Enhancing the Biorefinery of Chestnut Burrs, Part II: Influence of Pretreatment with Choline Chloride–Urea-Diluted Deep Eutectic Solvent on Enzymatic Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of DES
2.2. Substrates of DES Pretreatment
- -
- Untreated CB
- -
- Washed CB (W-CB) obtained by mixing untreated CB with distilled water in a glass bottle with an SLR of 1:8 (w/v), followed by agitation in an orbital shaker (Optic Ivymen System, Comecta S.A., distributed by Scharlab, Madrid, Spain) at 50 °C, 150 rpm for 4 h. The solid fraction was recovered by filtration and dried at 50 °C (Celsius 2007, Memmert, Schwabach, Germany).
- -
- CB from prehydrolysis (PreH). Untreated CB was pretreated with diluted H2SO4 under the conditions reported by Costa-Trigo et al. [4]. The solid fraction was recovered, washed with distilled water until neutral pH, and dried at 50 °C (Celsius 2007, Memmert, Schwabach, Germany).
2.3. DES Pretreatment
2.4. Enzymatic Hydrolysis
2.5. Analytical Methods
2.5.1. Lignocellulosic Composition
2.5.2. Attenuated Total Reflectance Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
2.5.3. X-Ray Diffraction (XRD)
2.5.4. Field Emission Scanning Electron Microscopy (FE-SEM)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Crystallinity of Lignocellulosic Starting Materials
3.2. Influence of DES Pretreatment on Different Biomasses
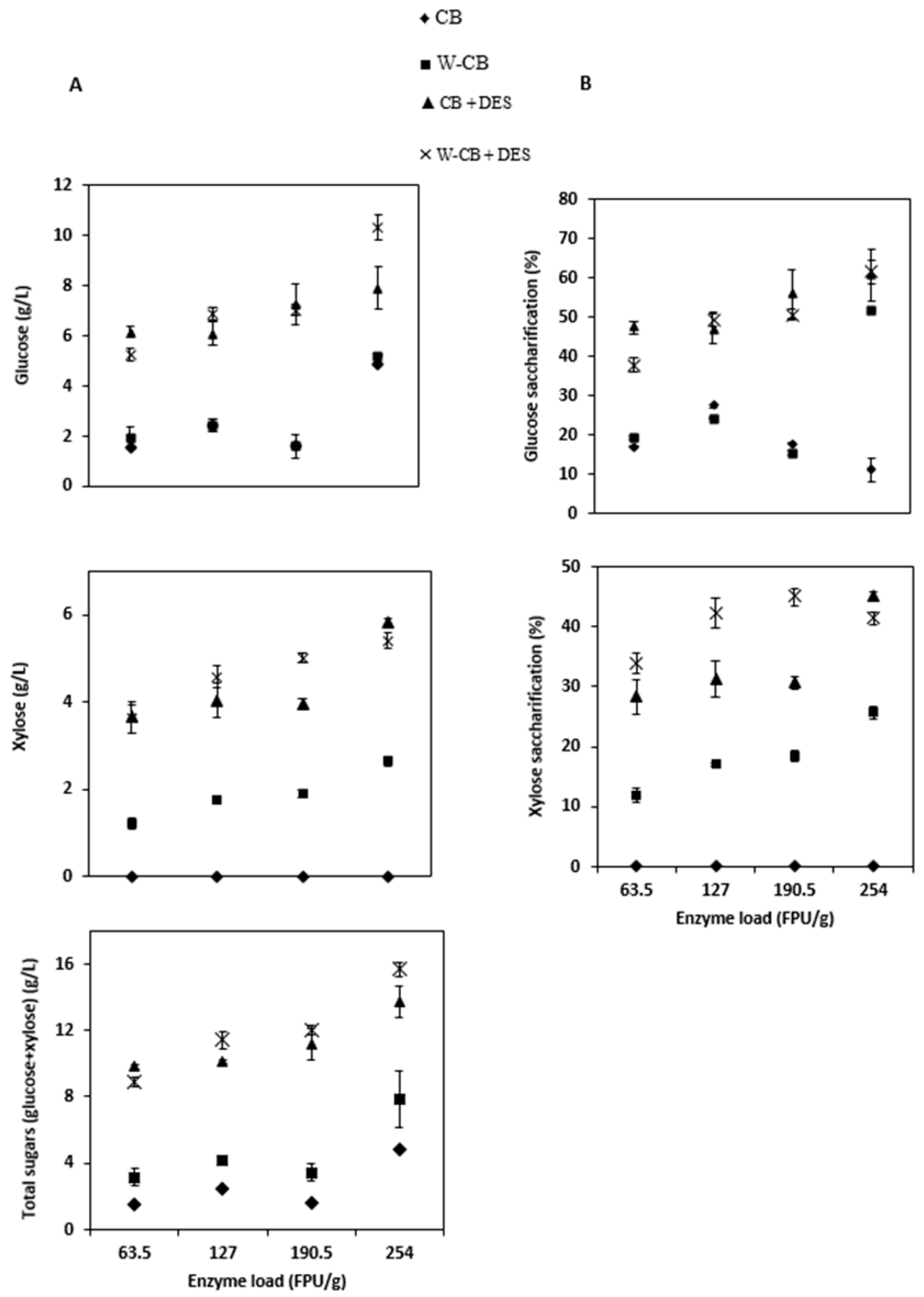
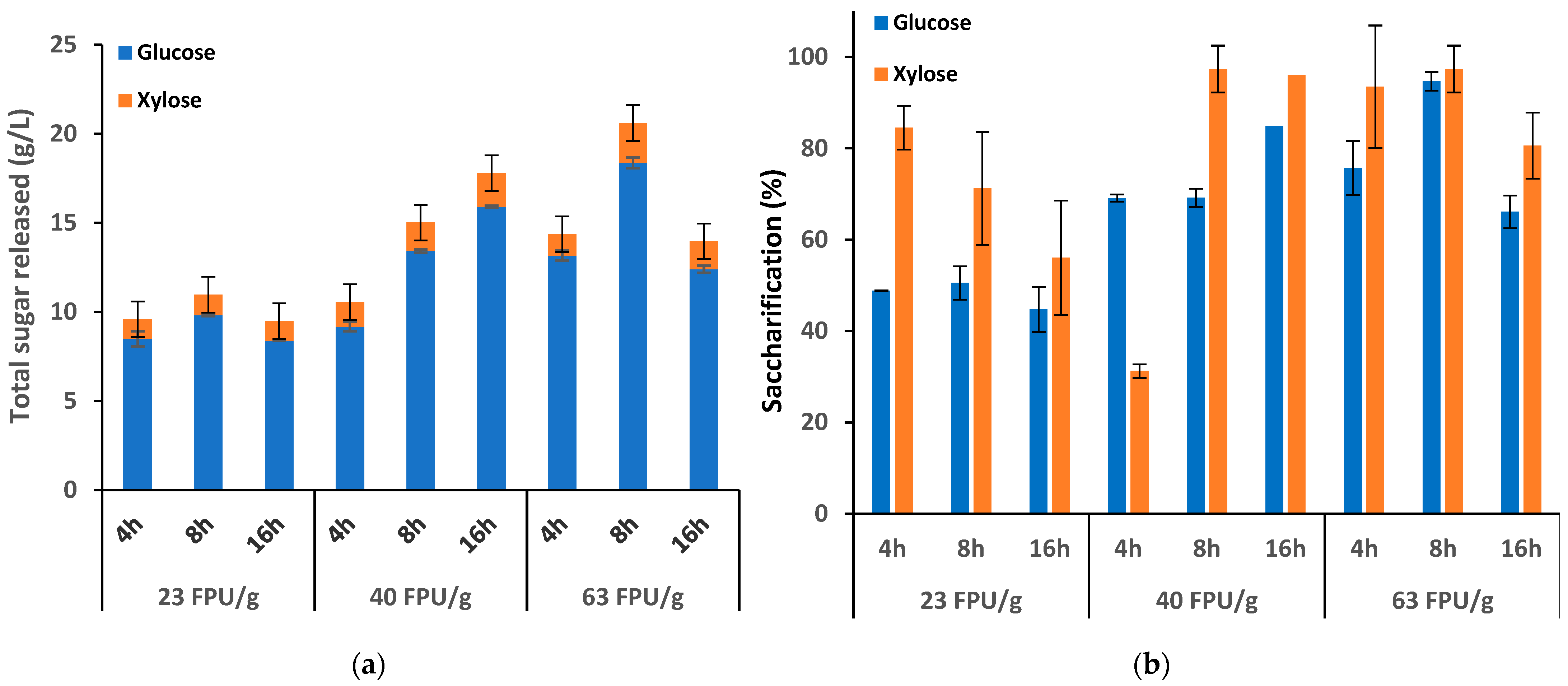
3.3. Enzymatic Hydrolysis
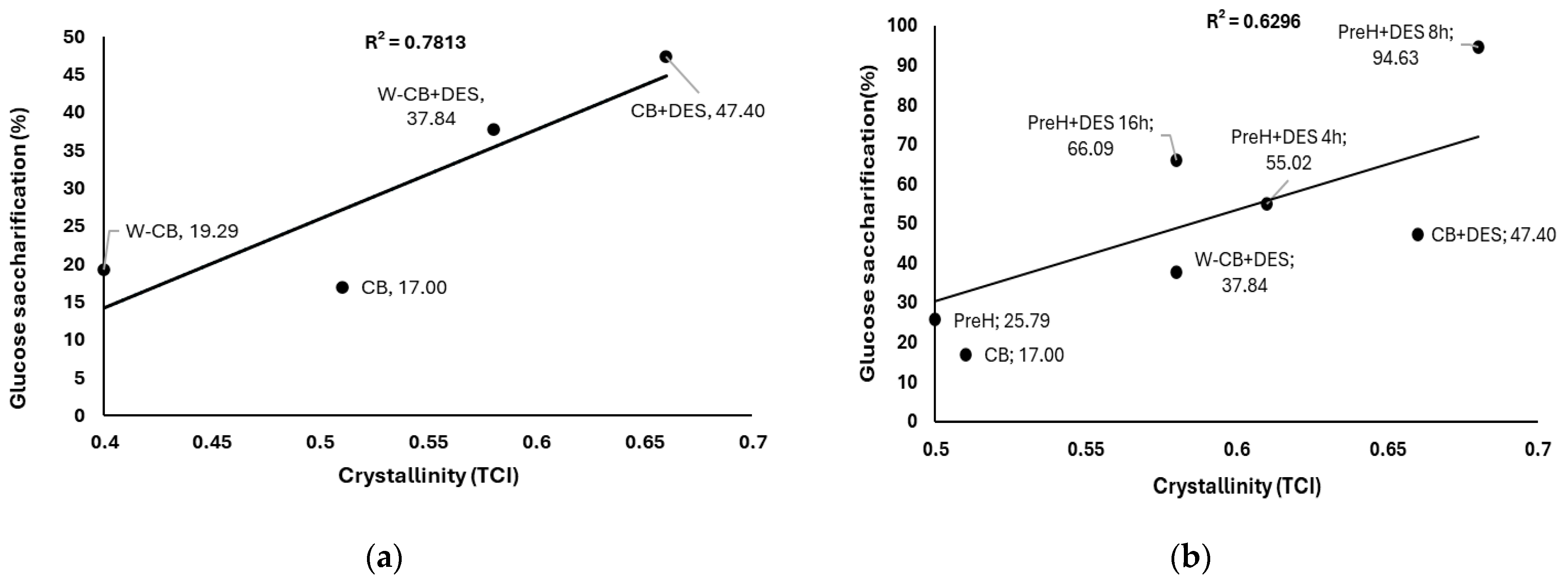
3.4. Relation Between Cellulose Crystallinity and Enzymatic Saccharification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pino-Hernández, E.; Pinto, C.A.; Abrunhosa, L.; Teixeira, J.A.; Saraiva, J.A. Hydrothermal and High-Pressure Processing of Chestnuts—Dependence on the Storage Conditions. Postharvest Biol. Technol. 2022, 185, 111773. [Google Scholar] [CrossRef]
- Eurostat Eurostat. Available online: https://ec.europa.eu/eurostat/databrowser/view/apro_cpsh1__custom_19005194/default/table (accessed on 21 November 2025).
- Andika, A.; Perdana, T.; Chaerani, D.; Utomo, D.S. Transitioning towards Zero Waste in the Agri-Food Supply Chain: A Review of Sustainable Circular Agri-Food Supply Chain. Sustain. Futur. 2025, 10, 100917. [Google Scholar] [CrossRef]
- Costa-Trigo, I.; Otero-Penedo, P.; Outeiriño, D.; Paz, A.; Domínguez, J.M. Valorization of Chestnut (Castanea Sativa) Residues: Characterization of Different Materials and Optimization of the Acid-Hydrolysis of Chestnut Burrs for the Elaboration of Culture Broths. Waste Manag. 2019, 87, 472–484. [Google Scholar] [CrossRef]
- Costa-Trigo, I.; Paz, A.; Morán-Aguilar, M.G.; Pérez Guerra, N.; Pinheiro de Souza Oliveira, R.; Domínguez, J.M. Enhancing the Biorefinery of Chestnut Burrs. Part I. Study of the Pretreatment with Choline Chloride Urea Diluted Deep Eutectic Solvent. Biomass Bioenergy 2023, 173, 106786. [Google Scholar] [CrossRef]
- Huang, C.; Jiang, X.; Shen, X.; Hu, J.; Tang, W.; Wu, X.; Ragauskas, A.; Jameel, H.; Meng, X.; Yong, Q. Lignin-Enzyme Interaction: A Roadblock for Efficient Enzymatic Hydrolysis of Lignocellulosics. Renew. Sustain. Energy Rev. 2022, 154, 111822. [Google Scholar] [CrossRef]
- Haldar, D.; Purkait, M.K. A Review on the Environment-Friendly Emerging Techniques for Pretreatment of Lignocellulosic Biomass: Mechanistic Insight and Advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef] [PubMed]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent Advances in Lignocellulosic Biomass for Biofuels and Value-Added Bioproducts—A Critical Review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, H.; Wang, Y.; He, B.; Lu, J.; Zhu, W.; Peng, L.; Wang, Y. Challenges and Perspectives of Green-like Lignocellulose Pretreatments Selectable for Low-Cost Biofuels and High-Value Bioproduction. Bioresour. Technol. 2023, 369, 128315. [Google Scholar] [CrossRef]
- Xu, H.; Kong, Y.; Peng, J.; Song, X.; Liu, Y.; Su, Z.; Li, B. Comprehensive Analysis of Important Parameters of Choline Chloride-Based Deep Eutectic Solvent Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2021, 319, 124209. [Google Scholar] [CrossRef]
- Gomes, M.G.; Gurgel, L.V.A.; Baffi, M.A.; Pasquini, D. Pretreatment of Sugarcane Bagasse Using Citric Acid and Its Use in Enzymatic Hydrolysis. Renew. Energy 2020, 157, 332–341. [Google Scholar] [CrossRef]
- Roy Choudhury, A.K. 4-Introduction to Enzymes. In Sustainable Technologies for Fashion and Textiles; Woodhead Publishing Series in Textiles; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081028674. [Google Scholar] [CrossRef]
- Wu, M.; Gong, L.; Ma, C.; He, Y.C. Enhanced Enzymatic Saccharification of Sorghum Straw by Effective Delignification via Combined Pretreatment with Alkali Extraction and Deep Eutectic Solvent Soaking. Bioresour. Technol. 2021, 340, 125695. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Cheng, H.; Zhou, H. Hemicellulose Degradation: An Overlooked Issue in Acidic Deep Eutectic Solvents Pretreatment of Lignocellulosic Biomass. Ind. Crops Prod. 2022, 187, 115335. [Google Scholar] [CrossRef]
- Procentese, A.; Rehmann, L. Fermentable Sugar Production from a Coffee Processing By-Product after Deep Eutectic Solvent Pretreatment. Bioresour. Technol. Rep. 2018, 4, 174–180. [Google Scholar] [CrossRef]
- Ma, C.; Peng, X.; Sun, S.; Wen, J.; Yuan, T. Short-Time Deep Eutectic Solvents Pretreatment Enhanced Production of Fermentable Sugars and Tailored Lignin Nanoparticles from Abaca. Int. J. Biol. Macromol. 2021, 192, 417–425. [Google Scholar] [CrossRef]
- Guo, Z.; Mao, J.; Zhang, Q.; Xu, F. Integrated Biorefinery of Bamboo for Fermentable Sugars, Native-like Lignin, and Furfural Production by Novel Deep Eutectic Solvents Treatment. Ind. Crops Prod. 2022, 188, 115453. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Li, D.; Zhang, J.; Nawaz, H.; You, T.; Xu, F. Highly-Efficient Pretreatment Using Alkaline Enhanced Aqueous Deep Eutectic Solvent to Unlock Poplar for High Yield of Fermentable Sugars: Synergistic Removal of Lignin and Mannan. Bioresour. Technol. 2022, 351, 126993. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical Properties of Deep Eutectic Solvents: A Review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Loow, Y.; Yeong, T.; Hoa, G.; Yang, L.; Kein, E.; Fong, L.; Jahim, J.; Wahab, A.; Hui, W. Deep Eutectic Solvent and Inorganic Salt Pretreatment of Lignocellulosic Biomass for Improving Xylose Recovery. Bioresour. Technol. 2018, 249, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Morán-Aguilar, M.G.; Calderón-Santoyo, M.; de Souza Oliveira, R.P.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Deconstructing Sugarcane Bagasse Lignocellulose by Acid-Based Deep Eutectic Solvents to Enhance Enzymatic Digestibility. Carbohydr. Polym. 2022, 298, 120097. [Google Scholar] [CrossRef]
- Costa-Trigo, I.; Paz, A.; Otero-penedo, P.; Outeiriño, D.; Pinheiro, R.; Oliveira, D.S.; Manuel, J. Detoxification of Chestnut Burrs Hydrolyzates to Produce Biomolecules. Biochem. Eng. J. 2020, 159, 107599. [Google Scholar] [CrossRef]
- Costa-Trigo, I.; Paz, A.; Otero-Penedo, P.; Outeiriño, D.; Pérez Guerra, N.; Domínguez, J.M. Enhancing the Saccharification of Pretreated Chestnut Burrs to Produce Bacteriocins. J. Biotechnol. 2021, 329, 13–20. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. In Biomass Analysis Technology Team Laboratory Analytical Procedure; Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP) (Revised July 2011); National Renewable Energy Laboratory: Applewood, CO, USA, 2011; pp. 1–14. Available online: https://docs.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 25 November 2025).
- Azizan, A.; Jusri, N.A.A.; Azmi, I.S.; Abd Rahman, M.F.; Ibrahim, N.; Jalil, R. Emerging Lignocellulosic Ionic Liquid Biomass Pretreatment Criteria/Strategy of Optimization and Recycling Short Review with Infrared Spectroscopy Analytical Know-How. Mater. Today Proc. 2022, 63, S359–S367. [Google Scholar] [CrossRef]
- Poletto, M.; Júnior Ornaghi, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 6105–6119. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1958, 29, 786–794. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, L.; Zhang, R.; Liu, G.; Cheng, G. Understanding Changes in Cellulose Crystalline Structure of Lignocellulosic Biomass during Ionic Liquid Pretreatment by XRD. Bioresour. Technol. 2014, 151, 402–405. [Google Scholar] [CrossRef]
- Kljun, A.; Benians, T.A.S.; Goubet, F.; Meulewaeter, F.; Knox, J.P.; Blackburn, R.S. Comparative Analysis of Crystallinity Changes in Cellulose i Polymers Using ATR-FTIR, X-Ray Diffraction, and Carbohydrate-Binding Module Probes. Biomacromolecules 2011, 12, 4121–4126. [Google Scholar] [CrossRef]
- Haykiri Acma, H.; Yaman, S. Treating Lignocellulosic Biomass with Dilute Solutions at Ambient Temperature: Effects on Cellulose Crystallinity. Biomass Convers. Biorefin 2022, 14, 9967–9981. [Google Scholar] [CrossRef]
- Ma, X.J.; Cao, S.L.; Lin, L.; Luo, X.L.; Hu, H.C.; Chen, L.H.; Huang, L.L. Hydrothermal Pretreatment of Bamboo and Cellulose Degradation. Bioresour. Technol. 2013, 148, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Huong, V.T.T.; Atjayutpokin, T.; Chinwatpaiboon, P.; Smith, S.M.; Boonyuen, S.; Luengnaruemitchai, A. Two-Stage Acid-Alkali Pretreatment of Vetiver Grass to Enhance the Subsequent Sugar Release by Cellulase Digestion. Renew. Energy 2022, 195, 755–765. [Google Scholar] [CrossRef]
- Santos, C.C.; de Souza, W.; Sant Anna, C.; Brienzo, M. Elephant Grass Leaves Have Lower Recalcitrance to Acid Pretreatment than Stems, with Higher Potential for Ethanol Production. Ind. Crops Prod. 2018, 111, 193–200. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, H.; Lin, X.C.; Tang, L.; Chen, J.J.; Mo, J.W.; Yu, R.S.; Shen, X.J. Novel Recyclable Deep Eutectic Solvent Boost Biomass Pretreatment for Enzymatic Hydrolysis. Bioresour. Technol. 2020, 307, 123237. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.L.; Chen, C.W.; Sun, P.P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C. Di Deep Eutectic Solvents as Promising Pretreatment Agents for Sustainable Lignocellulosic Biorefineries: A Review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, J.; Zhang, Z.; Li, A.; Li, J.; Zhang, W.; Chen, K. Efficient and Sustainable Fractionation of Eucalyptus Biomass into High Quality Cellulose and Lignin Using a Novel Ternary Deep Eutectic Solvent. Chem. Engin J. 2025, 518, 164829. [Google Scholar] [CrossRef]
- Shen, X.J.; Wen, J.L.; Mei, Q.Q.; Chen, X.; Sun, D.; Yuan, T.Q.; Sun, R.C. Facile Fractionation of Lignocelluloses by Biomass-Derived Deep Eutectic Solvent (DES) Pretreatment for Cellulose Enzymatic Hydrolysis and Lignin Valorization. Green. Chem. 2019, 21, 275–283. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Aguilar-Uscanga, M.G.; Pinheiro de Souza Oliveira, R.; Domínguez, J.M. Enhancing the Biorefinery of Brewery Spent Grain by Deep Eutectic Solvent Pretreatment: Optimisation of Polysaccharide Enrichment through a Response Surface Methodology. J. Ind. Eng. Chem. 2025, 145, 693–704. [Google Scholar] [CrossRef]
- Toscano, G.; Maceratesi, V.; Leoni, E.; Stipa, P.; Laudadio, E.; Sabbatini, S. FTIR Spectroscopy for Determination of the Raw Materials Used in Wood Pellet Production. Fuel 2022, 313, 123017. [Google Scholar] [CrossRef]
- Suopajärvi, T.; Ricci, P.; Karvonen, V.; Ottolina, G.; Liimatainen, H. Acidic and Alkaline Deep Eutectic Solvents in Delignification and Nanofibrillation of Corn Stalk, Wheat Straw, and Rapeseed Stem Residues. Ind. Crop. Prod. 2020, 145, 111956. [Google Scholar] [CrossRef]
- Bilatto, S.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Lignocellulose Nanocrystals from Sugarcane Straw. Ind. Crop. Prod. 2020, 157, 112938. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep Eutectic Solvent Pretreatment and Subsequent Saccharification of Corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Pan, M.; Zhao, G.; Ding, C.; Wu, B.; Lian, Z.; Lian, H. Physicochemical Transformation of Rice Straw after Pretreatment with a Deep Eutectic Solvent of Choline Chloride/Urea. Carbohydr. Polym. 2017, 176, 307–314. [Google Scholar] [CrossRef]
- Hou, X.D.; Feng, G.J.; Ye, M.; Huang, C.M.; Zhang, Y. Significantly Enhanced Enzymatic Hydrolysis of Rice Straw via a High-Performance Two-Stage Deep Eutectic Solvents Synergistic Pretreatment. Bioresour. Technol. 2017, 238, 139–146. [Google Scholar] [CrossRef]
- Ong, V.Z.; Wu, T.Y.; Chu, K.K.L.; Sun, W.Y.; Shak, K.P.Y. A Combined Pretreatment with Ultrasound-Assisted Alkaline Solution and Aqueous Deep Eutectic Solvent for Enhancing Delignification and Enzymatic Hydrolysis from Oil Palm Fronds. Ind. Crops Prod. 2021, 160, 112974. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, L.; Zhou, X.; Ouyang, S. Comparative Evaluation of Dilute Acid, Alkaline, and Deep Eutectic Solvent Pretreatments on Enzymatic Hydrolysis of Sunflower Stalk Bark. Appl. Biochem. Biotechnol. 2025, 197, 5774–5789. [Google Scholar] [CrossRef]
- Auxenfans, T.; Buchoux, S.; Husson, E.; Sarazin, C. Efficient Enzymatic Saccharification of Miscanthus: Energy-Saving by Combining Dilute Acid and Ionic Liquid Pretreatments. Biomass Bioenergy 2014, 62, 82–92. [Google Scholar] [CrossRef]
- Thi, S.; Lee, K.M. Comparison of Deep Eutectic Solvents (DES) on Pretreatment of Oil Palm Empty Fruit Bunch (OPEFB): Cellulose Digestibility, Structural and Morphology Changes. Bioresour. Technol. 2019, 282, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, G.; Wang, J. Comparative Insight into Biomass Pretreatment by Choline Chloride-Based Deep Eutectic Solvents in Relation to Their Physicochemical Characteristics. J. Environ. Chem. Eng. 2025, 13, 118256. [Google Scholar] [CrossRef]
- Lee, K.M.; Quek, J.D.; Tey, W.Y.; Lim, S.; Kang, H.S.; Quen, L.K.; Mahmood, W.A.W.; Jamaludin, S.I.S.; Teng, K.H.; Khoo, K.S. Biomass Valorization by Integrating Ultrasonication and Deep Eutectic Solvents: Delignification, Cellulose Digestibility and Solvent Reuse. Biochem. Eng. J. 2022, 187, 108587. [Google Scholar] [CrossRef]
- Panakkal, E.J.; Cheng, Y.-S.; Kuan-Shiong, K.; Pau-Loke, S.; Tantayotai, P.; Sriariyanun, M. Bioconversion of Napier Grass to 2-Phenylethanol: Unveiling the Synergy of Pretreatment with Deep Eutectic Solvents and Yeasts. Int. J. Appl. Sci. Eng. 2024, 21, 1–13. [Google Scholar] [CrossRef]
- Ji, G.; Han, L.; Gao, C.; Xiao, W.; Zhang, Y.; Cao, Y. Quantitative Approaches for Illustrating Correlations among the Mechanical Fragmentation Scales, Crystallinity and Enzymatic Hydrolysis Glucose Yield of Rice Straw. Bioresour. Technol. 2017, 241, 262–268. [Google Scholar] [CrossRef]
- Xu, H.; Che, X.; Ding, Y.; Kong, Y.; Li, B.; Tian, W. Effect of Crystallinity on Pretreatment and Enzymatic Hydrolysis of Lignocellulosic Biomass Based on Multivariate Analysis. Bioresour. Technol. 2019, 279, 271–280. [Google Scholar] [CrossRef]
- Kumar, N.; Muley, P.D.; Boldor, D.; Coty, G.G.; Lynam, J.G. Pretreatment of Waste Biomass in Deep Eutectic Solvents: Conductive Heating versus Microwave Heating. Ind. Crops Prod. 2019, 142, 111865. [Google Scholar] [CrossRef]
- Ling, Z.; Guo, Z.; Huang, C.; Yao, L.; Xu, F. Deconstruction of Oriented Crystalline Cellulose by Novel Levulinic Acid Based Deep Eutectic Solvents Pretreatment for Improved Enzymatic Accessibility. Bioresour. Technol. 2020, 305, 123025. [Google Scholar] [CrossRef]
- Zhou, M.; Lv, M.; Cai, S.; Tian, X. Effects of Enzymatic Hydrolysis and Physicochemical Properties of Lignocellulose Waste through Different Choline Based Deep Eutectic Solvents (DESs) Pretreatment. Ind. Crop. Prod. 2023, 195, 116435. [Google Scholar] [CrossRef]
- Mehta, J.P.; Metre, A.V.; Bhakhar, M.S.; Vetal, A.S. Comparative Study of Alkaline, Acidic, and Deep Eutectic Solvent Pretreatments on Lignocellulosic Biomass for Enhanced Enzymatic Hydrolysis. Biomass Convers. Biorefin 2025, 15, 14619–14631. [Google Scholar] [CrossRef]
- Udayakumar, V.; Anguraj, B.L. Optimized Deep Eutectic Solvent Cocktail Assisted Sequential Pretreatment to Enhance Enzymatic Hydrolysis of Arachis Hypogaea L Biomass. Int. Biodeterior. Biodegradation 2026, 206, 106207. [Google Scholar] [CrossRef]
- Wang, H.; Chen, T.; Yao, S.; Tang, Y. Comparison of Polyol-Based Deep Eutectic Solvents (DESs) on Pretreatment of Moso Bamboo (Phyllostachys Pubescens) for Enzymatic Hydrolysis. Ind. Crops Prod. 2022, 189, 115767. [Google Scholar] [CrossRef]
- Gundupalli, M.P.; Tantayotai, P.; Panakkal, E.J.; Chuetor, S.; Kirdponpattara, S.; Thomas, A.S.S.; Sharma, B.K.; Sriariyanun, M. Hydrothermal Pretreatment Optimization and Deep Eutectic Solvent Pretreatment of Lignocellulosic Biomass: An Integrated Approach. Bioresour. Technol. Rep. 2022, 17, 100957. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.J.; Yang, J.Y.; Li, M.F.; Peng, F.; Bian, J. Efficient Fractionation of Woody Biomass Hemicelluloses Using Cholinium Amino Acids-Based Deep Eutectic Solvents and Their Aqueous Mixtures. Bioresour. Technol. 2022, 354, 127139. [Google Scholar] [CrossRef]
- Sirviö, J.A.; Visanko, M.; Liimatainen, H. Deep Eutectic Solvent System Based on Choline Chloride-Urea as a Pre-Treatment for Nanofibrillation of Wood Cellulose. Green. Chem. 2015, 17, 3401–3406. [Google Scholar] [CrossRef]
- Chávez-Guerrero, L.; Silva-Mendoza, J.; Sepúlveda-Guzmán, S.; Medina-Aguirre, N.A.; Vazquez-Rodriguez, S.; Cantú-Cárdenas, M.E.; García-Gómez, N.A. Enzymatic Hydrolysis of Cellulose Nanoplatelets as a Source of Sugars with the Concomitant Production of Cellulose Nanofibrils. Carbohydr. Polym. 2019, 210, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ahola, S.; Turon, X.; Österberg, M.; Laine, J.; Rojas, O.J. Enzymatic Hydrolysis of Native Cellulose Nanofibrils and Other Cellulose Model Films: Effect of Surface Structure. Langmuir 2008, 24, 11592–11599. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Badillo, J.A.; Gallo, M.; Rutiaga-Quiñones, J.G.; Garza, J.; López-Albarrán, P. Insights on the Cellulose Pretreatment at Room Temperature by Choline-Chloride-Based Deep Eutectic Solvents: An Atomistic Study. Cellulose 2022, 29, 6517–6548. [Google Scholar] [CrossRef]



| CB | W-CB | PreH | |
|---|---|---|---|
| Moisture | 10.97 ± 0.11 a | 2.66 ± 0.02 b | 4.07 ± 0.05 c |
| CrI (%) | 19.90 | 44.90 | 56.14 |
| LOI (A1430/A898) | 1.96 | 2.15 | 3.08 |
| TCI (A1372/A2900) | 0.51 | 0.40 | 0.50 |
| HBI (A3400/A1320) ** | 2.60 | 2.30 | 2.11 |
| (%) | CB+DES (16 h) • | W-CB+DES (16 h) • | PreH+DES (4 h) | PreH+DES (8 h) | PreH+DES (16 h) • |
|---|---|---|---|---|---|
| AIL | 17.92 ± 0.68 a | 22.61 ± 0.95 b | 35.25 ± 0.30 c | 33.25 ± 1.02 c,d | 34.42 ± 0.70 c,d |
| ASL | 2.73 ± 0.14 c | 4.04 ± 0.12 d | 1.92 ± 0.15 b | 1.02 ± 0.00 a | 2.43 ± 0.11 c |
| Total Lignin | 20.65 ± 0.82 a | 26.86 ± 0.98 b | 37.17 ± 0.15 c | 37.21 ± 2.64 c | 36.86 ± 0.60 c |
| Delignification | 39.74 ± 2.40 d | 19.19 ± 2.95 a | 27.31 ± 0.30 b | 27.63 ± 5.15 b | 28.31 ± 1.16 b |
| Glucan | 38.87 ± 0.35 b | 34.67 ± 1.78 a | 46.98 ± 2.04 c | 52.43 ± 2.25 d | 50.63 ± 2.52 c,d |
| Xylan | 31.74 ± 0.47 c | 33.55 ± 2.32 c | 3.41 ± 0.12 a | 4.51 ± 0.08 b | 3.68 ± 1.00 a,b |
| Arabinan | 4.78 ± 0.11 a | 3.55 ± 1.09 a | n.d. | n.d. | n.d. |
| Total polysaccharides | 75.38 ± 0.71 d | 70.77 ± 3.01 c | 50.39 ± 2.17 a | 56.75 ± 2.45 b | 55.79 ± 2.04 a,b |
| CrI (%) | 48.40 | 53.75 | 37.04 | 54.65 | 38.67 |
| LOI (A1430/A898) | 1.79 | 2.62 | 2.02 | 3.43 | 2.57 |
| TCI (A1372/A2900) | 0.66 | 0.58 | 0.61 | 0.68 | 0.58 |
| HBI (A3400/A1320) ° | 3.54 | 4.07 | 3.21 | 2.76 | 3.04 |
| Biomass | DES | Pretreatment Conditions | Enzymatic Parameters | Enzyme Load | Saccharification | Reference |
|---|---|---|---|---|---|---|
| Rice straw | ChCl/urea (1:2) | 110 °C, 8 h, LSR 20:1 w/w | T: 50 °C, t: 48 h, LSR: 10/1 w/v | 100 µL cellulase/g substrate | Glucose: 0.78% Xylose: 0.26% | [44] |
| OPEFB * | ChCl/urea (1:2) | 120 °C, 3 h, LSR 10:1 w/w | T: 50 °C, t: 48 h, LSR: 2/1 v/w | 10 mg of Trichoderma viride cellulase/mL | Total reducing sugars: 19.60% | [49] |
| Wheat straw | ChCl/urea (1:2) | 120 °C, 3 h, LSR 30:1 w/w | T: 50 °C, t: 72 h, LSR: 50/1 v/w | 15 FPU CellicCtec2/g substrate | Glucose: 40% Xylose: 20% | [50] |
| Napier grass | ChCl/urea (1:2) | 130 °C, 5 h, LSR 20:1 w/w | T: 50 °C, t: 72 h, LSR; 40:1 v/w | 30 FPU CTech 2/g biomass | Glucose: 120 mg/g Xylose: 10 mg/g | [52] |
| OPEFB * | Ultrasounds assisted ChCl/urea (1:2) | 50 °C, 30′, 53 KHz, 210 W, LSR 10:1 w/v | T: 50 °C, t: 72 h, LSR: 40:1 v/w | Trichoderma viride cellulase 30–50 FPU/g | Total reducing sugars: 35% | [51] |
| Chestnut burrs | Diluted ChCl/urea (1:2) | 100 °C, 16 h, LSR 20:1 w/w | T: 50 °C, t: 72 h LSR: 30:1 v/w | 23 FPU Cellic Ctec2/g substrate | Glucose: 46% Xylose: 35% | This study |
| Chestnut burrs are pretreated after prehydrolysis | Diluted acid-ChCl/urea (1:2) | 100 °C, 8 h, LSR 20:1 w/w | T: 50 °C, t: 72 h LSR: 20:1 v/w | 23 FPU Cellic Ctec2/g substrate | Glucose: 60% Xylose: 74% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Trigo, I.; Morán-Aguilar, M.G.; Pérez Guerra, N.; Pinheiro de Souza Oliveira, R.; Domínguez, J.M. Enhancing the Biorefinery of Chestnut Burrs, Part II: Influence of Pretreatment with Choline Chloride–Urea-Diluted Deep Eutectic Solvent on Enzymatic Hydrolysis. Processes 2025, 13, 4090. https://doi.org/10.3390/pr13124090
Costa-Trigo I, Morán-Aguilar MG, Pérez Guerra N, Pinheiro de Souza Oliveira R, Domínguez JM. Enhancing the Biorefinery of Chestnut Burrs, Part II: Influence of Pretreatment with Choline Chloride–Urea-Diluted Deep Eutectic Solvent on Enzymatic Hydrolysis. Processes. 2025; 13(12):4090. https://doi.org/10.3390/pr13124090
Chicago/Turabian StyleCosta-Trigo, Iván, María Guadalupe Morán-Aguilar, Nelson Pérez Guerra, Ricardo Pinheiro de Souza Oliveira, and José Manuel Domínguez. 2025. "Enhancing the Biorefinery of Chestnut Burrs, Part II: Influence of Pretreatment with Choline Chloride–Urea-Diluted Deep Eutectic Solvent on Enzymatic Hydrolysis" Processes 13, no. 12: 4090. https://doi.org/10.3390/pr13124090
APA StyleCosta-Trigo, I., Morán-Aguilar, M. G., Pérez Guerra, N., Pinheiro de Souza Oliveira, R., & Domínguez, J. M. (2025). Enhancing the Biorefinery of Chestnut Burrs, Part II: Influence of Pretreatment with Choline Chloride–Urea-Diluted Deep Eutectic Solvent on Enzymatic Hydrolysis. Processes, 13(12), 4090. https://doi.org/10.3390/pr13124090








