Abstract
Pharmaceuticals are emerging contaminants of global concern, but their occurrence and removal in semi-arid regions such as Algeria remain poorly documented. This study provides the first systematic evaluation of pharmaceutical and physicochemical parameters in two wastewater treatment plants (WWTPs) in Mascara: an activated sludge system (WWTP-1) and an aerated lagoon system (WWTP-2). Ten pharmaceuticals of different therapeutic classes were quantified using UPLC-HR-QTOF-MS in influent, effluent, and sludge samples, and removal efficiencies were compared using ANOVA and Principal Component Analysis (PCA). WWTP-1 showed higher efficiency, with >90% removal of COD, BOD5, and ammonium, and near-complete elimination of sulfamethoxazole (99.9%) and atenolol (94%). In contrast, WWTP-2 achieved only moderate reductions (69% COD, 51% BOD5) and low pharmaceutical removal, with negative efficiencies for persistent compounds such as carbamazepine, diclofenac, and ibuprofen. Weak correlations between macro- and micropollutants indicated that traditional indicators cannot predict pharmaceutical behavior. This work is the first to integrate physicochemical monitoring, pharmaceutical profiling, and multivariate analysis in Algerian WWTPs. The findings highlight the limitations of conventional treatment in semi-arid conditions and provide a critical baseline for adopting advanced technologies to mitigate pharmaceutical pollution in North Africa.
1. Introduction
Water plays a fundamental role in the socio-economic development of nations, as a reliable supply of freshwater is essential for sustaining permanent communities and ensuring long-term sustainability [1]. However, global population growth, coupled with increasing demand for water, has led to more frequent episodes of water scarcity, especially in regions already under hydric stress [2]. Algeria, located in an arid and semi-arid climatic zone, is particularly vulnerable due to irregular rainfall patterns and elevated evapotranspiration rates [3,4], which significantly complicate water resource management [3,4]. In such areas, the reuse of treated wastewater has been proposed as a strategic solution to mitigate water shortages and enhance agricultural productivity [5].
In recent decades, the occurrence of pharmaceutically active compounds (PhACs) in aquatic environments has emerged as a critical environmental concern [6]. These substances are continuously introduced into the environment through diverse pathways, including human and veterinary excretion, effluents from hospitals and pharmaceutical industries, and the improper disposal of unused medications [7,8,9]. Globally, it is estimated that over 50,000 pharmaceutical products are manufactured, with annual consumption exceeding 30 million tons [10]. Once released into wastewater, many of these compounds persist through conventional treatment processes, leading to their detection in surface waters, sediments, and even drinking water supplies [11,12]. Their persistence is problematic because, even at trace concentrations, PhACs may contribute to antibiotic resistance [13], disrupt endocrine functions [14], or bioaccumulate in aquatic food webs, thereby posing risks to ecosystems and human health [15].
The environmental fate of PhACs depends on multiple factors, including compound-specific physicochemical properties, environmental conditions, and microbial activity, which may lead to transformation or incomplete degradation [16,17]. Nevertheless, conventional wastewater treatment processes, such as activated sludge or aerated lagoons, are often ineffective in ensuring their complete removal [18,19]. This has prompted growing international concern and led to the inclusion of several PhACs in the European Union Water Framework Directive (2000/60/EC) and its subsequent amendments, with diclofenac, in particular, identified as a priority compound for monitoring [20,21,22].
In Algeria, where pharmaceutical consumption is rapidly increasing due to demographic and health-related factors [23], there is limited research on the occurrence and removal of PhACs in wastewater treatment plants (WWTPs). Most studies to date have been confined to surface waters or isolated case studies of single plants [24,25]. Furthermore, little is known about the comparative performance of different wastewater treatment technologies under semi-arid conditions. This is a crucial knowledge gap, given that lagoon systems remain widely used in Algeria due to their low operational costs, despite uncertainties regarding their efficiency in removing micropollutants [26].
In this context, the present study aims to (i) quantify a set of ten representative PhACs in both liquid and solid fractions of wastewater; (ii) evaluate their removal efficiencies in two WWTPs operating with different biological treatment processes (conventional activated sludge and aerated lagoon) in Mascara, Algeria; and (iii) explore correlations between PhACs and conventional water quality parameters. To our knowledge, this is the first comparative study in Algeria assessing both conventional pollutants and micropollutants across distinct treatment configurations, thereby providing new insights into wastewater management in semi-arid environments and identifying critical aspects for regulatory and technological improvement.
2. Materials and Methods
2.1. Study Sites
This study was conducted in two wastewater treatment plants (WWTPs) located in the Mascara department (Algeria). Both facilities are strategically important for wastewater management in the region and receive inputs from urban areas and hospitals, with WWTP-2 also collecting agricultural runoff. The comparative analysis of these plants enables the evaluation of pharmaceutical removal efficiencies under different operational conditions. The schematic layouts of the two plants, including the main treatment stages and sampling points, are presented in Figure 1a–d.
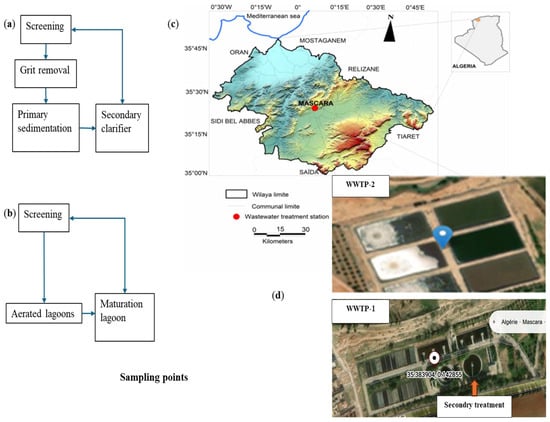
Figure 1.
Schematic diagrams of (a) WWTP-1 (conventional activated sludge) and (b) WWTP-2 (aerated lagoon), including treatment, (c) location, and (d) images of WWTP-1 and WWTP-2 [27].
2.1.1. Wastewater Treatment Plants El Kouayer (WWTP-1)
The El Kouayer WWTP (WWTP-1) is situated 3.2 km from Mascara city center (35°23′02.6″ N, 0°08′36.4″ E). Commissioned in 1994, it operates with a conventional activated sludge system and has a treatment capacity of 13,000 m3/day. The influent consists mainly of municipal and hospital wastewater, together with minor industrial discharges.
The treatment line comprises (i) pretreatment (screening and grit removal), (ii) biological treatment using a low-loaded activated sludge process (extended aeration), and (iii) sludge treatment in drying beds. The sludge retention time is approximately 14 days, while the hydraulic retention time (HRT) up to the secondary treatment stage is ~12 h. This is consistent with extended aeration systems, which typically require longer HRT (12–24 h) compared with conventional activated sludge.
2.1.2. Lagoon of Ghriss (WWTP-2)
The Ghriss WWTP (WWTP-2) is located 18 km from Mascara and 7 km from the Ghriss department (35°14′53″ N, 0°09′41″ E). It began operating in 2006 and uses an aerated lagoon system with a treatment capacity of 48,000 m3/day. This plant receives mixed wastewater inputs, including municipal, hospital, and agricultural effluents.
The treatment configuration consists of (i) preliminary screening and grit removal, followed by (ii) a series of aerated and maturation lagoons that enhance organic matter degradation through mechanical aeration and natural processes. The hydraulic retention time is ~34 days, which is characteristic of lagoon systems and provides sufficient residence time for biological self-purification.
The final effluent is reused for irrigation across 1095 ha of agricultural land in the Ghriss plain, contributing to water resource management in this semi-arid region.
2.2. Sampling Strategy
Sampling campaigns were carried out in November 2023, following standardized procedures ISO [28] to ensure representativeness and reproducibility. A total of eleven samples were collected from both WWTPs at regular intervals during daily operation. For each day, subsamples taken over an 8 h working period were pooled to obtain a composite sample representative of daily influent and effluent conditions.
At WWTP-1, samples were obtained from four critical points: influent, secondary effluent (after biological treatment), final effluent (discharge point), and sludge (biological sludge collected from drying beds). Two additional samples of influent and effluent were analyzed for physicochemical parameters.
At WWTP-2, samples were collected at three points: influent, secondary effluent (within the lagoon), and final effluent (outlet). In addition, influent and effluent samples were analyzed for physicochemical parameters.
All samples were collected in sterile high-density polyethylene bottles and immediately preserved at 4 °C during transport to the laboratory. This precaution minimized microbial degradation and chemical changes that could alter the concentrations of target compounds. Laboratory handling followed ISO recommendations to avoid cross-contamination and ensure analytical reliability [28].
2.3. Physicochemical Characterization
To comprehensively evaluate the performance of the two WWTPs and to support the interpretation of pharmaceutical removal efficiencies, a set of physicochemical parameters was systematically monitored in influent and effluent samples. These parameters included conventional water quality indicators widely used to assess wastewater treatment efficiency. The following variables were measured:
pH, recorded with a calibrated pH meter (Model 275-K, WTW, Troistedt, Germany). pH is an essential factor in biological treatment processes, as microbial activity and the solubility of many pollutants depend on it.
Temperature (air and water), determined using a combined electrode thermometer (Model OX 315 I). Temperature is particularly relevant in semi-arid regions such as Mascara, where fluctuations may affect microbial growth rates and enzymatic reactions involved in pollutant degradation.
Electrical conductivity (EC), measured with an OHAUS Starter 3100C conductimeter. EC serves as an indicator of ionic strength and salinity, both of which may influence adsorption processes and microbial activity.
Dissolved oxygen (DO), quantified with an optical probe (WTW Multi 3430). Monitoring DO is critical for evaluating the performance of aerobic biological treatment systems, since low oxygen concentrations can limit the efficiency of organic matter and micropollutant degradation.
Suspended solids (SS), chemical oxygen demand (COD), and biochemical oxygen demand (BOD5) were determined following the Standard Methods for the Examination of Water and Wastewater [29]. These parameters are widely recognized as key indicators of organic matter removal efficiency. COD provides information on the total oxidizable matter, while BOD5 reflects the fraction of biodegradable organic material available for microbial degradation.
Total nitrogen (TN) and total phosphorus (TP), analyzed spectrophotometrically after digestion according to APHA protocols [29] and national Algerian standards [30]. These nutrients are fundamental to evaluating the potential for eutrophication in receiving waters, as well as to exploring correlations with micropollutant behavior.
2.4. Quantitative Analysis of Pharmaceuticals
2.4.1. Chemicals and Standards
Ten pharmaceuticals belonging to different therapeutic classes (NSAIDs, analgesics, antibiotics, antidepressants, beta-blockers, and antihistamines) were selected (Table 1) based on their high consumption in Algeria and environmental relevance [11]. Analytical standards with purity >97% (Sigma-Aldrich, St. Louis, MO, USA) were used. Stock solutions (0.5 mg/mL) were prepared in methanol and stored at −20 °C in amber glass vials. Working solutions were freshly prepared by dilution with ultrapure water.

Table 1.
Main characteristics of the 10 pharmaceuticals. Chemical Abstract Service (CAS) registry number, a logarithm of the octanol–water partition coefficient (LogKow), and b dissociation constant (pKa) [31].
2.4.2. Sample Preservation and Preparation
Immediately after collection, water samples were filtered through pre-combusted glass fiber filters (0.7 μm, Whatman GF/F). Filtrates were stored at 4 °C and extracted within 48 h. Sludge samples were freeze-dried and homogenized before analysis. All procedures followed QA/QC guidelines to prevent contamination, including blanks and pre-cleaned glassware [32].
2.4.3. Solid-Phase Extraction (SPE)
SPE was performed using OASIS HLB cartridges (60 mg, Waters, Milford, MA, USA). Cartridges were conditioned with 3 mL of methyl tert-butyl ether (MTBE), 3 mL of methanol, and ultrapure water. Samples (100 mL) were loaded at~3 mL/min, followed by washing with ultrapure water and diluted methanol. Elution was performed with ethyl acetate/acetone (1:1, v/v). Extracts were evaporated under nitrogen and reconstituted in methanol. Method blanks and spiked controls were analyzed to verify recovery [33].
2.4.4. Instrumental Analysis (UPLC-HR-QTOF-MS)
Analyses were carried out with an Ultra-Performance Liquid Chromatography system coupled with High-Resolution Quadrupole Time-of-Flight Mass Spectrometry (UPLC-HR-QTOF-MS, Bruker Daltonik GmbH, Bremen, Germany). Separation was achieved on a BEH C18 column (50 × 2.1 mm, 1.7 μm). The mobile phases consisted of (A) ultrapure water with 0.1% formic acid and (B) methanol, with a gradient at 0.5 mL/min. The MS operated in positive electrospray ionization (ESI+), acquiring both full scan and MS/MS spectra for confirmation [34].
2.4.5. Method Validation and Quality Control
Linearity, accuracy, precision, and sensitivity were evaluated in accordance with ISO/IEC [35] standards. Calibration curves prepared in matrix-matched solutions yielded R2 > 0.98. LODs (S/N = 3) ranged from 0.04 to 0.55 ng/mL and LOQs (S/N = 10) from 0.10 to 1.33 ng/mL (Table 2). Recovery values ranged from 80% to 110%, and RSDs were <10%. Field blanks, procedural blanks, and duplicates ensured method reliability. These results are in agreement with previously published works using UPLC-HR-QTOF-MS [25,26]. The method adopted in this study is depicted in Figure 2.

Table 2.
Limit of detection (LOD) and limit of quantification (LOQ).
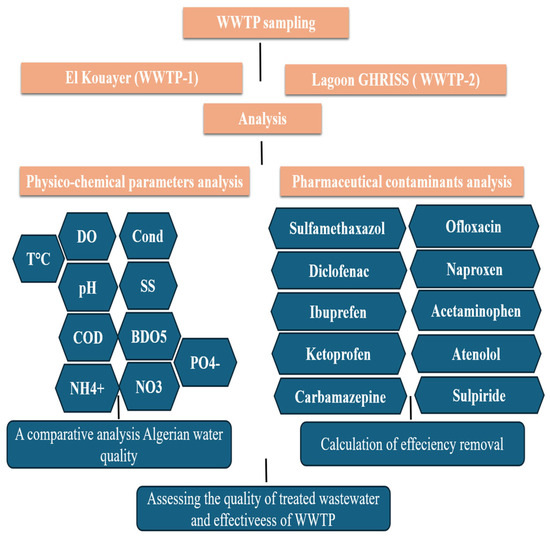
Figure 2.
The method adopted in this study for both WWTPs (WWTP-1 and WWTP-2).
2.4.6. Estimation of Removal Efficiency
Removal efficiency (RE) was calculated using Equation (1):
Negative removal values were observed when effluent concentrations exceeded influent concentrations, indicating processes such as desorption from sludge or deconjugation of metabolites during treatment [36].
where Ci and Ce represent the average concentrations in influent and effluent samples, respectively. In cases, for calculating RE, the compound was detected but below the LOQ, a concentration equivalent to half the LOQ value was used [37].
2.5. Data Analysis
Statistical Analysis
To compare the mean concentrations of PhACs in influent and effluent samples from WWTP-1 and WWTP-2, statistical analyses were conducted to ensure robust interpretation of the results. An analysis of variance (ANOVA) was performed with a significance level set at p < 0.05. This test was used to determine whether there were statistically significant differences in PhAC concentrations between the two WWTPs and across the treatment stages. When significant differences were detected, post hoc tests (Tukey HSD) were applied to identify specific groups that differed from each other. These statistical procedures were used to identify significant differences in pharmaceuticals between the two studied treatments.
In addition to univariate statistical analysis, a multivariate approach using Principal Component Analysis (PCA) and HAC were conducted. PCA was employed to explore potential associations between the type of WWTP, treatment stages, and PhAC concentrations. PCA is a multidimensional statistical method used to establish, on one hand, a similarity assessment among samples (samplings), and on the other hand, an assessment of the relationships between variables [38]. The fundamental concept of PCA is to reduce a large dataset with correlated variables into a smaller set of uncorrelated variables while maintaining the largest amount of information relating to the variation between the variables in the original dataset [39]. However, the HAC method is primarily used to complement the results obtained by PCA for organizing a large set of databases into clusters based on a given set of characteristics [40], to facilitates the classification of observations or variables into groups or subgroups based to their similarities or differences [41]. In this study, the HAC represented by a dendrogram which obtained by performing Ward’s method using squared Euclidean distance as a measure of similarity
Moreover, a correlation test was performed to evaluate the possible associations between micropollutants and physicochemical indicators. Therefore, considering the number of samples (<20) and the fact that most of the data failed the normality test (Pearson), coefficients R2 were used to evaluate the correlations between the parameters with a confidence level of p < 0.05. Graphs of the results and descriptive statistics, including ANOVA, PCA and correlation test were generated in Excel using Xlstat 2024.3.0.
3. Results
3.1. Physicochemical Parameters
The physicochemical characteristics of influent and effluent samples from WWTP-1 and WWTP-2 were analyzed to assess the performance of both treatment processes. These indicators provide critical insight into the efficiency of pollutant removal and the final quality of treated water. A summary of the results is presented in Table 3, while the main observations and removal trends are discussed below.

Table 3.
Physicochemical properties of influent and effluent samples from WWTP-1 and WWTP-2.
In WWTP-1, water and air temperatures remained stable, while pH values showed a slight decrease, remaining within the optimal range for biological treatment. Dissolved oxygen levels increased after treatment, reflecting microbial respiration and aeration efficiency. Electrical conductivity showed a moderate decline, consistent with partial removal of ionic substances, as expected in activated sludge systems.
Nutrients also displayed clear reductions: Ammonium and nitrite decreased markedly, confirming effective nitrification, while total phosphorus and orthophosphate exhibited moderate decreases, in line with conventional activated sludge systems that do not include enhanced nutrient removal. Suspended solids were substantially reduced, and both COD and BOD5 showed removal efficiencies above 90%, underscoring the strong capacity of this system for organic matter elimination. These results demonstrate that WWTP-1 achieves effluent quality that meets most Algerian standards.
At WWTP-2, water temperatures decreased slightly, while pH increased, remaining within acceptable discharge limits—likely due to aeration and carbonate equilibrium effects; dissolved oxygen in lagoon systems. Electrical conductivity showed only a minor reduction, indicating limited removal of dissolved ions.
Nutrient removal was moderate: Ammonium and nitrite decreased slightly, and phosphorus reduction remained limited. Suspended solids removal was high, highlighting the efficiency of natural settling in lagoon systems. However, organic matter removal was weaker than in WWTP-1, with COD and BOD5 reductions not sufficient to comply with national discharge standards. Overall, WWTP-2 offers cost-effective treatment but with notable limitations compared with activated sludge.
3.2. Pharmaceutical Concentration
The analysis confirmed significant contamination with pharmaceuticals across all treatment stages, with concentrations ranging from the ng/L to µg/L scale. Compounds were detected not only in influents and effluents but also in sludge, demonstrating their persistence even after conventional wastewater treatment. The ten pharmaceuticals investigated covered multiple therapeutic classes (antibiotics, NSAIDs, analgesics, beta-blockers, anticonvulsants, and antihistamines), underscoring the complexity of pharmaceutical pollution in municipal wastewaters.
The chromatographic profiles obtained by UPLC-HR-QTOF-MS are presented in Figure A1 (Appendix A), showing retention times and compound-specific peaks.
Influent concentrations in WWTP-1 varied widely, with ibuprofen (2.34 µg/L) and diclofenac (1.29 µg/L) standing out as the dominant compounds, followed by naproxen and acetaminophen at lower levels. Among antibiotics, sulfamethoxazole was present (~32 ng/L), while ofloxacin was not detected. Psychotropic drugs such as carbamazepine (~20 ng/L), sulpiride, and ketotifen were consistently detected but at lower concentrations.
In the effluent, some pharmaceuticals showed effective removal, with sulfamethoxazole and atenolol often falling below quantification limits. However, others persisted at notable levels. Ibuprofen even increased significantly, reaching 6.43 µg/L (p < 0.001), likely due to desorption from sludge or reconversion of conjugated metabolites. Diclofenac (~270 ng/L) and carbamazepine (~45 ng/L) also remained detectable, confirming their recalcitrant nature.
Secondary effluents still contained multiple compounds, with ibuprofen being the most abundant, followed by naproxen and diclofenac. In sludge, diclofenac (19.3 µg/L) and ibuprofen (8.9 µg/L) showed high sorption, while antibiotics like sulfamethoxazole were also concentrated. This highlights the dual role of sludge as both a sink and a potential secondary source of pharmaceuticals. Detailed results are illustrated in Figure 3.
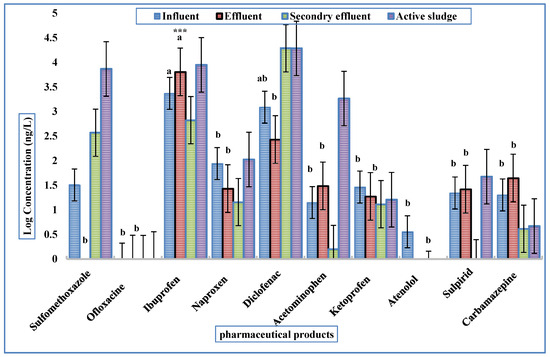
Figure 3.
Mean concentrations of PhACs detected in WWTP-1 (*** significant difference at p < 0.001, a and b significant differences among treatments according to Tukey’s HSD test at p < 0.001).
In the influent of WWTP-2, diclofenac (705 ng/L) was the most prominent compound, followed by ibuprofen (234 ng/L), acetaminophen (~96 ng/L), and naproxen. Antibiotics such as sulfamethoxazole (~6 ng/L) were present, whereas ofloxacin was not detected. Carbamazepine and sulpiride appeared consistently, with moderate concentrations compared to NSAIDs.
Effluent samples showed that several pharmaceuticals persisted or even increased. Diclofenac reached almost 1 µg/L, while ibuprofen and naproxen remained in the range of hundreds of ng/L. In some cases, concentrations in effluents were higher than in influents, pointing to desorption or transformation processes during lagoon treatment.
Secondary effluents confirmed limited removal: NSAIDs (diclofenac, naproxen, ibuprofen) remained dominant, while hydrophilic compounds like acetaminophen were reduced more effectively. Nevertheless, the persistence of carbamazepine and sulpiride indicates the limitations of lagoon systems in tackling recalcitrant pharmaceuticals. The results for WWTP-2 are shown in Figure 4.
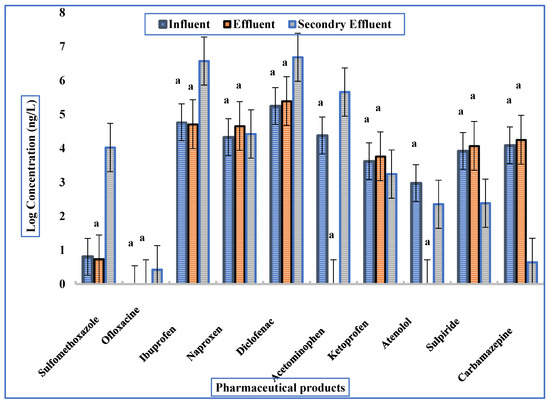
Figure 4.
Mean concentrations of PhACs detected in WWTP-2. Difference letters significant differences among treatments according to Tukey’s HSD test at p < 0.001.
3.3. Estimation of Removal Efficiency
The persistence of certain PhACs in effluent samples and active sludge after treatment highlights the challenges of achieving complete removal during wastewater treatment. The variability in removal efficiencies (RE) calculated for each compound is summarized in Figure 5 and Figure 6.
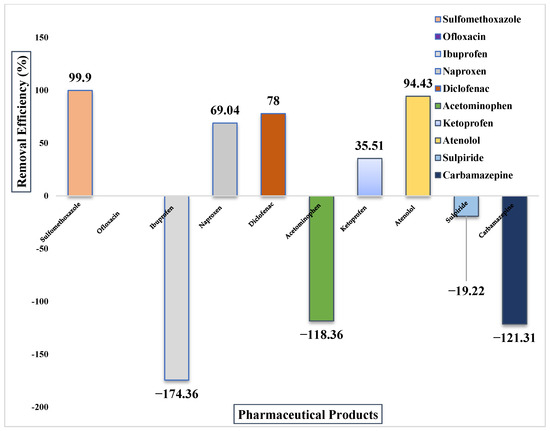
Figure 5.
Removal efficiency of PhACs detected in WWTP-1.
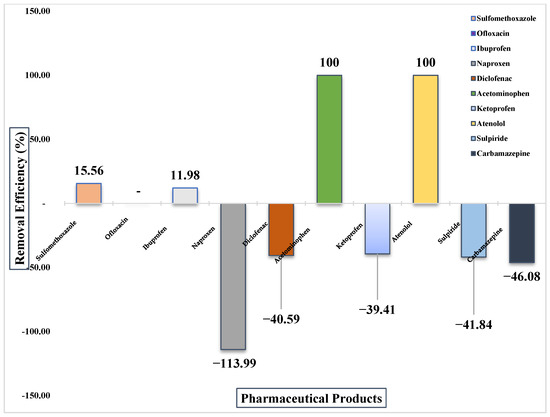
Figure 6.
Removal efficiency of PhACs detected in WWTP-2.
WWTP-1 showed highly variable removal performance. Some antibiotics achieved near-complete elimination, with sulfamethoxazole reaching ~100% removal and atenolol >90%. Similarly, ofloxacin was fully removed from the influent.
In contrast, several compounds exhibited negative removal efficiencies. Ibuprofen presented the most extreme case, with a RE of −174%, while acetaminophen and carbamazepine also increased in effluent compared to influent, consistent with desorption or reconversion of metabolites.
Diclofenac and naproxen achieved moderate removals (~78% and ~64%, respectively), while ketoprofen was only partially reduced (~36%). These findings confirm that although activated sludge can effectively remove biodegradable pharmaceuticals, persistent compounds such as carbamazepine and certain NSAIDs remain problematic. Detailed results are shown in Figure 5.
At WWTP-2, removal performance was even more inconsistent. Some compounds, including atenolol, acetaminophen, and ofloxacin, showed 100% removal, confirming the potential of lagoon systems for easily biodegradable substances.
However, many target compounds presented negative RE values, indicating higher concentrations in effluents than in influents. Diclofenac (−41%), naproxen (−114%), carbamazepine (−46%), and sulpiride (−42%) exemplified this pattern, reflecting the persistence and possible transformation of conjugated metabolites back into parent compounds.
Sulfamethoxazole and ibuprofen showed only modest removals (~16% and ~12%, respectively), with significant residual concentrations in the final effluent. These results highlight the lagoon system’s limited ability to address pharmaceuticals, particularly those with low biodegradability. The results are presented in Figure 6.
3.4. Comparison and Correlation of PhACs Between WWTP-1 and WWTP-2
3.4.1. Comparison Between Concentrations
All ten pharmaceuticals were detected in both WWTPs, though with different behaviors. Concentrations in WWTP-2 were generally higher than in WWTP-1, except for sulfamethoxazole, ibuprofen, and diclofenac, which were more prominent in the activated sludge system. Secondary effluents from WWTP-2 consistently exhibited elevated levels of most compounds, with carbamazepine and naproxen being similar across both plants.
In both facilities, the most abundant compounds belonged to NSAIDs (diclofenac, ibuprofen, and naproxen), followed by acetaminophen, the antibiotic sulfamethoxazole, and psychotropic drugs such as carbamazepine and sulpiride. Ofloxacin was not detected in influents, suggesting limited use or discharge into these systems.
Statistical analyses confirmed significant differences (p < 0.001) between influent and effluent concentrations at WWTP-1. Ibuprofen stood out with the highest influent levels and a persistent presence in the effluent, while diclofenac showed moderately persistent behavior. In WWTP-2, several compounds presented negative removal efficiencies, particularly diclofenac, naproxen, ketoprofen, carbamazepine, and sulpiride, suggesting re-release of conjugated metabolites, internal recirculation within lagoons, or additional external contamination.
Overall, WWTP-1 demonstrated more consistent removal, while WWTP-2 struggled with several persistent or recalcitrant compounds. These findings emphasize the inadequacy of conventional processes alone to address pharmaceutical pollution and highlight the need for advanced technologies such as ozonation, activated carbon, or membrane filtration.
3.4.2. Analysis Using Principal Component Analysis (PCA)
Principal Component Analysis (PCA) was used to explore similarities and differences in pharmaceutical concentrations across influent and effluent samples from both WWTPs.
At WWTP-1 (Figure 7A), PC1 explained 94.75% of the variance, mainly driven by high concentrations of diclofenac and ibuprofen in influent samples. Effluents showed more homogeneous compositions, with most compounds reduced but still persistent. Ibuprofen was unusual in displaying higher effluent than influent concentrations, consistent with reconversion from conjugates. The clustering analysis (Figure 7B) grouped carbamazepine, sulpiride, and ibuprofen, highlighting their resistance to removal.
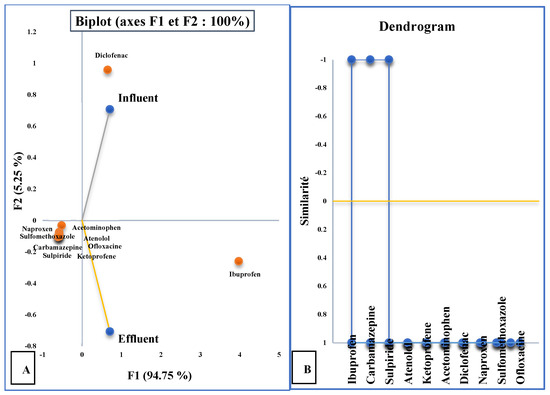
Figure 7.
(A) Graphical representation of the “molecule” variables in the different types of water in WWTP-1 and (B) cluster analysis dendrogram ( samples;
samples;  Pharmaceutical contaminants).
Pharmaceutical contaminants).
 samples;
samples;  Pharmaceutical contaminants).
Pharmaceutical contaminants).
In WWTP-2 (Figure 8A), PC1 explained 99% of the variance, strongly influenced by sulfamethoxazole, diclofenac, ketoprofen, atenolol, carbamazepine, and sulpiride. Effluent concentrations showed limited differentiation from influents, indicating poor overall removal. Diclofenac was particularly distinct, separating clearly in PCA space due to its persistence. PC2 (1% variance) was more associated with ibuprofen, naproxen, and acetaminophen, which showed moderate reductions. Cluster analysis (Figure 8B) grouped carbamazepine and sulpiride together, reflecting their similar persistence and resistance to degradation.
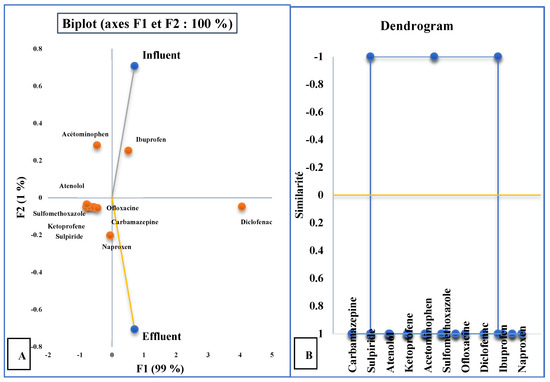
Figure 8.
(A) Graphical representation of the “molecule” variables in the different types of water in WWTP-2 and (B) cluster analysis dendrogram ( samples;
samples;  Pharmaceutical contaminants).
Pharmaceutical contaminants).
 samples;
samples;  Pharmaceutical contaminants).
Pharmaceutical contaminants).
3.4.3. Correlation Between Macropollution and Micropollution
Correlation analysis between pharmaceuticals and physicochemical parameters (COD, BOD5, NH4+, PO43−) revealed weak relationships at both plants. The correlation coefficients were 0.050 for WWTP-1 and 0.051 for WWTP-2, suggesting that micropollutant removal is largely independent of conventional water quality parameters. Although a slight trend indicated that some pharmaceuticals decreased as organic load increased, this relationship was negligible. These results confirm the need for targeted treatment processes to specifically remove micropollutants (Figure 9).

Figure 9.
Analysis of correlations between physicochemical parameters and outgoing macropollution (COD, BOD, NH4+, PO4−, Pt) and micropollutants (PhACs) of (WWTP-1 and WWTP-2).
4. Discussion
In the current study, the results of the temperature measurements in November 2023 conformed to the Algerian norms for wastewater, which specify a maximum temperature of 30 °C [26]. Seasonal variations can strongly influence wastewater temperature, as reported by El Morhit et al. [42], affecting the kinetics of organic matter degradation, mineralization [43], and accelerating contaminant decomposition [44].
The neutral pH values observed in wastewater samples were within the threshold re-quired for effluent discharge into receiving waters, consistent with JORA [26]. Previous studies have shown that pH varies across treatment stages [17] but generally remains within the range that supports bacterial growth essential for the biological breakdown of organic pollutants [45].
Changes in dissolved oxygen levels between influent and effluent are mainly due to differences in oxygen consumption, microbial respiration limitations, and temperature fluctuations [46,47]. Oxygen availability also plays an important role in abiotic decomposition, since processes like chemical oxidation can degrade organic com-pounds even in the absence of biological activity [44,48].
No significant changes in electrical conductivity were observed between influent and effluent at either WWTP, consistent with the findings of Sadik et al. [49]. This suggests that the ionic composition of treated water remains stable, with minor variations possible linked to specific pollutant inputs [50].
Regarding conventional pollution indicators, both plants achieved significant removal efficiencies for SS, COD, and BOD, demonstrating satisfactory organic matter degradation [51]. However, total phosphorus concentrations exceeded national and international standards (<2 mg/L), confirming the limited nutrient removal expected in conventional systems. Reductions in ammonium concentrations were mainly driven by nitrification, influent load, and treatment performance, corroborating the role of biological processes in nitrogen removal [52,53].
Recent analytical advances have enabled the detection of trace concentrations of PhACs in wastewater and other environmental matrices [54]. In this study, ten PhACs from major therapeutic classes—including antibiotics, NSAIDs, analgesics, antiepileptics, beta-blockers, and antihistamines—were detected at levels ranging from ng/L to µg/L. These results are consistent with those of Bodík et al. [55], who reported comparable global occurrences of PhACs in wastewater. Monitoring studies across Europe and Africa confirm that these therapeutic groups are the most frequently detected in WWTP effluents [56,57].
The presence of PhACs in both WWTPs can be explained by their shared inputs of hospital and domestic wastewater, as also observed by Gewurtz et al. [54]. WWTP-2 additionally receives agricultural runoff, which increases its pollutant load. Mourad et al. [58] found similar results in Fresnoy lagoons, where agricultural inputs caused significantly elevated pharmaceutical concentrations.
Among antibiotics, sulfamethoxazole was detected in both influent and effluent samples, consistent with the findings of Moslah et al. [59]. Previous studies confirm that sulfonamides and their metabolites are highly prevalent in wastewater worldwide [60,61]. Concentrations reported elsewhere vary widely, from hundreds of ng/L in Europe to tens of µg/L in African countries [62,63]. This antibiotic was detected in the order of 3600 ng/L in Ghana [64], 53.86–56.6 μg/L in Mozambique and Kenya [65,66], 5600 ng/L in Uganda [66], 1013.2 ng/L in Kwa-Zulu Natal, South Africa [66], and in concentrations reaching 77 μg/L in WWTPs of the Eastern Cape province of South Africa [66]. In this study, WWTP-1 achieved almost complete elimination of sulfamethoxazole (>99%), while WWTP-2 showed only partial removal (~16%), in agreement with reports that lagoon systems are less efficient for antibiotics [67,68]. By contrast, ofloxacin was not detected, which aligns with previous studies where its concentrations were typically near or below the LOQ [58,69].
NSAIDs were the most frequently detected compounds, with ibuprofen, diclofenac, and naproxen dominating influents and effluents, as observed in other studies [25,70]. Ibuprofen exhibited the highest concentrations, reflecting its global consumption and incomplete biodegradation in WWTPs [71]. Reported levels vary widely, from 10 µg/L in South African WWTPs [72] to 28 µg/L in Ghana [73]. The concentration of diclofenac was detected at 27.20 µg/L in Ghana [73] and at 9.68 μg/L in WWTP from Pretoria (South Africa) [74], Diclofenac, which was included in the EU Water Framework Directive Watch List, is particularly recalcitrant and has been repeatedly observed in treated effluents [25,75]. Naproxen also persisted in WWTP-2, likely due to its ionization at neutral pH and weak sorption onto sludge, as reported previously [76,77]. The concentration of naproxen was detected at 7.50 μg/L in Gauteng WWTP (South Africa) [74], 9.68 µg/L in WWTP from Pretoria (South Africa) [74], 20.8 µg/L in WWTP samples from KwaZulu-Natal (South Africa) [78], and between 0.079 and 3.6 in WWTPs from Assiut governorate (Egypt) [79]. This class of drugs is particularly persistent and partially removed by conventional systems [80]. Our findings regarding NSAIDs showed that they were the most commonly detected compounds in wastewater samples. Similar to the findings reported by Stec et al. [81] and Ashiwaju et al. [82], it was concluded that Ibuprofen, Diclofenac, and Naproxen were frequently found in both WWTPs. To our knowledge, there are no statistical studies concerning the consumption rates of pharmaceutical products in Algeria. However, according to the global consumption figures, NSAIDs are the most widely consumed, which should be the same for Algeria.
Acetaminophen was detected at µg/L levels in WWTP-1, similar to findings from Togola et al. [83] and Gros et al. [84]. In samples of wastewater from Ghana, paracetamol reached 22.03 µg/L [73] and 15.9 µg/L in the WWTP of Assiut governorate (Egypt) [79]. In WWTP-2, however, it showed negative removal, while WWTP-1 completely eliminated it. This is consistent with studies showing high variability in acetaminophen removal, from 38% to complete elimination, depending on treatment conditions [85]. Activated sludge systems are generally more effective in achieving >90% removal [86].
Ketoprofen was present at low ng/L levels but displayed negative removal in WWTP-2. Similar results have been reported in Algeria [77] and South Africa [74], confirming the difficulty of removing this compound in lagoons. Naproxen and diclofenac also exhibited negative removal efficiencies, which can be explained by deconjugation of metabolites or desorption processes [87,88].
Carbamazepine is well known as a refractory compound, with removal rates typically <10% in conventional plants [89]. Sulpiride was also poorly eliminated, in agreement with earlier findings [90].
Beta-blockers such as atenolol showed better removal in WWTP-1 (>90%), consistent with literature reports, although removal efficiencies vary widely depending on operational conditions [79]. Consequently, the removal of Atenolol in the present study showed similar results to those found by Hernando et al. [91], Maurer et al. [92], and Scheurer et al. [93], indicating that it can be detected in effluents, with removal rates ranging from 44.43% to 100%. In these cases, the removal could be influenced by their physicochemical properties, as literature suggests that the compounds with logKow values between 2.5 and 5 and high pKa values result in higher removal due to the excess sludge withdrawals [94]. However, its removal rate varies between 0% and 31% in other studies [95].
In this study, its removal efficiency was 99.98% by WWTP-1 and 15.56% by WWTP-2. The variation in removal efficiencies among PhACs indicates incomplete removal and varying degrees of biological transformation in WWTPs. Previous studies have report-ed low removal efficiencies for PhACs in conventional activated sludge processes [96,97]. Moreover, the removal rates of pharmaceuticals vary widely, from 35.52% to more than 90%, depending on the properties of the target molecule and the treatment applied [98,99]. Existing wastewater treatment processes are generally ineffective at removing most pharmaceutical contaminants, with removal rates ranging from 12.5% to 100% [100,101]. However, it was negatively removed in WWTP-2 com-pared to WWTP-1, where it was completely removed from effluent samples. In this study, acidic drugs such as Ibuprofen showed negative removal efficiencies in WWTP-1, and Naproxen, Diclofenac, and Ketoprofen in WWTP-2. Comparing our results to those of Kermia et al. [77] on samples from WWTP in Algeria, who reported that Ibuprofen can be eliminated up to 80%, Naproxen (73%), and Diclofenac (−174%). Thus, some products are eliminated from the human body unchanged, such as Aspirin, Acetaminophen, Ibuprofen, Naproxen, Ketoprofen, and Diclofenac, which are excreted at 14, 53, 10, 70, 80, and 10%, respectively [101]. Their degradation in wastewater treatment plants depends on the effectiveness of the biological treatment [81]. In general, Diclofenac is more detected in wastewater due to the low removal efficiency of wastewater treatment plants, as conventional activated sludge treatment processes [102,103].
Negative removal rates observed for certain compounds can result from metabolite deconjugation, desorption from sludge, or measurement uncertainties when concentrations are near detection limits [87,104]. These processes are particularly relevant for NSAIDs and psychotropic drugs, as supported by several mechanistic studies [105,106]. The persistence of these compounds highlights the limitations of conventional WWTPs, which are not designed to eliminate micropollutants [107]. In the present study, it is found that the behavior of acid PhACs (NSAIDs) such as naproxen, diclofenac, and the antihistamine (Ketoprofen) depend on their pKa and pH, which can transform these compounds between ionized and non-ionized forms, influencing their solubility, adsorption, and final concentration in the effluent. Therefore, both sorption and degradation affect the presence of this drug in the environment [108], although microbial transformation and deconjugation of PhAC glucuronides and active metabolites negatively impact treatment efficiency [109]; moreover, the conjugation–deconjugation phenomena of metabolites during treatment [110] result in their return to parent compounds. This phenomenon is supported by the decrease in the metabolite concentrations in the influent and the reappearance of their original compounds in the effluent [111], or incomplete degradation of PhAC residues can lead to the formation of intermediate metabolites [112]. Consequently, conjugated metabolites can release active substances back into the environment [113]; conversely, an underestimation of the actual quantities of pharmaceuticals adsorbed by the particulate phase and then eliminated by the filtration step and/or by their desorption during treatment in these WWTPs [114]. In addition, the effluent concentrations exceeding influent values may be produced via other factors are such as relationships with measurement errors, un-certainties in the data analysis, exacerbated by the prevalence of values falling below the detection limit (limit of quantification, LQ), sample contamination, or secondary release from biofilms/sludge. Moreover, the complexity of the influent matrix, often referred to as “matrix dirt”, amplifies the LQ, potentially leading to the recording of negative removal efficiencies. These underestimates of the interactions between the micropollutants and the wastewater matrix, particularly the solubility and sorption–desorption processes involving the wastewater solids and activated sludge [115]. Also, variations in the WWTP system (sludge retention time, hydraulic retention time, redox conditions, and pH) play a fundamental role in the efficiency of elimination processes [116]. This allowed for the evaluation of the performance of these two WWTPs in re-moving the investigated pharmaceuticals during the wastewater treatment process.
Factors affecting removal efficiency include the chemical structure and properties of the pharmaceutical compounds, the specific treatment processes employed, and wastewater residence time at different WWTPs [114,117]. In addition, the high concentrations of ibuprofen and naproxen in effluents are the consequence of the wide consumption of these molecules with or without prescription, leading to their permanent presence in the effluents, which is considered as pseudo-persistence because of their continual discharge [118].
The detection of PhACs in sludge confirms their sorption capacity, especially for hydrophobic molecules such as diclofenac and carbamazepine [119,120]. This creates a secondary risk of environmental release if sludge is not properly managed. The partitioning of pharmaceuticals between liquid and solid phases depends on hydrophobicity (logKow), ionization, and electrostatic interactions [121]. In this case, a significant part, the fraction adsorbed by the sludge, was estimated due to their high adsorption property on the particles, and this was the case for some hydrophobic compounds that produce electrostatic interactions with particles and microorganisms. In this study, the presence of PhACs in this fraction is higher compared to those found in the study re-ported by Kermia et al. [77] and performed at the Beni Messous WWTP station in Alge-ria for Diclofenac (388 ng/L) and Naproxen (13.35 ng/g) in WWTPs of the Eastern Cape province of South Africa [66]. Acetaminophen (855 ng/L), and for the other drugs, namely, Ibuprofen, Ketoprofen, and Naproxen, it was indicated that their absence was in the particulate phase.
Various studies worldwide have used multivariate statistical analyses, such as Principal Component Analysis (PCA) and Hierarchical Ascendant Classification (HAC), to gain insights into the relationships between quality parameters and pollution sources in wastewater treatment data that influence wastewater quality [122,123]. This technique helps establish a relationship between WWTP and micropollution in wastewater treatment data [123]. It identifies the variables that con-tribute the most to effluent quality, providing crucial information for operators and decision-makers to modify treatment processes accordingly [123].
Multivariate statistical analyses, such as PCA, provided further insights. In WWTP-1, diclofenac and ibuprofen contributed most to influent–effluent differentiation, clustering with carbamazepine and sulpiride as persistent pollutants. In WWTP-2, PCA con-firmed the general persistence of pharmaceuticals and limited differentiation between influent and effluent. These findings are in line with previous applications of PCA to wastewater datasets, which have highlighted the role of physicochemical properties and treatment configuration in removal variability [66,118]. The variation in removal efficiency between PhACs found in the present study means that the incomplete removal and magnitude of the biological transformation of PhACs in WWTPs vary considerably from one compound to another. As a result, the presence of certain PhACs at the outlet of the WWTP indicates that the elimination of the dis-solved phase is incomplete [101].
The presence of different concentrations of PhACs analyzed between WWTP-1 and WWTP-2 in the present study can be explained by the fact that the treatment processes are not the same. The treatment of these two wastewater treatment plants has proven to be ineffective in treating certain types of micropollutants, as several studies have shown [122,123].
Concentrations similar to those presented here have been reported by several monitoring programs conducted in different countries where they analyzed urban wastewater [69]. Concisely, the differences in PhAC concentrations between WWTP-1 and WWTP-2 can be attributed to variations in treatment processes. General approaches for wastewater treatment include physicochemical and aerobic/anaerobic biological treatments [124,125], which are often insufficient for removing pharmaceuticals, including antibiotics. Combining different treatment technologies may be necessary to improve removal efficiency [126,127].
Overall, the persistence of pharmaceuticals across both treatment systems under-scores the urgent need for improved wastewater management strategies. Activated sludge systems (WWTP-1) outperformed lagoons (WWTP-2), but neither achieved complete elimination. Advanced treatment technologies such as ozonation, activated carbon, or membrane filtration are increasingly recognized as necessary to achieve ad-equate removal [128].
5. Limitations and Future Perspectives
This study provides valuable insights into the occurrence and removal efficiency of pharmaceutical compounds in two wastewater treatment plants located in Mascara, Algeria. However, several limitations should be acknowledged. First, the monitoring campaign was limited to a single sampling period (November 2023), which restricts the interpretation of seasonal variability. Future studies should include multi-seasonal monitoring to better capture the influence of temperature and hydrological changes on treatment performance.
Second, the analysis was restricted to ten representative pharmaceuticals belonging to different therapeutic classes. While these compounds provide an informative overview, expanding the monitoring to a broader range of pharmaceuticals, including metabolites and transformation products, would give a more comprehensive understanding of micropollutant dynamics.
Third, although removal efficiencies were evaluated, the study did not include an ecotoxicological risk assessment. Future research should integrate bioassays and risk-based approaches to better assess the environmental and human health implications of residual pharmaceutical loads.
Finally, the study focused on conventional technologies (activated sludge and aerated lagoon) that were not specifically designed for micropollutant removal. Future investigations should explore the application of advanced and hybrid treatment systems—such as ozonation, activated carbon adsorption, and membrane filtration—to enhance removal efficiency. Combining conventional processes with these emerging techniques will be essential to meet stricter future regulations on micropollutants in wastewater effluents.
6. Conclusions
This study presents the first comparative evaluation of pharmaceutical and conventional pollutants in two wastewater treatment plants (WWTPs) in Mascara, Algeria—a semi-arid region where data remain scarce. By combining physicochemical characterization, targeted pharmaceutical analysis, and multivariate statistics, the work provides new insights into the performance and limitations of two representative treatment technologies: activated sludge (WWTP-1) and aerated lagoons (WWTP-2).
The results show that WWTP-1 achieved higher removal efficiencies for most macropollutants and certain pharmaceuticals, particularly sulfamethoxazole and atenolol. However, both systems exhibited limited or even negative removal of persistent compounds such as carbamazepine, diclofenac, and ibuprofen, which remained in effluents and sludge. These findings confirm that conventional treatments alone are insufficient for tackling recalcitrant pharmaceuticals and may contribute to secondary contamination pathways.
This study was restricted to ten PhACs and one sampling campaign. Broader monitoring, including seasonal variations and a wider range of compounds, is necessary. Future research should also assess ecotoxicological risks and evaluate advanced technologies such as ozonation, activated carbon, and membrane filtration under local conditions.
Author Contributions
S.S.: conceptualization, methodology, investigation, visualization, validation, data curation, and writing—original draft preparation. S.M.: supervision. I.M.-A.: conceptualization, methodology, and reviewing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Universidad Católica de Murcia (UCAM) for facilitating the short stay and extend our gratitude to Lissette Diaz Gamboa from UCAM for her invaluable assistance in the laboratory. Our sincere appreciation goes to José Enrique Yuste of the Centre of Biology (CEBAS), Murcia, Spain, for his expert assistance with the sample analysis using UPLC-HR-QTOF-MS.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| JORA | Official Journal of Reject Algerian |
| LOD | Limit of detection |
| LogKow | Octanol–water partition coefficient |
| LOQ | Limit of quantification |
| NSAIDs | Anti-inflammatory non-steroidal |
| PhACs | Pharmaceutically active compounds |
| SPE | Solid-phase extraction |
| UPLC-HR-QTOF-MS | Ultra-Performance Liquid Chromatography coupled with High-Resolution Quadrupole Time-of-Flight Mass Spectrometry |
| WWTP | Wastewater treatment plant |
Appendix A
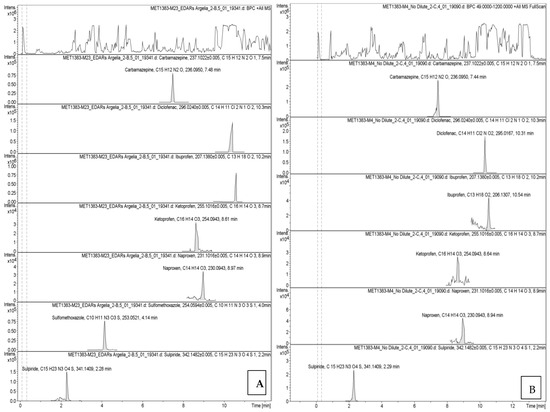
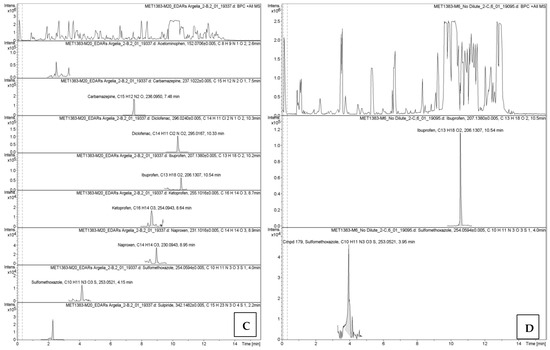
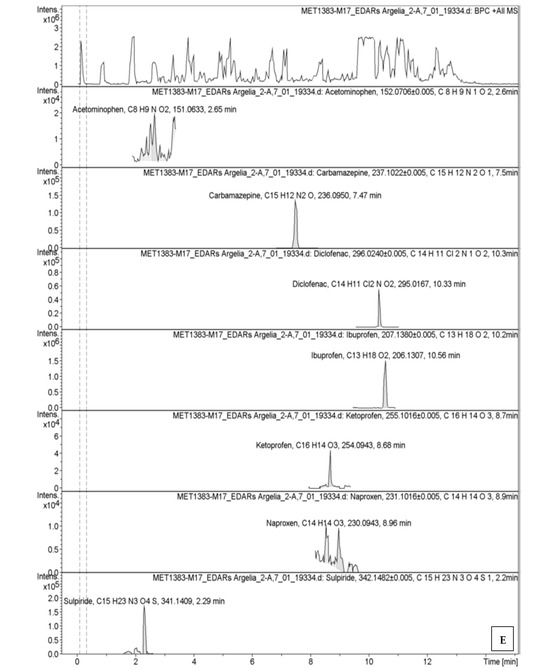
Figure A1.
Graphical display of absorbance versus retention time for pharmaceuticals and their respective peaks from UPLC-HR-QTOF-MS analysis: (A) influent (WWTP-1), (B) effluent (WWTP-1), (C) influent (WWTP-2), (D) effluent (WWTP-2), and (E) sludge (WWTP-1).
References
- Hashem, M.S.; Qi, X. Treated wastewater irrigation—A review. Water 2021, 13, 1527. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Hoekstra, A.Y. Four billion people facing severe water scarcity. Sci. Adv. 2022, 8, e1500323. [Google Scholar] [CrossRef]
- Guergueb, M.; Ferhat, F. la gestion des eaux en Algérie: Vers un nouveau paradigme. J. Adv. Econ. Res. 2021, 6, 304–315. [Google Scholar] [CrossRef]
- Derdour, A.; Bouanani, A.; Kaid, N.; Mukdasai, K.; Algelany, A.; Ahmad, H.; Menni, Y.; Ameur, H. Groundwater Potentiality Assessment of Ain Sefra Region in UpperWadi Namous Basin, Algeria Using Integrated Geospatial Approaches. Sustainability 2022, 14, 4450. [Google Scholar] [CrossRef]
- Foglia, A.; González-Camejo, J.; Radini, S.; Sgroi, M.; Li, K.; Eusebi, A.L.; Fatone, F. Transforming Wastewater Treatment Plants into Reclaimed Water Facilities in Water-Unbalanced Regions. An Overview of Possibilities and Recommendations Focusing on the Italian Case. J. Clean. Prod. 2023, 410, 137264. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2012, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Benstaali, I.; Talia, A.; Benadela, L. Optimized wastewater management and pharmaceutical removal in Algeria. Sustainability 2024, 16, 1765. [Google Scholar] [CrossRef]
- Awathale, S.N.; Kokare, D.M. Pharmaceutical waste: A health risk for humans. In 360-Degree Waste Management; Elsevier: Amsterdam, The Netherlands, 2023; Volume 2, pp. 81–95. [Google Scholar] [CrossRef]
- Nebot, C.A.L.; Falcón, R.; Boyd, K.G.; Gibb, S.W. Introduction of human pharmaceuticals from wastewater treatment plants into the aquatic environment: A rural perspective. Environ. Sci. Pollut. Res. 2015, 22, 10559–10568. [Google Scholar] [CrossRef]
- Liu, N.; Jin, X.; Feng, C.; Wang, Z.; Wu, F.; Johnson, A.C.; Xiao, H.; Hollert, H.; Giesy, J.P. Ecological risk assessment of fifty pharmaceuticals and personal care products (PPCPs) in Chinese surface waters: A proposed multiple-level system. Environ. Int. 2020, 136, 105454. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116–455. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical pollution in aquatic environments: A concise review of environmental impacts and bioremediation systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Meyer, C.; Stravs, M.A.; Hollender, J. How wastewater reflects human metabolism suspect screening of pharmaceutical metabolites in wastewater influent. Environ. Sci. Technol. 2024, 58, 9828–9839. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.; Danish, W.; Waheed, M.; Sattar, M.; Ali, M.; Sudheer, N.; Zahra, M.; Mehmood, M. Relationship between zoonotic diseases and food safety. Int. J. Agric. Biosci. Zoonosis 2023, 1, 338–347. [Google Scholar] [CrossRef]
- El Marghani, A.; Pradhan, A.; Seyoum, A.; Khalaf, H.; Ros, T.; Forsberg, L.-H.; Nermark, T.; Osterman, L.; Wiklund, U.; Ivarsson, P.; et al. Contribution of pharmaceuticals, fecal bacteria and endotoxin to the inflammatory responses to inland waters. Sci. Total Environ. 2014, 488–489, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A review on photocatalysis used for wastewater treatment: Dye degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Ayilara, M.S.; Babalola, O.O. Bioremediation of Environmental Wastes: The Role of Microorganisms. Front. Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Paíga, P.; Santos, L.H.; Ramos, S.; Jorge, S.; Silva, J.G.; Delerue-Matos, C. Presence of pharmaceuticals in the Lis River (Portugal): Sources, fate and seasonal variation. Sci. Total Environ. 2016, 573, 164–177. [Google Scholar] [CrossRef]
- ISO 11352:2012; Water Quality—General Rules for the Establishment of Performance Characteristics of Analytical Methods. International Organization for Standardization: Geneva, Switzerland, 2012.
- Water Framework Directive. Common Implementation Strategy for the Water Framework Directive (2000/60/EC). Guidance Document. 2003; Volume 7. Available online: https://op.europa.eu/publication-detail/-/publication/95072480-dbe7-46cb-9d4f-d3e6e559ed87 (accessed on 16 September 2025).
- European Commission. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field ofWater Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission. Off. J. Eur. Union L. 2018, 141, 9–12. [Google Scholar]
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. 2013/39/EC. Available online: https://eur-lex.europa.eu/eli/dir/2013/39/oj/eng (accessed on 16 September 2025).
- Bouabdellah, Z.; Ziani, N. La Gestion des Stocks des Produits Pharmaceutiques dans un Établissement Public de Santé en Algérie: Une Réponse aux Attentes et Besoins des Malades. Cas du CHU de TIZI-OUZOU. Ph.D. Thesis, Université Mouloud Mammeri, Tizi Ouzou, Algeria, 2017. [Google Scholar]
- Gorito, A.M.; Silva, A.R.L.; Ribeiro, A.R.L.; Silva, A.M.T. Occurrence of micropollutants in surface waters: Monitoring of Portuguese Lima and Douro River estuaries and interconnecting northwest coast. Mar. Pollut. Bull. 2024, 209, 117140. [Google Scholar] [CrossRef]
- Vieno, N.; Tuhkanen, T.; Kronberg, L. Elimination of pharmaceuticals in sewage treatment plants in Finland. Water Res. 2007, 41, 1001–1012. [Google Scholar] [CrossRef]
- JORA. Journal Officiel de la République Algérienne; Normes Algériennes de Rejet des Eaux Résiduaires. Décret Exécutif n° 93-160 du 10 Juillet 1993 Fixant les Normes de Qualité des Eaux Usées. Alger, Algérie. 1993. Available online: https://www.joradp.dz/FTP/JO-FRANCAIS/2016/F2016015.pdf (accessed on 16 September 2025).
- ONA, Office National de L’assainissement, 2019, Algérie. Available online: https://apex.ona-dz.com/ords/r/dpmg/o-n-a/home (accessed on 16 September 2025).
- ISO 5667-10:2020; Water Quality—Sampling—Part 10: Guidance on Sampling of Waste Water. International Organization for Standardization: Geneva, Switzerland, 2020.
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Algerian Ministry of Water Resources. Normes Algériennes de Qualité des Eaux Destinées à la Consommation Humaine. Off. J. Alger. 2012. [Google Scholar]
- National Center for Biotechnology Information. PubChem. U.S. National Library of Medicine. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 16 September 2025).
- Riva, F.; Zuccato, E.; Castiglioni, S. A multi-residue analytical method for extraction and analysis of pharmaceuticals and other selected emerging contaminants in sewage sludge. Anal. Methods 2021, 13, 526–535. [Google Scholar] [CrossRef]
- Jewell, K.S.; Wick, A.; Ternes, T.A. Comparisons between abiotic and biotic transformation of pharmaceuticals in wastewater treatment. Environ. Sci. Technol. 2016, 50, 6231–6241. [Google Scholar] [CrossRef]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017, 316, 332–340. [Google Scholar] [CrossRef]
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization/International Electrotechnical Committee: Geneva, Switzerland, 2006.
- Carvalho, G. Bioaugmentation for the Removal of the Antibiotic Sulfamethoxazole in Wastewater Treatment Plants. Ph.D. Thesis, Faculdade de Ciências e Technologia, Universidade Nova de Lisboa, Lisbon, Portugal, 2018. Available online: https://run.unl.pt/bitstream/10362/45215/1/Nguyen_2018.pdf (accessed on 16 September 2025).
- Díaz-Gamboa, L.; Martínez-López, S.; Ayuso-García, L.M.; Lahora, A.; Martínez-Alcalá, I. Can Lagoons Serve as a Quaternary Treatment for Micropollutants in Wastewater Treatment Plants? Recent Implications for Compliance with the New Urban Wastewater Treatment Directive. Environments 2024, 11, 105. [Google Scholar] [CrossRef]
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Mouhtady, O.; Obeid, E.; Abu-Samha, M.; Younes, K.; Murshid, N. Evaluation of the adsorption efficiency of graphene oxide hydrogels in wastewater Dye removal: Application of principal component analysis. Gels 2022, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Lata, R.; Thakur, N.; Bajala, V.; Chandra Kuniyal, J.; Kumar, K. Application of multivariate statistical analysis and water quality index for quality characterization of Parbati river, Northwestern Himalaya. Discov. Water. 2021, 1, 5. [Google Scholar] [CrossRef]
- Hussain, M.T. Hydrochemical evaluation of groundwater in the Blue Nile Basin, eastern Sudan, using conventional and multivariate techniques. Hydrogeol. J. 2004, 12, 144–158. [Google Scholar] [CrossRef]
- Morhit, M.; El Blidi, S.; Moukrim, A.; Belghyti, D. Étude de l’évolution spatio-temporelle des paramètres hydrologiques caractérisant la qualité des eaux de l’estuaire du Loukkos (Maroc). Bull. L’Inst. Sci. 2012, 34, 151–162. [Google Scholar]
- Moussaoui, T.; Derdour, A.; Hosni, A.; Ballesta-de los Santos, M.; Legua, P.; Pardo-Picazo, M.Á. Assessing the quality of treated wastewater for irrigation: A case study of Ain Sefra Wastewater treatment plant. Sustainability 2023, 15, 11133. [Google Scholar] [CrossRef]
- Alisawi, H.A.O. Performance of Wastewater Treatment during Variable Temperature. Appl. Water Sci. 2020, 10, 89. [Google Scholar] [CrossRef]
- Kadouche, S.; Hammoum, H.; Ghedamsi, H.; Si Tahar, L. Assessment of purifying performance of a wastewater filtration basin—Case study. J. Water Sci. 2018, 31, 387–398. [Google Scholar] [CrossRef][Green Version]
- Nouha, K.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. EPS producing microorganisms from municipal wastewater activated sludge. Civ. Eng. 2016, 7, 2. [Google Scholar] [CrossRef]
- Xu, S.; Yao, J.; Ainiwaer, M.; Hong, Y.; Zhang, Y. Analysis of bacterial community structure of activated sludge from wastewater treatment plants in winter. Biomed Res. Int. 2018, 2018, 8278970. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M.N. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Berglund, B.; Fick, J.; Lindberg, R.; Järhult, J. Abundance and Dynamics of Antibiotic Resistance Genes and Integrons in Lake Sediment Microcosms. PLoS ONE 2014, 9, e108151. [Google Scholar] [CrossRef] [PubMed]
- Lara-Martín, P.A.; González-Mazo, E.; Petrovic, M.; Barceló, D.; Brownawell, B.J. Occurrence, Distribution and Partitioning of Nonionic Surfactants and Pharmaceuticals in the Urbanized Long Island Sound Estuary (NY). Mar. Pollut. Bull. 2014, 85, 710–719. [Google Scholar] [CrossRef]
- Henze, M.; van Loosdrecht, M.C.M.; Ekama, G.A.; Brdjanovic, D. Biological Wastewater Treatment: Principles, Modelling and Design; IWA Publishing: London, UK, 2008. [Google Scholar]
- Roy, D.; McEvoy, J.; Blonigen, M.; Amundson, M.; Khan, E. Seasonal variation and ex-situ nitrification activity of ammonia oxidizing archaea in biofilm-based wastewater treatment processes. Bioresour. Technol. 2017, 244, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Haidara, R.; Abdelbaki, C.; Badr, N. Feasibility of Water Reuse for Agriculture—Case Study of Ain Temouchent (Algeria). In Sustainable Energy-Water-Environment Nexus in Deserts, Proceeding of the First International Conference on Sustainable Energy-Water; Springer Nature: Berlin, Germany, 2022. [Google Scholar] [CrossRef]
- Gewurtz, S.B.; Auyeung, A.S.; Teslic, S.; Smyth, S.A. Pharmaceuticals and personal care products in Canadian municipal wastewater and biosolids: Occurrence, fate, and time trends 2010–2013 to 2022. Environ. Sci. Pollut. Res. 2025, 32, 5022–5039. [Google Scholar] [CrossRef]
- Bodík, I.; Mackuľak, T.; Fáberová, M.; Ivanová, L. Pharmaceuticals and illicit drugs in wastewater: Occurrence, removal and environmental risk in Slovakia. Environ. Sci. Pollut. Res. 2016, 23, 1144–1153. [Google Scholar] [CrossRef]
- Song, J.; Qu, B.; Li, X.; Yuan, H.; Li, N.; Duan, L. Carbon sinks/sources in the Yellow and East China Seas—Air-sea interface exchange, dissolution in seawater, and burial in sediments. Sci. China Earth Sci. 2018, 61, 1583–1593. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Moslah, B.; Hapeshi, E.; Jrad, A.; Fatta-Kassinos, D.; Hedhili, A. Pharmaceuticals and illicit drugs in wastewater samples in north-eastern Tunisia. Environ. Sci. Pollut. Res. 2017, 25, 18226–18241. [Google Scholar] [CrossRef]
- Mourad, S.; Ayoub, G.M.; Al Hindi, M.; Zayyat, R.M. Occurrence and hazard assessment of natural radioactivity in drinking water in South Lebanon. Environ. Monit. Assess. 2021, 193, 6. [Google Scholar] [CrossRef]
- Yuan, F.; Hu, C.; Hu, X.; Wei, D.; Chen, Y.; Qu, J. Photodegradation and toxicity changes of antibiotics in UV and UV/H2O2 process. J. Hazard. Mater. 2011, 185, 1256–1263. [Google Scholar] [CrossRef]
- Rolbiecki, D.; Harnisz, M.; Korzeniewska, E.; Jałowiecki, Ł.; Płaza, G. Occurrence of fluoroquinolones and sulfonamides resistance genes in wastewater and sludge at different stages of wastewater treatment: A preliminary case study. Appl. Sci. 2020, 10, 5816. [Google Scholar] [CrossRef]
- Lorenzo, P.; Adriana, A.; Jéssica, S.; Carles, B.; Marinella, F.; Marta, L.; Luis, B.J.; Pierre, S. Antibiotic resistance in urban and hospital wastewaters and their impact on a receiving freshwater ecosystem. Chemosphere 2018, 206, 70–82. [Google Scholar] [CrossRef]
- Mutiyar, P.K.; Mittal, A.K. Risk assessment of antibiotic residues in different water matrices in India: Key issues and challenges. Environ. Sci. Pollut. Res. 2014, 21, 7723–7736. [Google Scholar] [CrossRef]
- Azanu, D.; Styrishave, B.; Darko, G.; Weisser, J.J.; Abaidoo, R.C. Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Sci. Total Environ. 2018, 622, 293–305. [Google Scholar] [CrossRef]
- K’oreje, K.O.; Demeestere, K.; De Wispelaere, P.; Vergeynst, L.; Dewulf, J.; Van Langenhove, H. From multi-residue screening to target analysis of pharmaceuticals in water: Development of a new approach based on magnetic sector mass spectrometry and application in the Nairobi River basin, Kenya. Sci. Total Environ. 2012, 437, 153–164. [Google Scholar] [CrossRef]
- Netshithothole, R.; Madikizela, L.M. Occurrence of selected pharmaceuticals in the East London coastline encompassing major rivers, estuaries, and seawater in the Eastern Cape province of South Africa. ACS Meas. Sci. Au 2024, 4, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Bürgmann, H.; Sørum, H.; Norström, M.; Pons, M.-N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Tahrani, L.; Van Loco, J.; Mansour, H.B.; Reyns, T. Occurrence of antibiotics in pharmaceutical industrial wastewater, wastewater treatment plant and sea waters in Tunisia. J. Water Health 2016, 14, 208–213. [Google Scholar] [CrossRef]
- Oluwalana, A.E.; Musvuugwa, T.; Sikwila, S.T.; Sefadi, J.S.; Whata, A.; Nindi, M.M.; Chaukura, N. The screening of emerging micropollutants in wastewater in sol plaatje municipality, northern cape, South Africa. Environ. Pollut. 2022, 314, 120275. [Google Scholar] [CrossRef] [PubMed]
- Waleng, N.J.; Nomngongo, P.N. Occurrence of pharmaceuticals in the environmental waters: African and Asian perspectives. Environ. Chem. Ecotoxicol. 2022, 4, 50–66. [Google Scholar] [CrossRef]
- Zuriaga, E.; Lomba, L.; German, B.; Lanuza, P.M.; Aldea, L.; Ribate, M.P.; García, C.B.; Giner, B. Ecotoxicity in Aliivibrio fischeri of ibuprofen, omeprazole and their mixtures. Chem. Ecol. 2019, 35, 102–114. [Google Scholar] [CrossRef]
- Faleye, A.C.; Adegoke, A.A.; Ramluckan, K.; Fick, J.; Bux, F.; Stenstrom, T.A. Concentration and reduction of antibiotic residues in selected wastewater treatment plants and receiving waterbodies in Durban, South Africa. Sci. Total Environ. 2019, 678, 10–20. [Google Scholar] [CrossRef]
- Sackey, L.N.A.; Okobeng, A.; Obidieh, P.Y.; Ngala, F.-M.M.; Otoo, E.B.; Quartey, J.; Bentil, J.A.; Azanu, D.; Etikala, B. Risk Assessment of Pharmaceutical Contaminants in Pharmaceutical Wastewater. Sci. World J. 2024, 2024, 5538398. [Google Scholar] [CrossRef] [PubMed]
- Akawa, M.N.; Dimpe, K.M.; Nomngongo, P.N. Ultrasonic assisted magnetic solid phase extraction based on the use of magnetic waste-tyre derived activated carbon modified with methyltrioctyl ammonium chloride adsorbent for the preconcentration and analysis of non-steroidal anti-inflammatory drugs in wastewater. Arab. J. Chem. 2021, 14, 103329. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on pharmaceuticals in the aquatic environment: Occurrence, distribution and removal. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Kermia, A.E.; Fouial-Djebbar, D.; Trari, M. Occurrence, fate and removal efficiencies of pharmaceuticals in wastewater treatment plants: Case of Algeria. Environ. Monit. Assess. 2016, 188, 548. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466–467, 421–438. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Occurrence of ibuprofen, diclofenac and naproxen in wastewater and river water of KwaZulu-Natal Province in South Africa. Environ. Sci. Pollut. Res. 2017, 24, 24443–24459. [Google Scholar] [CrossRef]
- Stec, M.; Astel, A. Occurrence and toxicological assessment of six non-steroidal anti-inflammatory drugs (NSAIDs) in a wastewater treatment plant in Słupsk (Poland). Ecohydrol. Hydrobiol. 2024, 24, 523–534. [Google Scholar] [CrossRef]
- Ashiwaju, B.I.; Uzougbo, C.G.; Orikpete, O.F. Environmental Impact of Pharmaceuticals: A Comprehensive Review. Matrix Sci. Pharma 2023, 7, 85–94. [Google Scholar] [CrossRef]
- Togola, A.; Budzinski, H. Analytical development for analysis of pharmaceuticals in water samples by SPE and GC-MS. Anal. Bioanal. Chem. 2007, 388, 627–635. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Barceló, D. Tracing pharmaceutical residues of different therapeutic classes in environmental waters by using liquid chromatography/quadrupole-linear ion trap mass spectrometry and automated library searching. Anal. Chem. 2009, 81, 898–912. [Google Scholar] [CrossRef]
- Thiebault, T. L’adsorption des Produits Pharmaceutiques par Interactions Organominerales: Processus et Applications Environnementales. Ph.D. Thesis, Universite d’Orleans, Orléans, France, 2015. [Google Scholar]
- Sim, W.J.; Lee, J.W.; Oh, J.E. Occurrence and Fate of Pharmaceuticals in Wastewater Treatment Plants and Rivers in Korea. Environ. Pollut. 2010, 158, 1938–1947. [Google Scholar] [CrossRef]
- Yu, J.T.; Bouwer, E.J.; Coelhan, M. Occurrence and biodegradability studies of selected pharmaceuticals and personal care products in sewage effluent. Agric. Water Manag. 2006, 86, 72–80. [Google Scholar] [CrossRef]
- Ternes, T.A.; Joss, A.; Siegrist, H. Scrutinizing pharmaceuticals and personal care products in wastewater treatment. Environ. Sci. Technol. 2004, 38, 392A–399A. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plants—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef]
- Heberer, T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175–189. [Google Scholar] [CrossRef]
- Hernando, M.D.; Mezcua, M.; Fernández-Alba, A.R.; Barceló, D. Environmental risk assessment of pharmaceutical residues in wastewater effluents, surface waters and sediments. Talanta 2006, 69, 334–342. [Google Scholar] [CrossRef]
- Maurer, M.; Escher, B.I.; Richle, P.; Schaffner, C.; Alder, A.C. Elimination of beta blockers in sewage treatment plants. Water Res. 2007, 41, 1614–1622. [Google Scholar] [CrossRef]
- Scheurer, M.; Ramil, M.; Metcalfe, C.D.; Groh, S.; Ternes, T.A. The challenge of analyzing beta-blocker drugs in sludge and wastewater. Anal. Bioanal. Chem. 2010, 396, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Choubert, J.M.; Martin-Ruel, S.; Budzinski, H.; Miege, C.; Esperanza, M.; Soulier, C.; Lagarrigue, C.; Coquery, M. Evaluer les rendements des stations d’épuration. Tech. Sci. Méthodes 2011, 1, 44–62. [Google Scholar] [CrossRef]
- Mansouri, F.; Chouchene, K.; Roche, N.; Ksibi, M. Removal of pharmaceuticals from water by adsorption and advanced oxidation processes: State of the art and trends. Appl. Sci. 2021, 11, 6659. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. Illicit drugs and pharmaceuticals in the environment–Forensic applications of environmental data. Part 1: Estimation of the usage of drugs in local communities. Environ. Pollut. 2009, 157, 1773–1777. [Google Scholar] [CrossRef]
- Bartha, B.; Huber, C.; Schröder, P. Uptake and metabolism of diclofenac in Typha latifolia–how plants cope with human pharmaceutical pollution. Plant Sci. 2014, 227, 12–20. [Google Scholar] [CrossRef]
- Osorio, V.; Imbert-Bouchard, M.; Zonja, B.; Abad, J.-L.; Pérez, S.; Barceló, D. Simultaneous determination of diclofenac, its human metabolites and microbial nitration/nitrosation transformation products in wastewaters by liquid chromatography/quadrupole-linear ion trap mass spectrometry. J. Chromatogr. A 2014, 1347, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR at different sludge retention times. Water Res. 2011, 45, 243–250. [Google Scholar] [CrossRef]
- Tran, N.H.; Li, J.; Hu, J.; Ong, S.L. Occurrence and suitability of pharmaceuticals as markers of wastewater contamination in surface water and groundwater. Environ. Sci. Pollut. Res. 2014, 21, 4727–4740. [Google Scholar] [CrossRef]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.A.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Xia, Z.; Wang, Y.; Wu, Y.; Gong, Z. Rapid determination of phytosterols by NIRS and chemometric methods. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2019, 211, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Balakrishna, K.; Sinha, R.K.; Yamashita, N.; Balasubramanian, V.G.; Kannan, K. Mass loading and removal of pharmaceuticals and personal care products, including psychoactive and illicit drugs and artificial sweeteners, in five sewage treatment plants in India. J. Environ. Chem. Eng. 2015, 3, 2882–2891. [Google Scholar] [CrossRef]
- Ebele, A.J.; Oluseyi, T.; Drage, D.S.; Harrad, S.; Abdallah, M.A.E. Occurrence, seasonal variation and human exposure to pharmaceuticals and personal care products in surface water, groundwater and drinking water in Lagos State, Nigeria. Emerg. Contam. 2020, 6, 124–132. [Google Scholar] [CrossRef]
- Angeles, L.F.; Mullen, R.A.; Huang, I.J.; Wilson, C.; Khunjar, W.; Sirotkin, H.I.; McElroy, A.E.; Aga, D.S. Assessing Pharmaceutical Removal and Reduction in Toxicity Provided by Advanced Wastewater Treatment Systems. Environ. Sci. Water Res. Technol. 2019, 6, 62–77. [Google Scholar] [CrossRef]
- Heidler, J.; Halden, R. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ. Sci. Technol. 2008, 42, 6324–6332. [Google Scholar] [CrossRef]
- Brown, D.M.; Hughes, C.B.; Spence, M.; Bonte, M.; Whale, G. Assessing the suitability of a manometric test system for determining the biodegradability of volatile hydrocarbons. Chemosphere 2018, 195, 381–389. [Google Scholar] [CrossRef]
- Gulkowska, A.; Leung, H.; So, M.; Taniyasu, S.; Yamashita, N.; Yeung, L.W.; Richardson, B.J.; Lei, A.; Giesy, J.; Lam, P.K. Removal of antibiotics from wastewater by sewage treatment facilities in Hong Kong and Shenzhen, China. Water Res. 2008, 42, 395–403. [Google Scholar] [CrossRef]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the Degradation, Sorption, and Negative Mass Balances of Pharmaceuticals and Personal Care Products during Wastewater Treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef]
- Ejhed, H.; Fang, J.; Hansen, K.; Graae, L.; Rahmberg, M.; Magnér, J.; Dorgeloh, E.; Plaza, G. The Effect of Hydraulic Retention Time in Onsite Wastewater Treatment and Removal of Pharmaceuticals, Hormones and Phenolic Utility Substances. Sci. Total Environ. 2018, 618, 250–261. [Google Scholar] [CrossRef]
- Carmona, E.; Andreu, V.; Pico, Y. Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: From waste to drinking water. Sci. Total Environ. 2014, 484, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Coxon, S. Assessment of the Presence of Pharmaceuticals in, and Removal from, Municipal Wastewater in Aotearoa New Zealand. 2024. Available online: https://www.phfscience.nz/media/yb1lpzwm/esr-health-risk-assessment-pharmaceuticals-new-zealand-wastewater.pdf (accessed on 16 September 2025).
- Joss, A.; Zabczynski, S.; Göbel, A.; Hoffmann, B.; Löffler, D.; McArdell, C.S.; Ternes, T.A.; Thomsen, A.; Siegrist, H. Biological degradation of pharmaceuticals in municipal wastewater treatment: Proposing a classification scheme. Water Res. 2006, 40, 1686–1696. [Google Scholar] [CrossRef]
- Göbel, A.; McArdell, C.S.; Joss, A.; Siegrist, H.; Ternes, T.A. Fate of sulfonamides, macrolides, and trimethoprim in different wastewater treatment technologies. Sci. Total Environ. 2007, 372, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- El Aatik, A.; Navarro, J.M.; Martínez, R.; Vela, N. Estimation of global water quality in four municipal wastewater treatment plants over time based on statistical methods. Water 2023, 15, 1520. [Google Scholar] [CrossRef]
- Newhart, S. Psychometric Properties of the Family Adaptability and Cohesion Scale IV with College Students in the United States. J. Child Fam. Stud. 2024, 33, 3920–3932. [Google Scholar] [CrossRef]
- Rahmat, M.A.A.; Hamid, A.S.A.; Lu, Y.; Ishak, M.A.A.; Suheel, S.Z.; Fazlizan, A.; Ibrahim, A. An analysis of renewable energy technology integration investments in Malaysia using HOMER pro. Sustainability 2022, 14, 13684. [Google Scholar] [CrossRef]
- Abba, S.; Elkiran, G.; Nourani, V. Improving novel extreme learning machine using PCA algorithms for multi-parametric modeling of the municipal wastewater treatment plant. Desalination Water Treat. 2021, 215, 414–426. [Google Scholar] [CrossRef]
- Jelic, A.; Petrovic, M.; Barceló, D. Multi-residue method for trace level determination of pharmaceuticals in solid samples using pressurized liquid extraction followed by liquid chromatography/quadrupole-linear ion trap mass spectrometry. Talanta 2009, 80, 363–371. [Google Scholar] [CrossRef]
- Chang, S.H. Utilization of green organic solvents in solvent extraction and liquid membrane for sustainable wastewater treatment and resource recovery—A review. Environ. Sci. Pollut. Res. 2010, 27, 32371–32388. [Google Scholar] [CrossRef] [PubMed]
- Altaf, Q.-A.; Barnett, A.H.; Et Tahrani, A.A. Novel therapeutics for type 2 diabetes: Insulin resistance. Diabetes Obes. Metab. 2015, 17, 319–334. [Google Scholar] [CrossRef]
- Peng, X. China’s demographic history and future challenges. Science 2011, 333, 581–587. [Google Scholar] [CrossRef]
- Benedetti, L.; Langeveld, J.; Comeau, A.; Corominas, L.; Daigger, G.; Martin, C.; Mikkelsen, P.S.; Vezzaro, L.; Weijers, S.; Vanrolleghem, P.A. Modelling and monitoring of integrated urban wastewater systems: Review on status and perspectives. Water Sci. Technol. 2013, 68, 1203–1215. [Google Scholar] [CrossRef]
- Hou, J.; Chen, Z.; Gao, J.; Xie, Y.; Li, L.; Qin, S.; Wang, Q.; Mao, D.; Luo, Y. Simultaneous removal of antibiotics and antibiotic resistance genes from pharmaceutical wastewater using the combinations of up-flow anaerobic sludge bed, anoxic-oxic tank, and advanced oxidation technologies. Water Res. 2019, 159, 511–520. [Google Scholar] [CrossRef]
- Guo, X.; Pang, J.; Chen, S.; Jia, H. Sorption properties of tylosin on four different microplastics. Chemosphere 2018, 209, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.P.; von Gunten, U.; Siegrist, H. Elimination of organic micropollutants in a municipal wastewater treatment plant upgraded with a full-scale post-ozonation followed by sand filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).