Red Beet and Tarragon Microgreens: Phytochemical Composition, Antioxidant Activity, and Sensory Properties of Cold-Pressed Juices
Abstract
1. Introduction
2. Materials and Methods
2.1. Microgreen Samples
2.2. Preparation of Cold-Pressed Microgreen Juices
2.3. Preparation of Microgreens for Chromatographic Analysis
2.4. Preparation of Cold-Pressed Microgreen Juices for Chromatographic Analysis
2.5. UHPLC-Q-ToF MS Analysis of Microgreens
2.6. Total Phenolics, Flavonoids, Betalains, and Chlorophyll Content in Microgreen Juices
2.7. Antioxidant Properties of Cold-Pressed Microgreen Juices
2.8. Sensory Properties of Cold-Pressed Microgreen Juices
2.9. Physicochemical Analyses
2.10. Statistical Analysis
3. Results and Discussion
3.1. UHPLC-Q-ToF MS Profile of Bioactive Compounds of Beet and Tarragon Microgreens
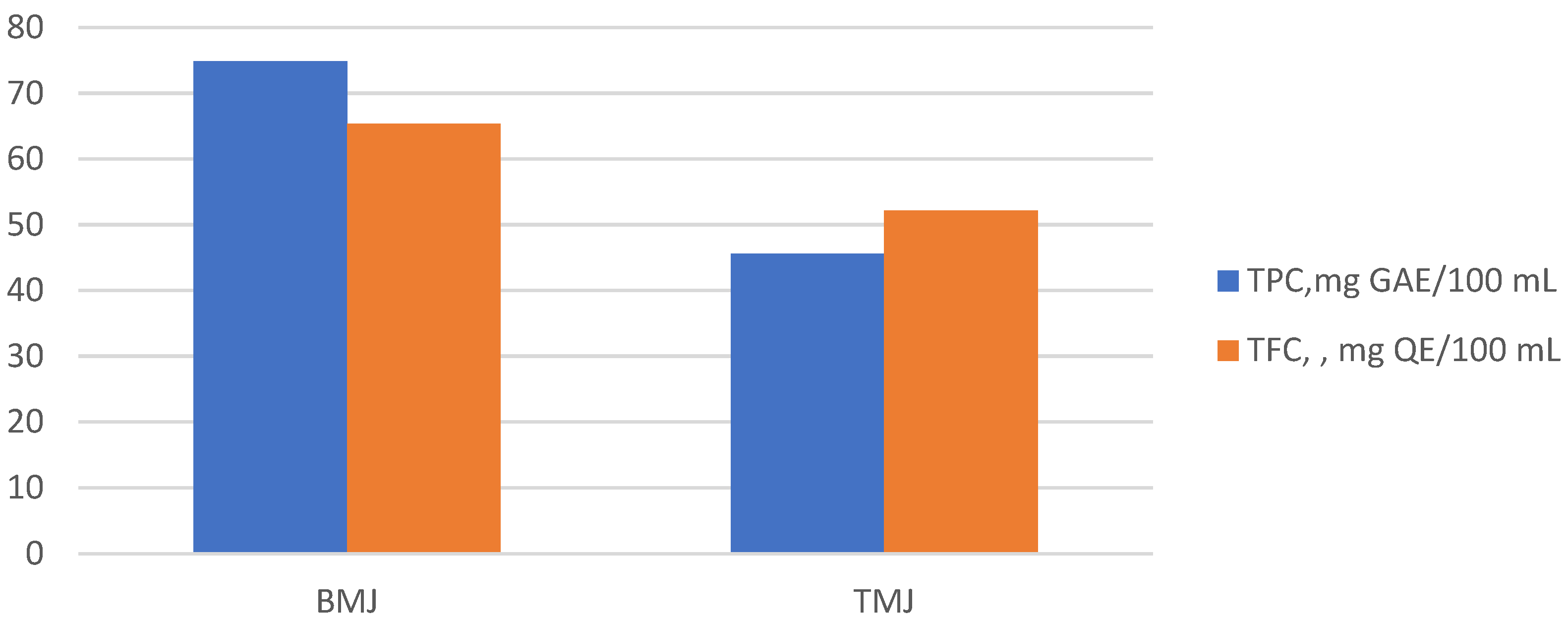
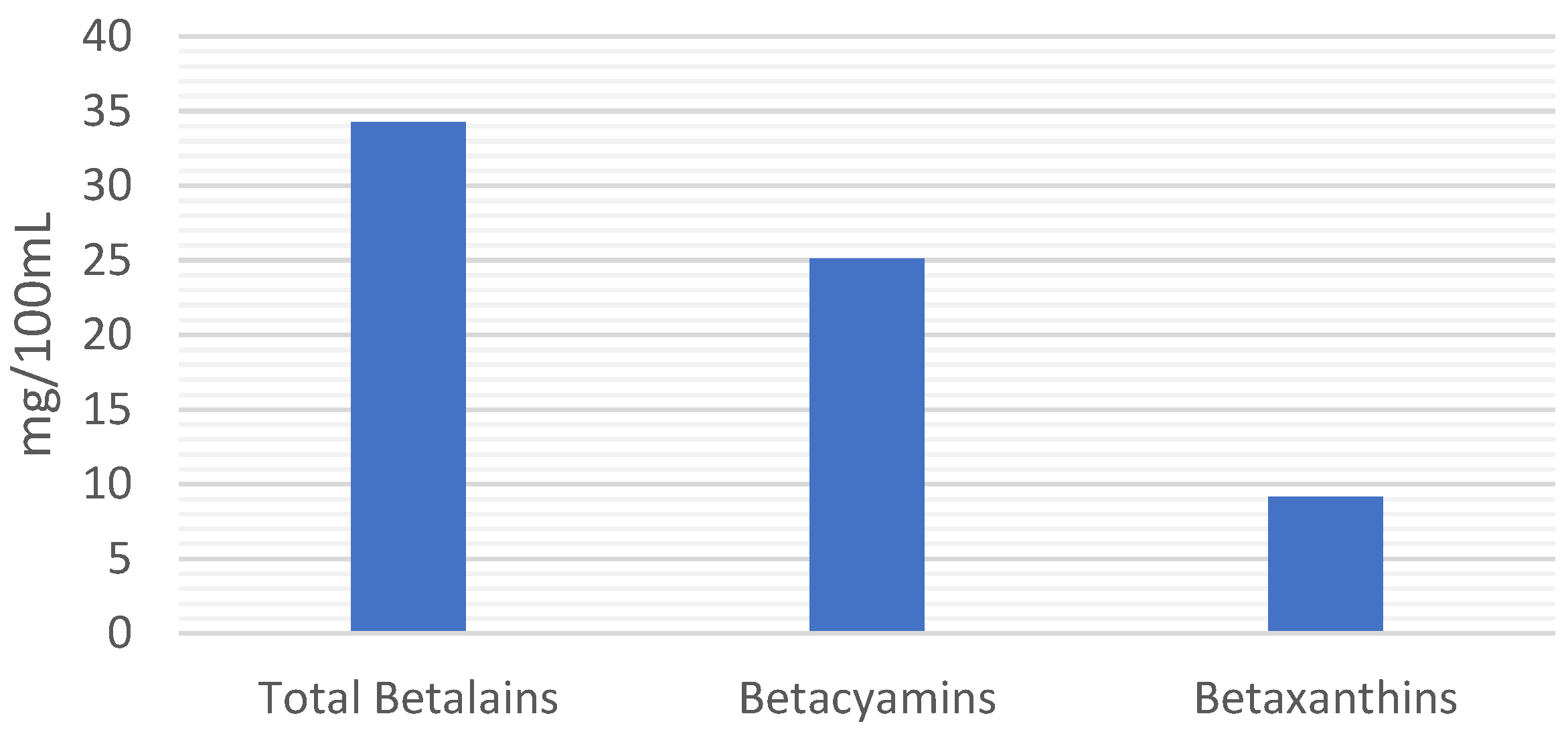
3.2. Total Phenolic, Flavonoid, Betalain, and Chlorophyll Content in Cold-Pressed Microgreen Juices
3.3. Antioxidant Properties of the Cold-Pressed Microgreen Juices
3.4. Sensory Properties of Cold-Pressed Microgreen Juices
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galieni, A.; Falcinelli, B.; Stagnari, F.; Datti, A.; Benincasa, P. Sprouts and microgreens: Trends, opportunities and perspectives for the agronomic sector. Agronomy 2020, 10, 1424. [Google Scholar] [CrossRef]
- Dubey, S.; Harbourne, N.; Harty, M.; Hurley, D.; Elliott-Kingston, C. Microgreens production: Exploiting environmental and growth conditions toward nutritional and phytochemical enhancement—A review. Plants 2024, 13, 2631. [Google Scholar] [CrossRef]
- Valisakkagari, H.; Chaturvedi, C.; Rupasinghe, H.P.V. Green Extraction of Phytochemicals from Fresh Vegetable Waste and Their Potential Application as Cosmeceuticals for Skin Health. Processes 2024, 12, 742. [Google Scholar] [CrossRef]
- Stajčić, S.; Ćetković, G.; Tumbas Šaponjac, V.; Travičić, V.; Ilić, P.; Brunet, S.; Tomić, A. Bioactive compounds and the antioxidant activity of selected vegetable microgreens: A correlation study. Processes 2024, 12, 1743. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food, and in human nutrition. Trends Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Carreón-Hidalgo, J.P.; Franco-Vásquez, D.C.; Gómez-Linton, D.R.; Pérez-Flores, L.J. Betalain plant sources, biosynthesis, extraction, stability enhancement methods, bioactivity, and applications. Food Res. Int. 2022, 151, 110821. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and microgreens—Novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Rani, S.; Mishra, A. Cruciferous microgreens: Growing performance and their scope as super foods at high altitude locations. Progress. Hortic. 2019, 51, 41. [Google Scholar] [CrossRef]
- Can Gerçek, Y.; Kutlu, N.; Çelik, S.; Gıdık, B.; Bayram, S.; Bayram, N.E. Extraction of Functional Compounds from Tarragon (Artemisia dracunculus L.) by Deep Eutectic Solvents at Different Properties. Chem. Biodivers. 2023, 20, e202300417. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.-O. Antioxidant capacities of individual and combined phenolics in a model system. Food Chem. 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Farhan, M.; Ahmad, Z.; Waseem, M.; Mehmood, T.; Javed, M.R.; Ali, M.; Manzoor, M.F.; Goksen, G. Assessment of Beetroot powder as nutritional, antioxidant, and sensory evaluation in candies. J. Agric. Food Res. 2024, 15, 101023. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Betalains and their applications in food: The current state of processing, stability and future opportunities in the industry. Food Chem. Mol. Sci. 2022, 4, 100089. [Google Scholar] [CrossRef] [PubMed]
- Koul, B.; Taak, P. The Artemisia genus: A review on traditional uses, phytochemical constituents, pharmacological properties and germplasm conservation. J. Glycom. Lipidom. 2017, 7, 142. [Google Scholar] [CrossRef]
- Sokolova, D.V.; Shvachko, N.A.; Mikhailova, A.S.; Popov, V.S.; Solovyeva, A.E.; Khlestkina, E.K. Characterization of Betalain Content and Antioxidant Activity Variation Dynamics in Table Beets (Beta vulgaris L.) with Differently Colored Roots. Agronomy 2024, 14, 999. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (Tarragon): A Critical Review of Its Traditional Use, Chemical Composition, Pharmacology, and Safety. J. Agric. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, G.; Assatarakul, K.; Sirikantaramas, S. Effect of cold-pressed and normal centrifugal juicing on quality attributes of fresh juices: Do cold-pressed juices harbor a superior nutritional quality and antioxidant capacity? Heliyon 2019, 5, e01917. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Miao, W.; Li, Y.; Ma, S.; Jiang, J.; Liu, H.; Cai, X.; Qin, Z.; Wang, X. Effects of cold-pressing conditions on physicochemical and functional properties of cold-pressed tigernut oil and starch isolated from press-cake. Int. J. Food Sci. Technol. 2021, 57, 662–675. [Google Scholar] [CrossRef]
- Miao, W.-B.; Ning, Y.-Y.; Huang, H.-R.; Liu, H.-M.; Cai, X.-S.; Wang, X.-D. Effect of dry heat modification and the addition of Chinese quince seed gum on the physicochemical properties and structure of tigernut tuber starch. Arab. J. Chem. 2021, 14, 103407. [Google Scholar] [CrossRef]
- Rusu, I.-C.; Pascariu, O.-E.; Popa (Burlacu), A.; Diguță, C.-F.; Apostol, L.; Nicolcioiu, M.-B.; Zăgrean, A.V.; Israel-Roming, F. The Influence of Substrate Composition on Nutritional Content and Biological Activity of Some Pleurotus Mushrooms Extracts. Agriculture 2025, 15, 791. [Google Scholar] [CrossRef]
- Dufossé, L.; Fouillaud, M.; Caro, Y. Fungi and Fungal Metabolites for the Improvement of Human and Animal Nutrition and Health. J. Fungi 2021, 7, 274. [Google Scholar] [CrossRef]
- Caracciolo, F.; El-Nakhel, C.; Raimondo, M.; Kyriacou, M.C.; Cembalo, L.; De Pascale, S.; Rouphael, Y. Sensory Attributes and Consumer Acceptability of 12 Microgreens Species. Agronomy 2020, 10, 1043. [Google Scholar] [CrossRef]
- Kardas, M.; Rakuła, M.; Kołodziejczyk, A.; Staśkiewicz-Bartecka, W. Consumer Preferences, Sensory Evaluation, and Color Analysis of Beetroot and Tomato Juices: Implications for Product Development and Marketing in Health-Promoting Beverages. Foods 2024, 13, 4059. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo Muniz, V.R.G.; Ribeiro, I.S.; Beckmam, K.R.L.; de Godoy, R.C.B. The impact of color on food choice. Braz. J. Food Technol. 2023, 26, e2022088. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef]
- Kolarević, T.; Milinčić, D.D.; Vujović, T.; Gašić, U.M.; Prokić, L.; Kostić, A.Ž.; Cerović, R.; Stanojevic, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic Compounds and Antioxidant Properties of Field-Grown and In Vitro Leaves, and Calluses in Blackberry and Blueberry. Horticulturae 2021, 7, 420. [Google Scholar] [CrossRef]
- Rocchetti, G.; Tomas, M.; Zhang, L.; Zengin, G.; Lucini, L.; Capanoglu, E. Red beet (Beta vulgaris) and amaranth (Amaranthus sp.) microgreens: Effect of storage and in vitro gastrointestinal digestion on the untargeted metabolomic profile. Food Chem. 2020, 332, 127415. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and Their In vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Pintać, D.; Majkić, T.; Torović, L.; Orčić, D.; Beara, I.; Simin, N.; Mimica-Dukić, N.; Lesjak, M. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind. Crops Prod. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Milinčić, D.D.; Špirović Trifunović, B.; Nedić, N.; Gašić, U.M.; Tešić, Ž.L.; Stanojević, S.P.; Pešić, M.B. Monofloral Corn Poppy Bee-Collected Pollen—A Detailed Insight into Its Phytochemical Composition and Antioxidant Properties. Antioxidants 2023, 12, 1424. [Google Scholar] [CrossRef] [PubMed]
- Kostić, A.Ž.; Milinčić, D.D.; Nedić, N.; Gašić, U.M.; Špirović Trifunović, B.; Vojt, D.; Tešić, Ž.L.; Pešić, M.B. Phytochemical Profile and Antioxidant Properties of Bee-Collected Artichoke (Cynara scolymus) Pollen. Antioxidants 2021, 10, 1091. [Google Scholar] [CrossRef]
- Ramirez-Velasquez, I.M.; Velez, E.; Bedoya-Calle, A.; Caro-Lopera, F.J. Mechanism of Antioxidant Activity of Betanin, Betanidin and Respective C15-Epimers via Shape Theory, Molecular Dynamics, Density Functional Theory and Infrared Spectroscopy. Molecules 2022, 27, 2003. [Google Scholar] [CrossRef]
- Ali, N.; Popović, V.; Koutchma, T.; Warriner, K.; Zhu, Y. Effect of thermal, high hydrostatic pressure, and ultraviolet-C processing on the microbial inactivation, vitamins, chlorophyll, antioxidants, enzyme activity, and color of wheatgrass juice. J. Food Process Eng. 2019, 43, e13036. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis: General Guidance for Design of Test Rooms. ISO: Genèva, Switzerland, 2007. Available online: https://www.iso.org/standard/36385.html (accessed on 1 August 2025).
- ISO 8586:2023; Sensory Analysis—Selection and Training of Sensory Assessors. ISO: Genèva, Switzerland, 2023. Available online: https://www.iso.org/standard/76667.html (accessed on 1 August 2025).
- ISO 11136:2014; Sensory Analysis—Methodology—General Guidance for Conducting Hedonic Tests with Consumers in a Controlled Area. ISO: Genèva, Switzerland, 2014. Available online: https://www.iso.org/standard/50125.html (accessed on 1 August 2025).
- Peng, C.; Ren, Y.; Ye, Z.; Zhu, H.; Liu, X.; Chen, X.; Hou, R.; Granato, D.; Cai, H. A comparative UHPLC-Q/TOF-MS-based metabolomics approach coupled with machine learning algorithms to differentiate Keemun black teas from narrow-geographic origins. Food Res. Int. 2022, 158, 111512. [Google Scholar] [CrossRef]
- Tsugawa, H.; Ikeda, K.; Takahashi, M.; Satoh, A.; Mori, Y.; Uchino, H.; Okahashi, N.; Yamada, Y.; Tada, I.; Bonini, P.; et al. MS-DIAL 4: Accelerating lipidomics using an MS/MS, CCS, and retention time atlas. bioRxiv 2020. [Google Scholar] [CrossRef]
- Niroula, S.; Adhikari, B. Effect of Processing Methods on Bioactive Components and Antioxidant Activity of Beetroot (Beta vulgaris L.). Tribhuvan Univ. J. Food Sci. Technol. 2022, 1, 46–56. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Akbari, A.; Izadi-Darbandi, A.; Bahmani, K.; Farhadpour, M.; Ebrahimi, M.; Ramshini, H.; Esmaeili, Z. Assessment of phenolic profile, and antioxidant activity in developed breeding populations of fennel (Foeniculum vulgare Mill). Biocatal. Agric. Biotechnol. 2023, 48, 102639. [Google Scholar] [CrossRef]
- Petrović, M.; Veljović, S.; Tomić, N.; Zlatanović, S.; Tosti, T.; Vukosavljević, P.; Gorjanović, S. Formulation of Novel Liqueurs from Juice Industry Waste: Consumer Acceptance, Phenolic Profile and Preliminary Monitoring of Antioxidant Activity and Colour Changes During Storage. Food Technol. Biotechnol. 2021, 59, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Vieira Teixeira da Silva, D.; dos Santos Baião, D.; de Oliveira Silva, F.; Alves, G.; Perrone, D.; Mere Del Aguila, E.; MFlosi Paschoalin, V. Betanin, a Natural Food Additive: Stability, Bioavailability, Antioxidant and Preservative Ability Assessments. Molecules 2019, 24, 458. [Google Scholar] [CrossRef]
- Martinez, R.M.; Melo, C.P.B.; Pinto, I.C.; Mendes-Pierotti, S.; Vignoli, J.A.; Verri, W.A.; Casagrande, R. Betalains: A Narrative Review on Pharmacological Mechanisms Supporting the Nutraceutical Potential Towards Health Benefits. Foods 2024, 13, 3909. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Wittek, L.; Sagmeister, M.; Kruse, M.; Krüger, J.; Sachse, B.; Menz, J.; Götz, M.E.; Schäfer, B. Comparative Analysis of Estragole, Methyleugenol, Myristicin, and Elemicin Regarding Micronucleus Formation in V79 Cells. Molecules 2025, 30, 806. [Google Scholar] [CrossRef]
- da Costa, R.H.S.; Martins, A.O.B.P.B.; Pessoa, R.T.; Alshehri, S.A.; Wahab, S.; Ahmad, M.F.; Suliman, M.; da Silva, L.Y.S.; Alcântara, I.S.; Ramos, A.G.B.; et al. Mechanisms of Actions Involved in The Antinociceptive Effect of Estragole and its β-Cyclodextrin Inclusion Complex in Animal Models. Plants 2022, 11, 2854. [Google Scholar] [CrossRef]
- Hernández-Adasme, C.; Palma-Dias, R.; Escalona, V.H. The Effect of Light Intensity and Photoperiod on the Yield and Antioxidant Activity of Beet Microgreens Produced in an Indoor System. Horticulturae 2023, 9, 493. [Google Scholar] [CrossRef]
- Zin, M.M.; Borda, F.; Márki, E.; Bánvölgyi, S. Betalains, total polyphenols, and antioxidant contents in red beetroot peel (Cylindra type). Prog. Agric. Eng. Sci. 2021, 16, 27–36. [Google Scholar] [CrossRef]
- Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patané, C. Effects of Different Light Spectra on Final Biomass Production and Nutritional Quality of Two Microgreens. Plants 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Vučetić, A.; Šovljanski, O.; Pezo, L.; Gligorijević, N.; Kostić, S.; Vulić, J.; Čanadanović-Brunet, J. A Comprehensive Antioxidant and Nutritional Profiling of Brassicaceae Microgreens. Antioxidants 2025, 14, 191. [Google Scholar] [CrossRef]
- Gouws, C.A.; Georgouopoulou, E.; Mellor, D.D.; Naumovski, N. The Effect of Juicing Methods on the Phytochemical and Antioxidant Characteristics of the Purple Prickly Pear (Opuntia ficus indica)—Preliminary Findings on Juice and Pomace. Beverages 2019, 5, 28. [Google Scholar] [CrossRef]
- Gunjal, M.; Singh, J.; Kaur, J.; Kaur, S.; Nanda, V.; Mehta, C.M.; Bhadariya, V.; Rasane, P. Comparative analysis of morphological, nutritional, and bioactive properties of selected microgreens in alternative growing medium. S. Afr. J. Bot. 2024, 165, 188–201. [Google Scholar] [CrossRef]
- Podsędek, A.; Frąszczak, B.; Kajszczak, D.; Sosnowska, D. Evaluation of Bioactive Compounds and Antioxidant Activity of Green and Red Kale (Brassica oleracea L. var. acephala) Microgreens Grown Under White, Red, and Blue LED Combinations. Agronomy 2024, 14, 2454. [Google Scholar] [CrossRef]
- Fernandes, V.C.; Queiroz CRAdos, A.; Almeida, E.S.; Melo, C.M.T. Phenolic content and antioxidant activity of medicinal plants. Res. Soc. Dev. 2023, 12, e16212340475. [Google Scholar] [CrossRef]
- Dhaka, A.S.; Dikshit, H.K.; Mishra, G.P.; Tontang, M.T.; Meena, N.L.; Kumar, R.R.; Ramesh, S.V.; Narwal, S.; Aski, M.; Thimmegowda, V.; et al. Evaluation of Growth Conditions, Antioxidant Potential, and Sensory Attributes of Six Diverse Microgreens Species. Agriculture 2023, 13, 676. [Google Scholar] [CrossRef]
- Ramteke, P.W. Sensory and Nutritional Study of Locally Available Fresh and Processed Fruit and Vegetable Juices in Allahabad City. 2017. Available online: https://www.semanticscholar.org/paper/Sensory-and-Nutritional-study-of-locally-available-Ramteke/d1f4c615c4f61af1894030cb394d952014d415a8 (accessed on 1 August 2025).



| No | Compound Name | RT | Formula | Calculated Mass | m/z | Exact Mass | mDa | MS Fragments (% Base Peak) | Beet (mg/100 g) | Tarragon (mg/100 g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Betanin (betacyanin) | 2.15 | C24H26N2O13 | 562.1379 | 551.1502 | 562.1427 | −4.80 | 389 (100), 307, 345, 178 | 82.45 ± 1.12 | - |
| 2 | Caffeic acid hexoside | 2.83 | C15H14O9 | 354.0583 | 355.0667 | 354.0615 | −3.20 | 179 (100), 161, 135, 119 | 12.34 ± 0.26 | 8.91 ± 0.15 |
| 3 | Ferulic acid hexoside | 3.47 | C16H18O9 | 370.0896 | 371.0979 | 370.0924 | −2.75 | 193 (100), 178, 149 | 7.84 ± 0.19 | 6.12 ± 0.14 |
| 4 | p-Coumaric acid | 4.12 | C9H8O4 | 180.0423 | 181.0506 | 180.0450 | −3.95 | 163 (100), 145, 119 | 4.65 ± 0.08 | 5.03 ± 0.11 |
| 5 | Quercetin-3-O-glucoside | 4.76 | C21H20O12 | 464.0955 | 463.0882 | 464.0955 | −4.22 | 301 (100), 179, 151 | 9.44 ± 0.16 | 11.02 ± 0.22 |
| 6 | Kaempferol-3-O-rutinoside | 5.54 | C27H30O15 | 594.1587 | 593.1512 | 594.1587 | −4.60 | 285 (100), 255, 227 | 6.25 ± 0.13 | 4.92 ± 0.10 |
| 7 | Estragole (methyl chavicol) | 6.02 | C10H12O | 164.0837 | 149.0969 | 178.0990 | −2.90 | 164 (100), 149, 135 | - | 34.18 ± 0.54 |
| 8 | Chlorogenic acid | 6.45 | C16H18O9 | 354.0951 | 197.0818 | 196.0770 | −3.30 | 179 (100), 161, 135 | 10.91 ± 0.17 | 7.45 ± 0.14 |
| 9 | Isorhamnetin-3-O-glucoside | 5.89 | C22H22O12 | 478.1112 | 479.1195 | 478.1112 | −3.10 | 315 (100), 300, 271 | 3.87 ± 0.06 | 2.45 ± 0.05 |
| 10 | Rutin (quercetin-3-O-rutinoside) | 5.72 | C27H30O16 | 610.1536 | 611.1609 | 610.1536 | −2.85 | 301 (100), 271, 179 | 5.63 ± 0.12 | 8.32 ± 0.16 |
| 11 | Apigenin-7-O-glucoside | 4.88 | C21H20O10 | 432.1056 | 433.1139 | 432.1056 | −3.00 | 271 (100), 253, 151 | 2.94 ± 0.05 | 6.15 ± 0.11 |
| 12 | Luteolin-7-O-glucoside | 4.94 | C21H20O11 | 448.1005 | 449.1088 | 448.1005 | −3.40 | 285 (100), 255, 151 | 3.18 ± 0.06 | 4.09 ± 0.07 |
| 13 | Gallic acid | 3.12 | C7H6O5 | 170.0215 | 171.0298 | 170.0215 | −3.70 | 125 (100), 107, 79 | 1.86 ± 0.03 | 2.11 ± 0.04 |
| 14 | Syringic acid | 3.26 | C9H10O5 | 198.0520 | 199.0603 | 198.0520 | −3.60 | 183 (100), 155, 127 | 1.94 ± 0.03 | 2.68 ± 0.05 |
| 15 | Sinapic acid | 3.78 | C11H12O5 | 224.0630 | 225.0713 | 224.0630 | −3.25 | 209 (100), 181, 153 | 2.55 ± 0.04 | 2.13 ± 0.04 |
| 16 | Vanillic acid | 3.08 | C8H8O4 | 168.0423 | 169.0506 | 168.0423 | −3.80 | 153 (100), 125, 97 | 2.21 ± 0.04 | 3.02 ± 0.05 |
| 17 | Myricetin-3-O-glucoside | 4.84 | C21H20O13 | 480.0853 | 481.0936 | 480.0853 | −3.15 | 317 (100), 299, 271 | 2.12 ± 0.04 | 3.45 ± 0.06 |
| 18 | Naringenin | 4.66 | C15H12O5 | 272.0685 | 273.0768 | 272.0685 | −3.50 | 153 (100), 119, 93 | 1.45 ± 0.03 | 2.74 ± 0.05 |
| 19 | Rosmarinic acid | 5.02 | C18H16O8 | 360.0846 | 361.0929 | 360.0846 | −3.40 | 179 (100), 161, 135 | 1.98 ± 0.03 | 7.92 ± 0.12 |
| 20 | Diosmetin-7-O-glucoside | 5.31 | C22H22O11 | 462.1163 | 463.1246 | 462.1163 | −3.20 | 301 (100), 286, 258 | 1.22 ± 0.02 | 2.54 ± 0.04 |
| 21 | Vitexin (apigenin-8-C-glucoside) | 4.43 | C21H20O10 | 432.1056 | 433.1139 | 432.1056 | −3.00 | 311 (100), 283, 255 | 2.84 ± 0.05 | 3.62 ± 0.07 |
| 22 | Isovitexin (apigenin-6-C-glucoside) | 4.39 | C21H20O10 | 432.1056 | 433.1139 | 432.1056 | −3.00 | 311 (100), 283, 255 | 2.41 ± 0.05 | 3.11 ± 0.06 |
| Attribute | Beet (BMJ) | Tarragon (TMJ) |
|---|---|---|
| Appearance | 4.9 ± 0.3 | 4.1 ± 0.4 |
| Odor | 4.2 ± 0.2 | 4.4 ± 0.3 |
| Texture | 4.3 ± 0.2 | 4.8 ± 0.2 |
| Taste | 3.4 ± 0.3 | 3.9 ± 0.3 |
| Overall quality | 3.6 ± 0.2 | 4.3 ± 0.3 |
| Overall acceptability (hedonic) | 5.2 ± 0.4 | 6.1 ± 0.3 |
| Parameter | Beet (BMJ) | Tarragon (TMJ) | p-Value |
|---|---|---|---|
| Moisture (%) | 91.2 ± 0.4 | 89.5 ± 0.5 | 0.012 |
| TSS (°Brix) | 6.8 ± 0.2 | 7.3 ± 0.3 | 0.021 |
| pH | 5.42 ± 0.05 | 5.18 ± 0.04 | 0.009 |
| Titratable acidity (g citric acid/100 mL) | 0.34 ± 0.02 | 0.41 ± 0.03 | 0.033 |
| Total sugars (g/100 mL) | 5.8 ± 0.2 | 6.2 ± 0.3 | 0.087 |
| Glucose (g/100 mL) | 2.1 ± 0.1 | 2.4 ± 0.1 | 0.041 |
| Fructose (g/100 mL) | 1.9 ± 0.1 | 2.0 ± 0.1 | 0.164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shoman, A.; Tokysheva, G.; Tultabayeva, T.; Konysbayeva, D.; Dairova, K.; Makangali, K.; Muldasheva, A. Red Beet and Tarragon Microgreens: Phytochemical Composition, Antioxidant Activity, and Sensory Properties of Cold-Pressed Juices. Processes 2025, 13, 3284. https://doi.org/10.3390/pr13103284
Shoman A, Tokysheva G, Tultabayeva T, Konysbayeva D, Dairova K, Makangali K, Muldasheva A. Red Beet and Tarragon Microgreens: Phytochemical Composition, Antioxidant Activity, and Sensory Properties of Cold-Pressed Juices. Processes. 2025; 13(10):3284. https://doi.org/10.3390/pr13103284
Chicago/Turabian StyleShoman, Aruzhan, Gulzhan Tokysheva, Tamara Tultabayeva, Damilya Konysbayeva, Kalamkas Dairova, Kadyrzhan Makangali, and Aknur Muldasheva. 2025. "Red Beet and Tarragon Microgreens: Phytochemical Composition, Antioxidant Activity, and Sensory Properties of Cold-Pressed Juices" Processes 13, no. 10: 3284. https://doi.org/10.3390/pr13103284
APA StyleShoman, A., Tokysheva, G., Tultabayeva, T., Konysbayeva, D., Dairova, K., Makangali, K., & Muldasheva, A. (2025). Red Beet and Tarragon Microgreens: Phytochemical Composition, Antioxidant Activity, and Sensory Properties of Cold-Pressed Juices. Processes, 13(10), 3284. https://doi.org/10.3390/pr13103284







