Misumi, S.; Oyama, H.; Tokusada, K.; Hinokuma, S.; Machida, M. Structures and Catalytic Properties of Cr–Cu Embedded CeO2 Surfaces with Different Cr/Cu Ratios. J. Phys. Chem. C 2016, 120, 26852–26863. [Google Scholar] [CrossRef]
- Machida, M. Heat- and corrosion-resistant catalytic materials for environmental and energy applications. J. Ceram. Soc. Jpn. 2021, 129, 234–240. [Google Scholar] [CrossRef]
- Hirakawa, T.; Shimokawa, Y.; Tokuzumi, W.; Sato, T.; Tsushida, M.; Yoshida, H.; Ohyama, J.; Machida, M. Multicomponent 3d Transition-Metal Nanoparticles as Catalysts Free of Pd, Pt, or Rh for Automotive Three-Way Catalytic Converters. ACS Appl. Nano Mater. 2020, 3, 9097–9107. [Google Scholar] [CrossRef]
- Pacella, M.; Garbujo, A.; Fabro, J.; Guiotto, M.; Xin, Q.; Natile, M.M.; Canu, P.; Cool, P.; Glisenti, A. PGM-free CuO/LaCoO3 nanocomposites: New opportunities for TWC application. Appl. Catal. B Environ. 2018, 227, 446–458. [Google Scholar] [CrossRef]
- Glisenti, A.; Pacella, M.; Guiotto, M.; Natile, M.M.; Canu, P. Largely Cu-doped LaCo1−Cu O3 perovskites for TWC: Toward new PGM-free catalysts. Appl. Catal. B Environ. 2016, 180, 94–105. [Google Scholar] [CrossRef]
- Ueda, K.; Ohyama, J.; Satsuma, A. Investigation of Reaction Mechanism of NO–C3H6–CO–O2 Reaction over NiFe2O4 Catalyst. ACS Omega 2017, 2, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hirakawa, T.; Oyama, H.; Nakashima, R.; Hinokuma, S.; Machida, M. Effect of Thermal Aging on Local Structure and Three-Way Catalysis of Cu/Al2O3. J. Phys. Chem. C 2019, 123, 10469–10476. [Google Scholar] [CrossRef]
- Copper Metal Prices. Available online: https://tradingeconomics.com/commodity/copper (accessed on 5 February 2025).
- Du, Y.; Gao, F.; Zhou, Y.; Yi, H.; Tang, X.; Qi, Z. Recent advance of CuO-CeO2 catalysts for catalytic elimination of CO and NO. J. Environ. Chem. Eng. 2021, 9, 106372. [Google Scholar] [CrossRef]
- Sun, J.; Ge, C.; Yao, X.; Zou, W.; Hong, X.; Tang, C.; Dong, L. Influence of different impregnation modes on the properties of CuO-CeO2/γ-Al2O3 catalysts for NO reduction by CO. Appl. Surf. Sci. 2017, 426, 279–286. [Google Scholar] [CrossRef]
- Luo, M.-F.; Fang, P.; He, M.; Xie, Y.-L. In situ XRD, Raman, and TPR studies of CuO/Al2O3 catalysts for CO oxidation. J. Mol. Catal. A Chem. 2005, 239, 243–248. [Google Scholar] [CrossRef]
- Artizzu, P.; Garbowski, E.; Primet, M.; Brulle, Y.; Saint-Just, J. Catalytic combustion of methane on aluminate-supported copper oxide. Catal. Today 1999, 47, 83–93. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, L.; Shen, M.; Liu, D.; Wang, J.; Ding, W.; Chen, Y. Influence of supports on the activities of copper oxide species in the low-temperature NO+CO reaction. Appl. Catal. B Environ. 2001, 31, 61–69. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Liu, Y.; Lian, D.; Chen, M.; Ji, Y.; Xing, L.; Wu, K.; Liu, S. Recent Advances of Cu-Based Catalysts for NO Reduction by CO under O2-Containing Conditions. Catalysts 2022, 12, 1402. [Google Scholar] [CrossRef]
- Papagianni, S.; Moschovi, A.-M.; Polyzou, E.; Yakoumis, I. Platinum Recovered from Automotive Heavy-Duty Diesel Engine Exhaust Systems in Hydrometallurgical Operation. Metals 2022, 12, 31. [Google Scholar] [CrossRef]
- Yakoumis, I.; Moschovi, A.; Panou, M.; Panias, D. Single-Step Hydrometallurgical Method for the Platinum Group Metals Leaching from Commercial Spent Automotive Catalysts. J. Sustain. Metall. 2020, 6, 259–268. [Google Scholar] [CrossRef]
- Moschovi, A.M.; Giuliano, M.; Kourtelesis, M.; Nicol, G.; Polyzou, E.; Parussa, F.; Yakoumis, I.; Sgroi, M.F. First of Its Kind Automotive Catalyst Prepared by Recycled PGMs-Catalytic Performance. Catalysts 2021, 11, 942. [Google Scholar] [CrossRef]
- Yakoumis, I. PROMETHEUS: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part I: Synthesis and Characterization. Materials 2021, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Yakoumis, I.; Polyzou, E.; Moschovi, A. Prometheus: A Copper-Based Polymetallic Catalyst for Automotive Applications. Part II: Catalytic Efficiency an Endurance as Compared with Original Catalysts. Materials 2021, 14, 2226. [Google Scholar] [CrossRef]
- Yakoumis, I. Copper and Noble Metal Polymetallic Catalysts for Engine Exhaust Gas Treatment. European Patent EP3569309A1, 20 November 2019. [Google Scholar]
- Soto Beobide, A.; Moschovi, A.M.; Mathioudakis, G.N.; Kourtelesis, M.; Lada, Z.G.; Andrikopoulos, K.S.; Sygellou, L.; Dracopoulos, V.; Yakoumis, I.; Voyiatzis, G.A. High Catalytic Efficiency of a Nanosized Copper-Based Catalyst for Automotives: A Physicochemical Characterization. Molecules 2022, 27, 7402. [Google Scholar] [CrossRef]
- Lu, C.-Y.; Chang, W.-C.; Wey, M.-Y. CuO/CeO2 catalysts prepared with different cerium supports for CO oxidation at low temperature. Mater. Chem. Phys. 2013, 141, 512–518. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, Y.; Ding, S.; Sattler, J.J.H.B.; Borodina, E.; Zhang, L.; Weckhuysen, B.M.; Su, H. Active sites over CuO/CeO2 and inverse CeO2/CuO catalysts for preferential CO oxidation. J. Power Sources 2014, 256, 301–311. [Google Scholar] [CrossRef]
- Tang, C.; Sun, J.; Yao, X.; Cao, Y.; Liu, L.; Ge, C.; Gao, F.; Dong, L. Efficient fabrication of active CuO-CeO2/SBA-15 catalysts for preferential oxidation of CO by solid state impregnation. Appl. Catal. B Environ. 2014, 146, 201–212. [Google Scholar] [CrossRef]
- Yu Yao, Y.-F. The oxidation of CO and C2H4 over metal oxides: V. SO2 effects. J. Catal. 1975, 39, 104–114. [Google Scholar] [CrossRef]
- Martínez-Arias, A.; Fernández-García, M.; Gálvez, O.; Coronado, J.M.; Anderson, J.A.; Conesa, J.C.; Soria, J.; Munuera, G. Comparative Study on Redox Properties and Catalytic Behavior for CO Oxidation of CuO/CeO2 and CuO/ZrCeO4 Catalysts. J. Catal. 2000, 195, 207–216. [Google Scholar] [CrossRef]
- Yang, Z.; He, B.; Lu, Z.; Hermansson, K. Physisorbed, Chemisorbed, and Oxidized CO on Highly Active Cu−CeO2(111). J. Phys. Chem. C 2010, 114, 4486–4494. [Google Scholar] [CrossRef]
- Jia, A.-P.; Jiang, S.-Y.; Lu, J.-Q.; Luo, M.-F. Study of Catalytic Activity at the CuO−CeO2 Interface for CO Oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. [Google Scholar] [CrossRef]
- Mrabet, D.; Abassi, A.; Cherizol, R.; Do, T.-O. One-pot solvothermal synthesis of mixed Cu-Ce-Ox nanocatalysts and their catalytic activity for low temperature CO oxidation. Appl. Catal. A Gen. 2012, 447–448, 60–66. [Google Scholar] [CrossRef]
- Guo, Y.; Li, C.; Lu, S.; Zhao, C. Low temperature CO catalytic oxidation and kinetic performances of KOH–Hopcalite in the presence of CO2. RSC Adv. 2016, 6, 7181–7188. [Google Scholar] [CrossRef]
- Vinodkumar, T.; Durgasri, D.N.; Maloth, S.; Reddy, B.M. Tuning the structural and catalytic properties of ceria by doping with Zr4+, La3+ and Eu3+ cations. J. Chem. Sci. 2015, 127, 1145–1153. [Google Scholar] [CrossRef]
- Dey, S.; Dhal, G.C. Highly Active Palladium Nanocatalysts for Low-Temperature Carbon Monoxide Oxidation. Polytechnica 2020, 3, 1–25. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Felpin, F.X.; Asefa, T.; Huang, X.; Silva, R.; Zou, X.; Zboril, R.; Varma, R.S. Cu and Cu-Based Nanoparticles: Synthesis and Applications in Catalysis. Chem. Rev. 2016, 116, 3722–3811. [Google Scholar] [CrossRef]
- Xu, F.; Mudiyanselage, K.; Baber, A.E.; Soldemo, M.; Weissenrieder, J.; White, M.G.; Stacchiola, D.J. Redox-Mediated Reconstruction of Copper during Carbon Monoxide Oxidation. J. Phys. Chem. C 2014, 118, 15902–15909. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Liu, P.; Wang, X.; Wen, W.; Hanson, J.; Hrbek, J.; Pérez, M.; Evans, J. Water-gas shift activity of Cu surfaces and Cu nanoparticles supported on metal oxides. Catal. Today 2009, 143, 45–50. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Q.; Stephanopoulos, M.F. Preferential oxidation of CO in H2 over CuO-CeO2 catalysts. Catal. Today 2004, 93–95, 241–246. [Google Scholar] [CrossRef]
- Oh, S.H.; Sinkevitch, R.M. Carbon Monoxide Removal from Hydrogen-Rich Fuel Cell Feedstreams by Selective Catalytic Oxidation. J. Catal. 1993, 142, 254–262. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Aljarash, A.; El-Shall, M.S.; Al Othman, Z.A.; Alghamdi, A.H. Microwave Synthesis of Bimetallic Nanoalloys and CO Oxidation on Ceria-Supported Nanoalloys. Chem. Mater. 2009, 21, 2825–2834. [Google Scholar] [CrossRef]
- Hungría, A.B.; Iglesias-Juez, A.; Martínez-Arias, A.; Fernández-García, M.; Anderson, J.A.; Conesa, J.C.; Soria, J. Effects of Copper on the Catalytic Properties of Bimetallic Pd–Cu/(Ce,Zr)Ox/Al2O3 and Pd–Cu/(Ce,Zr)Ox Catalysts for CO and NO Elimination. J. Catal. 2002, 206, 281–294. [Google Scholar] [CrossRef]
- Fernandez-García, M.; Martínez-Arias, A.; Anderson, J.A.; Conesa, J.C.; Soria, J. CO and NO elimination over Pd-Cu catalysts. In Studies in Surface Science and Catalysis; Corma, A., Melo, F.V., Mendioroz, S., Fierro, J.L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 130, pp. 1325–1330. [Google Scholar]
- Fernandez-García, M.; Martínez-Arias, A.; Belver, C.; Anderson, J.A.; Conesa, J.C.; Soria, J. Behavior of Palladium–Copper Catalysts for CO and NO Elimination. J. Catal. 2000, 190, 387–395. [Google Scholar] [CrossRef]
- Cai, F.; Yang, L.; Shan, S.; Mott, D.; Chen, B.H.; Luo, J.; Zhong, C.-J. Preparation of PdCu Alloy Nanocatalysts for Nitrate Hydrogenation and Carbon Monoxide Oxidation. Catalysts 2016, 6, 96. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; He, D. Catalytic oxidation of low-concentration CO at ambient temperature over supported Pd—Cu catalysts. Environ. Technol. 2014, 35, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, K.; Zhang, H.; Dong, Y.; Wang, T.; He, D. Low temperature CO catalytic oxidation over supported Pd–Cu catalysts calcined at different temperatures. Chem. Eng. J. 2014, 242, 10–18. [Google Scholar] [CrossRef]
- Wang, F.; Lu, G. Hydrogen feed gas purification over bimetallic Cu–Pd catalysts—Effects of copper precursors on CO oxidation. Int. J. Hydrogen Energy 2010, 35, 7253–7260. [Google Scholar] [CrossRef]
- Heo, I.; Wiebenga, M.H.; Gaudet, J.R.; Nam, I.-S.; Li, W.; Kim, C.H. Ultra low temperature CO and HC oxidation over Cu-based mixed oxides for future automotive applications. Appl. Catal. B Environ. 2014, 160–161, 365–373. [Google Scholar] [CrossRef]
- Singhania, A.; Gupta, S.M. Low-Temperature CO Oxidation: Effect of the Second Metal on Activated Carbon Supported Pd Catalysts. Catal. Lett. 2018, 148, 946–952. [Google Scholar] [CrossRef]
- Zedan, A.F.; Gaber, S.; AlJaber, A.S.; Polychronopoulou, K. CO Oxidation at Near-Ambient Temperatures over TiO2-Supported Pd-Cu Catalysts: Promoting Effect of Pd-Cu Nanointerface and TiO2 Morphology. Nanomaterials 2021, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Kugai, J.; Moriya, T.; Seino, S.; Nakagawa, T.; Ohkubo, Y.; Nitani, H.; Daimon, H.; Yamamoto, T.A. CeO2-supported Pt–Cu alloy nanoparticles synthesized by radiolytic process for highly selective CO oxidation. Int. J. Hydrogen Energy 2012, 37, 4787–4797. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Golubina, E.V.; Shilina, M.I. The effect of H2 treatment at 423–573K on the structure and synergistic activity of Pd–Cu alloy catalysts for low-temperature CO oxidation. Appl. Catal. B Environ. 2017, 208, 116–127. [Google Scholar] [CrossRef]
- Yao, Y.-F.Y. The oxidation of CO and hydrocarbons over noble metal catalysts. J. Catal. 1984, 87, 152–162. [Google Scholar] [CrossRef]
- Nguyen, T.-S.; Morfin, F.; Aouine, M.; Bosselet, F.; Rousset, J.-L.; Piccolo, L. Trends in the CO oxidation and PROX performances of the platinum-group metals supported on ceria. Catal. Today 2015, 253, 106–114. [Google Scholar] [CrossRef]
- Choi, K.I.; Vannice, M.A. CO oxidation over Pd and Cu catalysts III. Reduced Al2O3-supported Pd. J. Catal. 1991, 131, 1–21. [Google Scholar] [CrossRef]
- Nibbelke, R.H.; Campman, M.A.J.; Hoebink, J.H.B.J.; Marin, G.B. Kinetic Study of the CO Oxidation over Pt/γ-Al2O3and Pt/Rh/CeO2/γ-Al2O3in the Presence of H2O and CO2. J. Catal. 1997, 171, 358–373. [Google Scholar] [CrossRef]
- Oran, U.; Uner, D. Mechanisms of CO oxidation reaction and effect of chlorine ions on the CO oxidation reaction over Pt/CeO2 and Pt/CeO2/γ-Al2O3 catalysts. Appl. Catal. B Environ. 2004, 54, 183–191. [Google Scholar] [CrossRef]
- Liu, W.; Flytzanistephanopoulos, M. Total Oxidation of Carbon-Monoxide and Methane over Transition Metal Fluorite Oxide Composite Catalysts: II. Catalyst Characterization and Reaction-Kinetics. J. Catal. 1995, 153, 317–332. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Kinetics of CO and H2 oxidation over CuO–CeO2 and CuO catalysts. Chem. Eng. J. 2011, 176–177, 14–21. [Google Scholar] [CrossRef]
- Moreno, M.; Baronetti, G.T.; Laborde, M.A.; Mariño, F.J. Kinetics of preferential CO oxidation in H2 excess (COPROX) over CuO/CeO2 catalysts. Int. J. Hydrogen Energy 2008, 33, 3538–3542. [Google Scholar] [CrossRef]
- Marbán, G.; Fuertes, A.B. Highly active and selective CuOx/CeO2 catalyst prepared by a single-step citrate method for preferential oxidation of carbon monoxide. Appl. Catal. B Environ. 2005, 57, 43–53. [Google Scholar] [CrossRef]
- Sedmak, G.; Hočevar, S.; Levec, J. Kinetics of selective CO oxidation in excess of H2 over the nanostructured Cu0.1Ce0.9O2−y catalyst. J. Catal. 2003, 213, 135–150. [Google Scholar] [CrossRef]
- Tangpakonsab, P.; Genest, A.; Yang, J.; Meral, A.; Zou, B.; Yigit, N.; Schwarz, S.; Rupprechter, G. Kinetic and Computational Studies of CO Oxidation and PROX on Cu/CeO2 Nanospheres. Top. Catal. 2023, 66, 1129–1142. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.I.; Vannice, M.A. CO oxidation over Pd and Cu catalysts IV. Prereduced Al2O3-supported copper. J. Catal. 1991, 131, 22–35. [Google Scholar] [CrossRef]
- Fernández-García, M.; Conesa, J.C.; Clotet, A.; Ricart, J.M.; López, N.; Illas, F. Study of the Heterometallic Bond Nature in PdCu(111) Surfaces. J. Phys. Chem. B 1998, 102, 141–147. [Google Scholar] [CrossRef]
- Kamiuchi, N.; Haneda, M.; Ozawa, M. Propene oxidation over palladium catalysts supported on zirconium rich ceria–zirconia. Catal. Today 2015, 241, 100–106. [Google Scholar] [CrossRef]
- Kareem, H.; Shan, S.; Wu, Z.-P.; Velasco, L.; Moseman, K.; O’Brien, C.P.; Tran, D.T.; Lee, I.C.; Maswadeh, Y.; Yang, L.; et al. Catalytic oxidation of propane over palladium alloyed with gold: An assessment of the chemical and intermediate species. Catal. Sci. Technol. 2018, 8, 6228–6240. [Google Scholar] [CrossRef]
- Holder, R.; Bollig, M.; Anderson, D.R.; Hochmuth, J.K. A discussion on transport phenomena and three-way kinetics of monolithic converters. Chem. Eng. Sci. 2006, 61, 8010–8027. [Google Scholar] [CrossRef]
- Courtois, X.; Perrichon, V.; Primet, M. Three-way catalytic activity of alumina-supported copper catalysts modified by rhodium. Comptes Rendus De L’académie Des Sci. Ser. IIC Chem. 2000, 3, 429–436. [Google Scholar] [CrossRef]
- Courtois, X.; Perrichon, V. Distinct roles of copper in bimetallic copper–rhodium three-way catalysts deposited on redox supports. Appl. Catal. B Environ. 2005, 57, 63–72. [Google Scholar] [CrossRef]
- Ferri, D.; Elsener, M.; Kröcher, O. Methane oxidation over a honeycomb Pd-only three-way catalyst under static and periodic operation. Appl. Catal. B Environ. 2018, 220, 67–77. [Google Scholar] [CrossRef]
- Jiang, D.; Khivantsev, K.; Wang, Y. Low-Temperature Methane Oxidation for Efficient Emission Control in Natural Gas Vehicles: Pd and Beyond. ACS Catal. 2020, 10, 14304–14314. [Google Scholar] [CrossRef]
- Trivedi, S.; Prasad, R.; Mishra, A.; Kalam, A.; Yadav, P. Current scenario of CNG vehicular pollution and their possible abatement technologies: An overview. Environ. Sci. Pollut. Res. 2020, 27, 39977–40000. [Google Scholar] [CrossRef]
- Xi, Y.; Ottinger, N.; Liu, Z.G. The Dynamics of Methane and NOx Removal by a Three-Way Catalyst: A Transient Response Study. SAE Int. J. Engines 2018, 11, 1331–1341. [Google Scholar] [CrossRef]
- Raj, B.A. Methane Emission Control. Johns. Matthey Technol. Rev. 2016, 60, 228–235. [Google Scholar] [CrossRef]
- Dimaratos, A.; Toumasatos, Z.; Doulgeris, S.; Triantafyllopoulos, G.; Kontses, A.; Samaras, Z. Assessment of CO2 and NOx Emissions of One Diesel and One Bi-Fuel Gasoline/CNG Euro 6 Vehicles During Real-World Driving and Laboratory Testing. Front. Mech. Eng. 2019, 5, 62. [Google Scholar] [CrossRef]
- Dimaratos, A.; Toumasatos, Z.; Triantafyllopoulos, G.; Kontses, A.; Samaras, Z. Real-world gaseous and particle emissions of a Bi-fuel gasoline/CNG Euro 6 passenger car. Transp. Res. Part D Transp. Environ. 2020, 82, 102307. [Google Scholar] [CrossRef]
- Wang, M.; Dimopoulos Eggenschwiler, P.; Franken, T.; Ferri, D.; Kröcher, O. Reaction pathways of methane abatement in Pd-Rh three-way catalyst in heavy duty applications: A combined approach based on exhaust analysis, model gas reactor and DRIFTS measurements. Chem. Eng. J. 2021, 422, 129932. [Google Scholar] [CrossRef]
- Stoian, M.; Rogé, V.; Lazar, L.; Maurer, T.; Védrine, J.C.; Marcu, I.-C.; Fechete, I. Total Oxidation of Methane on Oxide and Mixed Oxide Ceria-Containing Catalysts. Catalysts 2021, 11, 427. [Google Scholar] [CrossRef]
- Najbar, M.; Barańska, M.; Jura, W. Low temperature oxidation of light hydrocarbons over silica supported noble metal catalysts. Catal. Today 1993, 17, 201–208. [Google Scholar] [CrossRef]
- Lampert, J.K.; Kazi, M.S.; Farrauto, R.J. Palladium catalyst performance for methane emissions abatement from lean burn natural gas vehicles. Appl. Catal. B Environ. 1997, 14, 211–223. [Google Scholar] [CrossRef]
- Farrauto, R.J.; Hobson, M.C.; Kennelly, T.; Waterman, E.M. Catalytic chemistry of supported palladium for combustion of methane. Appl. Catal. A Gen. 1992, 81, 227–237. [Google Scholar] [CrossRef]
- Domingos, D.; Simplício, L.M.T.; Estrela, G.l.S.; Prazeres, M.A.G.d.; Brandão, S.T. Catalytic combustion of methane over pdo-ceO2/al2O3 and pdo-ceO2/zrO2 catalysts. In Studies in Surface Science and Catalysis; Bellot Noronha, F., Schmal, M., Falabella Sousa-Aguiar, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 167, pp. 7–12. [Google Scholar]
- Lapisardi, G.; Urfels, L.; Gélin, P.; Primet, M.; Kaddouri, A.; Garbowski, E.; Toppi, S.; Tena, E. Superior catalytic behaviour of Pt-doped Pd catalysts in the complete oxidation of methane at low temperature. Catal. Today 2006, 117, 564–568. [Google Scholar] [CrossRef]
- Reyes, P.; Figueroa, A.; Pecchi, G.; Fierro, J.L.G. Catalytic combustion of methane on Pd–Cu/SiO2 catalysts. Catal. Today 2000, 62, 209–217. [Google Scholar] [CrossRef]
- Stotz, H.; Maier, L.; Deutschmann, O. Methane Oxidation over Palladium: On the Mechanism in Fuel-Rich Mixtures at High Temperatures. Top. Catal. 2017, 60, 83–109. [Google Scholar] [CrossRef]
- Chrzan, M.; Chlebda, D.; Jodłowski, P.; Salomon, E.; Kołodziej, A.; Gancarczyk, A.; Sitarz, M.; Łojewska, J. Towards Methane Combustion Mechanism on Metal Oxides Supported Catalysts: Ceria Supported Palladium Catalysts. Top. Catal. 2019, 62, 403–412. [Google Scholar] [CrossRef]
- He, L.; Fan, Y.; Bellettre, J.; Yue, J.; Luo, L. A review on catalytic methane combustion at low temperatures: Catalysts, mechanisms, reaction conditions and reactor designs. Renew. Sustain. Energy Rev. 2020, 119, 109589. [Google Scholar] [CrossRef]
- Au-Yeung, J.; Chen, K.; Bell, A.T.; Iglesia, E. Isotopic Studies of Methane Oxidation Pathways on PdO Catalysts. J. Catal. 1999, 188, 132–139. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Oh, D.G.; Aleksandrov, H.A.; Kim, H.; Koleva, I.Z.; Khivantsev, K.; Vayssilov, G.N.; Kwak, J.H. Understanding of Active Sites and Interconversion of Pd and PdO during CH4 Oxidation. Molecules 2023, 28, 1957. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jiang, M.; Yang, L.; Li, Z.; Tian, F.-X.; He, Y. Recent progress of catalytic methane combustion over transition metal oxide catalysts. Front. Chem. 2022, 10, 959422. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-F.; Chou, F.-C.; Lee, F.-C.; Lin, C.-Y.; Tsai, D.-H. Synergistic Catalysis of Methane Combustion Using Cu–Ce–O Hybrid Nanoparticles with High Activity and Operation Stability. J. Phys. Chem. C 2016, 120, 27389–27398. [Google Scholar] [CrossRef]
- Ribeiro, F.H.; Chow, M.; Dallabetta, R.A. Kinetics of the Complete Oxidation of Methane over Supported Palladium Catalysts. J. Catal. 1994, 146, 537–544. [Google Scholar] [CrossRef]
- Anderson, R.B.; Stein, K.C.; Feenan, J.J.; Hofer, L.J.E. Catalytic Oxidation of Methane. Ind. Eng. Chem. 1961, 53, 809–812. [Google Scholar] [CrossRef]
- Cullis, C.F.; Willatt, B.M. Oxidation of methane over supported precious metal catalysts. J. Catal. 1983, 83, 267–285. [Google Scholar] [CrossRef]
- Yao, Y.-F.Y. Oxidation of Alkanes over Noble Metal Catalysts. Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 293–298. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Ji, K.; Sun, H.; Li, J. Pt Nanoparticles Embedded in Colloidal Crystal Template Derived 3D Ordered Macroporous Ce0.6Zr0.3Y0.1O2: Highly Efficient Catalysts for Methane Combustion. ACS Catal. 2015, 5, 1781–1793. [Google Scholar] [CrossRef]
- Bozo, C.; Guilhaume, N.; Garbowski, E.; Primet, M. Combustion of methane on CeO2–ZrO2 based catalysts. Catal. Today 2000, 59, 33–45. [Google Scholar] [CrossRef]
- Burch, R.; Loader, P.K. Investigation of Pt/Al2O3 and Pd/Al2O3 catalysts for the combustion of methane at low concentrations. Appl. Catal. B Environ. 1994, 5, 149–164. [Google Scholar] [CrossRef]
- Kumar, R.S.; Mmbaga, J.P.; Semagina, N.; Hayes, R.E. Methane Combustion Kinetics over Palladium-Based Catalysts: Review and Modelling Guidelines. Catalysts 2024, 14, 319. [Google Scholar] [CrossRef]
- Kundakovic, L.; Flytzani-Stephanopoulos, M. Reduction characteristics of copper oxide in cerium and zirconium oxide systems. Appl. Catal. A Gen. 1998, 171, 13–29. [Google Scholar] [CrossRef]
- Iamarino, M.; Chirone, R.; Lisi, L.; Pirone, R.; Salatino, P.; Russo, G. Cu/γ-Al2O3 catalyst for the combustion of methane in a fluidized bed reactor. Catal. Today 2002, 75, 317–324. [Google Scholar] [CrossRef]
- Águila, G.; Gracia, F.; Cortés, J.; Araya, P. Effect of copper species and the presence of reaction products on the activity of methane oxidation on supported CuO catalysts. Appl. Catal. B Environ. 2008, 77, 325–338. [Google Scholar] [CrossRef]
- DiGiulio, C.D.; Pihl, J.A.; Ii, J.E.P.; Amiridis, M.D.; Toops, T.J. Passive-ammonia selective catalytic reduction (SCR): Understanding NH3 formation over close-coupled three way catalysts (TWC). Catal. Today 2014, 231, 33–45. [Google Scholar] [CrossRef]
- Forzatti, P.; Castoldi, L.; Nova, I.; Lietti, L.; Tronconi, E. NOx removal catalysis under lean conditions. Catal. Today 2006, 117, 316–320. [Google Scholar] [CrossRef]
- Cheng, X.; Zhang, X.; Su, D.; Wang, Z.; Chang, J.; Ma, C. NO reduction by CO over copper catalyst supported on mixed CeO2 and Fe2O3: Catalyst design and activity test. Appl. Catal. B Environ. 2018, 239, 485–501. [Google Scholar] [CrossRef]
- Yao, X.; Yu, Q.; Ji, Z.; Lv, Y.; Cao, Y.; Tang, C.; Gao, F.; Dong, L.; Chen, Y. A comparative study of different doped metal cations on the reduction, adsorption and activity of CuO/Ce0.67M0.33O2 (M=Zr4+, Sn4+, Ti4+) catalysts for NO+CO reaction. Appl. Catal. B Environ. 2013, 130–131, 293–304. [Google Scholar] [CrossRef]
- Liu, L.; Liu, B.; Dong, L.; Zhu, J.; Wan, H.; Sun, K.; Zhao, B.; Zhu, H.; Dong, L.; Chen, Y. In situ FT-infrared investigation of CO or/and NO interaction with CuO/Ce0.67Zr0.33O2 catalysts. Appl. Catal. B Environ. 2009, 90, 578–586. [Google Scholar] [CrossRef]
- Liu, L.; Yao, Z.; Liu, B.; Dong, L. Correlation of structural characteristics with catalytic performance of CuO/CexZr1−xO2 catalysts for NO reduction by CO. J. Catal. 2010, 275, 45–60. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Hu, Z.; Yao, M.; Li, Y. A Review on the Pd-Based Three-Way Catalyst. Catal. Rev. 2015, 57, 79–144. [Google Scholar] [CrossRef]
- Asakura, H.; Hosokawa, S.; Ina, T.; Kato, K.; Nitta, K.; Uera, K.; Uruga, T.; Miura, H.; Shishido, T.; Ohyama, J.; et al. Dynamic Behavior of Rh Species in Rh/Al2O3 Model Catalyst during Three-Way Catalytic Reaction: An Operando X-ray Absorption Spectroscopy Study. J. Am. Chem. Soc. 2018, 140, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Burch, R.; Breen, J.P.; Meunier, F.C. A review of the selective reduction of NOx with hydrocarbons under lean-burn conditions with non-zeolitic oxide and platinum group metal catalysts. Appl. Catal. B Environ. 2002, 39, 283–303. [Google Scholar] [CrossRef]
- Burch, R.; Shestov, A.A.; Sullivan, J.A. A Steady-State Isotopic Transient Kinetic Analysis of the NO/O2/H2 Reaction over Pt/SiO2 Catalysts. J. Catal. 1999, 188, 69–82. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx Abatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, S.; Chafik, T.; Ukisu, Y.; Miyadera, T. Reactivity of surface isocyanate species with NO, O2 and NO+O2 in selective reduction of NOχ over Ag/Al2O3 and Al2O3 catalysts. Catal. Lett. 1998, 55, 211–215. [Google Scholar] [CrossRef]
- Liang, J.; Wang, H.P.; Spicer, L.D. FT-IR study of nitric oxide chemisorbed on rhodium/alumina. J. Phys. Chem. 1985, 89, 5840–5845. [Google Scholar] [CrossRef]
- Almusaiteer, K.A.; Chuang, S.S.C. Infrared Characterization of Rh Surface States and Their Adsorbates during the NO−CO Reaction. J. Phys. Chem. B 2000, 104, 2265–2272. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Chafik, T.; Verykios, X.E. Catalytic Reduction of NO by CO over Rhodium Catalysts: 2. Effect of Oxygen on the Nature, Population, and Reactivity of Surface Species Formed under Reaction Conditions. J. Catal. 2000, 191, 147–164. [Google Scholar] [CrossRef]
- Gayen, A.; Priolkar, K.R.; Sarode, P.R.; Jayaram, V.; Hegde, M.S.; Subbanna, G.N.; Emura, S. Ce1-xRhxO2-δ Solid Solution Formation in Combustion-Synthesized Rh/CeO2 Catalyst Studied by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2004, 16, 2317–2328. [Google Scholar] [CrossRef]
- Frank, B.; Renken, A. Kinetics and Deactivation of the NO Reduction by CO on Pt-Supported Catalysts. Chem. Eng. Technol. 1999, 22, 490–494. [Google Scholar] [CrossRef]
- Oh, S.H.; Fisher, G.B.; Carpenter, J.E.; Goodman, D.W. Comparative kinetic studies of CO-O2 and CO-NO reactions over single crystal and supported rhodium catalysts. J. Catal. 1986, 100, 360–376. [Google Scholar] [CrossRef]
- Oh, S.H. Effects of cerium addition on the CO+NO reaction kinetics over alumina-supported rhodium catalysts. J. Catal. 1990, 124, 477–487. [Google Scholar] [CrossRef]
- Fu, Y.; Tian, Y.; Lin, P. A low-temperature IR spectroscopic study of selective adsorption of NO and CO on CuO/γ-Al2O3. J. Catal. 1991, 132, 85–91. [Google Scholar] [CrossRef]
- London, J.W.; Bell, A.T. A simultaneous infrared and kinetic study of the reduction of nitric oxide by carbon monoxide over copper oxide. J. Catal. 1973, 31, 96–109. [Google Scholar] [CrossRef]
- Li, P.; Feng, L.; Yuan, F.; Wang, D.; Dong, Y.; Niu, X.; Zhu, Y. Effect of Surface Copper Species on NO + CO Reaction over xCuO-Ce0.9Zr0.1O2 Catalysts: In Situ DRIFTS Studies. Catalysts 2016, 6, 124. [Google Scholar] [CrossRef]
- Zhang, R.; Teoh, W.Y.; Amal, R.; Chen, B.; Kaliaguine, S. Catalytic reduction of NO by CO over Cu/CexZr1−xO2 prepared by flame synthesis. J. Catal. 2010, 272, 210–219. [Google Scholar] [CrossRef]
- Yao, X.; Gao, F.; Yu, Q.; Qi, L.; Tang, C.; Dong, L.; Chen, Y. NO reduction by CO over CuO–CeO2 catalysts: Effect of preparation methods. Catal. Sci. Technol. 2013, 3, 1355–1366. [Google Scholar] [CrossRef]
- Ukisu, Y.; Sato, S.; Muramatsu, G.; Yoshida, K. Surface isocyanate intermediate formed during the catalytic reduction of nitrogen oxide in the presence of oxygen and propylene. Catal. Lett. 1991, 11, 177–181. [Google Scholar] [CrossRef]
- Sun, R.; Yu, F.; Wan, Y.; Pan, K.; Li, W.; Zhao, H.; Dan, J.; Dai, B. Reducing N2O Formation over CO-SCR Systems with CuCe Mixed Metal Oxides. ChemCatChem 2021, 13, 2709–2718. [Google Scholar] [CrossRef]
- Song, J.; Choi, M.; Lee, J.; Kim, J.M. Improvement of Fuel Economy and Greenhouse Gases Reduction in Gasoline Powered Vehicles Through the TWC-NOx Trap Catalyst. Int. J. Automot. Technol. 2020, 21, 441–449. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Li, N.; Tan, J.; Chen, D. Research on ammonia emissions from three-way catalytic converters based on small sample test and vehicle test. Sci. Total Environ. 2021, 795, 148926. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Bertoa, R.; Pechout, M.; Vojtíšek, M.; Astorga, C. Regulated and Non-Regulated Emissions from Euro 6 Diesel, Gasoline and CNG Vehicles under Real-World Driving Conditions. Atmosphere 2020, 11, 204. [Google Scholar] [CrossRef]
- Hakeem, K.R.; Sabir, M.; Ozturk, M.; Akhtar, M.S.; Ibrahim, F.H. Nitrate and Nitrogen Oxides: Sources, Health Effects and Their Remediation. Rev. Environ. Contam. Toxicol. 2017, 242, 183–217. [Google Scholar] [CrossRef]
- Pinder, R.W.; Gilliland, A.B.; Dennis, R.L. Environmental impact of atmospheric NH3 emissions under present and future conditions in the eastern United States. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef]
- Wyer, K.E.; Kelleghan, D.B.; Blanes-Vidal, V.; Schauberger, G.; Curran, T.P. Ammonia emissions from agriculture and their contribution to fine particulate matter: A review of implications for human health. J. Environ. Manag. 2022, 323, 116285. [Google Scholar] [CrossRef] [PubMed]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Ma, R.; Li, K.; Guo, Y.; Zhang, B.; Zhao, X.; Linder, S.; Guan, C.; Chen, G.; Gan, Y.; Meng, J. Mitigation potential of global ammonia emissions and related health impacts in the trade network. Nat. Commun. 2021, 12, 6308. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.E.; Tsigaridis, K.; Miller, R. Significant atmospheric aerosol pollution caused by world food cultivation. Geophys. Res. Lett. 2016, 43, 5394–5400. [Google Scholar] [CrossRef]
- Bae, W.B.; Kim, D.Y.; Byun, S.W.; Hazlett, M.; Yoon, D.Y.; Jung, C.; Kim, C.H.; Kang, S.B. Emission of NH3 and N2O during NO reduction over commercial aged three-way catalyst (TWC): Role of individual reductants in simulated exhausts. Chem. Eng. J. Adv. 2022, 9, 100222. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, M.; Wang, J.; Wang, X.; Wang, J. N2O formation during NOx storage and reduction using C3H6 as reductant. Catal. Today 2017, 297, 92–103. [Google Scholar] [CrossRef]
- Dimaratos, A.; Kontses, D.; Kontses, A.; Saltas, E.; Raptopoulos-Chatzistefanou, A.; Andersson, J.; Aakko-Saksa, P.; Samaras, Z. Emissions of currently non-regulated gaseous pollutants from modern passenger cars. Transp. Res. Procedia 2023, 72, 3078–3085. [Google Scholar] [CrossRef]
- Dégeilh, P.; Kermani, J.; Sery, J.; Michel, P. Study of Euro 6d-TEMP Emissions—IFPEN for DGEC; Summary Report; Ministère de l’Ecologie: Paris, France, 2020. [Google Scholar]
- Clairotte, M.; Suarez-Bertoa, R.; Zardini, A.A.; Giechaskiel, B.; Pavlovic, J.; Valverde, V.; Ciuffo, B.; Astorga, C. Exhaust emission factors of greenhouse gases (GHGs) from European road vehicles. Environ. Sci. Eur. 2020, 32, 125. [Google Scholar] [CrossRef]
- Macleod, N.; Isaac, J.; Lambert, R.M. A comparison of sodium-modified Rh/γ-Al2O3 and Pd/γ-Al2O3 catalysts operated under simulated TWC conditions. Appl. Catal. B Environ. 2001, 33, 335–343. [Google Scholar] [CrossRef]
- Obuchi, A.; Naito, S.; Onishi, T.; Tamaru, K. Mechanism of catalytic reduction of NO by H2 or CO on a Pd foil; Role of chemisorbed nitrogen on Pd. Surf. Sci. 1982, 122, 235–255. [Google Scholar] [CrossRef]
- Obuchi, A.; Naito, S.; Onishi, T.; Tamaru, K. Mechanism of NO+H2 reaction over Rh foil and the role of chemisorbed nitrogen atoms. Surf. Sci. 1983, 130, 29–40. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Valverde, V.; Kontses, A.; Suarez-Bertoa, R.; Selleri, T.; Melas, A.; Otura, M.; Ferrarese, C.; Martini, G.; Balazs, A.; et al. Effect of Extreme Temperatures and Driving Conditions on Gaseous Pollutants of a Euro 6d-Temp Gasoline Vehicle. Atmosphere 2021, 12, 1011. [Google Scholar] [CrossRef]
- Wang, C.; Tan, J.; Harle, G.; Gong, H.; Xia, W.; Zheng, T.; Yang, D.; Ge, Y.; Zhao, Y. Ammonia Formation over Pd/Rh Three-Way Catalysts during Lean-to-Rich Fluctuations: The Effect of the Catalyst Aging, Exhaust Temperature, Lambda, and Duration in Rich Conditions. Environ. Sci. Technol. 2019, 53, 12621–12628. [Google Scholar] [CrossRef] [PubMed]
- Heeb, N.V.; Forss, A.-M.; Brühlmann, S.; Lüscher, R.; Saxer, C.J.; Hug, P. Three-way catalyst-induced formation of ammonia—Velocity- and acceleration-dependent emission factors. Atmos. Environ. 2006, 40, 5986–5997. [Google Scholar] [CrossRef]
- Heeb, N.V.; Saxer, C.J.; Forss, A.-M.; Brühlmann, S. Trends of NO-, NO2-, and NH3-emissions from gasoline-fueled Euro-3- to Euro-4-passenger cars. Atmos. Environ. 2008, 42, 2543–2554. [Google Scholar] [CrossRef]
- Hirano, H.; Yamada, T.; Tanaka, K.I.; Siera, J.; Cobden, P.; Nieuwenhuys, B.E. Mechanisms of the various nitric oxide reduction reactions on a platinum-rhodium (100) alloy single crystal surface. Surf. Sci. 1992, 262, 97–112. [Google Scholar] [CrossRef]
- Kobylinski, T.P.; Taylor, B.W. The catalytic chemistry of nitric oxide: II. Reduction of nitric oxide over noble metal catalysts. J. Catal. 1974, 33, 376–384. [Google Scholar] [CrossRef]
- Nova, I.; Lietti, L.; Forzatti, P.; Prinetto, F.; Ghiotti, G. Experimental investigation of the reduction of NOx species by CO and H2 over Pt–Ba/Al2O3 lean NOx trap systems. Catal. Today 2010, 151, 330–337. [Google Scholar] [CrossRef]
- Borsari, V.; Assunção, J.V.d. Ammonia emissions from a light-duty vehicle. Transp. Res. Part D Transp. Environ. 2017, 51, 53–61. [Google Scholar] [CrossRef]
- Breen, J.P.; Burch, R.; Lingaiah, N. An Investigation of Catalysts for the On Board Synthesis of NH3. A Possible Route to Low Temperature NOx Reduction for Lean-Burn Engines. Catal. Lett. 2002, 79, 171–174. [Google Scholar] [CrossRef]
- Schlatter, J.C.; Taylor, K.C. Platinum and palladium addition to supported rhodium catalysts for automotive emission control. J. Catal. 1977, 49, 42–50. [Google Scholar] [CrossRef]
- Wunsch, R.; Schön, C.; Frey, M.; Tran, D.; Proske, S.; Wandrey, T.; Kalogirou, M.; Schäffner, J. Detailed experimental investigation of the NOx reaction pathways of three-way catalysts with focus on intermediate reactions of NH3 and N2O. Appl. Catal. B Environ. 2020, 272, 118937. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Astorga, C. Isocyanic acid and ammonia in vehicle emissions. Transp. Res. Part D Transp. Environ. 2016, 49, 259–270. [Google Scholar] [CrossRef]
- Shelef, M.; Graham, G.W. Why Rhodium in Automotive Three-Way Catalysts? Catal. Rev. 1994, 36, 433–457. [Google Scholar] [CrossRef]
- Goula, G.; Botzolaki, G.; Osatiashtiani, A.; Parlett, C.M.A.; Kyriakou, G.; Lambert, R.M.; Yentekakis, I.V. Oxidative Thermal Sintering and Redispersion of Rh Nanoparticles on Supports with High Oxygen Ion Lability. Catalysts 2019, 9, 541. [Google Scholar] [CrossRef]
- Nagai, Y.; Hirabayashi, T.; Dohmae, K.; Takagi, N.; Minami, T.; Shinjoh, H.; Matsumoto, S.i. Sintering inhibition mechanism of platinum supported on ceria-based oxide and Pt-oxide–support interaction. J. Catal. 2006, 242, 103–109. [Google Scholar] [CrossRef]
- Taylor, K.C. Nitric Oxide Catalysis in Automotive Exhaust Systems. Catal. Rev. 1993, 35, 457–481. [Google Scholar] [CrossRef]
- WHO. Air Quality Guidelines for Europe, 2nd ed.; World Health Organization: Geneva, Switzerland, 2000.
- Smit, R.; Zeise, K.; Caffin, A.; Anyon, P. Dioxins Emissions from Motor Vehicles in Australia, National Dioxins Program Technical Report No. 2; Department of Climate Change, Energy, the Environment and Water: Canberra, Australia, 2004; pp. 1–55. [Google Scholar]
- Srogi, K. Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: A review. Environ. Chem. Lett. 2007, 6, 1–28. [Google Scholar] [CrossRef]
- Dyke, P.H.; Sutton, M.; Wood, D.; Marshall, J. Investigations on the effect of chlorine in lubricating oil and the presence of a diesel oxidation catalyst on PCDD/F releases from an internal combustion engine. Chemosphere 2007, 67, 1275–1286. [Google Scholar] [CrossRef]
- Laroo, C.A.; Schenk, C.R.; Sanchez, L.J.; McDonald, J. Emissions of PCDD/Fs, PCBs, and PAHs from a modern diesel engine equipped with catalyzed emission control systems. Envrion. Sci Technol 2011, 45, 6420–6428. [Google Scholar] [CrossRef]
- Liu, Z.G.; Wall, J.C.; Barge, P.; Dettmann, M.E.; Ottinger, N.A. Investigation of PCDD/F emissions from mobile source diesel engines: Impact of copper zeolite SCR catalysts and exhaust aftertreatment configurations. Envrion. Sci Technol 2011, 45, 2965–2972. [Google Scholar] [CrossRef]
- Olie, K.; Addink, R.; Schoonenboom, M. Metals as Catalysts during the Formation and Decomposition of Chlorinated Dioxins and Furans in Incineration Processes. J. Air Waste Manag. Assoc. 1995 1998, 48, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Bremmer, H.J.; Troost, L.M.; Kuipers, G.; de Koning, J.; Sein, A.A. Emissions of Dioxins in the Netherlands; National Institute for Public Health and the Environment RIVM: Utrecht, The Netherlands, 1994. [Google Scholar]
- National Atomospheric Emissions Inventory. Dioxins (PCDD/F) Emission Summary Data. 2021. Available online: https://naei.energysecurity.gov.uk/node/25 (accessed on 7 February 2025).
- Environmental Protection Agency. An Inventory of Sources and Environmental Releases of Dioxin-like Compounds in the United States for the Years 1987, 1995, and 2000; National Center for Environmental Assessment: Washington, DC, USA, 2006.
- Heeb, N.V.; Zennegg, M.; Gujer, E.; Honegger, P.; Zeyer, K.; Gfeller, U.; Wichser, A.; Kohler, M.; Schmid, P.; Emmenegger, L.; et al. Secondary Effects of Catalytic Diesel Particulate Filters: Copper-Induced Formation of PCDD/Fs. Environ. Sci. Technol. 2007, 41, 5789–5794. [Google Scholar] [CrossRef] [PubMed]
- Laroo, C.A.; Schenk, C.; Sanchez, J.; McDonald, J.; Smith, P. Emissions of PCDD/Fs, PCBs, and PAHs from a Modern Diesel Engine Equipped with Selective Catalytic Reduction Filters. SAE Int. J. Engines 2013, 6, 1311–1339. [Google Scholar] [CrossRef]
- Clunies-Ross, C.; Stanmore, B.; Millar, G. Dioxins in diesel exhaust. Nature 1996, 381, 379. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Yeh, J.W.; Chein, H.M.; Hsu, L.Y.; Chi, K.H.; Chang, M.B. PCDD/F Adsorption and Destruction in the Flue Gas Streams of MWI and MSP via Cu and Fe Catalysts Supported on Carbon. Environ. Sci. Technol. 2008, 42, 5727–5733. [Google Scholar] [CrossRef] [PubMed]
- Gullett, B.K.; Ryan, J.V. On-Road Emissions of PCDDs and PCDFs from Heavy Duty Diesel Vehicles. Environ. Sci. Technol. 2002, 36, 3036–3040. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).